Keywords: nerve regeneration, spinal cord injury, neuroprotection; methylprednisolone, apoptosis, locomotor function, caspase-3, caspase-9, Bax, Bcl-2, neural regeneration
Abstract
Some studies have indicated that the Wnt/β-catenin signaling pathway is activated following spinal cord injury, and expression levels of specific proteins, including low-density lipoprotein receptor related protein-6 phosphorylation, β-catenin, and glycogen synthase kinase-3β, are significantly altered. We hypothesized that methylprednisolone treatment contributes to functional recovery after spinal cord injury by inhibiting apoptosis and activating the Wnt/β-catenin signaling pathway. In the current study, 30 mg/kg methylprednisolone was injected into rats with spinal cord injury immediately post-injury and at 1 and 2 days post-injury. Basso, Beattie, and Bresnahan scores showed that methylprednisolone treatment significantly promoted locomotor functional recovery between 2 and 6 weeks post-injury. The number of surviving motor neurons increased, whereas the lesion size significantly decreased following methylprednisolone treatment at 7 days post-injury. Additionally, caspase-3, caspase-9, and Bax protein expression levels and the number of apoptotic cells were reduced at 3 and 7 days post-injury, while Bcl-2 levels at 7 days post-injury were higher in methylprednisolone-treated rats compared with saline-treated rats. At 3 and 7 days post-injury, methylprednisolone up-regulated expression and activation of the Wnt/β-catenin signaling pathway, including low-density lipoprotein receptor related protein-6 phosphorylation, β-catenin, and glycogen synthase kinase-3β phosphorylation. These results indicate that methylprednisolone-induced neuroprotection may correlate with activation of the Wnt/β-catenin signaling pathway.
Introduction
Spinal cord injury (SCI), including primary and secondary injury, is a worldwide medical problem (Wyndaele and Wyndaele, 2006). Secondary injury plays a critical role in SCI and is induced by various factors, such as apoptosis, oxidative stress, and the inflammatory immune response. In particular, neuronal apoptosis, which also affects microglia and oligodendrocytes and inhibits recovery of white matter and neurological functions, prevents functional recovery after SCI (Yuan and Yankner, 2000; Beattie et al., 2002). Secondary damage pathways offer potential therapeutic targets for treating SCI to enable greater functional recovery (Profyris et al., 2004; Zhang et al., 2015a). Methylprednisolone (MP), a synthetic glucocorticoid used to treat inflammatory diseases, is clinically utilized to treat SCI. Several studies have shown that MP inhibits inflammation, oxidative stress, and neuronal apoptosis, contributing to the neuroprotective effect on functional recovery after SCI (Vaquero et al., 2006; Gao et al., 2014; Gocmez et al., 2015). However, some reports have started to question the neuroprotective effects of MP after SCI in recent years, stating that MP failed to exert a neuroprotective effect and additionally increased complications in SCI, such as infection, pulmonary embolism, and even death (Hall and Springer, 2004; Pereira et al., 2009; Wilson and Fehlings, 2011; Fehlings et al., 2014; Hurlbert et al., 2015). Hence, further verification of the effect and investigation of the specific molecular mechanisms of MP is of utmost importance to justify continued clinical application of MP in SCI.
The Wnt signaling pathways, particularly the canonical Wnt/β-catenin pathway, were first identified for their role in carcinogenesis. However, accumulating evidence has shown that Wnt/β-catenin signaling plays a critical role in embryonic development. Wnt/β-catenin has been implicated in neural development, axonal guidance, neuropathic pain remission, neuronal survival, and SCI (Zhang et al., 2013b; Ziaei et al., 2015). Furthermore, in some diseases, apoptosis is induced following inhibition of the Wnt/β-catenin signaling pathway (Hsu et al., 2014; Li et al., 2015). Apoptosis of neuronal cells is an important pathological feature of several central nervous system diseases, including neurodegeneration and SCI (Yuan and Yankner, 2000; Tang et al., 2014; Zhang et al., 2015b). Neuronal apoptosis is enhanced following SCI, which leads to suppression of functional recovery, thereby providing a potential therapeutic target for SCI.
We hypothesized that the molecular mechanism underlying the inhibitory effect of MP on apoptosis and related restoration of neurological function after SCI occurs via regulation of the Wnt/β-catenin signaling pathway. Therefore, the goal of the present study was to further confirm the neuroprotective effect of MP after SCI by examining recovery of locomotor function and the injured spinal cord, neuronal survival in the spinal cord anterior horn, and anti-apoptotic activity, as well as to correlate MP activity with alterations in the Wnt/β-catenin signaling pathway, as a potential molecular mechanism.
Materials and Methods
Animals
A total of 105 adult, male, 8–12-week-old, Sprague-Dawley rats, weighing 260–300 g, were purchased from the Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China, license No. SCXK (Jing) 2012-0001) and were raised in the specific-pathogen-free Laboratory Animal Center. The rats were maintained at 23.0 ± 0.5°C with an alternating 12-hour light/dark cycle. The rats were randomly divided into the following groups: MP (n = 35; SCI + MP), saline (n = 35; SCI + saline), and sham (n = 35).
All procedures were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animal (NIH Publication No. 85-23, revised 1986). The protocols were approved by the Animal Ethics Committee of Jinzhou Medical University of China. All efforts to minimize the number of animals used and their suffering were made.
Rat SCI model and MP administration
The rat SCI model was established as previously described (Yacoub et al., 2014). Briefly, the rats were anesthetized via intraperitoneal injection of 10% chloral hydrate (0.33 mL/kg), and the T9–10 of the spinal cord was exposed. An impounder (diameter: 2.0 mm; weight: 10 g) was dropped from a height of 25.0 mm above the spinal cord. Congestion in the injured spinal cord was immediately observed followed by rapid withdrawal of the hind limbs. Congestion at the injury site, rapid contraction, tremor of the lower limbs, and incontinence confirmed successful model establishment. Rats with unsuccessful SCI induction were not selected for further experimentation. After SCI, the surgical wounds were cleaned with warm saline and were sutured. The bladder was massaged three times daily to improve functional recovery of automatic micturition, and penicillin was administered for 3 consecutive days.
MP (30 mg/kg) and saline (1 mL/kg) were injected intravenously via the tail immediately post-SCI and 1 and 2 days post-SCI, and then once daily for two days. The sham group underwent laminectomy only.
Analysis of locomotor activity in the SCI rat model
The Basso, Beattie, and Bresnahan (BBB) open-field locomotor rating scale was used to evaluate locomotor function prior to injury and recovery at 1 and 3 days, as well as at 1, 2, 3, 4, 5, and 6 weeks post-SCI (Basso et al., 1995). Briefly, the BBB scores ranged from 0 (complete paralysis) to 21 (unimpaired locomotion) and were assessed by three independent examiners in a blinded fashion.
Tissue preparation
At 7 days post-SCI, the rats were intraperitoneally anesthetized with 10% chloral hydrate (0.3 mL/kg), and then perfused with 0.9% saline and 4% paraformaldehyde (Xue et al., 2013; Zhang et al., 2013a). The T8–12 spinal cord segments (including the epicenter) were removed and immersed in 4% paraformaldehyde for 3 days, and then dehydrated in 30% sucrose. Using a cryostat microtome (Leica CM3050S; Heidelberg, Germany), 5-μm-thick cross sections (3 mm rostral to the epicenter) were prepared for terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) and immunofluorescence staining, and 20-μm-thick cross sections (3 mm rostral to the epicenter) were used for Nissl staining.
Nissl staining of spinal cord tissue following SCI
First, 20-μm-thick coronal sections (3 mm rostral to the epicenter) were placed in mixing solution (alcohol/chloroform, 1:1) overnight at room temperature. The following day, sections were consecutively placed in 100% alcohol, 95% alcohol, 70% alcohol, and distilled water. Subsequently, the sections were stained in 0.05% cresyl violet (pH 3.0, Sigma-Aldrich, St. Louis, MO, USA) for 10 minutes at 40°C after which sections were differentiated in 95% alcohol, dehydrated in 100% alcohol, and cleared in xylene. The images were captured by a light microscope (Leica, Heidelberg, Germany). The large and Nissl-stained anterior horn cells in the spinal cord tissue were recognized as motor neurons. Five Nissl-stained sections in every experimental rat were randomly selected for evaluating the average number of surviving neurons in the spinal cord anterior horn. The total and residual white-matter areas were measured using a BZ-Analyzer (Keyence) to measure lesion size in the spinal cord tissue in all groups (Xue et al., 2013; Zhang et al., 2013a).
Western blot assay of spinal cord tissue following SCI
At 3 and 7 days post-SCI, the rats were first anesthetized with 10% chloral hydrate (0.33 mL/kg) via intraperitoneal injection and then euthanized. T9–11 spinal cord tissues (3 mm cephalad and caudal from the lesion epicenter) were separated and homogenized in radioimmune precipitation assay (RIPA) lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% Na-deoxycholate, 1 mM EDTA). Protein homogenates (40 µg) were subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Subsequently, the proteins were transferred to polyvinylidene difluoride membranes and incubated with the following primary antibodies at 4°C overnight: rabbit anti-phosphorylated low-density lipoprotein receptor related protein-6 (p-LRP-6) polyclonal antibody (1:500; Cell Signaling Technology, Inc., Boston, MA, USA), rabbit anti-β-catenin polyclonal antibody (1:500; Cell Signaling Technology), rabbit anti-phosphorylated glycogen synthase kinase-3β (p-GSK-3β) polyclonal antibody (1:300; Cell Signaling Technology), rabbit anti-active-caspase-3 polyclonal antibody (1:1,000; Abcam, Cambridge, UK), rabbit anti-active-caspase-9 polyclonal antibody (1:1,000; Abcam), and rabbit anti-β-actin polyclonal antibody (1:1,000; Abcam). The following day, the membranes were incubated with secondary antibodies (goat anti-rabbit IgG; 1:2,000; Abcam) for 2 hours at room temperature and immunoreactive bands were visualized with the ChemiDoc-It™TS2 Imager (UVP LLC, Upland, CA, USA). Finally, ImageJ2x software (National Institutes of Health, Bethesda, MD, USA) was utilized to measure the relative optical density of protein bands. The optical density of protein bands was normalized to β-actin controls.
TUNEL assay of spinal cord tissue following SCI
TUNEL assay was utilized to detect DNA fragmentation as a measure of cell apoptosis in the spinal cord anterior horn after SCI. Briefly, the 5-µm-thick coronal sections (3 mm rostral to the epicenter) were fixed with fixation solution (4% paraformaldehyde in PBS, pH 7.4) for 30 minutes. Afterwards, the tissues were washed with PBS for 30 minutes at room temperature. The tissues were incubated in permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) at 4°C for 2 minutes and then incubated with TUNEL reaction mixture (In Situ Cell Death Detection Kit, TMR red, Roche, Mannheim, Germany) at 37°C for 1 hour. Subsequently, the tissues were subjected to 4′,6-diamidino-2-phenylindole (DAPI) (1:1,000) staining. After mounting, all samples were analyzed using fluorescence microscopy (Leica, Heidelberg, Germany). The double-stained cells (red and blue) were considered to be TUNEL-positive cells. The number of TUNEL-positive cells was quantified by randomly selecting four sections in each experimental group.
Immunofluorescence staining of spinal cord tissue following SCI
Briefly, coronal sections (5 µm) were placed in 0.01 M citric acid (pH 6.0) for 15 minutes for antigen retrieval. The sections were blocked with blocking buffer (5% goat serum, 0.1% Triton X-100 in PBS) for 1 hour at 4°C and incubated with the primary polyclonal antibody overnight at 4°C (rabbit anti-Bcl-2 polyclonal antibody, 1:500; rabbit anti-Bax polyclonal antibody, 1:500, Novus Biologicals, Littleton, CO, USA). The following day, the sections were incubated with fluorescein isothiocyanate-coupled secondary antibody (goat anti-rabbit IgG; 1:400, Abcam) for 2 hours at room temperature, and the nuclei were counterstained using DAPI (1:1,000). The mounted sections were imaged using fluorescence microscopy (Leica 4000B, Heidelberg, Germany). The fluorescence intensity of Bcl-2 and Bax immunoreactivity in the spinal cord anterior horn was analyzed using the ImageJ2x software.
Statistical analysis
All data were analyzed using GraphPad Prism version 5.0 for Windows software (GraphPad Software, La Jolla, CA, USA) and expressed as the mean ± SD. Discrepancies among multiple groups were detected using one-way analysis of variance and least significant difference test, and the BBB scores were analyzed using the Mann-Whitney U test. P-values < 0.05 were considered statistically significant.
Results
MP promoted locomotor functional recovery after SCI
The BBB scores were utilized to determine locomotor functional recovery before surgery and at 1 and 3 days, and 1, 2, 3, 4, 5, and 6 weeks post-SCI (Figure 1). BBB scores were 21 points before injury and 0 points at 1 day post-injury in MP and saline groups. The BBB scores in the two groups gradually increased from 1 day to 2 weeks, but no statistically significant difference was detected between the MP and saline groups at 3 and 7 days (P > 0.05). However, the BBB scores were significantly higher in the MP group compared with the saline group from 2 to 6 weeks post-injury (P < 0.05). The BBB scores reached a peak between 5 and 6 weeks, indicating that the neurological function related to locomotor activity improved with MP intervention post-SCI.
Figure 1.
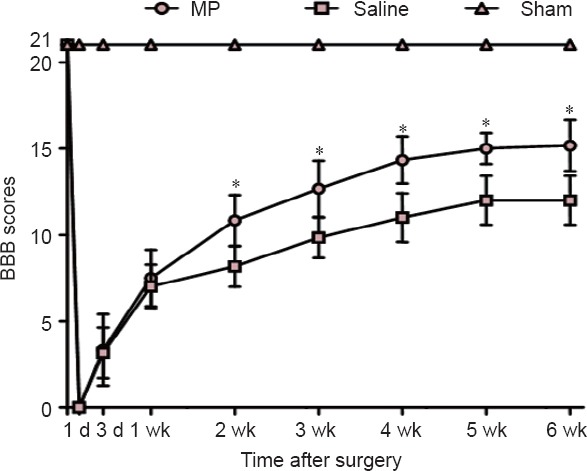
BBB locomotor rating scale scores in a rat model of SCI.
BBB scores were used to assess neurological function of rats in the MP (SCI + MP), saline (SCI + saline), and sham (laminectomy alone) groups at 1 and 3 days (d), and 1, 2, 3, 4, 5, and 6 weeks (wk) post-SCI. Higher scores indicate better neurological function. *P < 0.05, vs. saline group (mean ± SD, n = 6, Mann-Whitney U test). BBB: Basso, Beattie & Bresnahan; SCI: spinal cord injury; MP: methylprednisolone.
MP treatment improved pathological recovery post-SCI
Nissl staining was performed to detect pathological morphology in the spinal cord of SCI rats (Figure 2A). The number of Nissl-positive cells in the anterior horn of the spinal cord was less in the saline group compared with the sham group. Conversely, MP intervention dramatically increased the number of large-diameter Nissl-positive neurons, morphologically appearing as alpha-motor neurons, in the spinal cord anterior horn compared with the saline group (Figure 2A). Concomitantly, the number of surviving neurons in the spinal cord anterior horn was also clearly augmented by MP treatment post-SCI (Figure 2B; P < 0.01). Furthermore, compared with the saline group, the proportion of lesion size was smaller in the MP group after treatment with MP (Figure 2C; P < 0.01). These results illustrated significantly improved pathological morphology of the spinal cord with MP treatment post-SCI.
Figure 2.
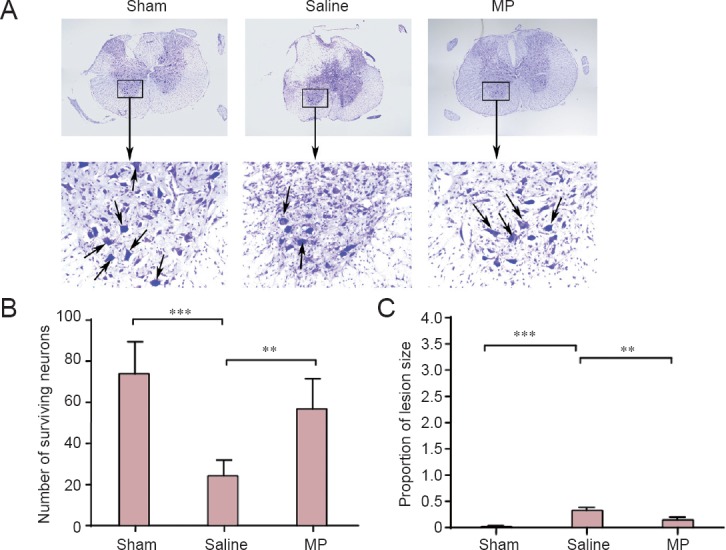
Nissl staining of neuronal pathology in the spinal cord anterior horn in a rat SCI model.
(A) Nissl staining of a coronal section of the spinal cord of MP-treated (SCI + MP), saline-treated (SCI + saline), and sham-operated (laminectomy alone) SCI rats. Arrows indicate healthy large-diameter Nissl-positive neurons in the anterior horn of the spinal cord. Original magnification: upper: × 50; lower, × 400. The numbers of surviving neurons in the spinal cord anterior horn (B) and proportion of lesion size (C) in rats of the three groups were evaluated at 7 days post-SCI. All data are expressed as the mean ± SD (n = 5; one-way analysis of variance and the least significant difference test). **P < 0.01, ***P < 0.001. MP: Methylprednisolone; SCI: spinal cord injury.
MP activated Wnt/β-catenin signaling pathway after SCI
To examine the molecular mechanisms underlying the role of MP in functional recovery, western blot assay was used in spinal cord tissue from the lower thoracic region, cephalad and caudal from the lesion epicenter, to analyze alterations of the Wnt/β-catenin signal pathway, which is known to inhibit neuronal apoptosis, at 3 and 7 days post-SCI in the three groups (Figure 3A). The β-catenin protein expression levels were significantly increased in the saline group compared with the sham group, and the β-catenin expression was higher in the MP group compared with the saline group (Figure 3C) (3 days: P < 0.001; 7 days: P < 0.01). Additionally, compared with the sham group, expression levels of p-LRP-6 and GSK-3β increased after SCI, and were even higher after MP treatment (Figure 3B; 3 days: P < 0.001; 7 days: P < 0.001); (Figure 3D; 3 days: P < 0.05; 7 days: P < 0.01). These results demonstrated that the Wnt/β-catenin signaling pathway was significantly activated after SCI, potentially as part of the endogenous repair mechanism, and was further enhanced by MP treatment in the spinal cord of SCI rats.
Figure 3.
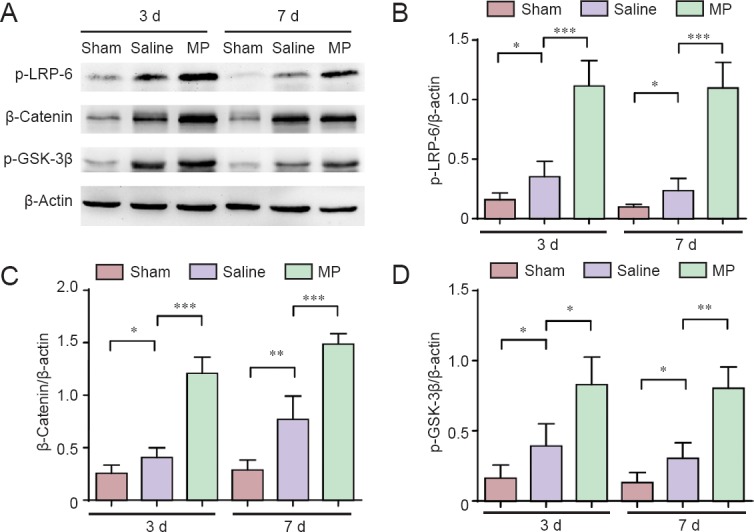
Western blot assay of expression of the canonical Wnt/β-catenin signaling pathway in spinal cord tissue in a rat model of spinal cord injury (SCI).
(A) The expression of phosphorylated LRP-6 (p-LRP-6), β-catenin, and phosphorylated GSK-3β (p-GSK-3β) as key components of the canonical Wnt/β-catenin signaling pathway at 3 and 7 days (d) post-SCI in methylprednisolone (MP)-treated (SCI + MP), saline-treated (SCI + saline), and sham-operated (laminectomy alone) rats; (B) quantification of phosphorylation levels of LRP-6; (C) expression of β-catenin; (D) and p-GSK-3β protein expression. The optical density of protein bands was normalized to the β-actin control. All data are expressed as the mean ± SD (n = 4; one-way analysis of variance and the least significant difference test). *P < 0.05, **P < 0.01, ***P < 0.001. d: Days.
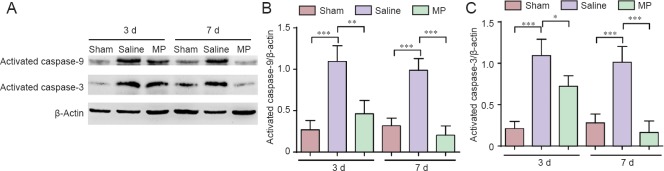
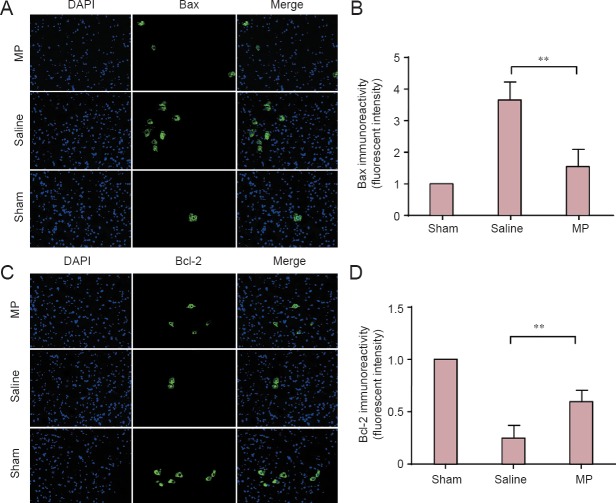
Cell apoptosis inhibited by MP following SCI
Some studies have stated that neuron cell apoptosis increases from 1 to 14 days post-SCI, and the variation tendency shows more significant alterations at 3 and 7 days post-SCI (Rios et al., 2015; Yan et al., 2015). In our study, western blot analysis was used to examine the anti-apoptotic effect of MP at 3 and 7 days post-SCI (Figure 4A). Interestingly, expression of active caspase-9 and caspase-3 in the spinal cord cephalad and caudal tissue from the lesion epicenter was decreased by MP after SCI compared with elevated levels in the saline group (Figure 4B, C; 3 days: P < 0.01 or P < 0.05; 7 days: P < 0.001). Concomitantly, the number of TUNEL-positive cells was reduced by MP treatment compared with the saline group after SCI (Figure 5A, B; 7 days: P < 0.001). Furthermore, immunofluorescence analysis was utilized to detect expression of the apoptotic and anti-apoptotic proteins Bax and Bcl-2 in the spinal cord anterior horn at 7 days post-SCI (Figure 6A, C). Results showed increased Bax levels, whereas Bcl-2 levels were decreased in the saline group compared with the sham group. Conversely, MP treatment inhibited Bax expression and elevated Bcl-2 expression after SCI compared with the saline group (Figure 6B, D; 7 days: P < 0.01). Our data indicate that MP treatment inhibited neuronal apoptosis, with a potentially significant neuroprotective effect on SCI.
Figure 4.
Western blot assay of expression of activated caspase-9 and caspase-3 as markers for apoptosis in spinal cord tissue in a SCI rat model.
(A) Western blots of activated caspase-9 and caspase-3 at 3 and 7 d in the spinal cord of MP-treated (SCI + MP), saline-treated (SCI + saline), and sham-operated (laminectomy alone) rats. (B, C) Quantification of activated caspase-9 (B) and caspase-3 (C) at 3 and 7 d post-SCI. The optical density of protein bands was normalized to the β-actin control. All data are expressed as the mean ± SD (n = 4; one-way analysis of variance and the least significant difference test). *P < 0.05, **P < 0.01, ***P < 0.001. MP: Methylprednisolone; SCI: spinal cord injury; d: days.
Figure 5.
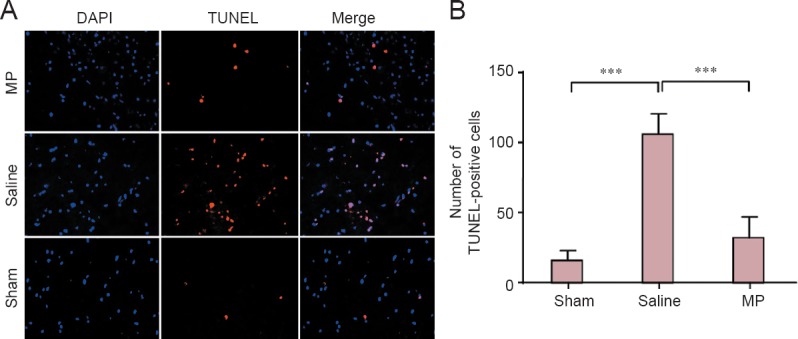
Apoptosis in the spinal cord anterior horn in a rat SCI model.
(A) TUNEL, DAPI, and merged staining of MP-treated (SCI + MP), saline-treated (SCI + saline) and sham-operated (laminectomy alone) rats at 7 days post-surgery (× 400). (B) Quantification of TUNEL-positive cells in the three rat groups. All data are expressed as the mean ± SD (n = 4; one-way analysis of variance and the least significant difference test). ***P < 0.001. Blue: Cell nucleus; red: apoptotic cells. MP: Methylprednisolone; TUNEL: terminal deoxynucleotidyl transferase dUTP nick-end labeling; DAPI: 4′,6-diamidino-2-phenylindole; SCI: spinal cord injury.
Figure 6.
Immunofluorescence of Bax and Bcl-2 in the spinal cord anterior horn in a rat SCI model.
(A) DAPI, Bax, and merged staining in spinal cord sections of MP-treated (SCI + MP), saline-treated (SCI + saline) and sham-operated (laminectomy alone) SCI rats at 7 days following SCI (× 400). (C) DAPI, Bcl-2, and merged staining in spinal cord sections of the three rat groups at 7 days post-SCI (× 400). (B, D) The fluorescence intensity of Bax (B) and Bcl-2 (D) was assessed using Image J2x software. The fluorescence intensity of Bax and Bcl-2 in the MP and saline groups was normalized to the sham group. All data are expressed as the mean ± SD (n = 4; one-way analysis of variance and the least significant difference test). **P < 0.01. Blue: Cell nucleus; green: interest protein. MP: Methylprednisolone; SCI: spinal cord injury; DAPI: 4′,6-diamidino-2-phenylindole.
Discussion
To evaluate the effect of MP on functional recovery in a rat SCI model, BBB scores were measured to determine open-field locomotor function. MP treatment significantly promoted locomotor functional recovery, decreased lesion size, and increased the number of surviving neurons in the spinal cord anterior horn in SCI rats. The molecular and cellular mechanisms underlying these neuroprotective effects likely involved a reduction in the number of apoptotic cells, which was accompanied by reduced levels of activated caspase-3, caspase-9, and Bax protein expression, while the anti-apoptotic Bcl-2 levels were higher in the spinal cord anterior horn of MP-treated rats. Interestingly, MP further up-regulated the expression levels and activation of the Wnt/β-catenin signaling pathway, including LRP-6 phosphorylation, β-catenin, and GSK3-β phosphorylation, following SCI. Thus, the neuroprotective effect of MP treatment suppressing apoptosis may be correlated with the activation of the Wnt/β-catenin signaling pathway.
Recent studies have tried to identify the molecular mechanisms of primary and secondary injury in SCI (Estrada and Muller, 2014). Primary injury, leading to irreparable damage, is caused by various factors, such as initial mechanical impact, compression, and contusion, resulting in damage to nerve cells, myelin, blood vessels, and supporting bone structures in SCI. Secondary injury plays a more important long-term role in SCI. The traumatic injury causes swelling and hemorrhage, which lead to increased free radicals and decreased blood flow, causing cell membrane dysfunction, oxidative stress, inflammation, and apoptosis (Kang et al., 2007; Haider et al., 2015; Saxena et al., 2015). Apoptosis is considered as a significant therapeutic target in secondary injury. The application of anti-apoptotic drugs potentially promotes functional recovery in SCI.
The synthetic glucocorticoid MP is widely used for the clinical treatment of SCI. Initially, MP had been observed to inhibit secondary injury and improve functional recovery by intercalation into the cell membrane and inhibition of the propagation of peroxidative reactions. It was believed to inhibit posttraumatic spinal cord lipid peroxidation after SCI (Hall et al., 1987; Hall, 1993). Additionally, multiples effects of MP on spinal cord injury, such as anti-inflammatory, anti-oxidative, and autophagy-suppressive activity, have been reported (Botelho et al., 2009; Chen et al., 2012; Chengke et al., 2013; Boyaci et al., 2014). However, some studies have started to question the neuroprotective effect of MP after SCI (Liu et al., 2009; Tesiorowski et al., 2013; Harrop, 2014). Although some studies have shown that a mega dose of MP within 8 hours after SCI improves prognosis and promotes functional recovery after SCI (Bracken, 2012), other reports indicate that MP fails to exert a neuroprotective effect and actually increases complications in SCI, such as infection, pulmonary embolism, and death (Pereira et al., 2009; Hurlbert et al., 2015). In the present rat SCI model, Nissl staining results showed that MP treatment significantly decreased the proportion of lesion size after SCI and increased the number of surviving neurons in the anterior horns of the spinal cord. Notably, these Nissl-positive, large-diameter neurons in the spinal cord anterior horn appear morphologically as alpha-motor neurons, which contribute to functional recovery after SCI (Zhang et al., 2013a). The concomitant BBB scores for functional locomotor recovery were higher in the MP group compared with the saline group after SCI. These results illustrated that MP exhibits neuroprotective properties in vivo leading to functional recovery after SCI.
Some studies have demonstrated that increased apoptosis especially during a later phase, affects microglia and oligodendrocytes and inhibits white matter recovery, leading to neuronal loss and inhibition of functional recovery after SCI (Yuan and Yankner, 2000; Beattie et al., 2002). The expression levels of caspases and Bax increase, whereas anti-apoptotic Bcl-2 levels decrease after apoptosis induction. These results provide potential therapeutic targets for promoting neurological function recovery after SCI (Yuan and Yankner, 2000; Tang et al., 2014; Zhang et al., 2015b). To study apoptosis in SCI, expression levels of Bcl-2 and Bax protein were detected by immunofluorescence analysis. Western blot assay was employed to detect expression of two other apoptotic markers, activated caspase-3 and caspase-9, after SCI. Results showed that expressions of activated caspase-3, caspase-9, and Bax expression were inhibited by MP, but levels of the anti-apoptotic protein Bcl-2 were enhanced. Concomitantly, the number of TUNEL-positive cells was also reduced by MP treatment following SCI. These results indicate that neuronal apoptosis was significantly suppressed by MP treatment following SCI, and further supported the neuroprotective activity of MP and effect on functional recovery. However, the explicit molecular mechanisms underlying the neuroprotective effects of MP remain to be shown to develop novel therapeutic strategies for SCI.
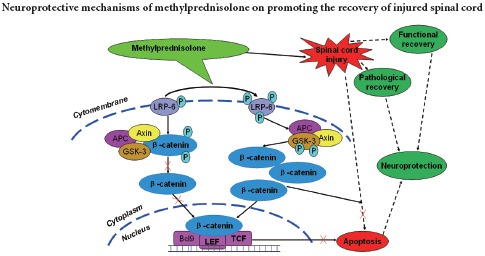
Wnts are a well-characterized family of glycoproteins and play an important role in embryonic development, influencing processes such as proliferation, composition, and survival of neurons (Hollis and Zou, 2012; Gonzalez-Fernandez et al., 2014). Wnt signaling pathways are mainly divided into three different signaling pathways: the canonical Wnt/β-catenin pathway, the non-canonical planar cell polarity (Wnt-JNK) pathway, and the Wnt-Ca2+ pathway (Fernandez-Martos et al., 2011). In the canonical wnt/β-catenin signal transduction pathway, binding of the wnt protein to the receptors Frizzled and LRP-5/6 induces LRP-6 phosphorylation, which in turn induces GSK-3β phosphorylation, improves stabilization of β-catenin, and induces dissociation of β-catenin from a protein complex consisting of adenomatous polyposis coli, Axin, and GSK3-β. Subsequently, β-catenin is translocated into the nucleus where it binds to the TCF/LEF transcription factor, thereby regulating cell proliferation, cell apoptosis, or differentiation (Reya and Clevers, 2005; MacDonald et al., 2009). Recent studies have shown that the activated Wnt/β-catenin signaling pathway promotes regeneration of axons and functional recovery after SCI (Sun et al., 2013; Yang et al., 2013). Other reports demonstrate that suppression of the Wnt/β-catenin singaling pathway induces apoptosis (Wang et al., 2002; Bilir et al., 2013). However, the effect of MP on the Wnt/β-catenin signaling pathway after SCI has not been previously reported. Western blot results in the present study demonstrated enhanced phosphorylation levels of LRP-6 and GSK-3β, as well as increased β-catenin protein expression, indicating increased activation of the canonical Wnt/β-catenin signaling pathway by MP after SCI. These findings most likely indicate an increase in endogenous repair mechanisms regulated by the Wnt/β-catenin signaling following SCI. Furthermore, MP inhibited neuronal apoptosis, which may imply that the anti-apoptotic effect was mediated by further activation of the Wnt/β-catenin signaling pathway post-SCI. Thus, the neuroprotective activity of MP on SCI may be attributed to the activation of the Wnt/β-catenin signaling pathway, providing a novel molecular mechanism underlying the role of MP in SCI treatment.
In our study, MP treatment increased the number of surviving neurons in the spinal cord anterior horn, suppressed neuronal apoptosis, and improved pathological and locomotor functional recovery following SCI in rats. In line with these results, MP treatment further activated the wnt/β-catenin signaling pathway close to the lesion epicenter after SCI, which is known to attenuate neuronal apoptosis. Nevertheless, the effect of MP has been studied only on neurons in our study, and it will be necessary to further observe the effects of MP on the Wnt/β-catenin signaling pathway and recovery of other neural cells post-SCI through in vivo and in vitro experiments. Further studies are needed to verify the various effects of MP on the Wnt/β-catenin signaling pathway and neuroprotective effects on functional recovery at additional time points in SCI rats to determine the molecular mechanisms of Wnt/β-catenin signaling in vivo and in vitro to further support the potential advantages of clinical application of MP in SCI treatment.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 81471854.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Cooper C, Robens J, Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- Bilir B, Kucuk O, Moreno CS. Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple-negative breast cancer cells. J Transl Med. 2013;11:280. doi: 10.1186/1479-5876-11-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho RV, Daniel JW, Boulosa JL, Colli BO, Farias Rde L, Moraes OJ, Pimenta WE, Jr, Ribeiro CH, Ribeiro FR, Taricco MA, Carvalho MV, Bernardo WM. Effectiveness of methylprednisolone in the acute phase of spinal cord injuries-a systematic review of randomized controlled trials. Rev Assoc Med Bras. 2009;55:729–737. doi: 10.1590/s0104-42302009000600019. [DOI] [PubMed] [Google Scholar]
- Boyaci MG, Eser O, Kocogullari CU, Karavelioglu E, Tokyol C, Can Y. Neuroprotective effect of alpha-lipoic acid and methylprednisolone on the spinal cord ischemia/reperfusion injury in rabbits. Br J Neurosurg. 2014:1–6. doi: 10.3109/02688697.2014.954986. [DOI] [PubMed] [Google Scholar]
- Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2012;1:Cd001046. doi: 10.1002/14651858.CD001046.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Fong TH, Lee AW, Chiu WT. Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine. 2012;37:470–475. doi: 10.1097/BRS.0b013e318221e859. [DOI] [PubMed] [Google Scholar]
- Chengke L, Weiwei L, Xiyang W, Ping W, Xiaoyang P, Zhengquan X, Hao Z, Penghui Z, Wei P. Effect of infliximab combined with methylprednisolone on expressions of NF-kappaB, TRADD, and FADD in rat acute spinal cord injury. Spine. 2013;38:E861–869. doi: 10.1097/BRS.0b013e318294892c. [DOI] [PubMed] [Google Scholar]
- Estrada V, Muller HW. Spinal cord injury: there is not just one way of treating it. F1000Prime Rep. 2014;6:84. doi: 10.12703/P6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery. 2014;61(Suppl 1):36–42. doi: 10.1227/NEU.0000000000000412. [DOI] [PubMed] [Google Scholar]
- Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ. Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One. 2011;6:e27000. doi: 10.1371/journal.pone.0027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Ding J, Xiao HJ, Li ZQ, Chen Y, Zhou XS, Wang JE, Wu J, Shi WZ. Anti-inflammatory and anti-apoptotic effect of combined treatment with methylprednisolone and amniotic membrane mesenchymal stem cells after spinal cord injury in rats. Neurochem Res. 2014;39:1544–1552. doi: 10.1007/s11064-014-1344-9. [DOI] [PubMed] [Google Scholar]
- Gocmez C, Celik F, Kamasak K, Kaplan M, Uzar E, Arikanoglu A, Evliyaoglu O. Effects of intrathecal caffeic acid phenethyl ester and methylprednisolone on oxidant/antioxidant status in traumatic spinal cord injuries. J Neurol Surg A Cent Eur Neurosurg. 2015;76:20–24. doi: 10.1055/s-0034-1371513. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Fernandez C, Fernandez-Martos CM, Shields SD, Arenas E, Javier Rodriguez F. Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma. 2014;31:565–581. doi: 10.1089/neu.2013.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider T, Hoftberger R, Ruger B, Mildner M, Blumer R, Mitterbauer A, Buchacher T, Sherif C, Altmann P, Redl H, Gabriel C, Gyongyosi M, Fischer MB, Lubec G, Ankersmit HJ. The secretome of apoptotic human peripheral blood mononuclear cells attenuates secondary damage following spinal cord injury in rats. Exp Neurol. 2015;267:230–242. doi: 10.1016/j.expneurol.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Hall ED. Neuroprotective actions of glucocorticoid and nonglucocorticoid steroids in acute neuronal injury. Cell Mol Neurobiol. 1993;13:415–432. doi: 10.1007/BF00711581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, McCall JM, Chase RL, Yonkers PA, Braughler JM. A nonglucocorticoid steroid analog of methylprednisolone duplicates its high-dose pharmacology in models of central nervous system trauma and neuronal membrane damage. J Pharmacol Exp Ther. 1987;242:137–142. [PubMed] [Google Scholar]
- Harrop JS. Spinal cord injury: debating the efficacy of methylprednisolone. Neurosurgery. 2014;61(Suppl 1):30–31. doi: 10.1227/NEU.0000000000000391. [DOI] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Zou Y. Expression of the Wnt signaling system in central nervous system axon guidance and regeneration. Front Mol Neurosci. 2012;5:5. doi: 10.3389/fnmol.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HC, Liu YS, Tseng KC, Tan BC, Chen SJ, Chen HC. LGR5 regulates survival through mitochondria-mediated apoptosis and by targeting the Wnt/beta-catenin signaling pathway in colorectal cancer cells. Cell Signal. 2014;26:2333–2342. doi: 10.1016/j.cellsig.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Hurlbert RJ, Hadley MN, Walters BC, Aarabi B, Dhall SS, Gelb DE, Rozzelle CJ, Ryken TC, Theodore N. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2015;76(Suppl 1):S71–83. doi: 10.1227/01.neu.0000462080.04196.f7. [DOI] [PubMed] [Google Scholar]
- Kang SK, Yeo JE, Kang KS, Phinney DG. Cytoplasmic extracts from adipose tissue stromal cells alleviates secondary damage by modulating apoptosis and promotes functional recovery following spinal cord injury. Brain Pathol (Zurich, Switzerland) 2007;17:263–275. doi: 10.1111/j.1750-3639.2007.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Zeng M, He W, Huang X, Luo L, Zhang H, Deng DY. Ghrelin protects alveolar macrophages against lipopolysaccharide-induced apoptosis through growth hormone secretagogue receptor 1a-dependent c-Jun N-terminal kinase and Wnt/beta-catenin signaling and suppresses lung inflammation. Endocrinology. 2015;156:203–217. doi: 10.1210/en.2014-1539. [DOI] [PubMed] [Google Scholar]
- Liu JC, Patel A, Vaccaro AR, Lammertse DP, Chen D. Methylprednisolone after traumatic spinal cord injury: yes or no? PM R. 2009;1:669–673. doi: 10.1016/j.pmrj.2009.06.002. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JE, Costa LM, Cabrita AM, Couto PA, Filipe VM, Magalhaes LG, Fornaro M, Di Scipio F, Geuna S, Mauricio AC, Varejao AS. Methylprednisolone fails to improve functional and histological outcome following spinal cord injury in rats. Exp Neurol. 2009;220:71–81. doi: 10.1016/j.expneurol.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15:415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Rios C, Orozco-Suarez S, Salgado-Ceballos H, Mendez-Armenta M, Nava-Ruiz C, Santander I, Baron-Flores V, Caram-Salas N, Diaz-Ruiz A. Anti-apoptotic effects of dapsone after spinal cord injury in rats. Neurochem Res. 2015;40:1243–1251. doi: 10.1007/s11064-015-1588-z. [DOI] [PubMed] [Google Scholar]
- Saxena T, Loomis KH, Pai SB, Karumbaiah L, Gaupp E, Patil K, Patkar R, Bellamkonda RV. Nanocarrier-mediated inhibition of macrophage migration inhibitory factor attenuates secondary injury after spinal cord injury. ACS Nano. 2015;9:1492–1505. doi: 10.1021/nn505980z. [DOI] [PubMed] [Google Scholar]
- Sun L, Pan J, Peng Y, Wu Y, Li J, Liu X, Qin Y, Bauman WA, Cardozo C, Zaidi M, Qin W. Anabolic steroids reduce spinal cord injury-related bone loss in rats associated with increased Wnt signaling. J Spinal Cord Med. 2013;36:616–622. doi: 10.1179/2045772312Y.0000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, He C, Du H, Zhang L. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49:276–287. doi: 10.1007/s12035-013-8518-3. [DOI] [PubMed] [Google Scholar]
- Tesiorowski M, Potaczek T, Jasiewicz B, Sapa J, Zygmunt M. Methylprednisolone-acute spinal cord injury, benefits or risks? Postepy Hig Med Dosw (Online) 2013;67:601–609. doi: 10.5604/17322693.1054873. [DOI] [PubMed] [Google Scholar]
- Vaquero J, Zurita M, Oya S, Aguayo C, Bonilla C. Early administration of methylprednisolone decreases apoptotic cell death after spinal cord injury. Histol Histopathol. 2006;21:1091–1102. doi: 10.14670/HH-21.1091. [DOI] [PubMed] [Google Scholar]
- Wang X, Xiao Y, Mou Y, Zhao Y, Blankesteijn WM, Hall JL. A role for the beta-catenin/T-cell factor signaling cascade in vascular remodeling. Circ Res. 2002;90:340–347. doi: 10.1161/hh0302.104466. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Fehlings MG. Emerging approaches to the surgical management of acute traumatic spinal cord injury. Neurotherapeutics. 2011;8:187–194. doi: 10.1007/s13311-011-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature, survey? Spinal Cord. 2006;44:523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- Xue H, Zhang XY, Liu JM, Song Y, Liu TT, Chen D. NDGA reduces secondary damage after spinal cord injury in rats via anti-inflammatory effects. Brain Res. 2013;1516:83–92. doi: 10.1016/j.brainres.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Hajec MC, Stanger R, Wan W, Young H, Mathern BE. Neuroprotective effects of perflurocarbon (oxycyte) after contusive spinal cord injury. J Neurotrauma. 2014;31:256–267. doi: 10.1089/neu.2013.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Liu YW, Shao W, Mao XG, Yang M, Ye ZX, Liang W, Luo ZJ. EGb761 improves histological and functional recovery in rats with acute spinal cord contusion injury. Spinal Cord. 2015;54:259–265. doi: 10.1038/sc.2015.156. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wu Y, Zheng L, Zhang C, Yang J, Shi M, Feng D, Wu Z, Wang YZ. Conditioned medium of Wnt/beta-catenin signaling-activated olfactory ensheathing cells promotes synaptogenesis and neurite growth in vitro. Cell Mol Neurobiol. 2013;33:983–990. doi: 10.1007/s10571-013-9966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhang G, Rong W, Wang A, Wu C, Huo X. Early applied electric field stimulation attenuates secondary apoptotic responses and exerts neuroprotective effects in acute spinal cord injury of rats. Neuroscience. 2015a;291:260–271. doi: 10.1016/j.neuroscience.2015.02.020. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wang ZG, Wu FZ, Kong XX, Yang J, Lin BB, Zhu SP, Lin L, Gan CS, Fu XB, Li XK, Xu HZ, Xiao J. Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neurobiol. 2013a;48:452–464. doi: 10.1007/s12035-013-8432-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cui Z, Feng G, Bao G, Xu G, Sun Y, Wang L, Chen J, Jin H, Liu J, Yang L, Li W. RBM5 and p53 expression after rat spinal cord injury: implications for neuronal apoptosis. Int J Biochem Cell Biol. 2015b;60:43–52. doi: 10.1016/j.biocel.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Zhang YK, Huang ZJ, Liu S, Liu YP, Song AA, Song XJ. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest. 2013b;123:2268–2286. doi: 10.1172/JCI65364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaei A, Ardakani MR, Hashemi MS, Peymani M, Ghaedi K, Baharvand H, Nasr-Esfahani MH. Acute course of deferoxamine promoted neuronal differentiation of neural progenitor cells through suppression of Wnt/beta-catenin pathway: a novel efficient protocol for neuronal differentiation. Neurosci Lett. 2015;590:138–144. doi: 10.1016/j.neulet.2015.01.083. [DOI] [PubMed] [Google Scholar]