Keywords: nerve regeneration, RGC-5 cells, palmitic acid, nerve growth factor, apoptosis, PI3K, Akt, FoxO1, ERK1/2, neural regeneration
Abstract
Accumulating evidence supports an important role for nerve growth factor (NGF) in diabetic retinopathy. We hypothesized that NGF has a protective effect on rat retinal ganglion RGC-5 cells injured by palmitic acid (PA), a metabolic factor implicated in the development of diabetes and its complications. Our results show that PA exposure caused apoptosis of RGC-5 cells, while NGF protected against PA insult in a concentration-dependent manner. Additionally, NGF significantly attenuated the levels of reactive oxygen species (ROS) and malondialdehyde (MDA) in RGC-5 cells. Pathway inhibitor tests showed that the protective effect of NGF was completely reversed by LY294002 (PI3K inhibitor), Akt VIII inhibitor, and PD98059 (ERK1/2 inhibitor). Western blot analysis revealed that NGF induced the phosphorylation of Akt/FoxO1 and ERK1/2 and reversed the PA-evoked reduction in the levels of these proteins. These results indicate that NGF protects RGC-5 cells against PA-induced injury through anti-oxidation and inhibition of apoptosis by modulation of the PI3K/Akt and ERK1/2 signaling pathways.
Introduction
Diabetic retinopathy (DR) is a severe complication of diabetes mellitus and the leading cause of blindness worldwide (Zheng et al., 2012). Accumulating evidence suggests that excessive plasma levels of saturated fatty acids, such as palmitic acid, are caused by a high-fat diet. This can lead to insulin resistance and its associated complications, including DR (Kulacoglu et al., 2003; Shen et al., 2014; Kumar et al., 2015; Sasaki et al., 2015). Progressive loss of the retinal cells responsible for communication between the eye and brain contributes to early pathogenic events in DR, and can explain some of the vision defects that occur soon after the onset of diabetes (Barber et al., 2011; van Dijk et al., 2012; Pelikánová, 2016). Traditional treatments such as photocoagulation, vitrectomy and anti-vascular endothelial growth factor therapy can be effective, but are limited and can have considerable side effects (Yam and Kwok, 2007; Wilkinson-Berka, 2008). Novel approaches are, therefore, being sought that can prevent or delay retinal cell death and maintain normal neuronal functions.
Nerve growth factor (NGF), discovered in 1948 (Bradshaw et al., 1984), prevents neuronal apoptosis in primary cultured neurons and reduces neuronal degeneration in animal models of neurodegenerative diseases (Wiese et al., 1999; Sofroniew et al., 2001). In the retina, NGF is produced and utilized by retinal ganglion cell (RGCs) and glial cells in a paracrine and autocrine fashion (Turner et al., 1980; Mysona et al., 2014). Restoring NGF signaling has been reported to be a potential therapeutic strategy to overcome retinal degenerative diseases, including DR (Colafrancesco et al., 2011; Abu El-Asrar et al., 2013; Mysona et al., 2013, 2015; Mantelli et al., 2014; Wang et al., 2015b; Zhang and Zhou, 2015). NGF can prevent early retinal cell apoptosis and development of cellular occluded capillaries (Hammes et al., 1995), while an anti-NGF antibody increased RGC loss in experimental diabetic rats (Mantelli et al., 2014). Furthermore, NGF had a neuroprotective effect on RGCs after retinal ischemia/reperfusion injury (Chen et al., 2015), while administration of NGF eye drops restored TrkA levels in the retina, and protected RGCs from degeneration in an experimental diabetic model and a glaucoma rat model (Lambiase et al., 2009; Colafrancesco et al., 2011). A large number of studies, including from our group, show that NGF confers its neuroprotection via PI3K/Akt and ERK1/2 signaling pathways in primary neurons and cell lines (Gan et al., 2005; Lambiase et al., 2009; Wen et al., 2011). The PI3k/Akt and ERK1/2 signaling pathways are the two main pathways involved in cell survival and apoptosis (Schmitz et al., 2007). They are activated by growth factors, drugs and hormones but play different neuroprotective roles under different conditions (Ahn, 2014; Li et al., 2014). Members of the FoxO (forkhead box, O class) Forkhead transcription factor family, including FoxO1, 3, 4, and 6, are downstream targets of PI3K/Akt and phosphorylation decreases their transcriptional activity, resulting in their redistribution to the cytoplasm (Dobson et al., 2011). FoxO1 has a crucial role in apoptosis and survival of different cells (Zhang et al., 2011).
The retinal ganglion RGC-5 cell line, derived from post-natal rat retina, has characteristic retinal progenitor markers and can be used to study cellular and molecular mechanisms of RGC-associated eye diseases (Maher and Hanneken, 2005). However, whether NGF retains its protective action in RGC-5 cells against PA insult remains unclear. This study aimed to explore the neuroprotective effect of NGF on PA-induced RGC-5 injury.
Materials and Methods
Cell culture
RGC-5 cells were provided by Sun Yat-sen University, Guangzhou, Guangdong Province, China. Cells were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) (Invitrogen), streptomycin (100 μg/mL; Invitrogen) and penicillin (100 U/mL; Invitrogen) at 37°C in a 5% CO2 humidified atmosphere. Medium was changed every 3 days and 25% of cells were passaged weekly.
MTT assay
Cell viability was assessed using the MTT assay as described previously (Wang et al., 2013; Zeng et al., 2016a). Briefly, RGC-5 cells were seeded in 96-well plates at a density of 2 × 105 cells/well. Cultures were incubated with 100 μM PA (Sigma-Aldrich, St. Louis, MO, USA) or pretreated with 25-100 ng/mL NGF for 24 hours, and were then incubated with MTT (0.5 mg/mL; Sigma-Aldrich) for another 3 hours. Medium was removed and dimethyl sulfoxide (DMSO; 200 μL) added to each well. The optical density (OD) of each well was measured at a wavelength of 570 nm using a Multiskan Ascent Revelation Plate Reader (Thermo Fisher Scientific, Waltham, MA, USA) and the data are presented as a percentage relative to the control. Assays were repeated three to six times.
To evaluate the role of PI3K/Akt and Erk1/2 pathways on the survival promoting effect of NGF on cell apoptosis induced by PA, the cultures were pretreated with NGF (50 ng/mL) in the presence of preincubated with the PI3K inhibitor LY294002 (10 μM; Calbiochem, La Jolla, CA, USA), AktVIII (5 μM; Calbiochem) and the Erk1/2 inhibitor PD98059 (10 μM; Calbiochem) for 30 minutes then PA treated for another 24 hours, and the viability of cells was determined by the MTT assay.
Annexin V-FITC/PI staining to evaluate apoptosis
RGC-5 cells were treated for 16 hours with 100 μM PA with or without 50 ng/mL NGF pretreatment. Cells were then digested, washed twice with ice-cold phosphate buffered saline (PBS) then centrifuged for 5 minutes and re-suspended in 195 μL Annexin V-FITC binding buffer (Beijing 4A Biotech, China) as described previously (Zeng et al., 2016b). Annexin V-FITC (20 μg/mL) was added and the cells incubated away from light at 20–25°C for 10 minutes. Then cells were then washed with ice-cold PBS and resuspended in binding buffer. Propidium iodide (PI) (1 mg/mL) (Beijing 4A Biotech) was then added and the cells incubated in darkness. Apoptosis was quantified by flow cytometry using Cell Quest Pro software (Beckman Coulter, Brea, CA, USA).
Measurement of reactive oxygen species (ROS)
Intracellular ROS accumulation was measured using H2DCF-DA (Wang et al., 2015a). Briefly, after treatment, RGC-5 cells were washed and then stained with 10 μM H2DCF-DA (Sigma-Aldrich) in serum-free medium for 30 minutes at 37°C in the dark. The cells were photographed using a fluorescence microscope (Olympus, Tokyo, Japan).
Estimation of malondialdehyde (MDA)
MDA reacts with thiobarbituric acid (TBA) to produce a fluorescent product (Wang et al., 2015a) that can be measured using a pectrofluorometer microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) at a wavelength of 535 nm. Therefore, RGC-5 cells in 6-well plates were exposed to 100 μM PA with or without 50 ng/mL NGF pretreatment and cultured to more than 90% confluence. Cells were harvested and MDA levels were determined using an MDA detection kit from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer's instructions.
Western blot assay
Following treatment, RGC-5 cells were lysed with ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer as described previously (Zheng and Quirion, 2009). Protein concentration was determined with a protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer's instructions. Samples with equal amounts of protein were separated on 10% polyacrylamide gels, then transferred to polyvinylidene fluoride (PVDF) membranes and probed with selective anti-phospho Akt (Ser473), FoxO1 or ERK1/2 antibodies or a total Akt/FoxO1/ERK1/2 antibody, at 4°C overnight. Anti-phospho-Akt (Ser473) antibody (1:1,000), anti-Akt antibody (1:1,000), anti-phospho-FoxO1 antibody (1:1,000), anti-FoxO1 antibody (1:1,000), anti-phospho ERK1/2 antibody (1:1,000), and anti-ERK1/2 antibody (1:1,000) were obtained from Cell Signaling Technology (Woburn, MA, USA). Membranes were then washed twice with Tris-buffered saline containing Tween (TBST) and incubated at room temperature for 1 hour with appropriate secondary antibodies conjugated with horseradish peroxidase (Cell Signaling Technology). Membranes were finally washed several times with TBST to remove unbound secondary antibodies and visualized using enhanced chemiluminescence (ECL) as described by the manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA, USA). Blots were subsequently stripped of antibodies and re-probed with the pan antibody to confirm equal protein loading. Band intensity was quantified in the linear range by densitometry using image J software (NIH, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± SEM or mean ± SD. Variation between groups was analyzed using one-way analysis of variance and least significant difference post hoc test. P < 0.05 was considered statistically significant. Statistical analyses were conducted with SPSS 13.0 (SPSS, Chicago, IL, USA).
Results
NGF attenuated PA-induced cell death in RGC-5 cells
MTT assays showed that RGC-5 cells pretreated with NGF for 30 minutes were protected from PA-induced insult in a concentration-dependent manner (Figure 1A). A significant inhibition effect of NGF commenced at 50 and 100 ng/mL. Flow cytometry indicated that 100 μM PA caused apoptosis of RGC-5 cells, while NGF (50 ng/mL) pretreatment reversed the effect (Figure 1B, C).
Figure 1.
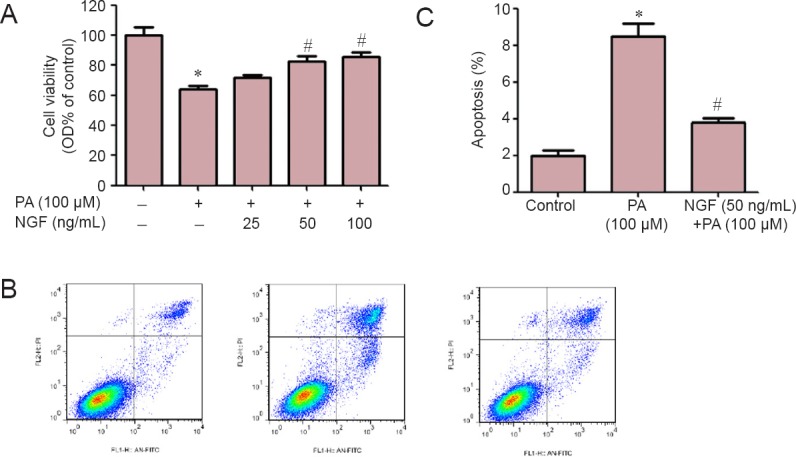
NGF attenuated PA-induced cell death in RGC-5 cells.
(A) Cell viability was determined by MTT assays; (B, C) apoptosis of cells was measured by the Annexin V-FITC/PI assay. Results are shown as the mean ± SEM and represent three independent experiments (n = 6 for MTT, n = 3 for flow cytometry, one-way analysis of variance and least significant difference post hoc test). *P < 0.05, vs. control group; #P < 0.05, vs. PA group. OD: Optical density; PA: palmitic acid; NGF: nerve growth factor.
NGF inhibited the levels of ROS and MDA in RGC-5 cells
PA produces oxidative stress in cells (Wong et al., 2014). MDA, formed by the degradation of polyunsaturated lipids by ROS is used as a marker of oxidative stress (Clarkson and Thompson, 2000). As shown in Figure 2, NGF diminished the elevation of ROS and MDA caused by PA.
Figure 2.
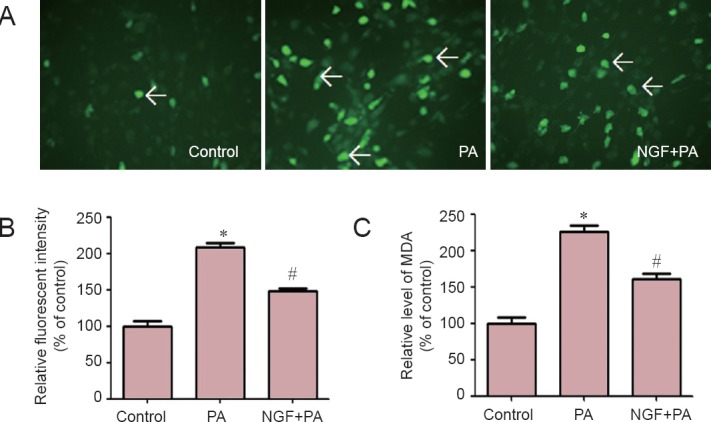
NGF inhibited the levels of ROS and MDA elevated by PA in RGC-5 cells.
(A) RGC-5 cells were pretreated with NGF (50 ng/mL), and then incubated with 100 μM PA for 12 hours (magnification × 200). The fluorescence intensity of DCFH-DA showed that NGF reduced the accumulation of ROS (arrows) caused by PA. (B) ROS levels in RGC-5 cells after exposure to PA with or without NGF pretreatment. (C) MDA levels in RGC-5 cells. ROS and MDA levels are presented as a percentage of control (n = 3, one-way analysis of variance and least significant difference post hoc test). *P < 0.05, vs. control group; #P < 0.05, vs. PA group. PA: Palmitic acid; NGF: nerve growth factor; ROS: reactive oxygen species; MDA: malondialdehyde.
PI3K/Akt and ERK1/2 signaling pathways mediated the protective effect of NGF in RGC-5 cells
We have previously shown that NGF stimulates PI3K/Akt and ERK1/2 pathways in PC12 cells (Wen et al., 2011). MTT assays showed that the protective effect of NGF was diminished in the presence of the PI3K inhibitor, LY294002, the Akt inhibitor, AktVIII, and the ERK1/2 inhibitor, PD98059 (Figure 3). The concentrations of inhibitors used (LY294002, 10 µM; AktVIII, 5 μM; and PD98059, 5 µM) had no effect on cell death itself, as previously reported (Wang et al., 2015b). Thus, both PI3K/Akt and ERK1/2 pathways mediated the protective effect of NGF.
Figure 3.
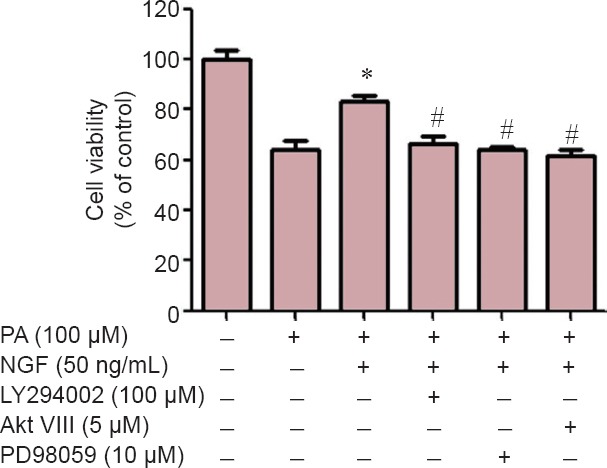
Both Akt and ERK1/2 signaling pathways mediated the protective effect of NGF in RGC-5 cells.
RGC-5 cells pretreated with PI3K inhibitor LY294002, Akt inhibitor VIII or ERK1/2 inhibitor PD98059 were treated with NGF and PA for 24 hours. Cell viability was determined by the MTT assay. Data are expressed as a percentage of the corresponding control, which was set at 100%. Results are shown as the mean ± SEM and represent assays from three independent experiments (n = 6, one-way analysis of variance and least significant difference post hoc test). #P < 0.05, vs. PA group; †P < 0.05, vs. NGF + PA group. PA: Palmitic acid; NGF: nerve growth factor.
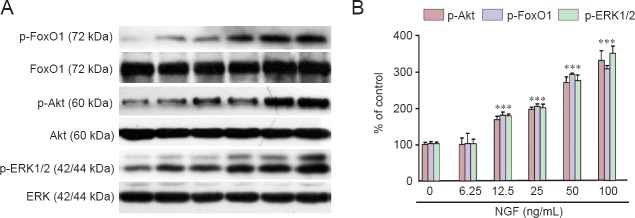
NGF stimulated the phosphorylation of Akt, FoxO1, and ERK1/2 in RGC-5 cells in a concentration-dependent manner
As shown in Figure 4, NGF increased the phosphorylation of p-Akt (Ser473), p-FoxO1 and p-ERK1/2 in RGC-5 cells in a dose-dependent fashion after 10 minutes of stimulation.
Figure 4.
NGF stimulated the phosphorylation of Akt, FoxO1, and ERK1/2 in RGC-5 cells.
(A) RGC-5 cells were treated with different concentrations of NGF for 10 minutes. Levels of phosphorylated Akt, FoxO1 and ERK1/2 were analyzed by immunoblotting. (B) Relative levels of p-Akt versus total Akt, p-FoxO1 versus total FoxO1 and p-ERK1/2 versus total ERK1/2 in each sample were determined by densitometry and are expressed as a percentage of control, which was set at 100%. Results are shown as the mean ± SEM and represent three independent experiments (n = 3, one-way analysis of variance and least significant difference post hoc test). *P < 0.05, vs. control group (0 ng/mL NGF). NGF: Nerve growth factor.
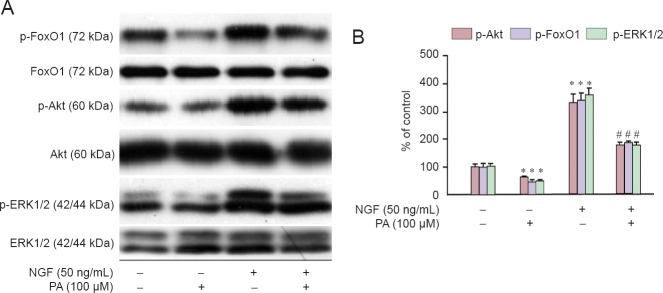
NGF reversed the down-regulation of Akt/FoxO1 and ERK1/2 phosphorylation induced by PA
Cells incubated with NGF (50 ng/mL) for 30 minutes were exposed to PA for 4 hours and the phosphorylation of Akt/FoxO1 and ERK1/2 were analyzed. PA decreased the phosphorylation of Akt/FoxO1 and ERK1/2 in RGC-5 cells, while NGF prevented this effect (Figure 5).
Figure 5.
NGF reversed the down-regulation of Akt/FoxO1 and ERK1/2 phosphorylation induced by PA.
(A) RGC-5 cells were treated with 50 ng/mL NGF for 30 minutes, and then incubated with 100 μM PA for 4 hours. Expression of phosphorylated Akt, FoxO1 and ERK1/2 was analyzed by immunoblotting. (B) Relative levels of p-Akt versus total Akt, p-FoxO1 versus total FoxO1 and p-ERK1/2 versus total ERK1/2 in each sample were determined by densitometry and are expressed as a percentage of the control, which was set at 100%. Results are shown as the mean ± SEM and represent three independent experiments (n = 3, one-way analysis of variance and least significant difference post hoc test). *P < 0.05, vs. control group (0 ng/mL NGF + 0 μM PA); #P < 0.05, vs. PA (100 μM) group. PA: Palmitic acid; NGF: nerve growth factor.
Discussion
DR, a major ocular complication of diabetes, is a leading cause of blindness in working age adults worldwide and limited treatments are available (Mysona et al., 2014). In addition to microcirculation abnormalities, neurodegenerative changes appear in the retina at an early stage of DR (van Dijk et al., 2012). Recently, increased apoptosis of RGCs was demonstrated in humans with diabetes, which leads to the progressive loss of retinal neurons and functional deficits in vision (Ng et al., 2016). Nevertheless, the effect of excessive PA on apoptosis of RGCs is unknown. In line with a previous report (Wang et al., 2016), we show that PA exposure induced dramatic apoptosis of RGC-5 cells but at a lower PA concentration. Thus, our data support high levels of saturated fatty acids as an important metabolic risk factor associated with the increased apoptosis of RGCs at the onset of DR.
The widespread involvement of NGF in retinal dysfunction is based on a diabetes-induced proNGF/NGF imbalance and alterations in TrkA and p75NTR receptor function and expression (Mohamed and El-Remessy, 2015). Reduction of trophic support due to decreased NGF expression contributes to diabetes-induced RGC death. The importance of NGF in RGC survival is illustrated by recent studies, in which NGF supplementation reduced diabetes-induced RGC death (Hammes et al., 1995; Mantelli et al., 2014). In our study, we discovered that NGF was able to protect against PA-induced death of RGC-5 cells, which further indicated that NGF can block diabetes-induced RGC death. In the development of diabetic retinopathy, increased oxidative stress is another early event (Wu et al., 2014). Our findings suggest that decreasing oxidative stress caused by PA might be another mechanism by which NGF ameliorates the PA insult.
The PI3K/Akt and ERK1/2 pathways are the two main pathways involved in the survival and adaptive protection of various cell types that are activated by growth factors, hormones and drugs. They also play different roles in neuroprotection observed under different conditions (Schmitz et al., 2007). Previous reports indicate that NGF exerts a neuroprotective effect on RGCs against retinal ischemia/reperfusion injury by regulating the PI3K/Akt signaling pathway (Chen et al., 2015). Similarly, we show here that the protective effect of NGF was completely abolished in the presence of the PI3K inhibitor, LY294002, Akt inhibitor VIII, as well as the ERK1/2 inhibitor, PD98059, indicating that NGF elicits its protective effects via PI3K/Akt and ERK1/2 signaling pathways. In addition, treatment of RGC-5 cells with NGF leads to the phosphorylation of Akt and ERK1/2, as seen in our previous report (Wen et al., 2011), while PA decreases Akt and Erk1/2 phosphorylation. In general, these data suggest that NGF promotes RGC-5 cell survival and protects cells from the toxic effects of PA insult by specifically activating the pro-survival PI3K/Akt and ERK1/2 pathways.
The FoxO1 transcription factor is important in the cell cycle as its nuclear localization causes apoptosis (Zhang et al., 2011). We have previously reported that NGF induced the phosphorylation of FoxO1 in cultured PC12 cells (Wen et al., 2011). Interestingly, FoxO1 is a direct downstream target of Akt; thus, we examined the potential role of FoxO1 in the NGF promotion of RGC-5 cell survival. We found that PA inhibited the level of phosphorylated FoxO1, in contrast to the increase caused by NGF.
In summary, the present study demonstrates that the protective effect of NGF against apoptosis of RGC-5 cells is mediated through stimulation of the PI3K/Akt and ERK1/2 pathways. Most importantly, the present study also illustrates that inhibition of oxidative stress and FoxO1 are involved in these events. However, the effects of NGF in vivo and its specific mechanisms of action require further detailed investigation.
Acknowledgments
We are very grateful to the Wen-hua Zheng from the Sun Yat-sen University, China for providing RGC-5 cells.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Allen J, Frenchman B, Yan PS, Yu J, Li CH, Li JY, Song LP, Zhao M
References
- Abu El-Asrar AM, Mohammad G, De Hertogh G, Nawaz MI, Van Den Eynde K, Siddiquei MM, Struyf S, Opdenakker G, Geboes K. Neurotrophins and neurotrophin receptors in proliferative diabetic retinopathy. PLoS One. 2013;8:e65472. doi: 10.1371/journal.pone.0065472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JY. Neuroprotection signaling of nuclear akt in neuronal cells. Exp Neurobiol. 2014;23:200–206. doi: 10.5607/en.2014.23.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Visual Sci. 2011;52:1156–1163. doi: 10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw RA, Dunbar JC, Isackson PJ, Kouchalakos RN, Morgan CJ. Nerve growth factor: mechanism of action. Symp Fundam Cancer Res. 1984;37:87–101. [PubMed] [Google Scholar]
- Chen Q, Wang H, Liao S, Gao Y, Liao R, Little PJ, Xu J, Feng ZP, Zheng Y, Zheng W. Nerve growth factor protects retinal ganglion cells against injury induced by retinal ischemia-reperfusion in rats. Growth Factors. 2015;33:149–159. doi: 10.3109/08977194.2015.1010642. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Thompson HS. Antioxidants: what role do they play in physical activity and health? Am J Clin Nutr. 2000;72:637S–646S. doi: 10.1093/ajcn/72.2.637S. [DOI] [PubMed] [Google Scholar]
- Colafrancesco V, Coassin M, Rossi S, Aloe L. Effect of eye NGF administration on two animal models of retinal ganglion cells degeneration. Ann Ist Super Sanita. 2011;47:284–289. doi: 10.4415/ANN_11_03_08. [DOI] [PubMed] [Google Scholar]
- Dobson M, Ramakrishnan G, Ma S, Kaplun L, Balan V, Fridman R, Tzivion G. Bimodal regulation of FoxO3 by AKT and 14-3-3. Biochim Biophys Acta. 2011;1813:1453–1464. doi: 10.1016/j.bbamcr.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Zheng W, Chabot JG, Unterman TG, Quirion R. Nuclear/cytoplasmic shuttling of the transcription factor FoxO1 is regulated by neurotrophic factors. J Neurochem. 2005;93:1209–1219. doi: 10.1111/j.1471-4159.2005.03108.x. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Federoff HJ, Brownlee M. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med. 1995;1:527–534. [PMC free article] [PubMed] [Google Scholar]
- Kulacoglu DN, Kocer I, Kurtul N, Keles S, Baykal O. Alterations of fatty acid composition of erythrocyte membrane in type 2 diabetes patients with diabetic retinopathy. Jpn J Ophthalmol. 2003;47:551–556. doi: 10.1016/j.jjo.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Kumar B, Kowluru A, Kowluru RA. Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Invest Ophthalmol Visual Sci. 2015;56:2985–2992. doi: 10.1167/iovs.15-16466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Aloe L, Centofanti M, Parisi V, Mantelli F, Colafrancesco V, Manni GL, Bucci MG, Bonini S, Levi-Montalcini R. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: implications for glaucoma. Proc Natl Acad Sci U S A. 2009;106:13469–13474. doi: 10.1073/pnas.0906678106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Chen M, Liu H, Yang L, Yang T, He G. The dual role of ERK signaling in the apoptosis of neurons. Front Biosci (Landmark Ed) 2014;19:1411–1417. doi: 10.2741/4291. [DOI] [PubMed] [Google Scholar]
- Maher P, Hanneken A. The molecular basis of oxidative stress-induced cell death in an immortalized retinal ganglion cell line. Invest Ophthalmol Visual Sci. 2005;46:749–757. doi: 10.1167/iovs.04-0883. [DOI] [PubMed] [Google Scholar]
- Mantelli F, Lambiase A, Colafrancesco V, Rocco ML, Macchi I, Aloe L. NGF and VEGF effects on retinal ganglion cell fate: new evidence from an animal model of diabetes. Eur J Ophthalmol. 2014;24:247–253. doi: 10.5301/ejo.5000359. [DOI] [PubMed] [Google Scholar]
- Mohamed R, El-Remessy AB. Imbalance of the Nerve Growth Factor and Its Precursor: Implication in Diabetic Retinopathy. J Clin Exp Ophthalmol. 2015;6:483. doi: 10.4172/2155-9570.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysona BA, Shanab AY, Elshaer SL, El-Remessy AB. Nerve growth factor in diabetic retinopathy: beyond neurons. Expert Rev Ophthalmol. 2014;9:99–107. doi: 10.1586/17469899.2014.903157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysona BA, Al-Gayyar MM, Matragoon S, Abdelsaid MA, El-Azab MF, Saragovi HU, El-Remessy AB. Modulation of p75(NTR) prevents diabetes- and proNGF-induced retinal inflammation and blood–retina barrier breakdown in mice and rats. Diabetologia. 2013;56:2329–2339. doi: 10.1007/s00125-013-2998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysona BA, Matragoon S, Stephens M, Mohamed IN, Farooq A, Bartasis ML, Fouda AY, Shanab AY, Espinosa-Heidmann DG, El-Remessy AB. Imbalance of the nerve growth factor and its precursor as a potential biomarker for diabetic retinopathy. Biomed Res Int 2015. 2015 doi: 10.1155/2015/571456. 571456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DS, Chiang PP, Tan G, Cheung CG, Cheng CY, Cheung CY, Wong TY, Lamoureux EL, Ikram MK. Retinal ganglion cell neuronal damage in diabetes and diabetic retinopathy. Clin Exp Ophthalmol. 2016;44:243–250. doi: 10.1111/ceo.12724. [DOI] [PubMed] [Google Scholar]
- Pelikánová T. Diabetic retinopathy: pathogenesis and therapeutic implications. Vnitr Lek. 2016;62:620–628. [PubMed] [Google Scholar]
- Sasaki M, Kawasaki R, Rogers S, Man RE, Itakura K, Xie J, Flood V, Tsubota K, Lamoureux E, Wang JJ. The Associations of Dietary Intake of Polyunsaturated Fatty Acids With Diabetic Retinopathy in Well-Controlled DiabetesDietary PUFAs and Diabetic Retinopathy. Invest Ophthalmol Visual Sci. 2015;56:7473–7479. doi: 10.1167/iovs.15-17485. [DOI] [PubMed] [Google Scholar]
- Schmitz KJ, Lang H, Wohlschlaeger J, Sotiropoulos GC, Reis H, Schmid KW, Baba HA. AKT and ERK1/2 signaling in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2007;13:6470–6477. doi: 10.3748/wjg.v13.i48.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Bi YL, Das UN. Potential role of polyunsaturated fatty acids in diabetic retinopathy. Arch Med Sci. 2014;10:1167–1174. doi: 10.5114/aoms.2014.47826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Turner JE, Delaney RK, Johnson JE. Retinal ganglion cell response to nerve growth factor in the regenerating and intact visual system of the goldfish (Carassius auratus) Brain Res. 1980;197:319–330. doi: 10.1016/0006-8993(80)91118-x. [DOI] [PubMed] [Google Scholar]
- van Dijk HW, Verbraak FD, Kok PH, Stehouwer M, Garvin MK, Sonka M, DeVries JH, Schlingemann RO, Abràmoff MD. Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Visual Sci. 2012;53:2715–2719. doi: 10.1167/iovs.11-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Zhu HZ, Li SW, Yang JM, Xiao Y, Kang QR, Li CY, Zhao YS, Zeng Y, Li Y, Zhang J, He ZD, Ying Y. Crude saponins of panax notoginseng have neuroprotective effects to inhibit palmitate-triggered endoplasmic reticulum stress-associated apoptosis and loss of postsynaptic proteins in staurosporine differentiated rgc-5 retinal ganglion cells. J Agric Food Chem. 2016;64:1528–1539. doi: 10.1021/acs.jafc.5b05864. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou X, Huang J, Mu N, Guo Z, Wen Q, Wang R, Chen S, Feng ZP, Zheng W. The role of Akt/FoxO3a in the protective effect of venlafaxine against corticosterone-induced cell death in PC12 cells. Psychopharmacology. 2013;228:129–141. doi: 10.1007/s00213-013-3017-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Liao S, Geng R, Zheng Y, Liao R, Yan F, Thrimawithana T, Little PJ, Feng ZP, Lazarovici P, Zheng W. IGF-1 signaling via the PI3K/Akt pathway confers neuroprotection in human retinal pigment epithelial cells exposed to sodium nitroprusside insult. J Mol Neurosci. 2015a;55:931–940. doi: 10.1007/s12031-014-0448-7. [DOI] [PubMed] [Google Scholar]
- Wang R, Yang J, Peng L, Zhao J, Mu N, Huang J, Lazarovici P, Chen H, Zheng W. Gardenamide A attenuated cell apoptosis induced by serum deprivation insult via the ERK1/2 and PI3K/AKT signaling pathways. Neuroscience. 2015b;286:242–250. doi: 10.1016/j.neuroscience.2014.11.056. [DOI] [PubMed] [Google Scholar]
- Wen Q, Duan X, Liao R, Little P, Gao G, Jiang H, Lalit S, Quirion R, Zheng W. Characterization of intracellular translocation of Forkhead transcription factor O (FoxO) members induced by NGF in PC12 cells. Neurosci Lett. 2011;498:31–36. doi: 10.1016/j.neulet.2011.04.055. [DOI] [PubMed] [Google Scholar]
- Wiese S, Digby MR, Gunnersen JM, Götz R, Pei G, Holtmann B, Lowenthal J, Sendtner M. The anti-apoptotic protein ITA is essential for NGF-mediated survival of embryonic chick neurons. Nat Neurosci. 1999;2:978–983. doi: 10.1038/14777. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Berka JLM, A G. Update on the treatment of diabetic retinopathy. ScientificWorldJournal. 2008;8:98–120. doi: 10.1100/tsw.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KL, Wu YR, Cheng KS, Chan P, Cheung CW, Lu DY, Su TH, Liu ZM, Leung YM. Palmitic acid-induced lipotoxicity and protection by (+)-catechin in rat cortical astrocytes. Pharmacol Rep. 2014;66:1106–1113. doi: 10.1016/j.pharep.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Wu Y, Tang L, Chen B. Oxidative stress: implications for the development of diabetic retinopathy and antioxidant therapeutic perspectives. Oxid Med Cell Longev 2014. 2014 doi: 10.1155/2014/752387. 752387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam JC, Kwok AK. Update on the treatment of diabetic retinopathy. Hong Kong Med J. 2007;13:46–60. [PubMed] [Google Scholar]
- Zeng Z, Wang H, Shang F, Zhou L, Little PJ, Quirion R, Zheng W. Lithium ions attenuate serum-deprivation-induced apoptosis in PC12 cells through regulation of the Akt/FoxO1 signaling pathways. Psychopharmacology. 2016a;233:785–794. doi: 10.1007/s00213-015-4168-7. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Wang X, Bhardwaj SK, Zhou X, Little PJ, Quirion R, Srivastava LK, Zheng W. The atypical antipsychotic agent, clozapine, protects against corticosterone-induced death of PC12 cells by regulating the Akt/FoxO3a signaling pathway. Mol Neurobiol. 2016b doi: 10.1007/s12035-016-9904-4. doi:10.1007/s12035-016-9904-4. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhou Z. Combination of bevacizumab and NGF reduces the risk of diabetic retinopathy. Cell Biochem Biophys. 2015;73:79–85. doi: 10.1007/s12013-015-0564-1. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of, apoptosis. Biochim Biophys Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Zheng WH, Quirion R. Glutamate acting on N-methyl-D-aspartate receptors attenuates insulin-like growth factor-1 receptor tyrosine phosphorylation and its survival signaling properties in rat hippocampal neurons. J Biol Chem. 2009;284:855–861. doi: 10.1074/jbc.M807914200. [DOI] [PubMed] [Google Scholar]
- Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60:428–431. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]