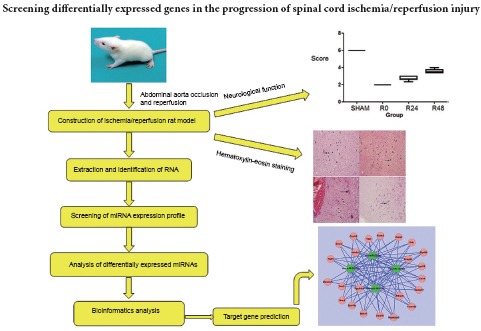
Keywords: nerve regeneration, spinal cord injury, ischemia/reperfusion injury, mRNA, microRNA, bioinformatics, Tmem69, Cxcl10, transcriptome, microRNA arrays, neural regeneration
Abstract
The temporal expression of microRNA after spinal cord ischemia/reperfusion injury is not yet fully understood. In the present study, we established a model of spinal cord ischemia in Sprague-Dawley rats by clamping the abdominal aorta for 90 minutes, before allowing reperfusion for 24 or 48 hours. A sham-operated group underwent surgery but the aorta was not clamped. The damaged spinal cord was removed for hematoxylin-eosin staining and RNA extraction. Neuronal degeneration and tissue edema were the most severe in the 24-hour reperfusion group, and milder in the 48-hour reperfusion group. RNA amplification, labeling, and hybridization were used to obtain the microRNA expression profiles of each group. Bioinformatics analysis confirmed four differentially expressed microRNAs (miR-22-3p, miR-743b-3p, miR-201-5p and miR-144-5p) and their common target genes (Tmem69 and Cxcl10). Compared with the sham group, miR-22-3p was continuously upregulated in all three ischemia groups but was highest in the group with no reperfusion, whereas miR-743b-3p, miR-201-5p and miR-144-5p were downregulated in the three ischemia groups. We have successfully identified the key genes expressed at different stages of spinal cord ischemia/reperfusion injury, which provide a reference for future investigations into the mechanism of spinal cord injury.
Introduction
Spinal cord injury (SCI) has a worldwide annual incidence of 11–60 cases per million people (Ackery et al., 2004) and is a major burden on patients and society. Spinal cord ischemia/reperfusion injury (SCIRI) refers to the damage caused by the recovery of the blood supply after SCI (Nieto-Diaz et al., 2014; Wang et al., 2016). The incidence of SCIRI is 3–18% (Wang et al., 2014) and usually has a poor prognosis, often leading to severe paralysis or even death (Richards et al., 1982; Krishnan et al., 1992). There is an urgent need for effective therapeutic and preventative strategies.
MicroRNA (miRNA) microarray studies can inform strategies for the development of more effective treatments for SCIRI, as they provide a broad view of the complex molecular and cellular changes that occur after injury. Bioinformatics analysis of the dynamic transcriptome and expression regulation may guide future research into the mechanisms underlying reperfusion injury. Establishing gene regulatory networks with mRNA data will lead to a better understanding of the pathogenesis of spinal cord ischemia/reperfusion injury.
Here, we explore the mRNA targets of miRNAs, using bioinformatics for high-order analysis of interconnected networks after SCI. Our data reveal affected pathways not previously identifiable with traditional techniques such as gene knock-in/out approaches or mRNA microarrays. The aim of the present study was to evaluate the use of miRNAs as a reliable and cost-effective biomarker for SCI or SCIRI, and identify further key disease-related genes.
Materials and Methods
Animals
We used 24 clean, healthy, adult male Sprague-Dawley rats, weighing 280–300 g (Beijing HFK Bioscience Co., Ltd., Beijing, China; animal license No. SCXK (Jing) 2009-0004). Rats were acclimated in individual cages under temperature-controlled conditions (23–25°C) and a 12-hour light/dark cycle, with free access to food and water. Surgical procedures were performed using standard sterile techniques under general anesthesia, and all efforts were made to minimize pain and distress in the animals. All experiments conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication No 85-23, revised 1996). This study was approved by the Institutional Animal Care and Use Committee, Jilin University, China.
Construction of SCIRI rat model
The rats were equally and randomly allocated to four groups: sham, 90-minute ischemia (I90R0), 90-minute ischemia + 24-hour reperfusion (I90R24), and 90-minute ischemia + 48-hour reperfusion (I90R48). Ischemia and reperfusion times were based on a preliminary study (Zhu et al, 2010). The SCIRI rat model was established by occluding the abdominal aorta, as described previously (Bowes et al, 1994). Surgical procedures were performed using a standard sterile technique under general anesthesia with chloral hydrate (0.3 mL/100 g). The abdominal aorta was exposed via a median longitudinal incision. In the I90R0, I90R24 and I90R48 groups, the abdominal aorta was blocked with a vascular clamp for 90 minutes; the left kidney changed color from bright to dark red, and the clamp was removed. The sham group underwent the same procedure without abdominal aorta occlusion. The incision was sutured after revascularization.
Neurological function assessment
Neurological function was assessed 24 or 48 hours after suturing the incision in the I90R24 and I90R48 groups, or when the rats in the sham and I90R0 groups had regained consciousness. Three investigators who had not participated in establishing the models performed hind limb motor function testing using the modified Tarlov score (Cheng et al., 1996), as follows: grade 0, no activity, totally paralyzed, no response to acupuncture; grade 1, no activity, totally paralyzed, response to acupuncture; grade 2, active, cannot load; grade 3, hind limb can load, cannot walk; grade 4, can walk, but unsteady, ataxia; grade 5, can walk, but not flexible, no ataxia; grade 6, normal walking. The mean value from the three investigators was calculated and recorded.
Histological staining
Spinal cords were harvested for hematoxylin-eosin staining 90 minutes after surgery in the sham and I90R0 groups, and after 24 and 48 hours of reperfusion in the I90R24 and I90R48 groups, respectively. A section of the spine at the L2–5 level was exposed and a 0.8-cm spinal segment (L3–4) was obtained. Part of this segment was fixed in 4% paraformaldehyde for 24 hours, dehydrated, embedded in paraffin wax, and cut into serial sections 4 µm thick. The sections were dewaxed with xylene and dehydrated through an alcohol gradient for hematoxylin–eosin staining and observed under a light microscope (BH-2; Olympus, Tokyo, Japan).
Extraction and identification of RNA
The remaining part of the spinal cord was triturated with a pestle and then homogenized with a Mini-Beadbeater-16 homogenizer (Biospec, Bartlesville, OK, USA) for 1–2 minutes. The samples were placed at room temperature for 5 minutes to completely dissociate the nucleic acid-protein complex, and then incubated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and chloroform. Total RNA from 700 µL samples was purified using the RNeasy Mini Kit (Qiagen cat. No. 74104, Germany) according to the manufacturer's instructions. Optical density (OD) values were measured at 230, 260 and 280 nm with a spectrophotometer (NanoDrop ND-1000; NanoDrop, Wilmington, DE, USA) in three samples from each group. OD ratios were calculated at 260 nm/280 nm and 260 nm/230 nm, and total RNA content and purity were measured to confirm that the extracted RNA satisfied the requirements of the subsequent experiments.
Rat SCIRI miRNA expression profile screening
The miRCURY LNA™ microRNA Array kit (V.16.0, cat. #208021, Exiqon, Denmark) was used to evaluate the miRNA expression profile for each group, and a GenePix 4000B microarray scanner (Axon Instruments, Union City, CA, USA) and GenePix Pro 6.0 (Axon Instruments) were used to quantify miRNA expression, according to the manufacturer's guidelines. The microarray analysis algorithm in the GenePix program was used for background correction, intra- and inter-microarray normalization, and expression signal calculation. Green signal strength calculation was performed after background subtraction, taking four replication sites per probe on the same chip. Data were normalized according to the following formula: normalized data = (green signal strength − background signal strength)/median value, where the median value is the miRNA strength (> 30), and background signal was normalized to the 50th percentile of all samples.
Preliminary analysis of differentially expressed miRNAs in rat models of SCIRI
We combined the miRNA expression profile data from the two spinal cord ischemia/reperfusion injury groups (I90R24 and I90R48 groups) to evaluate the total miRNA expression profile of rat models of SCIRI. GenePix Pro 6.0 was used to quantify miRNA array data, and miRNA expression was compared with the sham group using t-tests. Unsupervised hierarchical clustering was performed to analyze the miRNA expression profile, and regulatory pattern analysis of miRNAs with fold-changes ≥ 1.5 was carried out. Target prediction of these selected differentially expressed miRNAs was performed based on data provided in public databases including microRNA.org, Microcosm and miRanda. Bioinformatics analysis was carried out to identify the biological functions of the differentially expressed miRNAs. All data were analyzed using MATLAB 7.12 (MathWorks, Natick, MA, USA) to estimate the correlation coefficient (r) between differentially expressed miRNAs and target genes, with |r| values closer to 1 indicating a closer linear relationship between the two variables.
Statistical analysis
Measurement data are expressed as the mean ± SD, and analyzed using IBM SPSS 21.0 (IBM, Armonk, NY, USA). One-way analysis of variance and the Tukey-Kramer post hoc test were used to compare groups. P < 0.05 was considered statistically significant.
Results
Neurological function of rats with SCIRI
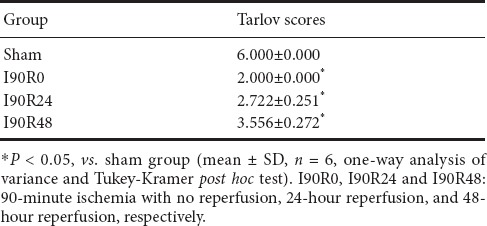
Limb function of rats in the I90R0, I90R24 and I90R48 groups was significantly impaired (Tarlov scores of 2–4) compared with the sham group (P < 0.05), but improved over time after reperfusion, with the first improvement noticeable after 24 hours (Table 1).
Table 1.
Neurological function in rats with spinal cord ischemia/reperfusion injury
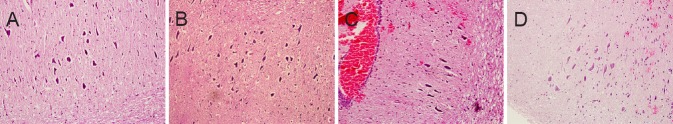
Histological changes of injured spinal cord in rats
In the sham group, neuronal processes were distinct; nuclei were darkly stained, intact and rounded; and no edema was found (Figure 1A). In the I90R0 group, cell bodies were irregular; the cytoplasm was slightly dense; nuclei began to swell and were weakly stained; and slight edema was observed (Figure 1B). In the I90R24 group, neurons were spindle-shaped; nuclei were displaced, small, and fragmented, and the degree of edema was greater than in the I90R0 group (Figure 1C). In the I90R48 group, neuronal cells were spindle-shaped; nuclei were fragmented or absent; edema was milder but still visible in interstitial tissue (Figure 1D).
Figure 1.
Histological changes in the injured spinal cord of rats (hematoxylin-eosin staining, light microscope, × 200).
(A) Nuclei were darkly stained, intact and rounded and no edema was observed. Cell bodies were irregular; the cytoplasm was slightly dense. (B) Nuclei began to swell and were weakly stained, and slight edema was observed. (C) Neurons were spindle-shaped; nuclei were displaced, small, and fragmented; edema was severe. (D) Neuronal cells were spindle-shaped; nuclei were fragmented or absent; edema was visible in interstitial tissue.
Extraction and identification of RNA
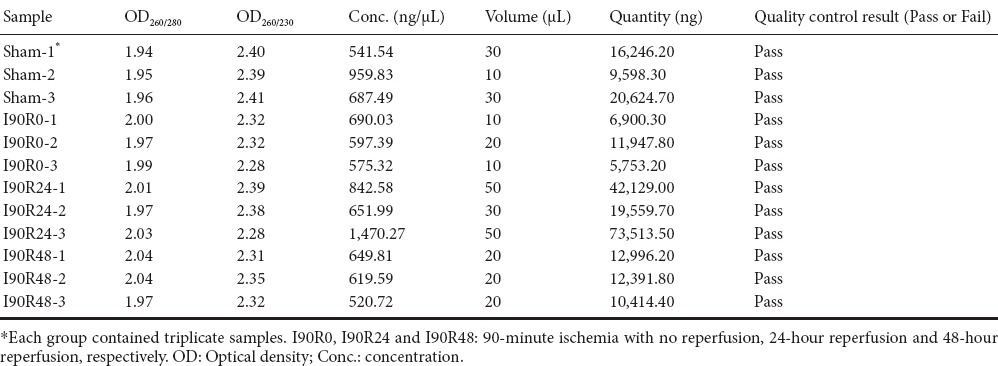
Total RNA values were > 1.8 in each group (Table 2), confirming that RNA extraction satisfied the requirements of the subsequent experiment.
Table 2.
Identification of RNA extracted from the injured spinal cord of rats
Screening of miRNA expression profile in rats with SCIRI
The miRNA expression profiles of the sham, I90R0, I90R24 and I90R48 groups were evaluated, and screenshots of the miRNA arrays were obtained (Figure 2).
Figure 2.
Scanning patterns of original miRNA chips (screenshot of Hy3™ fluorescent staining).
Microarray systematic error was reduced to an acceptable range by median normalization, allowing subsequent analysis of differentially expressed miRNAs. I90R0, I90R24 and I90R48: 90-minute ischemia with no reperfusion, 24-hour reperfusion and 48-hour reperfusion, respectively.
Preliminary analysis of differentially expressed miRNAs in rat models of SCIRI
Bioinformatics analysis confirmed that the group of genes whose expression levels changed with time after reperfusion was mainly regulated by four types of miRNA in the three ischemia groups. These were miR-22-3p, miR-743b-3p, miR-201-5p and miR-144-5p (Table 3). Furthermore, 2,698 different target genes were negatively correlated with these four miRNAs. Compared with the sham group, miR-22-3p was upregulated in all three experimental groups, with the highest expression in I90R0. miR-743b-3p, miR-201-5p and miR-144-5p were downregulated in the three experimental groups. There were 23 common target genes that were negatively regulated by all four miRNAs; among these were Tmem69 and Cxcl10. All 23 genes were used to construct a negatively correlated network of miRNA and mRNA interactions (Figure 3).
Table 3.
Negatively regulated interactions between miRNAs and target genes
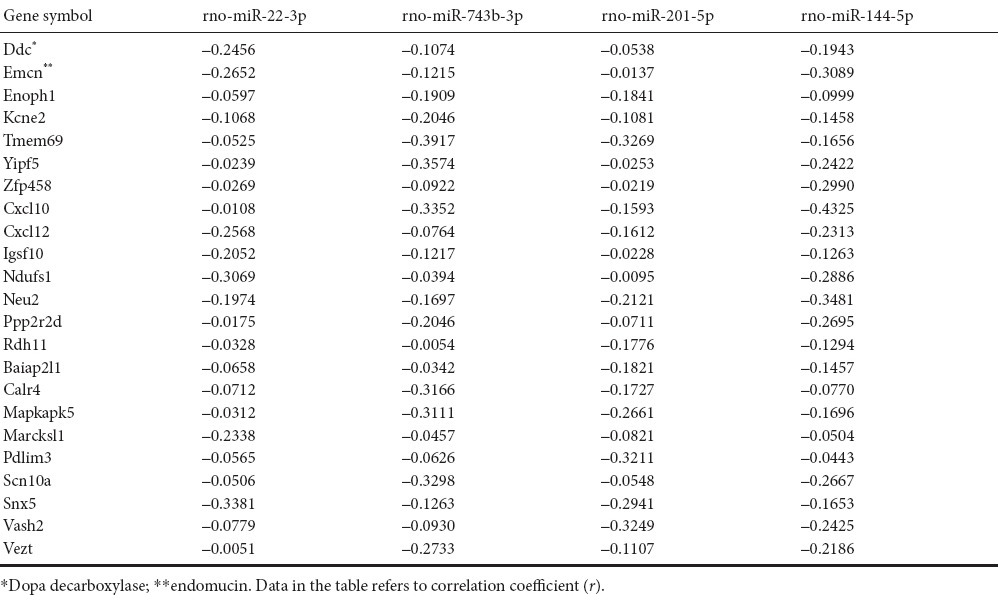
Figure 3.
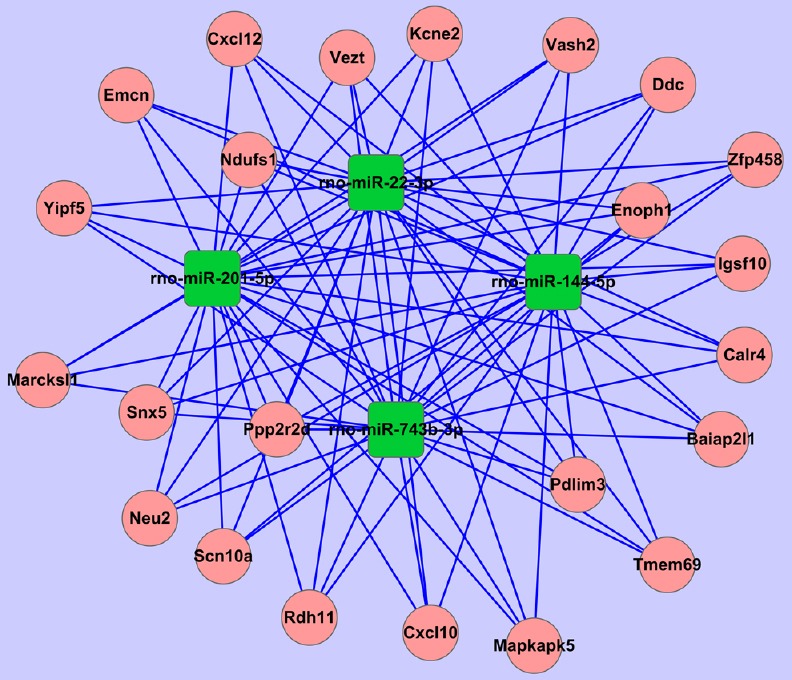
Negatively correlated miRNA gene network
Negatively regulated target genes common to all four miRNAs (miR-22-3p, miR-743b-3p, miR-201-5p and miR-144-5p).
Discussion
miRNAs are small, noncoding RNA fragments, approximately 22 nucleotides long (Gallego et al., 2012; Cheng et al., 2013, 2015; Van Giau et al., 2016), that modulate protein expression levels by antagonizing mRNA translation. miRNAs are emerging as powerful regulators of cellular function (Min et al., 2015; Lu et al., 2016; Stoicea et al., 2016) and important potential biotherapeutic targets for several diseases. The actions of miRNAs are thought to be widespread, as more than 1,500 miRNAs have been shown to affect the expression of many genes (Sheinerman et al., 2013; Cloutier et al., 2015). miRNA plays an important role in virtually all cellular functions, including proliferation, differentiation, and apoptosis (Siegel et al., 2012; Ksiazek-Winiarek et al., 2013).
SCIRI has long been known to follow SCI, but as yet, no clear definition, specific measurement index, or effective preventative measure or treatment for it has been identified. miRNA profiling may be helpful for early diagnosis of SCI or SCIRI, allowing for early prevention and timely treatment of many disorders. Neuronal cell injury in gray and white matter edema was observed immediately after ischemia, and markedly worsened 24 hours after reperfusion. At 48 hours after reperfusion, neuronal cell injury was still visible but edema had reduced. Sepramaniam et al. (2012) suggested that miR-130a, a strong transcriptional repressor of the AQP4 M1 isoform, could upregulate transcription of the AQP4 M1 transcript, causing a reduction in cerebral infarct size and promoting recovery. Zhi et al. (2014) confirmed the effect of miR-874 on the regulation of the intestinal barrier by targeting AQP3 in mice with intestinal ischemic injury. TICAM-2 is a novel target of miR-27a (Li et al., 2015), and upregulation of miR-27a attenuates irradiation-induced inflammatory damage to the blood-spinal cord barrier by negatively regulating TICAM-2 of the TLR4 signaling pathway and inhibiting the NF-κB/IL-1β pathway. Furthermore, miR-320a directly and functionally modulates AQP1 expression under in vitro and in vivo conditions (Li et al., 2016). Inhibition of AQP1 might provide a new therapeutic alternative for maintenance of blood spinal cord barrier integrity and treatment of spinal edema. These findings suggested that miRNAs could be used as potential regulators to modulate AQP1 in spinal cord edema after SCIRI.
In the present study, we found upregulation of miR-22-3p in all three experimental groups, with the highest expression in the I90R0 group. Conversely, miR-743b-3p, miR-201-5p and miR-144-5p were downregulated in all three groups. Our bioinformatics analysis confirmed that the group of differentially expressed genes involving damage evolution was mainly regulated by four types of miRNA (miR-22-3p, miR-743b-3p, miR-201-5p and miR-144-5p) and 2,698 different target genes were correlated negatively with the four miRNAs. We were then able to construct an miRNA–mRNA negative correlation network. These four miRNAs involved in the damage process play an important role in regulation. Together, the data provide a theoretical foundation for thorough analysis, research and verification of the transcriptome in spinal cord ischemia/reperfusion injury.
In a related study of premature ovarian failure, Dang et al. (2015) found that the expression of miR-22-3p was significantly lower than control values and that it caused disease progression, resulting in diminished ovarian reserve. Furthermore, overexpression of miR-22 was suggested to play a neuroprotective role by inhibiting apoptosis (Jovicic et al., 2013), and is a likely Huntington's disease-related initiation factor. miR-22 can target the regulation of tumor suppressor TET2 to promote hematopoietic stem cell self-renewal and transformation (Song et al., 2013; Danielson et al., 2015). Using bioinformatics analysis, we identified a highly conserved miR-434-3p binding site within the mRNA encoding eIF5A1. Consequently, miR-434-3p/eIF5A1 in muscle is a promising biotherapeutic for SCI repair (Wei et al., 2015; Shang et al., 2016). Bioinformatics analysis in our study showed that miR-22 expression in spinal cord tissue of rat models of SCIRI would play a reparative role by modulation of the apoptosis regulatory pathway. Despite the advantages of the miRNA analysis and analytical technology presented here, these techniques are not yet widely affordable or clinically viable as diagnostic tools, and require further standardization.
In conclusion, we have explored the prospective use of miRNA as a reliable and affordable biomarker for SCI or SCIRI. Our comparisons of healthy control spinal cord with spinal cord tissue exposed to ischemia/reperfusion injury provide an important reference for future studies.
Footnotes
Funding: This study was supported by a Grant from the National Natural Science Foundation of China, No. 81350013, 31572217, and 81672263.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Slone-Murphy J, Norman C, Li JA, Wang J, Li CH, Li JY, Song LP, Zhao M
References
- Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21:1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- Bowes MP, Masliah E, Otero DA, Zivin JA, Saitoh T. Reduction of neurological damage by a peptide segment of the amyloid beta/A4 protein precursor in a rabbit spinal cord ischemia model. Exp Neurol. 1994;129:112–119. doi: 10.1006/exnr.1994.1152. [DOI] [PubMed] [Google Scholar]
- Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Seience. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- Cheng L, Quek CY, Sun X, Bellingham SA, Hill AF. The detection of microRNA associated with Alzheimer's disease in biological fluids using next-generation sequencing technologies. Front Genet. 2013;4:150. doi: 10.3389/fgene.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Doecke JD, Sharples RA, Villemagne VL, Fowler CJ, Rembach A, Martins RN, Rowe CC, Macaulay SL, Masters CL, Hill AF. Prognostic serum miRNA biomarkers associated with Alzheimer's disease shows concordance with neuropsychological and neuroimaging assessment. Mol Psychiatry. 2015;20:1188–1196. doi: 10.1038/mp.2014.127. [DOI] [PubMed] [Google Scholar]
- Cloutier F, Marrero A, O'Connell C, Morin P., Jr MicroRNAs as potential circulating biomarkers for amyotrophic lateral sclerosis. J Mol Neurosci. 2015;56:102–112. doi: 10.1007/s12031-014-0471-8. [DOI] [PubMed] [Google Scholar]
- Dang Y, Zhao S, Qin Y, Han T, Li W, Chen ZJ. MicroRNA-22-3p is down-regulated in the plasma of Han Chinese patients with premature ovarian failure. Fertil Steril. 2015;103:802–807. doi: 10.1016/j.fertnstert.2014.12.106. [DOI] [PubMed] [Google Scholar]
- Danielson LS, Reavie L, Coussens M, Davalos V, Castillo-Martin M, Guijarro MV, Coffre M, Cordon-Cardo C, Aifantis I, Ibrahim S, Liu C, Koralov SB, Hernando E. Limited miR-17-92 overexpression drives hematologic malignancies. Leuk Res. 2015;39:335–341. doi: 10.1016/j.leukres.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego JA, Gordon ML, Claycomb K, Bhatt M, Lencz T, Malhotra AK. In vivo microRNA detection and quantitation in cerebrospinal fluid. J Mol Neurosci. 2012;47:243–248. doi: 10.1007/s12031-012-9731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicic A, Zaldivar Jolissaint JF, Moser R, Silva Santos Mde F, Luthi-Carter R. Micro RNA-22 (miR-22) overexpression is neuroprotective via general anti-apoptotic effects and may also target specific Huntington's disease-related mechanisms. PLoS One. 2013;8:e54222. doi: 10.1371/journal.pone.0054222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan KR, Glass CA, Turner SM, Watt JW, Fraser MH. Perceptual deprivation in the acute phase of spinal injury rehabilitation. J Am Paraplegia Soc. 1992;15:60–65. doi: 10.1080/01952307.1992.11735863. [DOI] [PubMed] [Google Scholar]
- Ksiazek-Winiarek DJ, Kacperska MJ, Glabinski A. MicroRNAs as novel regulators of neuroinflammation. Mediators Inflamm. 2013;2013:172351. doi: 10.1155/2013/172351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XQ, Fang B, Tan WF, Wang ZL, Sun XJ, Zhang ZL, Ma H. miR-320a affects spinal cord edema through negatively regulating aquaporin-1 of blood-spinal cord barrier during bimodal stage after ischemia reperfusion injury in rats. BMC Neurosci. 2016;17:10. doi: 10.1186/s12868-016-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li XQ, Lv HW, Wang ZL, Tan WF, Fang B, Ma H. MiR-27a ameliorates inflammatory damage to the blood-spinal cord barrier after spinal cord ischemia: reperfusion injury in rats by downregulating TICAM-2 of the TLR4 signaling pathway. J Neuroinflammation. 2015;12:25. doi: 10.1186/s12974-015-0246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Qin B, Hu H, Zhang J, Wang Y, Wang Q, Wang S. Integrative microRNA-gene expression network analysis in genetic hypercalcluric stone-forming rat kidney. Peer J. 2016;4:e1884. doi: 10.7717/peerj.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min XL, Wang TY, Cao Y, Liu J, Li JT, Wang TH. MicroRNAs: a novel promising therapeutic target for cerebral ischemia/reperfusion injury? Neural Regen Res. 2015;10:1799–1808. doi: 10.4103/1673-5374.170302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Diaz M, Esteban FJ, Reigada D, Muñoz-Galdeano T, Yunta M, Caballero-López M, Navarro-Ruiz R, Del Águila A, Maza RM. MicroRNA dysregulation in spinal cord injury: causes, consequences and therapeutics. Front Cell Neurosci. 2014;8:53. doi: 10.3389/fncel.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Hirt M, Melamed L. Spinal cord injury: a sensory restriction perspective. Arch Phys Med Rehabil. 1982;63:195–199. [PubMed] [Google Scholar]
- Sepramaniam S, Ying LK, Armugam A, Wintour EM, Jeyaseelan K. MicroRNA-130a represses transcriptional activity of aquaporin 4 M1 promoter. J Biol Chem. 2012;287:12006–12015. doi: 10.1074/jbc.M111.280701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang FF, Xia QJ, Liu W, Xia L, Qian BJ, You L, He M, Yang JL, Wang TH. miR-434-3p and DNA hypomethylation co-regulate eIF5A1 to increase AChRs and to improve plasticity in SCT rat skeletal muscle. Sci Rep. 2016;6:22884. doi: 10.1038/srep22884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinerman KS, Umansky SR. Circulating cell-free microRNA as biomarkers for screening, diagnosis and monitoring of neurodegenerative diseases and other neurologic pathologies. Front Cell Neurosci. 2013;7:150. doi: 10.3389/fncel.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel SR, Mackenzie J, Chaplin G, Jablonski NG, Griffiths L. Circulating microRNAs involved in multiple sclerosis. Mol Biol Rep. 2012;39:6219–6225. doi: 10.1007/s11033-011-1441-7. [DOI] [PubMed] [Google Scholar]
- Song SJ, Ito K, Ala U, Kats L, Webster K, Sun SM, Jongen-Lavrencic M, Manova-Todorova K, Teruya-Feldstein J, Avigan DE, Delwel R, Pandolfi PP. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell. 2013;13:87–101. doi: 10.1016/j.stem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoicea N, Du A, Lakis DC, Tipton C, Arias-Morales CE, Bergese SD. The MiRNA Journey from theory to practice as a CNS biomarker. Front Genet. 2016;7:11. doi: 10.3389/fgene.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Giau V, An SS. Emergence of exosomal miRNAs as a diagnostic biomarker for Alzheimer's disease. J Neurol Sci. 2016;360:141–152. doi: 10.1016/j.jns.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang F, Liu J, Zhao W, Zhao Q, He M, Qian BJ, Xu Y, Liu R, Liu SJ, Liu W, Liu J, Zhou XF, Wang TH. SNAP25 ameliorates sensory deficit in rats with spinal cord transection. Mol Neurobiol. 2014;50:290–304. doi: 10.1007/s12035-014-8642-8. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fang B, Tan Z, Zhang D, Ma H. Hypoxic preconditioning increases the protective effect of bone marrow mesenchymal stem cells on spinal cord ischemia/reperfusion injury. Mol Med Rep. 2016;13:1953–1960. doi: 10.3892/mmr.2016.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Li Z, Wang X, Wang J, Pang W, Yang G, Shen QW. microRNA-151-3p regulates slow muscle gene expression by targeting ATP2a2 in skeletal muscle cells. J Cell Physiol. 2015;230:1003–1012. doi: 10.1002/jcp.24793. [DOI] [PubMed] [Google Scholar]
- Zhi X, Tao J, Li Z, Jiang B, Feng J, Yang L, Xu H, Xu Z. MiR-874 promotes intestinal barrier dysfunction through targeting AQP3 following intestinal ischemic injury. FEBS Lett. 2014;588:757–763. doi: 10.1016/j.febslet.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Zhu BQ, Yang XY, Gu R, Qiu BT. Difference in proteome Maps between spinal cord ischemia reperfusion injury (IR) and normal rabbit spinal cord. Zhongguo Shiyan Zhenduanxue. 2010;4:487–489. [Google Scholar]