Abstract
RBP4 is produced mainly by hepatocytes. In type 2 diabetes and obesity, circulating RBP4 is increased and may act systemically to cause insulin resistance and glucose intolerance. Observations that adipocyte RBP4 mRNA increases in parallel with circulating RBP4 in these conditions, whereas liver RBP4 mRNA does not, led to a widely held hypothesis that elevated circulating RBP4 is a direct result of increased production by adipocytes. To test this, we generated mice with hepatocyte-specific deletion of RBP4 (liver RBP4 knockout or LRKO mice). Adipose tissue RBP4 expression and secretion remained intact in LRKO mice and increased as expected in the setting of diet-induced insulin resistance. However, circulating RBP4 was undetectable in LRKO mice. We conclude that adipocyte RBP4 is not a significant source of circulating RBP4, even in the setting of insulin resistance. Adipocyte RBP4, therefore, may have a more important autocrine or paracrine function that is confined within the adipose tissue compartment.
Introduction
Liver is the main organ for vitamin A (retinol) storage in vertebrates (1). Hepatocytes distribute retinol to other tissues through a circulating carrier protein, serum retinol-binding protein (RBP4) (2). RBP4 is expressed primarily in hepatocytes and to a lesser extent in adipocytes and other cell types and functions as the sole specific blood transport protein for retinol (2). RBP4 expressed in hepatocytes binds intracellular retinol and is secreted into the circulation as retinol-bound holo-RBP4, which delivers retinol to multiple tissues (2).
Circulating RBP4 concentrations are increased in humans and rodents with obesity and type 2 diabetes (3,4). Several lines of evidence suggest RBP4 acts systemically in a classic endocrine fashion to dysregulate insulin-glucose homeostasis in these conditions (3,4). In mice, increasing serum RBP4 by genetic or pharmacological approaches causes insulin resistance and glucose intolerance, whereas lowering serum RBP4 improves insulin sensitivity and enhances glucose tolerance (3). Elevated serum RBP4 induces the gluconeogenic enzyme phosphoenolpyruvate carboxykinase in liver and impairs insulin signaling in skeletal muscle (3). RBP4 has been suggested to play a key role in coordinating cellular immunity to induce adipose tissue inflammation in obesity (5).
Extensive work in human subjects (children and adults) from diverse clinical contexts, resulting in hundreds of publications over the past decade, has confirmed strong correlations of serum RBP4 with diabetes and diabetic complications, insulin resistance, metabolic syndrome, polycystic ovarian syndrome, metabolic complications of pregnancy, atherosclerosis, heart disease, stroke, and certain cancers (6–13). Moreover, humans harboring a genetic polymorphism (−803G>A) of Rbp4, which regulates transcription factor binding to the Rbp4 promoter (14), exhibit elevated serum RBP4 (15) and up to a 2.7-fold greater risk of incident diabetes (16). Nevertheless, how serum RBP4 regulates insulin-glucose homeostasis remains poorly understood, including which tissues contribute to elevated serum RBP4 during insulin resistance.
To our knowledge, the contribution of RBP4 produced within adipose tissue to the circulating RBP4 pool has never been formally tested. We determined the relative contributions of adipose and other extrahepatic tissues to circulating RBP4 concentrations in vivo by generating and characterizing mice with hepatocyte-specific genetic deletion of Rbp4 (liver-specific RBP4 knockout [LRKO] mice). We report the unexpected finding that LRKO mice lack detectable RBP4 in circulation.
Research Design and Methods
Animal Husbandry, Diets, and Genotyping
Rbp4(loxP/loxP) mice on mixed C57BL/6J × 129Sv background (17) (a gift from P. Chambon, University of Strasbourg), were crossed with albumin promoter (Alb)-Cre transgenic mice (18) (a gift from M. Magnuson, The Jackson Laboratory, Bar Harbor, ME; strain #003574) to produce Rbp4(loxP/+)/Alb-Cre(Tg/0) mice, which were interbred to produce F2 mice with hepatocyte-specific genetic knockout (KO) of Rbp4 (i.e., Rbp4(loxP/loxP)/Alb-Cre(Tg/0) mice, called LRKO mice). Littermates with genotypes Rbp4(+/+)/Alb-Cre(0/0), Rbp4(+/+)/Alb-Cre(Tg/0), and Rbp4(loxP/loxP)/Alb-Cre(0/0) served as controls. The various genotypes used for controls showed no statistically significant differences in metabolic parameters or serum RBP4 levels. Mice were maintained in an American Association of Laboratory Animal Care–accredited temperature and humidity–controlled vivarium with 12-h light/dark cycles. Studies were approved by the Institutional Animal Care and Use Committee at the University of Utah. Standard chow diet (Teklad 2920×; Harlan-Teklad) or high-fat (HF) diet (55% fat calories) (Teklad 93075) were administered from weaning until sacrifice at 20–22 weeks. The irradiated chow diet contained 15 International Units/g vitamin A as retinyl acetate. The γ-irradiated HF/high-sucrose (HS) diets contained 26 International Units/g retinyl acetate (before γ-irradiation for sterilization); according to the manufacturer, the dose of γ-irradiation used causes a 35–45% reduction in bioavailable vitamin A in rodent diets. Genotyping for Rbp4 alleles and Alb-Cre transgene was performed as previously described (17,18).
Quantitative RT-PCR
Commercial reagents (miRNeasy; QIAGEN) were used to isolate purified mRNA, and cDNA was immediately generated by Transcriptor First-Strand cDNA Synthesis Kit (Roche). For quantitative RT-PCR, RBP4 transcripts were amplified using primers 5′-GACAAGGCTCGTTTCTCTGG-3′ and 5′-AAAGGAGGCTACACCCCAGT-3′ (University of Utah Genomics Core) and detected with the FastStart Universal SYBR Green Master (Rox) kit (Roche) on a 7900HT Fast Real-Time PCR System (Applied Biosystems).
Hepatic Portal Vein Blood Sampling
Mice were anesthetized with 3–5% isofluorane before exposing the hepatic portal vein with a ventral incision. Hepatic portal blood was then collected with a 27-gauge insulin syringe. Blood was allowed to clot for 15 min and centrifuged at 1,500g for 10 min. Serum was then collected and analyzed by Western blotting. The protocol was adapted for mice (19).
Western Blotting
Serum was analyzed by SDS-PAGE and Western blotting for RBP4 as previously described (3,20). Commercially available purified RBP4 isolated from pooled human donor plasma (Hytest) was used as a positive control. For explant analysis, one-half perigonadal fat pad per animal was isolated, weighed, minced with fine scissors, washed by buoyancy centrifugation, and incubated overnight in media (DMEM; Gibco). The media volumes were adjusted so that total explant tissue weight per volume of media was equal for every sample. RBP4 was isolated by immunoprecipitation (by using polyclonal rabbit anti-human RBP4 [A0040; Dako]) from the conditioned media and then detected as above; the upper part of the SDS-PAGE gel was cut to prevent interference in Western blotting from the heavy chain of the immunoprecipitating antibody. This technique produced total precipitation of RBP4 in the serum of control mice (Supplementary Fig. 4). Transferrin antibody was from Bethyl Laboratories (Cat. A80-128) and transthyretin (TTR) (prealbumin) antibody was purchased Santa Cruz Biotechnology (Cat. sc-377517).
Metabolic Measurements
Serum insulin was measured by commercial ELISA (Crystal Chem). Serum glucose was measured in whole blood by glucometer (Bayer). Intraperitoneal glucose tolerance testing (1 g/kg body weight) and insulin tolerance testing (1 unit/kg body weight insulin aspart; Novo Nordisk) were performed as previously described (3). Urine RBP4 levels were determined by ELISA by using a monoclonal IgA-based assay (21) according to the manufacturer’s instructions (Adipogen). Body composition was measured by whole-body nuclear magnetic resonance (minispec; Bruker). Retinol and retinyl esters (REs) were measured as previously described for serum, tissue, and cells. The RE fraction contains the summed levels of retinyl palmitate, retinyl oleate, retinyl stearate, and retinyl linoleate. These four REs account for >95% of the RE present in liver.
Statistical Analysis
Two-way ANOVA was used to test for significant differences in groupings based on the independent factors of genotype and diet. Insulin tolerance test (ITT) area over the curve and glucose tolerance test (GTT) area under the curve were calculated as the change in glucose (mg/dL) from the baseline (time 0) value integrated over time (min).
Results
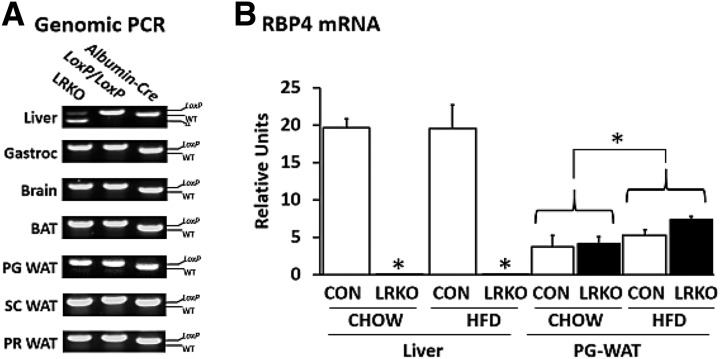
LRKO mice were born at expected Mendelian frequencies and did not differ from littermate controls in growth, body weight, or adiposity on normal chow diet (Table 1). Cre-mediated LoxP site recombination of Rbp4 was detected in the liver of LRKO mice but not in brain, skeletal muscle, brown adipose tissue, or any of three different white adipose tissue depots surveyed (Fig. 1A). Hepatocyte-specific Rbp4 recombination diminished RBP4 expression in liver without affecting RBP4 expression in fat (Fig. 1B).
Table 1.
Metabolic characterization of LRKO mice
| Control chow | LRKO chow | Control HF/HS | LRKO HF/HS | |
|---|---|---|---|---|
| Body composition | ||||
| Body weight (g) | 31.6 ± 0.9 | 29.2 ± 0.6 | 31.6 ± 0.7 | 35.4 ± 1.1*† |
| Change in weight after weaning (g) | 22.0 ± 0.8 | 19.8 ± 0.6 | 22.5 ± 0.7 | 25.1 ± 1.3*† |
| Percent fat mass (%) | 19.1 ± 3.0 | 14.3 ± 2.5 | 23.8 ± 2.6 | 29.2 ± 3.4*† |
| Metabolic phenotype | ||||
| Food intake (g/body weight/day) | 0.102 ± 0.002 | 0.099 ± 0.002 | 0.127 ± 0.025* | 0.131 ± 0.031* |
| Fasted glucose (mg/dL) | 62.5 ± 4.1 | 62.75 ± 1.9 | 61.8 ± 3.5 | 61.3 ± 0.4 |
| Fed glucose (mg/dL) | 111.8 ± 7.5 | 119.3 ± 12.6 | 120.3 ± 9.3 | 106.5 ± 4.8 |
| Fasted insulin (ng/mL) | 0.30 ± 0.04 | 0.30 ± 0.02 | 0.31 ± 0.05 | 0.35 ± 0.06 |
| Fed insulin (ng/mL) | 0.53 ± 0.07 | 0.78 ± 0.21 | 1.00 ± 0.24* | 1.23 ± 0.15* |
| ITT (area over the curve) | 11,944 ± 625 | 11,833 ± 730 | 8,678 ± 1,335* | 7,980 ± 1,191* |
| GTT (area under the curve) | 27,030 ± 1,445 | 26,009 ± 1,558 | 37,542 ± 1,863* | 37,490 ± 2,026* |
| Retinoid phenotype | ||||
| Serum RBP4 by ELISA (μg/mL) | 100.7 ± 9.6 | ND | 115.58 ± 8.2 | ND |
| Serum ROH (μg/dL) | 25.9 ± 2.3 | 1.7 ± 0.2† | ||
| Liver ROH (nmol/g) | 126 ± 12 | 125 ± 19 | ||
| Liver RE (nmol/g) | 4,818 ± 166 | 4,392 ± 285 | ||
| Hepatocyte ROH (nmol/106 cells) | 0.0600 ± 0.004 | 0.0478 ± 0.004 | ||
| Hepatocyte RE (nmol/106 cells) | 9.5 ± 1.6 | 6.9 ± 0.8 | ||
| HSC RE (nmol/106 cells) | 138.8 ± 18.6 | 98.6 ± 34.0 |
Data are mean ± SEM. Male mice; ages 20–22 weeks; chow or HF/HS diets started at weaning. n = 9–10 per group for RBP4 ELISA; n = 5–6 per group for other studies. Units for ITT and GTT area over/under the curve are mg ⋅ dL−1 ⋅ min−1. Maximum sensitivity of assay is 0.06 ng/mL. HSC, hepatic stellate cell; ND, not detected; ROH, retinol.
*P < 0.05 vs. chow; †P < 0.05 vs. control on same diet, by two-way ANOVA.
Figure 1.
LRKO mice. A: Representative PCR analysis of genomic DNA prepared from tissues of LRKO, Rbp4(fl/fl), and albumin promoter-Cre(Tg/0) transgenic mice. The lower–molecular-weight Cre-LoxP recombinant allele (Δ) is observed solely in the liver of LRKO mice and not in other tissues surveyed. B: Measurement of adipose tissue RBP4 mRNA by quantitative RT-PCR demonstrates a reduction of liver RBP4 mRNA to levels near the limit of detection in LRKO mice and induction of adipose tissue RBP4 mRNA by HF/HS feeding independently of genotype. Data are mean ± SEM (n = 5 per group). Statistical testing for liver and adipose tissue, respectively: *P < 0.05 vs. control and *P < 0.05 for chow-fed vs. HF/HS-fed groups (as indicated by brackets), by two-way ANOVA. BAT, brown adipose tissue; CON, control; Gastroc, gastrocnemius; HFD, high-fat diet; PG, perigonadal; PR, perirenal; SC, subcutaneous; WAT, white adipose tissue; WT, wild type.
Because adipose RBP4 is upregulated in insulin-resistant states (3,4), we fed LRKO and control mice an HF/HS diet to induce insulin resistance. Food intake did not differ between genotypes. Control mice fed HF/HS exhibited resistance to diet-induced obesity, consistent with their 129Sv mixed genetic background (22), but still developed insulin resistance and glucose intolerance (ITT response decreased 28%, and GTT response increased 39%; P < 0.05 for each result) (Table 1 and Supplementary Fig. 2A and B). Conversely, LRKO mice fed HF/HS exhibited small, but significant gains in body weight (12% increase over chow-fed LRKO mice; P < 0.05) and greater adiposity (29.2 ± 3.4% fat mass in LRKO mice vs. 23.8 ± 2.6% fat mass in control mice; P < 0.05) (Table 1) yet developed similar degrees of insulin resistance and glucose intolerance (ITT response decreased 33%, and GTT increased 41%; P < 0.05 vs. chow-fed LRKO mice and nonsignificant vs. control mice for each result) (Table 1 and Supplementary Fig. 2A and B). HF/HS feeding increased adipose tissue RBP4 mRNA independently of genotype (48% greater in HF/HS-fed vs. chow-fed mice; P < 0.05) (Fig. 1B), which is consistent with other mouse models of insulin resistance (3). Similar to the phenotype of germline Rbp4 KO mice (total-body Rbp4 KO mice) reported previously (23), LRKO mice exhibited greatly reduced serum retinol (<7% of control mouse levels; P < 0.05) (Table 1). However, liver retinol and RE content did not differ between LRKO and control mice when analyzed in whole tissue or in isolated parenchymal hepatocytes and hepatic stellate cells (Table 1), and liver weight did not differ between genotypes (data not shown).
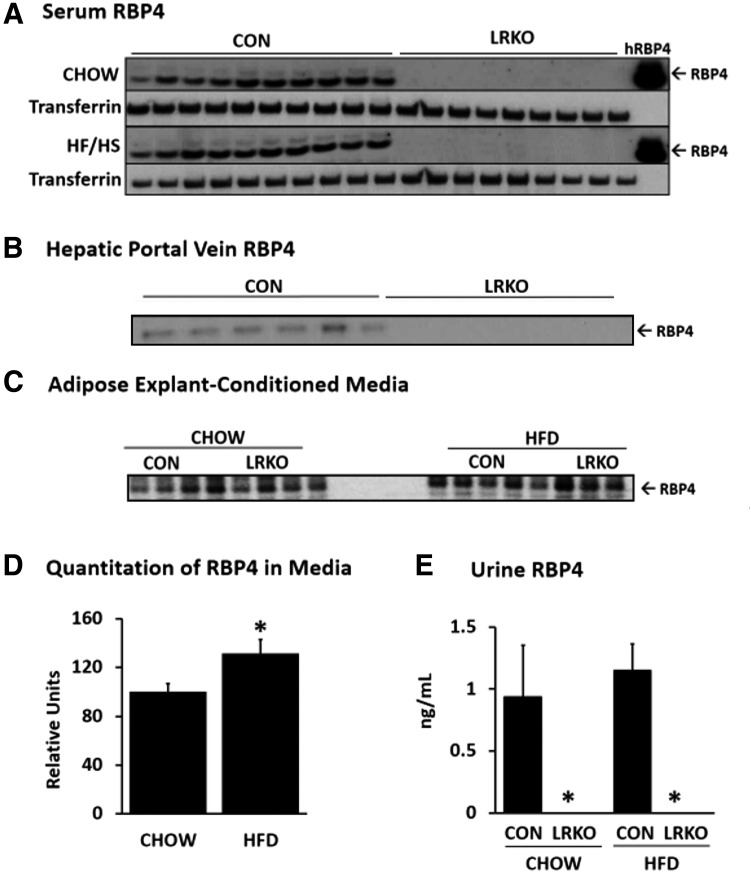
Of note, RBP4 was not detected in serum from the general or portal circulation of LRKO mice, even in response to HF/HS feeding–induced insulin resistance (Fig. 2A and B). Perigonadal white adipose tissue explants taken from LRKO and control mice secreted equal amounts of RBP4 into culture media (Fig. 2C and Supplementary Fig. 3A). Explant secretion was increased by HF/HS feeding independently of genotype (Fig. 2D). In the absence of its hepatocyte-secreted binding partner TTR, serum RBP4 is filtered through the glomerulus and excreted into urine (2). Serum TTR did not differ between genotypes (Supplementary Fig. 4). However, adipocytes do not express TTR, so we reasoned that RBP4 produced in its absence would undergo more rapid renal clearance. To test this, we measured urine RBP4 levels. However, RBP4 was detected in urine of control mice but not in urine of LRKO mice (Fig. 2E).
Figure 2.
RBP4 is secreted by adipose tissue explants but is not detected in serum or urine of LRKO mice. A: RBP4 protein levels are undetectable in serum of LRKO mice fed either a normal chow or an HF/HS diet as determined by Western blotting. Transferrin was used as a loading control. The hRBP4 lane contains 10 ng purified human RBP4. No immunoreactivity is evident in LRKO serum. B: RBP4 protein levels are undetectable in serum collected from the hepatic portal vein as determined from Western blotting. C: RBP4 protein secretion by adipose explants of LRKO and control littermates fed a normal chow or an HF/HS diet. D: Quantitation of Western blot data in adipose explant–conditioned media shown in panel C. HF/HS feeding caused an ∼25% increase in explant RBP4 secretion, independently of genotype. *P < 0.05 vs. chow-feeding (n = 4 per group), by two-way ANOVA. E: RBP4 protein is not detected in urine of LRKO mice by ELISA. *P < 0.05 vs. control (n = 5 per group), by two-way ANOVA. Data are mean ± SEM. CON, control; HFD, high-fat diet.
Discussion
Together these findings indicate that mice lacking hepatocyte-derived RBP4 exhibit no detectable circulating RBP4, which is not due to increased renal excretion of RBP4 or the absence of adipocyte RBP4 expression/secretion. Therefore, hepatocytes appear to be the principal, and perhaps sole, source of circulating RBP4 in mice. The clear dissociation between adipose RBP4 production and circulating RBP4 in LRKO mice indicates that adipocyte-secreted RBP4 is most likely confined to autocrine or paracrine actions within adipose tissue, even in the setting of insulin resistance.
Of note, LRKO mice fed chow do not exhibit the enhanced insulin sensitivity and glucose tolerance previously observed in total-body RBP4 KO mice (3), suggesting that an additional loss of RBP4 expressed in extrahepatic tissues (possibly adipocyte RBP4) is necessary for the favorable metabolic phenotype of the total-body RBP4 KO mice. However, these observations are complicated by the obesity-resistant, 129Sv mixed background of the mice studied. In future studies, characterization of the metabolic phenotype of adipocyte-specific RBP4 KO mice compared with LRKO mice will be important, ideally on a homogenous genetic background. Furthermore, although total-body RBP4 KO mice acquire increased RE and retinol stores with aging (24), this was not apparent in our 5-month-old LRKO mice (Table 1). Otherwise, phenotypes of LRKO mice and total-body RBP4 KO mice are similar; neither line exhibited protection from diet-induced insulin resistance or hyperglycemia (Table 1). Of note, LRKO mice unexpectedly developed slightly greater diet-induced weight gain and adiposity than littermate controls (Table 1), despite the obesity-resistant 129Sv mixed genetic background. Further study will clarify whether the observed increase in adiposity is a consistent feature of the LRKO phenotype in other genetic backgrounds.
Why does RBP4 secretion by adipocytes not produce circulating RBP4 in LRKO mice? Adipocyte RBP4 might be degraded rapidly within adipose tissue in vivo by undetermined mechanisms. However, we did not detect RBP4 proteolytic products in serum, adipose tissue, or adipose explant-conditioned media of LRKO or control mice by Western blotting with various combinations of N- or C-terminus–directed antibodies (data not shown). Work by Moraes-Vieira et al. (5) in mice expressing human RBP4 ectopically in muscle (muscle creatine kinase promoter-hRBP4 transgenic mice) suggested an alternative explanation: hRBP4 produced in muscle, which can be differentiated from endogenous mouse RBP4, accumulates in adipose tissue in vivo, where it activates CD11c+ cells (macrophage antigen-presenting cells and dendritic cells) and produces adipose tissue inflammation and systemic insulin resistance. Moreover, recent work by Lee et al. (25) showed that overexpression of hRBP4 in adipocytes causes increased levels of adipose tissue RBP4 but does not increase total circulating RBP4. Taken together, these findings suggest that circulating RBP4 may bind and accumulate in fat through yet-to-be-determined binding sites, and adipocyte-secreted RBP4 primarily remains within the adipose compartment. A survey of gene expression in adipose tissue of LRKO mice revealed no statistically significant differences between genotypes in mRNA levels of the RBP4 receptors Stra6 and Stra6L, which could potentially mediate RBP4 binding in fat, or in mRNA of classic retinoic acid target genes Rarβ, Crbp1, and Cyp26a1 (Supplementary Fig. 5A); additionally, no differences in mRNA of proinflammatory cytokines tumor necrosis factor-α and Mcp1 or in markers of macrophage infiltration Cd68 and F4/80 were found (Supplementary Fig. 5B).
There are several considerations in interpreting these findings. First, studies should be designed to determine whether the relative contributions of adipose and other extrahepatic tissues to circulating RBP4 levels differ between mice and humans. Second, although LRKO mice with diet-induced obesity exhibit no circulating RBP4, adipocyte RBP4 could make a greater contribution to circulating levels in the setting of severe obesity, as observed in ob/ob or db/db mice (26) or in morbidly obese humans, although the current findings suggest that this contribution might be minor. Third, adipocyte RBP4 could regulate serum RBP4 indirectly by altering hepatocyte RBP4 secretion or renal RBP4 clearance through as yet undetermined mechanisms. Finally, the finding that RBP4 does not function as a circulating adipokine in the classic endocrine sense does not negate or otherwise contradict the hundreds of publications that link elevated serum RBP4 to clinical features of insulin resistance, type 2 diabetes, dyslipidemia, cardiovascular disease, and cancer in humans; however, it does suggest that other parameters such as RBP4 production by hepatocytes, affinity of RBP4-TTR interactions, and RBP4 renal clearance may be more important determinants of RBP4 concentrations in these conditions. Further studies will clarify the dynamics of RBP4 secretion and action within the adipose tissue compartment and determine the extent to which (and mechanisms by which) adipocyte RBP4 and other extrahepatic RBP4 sources contribute to systemic insulin-glucose homeostasis.
Supplementary Material
Article Information
Acknowledgments. The authors thank Sihem Boudina, John David Symons, Corrine Welt, Thunder Jalili, Micah Drummond, and Anandh Babu Pon Velayutham of the University of Utah for intellectual contributions to the discussion of the study design and data interpretation.
Funding. This work was supported by a Howard Hughes Medical Institute Med into Grad Award (to A.S.); by an American Heart Association Predoctoral Fellowship award (to R.S. and T.E.G.); and by U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Merit Review Award BX000937, American Diabetes Association Core Basic Science Award 7-13-BS-05, and National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK-100826 (to T.E.G.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.J.T., A.S., and T.E.G. designed and executed the experiments, interpreted the data, and wrote the manuscript. S.-A.L., J.J.Y., and W.S.B. measured the retinol and REs in whole liver and isolated hepatocytes and hepatic stellate cells. J.C. and R.S. contributed to the data analysis of metabolic phenotype and RBP4 mRNA measurements. N.G. and M.M. developed and shared the flox-RBP4 mice for these studies. T.E.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0286/-/DC1.
References
- 1.Blaner WS, Olson JA. Retinol and Retinoic Acid Metabolism. New York, NY, Raven Press, 1994 [Google Scholar]
- 2.Saprano DB, Blaner WS. Plasma Retinol-Binding Protein. New York, NY, Raven Press, 1994 [Google Scholar]
- 3.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005;436:356–362 [DOI] [PubMed] [Google Scholar]
- 4.Graham TE, Yang Q, Blüher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006;354:2552–2563 [DOI] [PubMed] [Google Scholar]
- 5.Moraes-Vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab 2014;19:512–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aigner E, Bachofner N, Klein K, et al. Retinol-binding protein 4 in polycystic ovary syndrome—association with steroid hormones and response to pioglitazone treatment. J Clin Endocrinol Metab 2009;94:1229–1235 [DOI] [PubMed] [Google Scholar]
- 7.Cho YM, Youn BS, Lee H, et al. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 2006;29:2457–2461 [DOI] [PubMed] [Google Scholar]
- 8.El-Mesallamy HO, Hamdy NM, Zaghloul AS, Sallam AM. Serum retinol binding protein-4 and neutrophil gelatinase-associated lipocalin are interrelated in pancreatic cancer patients. Scand J Clin Lab Invest 2012;72:602–607 [DOI] [PubMed] [Google Scholar]
- 9.Su YX, Hong J, Yan Q, et al. Increased serum retinol-binding protein-4 levels in pregnant women with and without gestational diabetes mellitus. Diabetes Metab 2010;36:470–475 [DOI] [PubMed] [Google Scholar]
- 10.Kadoglou NP, Lambadiari V, Gastounioti A, et al. The relationship of novel adipokines, RBP4 and omentin-1, with carotid atherosclerosis severity and vulnerability. Atherosclerosis 2014;235:606–612 [DOI] [PubMed] [Google Scholar]
- 11.Reinehr T, Stoffel-Wagner B, Roth CL. Retinol-binding protein 4 and its relation to insulin resistance in obese children before and after weight loss. J Clin Endocrinol Metab 2008;93:2287–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y, Liu C, Zhang N, Wang S, Zhang Z. Proteomics analysis for finding serum markers of ovarian cancer. Biomed Res Int 2014;2014:179040 [DOI] [PMC free article] [PubMed]
- 13.Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab 2007;92:1971–1974 [DOI] [PubMed] [Google Scholar]
- 14.Munkhtulga L, Nagashima S, Nakayama K, et al. Regulatory SNP in the RBP4 gene modified the expression in adipocytes and associated with BMI. Obesity (Silver Spring) 2010;18:1006–1014 [DOI] [PubMed] [Google Scholar]
- 15.Munkhtulga L, Nakayama K, Utsumi N, et al. Identification of a regulatory SNP in the retinol binding protein 4 gene associated with type 2 diabetes in Mongolia. Hum Genet 2007;120:879–888 [DOI] [PubMed] [Google Scholar]
- 16.van Hoek M, Dehghan A, Zillikens MC, Hofman A, Witteman JC, Sijbrands EJ. An RBP4 promoter polymorphism increases risk of type 2 diabetes. Diabetologia 2008;51:1423–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghyselinck NB, Vernet N, Dennefeld C, et al. Retinoids and spermatogenesis: lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn 2006;235:1608–1622 [DOI] [PubMed] [Google Scholar]
- 18.Postic C, Shiota M, Niswender KD, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem 1999;274:305–315 [DOI] [PubMed] [Google Scholar]
- 19.Scholtens E, Mulder GJ. A simple method of obtaining multiple blood samples from the portal vein and the hepatic vein in the rat in vivo. Experientia 1983;39:1176–1177 [DOI] [PubMed] [Google Scholar]
- 20.Mody N, Graham TE, Tsuji Y, Yang Q, Kahn BB. Decreased clearance of serum retinol-binding protein and elevated levels of transthyretin in insulin-resistant ob/ob mice. Am J Physiol Endocrinol Metab 2008;294:E785–E793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SE, Lee NS, Park JW, et al. Association of urinary RBP4 with insulin resistance, inflammation, and microalbuminuria. Eur J Endocrinol 2014;171:443–449 [DOI] [PubMed] [Google Scholar]
- 22.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol Behav 2001;72:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quadro L, Blaner WS, Salchow DJ, et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J 1999;18:4633–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik J, Vogel S, Quadro L, et al. Vitamin A: overlapping delivery pathways to tissues from the circulation. J Nutr 2004;134:276S–280S [DOI] [PubMed] [Google Scholar]
- 25.Lee SA, Yuen JJ, Jiang H, Kahn BB, Blaner WS. Adipocyte-specific overexpression of retinol-binding protein 4 causes hepatic steatosis in mice. Hepatology 2016;64:1534–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev 2014;10:131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.