Abstract
BACKGROUND & AIMS
Non-alcoholic steatohepatitis (NASH) is an independent risk factor for cardiovascular disease (CVD) morbidity after liver transplantation, but its impact on CVD mortality is unknown. We sought to assess the impact of NASH on CVD mortality after liver transplantation and to predict which NASH recipients are at highest risk of a CVD-related death following a liver transplant.
METHODS
Using the Organ Procurement and Transplantation Network database we examined associations between NASH and post liver transplant CVD mortality, defined as primary cause of death from thromboembolism, arrhythmia, heart failure, myocardial infarction, or stroke. A physician panel reviewed cause of death.
RESULTS
Of 48,360 liver transplants (2/2002–12/2011), 5,057 (10.5%) were performed for NASH cirrhosis. NASH recipients were more likely to be older, female, obese, diabetic, and have history of renal failure or prior CVD versus non-NASH (p<0.001 for all). Although there was no difference in overall all-cause mortality (log-rank p=0.96), both early (30-day) and long-term CVD-specific mortality was increased among NASH recipients (Odds ratio=1.30, 95% Confidence interval (CI): 1.02–1.66; Hazard ratio=1.42, 95% CI: 1.07–1.41, respectively). These associations were no longer significant after adjustment for pre-transplant diabetes, renal impairment or CVD. A risk score comprising age ≥ 55, male sex, diabetes and renal impairment was developed for prediction of post liver transplant CVD mortality (c-statistic 0.60).
CONCLUSION
NASH recipients have an increased risk of CVD mortality after liver transplantation explained by a high prevalence of co-morbid cardiometabolic risk factors that in aggregate identify those at highest risk of post-transplant CVD mortality.
Keywords: fatty liver, heart disease, mortality, NASH, NAFLD, liver transplant
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of early (30-day) mortality after liver transplantation (LT) in the United States[1] and has been shown to significantly impact long-term post-transplant morbidity and mortality[2, 3]. In addition, certain underlying causes of liver disease, such as non-alcoholic steatohepatitis (NASH), may adversely impact cardiac risk due to a high prevalence of co-morbid cardiometabolic risk factors[3]. NASH is quickly becoming a leading cause of cirrhosis necessitating transplantation in developed countries and its incidence is expected to continue to rise[4]. As we enter a new era of effective treatment for hepatitis C virus (HCV), transplantation rates for HCV are predicted to decline and therefore NASH will increase in relative, as well as absolute importance[4]. Patients with NASH are more likely to die of cardiovascular complications than from liver-related death[5]. Perhaps not surprisingly, as transplantation rates for NASH cirrhosis have risen in the United States, CVD-related mortality after LT has also increased in parallel (Supplemental Figure 1).
Interest in the potential role of NASH as an independent risk factor for CVD has steadily increased[6–9]. The plausibility of a direct association is supported by common pathophysiologic pathways as well as by mechanisms of injury in NASH that could adversely affect various aspects of CVD. NASH is associated with impaired endothelial function[10], a higher prevalence of vulnerable coronary plaques[11, 12], a pro-atherogenic lipid profile[13], and with markers of subclinical cardiovascular disease such as increased carotid intima media thickness[14] and increased coronary artery calcification[15, 16]. NASH has also been associated with several markers of subclinical myocardial dysfunction with a propensity towards the development of symptomatic diastolic dysfunction[17–22]. A recent meta-analysis also suggests that NASH may be associated with increased cardiovascular events independent of several traditional CVD risk factors[9].
Existing data demonstrate that the long-term survival for NASH patients undergoing LT is comparable to other causes of liver disease[4, 23, 24]. However, early mortality (e.g. less than 1 year) is higher in NASH patients primarily due to infectious and CVD complications [25, 26]. Yet, it is unknown whether the presence of NASH is an independent risk factor, beyond its associated traditional CVD risk factors, for CVD-specific mortality post-LT. We previously demonstrated that NASH is an independent risk factor for early (1-year) CVD morbidity after LT compared to those transplanted for alcohol-induced liver disease at two Chicago transplant centers[3]. However, this study was not appropriately powered to look at CVD-related mortality. Therefore, using a national database, in the current study we sought: 1) To examine whether NASH cirrhosis (compared to other causes of end-stage liver disease) is independently associated with CVD-related mortality after LT; and 2) To develop a risk score to predict CVD-related mortality after LT in NASH recipients. We hypothesized that NASH patients would have an increased incidence of CVD mortality independent of traditional CVD risk factors compared to all other causes of end stage liver disease (ESLD) based on prior published literature. Findings from this study will further our understanding of post-operative CVD risk in this burgeoning indication for LT and may identify a high-risk sub-population that could benefit from additional pre-transplant or early post-transplant interventions in order to improve patient outcomes.
PATIENTS AND METHODS
Study Population
Adults (age ≥ 18 years) who underwent LT between February 1, 2002 and December 31, 2011 were identified from the Organ Procurement Transplantation Network (OPTN) Standard Transplant Analysis and Research files (created on March 15, 2013, n=55,914). Follow up data was available through December 31, 2012. Those listed as status 1, who were undergoing re-transplantation, who were transplanted prior to the inception of the Model for End Stage Liver Disease (MELD) organ allocation system, who received simultaneous heart, lung, intestine or pancreas transplants or who had an unknown indication for liver transplantation were excluded (n= 7,443). Of the 48,360 remaining first LT recipients, 2,705 had a primary or secondary indication for transplant listed as NASH and 2,352 had probable NASH, defined as a diagnosis of cryptogenic cirrhosis with at least one component of the metabolic syndrome (e.g. diabetes, hypertension or obesity=Body Mass Index (BMI) ≥ 30). Diagnoses were assigned by transplant centers without prerequisite diagnostic or confirmatory criteria. There were minimal clinically significant differences found between those with definite NASH and probable NASH, therefore the groups were pooled (see Supplemental Table 1). The final NASH cohort consisted of 5,057 LT recipients representing 10.5% of all U.S. liver transplants. The Institutional Review Board of Northwestern University approved the study in accordance with the ethical standards outlined in the Declaration of Helsinki. The study met the requirements for waiver of informed consent in line with the United States’ Department of Health and Human Services Code of Federal Regulations (45 CFR 46.116(d)).
Definitions and Outcomes
Data collected and analyzed from the OPTN database included the following recipient factors: age, sex, race/ethnicity (Black, non-Hispanic White, Asian and Hispanic), socioeconomic status, BMI, history of CVD co-morbid conditions (e.g. peripheral vascular disease, angina, hypertension, stroke, pulmonary embolism), diabetes status, renal function, laboratory values at the time of transplant (creatinine, albumin, sodium, INR, alanine aminotransferase (ALT), bilirubin), hepatocellular carcinoma status, calculated MELD score at the time of transplant, wait list time, functional capacity prior to transplant, complications of ESLD (ascites, encephalopathy, portal vein thrombosis, etc.), hospitalization and ventilator status at transplant. Donor factors included age, sex, race, BMI, cause of death, donor type (living, deceased, donation after cardiac death), and donor risk index. Transplant-related variables included transplant center location and volume, region, organ allocation type, cold ischemia time, steroid induction, and use of a calcineurin inhibitor. Renal impairment was defined as an estimated glomerular filtration rate less than or equal to 60 mL/min/1.73 m2 or need for renal replacement therapy.
Recipient cause of death was determined by a physician's review (L.V.W. and D.L.J.) of primary and contributory causes of death (including all free text inputs) listed in the OPTN database and included death from infection, graft failure, renal failure, respiratory failure, CVD, hemorrhage, operative and unknown causes. Any potential case with death due to CVD[27], defined as primary cause of death from arrhythmia, heart failure, myocardial infarction, primary cardiac arrest, thromboembolism, and/or stroke, was then manually reviewed by an independent panel of four physicians (1 hepatologist, 2 cardiologists, 1 surgeon) who were blinded to the underlying indication for LT in order to attempt to adjudicate CVD case mortality. Since cardiac arrest is often a final common pathway of death, recipients with a primary cardiac arrest who had a secondary cause of death available were coded as non-CVD deaths (n=68). The primary study outcome was 30-day CVD mortality. This standardized time period was chosen based on prior literature describing a trend towards increased early mortality in NASH recipients in the perioperative period[23, 25, 26] and for its common use as an outcomes-based quality indicator[28]. The time period also allows a fair assessment of transplant outcomes across centers and minimizes differences in variations in length of post-transplant stay from affecting the measurement. Since some operative factors, such as electrolyte flux, are not captured within the OPTN dataset and may have a differential effect on cardiac events, analyses were also categorized into peri-operative CVD mortality, defined as CVD mortality within the first 24 hours of transplant, and early postoperative CVD mortality, defined as CVD mortality occurring between 1 and 30 days. Secondary outcomes included all-cause and CVD-related patient survival. Patients were censored at time of death, date of last follow up, time of re-transplantation or at 30 days.
Statistical Analysis
Clinical characteristics and causes of death of LT recipients from the 2002–2012 OPTN dataset were described using frequency counts and percentages for categorical variables and means ± standard deviations for continuous variables. Multivariate logistic regression models were used to quantify associations between the exposure, presence of NASH, and the primary outcome, CVD mortality within 30 days of transplantation. Covariates in the multivariable model were chosen a priori for clinical importance. Factors considered potential confounders included age, race, sex, transplant center, highest education level attained, pre-transplant diabetes, renal impairment and presence of at least one pre-transplant CVD co-morbid condition, defined as angina, hypertension, thromboembolism, peripheral vascular disease and/or stroke. Four models were fitted: Model 1: age, race, sex, transplant center, and educational level; Model 2: Model 1 + pre-transplant CVD; Model 3: Model 1 + pre-transplant diabetes; Model 4: Model 1 + pre-transplant renal impairment (estimated glomerular filtration rate ≤ 60 mL/min/1.73 m2). No significant interactions between NASH and early CVD mortality were observed according to sex, age, or race. Twelve candidate variables with p value <0.05 on univariate analysis were entered into a Cox regression for cardiac death post transplant in order to identify factors independently associated with an increased risk of CVD mortality among NASH recipients (Supplemental Table 2). Scores were assigned to multivariate predictors based on model coefficients. The cohort was then divided into 4 risk groups: low (score=0–1), intermediate (score=2), high (score=3–4) and very high (score=5) risk. The performance of the logistic regression model was then internally validated using 1000 bootstrap re-samples. Bootstrapping is a nonparametric method for assigning measures of accuracy to sample estimates. Bootstrapping essentially re-samples (multiple times) from the study population to approximate how precise statistical estimates (e.g. C statistic, confidence intervals) are related to the true population of interest. Kaplan-Meier analysis with log-rank test assessed time to graft and patient survival. All analyses were performed using SAS 9.3 software (SAS institute, Cary, NC).
RESULTS
Patient Characteristics
When compared with other indications for liver transplantation, NASH patients were older (58.1 vs. 52.7 years), more likely to be female (43.4% vs. 31.1%) and more likely to be white (81.5% vs. 72.8%, p<0.001 for all, Table 1). NASH recipients had slightly higher calculated MELD scores (21.0 vs. 19.7) likely driven by their somewhat higher creatinine levels (1.76 vs. 1.53 mg/dL) and increased requirement for renal replacement therapy (p<0.001 for all). They were also more likely to receive a simultaneous kidney transplant (8.6% vs. 5.8%), have higher pre-transplant BMI and nearly two-thirds met clinical criteria for obesity (p<0.001 for all). Not surprisingly, compared to non-NASH patients NASH recipients demonstrated an increased prevalence of pre-transplant CVD manifested by a higher prevalence of symptomatic CVD such as angina, stroke, and peripheral vascular disease and substantially higher prevalence of pre-existing hypertension (35.7% vs. 17.4%, p<0.001) and diabetes (57.1% vs. 20.8%, p<0.001, Table 1). There were also differences in the prevalence of several complications of ESLD (e.g. ascites, variceal bleed) in the NASH compared to non-NASH group (Table 1). In terms of donor factors, NASH recipient donors were older and had slightly higher BMI than non-NASH (p<0.001 for both). There was no statistically significant difference in rates of donation after cardiac death between recipient groups. Importantly there were also no clinically significant differences in graft cold ischemia time or in choice of immunosuppressant (Table 1).
Table 1.
Characteristics of 48,360 liver transplant recipients in the United States’ Organ Procurement and Transplantation Network (OPTN) database (2002–2011)
| Characteristic | NASH (N = 5,057) |
HCV (N = 14,820) |
Alcohol (N = 6,998) |
Othera (N = 21,485) |
P value |
|---|---|---|---|---|---|
| Recipient Age, mean ± SD, years | 58.1 ± 8.4 | 53.8 ± 7.3 | 53.9 ± 8.6 | 52.9 ± 10.9 | <0.001c,d.e |
| Recipient Female, No (%) | 2197 (43.4) | 4122 (27.8) | 1490 (21.3) | 7349 (34.2) | <0.001c,d,e |
| Recipient Race & Ethnicity, No (%) | |||||
| Non-Hispanic White | 4121 (81.5) | 10421 (70.3) | 5624 (80.4) | 15011 (69.9) | |
| Black | 160 (3.2) | 1694 (11.4) | 261 (3.7) | 2103 (9.8) | |
| Hispanic | 657 (13.0) | 2110 (14.2) | 969 (13.9) | 2617 (12.2) | <0.001c,e, 0.02d |
| Asian | 79 (1.6) | 463 (3.1) | 75 (1.1) | 1526 (7.1) | |
| Other | 40 (0.8) | 132 (0.9) | 69 (1.0) | 228 (1.1) | |
| Socioeconomic status | |||||
| Highest education < High School | 244 (4.8) | 632 (4.3) | 313 (4.5) | 951 (4.4) | 0.09c, 0.36d, 0.22e |
| Working for income | 917 (21.0) | 2890 (25.6) | 901 (16.2) | 5265 (30.7) | <0.001c,d,e |
| Calculated MELD score at transplant | 21.0 ± 9.4 | 18.9 ± 10.3 | 21.7 ± 9.6 | 17.6 ± 10.6 | <0.001c,d,e |
| Waitlist time, mean, days | 241.7 ± 415.3 | 278.4 ± 476.2 | 188.5 ± 389.3 | 304.9 ± 542.0 | <0.001c,d,e |
| Hepatocellular Carcinoma | 327 (6.5) | 2646 (17.9) | 541 (7.7) | 7485 (34.8) | <0.001c,e, 0.008d |
| Simultaneous kidney transplant | 435 (8.6) | 834 (5.6) | 515 (7.4) | 959 (4.5) | <0.001c,e, 0.01d |
| Recipient BMI (kg/m2) at transplant | 32.0 ± 5.8 | 28.3 ± 5.3 | 28.0 ± 5.4 | 27.3 ± 5.4 | <0.001c,d,e |
| Obesity (BMI ≥ 30) | 3237 (64.0) | 4969 (33.6) | 2249 (32.2) | 5698 (26.5) | <0.001c,d,e |
| Morbid obesity (BMI ≥ 40) | 437 (8.6) | 416 (2.8) | 177 (2.5) | 506 (2.4) | <0.001c,d,e |
| Labs at time of transplant | |||||
| Creatinine (mg/dL) | 1.76 ± 1.41 | 1.53 ± 1.37 | 1.65 ± 1.32 | 1.38 ± 1.24 | <0.001c,d,e |
| Total bilirubin (mg/dL) | 7.01 ± 9.39 | 6.56 ± 9.55 | 8.15 ± 9.74 | 7.70 ± 10.63 | 0.004c, <0.001d,e |
| INR | 1.82 ± 0.89 | 1.80 ± 0.91 | 1.95 ± 1.26 | 1.73 ± 1.16 | 0.11c, <0.001d,e |
| Sodium (mEq/L) | 135.7 ± 5.0 | 135.6 ± 5.2 | 135.0 ± 5.4 | 136.3 ± 5.0 | 0.24c, <0.001d,e |
| Pre-transplant CVD (composite) | 730 (37.7) | 1450 (19.8) | 775 (22.8) | 1623 (17.0) | <0.001c,d,e |
| Angina | 73 (7.0) | 94 (3.0) | 53 (3.2) | 119 (2.7) | <0.001c,d,e |
| Cerebrovascular Disease | 23 (1.2) | 36 (0.5) | 22 (0.7) | 53 (0.6) | <0.001c, 0.04d, 0.002e |
| Hypertension | 676 (35.7) | 1339 (18.6) | 696 (20.9) | 1454 (15.6) | <0.001c,d,e |
| Peripheral Vascular Disease | 33 (1.8) | 62 (0.9) | 42 (1.3) | 77 (0.8) | <0.001c,e, 0.15d |
| Pulmonary Embolism | 6 (0.3) | 16 (0.2) | 10 (0.3) | 19 (0.2) | 0.44c, 0.91d, 0.42e |
| Other Comorbid Conditions | |||||
| Diabetes | 2888 (57.1) | 3373 (22.8) | 1576 (22.5) | 4397 (20.5) | <0.001c,d,e |
| Respiratory failure on ventilator | 231 (4.6) | 566 (3.8) | 400 (5.7) | 753 (3.5) | 0.02c, 0.005d, <0.001e |
| Renal Failure requiring RRT | 681 (13.5) | 1465 (9.9) | 1000 (14.3) | 1708 (8.0) | <0.001c,e, 0.20d |
| Renal Impairmentb | 2887 (57.2) | 5660 (38.2) | 3202 (45.9) | 7022 (32.7) | <0.001c,d,e |
| Recipient Complications of ESLD | |||||
| Ascites at transplant | 4378 (87.6) | 11933 (81.5) | 6221 (89.9) | 15659 (73.9) | <0.001c,d,e |
| SBP | 149 (7.8) | 600 (8.3) | 359 (10.8) | 653 (7.0) | 0.48c, <0.001d, 0.19e |
| Encephalopathy | 3811 (76.0) | 10460 (71.1) | 5608 (80.8) | 13003 (61.1) | <0.001c,d,e |
| Portal Vein Thrombosis | 551 (11.0) | 940 (6.4) | 536 (7.7) | 1504 (7.1) | <0.001c,d,e |
| TIPS | 655 (13.1) | 1436 (9.8) | 1019 (14.7) | 1948 (9.2) | <0.001c,e, 0.01d |
| Variceal Bleed | 146 (7.8) | 353 (4.9) | 239 (7.2) | 558 (6.0) | <0.001c, 0.48d, 0.005e |
| Functional status at transplant | |||||
| Independent | 2081 (45.3) | 6859 (51.9) | 2790 (44.6) | 10240 (52.9) | |
| Partially dependent | 1227 (26.7) | 3267 (24.7) | 1577 (25.2) | 4676 (24.2) | <0.001c,e, 0.02d |
| Totally dependent | 1282 (27.9) | 3087 (23.4) | 1894 (30.2) | 4435 (22.9) | |
| Recipient length of stay | 17.8 ± 23.0 | 15.7 ± 20.2 | 17.4 ± 21.7 | 15.2 ± 21.6 | <0.001c,e, 0.46d |
| Donor Factors | |||||
| Age | 43.4 ± 17.5 | 40.4 ± 16.0 | 42.7 ± 17.6 | 41.3 ± 17.2 | <0.001c,e, 0.02d |
| BMI (kg/m2) | 27.5 ± 6.3 | 26.8 ± 6.3 | 27.2 ± 6.0 | 26.7 ± 5.9 | <0.001c,e, 0.06d |
| Donation after Cardiac Death | 487 (9.6) | 1487 (10.0) | 748 (10.7) | 1974 (9.2) | 0.41c, 0.06d, 0.33e |
| Living Donor | 190 (3.8) | 558 (3.8) | 202 (2.9) | 1159 (5.4) | 0.98c, 0.008d, <0.001e |
| Graft cold ischemia time, hours | 7.14 ± 3.73 | 7.06 ± 3.65 | 7.15 ± 3.37 | 6.99 ± 3.62 | 0.20c, 0.91d, 0.02e |
| Recipient Immunoprophylaxis | |||||
| Calcineurin Inhibitor | 4669 (92.3) | 13975 (94.3) | 6559 (93.7) | 20247 (94.2) | <0.001c,e, 0.003d |
| Steroids | 4030 (79.7) | 11605 (78.3) | 5630 (80.5) | 17659 (82.2) | 0.04c, 0.30d, <0.001e |
Other=drug-induced, autoimmune, other viral hepatitis, cholestatic, metabolic
eGFR ≤ 60 mL/min/1.73 m2
NASH vs. HCV
NASH vs. alcohol
NASH vs. other
Values are expressed as number (%) or mean ± standard deviation
Abbreviations: NASH, nonalcoholic steatohepatitis; No, number; MELD, model for end-stage liver disease; BMI, body mass index; INR, international normalized ratio; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; RRT, renal replacement therapy; ESLD, end-stage liver disease; SBP, spontaneous bacterial peritonitis; TIPS, transjugular intrahepatic portosystemic shut
30-day Post-transplant Survival
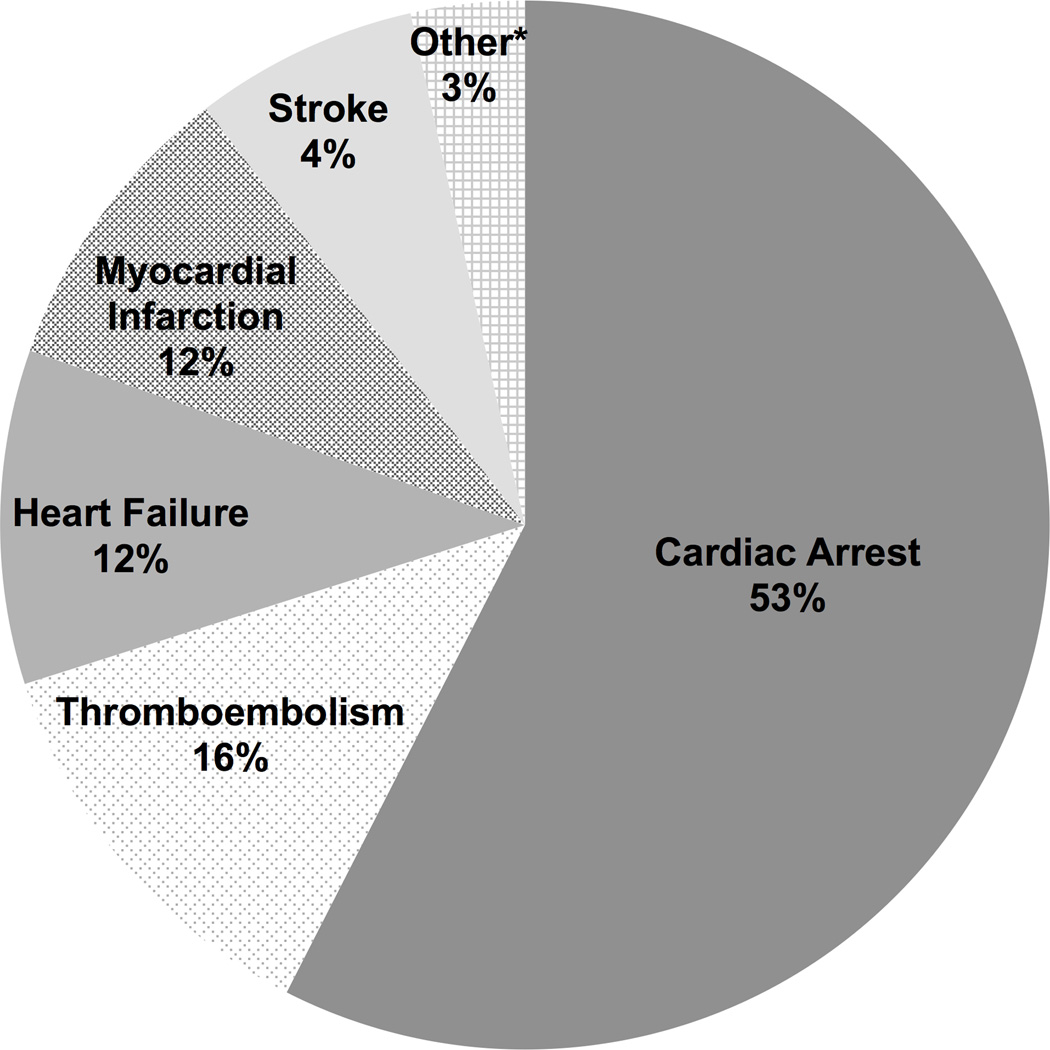
In univariate analysis, NASH was associated with greater early all-cause mortality with 192 (3.8%) NASH recipients dying within 30 days of transplant compared with 1021 (2.8%) non-NASH recipients (p<0.001). CVD-related mortality was the most common cause of early mortality in both groups, accounting for over 40% of deaths within 30 days. This was followed by infection (28%) and graft failure (12%). Compared with non-NASH patients, NASH recipients were more likely to have a CVD-related death within 30-days of LT (1.5% vs. 1.2%, p=0.04). Cardiac arrest accounted for the majority of early CVD-related deaths (53.3%) in the NASH group, followed by thromboembolism (16.0%), heart failure (12.0%) and myocardial infarction (12.0%), Figure 1. Notably, the proportion of cardiac arrest events in the NASH cohort (53%) was significantly higher than that from other causes of end stage liver disease (44%, p<0.01). In multivariable logistic regression models adjusted for age, sex, race, education, transplant center and pre-transplant cardiometabolic risk factors (CVD, diabetes, or renal impairment) NASH was no longer independently associated with early CVD-related mortality (Table 2). These associations did not significantly change when the analysis was stratified by those CVD deaths occurring in the peri-operative period (<24 hours) compared to the early postoperative period (1–30 days, Table 2). There were also no significant differences in early mortality when stratified by transplant region or center (data not shown).
Figure 1. Underlying cause of CVD-related mortality in 75 NASH recipients who died within 30 days of transplant.
*Other = arrhythmia, ruptured aortic aneurysm, hypertensive crisis, pulmonary hypertension
Table 2.
Association of NASH with any 30-day, perioperative (within 24 hours) or early postoperative (1–30 days) cardiovascular disease mortality following liver transplantation (OPTN, 2002–2011)
| Characteristic | Any 30-day CVD mortality (N=492) |
Perioperative CVD mortality (N=185) |
Early postoperative CVD mortality (N=307) |
|||
|---|---|---|---|---|---|---|
| OR (95% CI)a |
P value | OR (95% CI) |
P value | OR (95% CI) |
P value | |
| Unadjusted | 1.30 (1.02, 1.66) |
0.037 | 1.64 (1.14, 2.37) |
0.008 | 1.10 (0.80, 1.53) |
0.562 |
| Base Model | 1.23 (0.96, 1.59) |
0.108 | 1.43 (1.01, 2.01) |
0.041 | 1.11 (0.79, 1.56) |
0.537 |
| Base + Pre-transplant Composite CVD | 1.35 (0.93, 1.96) |
0.119 | 1.35 (0.84, 2.17) |
0.215 | 1.37 (0.84, 2.22) |
0.212 |
| Base + Pre-transplant Diabetes | 1.22 (0.93, 1.58) |
0.146 | 1.35 (0.94, 1.92) |
0.100 | 1.13 (0.80, 1.59) |
0.503 |
| Base + Pre-transplant Renal Impairmentb | 1.13 (0.87, 1.46) |
0.355 | 1.29 (0.92, 1.82) |
0.146 | 1.03 (0.73, 1.44) |
0.873 |
Logistic regression
eGFR ≤ 60 mL/min/1.73 m2
Base model: age, race, sex, education level, transplant center
Abbreviations: NASH, nonalcoholic steatohepatitis; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate
Post-transplant Survival beyond 30 days
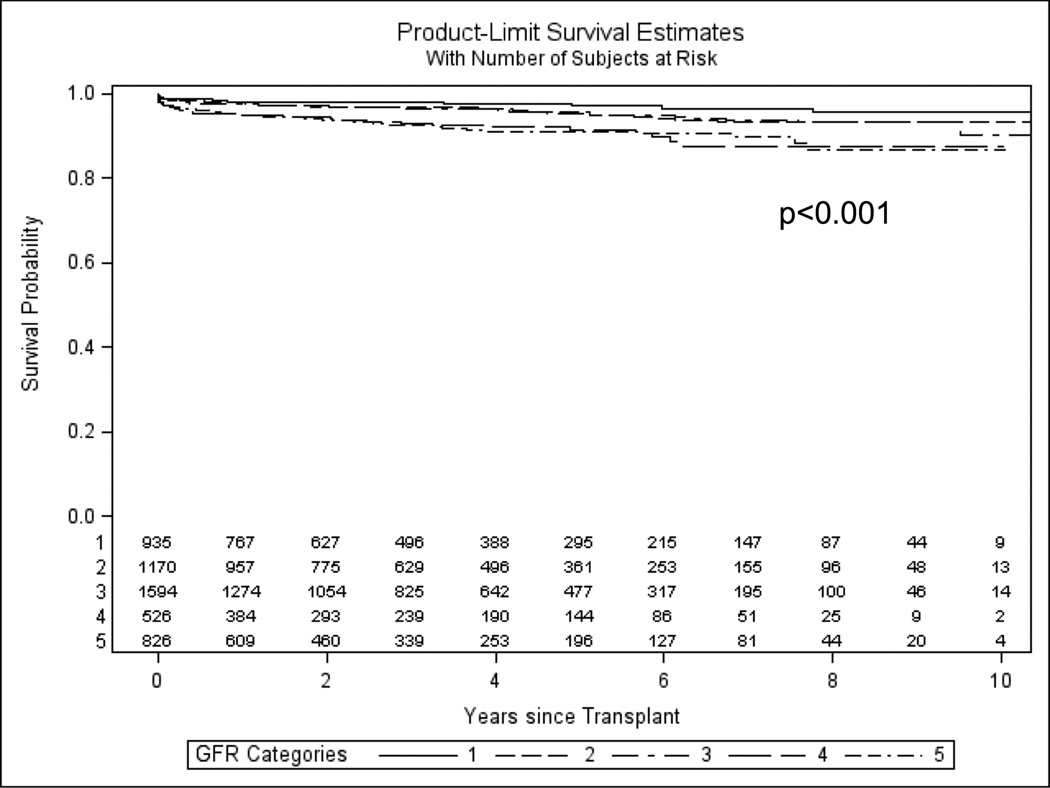
The median follow-up time for the study cohort was 3.2 years (interquartile range: 1.22–5.97 years). In univariate analysis, long-term graft and overall patient survival were slightly better in the NASH group (71.3% vs. 68.1% and 73.9 vs. 71.7%, respectively, p<0.001 for both). However, in survival analysis there was no statistically significant difference in overall patient survival between NASH and non-NASH recipients (Supplemental Figure 2, log-rank p=0.739). In contrast, NASH recipients were more likely to die from a CVD-related death (4.5% vs. 3.4%) compared to non-NASH patients over a mean of 1.62 ± 2.29 years of follow up (Supplemental Figure 3, log-rank p<0.001; HR=1.42, 95% CI: 1.23–1.63, p<0.001). Univariate analysis demonstrated the presence of renal impairment as an important predictor of post-transplant CVD-related mortality (Supplemental Table 2, p<0.0001). In Kaplan-Meier survival analysis, there was a dose response relationship between degree of renal impairment (as measured by GFR) and risk of CVD-related mortality after LT (Figure 2, log-rank p<0.0001). However, in multivariable cox regression analysis adjusted for age, sex, race, education, transplant center and pre-transplant cardiometabolic risk factors (CVD, diabetes, or renal impairment) NASH was no longer independently associated with CVD-related mortality (HR=1.04, 95% CI:0.84–1.27; p=0.75).
Figure 2. Long-term CVD-related survival stratified by stage of chronic kidney disease as measured by glomerular filtration rate.
Patients were censored at time of death or date of last follow up. Kaplan-Meier survival analysis, Log-rank, p<0.001
- >or= 90 (normal function) = 1
- 60–89 (stage 2 CKD) = 2
- 30–59 (stage 3 CKD) = 3
- 15–29 (stage 4 CKD) =4
- <15 or on renal replacement therapy at time of transplant (stage V CKD) =5
Prediction of post-transplant CVD mortality in NASH recipients
In univariate analysis, 12 factors predicted CVD-related mortality among NASH recipients (Supplemental Table 2). In multivariate analysis with backwards stepwise selection, four pre-transplant factors predicted long-term CVD mortality after liver transplant for NASH: age ≥ 55, male sex, diabetes, and renal impairment (Supplemental Table 3). A score of 1 was assigned to sex, age, and diabetes, and a score of 2 to renal impairment based on model coefficients (Supplemental Table 3). The cohort was divided into 4 risk groups: low (score=0–1), intermediate (score=2), high (score=3–4) and very high (score=5) risk (Table 3). Very high-risk recipients were twice as likely as low risk recipients to die from a CVD-related cause (incidence rate: 13.54 vs. 6.77 per 10 person-years; p<0.001). The model showed moderate discrimination (c-statistic 0.60, 95% CI: 0.59–0.62 after bootstrapping). There was no significant interaction between transplant region and predicted risk in our model (p=0.56) with regard to CVD mortality.
Table 3.
Risk of cardiovascular disease mortality after liver transplantation for NASH
| Risk Groupa | Population N (%) | Deaths N (%) | Hazard Ratio (95% CI) | P value |
|---|---|---|---|---|
| Low (0–1 point) | 1453 (28.7) | 42 (2.9) | Reference | |
| Intermediate (2 points) | 1984 (39.2) | 80 (4.0) | 1.42 (0.98, 2.07) | 0.064 |
| High (3–4 points) | 1461 (28.9) | 93 (6.4) | 2.36 (1.64, 3.40) | <0.001 |
| Very High (5 points) | 159 (3.1) | 13 (8.2) | 3.34 (1.79, 6.23) | <0.001 |
Each patient was classified according to how many risk factors they had pretransplant: Age ≥ 55 (1 point), male sex (1 point), diabetes (1 point), renal impairment (2 points), defined as estimated glomerular filtration rate (eGFR) ≤ 60 mL/min/1.73 m2 or need for renal replacement therapy
C-statistic = 0.60 (95% CI 0.59–0.62), after bootstrapping
DISCUSSION
Despite a large body of literature demonstrating an independent association between NASH and CVD, using a large national patient sample it does not appear that presence of NASH is independently associated with CVD-related mortality after LT. However, our results do demonstrate that NASH patients, compared to patients transplanted for other causes of liver disease, have a modest, but statistically significant, higher risk of both early (≤ 30 days) and late (> 30 days) post-LT CVD-related mortality. This appears to be largely attributable to the high prevalence of co-existing cardiometabolic risk factors present in NASH candidates prior to transplant and represents an opportunity for improved management of this high cardiac risk population.
In line with what others have shown, our data demonstrate that patients transplanted for NASH cirrhosis have comparable long-term survival to those transplanted for other etiologies of end-stage liver disease[4, 23, 24, 29]. A recent meta-analysis demonstrates that death caused by cardiovascular complications after LT in patients with NASH is increased compared with patients without NASH despite similar long-term outcomes[29]. We speculate that similar long-term survival, despite poor early outcomes, may be related to competing long-term mortality risk in other causes of liver disease such as graft failure (e.g. hepatitis C) and malignancy (e.g. hepatocellular carcinoma). With highly effective therapy for HCV and a subsequent reduction in HCV recurrence post-LT, we may see a relative decline in long-term outcomes for NASH recipients over time. Albeldawi et al.[30] demonstrated that CVD risk following LT varies with etiology of liver disease, and is higher for NASH cirrhosis than compared to those patients transplanted for primary biliary cirrhosis (PBC) or primary sclerosing cholangitis (PSC). In addition, we previously published that NASH recipients are more likely to experience CVD complications within 1 year of LT independent of traditional CVD risk factors[3]. In the current study NASH recipients have an increased risk of both early and late CVD-related mortality that appears attributable to a high prevalence of both modifiable and non-modifiable cardiometabolic risk factors. In aggregate, these results suggest the need for an individualized approach to cardiac assessment and risk stratification pre-transplant particularly among higher risk NASH candidates.
The strongest predictor of post-LT CVD mortality among NASH candidates was renal impairment. Chronic kidney disease is associated with a dramatic predisposition to cardiovascular events and death in the general population[31] and in small studies has been associated with increased cardiac events after LT[32]. We recently examined predictors of cardiac mortality in a large national sample of liver transplant recipients in the United States[1]. However, renal impairment did not remain a significant predictor in the final risk model suggesting that renal injury among NASH candidates may have more deleterious effects on cardiac function than in other causes of ESLD[1]. Due to the high prevalence of diabetes and hypertension among NASH candidates, renal dysfunction is a common co-morbid condition. Furthermore, NASH has been shown to be an independent risk factor for worsening renal dysfunction after LT[33]. In addition, the frequency of simultaneous liver-kidney transplants is increasing disproportionately among NASH patients when compared to other indications for liver transplantation with poorer outcomes[34]. Thus, close attention to minimization of nephrotoxins both pre- and post-transplant in NASH recipients, may play a role in reducing excess cardiac deaths. In addition, as a result of the increasing average age at which patients are now being transplanted, prevalent CVD co-morbidity at the time of transplantation is expected to rise[35]. Older age was also a significant predictor in our risk model highlighting concerns about increased cardiac risk associated with the aging transplant recipient population.
The leading mechanism for early CVD in the NASH cohort was cardiac arrest (53%), which can have a variety of underlying causes that unfortunately cannot be elucidated using available OPTN data. However, the proportion of cardiac arrest events in the NASH cohort was significantly higher than that from other causes of end stage liver disease. This is in line with recent research demonstrating that NASH is associated with an increased risk of atrial fibrillation[36], autonomic dysfunction[37], and impaired diastolic function[22, 38, 39]. These alterations predispose patients to worse outcomes if they suffer any other type of complication, such as sepsis or hemorrhage, thus highlighting their importance as markers of future cardiovascular morbidity and mortality.
There are limitations of our analysis. First, our analysis may be subject to reporting error or bias inherent to any large registry database. Second, the decision to pursue pre-operative cardiac testing is determined by the individual transplant centers leading to several possible sources of bias in our data, such as restricting transplant only to those with a low prevalence of co-morbid cardiac conditions. However, we did not observe significant differences in CVD-related mortality between centers, and all models were adjusted for transplant center thus strengthening the generalizability of our findings. We acknowledge that the current analysis may also be limited due to the lack of precise measurement of pre-operative CVD risk variables, such as pre-operative cardiovascular testing, laboratory values, medication use, and recipient family history of CVD, which are not currently available within the OPTN database. In addition, we are unable to determine the impact of duration of renal impairment as well as cause of renal impairment among NASH recipients using data available within the OPTN database. Despite these limitations, the strength of this analysis is that we have rigorously evaluated the available national data and provide important characterization regarding risk stratification of NASH candidates for post-LT CVD mortality.
In summary, our study demonstrates that patients transplanted for NASH cirrhosis have an increased risk of CVD-related mortality compared to patients with other causes of end stage liver disease attributable to a high prevalence of pre-existing cardiometabolic risk factors in this population. Although NASH recipients have similar long-term outcomes compared to non-NASH, CVD-specific survival is lower in NASH recipients. This observation highlights opportunities for secondary prevention post-liver transplant in a high metabolic risk population. Prospective studies are needed to assess the overall healthcare burden of increased CVD morbidity and mortality associated with transplantation for NASH cirrhosis. Such studies would provide the foundation for patient-specific cardiovascular risk stratification and aggressive management of NASH particularly or its’ associated metabolic co-morbidities prior to transplant that could modify post-transplant outcomes in this rapidly growing patient population.
Supplementary Material
KEY POINTS.
NASH liver transplant recipients have an increased risk of both early (≤ 30 days) and late (> 30 days) cardiovascular disease-related mortality compared to non-NASH recipients.
NASH recipients have a disproportionately high prevalence of pre-transplant cardiometabolic risk factors that predispose to cardiovascular disease mortality after liver transplantation.
Renal impairment is the strongest predictor of post-liver transplant cardiovascular disease mortality in NASH recipients.
A risk score comprised of age ≥ 55, male sex, diabetes, and renal impairment predicts post-transplant cardiovascular disease mortality after liver transplantation for NASH.
Acknowledgments
The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as a contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
GRANTS AND FINANCIAL SUPPORT:
Dr. VanWagner is supported by the National Institutes of Health (1 F32 HL116151-01), the American Liver Foundation (New York, NY), the American Association for the Study of Liver Diseases (AASLD) foundation and the Alpha Omega Alpha (AOA) Medical Honor Society Foundation. The sponsors played no role in the development of writing of this manuscript.
ABBREVIATIONS
- ALT
alanine aminotransferase
- BMI
body mass index
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- ESLD
end-stage liver disease
- HCV
hepatitis C virus
- LT
liver transplantation
- MELD
model for end-stage liver disease
- NASH
nonalcoholic steatohepatitis
- OPTN
organ procurement and transplantation network
Footnotes
RESPECTIVE CONTRIBUTIONS:
Lisa B. VanWagner, Study design, data analysis, manuscript preparation
Brittany Lapin, Statistical analysis, manuscript review
Anton I. Skaro, Study design, manuscript review
Donald M. Lloyd-Jones, Study design, data interpretation, manuscript review
Mary E. Rinella, Data interpretation, manuscript review
DISCLOSURES
The authors of this manuscript have no conflicts of interest to disclose.
REFERENCES
- 1.Vanwagner LB, Lapin B, Levitsky J, Wilkins JT, Abecassis MM, Skaro AI, et al. High early cardiovascular mortality after liver transplantation. Liver Transpl. 2014;20(11):1306–1316. doi: 10.1002/lt.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Therapondos G, Flapan AD, Plevris JN, Hayes PC. Cardiac morbidity and mortality related to orthotopic liver transplantation. Liver Transpl. 2004;10(12):1441–1453. doi: 10.1002/lt.20298. [DOI] [PubMed] [Google Scholar]
- 3.Vanwagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for nonalcoholic steatohepatitis (NASH) are at increased risk for post-operative cardiovascular events. Hepatology. 2012 doi: 10.1002/hep.25855. [DOI] [PubMed] [Google Scholar]
- 4.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 5.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33(10):1190–1200. doi: 10.1093/eurheartj/ehr453. [DOI] [PubMed] [Google Scholar]
- 7.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 8.Pacana T, Fuchs M. The cardiovascular link to nonalcoholic fatty liver disease: a critical analysis. Clin Liver Dis. 2012;16(3):599–613. doi: 10.1016/j.cld.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Lu H, Liu H, Hu F, Zou L, Luo S, Sun L. Independent Association between Nonalcoholic Fatty Liver Disease and Cardiovascular Disease: A Systematic Review and Meta-Analysis. International journal of endocrinology. 2013;2013:124958. doi: 10.1155/2013/124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42(2):473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 11.Akabame S, Hamaguchi M, Tomiyasu K, Tanaka M, Kobayashi-Takenaka Y, Nakano K, et al. Evaluation of vulnerable coronary plaques and non-alcoholic fatty liver disease (NAFLD) by 64-detector multislice computed tomography (MSCT) Circ J. 2008;72(4):618–625. doi: 10.1253/circj.72.618. [DOI] [PubMed] [Google Scholar]
- 12.Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology. 2010;254(2):393–400. doi: 10.1148/radiol.09090769. [DOI] [PubMed] [Google Scholar]
- 13.Defilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, et al. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227(2):429–436. doi: 10.1016/j.atherosclerosis.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volzke H, Robinson DM, Kleine V, Deutscher R, Hoffmann W, Ludemann J, et al. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol. 2005;11(12):1848–1853. doi: 10.3748/wjg.v11.i12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56(2):605–613. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanwagner LB, Ning H, Lewis CE, Shay CM, Wilkins J, Carr JJ, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: The Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235(2):599–605. doi: 10.1016/j.atherosclerosis.2014.05.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanwagner LB, Wilcox J, Colangelo LA, Lloyd-Jones D, Carr JJ, Lima JA, et al. Associations of Nonalcoholic Fatty Liver Disease with Subclinical Myocardial Dysfunction: The CARDIA Study. Circulation. 2014;129(Suppl 1):A52. [Google Scholar]
- 18.Pacifico L, Di Martino M, De Merulis A, Bezzi M, Osborn JF, Catalano C, et al. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology. 2014;59(2):461–470. doi: 10.1002/hep.26610. [DOI] [PubMed] [Google Scholar]
- 19.Karabay CY, Kocabay G, Kalayci A, Colak Y, Oduncu V, Akgun T, et al. Impaired left ventricular mechanics in nonalcoholic fatty liver disease: a speckle-tracking echocardiography study. Eur J Gastroenterol Hepatol. 2014;26(3):325–331. doi: 10.1097/MEG.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 20.Sert A, Aypar E, Pirgon O, Yilmaz H, Odabas D, Tolu I. Left ventricular function by echocardiography, tissue Doppler imaging, and carotid intima-media thickness in obese adolescents with nonalcoholic fatty liver disease. Am J Cardiol. 2013;112(3):436–443. doi: 10.1016/j.amjcard.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 21.Bonapace S, Perseghin G, Molon G, Canali G, Bertolini L, Zoppini G, et al. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35(2):389–395. doi: 10.2337/dc11-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fotbolcu H, Yakar T, Duman D, Karaahmet T, Tigen K, Cevik C, et al. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J. 2010;17(5):457–463. [PubMed] [Google Scholar]
- 23.Kennedy C, Redden D, Gray S, Eckhoff D, Massoud O, Mcguire B, et al. Equivalent survival following liver transplantation in patients with non-alcoholic steatohepatitis compared with patients with other liver diseases. HPB (Oxford) 2012;14(9):625–634. doi: 10.1111/j.1477-2574.2012.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Afzali A, Berry K, Ioannou GN. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transpl. 2012;18(1):29–37. doi: 10.1002/lt.22435. [DOI] [PubMed] [Google Scholar]
- 25.Bhagat V, Mindikoglu AL, Nudo CG, Schiff ER, Tzakis A, Regev A. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009;15(12):1814–1820. doi: 10.1002/lt.21927. [DOI] [PubMed] [Google Scholar]
- 26.Malik SM, Devera ME, Fontes P, Shaikh O, Ahmad J. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009;9(4):782–793. doi: 10.1111/j.1600-6143.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 27.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krumholz HM, Normand SL. Public reporting of 30-day mortality for patients hospitalized with acute myocardial infarction and heart failure. Circulation. 2008;118(13):1394–1397. doi: 10.1161/CIRCULATIONAHA.108.804880. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Li J, Riaz DR, Shi G, Liu C, Dai Y. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12(3):394.e391–402.e391. doi: 10.1016/j.cgh.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Albeldawi M, Ashish A, Madhwal S, Cywinski J, Lopez R, Eghtesad B, et al. Cumulative risk of cardiovascular events following orthotopic liver transplantation. Liver Transpl. 2011 doi: 10.1002/lt.22468. [DOI] [PubMed] [Google Scholar]
- 31.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 32.Josefsson A, Fu M, Bjornsson E, Castedal M, Kalaitzakis E. Pre-transplant renal impairment predicts posttransplant cardiac events in patients with liver cirrhosis. Transplantation. 2014;98(1):107–114. doi: 10.1097/01.TP.0000442781.31885.a2. [DOI] [PubMed] [Google Scholar]
- 33.Houlihan DD, Armstrong MJ, Davidov Y, Hodson J, Nightingale P, Rowe IA, et al. Renal function in patients undergoing transplantation for nonalcoholic steatohepatitis cirrhosis: Time to reconsider immunosuppression regimens? Liver Transplantation. 2011;17(11):1292–1298. doi: 10.1002/lt.22382. [DOI] [PubMed] [Google Scholar]
- 34.Singal AK, Salameh H, Kuo YF, Wiesner RH. Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation. 2014;98(2):216–221. doi: 10.1097/TP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 35.Annual Report of the U.S. Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR): Transplant Data 1998–2011. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2012. [Google Scholar]
- 36.Targher G, Valbusa F, Bonapace S, Bertolini L, Zenari L, Rodella S, et al. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One. 2013;8(2):e57183. doi: 10.1371/journal.pone.0057183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu YC, Hung CS, Wu YW, Lee YC, Lin YH, Lin C, et al. Influence of non-alcoholic fatty liver disease on autonomic changes evaluated by the time domain, frequency domain, and symbolic dynamics of heart rate variability. PLoS One. 2013;8(4):e61803. doi: 10.1371/journal.pone.0061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goland S, Shimoni S, Zornitzki T, Knobler H, Azoulai O, Lutaty G, et al. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol. 2006;40(10):949–955. doi: 10.1097/01.mcg.0000225668.53673.e6. [DOI] [PubMed] [Google Scholar]
- 39.Fallo F, Dalla Pozza A, Sonino N, Lupia M, Tona F, Federspil G, et al. Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis. 2009;19(9):646–653. doi: 10.1016/j.numecd.2008.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.