Summary
Numerous synergistic cancer immunotherapy combinations have been identified, but the effects of relative dose timing are rarely considered. In established syngeneic mouse tumor models, we found that staggering IFNα administration after, rather than before or simultaneously with, serum-persistent IL-2 and tumor-specific antibody significantly increased long-term survival. Successful combination therapy required IFNα-induced activation of cross-presenting CD8α+ DCs following release of antigenic tumor debris by the IL-2-and-antibody-mediated immune response. Due to decreased phagocytic ability post-maturation, DCs activated too early captured less antigen and could not effectively prime CD8+ T cells. Temporally programming DC activation to occur after tumoricidal activity enhanced tumor control by multiple distinct combination immunotherapies, highlighting dose schedule as an underappreciated factor that can profoundly affect the success of multi-component immunotherapies.
Introduction
Immunotherapy possesses unprecedented potential for cancer treatment, promoting antitumor host immune responses that can generate durable remissions. Many studies have demonstrated synergistic tumor control using various immunotherapies in combination with one another or with chemo- or radio- therapy (Melero et al., 2015). With major efforts focused on identifying treatment combinations that affect non-redundant immune pathways for maximal antitumor activity, less thought is given to the order in which therapeutic components are administered. Often, treatments are provided either concurrently for convenience or sequentially as patients are transitioned to a more promising drug; very rarely are concurrent and sequential combinations compared directly (Chen and Mellman, 2013; Melero et al., 2015). Moreover, the few studies documenting schedule-dependent synergy in combination therapies do not elucidate the mechanism underlying such synergy (Park et al., 2010; Reck et al., 2013; Schwartz et al., 1982), making it difficult to determine whether optimal dose timing can be rationally devised for drugs with known mechanisms of action.
To investigate the effect of dose schedule on antitumor efficacy in combination immunotherapy, we combined a well-characterized extended half-life interleukin-2 and tumor-specific antibody regimen (FcIL2 + TA99; Zhu et al., 2015) with interferon-α (IFNα), the only other FDA-approved cytokine for cancer treatment, in syngeneic solid tumor models. Since IL-2 and IFNα signal through distinct pathways, their synergistic potential has been assayed extensively, though clinical trials have failed to show a survival benefit from combination therapy over monotherapy (Cohen and Kaufman, 2007). However, since we had found serum-persistent FcIL2 to be more potent than IL-2 in delaying tumor progression together with TA99 (Zhu et al., 2015), we hypothesized that this regimen’s ability to mediate innate and adaptive immunity-dependent tumor cytotoxicity could be well complemented by IFNα’s pleiotropic effects. Endogenous or administered type I IFNs such as IFNα are respectively required for or enhance the antitumor activity of many cancer immunotherapies, including monoclonal antibodies and peptide vaccines (Sikora et al., 2009; Stagg et al., 2011), and are also necessary for spontaneous tumor rejection (Diamond et al., 2011; Fuertes et al., 2011).
We demonstrate here that FcIL2 + TA99 exhibits unexpectedly strong schedule-dependent antitumor synergy with IFNα, such that delaying IFNα injection with respect to FcIL2 + TA99 administration results in profoundly improved survival compared to simultaneous administration of all three components or injection of IFNα prior to FcIL2 + TA99. Furthermore, we find that the relative timing of IFNα-mediated CD8α+ DC activation ultimately determines the outcome of IFNα combination immunotherapy. We also show that the chronology of DC activation by various other combination immunotherapies significantly impacts antitumor responses, highlighting dose schedule as a crucial variable to consider when combining multiple immunomodulatory agents.
Results
IFNα Exhibits Potent Schedule-Dependent Antitumor Synergy with Serum-Persistent IL-2 and Tumor-Specific Antibody
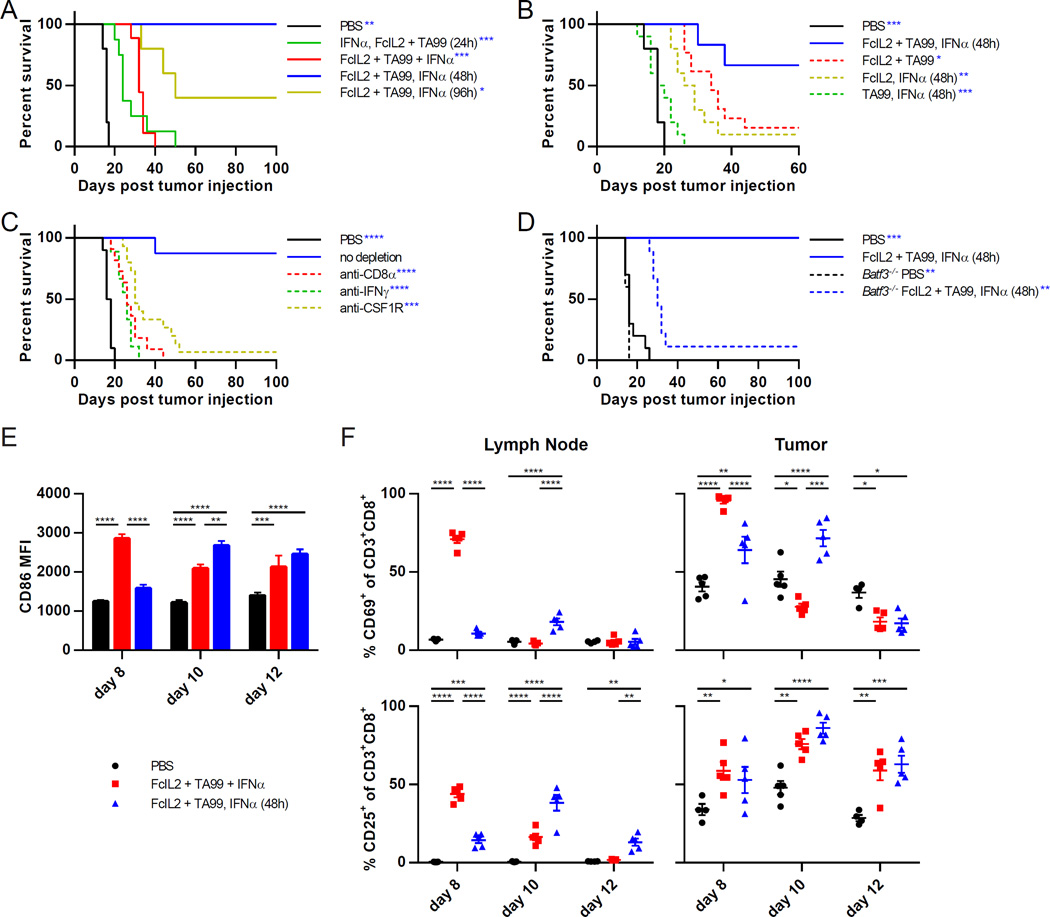
To test whether the relative timing of combination immunotherapy component administration affects antitumor efficacy, we used the poorly immunogenic B16F10 melanoma model, allowing subcutaneous tumors to establish in syngeneic C57BL/6 mice prior to treatment. Mice were treated with FcIL2 + TA99, which comprises an extended serum half-life IL-2 and an antitumor murine IgG2a antibody against TRP1 (Zhu et al., 2015). Murine IFNα was administered either 24 h before, concurrently with, or 48 to 96 h after FcIL2 + TA99 (Figure S1A). While injecting IFNα prior to or simultaneously with FcIL2 + TA99 did not induce durable remissions, staggering IFNα administration 48 h after FcIL2 + TA99 treatment resulted in cure rates ranging from 67% to 100% (Figures 1A, 1B, and S1B). All three immunotherapeutic agents were required for the long-term survival benefit conferred by staggered IFNα combination therapy, since omission of any agent significantly diminished antitumor efficacy (Figures 1B and S1C). Although synergistic tumor control depended greatly on the relative timing of IFNα and FcIL2 + TA99 administration, treatment outcomes were relatively unaffected by IFNα dosage (Figure S1D).
Figure 1. Relative Timing of Combination Immunotherapy Component Administration Determines Synergistic Antitumor Efficacy and Requires Specific Elements of Innate and Adaptive Immunity.
(A) Survival curves for mice injected s.c. with 106 B16F10 melanoma cells, then treated on days 6 and 12 with PBS or FcIL2 + TA99. Mice given FcIL2 + TA99 also received IFNα at indicated time points relative to FcIL2 + TA99 treatment. n = 5–9 per group.
(B) Survival curves for mice treated as described in (A), or with one of the three therapeutic components omitted. n = 5–13 per group.
(C) Survival curves for mice treated as described in (A). Mice given immunotherapy were also injected with the indicated depleting or neutralizing antibodies. n = 8–15 per group.
(D) Survival curves for wild-type or Batf3−/− mice treated as described in (A). n = 5–10 per group.
(E) MFI levels of CD86 expression by draining lymph node CD8α+ DCs (CD3−CD11chiPDCA-1−CD8α+) from immunotherapy-treated mice bearing established s.c. B16F10 tumors. n = 4–5 per group.
(F) Percentages of draining lymph node or intratumoral CD8+ T cells expressing CD69 or CD25. Cells were isolated from immunotherapy-treated mice bearing established s.c. B16F10 tumors. n = 4–5 per group.
Data represent mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 between the indicated pairs or versus the corresponding color group in the legend. See also Figures S1 and S2.
CD8+ T Cells, CD8α+ DCs, and IFNγ Are Required for Effective IFNα Combination Immunotherapy
We next sought to identify a mechanistic basis for the schedule-dependent antitumor synergy observed between IFNα and FcIL2 + TA99. IFNα can directly inhibit tumor cell proliferation and indeed demonstrated mild antiproliferative activity when incubated with several cancer cell lines (Figure S1E). However, if inhibition of tumor cell proliferation were IFNα’s major contribution, then earlier IFNα administration would be expected to result in better outcomes. Moreover, even cultured in the presence of an IFNα concentration twofold greater than peak serum levels following a therapeutic dose, B16F10 cells exhibited only a ~65% reduction in proliferation compared to untreated controls (Figure S1E). These data imply that IFNα’s antiproliferative effects play a minor role in tumor control mediated by the combination immunotherapy.
IFNα can also stimulate tumoricidal functions in a variety of immune effector cells. After immunotherapy, intratumoral levels of the chemokines IP-10, MIP-2, MIG, and MCP-1 were elevated (Figure S2A), likely contributing to the local recruitment of NK cells, T cells, neutrophils, and phagocytes (Figures S2D, S2E, and S3A). Strikingly, mice depleted of CD8+ T cells or macrophages, but not other immune effector cells, failed to respond to staggered IFNα combination therapy (Figures 1C, S2B, and S2C). While IFNγ-neutralizing antibodies significantly impaired the antitumor activity of the combination therapy, TNF-neutralizing antibodies did not (Figures 1C and S2C). Other studies have further identified a necessary role for type I IFNs in mediating antitumor cytotoxic T cell responses through the promotion of tumor antigen cross-presentation by the CD8α+ DC subset (Diamond et al., 2011; Fuertes et al., 2011). We found that the efficacy of the staggered IFNα combination therapy was severely attenuated in Batf3−/− mice lacking this DC subset (Figures 1D and S2F), revealing an additional requirement for CD8α+ DCs in therapy-induced tumor rejection.
Although the extent of tumor infiltration by immune effector cells appeared similar for both simultaneous and staggered IFNα combination therapies (Figures S2D, S2E, and S3A), the two treatment regimens exhibited marked differences in the timing of immune cell activation. Expression of the maturation marker CD86 by draining lymph node CD8α+ DCs closely trailed the time of IFNα dosing (Figure 1E), consistent with IFNα’s known ability to activate DCs (Luft et al., 1998). Since the CD8α+ DC subset is heavily involved in priming lymph node T cells that then traffic to the tumor (Diamond et al., 2011), after treatment we monitored the percentages of CD8+ T cells in both compartments that expressed the activation markers CD69 or CD25. Interestingly, in the lymph node, CD69 and CD25 expression peaked two days following IFNα administration. Expression of both markers quickly decreased thereafter (Figure 1F), reflecting transient induction in the case of CD69 and migration of activated T cells to the tumor in the case of CD25 (Fuertes et al., 2013). In the tumor, CD8+ T cells also strongly upregulated activation marker expression soon after IFNα dosing. The close correlation of IFNα dose timing with tumor-proximal CD8α+ DC and CD8+ T cell activation suggests that coordinating DC maturation with antigen uptake is a critical requirement for efficacy.
Antitumor Responses to IFNα Combination Immunotherapy Depend on Timing of CD8α+ DC Activation
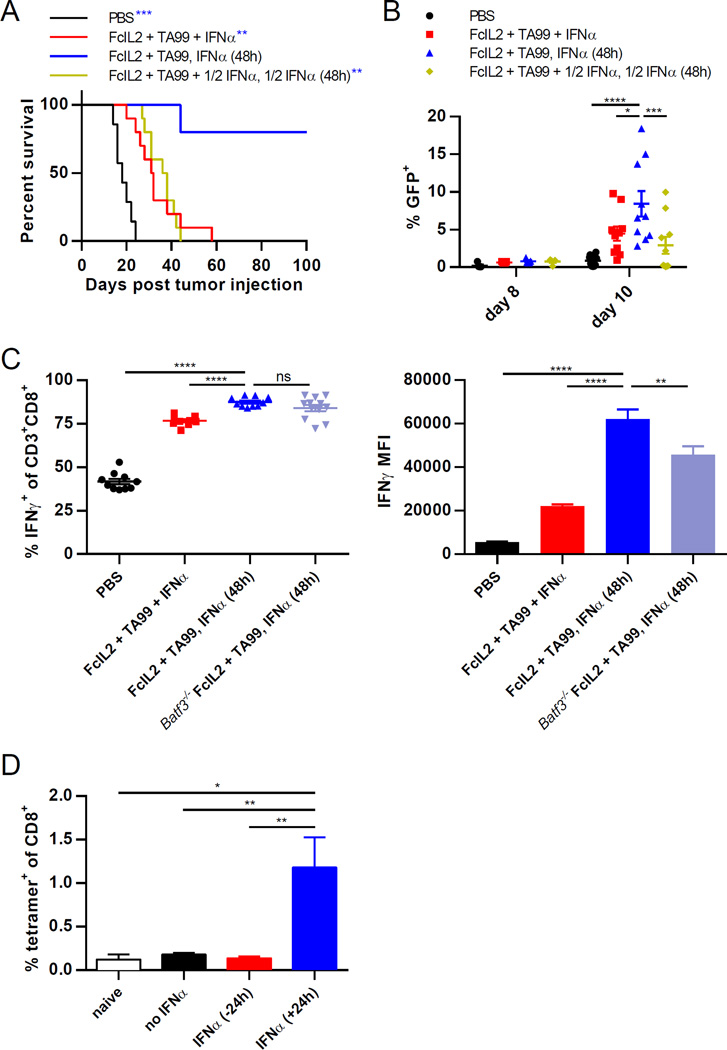
Upon activation and maturation, DCs not only upregulate a variety of costimulatory molecules, but also lose phagocytic capacity, relinquishing the ability to capture new antigens in favor of an increased ability to cross-prime CD8+ T cells specific for already internalized antigens (Wilson et al., 2006). We therefore hypothesized that the timing of DC activation relative to the release of immunogenic tumor antigens was a key determinant of therapeutic efficacy in this IFNα combination therapy. Previously, we showed that antitumor innate immunity, as characterized by granulocyte respiratory burst activity and inflammatory cytokine release, peaks two days after FcIL2 + TA99 administration (Zhu et al., 2015). If CD8α+ DCs became activated and poorly endocytic before the treatment-mediated immune response generated substantial antigenic tumor debris, then their ability to prime CD8+ T cells would be hampered due to insufficiency of internalized tumor antigens available for cross-presentation. To test this hypothesis, we administered IFNα both simultaneously with FcIL2 + TA99 therapy and again afterwards. Despite the total IFNα dose being the same, administration of a portion of that dose concurrently with antibody had a dominant negative effect, resulting in significantly worse tumor control than giving IFNα only after FcIL2 + TA99 (Figures 2A, S3C, S1D), presumably because IFNα-matured DCs became less phagocytic following the first IFNα dose. Furthermore, although CD8α+ DCs can play an essential role as producers of IL-12 and IL-15 (Ferlazzo et al., 2004), intratumorally injected IL-12 or IL-15 complex was unable to rescue the antitumor efficacy of staggered IFNα combination therapy in Batf3−/− mice (Figure S3B), consistent with the hypothesis that the cross-priming ability of CD8α+ DCs is the dominant contributor to tumor control in this context. To more directly evaluate DC phagocytic ability following immunotherapy, we treated mice bearing B16F10 tumors that stably expressed enhanced green fluorescent protein (GFP) and used GFP signal as a proxy for tumor antigen uptake by draining lymph node CD8α+ DCs (Figure 2B). A few days later, greater percentages of GFP+ CD8α+ and GFP+CD86+ CD8α+ DCs were encountered in mice treated with staggered IFNα combination therapy than in those treated with simultaneous or simultaneous + staggered IFNα combination therapies (Figures 2B and S3D).
Figure 2. Properly Timed CD8α+ DC Activation Is Necessary for Optimal CD8+ T Cell Priming in Combination Immunotherapy.
(A) Survival curves for mice injected s.c. with 106 B16F10 melanoma cells, then treated on days 6 and 12 with PBS or FcIL2 + TA99. Mice given FcIL2 + TA99 also received IFNα as indicated. n = 5–10 per group.
(B) Percentages of GFP+ draining lymph node CD8α+ DCs from mice treated as described in (A), except that B16F10-GFP instead of B16F10 cells were used. n = 5–10 per group.
(C) IFNγ expression by peripheral blood CD8+ T cells as represented by percentages of IFNγ+ cells or IFNγ MFI levels. On day 12, blood was collected from immunotherapy-treated wild-type or Batf3−/− mice bearing established s.c. B16F10 tumors and incubated for 6 h in the presence of brefeldin A and monensin with PMA/ionomycin restimulation prior to flow cytometric analysis. Background IFNγ expression levels detected using controls incubated without PMA/ionomycin were subtracted from the corresponding samples. n = 10–12 per group.
(D) Percentages of peripheral blood CD8+ T cells staining positive for H-2Kb/SIINFEKL tetramer. Mice were immunized s.c. with OVA and treated with IFNα either 24 h before or after immunization. 7 days later, blood was collected for analysis by flow cytometry. n = 10 per group.
Data represent mean ± SEM. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 between the indicated pairs or versus the corresponding color group in the legend. See also Figure S3.
To examine whether increased tumor antigen uptake by CD8α+ DCs corresponds to better CD8+ T cell priming, we evaluated CD8+ T cell function by analyzing IFNγ production in response to ex vivo restimulation. A significantly greater fraction of circulating CD8+ T cells expressed IFNγ after staggered versus simultaneous IFNα combination therapy, and CD8+ T cells from mice treated with the staggered IFNα combination generated more IFNγ per cell than those from mice treated with the simultaneous IFNα combination (Figure 2C). Intriguingly, administering the staggered IFNα combination therapy in Batf3−/− mice diminished the magnitude but not the frequency of IFNγ production by CD8+ T cells (Figure 2C), suggesting that CD8α+ DCs contribute to therapeutic efficacy by amplifying the level of IFNγ production per CD8+ T cell rather than by simply increasing the percentage of IFNγ-producing cells. Staggering IFNα administration also boosted expression of the degranulation marker CD107a by intratumoral CD8+ T cells compared to administering IFNα simultaneously with FcIL2 + TA99, although the fractions of IFNγ-producing CD8+ T cells were similar in both groups of mice at the analyzed time point (Figure S3E). Finally, to assess the effect of CD8α+ DC maturation timing on the priming of antigen-specific CD8+ T cells, we quantified the generation of ovalbumin (OVA)-specific T cells following subcutaneous OVA immunization in animals exposed to IFNα at different times. Whereas mice treated with IFNα after OVA injection showed a robust anti-OVA CD8+ T cell response, OVA-specific T cells were not detected in mice pre-exposed to IFNα (Figure 2D), again indicating that DC activation prior to antigen exposure can significantly obstruct the generation of an effective T cell response against that antigen. Together, these data accentuate the importance of delaying CD8α+ DC activation until an innate immune response has generated sufficient tumor antigenic debris, in order for maximal tumor antigen endocytosis and cytotoxic T cell priming by this DC subset to occur.
Effective IFNα Combination Immunotherapy Protects against Subsequent Tumor Rechallenge
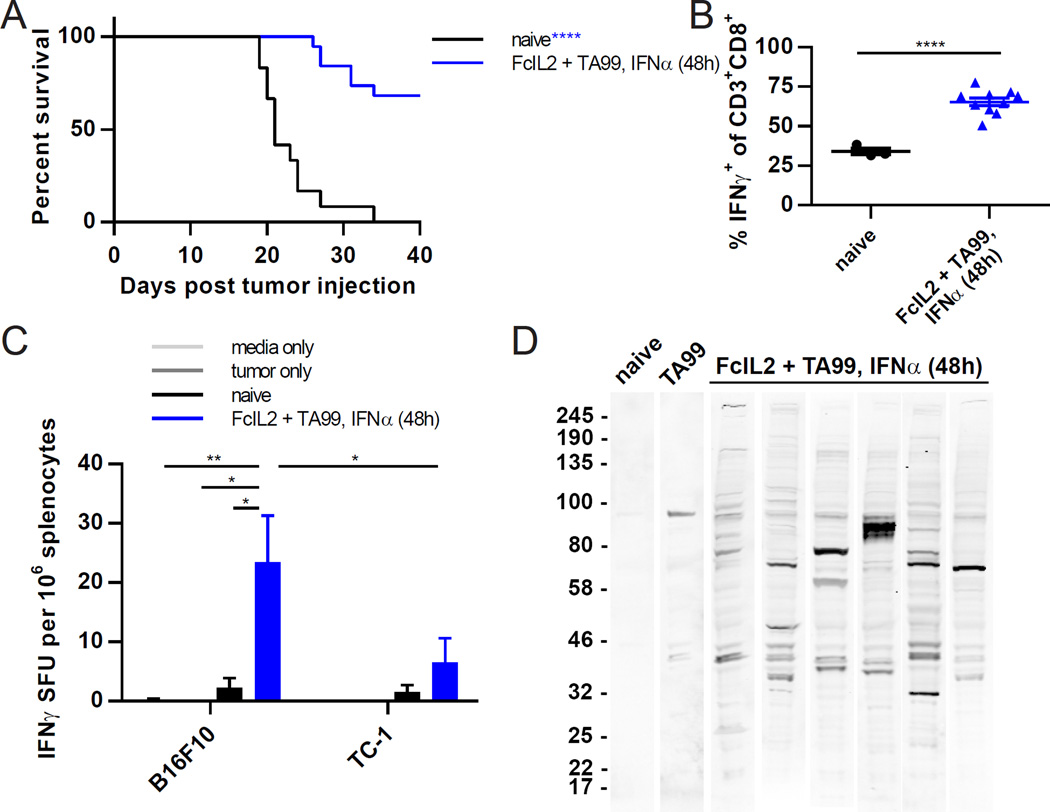
To determine whether mice treated with staggered IFNα combination therapy that survived B16F10 tumor challenge could reject subsequent challenge without additional treatment, we rechallenged surviving animals with B16F10 tumor cells at a distal site. More than two-thirds of these previously treated mice rejected the secondary challenge, whereas all of the control naïve animals exhibited rapid tumor outgrowth (Figures 3A and S4A). Furthermore, circulating CD8+ T cells from rechallenged, previously treated mice showed greater functional ability than those from naïve mice challenged with the same tumor inoculum (Figure 3B). The cellular response to rechallenge was tumor specific, since a higher frequency of IFNγ secretion was detected by ELISPOT upon the incubation of splenocytes from previously treated mice with B16F10 melanoma cells versus unrelated TC-1 lung cancer cells (Figure 3C). Additionally, immunoblots using sera from rechallenged, previously treated mice to probe B16F10 cell lysates revealed the presence of antitumor antibodies to multiple epitopes beyond the TRP1 protein targeted by TA99 (Figures 3D and S4B). Collectively, these findings confirm that staggered IFNα combination therapy elicits long-term protective cellular and humoral antitumor immunity.
Figure 3. Effective Combination Immunotherapy Elicits Protective Antitumor Immune Memory.
(A) Survival curves for mice treated with FcIL2 + TA99 and IFNα 48 h later that rejected initial challenge with 106 B16F10 melanoma cells s.c. and were rechallenged on day ~100 with 105 B16F10 cells s.c. As a control, the survival of naïve mice challenged with 105 B16F10 s.c. was also monitored. n = 12–19 per group.
(B) Percentages of peripheral blood CD8+ T cells expressing IFNγ following B16F10 tumor rechallenge. On day 8 post rechallenge, blood was collected from mice treated as described in (A) and incubated for 6 h in the presence of brefeldin A and monensin with PMA/ionomycin restimulation prior to flow cytometric analysis. Background IFNγ expression levels detected using controls incubated without PMA/ionomycin were subtracted from the corresponding samples. n = 3–10 per group.
(C) ELISPOT analysis of B16F10-specific IFNγ production by splenocytes isolated from mice treated as described in (A) on day 6 post rechallenge. 106 splenocytes and 2.5×104 irradiated tumor cells were co-incubated for 24 h prior to analysis. Nonspecific responses were quantified by co-incubation with the unrelated TC-1 tumor cell line. Background IFNγ expression levels detected using splenocytes incubated in the absence of tumor cells were subtracted from the corresponding samples. n = 3–7 per group.
(D) Endogenous antitumor antibody response following B16F10 tumor rechallenge as measured by immunoblot. 3–5 weeks post rechallenge, sera were obtained from mice treated as described in (A) and analyzed for antibodies reactive against B16F10 cell lysate. A control immunoblot using TA99 antibody against B16F10 cell lysate was also performed. Each lane represents pooled sera from three mice (naïve) or serum from one individual mouse (FcIL2 + TA99, IFNα 48 h).
Data represent mean ± SEM. *p < 0.05; **p < 0.01; ****p < 0.0001 between the indicated pairs or versus the corresponding color group in the legend. See also Figure S4.
Temporally Programmed Dose Schedule Effects Are Generalizable to Other DC-Activating Immunotherapies
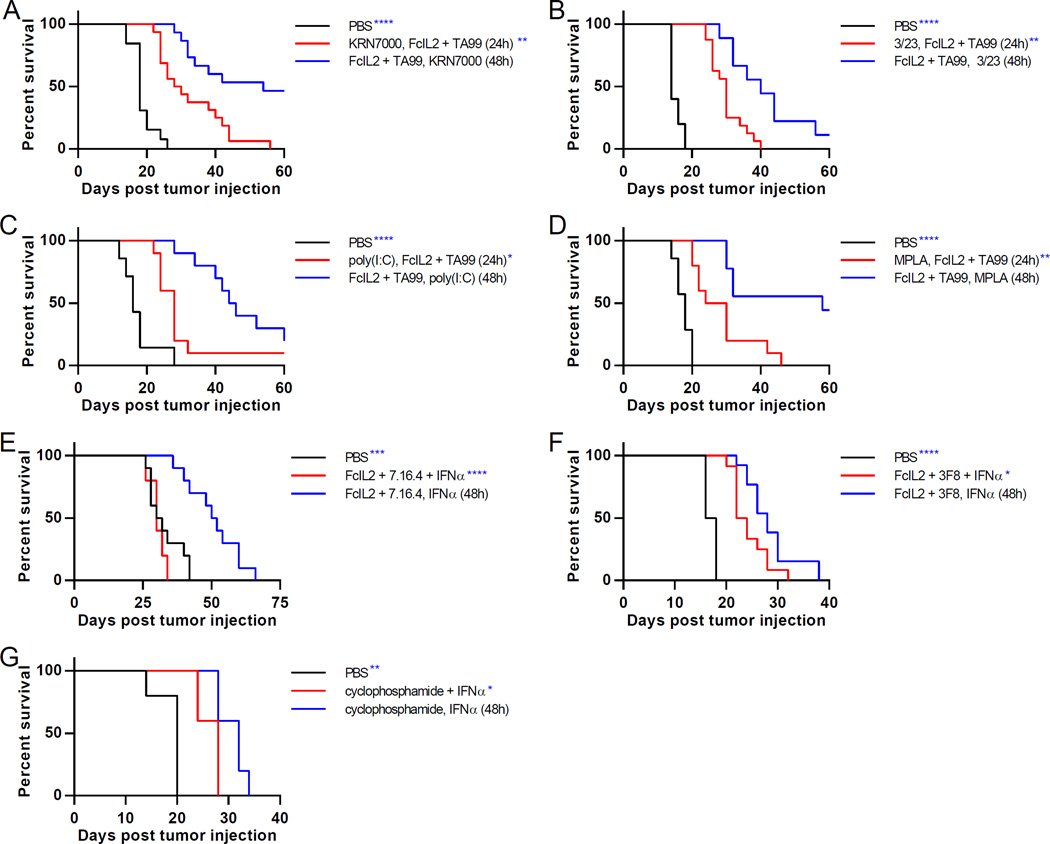
Many immunomodulators induce DC maturation, motivating us to investigate whether other DC-activating agents exhibit schedule-dependent synergies. We combined FcIL2 + TA99 with the synthetic α-galactosylceramide analog KRN7000, the agonistic anti-CD40 antibody 3/23, the nucleic acid analog poly(I:C), or the lipopolysaccharide derivative MPLA, which activate DCs indirectly via invariant NKT cell-based transactivation or directly through costimulatory or Toll-like receptors (Fujii et al., 2003; Hennessy et al., 2010; White et al., 2011). Strikingly, tumor-bearing mice treated with KRN7000, 3/23, poly(I:C), or MPLA after FcIL2 + TA99 therapy showed dramatically improved survival versus those treated with these DC activators prior to FcIL2 + TA99 (Figures 4A–D and S5A–D). Despite the vastly different biophysical properties and DC-activating mechanisms of the tested immunostimulatory agents, temporally programming DC activation to occur predominantly following antigen-generating tumoricidal activity led to more effective combination therapy in every case, emphasizing that component dosing order can strongly govern the efficacy of combination therapies.
Figure 4. Schedule-Dependent Synergy is Generalizable to a Wide Range of Combination Immunotherapies in Various Tumor Models.
(A–D) Survival curves for mice injected s.c. with 106 B16F10 melanoma cells, then treated on days 6 and 12 with PBS or FcIL2 + TA99. Mice given FcIL2 + TA99 also received KRN7000, 3/23, poly(I:C), or MPLA at indicated times. n = 13–15 per group.
(E) Survival curves for mice injected s.c. with 106 DD-Her2/neu breast cancer cells, then treated on days 6 and 12 with PBS or FcIL2 + 7.16.4. Mice given FcIL2 + 7.16.4 also received IFNα at indicated times. n = 5–10 per group.
(F) Survival curves for mice injected s.c. with 2.5×104 RM9 prostate cancer cells, then treated on days 6 and 12 with PBS or FcIL2 + 3F8. Mice given FcIL2 + 3F8 also received IFNα at indicated times. n = 12–13 per group.
(G) Survival curves for mice injected s.c. with 106 B16F10 melanoma cells, then treated on days 6 and 12 with i.p. PBS or cyclophosphamide. Mice given cyclophosphamide also received IFNα at indicated times. n = 5 per group.
*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 versus the corresponding color group in the legend. See also Figure S5.
For further generalization, we tested our combination immunotherapies in two alternate syngeneic tumor models, administering IFNα with FcIL2 + 7.16.4, an anti-Her2 murine IgG2a antibody, to BALB/c mice bearing established DD-Her2/neu breast tumors (Draganov et al., 2015) or with 3F8, an anti-GD2 murine IgG3 antibody, to C57BL/6 mice bearing established RM9 prostate tumors (Zhu et al., 2015). Staggering IFNα treatment after FcIL2 + antitumor antibody provided superior tumor control than giving all three components simultaneously (Figures 4E, 4F, S5E, and S5F). Lastly, we used the chemotherapeutic agent cyclophosphamide, which can induce immunogenic tumor cell death (Bezu et al., 2015), in lieu of FcIL2 + TA99 to generate tumor debris in the B16F10 model. IFNα given staggered after cyclophosphamide prolonged survival compared to simultaneous administration of chemotherapy and IFNα (Figures 4G and S5G), again most likely due to CD8α+ DC activation after, rather than concurrent with, tumoricidal activity (Figure S5G). Thus, the enhanced antitumor efficacy conferred by properly timed DC activation was also validated for combination therapies with an alternate means of tumor cell killing and in two additional tumor models using different mouse strains, demonstrating the broad applicability of this temporal programming approach.
Discussion
As cancer immunotherapy comes of age, much attention has focused on determining which drug classes exhibit synergistic antitumor activity (Chen and Mellman, 2013; Melero et al., 2015), while comparatively little effort has been directed towards considering the importance of dose schedules for these combinations. Here, we show that the relative timing of drug administration can play a pivotal role in dictating combination immunotherapy outcomes, using an aggressive syngeneic tumor model to characterize the mechanism by which such schedule-dependent antitumor synergy arises. Before treatment, the paucity of tumor-derived antigens in the immunosuppressive tumor microenvironment and draining lymph node results in poor CD8+ T cell priming by immature CD8α+ DCs. Administration of a tumoricidal regimen such as tumor-specific antibody with IL-2 support induces extensive tumor cell death (Zhu et al., 2015), generating tumor debris for capture and cross-presentation by the DCs, provided they receive a maturation signal only after tumor-derived antigens become available (Figure 5). Thus, in the subcutaneous B16F10 tumor model, a two-day delay in DC-activating IFNα administration following the injection of tumoricidal therapy yields a ~85% survival rate, in stark contrast to 0% long-term survivors when both therapies are given simultaneously. We further demonstrate with a variety of other combination therapies and tumor models that superior tumor control is achieved by temporally programming DC activation to occur after the culmination of tumoricidal activity, highlighting a general strategy for enhancing the therapeutic efficacy of many existing treatment combinations.
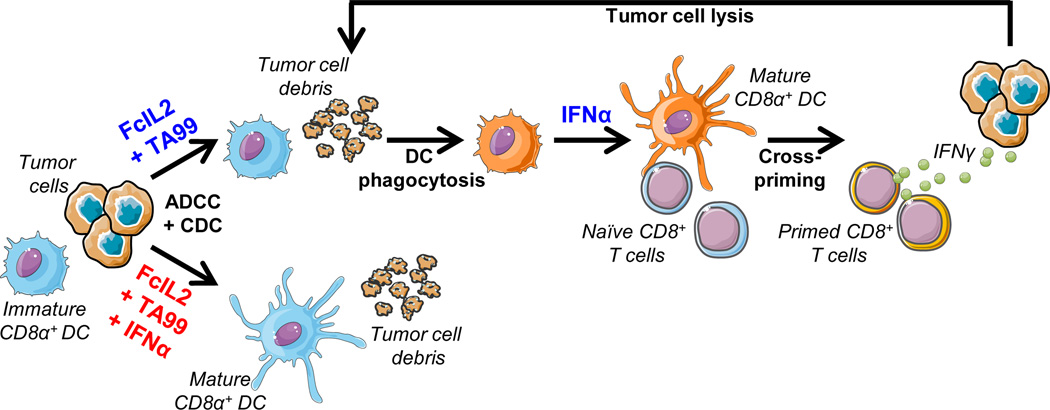
Figure 5. Model for the Differential Generation of Immune Responses Based on Chronology of CD8α+ DC Activation.
Prior to treatment, tumor-proximal CD8α+ DCs mostly exist in an immature, unactivated state due to lack of stimuli and/or the immunosuppressive microenvironment. The tumor cells overwhelm host immunity and produce little tumor debris for the DCs to sample. Administration of FcIL2 + TA99 activates a vigorous antitumor response that results in the generation of immunogenic tumor debris. Since this response takes time to mount, the timing of DC activation by IFNα is extremely important. Bottom: If IFNα is given simultaneously with FcIL2 + TA99, CD8α+ DCs mature too early and lose phagocytic activity before the immune response can generate tumor debris. These mature DCs are unable to ingest and cross-present the tumor-derived antigens that subsequently become available, and an antitumor CD8+ T cell response is not elicited. Top: If IFNα is given staggered after FcIL2 + TA99, CD8α+ DCs have the opportunity to sample tumor debris before receiving a maturation signal, leading to cross-presentation of tumor-derived antigens and cross-priming of tumor-specific CD8+ T cells in the draining lymph node. The primed CD8+ T cells traffic to the tumor, induce additional tumor cell death through IFNγ and direct cell lysis, and establish long-term antitumor immune memory.
Our unexpected finding that premature pharmacological CD8α+ DC maturation impedes the generation of a durable antitumor immune response nevertheless agrees with a prior observation that the systemic activation of CD8α+ DCs by malaria infection or microbial ligands greatly impaired subsequent cross-presentation and resulted in immunosuppression (Wilson et al., 2006). By contrast, we show that when triggered at an appropriate time, DC activation significantly improves the efficacy of cancer immunotherapy (Figures 1A and 2A). Activated CD8α+ DCs primed a robust CD8+ T cell response against tumor-derived antigens, leading to durable remissions and rejection of subsequent tumor challenge (Figure 3A). Although these CD8+ T cells exhibited specific reactivity to irradiated B16F10 tumor cells (Figure 3C), we did not detect T cells reactive to known B16F10 peptide epitopes including gp100, TRP1, TRP2, and p15E (Overwijk and Restifo, 2001; data not shown). More sensitive techniques such as cancer exome analysis or tandem minigene library screening might be needed to identify the precise antigen specificities of the CD8+ T cells mediating tumor regression in this study (Lu et al., 2014; Matsushita et al., 2012).
The near-total ablation of combination immunotherapy efficacy in mice deficient in CD8+ or Batf3-dependent cells (Figures 1C and 1D), along with published evidence that endogenous antitumor cytotoxic T cell responses selectively require type I IFN signaling in CD8α+ DCs (Diamond et al., 2011; Fuertes et al., 2011), prompted us to focus our investigation on the CD8α+ DC subset, which is considered to have the most potent CD8+ T cell cross-priming ability (den Haan and Bevan, 2002). It nonetheless is likely that alternate mechanisms also contribute to the schedule-dependent synergy observed in our combination immunotherapy, since IFNα can activate other DC subsets, including Batf3-independent CD8α− DCs (Diamond et al., 2011). Indeed, type I IFN signaling was shown to inhibit phagocytic capacity in CD8α− DCs, hindering Th1-dependent responses to malaria (Haque et al., 2014). In addition, wild-type mice treated with simultaneous IFNα combination therapy demonstrated weaker CD8+ T cell priming than Batf3−/− mice treated with staggered IFNα combination therapy (Figure 2C), indicating a deleterious effect of untimely IFNα exposure on Batf3-independent antitumor immunity. These data suggest that premature activation of CD8α− DCs, which can cross-present to CD8+ T cells under certain circumstances (den Haan and Bevan, 2002), could partially account for the decreased survival when DC maturation occurs concurrently with instead of after tumoricidal activity. Further work is necessary to definitively characterize the effects of IFNα dose timing on CD8α− DC numbers, activation status, and relationship to effective antitumor immunity.
Our work indicates that a strategy of administering tumoricidal therapy prior to activating DCs for enhanced antitumor synergy generalizes to combinations involving a wide spectrum of cytotoxic or DC-stimulating treatments and reveals several areas for further exploration. First, the ability of tumor-specific antibody to mediate tumor cell opsonization by DCs may contribute to treatment efficacy, since combinations with antibody had greater efficacy than those without; antibody isotype may also influence tumor control (Figure 4). Second, recent studies have linked the success of several anticancer therapies, including STAT3 inhibitors and stimulator of IFN gene (STING) agonists, with the potent induction of type I IFN signaling leading to tumor regression (Corrales et al., 2015; Yang et al., 2015), making these therapies promising candidates for synergistic DC activation in combination with tumoricidal agents. Last, the principle of temporal programming may extend to other steps in the generation of an antitumor immune response, including T cell activation, infiltration into tumors, and recognition of cancer cells (Chen and Mellman, 2013). For example, previous findings that injection of plasmid IL-2-immunoglobulin after, but not concurrently with, an HIV vaccine boosted immune responses (Barouch et al., 1998) and that pre-exposure to IL-2 impaired subsequent antigen-specific CD4+ T cell activation (Sckisel et al., 2015) suggest that administering T cell stimulants such as IL-2 only after antigen presentation and costimulation have occurred may recapitulate the temporal progression of endogenous immune responses and further augment the efficacy of combination cancer therapies.
In conclusion, we have uncovered a simple yet powerful approach to improve the efficacy of combination cancer immunotherapies, and characterized the biological mechanism underpinning this approach. Although studies to date have focused on identifying drug classes that act synergistically, we show that when designing combination therapies, careful attention should be paid not only to the nature of constituent drugs, but also to the relative timing of drug administration, as premature immune stimulation may paradoxically suppress rather than enhance antitumor activity. As our understanding of cancer biology increases, the concept of temporally programming immunological events to maximize the strength of an immune response will enable the optimized combinatorial usage of currently available immunomodulators, including immune checkpoint inhibitors, agonistic and antitumor antibodies, cytokines, and cancer vaccines.
Experimental Procedures
More detailed procedures are provided in the Supplemental Experimental Procedures.
Mice
C57BL/6 (Taconic or the Jackson Laboratory), BALB/c (Taconic), and Batf3−/− (B6.129S(C)-Batf3tm1Kmm/J; bred in-house from breeding pairs obtained from the Jackson Laboratory) mice were maintained under specific-pathogen free conditions and used at 6–10 weeks of age. All experiments were approved by the MIT Division of Comparative Medicine and performed in accordance with federal, state, and local regulations.
Tumor Treatment
For tumor induction, 106 B16F10 melanoma cells in 100 µl PBS were injected subcutaneously into the flanks of C57BL/6 or Batf3−/− mice. Mice were treated retroorbitally on days 6 and 12 after tumor inoculation with 25 µg FcIL2 and/or 100 µg TA99. Some mice also received IFNα before, concurrent with, and/or staggered after FcIL2 and/or TA99. Tumor length and width were measured using calipers, and mice were euthanized when tumors reached 200 mm2. Additional details, modifications, and models are described in the Supplemental Experimental Procedures.
Flow Cytometry
B16F10 tumors were induced as detailed above and treated with a single dose of combination therapy prior to the preparation, staining, and analysis of single-cell suspensions as described in the Supplemental Experimental Procedures.
Statistical Analysis
Results were analyzed using GraphPad Prism 6 software with comparisons performed as detailed in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank the staff of the Swanson Biotechnology Center at the Koch Institute for technical assistance. This work was funded by CA174795 and CA101830. A.T. was supported by a Ludwig Center for Molecular Oncology Graduate Fellowship and Siebel Scholarship. E.F.Z. and C.F.O. were supported by NSF Graduate Research Fellowships. K.D.M. was supported by a Hertz Foundation Fellowship.
Footnotes
Author Contributions
A.T. and K.D.W. designed research; A.T., M.J.K., E.F.Z., K.D.M., C.F.O., N.J.Y., N.M., and R.L.K. performed research; A.T., M.J.K., E.F.Z., K.D.M., G.L.S., W.W.O., D.J.I., and K.D.W. analyzed data; and A.T. and K.D.W. wrote the paper.
References
- Barouch DH, Santra S, Steenbeke TD, Zheng XX, Perry HC, Davies ME, Freed DC, Craiu A, Strom TB, Shiver JW, et al. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J Immunol. 1998;161:1875–1882. [PubMed] [Google Scholar]
- Bezu L, Gomes-de-Silva LC, Dewitte H, Breckpot K, Fucikova J, Spisek R, Galluzzi L, Kepp O, Kroemer G. Combinatorial strategies for the induction of immunogenic cell death. Front Immun. 2015;6:187. doi: 10.3389/fimmu.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Daniel S, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Kaufman HL. Combination cytokine therapy. In: Caligiuri MA, Lotze MT, editors. Cytokines in the Genesis and Treatment of Cancer. Humana Press; 2007. pp. 373–398. [Google Scholar]
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Repo. 2015;11:1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(−) dendritic cells in vivo. J Exp Med. 2002;196:817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganov D, Gopalakrishna-Pillai S, Chen YR, Zuckerman N, Moeller S, Wang C, Ann D, Lee PP. Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Scientific reports. 2015;5:16222. doi: 10.1038/srep16222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Munz C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8(+) T cell responses through CD8 alpha(+) dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34:67–73. doi: 10.1016/j.it.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S-i, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Best SE, Montes de Oca M, James KR, Ammerdorffer A, Edwards CL, de Labastida Rivera F, Amante FH, Bunn PT, Sheel M, et al. Type I IFN signaling in CD8– DCs impairs Th1-dependent malaria immunity. J Clin Invest. 2014;124:2483–2496. doi: 10.1172/JCI70698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy EJ, Parker AE, O'Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- Lu YC, Yao X, Crystal JS, Li YF, El-Gamil M, Gross C, Davis L, Dudley ME, Yang JC, Samuels Y, et al. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin Cancer Res. 2014;20:3401–3410. doi: 10.1158/1078-0432.CCR-14-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft T, Pang KC, Thomas E, Hertzog P, Hart DNJ, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS, Shea LK, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero I, Berman DM, Aznar MA, Korman AJ, Gracia JLP, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457–472. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- Overwijk WW, Restifo NP. B16 as a mouse model for human melanoma. Curr Protoc Immun. 2001;39:20.21.21–20.21.29. doi: 10.1002/0471142735.im2001s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Jiang ZJ, Mortenson ED, Deng LF, Radkevich-Brown O, Yang XM, Sattar H, Wang Y, Brown NK, Greene M, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Lu H, Cuillerot J-M, Lynch TJ. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- Schwartz SA, Morgenstern B, Capizzi RL. Schedule-dependent synergy and antagonism between high-dose 1-β-d-arabinofuranosylcytosine and asparaginase in the L5178Y murine leukemia. Cancer Res. 1982;42:2191–2197. [PubMed] [Google Scholar]
- Sckisel GD, Bouchlaka MN, Monjazeb AM, Crittenden M, Curti BD, Wilkins DE, Alderson KA, Sungur CM, Ames E, Mirsoian A, et al. Out-of-sequence signal 3 paralyzes primary CD4 T-cell-dependent immunity. Immunity. 2015;43:240–250. doi: 10.1016/j.immuni.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora AG, Jaffarzad N, Hailemichael Y, Gelbard A, Stonier SW, Schluns KS, Frasca L, Lou Y, Liu C, Andersson HA, et al. IFN-α enhances peptide vaccine-induced CD8+ T cell numbers, effector function, and antitumor activity. J Immunol. 2009;182:7398–7407. doi: 10.4049/jimmunol.0802982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Teng MW, Smyth MJ. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108:7142–7147. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AL, Chan HTC, Roghanian A, French RR, Mockridge CI, Tutt AL, Dixon SV, Ajona D, Verbeek JS, Al-Shamkhani A, et al. Interaction with FcγRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J Immunol. 2011;187:1754–1763. doi: 10.4049/jimmunol.1101135. [DOI] [PubMed] [Google Scholar]
- Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, et al. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- Yang H, Yamazaki T, Pietrocola F, Zhou H, Zitvogel L, Ma Y, Kroemer G. STAT3 inhibition enhances the therapeutic efficacy of immunogenic chemotherapy by stimulating type 1 interferon production by cancer cells. Cancer Res. 2015;75:3812–3822. doi: 10.1158/0008-5472.CAN-15-1122. [DOI] [PubMed] [Google Scholar]
- Zhu Eric F, Gai Shuning A, Opel Cary F, Kwan Byron H, Surana R, Mihm Martin C, Kauke Monique J, Moynihan Kelly D, Angelini A, Williams Robert T, et al. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell. 2015;27:489–501. doi: 10.1016/j.ccell.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.