Abstract
Acute megakaryoblastic leukemia (AMKL) in children without Down syndrome (DS) has an extremely poor outcome with 3-year survival of less than 40%, whereas AMKL in children with DS has an excellent survival rate. Recently, a novel recurrent translocation involving CBFA2T3 and GLIS2 was identified in about 30% of children with non-DS AMKL, and the fusion gene was reported as a strong poor prognostic factor in pediatric AMKL. We report the difficult clinical courses of pediatric patients with AMKL harboring the CBFA2T3-GLIS2 fusion gene.
Keywords: Acute megakaryoblastic leukemia without Down syndrome, CBFA2T3-GLIS2 fusion gene
Abstract
Down sendromu (DS) olmayan çocuklarda akut megakaryoblastik löseminin (AMKL) prognozu çok kötü ve 3 yıllık sağkalım %40’ın altında iken, DS’li çocuklarda AMKL’nin sağkalım oranı mükemmeldir. Yakın zamanda, DS olmayan AMKL’li çocukların yaklaşık %30’unda CBFA2T3 ve GLIS2’yi içeren yeni bir tekrarlayan translokasyon tanımlandı ve füzyon geninin pediatrik AMKL olgularında kötü prognoz ile ilişkili güçlü bir prognostik belirteç olduğu bildirildi. CBFA2T3-GLIS2 füzyon genini taşıyan AMKL tanılı pediatrik hastalarda sorunlu klinik seyri bildiriyoruz.
INTRODUCTION
Acute megakaryoblastic leukemia (AMKL) is classified as M7 in the FAB (French-American-British) classification. AMKL accounts for approximately 10% of pediatric acute myeloid leukemia (AML) cases and 1% of adult AML cases [1,2,3]. Pediatric AMKL is divided into two subgroups: AMKL arising in patients with Down syndrome (DS-AMKL), and AMKL arising in patients without DS (non-DS-AMKL). Although patients with DS-AMKL have an excellent survival rate, patients with non-DS-AMKL have an extremely poor outcome with 3-year survival of less than 40% [1,2,4]. Recently, two studies identified a novel recurrent translocation involving CBFA2T3 and GLIS2 in about 30% of children with non-DS-AMKL. The CBFA2T3-GLIS2 fusion gene was reported as a strong poor prognostic factor in pediatric AMKL [5,6]. We report the difficult clinical courses of two pediatric patients with AMKL harboring the CBFA2T3-GLIS2 fusion gene.
CASE PRESENTATION
Between 2003 and 2012, six patients were diagnosed with AMKL at the Department of Pediatrics of Yokohama City University Hospital. We analyzed the fusion gene, CBFA2T3-GLIS2, in the six leukemic samples at the time of diagnosis by reverse transcription polymerase chain reaction (PCR) and direct sequencing, according to a previous report [5]. We compared characteristics between the patients who were diagnosed with AMKL with or without the CBFA2T3-GLIS2 fusion gene.
Two patients had DS-AMKL harboring a GATA1 mutation and four had non-DS-AMKL. None of them had inv(16)/t(16;16) chromosomal abnormalities upon G-band karyotyping. Two patients with non-DS-AMKL (Patient 1 and Patient 3) had the CBFA2T3-GLIS2 fusion gene (Table 1). Reverse transcription PCR and direct sequencing revealed that exon 11 of CBFA2T3 was fused to exon 3 of GLIS2 in both cases (Figures 1A and 1B). Neither of them achieved complete remission (CR) after induction therapies. They died from the primary disease after stem cell transplantation (SCT). The other 4 patients remain alive in CR (Table 1).
Table 1. Patient details.
Figure 1. Clinical courses of two Acute megakaryoblastic leukemia patients with the CBFA2T3-GLIS2 fusion gene. A) Reverse transcription polymerase chain reaction for the CBFA2T3-GLIS2 fusion gene in our patients. Two patients with non-Down syndrome-acute megakaryoblastic leukemia (patients 1 and 3) had the CBFA2T3-GLIS2 fusion gene. NC: Negative control. B) Direct sequencing for the polymerase chain reaction product of the CBFA2T3-GLIS2 fusion gene in patient 1 revealed that exon 11 of CBFA2T3 was fused to exon 3 of GLIS2. C) Clinical course of patient 1. FLAG: Fludarabine, cytarabine, G-CSF; FK506: tacrolimus. D) Magnetic resonance imaging of patient 1 revealed an extramedullary lesion at the thoracic spinal cord (Th9). E) Clinical course of patient 3. CAG: Cytarabine, aclarubicin, G-CSF; GO: gemtuzumab ozogamicin; IDA: idarubicin; VPL: vincristine, prednisolone, L-asparaginase.
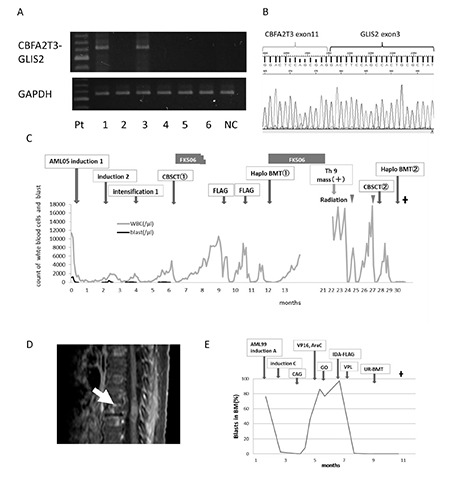
Patient 1 with the CBFA2T3-GLIS2 fusion gene was treated under the AML05 protocol of the Japanese Pediatric Leukemia/Lymphoma Study Group [7] and could not achieve CR after induction 1 therapy (Figure 1C). After induction 2 therapy, Patient 1 under non-CR conditions was treated with unrelated cord blood SCT (CBSCT) after a myeloablative conditioning regimen. Three months after CBSCT, her AMKL relapsed. She underwent two courses of chemotherapy. She received a haploidentical SCT (haplo-SCT) from her mother under non-CR conditions. After the second transplant, she had leg paralysis and bladder and rectal disturbance from an extramedullary lesion at the thoracic spinal cord (Th9) (Figure 1D). Although she underwent radiation therapy for the Th9 mass, the mass did not disappear. While she received a second CBSCT and haplo-SCT, she failed to engraft and died 30 months after the fourth SCT.
Patient 3 with the CBFA2T3-GLIS2 fusion gene was treated under the AML99 protocol [8] and could not achieve CR after induction A therapy (Figure 1E). She did not achieve CR even after several types of chemotherapy. Thereafter, she underwent chemotherapy with vincristine, prednisolone, and L-asparaginase (VPL), which is commonly used in therapy for acute lymphoblastic leukemia (ALL). After the VPL therapy, the percentage of blastic cells in the bone marrow decreased. She received unrelated bone marrow transplantation after a reduced-intensity conditioning regimen. She maintained remission for about 180 days and thereafter relapsed. Despite treatment with drugs including imatinib and L-asparaginase, she died 23 months after bone marrow transplantation.
DISCUSSION
It was reported that CBFA2T3-GLIS2 fusion gene-positive cases account for about 30% of pediatric patients with AMKL [5,6,9]. In addition, the overall survival rate and the event-free survival rate were lower in patients with the CBFA2T3-GLIS2 fusion gene than in those without this fusion gene [5,9,10,11]. There is little information about the clinical course of these patients. We encountered two AMKL patients with poor prognostics harboring the CBFA2T3-GLIS2 fusion gene, even though neither of them had inv(16)/t(16;16) chromosomal abnormalities upon G-band karyotyping. Therefore, evaluation of AMKL patients with this fusion gene without inv(16)/t(16;16) is needed.
CD56 was expressed in leukemic blasts of the two CBFA2T3-GLIS2-positive patients with AMKL but not in the two CBFA2T3-GLIS2-negative patients among the non-DS-AMKL patients in our cohort (Table 1). It was reported that CD41 and CD56 were positive and CD56 was drastically more highly expressed in patients with CBFA2T3-GLIS2-positive AMKL [6]. Higher expression of the CD56 antigen was reported as a poor prognostic marker [9,12,13,14,15,16,17]. Some investigators demonstrated that patients with CD56 positivity in blasts showed a higher incidence of extramedullary manifestations [12,13,14,18]. Among our patients with AMKL, CD56 was also more highly expressed in the two CBFA2T3-GLIS2-positive patients with AMKL with poor outcomes, and Patient 1 had extramedullary manifestation that did not regress after irradiation. High CD56 expression may be a surrogate marker of CBFA2T3-GLIS2 positivity in AMKL.
In Patient 3 with CBFA2T3-GLIS2-positive AMKL, chemotherapy regimens used to treat AML were not effective, but chemotherapy with VPL, commonly used to treat ALL, seemed to be more effective. When some of the treatment strategies commonly used to treat AML are not effective, the type of chemotherapy used to treat ALL might be effective in a subpopulation of patients with AMKL. There is a possibility that the conventional treatment commonly used to treat ALL may be effective for AMKL with this fusion gene. Eventually, the AMKL in both of the CBFA2T3-GLIS2-positive patients in our cohort became intractable to treatment, including SCT. Despite some chemotherapy regimens and SCT, the two patients with the CBFA2T3-GLIS2 fusion gene had poor prognosis. As previously reported, CBFA2T3-GLIS2 expression enhances BMP2/BMP4 signaling [5]. The development of treatments including novel targeted therapy drugs is desired.
CONCLUSION
Clinical courses of pediatric patients with AMKL harboring the CBFA2T3-GLIS2 fusion gene are poor due to resistance to chemotherapies and SCT. New treatment strategies are necessary.
Ethics
Ethics Committee Approval: The protocol of this survey and research plan has been approved by the Clinical Ethics Committee of Yokohama City University (A130725002), Informed Consent: It was taken from patients and/or their parents.
Footnotes
Concept: Masakatsu D.Yanagimachi; Design: Masakatsu D. Yanagimachi, Hiroaki Goto, Shumpei Yokota; Data Collection or Processing: Mayu Ishibashi, Tomoko Yokosuka, Masakatsu D. Yanagimachi, Fuminori Iwasaki, Shin-ichi Tsujimoto, Koji Sasaki, Masanobu Takeuchi, Reo Tanoshima, Hiromi Kato, Ryosuke Kajiwara, Fumiko Tanaka, Hiroaki Goto, Shumpei Yokota; Analysis or Interpretation: Mayu Ishibashi, Tomoko Yokosuka, Masakatsu D. Yanagimachi; Literature Search: Mayu Ishibashi, Tomoko Yokosuka, Masakatsu D. Yanagimachi, Fuminori Iwasaki, Shin-ichi Tsujimoto, Koji Sasaki, Masanobu Takeuchi, Reo Tanoshima, Hiromi Kato, Ryosuke Kajiwara, Fumiko Tanaka, Hiroaki Goto, Shumpei Yokota; Writing: Mayu Ishibashi, Tomoko Yokosuka, Masakatsu D. Yanagimachi, Fuminori Iwasaki, Shin-ichi Tsujimoto, Koji Sasaki, Masanobu Takeuchi, Reo Tanoshima, Hiromi Kato, Ryosuke Kajiwara, Fumiko Tanaka, Hiroaki Goto, Shumpei Yokota.
Conflict of Interest: The authors of this paper have no conflicts of interest, including specific financial interests, relationships, and/or affiliations relevant to the subject matter or materials included.
References
- 1.Athale UH, Razzouk BI, Raimondi SC, Tong X, Behm FG, Head DR, Srivastava DK, Rubnitz JE, Bowman L, Pui CH, Ribeiro RC. Biology and outcome of childhood acute megakaryoblastic leukemia: a single institution’s experience. Blood. 2001;97:3727–3732. doi: 10.1182/blood.v97.12.3727. [DOI] [PubMed] [Google Scholar]
- 2.Barnard DR, Alonzo TA, Gerbing RB, Lange B, Woods WG Children’s Oncology Group. Comparison of childhood myelodysplastic syndrome, AML FAB M6 or M7, CCG 2891: report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;49:17–22. doi: 10.1002/pbc.20951. [DOI] [PubMed] [Google Scholar]
- 3.Tallman MS, Neuberg D, Bennett JM, Francois CJ, Paietta E, Wiernik PH, Dewald G, Cassileth PA, Oken MM, Rowe JM. Acute megakaryocytic leukemia: the Eastern Cooperative Oncology Group experience. Blood. 2000;96:2405–2411. [PubMed] [Google Scholar]
- 4.Creutzig U, Zimmermann M, Ritter J, Reinhardt D, Hermann J, Henze G, Jurgens H, Kabisch H, Reiter A, Riehm H, Gadner H, Schellong G. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19:2030–2042. doi: 10.1038/sj.leu.2403920. [DOI] [PubMed] [Google Scholar]
- 5.Gruber TA, Larson Gedman A, Zhang J, Koss CS, Marada S, Ta HQ, Chen SC, Su X, Ogden SK, Dang J, Wu G, Gupta V, Andersson AK, Pounds S, Shi L, Easton J, Barbato MI, Mulder HL, Manne J, Wang J, Rusch M, Ranade S, Ganti R, Parker M, Ma J, Radtke I, Ding L, Cazzaniga G, Biondi A, Kornblau SM, Ravandi F, Kantarjian H, Nimer SD, Döhner K, Döhner H, Ley TJ, Ballerini P, Shurtleff S, Tomizawa D, Adachi S, Hayashi Y, Tawa A, Shih LY, Liang DC, Rubnitz JE, Pui CH, Mardis ER, Wilson RK, Downing JR. An Inv(16)(p13.3q24.3)-encoded CBFA2T3-GLIS2 fusion protein defines an aggressive subtype of pediatric acute megakaryoblastic leukemia. Cancer Cell. 2012;22:683–697. doi: 10.1016/j.ccr.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiollier C, Lopez CK, Gerby B, Ignacimouttou C, Poglio S, Duffourd Y, Guegan J, Rivera-Munoz P, Bluteau O, Mabialah V, Diop M, Wen Q, Petit A, Bauchet AL, Reinhardt D, Bornhauser B, Gautheret D, Lecluse Y, Landman-Parker J, Radford I, Vainchenker W, Dastugue N, Botton S, de, Dessen P, Bourquin JP, Crispino JD, Ballerini P, Bernard OA, Pflumio F, Mercher T. Characterization of novel genomic alterations and therapeutic approaches using acute megakaryoblastic leukemia xenograft models. J Exp Med. 2012;209:2017–2031. doi: 10.1084/jem.20121343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu HH, Zhang XH, Qin YZ, Liu DH, Jiang H, Chen H, Jiang Q, Xu LP, Lu J, Han W, Bao L, Wang Y, Chen YH, Wang JZ, Wang FR, Lai YY, Chai JY, Wang LR, Liu YR, Liu KY, Jiang B, Huang XJ. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood. 2013;121:4056–4062. doi: 10.1182/blood-2012-11-468348. [DOI] [PubMed] [Google Scholar]
- 8.Tsukimoto I, Tawa A, Horibe K, Tabuchi K, Kigasawa H, Tsuchida M, Yabe H, Nakayama H, Kudo K, Kobayashi R, Hamamoto K, Imaizumi M, Morimoto A, Tsuchiya S, Hanada R. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2009;27:4007–4013. doi: 10.1200/JCO.2008.18.7948. [DOI] [PubMed] [Google Scholar]
- 9.Alegretti AP, Bittar CM, Bittencourt R, Piccoli AK, Schneider L, Silla LM, Bo SD, Xavier RM. The expression of CD56 antigen is associated with poor prognosis in patients with acute myeloid leukemia. Rev Bras Hematol Hemoter. 2011;33:202–206. doi: 10.5581/1516-8484.20110054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masetti R, Pigazzi M, Togni M, Astolfi A, Indio V, Manara E, Casadio R, Pession A, Basso G, Locatelli F. CBFA2T3-GLIS2 fusion transcript is a novel common feature in pediatric, cytogenetically normal AML, not restricted to FAB M7 subtype. Blood. 2013;121:3469–3472. doi: 10.1182/blood-2012-11-469825. [DOI] [PubMed] [Google Scholar]
- 11.Masetti R, Togni M, Astolfi A, Pigazzi M, Manara E, Indio V, Rizzari C, Rutella S, Basso G, Pession A, Locatelli F. DHH-RHEBL1 fusion transcript: a novel recurrent feature in the new landscape of pediatric CBFA2T3-GLIS2-positive acute myeloid leukemia. Oncotarget. 2013;4:1712–1720. doi: 10.18632/oncotarget.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graf M, Reif S, Hecht K, Pelka-Fleischer R, Kroell T, Pfister K, Schmetzer H. High expression of costimulatory molecules correlates with low relapse-free survival probability in acute myeloid leukemia (AML) Ann Hematol. 2005;84:287–297. doi: 10.1007/s00277-004-0978-0. [DOI] [PubMed] [Google Scholar]
- 13.Iizuka Y, Aiso M, Oshimi K, Kanemaru M, Kawamura M, Takeuchi J, Horikoshi A, Ohshima T, Mizoguchi H, Horie T. Myeloblastoma formation in acute myeloid leukemia. Leuk Res. 1992;16:665–671. doi: 10.1016/0145-2126(92)90017-2. [DOI] [PubMed] [Google Scholar]
- 14.Kuwabara H, Nagai M, Yamaoka G, Ohnishi H, Kawakami K. Specific skin manifestations in CD56 positive acute myeloid leukemia. J Cutan Pathol. 1999;26:1–5. doi: 10.1111/j.1600-0560.1999.tb01782.x. [DOI] [PubMed] [Google Scholar]
- 15.Rego EM. The expression of the CD56 antigen is associated with poor prognosis in patients with acute myeloid leukemia. Rev Bras Hematol Hemoter. 2011;33:176–177. doi: 10.5581/1516-8484.20110048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang DH, Lee JJ, Mun YC, Shin HJ, Kim YK, Cho SH, Chung IJ, Seong CM, Kim HJ. Predictable prognostic factor of CD56 expression in patients with acute myeloid leukemia with t(8:21) after high dose cytarabine or allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82:1–5. doi: 10.1002/ajh.20739. [DOI] [PubMed] [Google Scholar]
- 17.Raspadori D, Damiani D, Michieli M, Stocchi R, Gentili S, Gozzetti A, Masolini P, Michelutti A, Geromin A, Fanin R, Lauria F. CD56 and PGP expression in acute myeloid leukemia: impact on clinical outcome. Haematologica. 2002;87:1135–1140. [PubMed] [Google Scholar]
- 18.Liang C, Chan KH, Yoon PJ, Lovell MA. Clinicopathological characteristics of extramedullary acute megakaryoblastic leukemia (AMKL): report of a case with initial mastoid presentation and review of literature to compare extramedullary AMKL and non-AMKL cases. Pediatr Dev Pathol. 2012;15:385–392. doi: 10.2350/11-12-1124-CR.1. [DOI] [PubMed] [Google Scholar]


