Abstract
Background
Eating frequency may influence obesity-related disease risk by attenuating post-prandial fluctuations in hormones involved in metabolism, appetite regulation, and inflammation.
Materials/Methods
This randomized crossover intervention trial tested the effects of eating frequency on fasting plasma insulin-like growth factor-I (IGF-1) and leptin. Fifteen participants (4 males, 11 females) completed two eucaloric intervention phases lasting 21 days each: low eating frequency (“low-EF”; 3 eating occasions/day) and high eating frequency (“high-EF”; 8 eating occasions/day). Participants were free-living and consumed their own meals using individualized structured meal plans with instruction from study staff. Participants completed fasting blood draws and anthropometry on the first and last day of each study phase. The GEE modification of linear regression tested the intervention effect on fasting serum insulin-like growth factor I (IGF-I) and leptin.
Results
Mean (± SD) age was 28.5 ± 8.70 years, and mean (± SD) BMI was 23.3 (3.4) kg/m2. We found lower mean serum IGF-1 following the high-EF condition compared to the low-EF condition (p<0.001). There was no association between EF and plasma leptin (p=0.83).
Conclusion
These results suggest that increased eating frequency may lower serum IGF-1, which is a hormonal biomarker linked to increased risk of breast, prostate and colorectal cancer.
Keywords: Eating frequency, biomarkers, inflammation, IGF-1, leptin
Introduction
The association of excess caloric intake with increased cancer risk has been well-documented (1–3). However, the diet-cancer risk relationship is more complex than just calorie or nutrient intake. Specifically, considerable interest has emerged in the study of dietary patterns, including the frequency and timing of eating, and disease risk (4,5). However, many of these studies have examined cardiometabolic risk factors or weight (5,6), while fewer data exist with respect to eating frequency or timing and cancer risk..(6).
While there are fewer investigations of eating frequency and cancer risk, the data to date are intriguing. Colorectal cancer has frequently been investigated because it is biologically plausible that eating frequency, bile acid production, and dietary components may interact to influence tumor development or progression specifically in the colon and rectum (7). The majority of studies report that more frequent eating is associated with greater CRC risk (8–17), although a small number of investigations have found an inverse relationship (18, 19).
Clinical trials do not provide clear evidence of a pattern or direction of relationship between eating frequency and biomarkers of cancer susceptibility, such as glucose, insulin, inflammatory factors and cytokines. For example, in one study Jenkins and colleagues found that mean 12-hour insulin concentrations were lowered in a small sample of males (n=7) who ate 17 times per day vs. three times per day (20). However, that study and others have found no significant effect of eating frequency on fasting serum glucose or insulin concentration (20–24), or glucose and insulin response curves (25, 26). Other studies have found associations between high EF and increased fasting blood glucose and decreased glucose tolerance (27) and increased serum insulin (23). Relatively few studies have measured the impact of eating frequency on biomarkers other than insulin or other cardiometabolic biomarkers or weight (5,6).
Insulin-like Growth Factor-1 (IGF-1) has been implicated in the development of cancer and tumor growth, as it is expressed in neoplastic tissue and prevents apoptosis (28). Hepatic production of IGF-1 is stimulated by pituitary production of Growth Hormone (GH), enhanced by hypoglycemia, and may also be increased by leptin (28, 29). It is possible that by moderating post-prandial fluctuations in blood glucose, the consumption of a high-EF diet may reduce production of IGF-1.
In addition to potentiating IGF-1, leptin’s role in intake regulation, energy metabolism, and the immune response (30, 31) make it important to consider in studies of eating frequency and cancer risk. A positive correlation between fasting leptin and insulin concentrations has been demonstrated; however, it is unclear from human in vivo studies whether increased insulin directly leads to increased leptin (32), with evidence indicating that glucose must be available in order for insulin to increase leptin secretion (31). Two previous clinical studies on eating frequency have measured leptin concentrations in the absence of caloric manipulation. Participants in one weight-maintenance trial who consumed all calories within a four-hour window each day (one meal/day) vs. consuming all calories over three eating occasions per day showed no difference in fasting morning plasma leptin concentrations after a period of eight weeks (27). In another trial, participants who regularly consumed four meals/day and switched to eating three meals/day had significantly increased leptin concentrations after 28 days. In another study, participants who regularly consumed three meals/day and switched to eating four meals/day showed no difference in leptin concentrations (23).
The purpose of the present study was to examine the influence of eating frequency on circulating IGF-1 and leptin, two hormonal indicators of nutritional status that have the potential to influence metabolic and inflammatory pathways related to carcinogenesis. Specifically, we conducted a randomized, crossover intervention to address whether a high-EF diet (eight eating occasions/day) vs. a low-EF diet (three eating occasions/day) for a period of 21 days each would decrease plasma IGF-1 and leptin concentrations.
Materials and methods
Participants
Participants were recruited for the Meals and Grazing Study using posters and online advertisements at the Fred Hutchinson Cancer Research Center (FHCRC) and the University of Washington campus in Seattle, WA. Participants were healthy 18–50 year-old males and females with a body mass index (BMI) greater than or equal to 18 kg/m2 (normal to obese). Exclusion criteria included diabetes, smoking, following a diet to gain or lose weight, athletes in training, non-normal blood cholesterol or blood pressure, taking prescribed medication other than oral contraceptives, and pregnancy or nursing (females). These exclusion criteria were assessed by self-report. All experimental protocols were approved by the Institutional Review Office at the Fred Hutchinson Cancer Research Center (FHCRC). All participants provided informed consent and were compensated $50.00 for their time.
Study Design
The overall study design for the Meals and Grazing Study is shown in Figure 1. Study recruitment and data collection were completed over the time period May 2011 through July 2012. Interested individuals were invited to an initial session at FHCRC in which eligibility criteria were verified and study procedures were explained in detail. Participants were free-living (e.g. not confined to a metabolic ward) and consumed their own food throughout the study using an individually tailored meal plan, which was designed using the following procedures. Eligible participants who enrolled in the study were provided detailed written and verbal instructions on keeping a seven-day food record. Participants then recorded details (type and quantity) for all food and beverages (except plain water) consumed for 7 consecutive days. Participants returned the completed food records to study staff via hand delivery or U.S. Post. Upon receipt, the study dietitian analyzed the seven-day food record for energy and macronutrient content using The Food Processor software (ESHA Research, Salem OR.) Keeping food items, energy, and macronutrient content of the diet constant, a low-EF “Meals” meal plan (providing all energy as three evenly spaced eating occasions per day) and a high-EF “Grazing” meal plan (providing all energy as eight evenly spaced eating occasions per day) were individually designed for each participant. The “Meals” condition was set to represent a “typical” day of intake (including breakfast, lunch and dinner), and to provide comparison to other studies in which three meals were provided as either the higher or the lower EF condition (6, 23, 26, 27). Eight eating occasions were provided in the “Grazing” condition in order to maintain the lowest possible participant burden and maximize the difference in the number of eating occasions between conditions. Weight-maintaining meal plans rotated every seven days and participants were instructed to eat only the foods on their individual eating plan at the specified hours. All food and beverages (including time of intake) was reported daily using an electronic meal plan checklist. Thirty-four participants attended the initial session, of which 32 were eligible to participate and enrolled. Of those, 15 completed the Meals and Grazing Study. Reasons for drop-out included loss to follow-up after the initial session (8), received medical advice not to participate after initial session (1), decided not to participate after completing seven-day food record (1), reason not indicated (1), loss to follow-up after the first clinic visit (3), and personal schedule conflicts with meal timing (3).
Figure 1. Study Procedures.
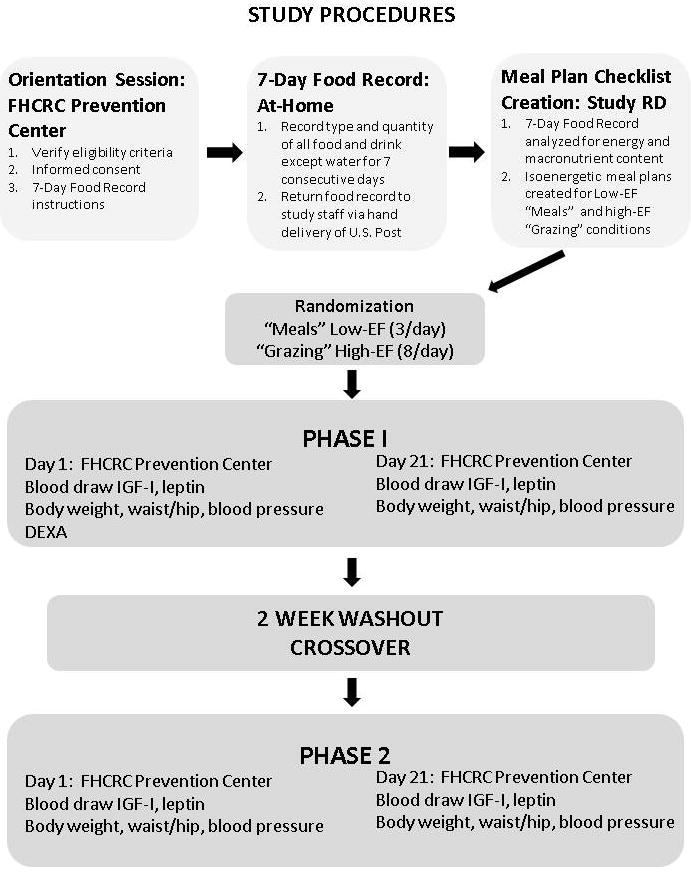
Participants attended one orientation session and four clinic visits at the FHCRC Prevention Center and all other study procedures were carried out at home. Order of conditions was randomly assigned.
This study used a randomized crossover design so that each participant served as his or her own control. Participants completed two 21-day study phases in random order, using a computer randomization program administered by the study dietitian (Phase 1 and Phase 2 in Figure 1), with a 14-day washout period during which they were instructed to consume their habitual diet (6). The goal of the washout period is to minimize or prevent carry-over effects from the prior period in a cross-over design and 14 days is sufficient for this purpose. Clinic visits and blood draws were completed at the beginning and end of each study phase. In both phases, the foods, energy, and macronutrient content of the participant’s diet were kept constant and were matched with the participant’s normal diet as reported in the seven-day food record. In both conditions, the first eating occasion of the day was usually timed at 8:00 am. In the low-EF “Meals” condition, foods were divided into three approximately equal eating occasions per day, spaced apart by 5.6 (SD 0.52) hours and spanning 11.0 (SD 1.0) hours on average. In the high-EF “Grazing” condition, foods were divided into eight approximately equal eating occasions per day, spaced apart by 1.77 (SD 0.25) hours and spanning 12.6 (SD 1.89) hours on average. Each day, participants received an automatically-generated email from the study, linking to an online Meal Plan Checklist that indicated the specific foods and serving sizes to consume and the times of each daily eating occasion (see Appendix 1). Participants were provided with verbal and written guidance on meal and food substitutions prior to beginning the study. For example, an appropriate substitution for a medium apple would be another medium fruit such as a pear or an orange. Participants were allowed to consume non-caloric beverages such as water, black coffee, unsweetened tea, and diet soda ad libitum in both study conditions. Meal Plan Checklists, specifying deviations including substituted foods, different portions, or missed/extra meals were submitted online daily to study staff. Participants were instructed to maintain the same level of physical activity throughout the study to ensure that energy needs would not vary between conditions. For example, if a participant normally walked for 30 minutes each day, they were instructed to maintain that pattern throughout both study conditions and physical activity was verified on the daily Meal Plan Checklist (see Appendix 1). Participants were encouraged to contact the study coordinator if they were not able to complete the assigned eating occasions or consume the foods on their Meal Plan Checklists.
Participants attended in-person clinic visits at 8:00 am on day one and day 21 of both phases for a total of four visits. Clinic visits were scheduled on the same day of the week whenever possible. All other study activities were completed at home. Participants were asked to consume nothing other than noncarbonated water for 12 hours prior to their appointment, and to refrain from drinking alcohol or eating or exercising outside of their normal routine. In addition, they were asked not to use any non-steroidal anti-inflammatory drugs (NSAIDS; such as ibuprofen) for 72 hours prior to their appointment. Any deviations from protocol, including use of NSAIDS and alcohol ingestion, were reported to study staff and recorded. Study sessions were rescheduled for a later date when possible to ensure protocol compliance. During all four clinic visits, body weight, height, waist and hip circumference, pulse, diastolic and systolic blood pressure were measured using standardized procedures by trained staff. During the first clinic visit, Dual energy X-ray absorptiometry (DEXA) using a GE Lunar DPX Pro (GE Healthcare Lunar, Madison, WI) was used to measure body fat percentage.
Blood was collected after a 12 hour fast and the beginning and end of each study phase for a total of four blood draws. All samples were processed immediately by trained staff using a standardized protocol, aliquoted into labeled cryovials and frozen immediately at −80°C until analysis. Leptin concentrations were measured using the Human Leptin ELISA (EMD Millipore, Inc., Billerica, MA). The lowest standard for the leptin assay was 0.25ng/ml. IGF-1 levels were measured using the Human IGF-I Quantikine ELISA Kit (R&D Systems, Minneapolis, MN). The lowest standard for the IGF-1 assay was 0.094 ng/ml and plasma samples were pre-treated according to manufacturer’s protocol resulting in a dilution factor of 100. Samples were run in duplicate, and the median duplicate intra-assay coefficients of variation (CVs) were 5.7% for leptin and 1.2% for IGF. Inter-assay CVs of quality control samples were 7.8% for leptin and 4.1% for IGF-1. The assays were performed on never-thawed samples. All samples from the same individual were run in the same batch.
Sample size, data analyses, and statistical tests
The primary goal of statistical analysis was to compare mean IGF-1 and leptin concentrations in the high-EF vs. low-EF conditions, adjusted for values at baseline and at the end of the washout. Logarithmic transformations were used to improve the normality of distributions. The generalized estimated equations (GEE) modification of linear regression with a working unstructured correlation was used to account for the correlation within individuals over time, and covariate adjustment. Regression analyses were adjusted for order of conditions, gender, and body fat percentage only if they altered the effect of condition by more than 10%. This did not occur in any of the models tested and so a more parsimonious model was used. Between-conditions differences in weight change, waist to hip ratio, blood pressure, and compliance to study diets were compared using GEE. Paired t-tests were used to compare average percent compliance on Meal Plan Checklists between conditions and to verify no difference in intake between normal and overweight participants. Diastolic blood pressure was imputed for one male participant whose diastolic blood pressure was recorded erroneously at baseline of the low-EF condition. Statistical significance was set at p<0.05. Statistical analyses were performed using Stata version 12.0 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP.)
Results
A total of 15 participants (4 males, 11 females) completed both study phases. Participant characteristics are shown in Table 1. Participant diet composition from the seven-day food record is shown in Table 2. Per day, participants consumed on average 2262 (SD 436) kcals, with 48.0% energy from carbohydrates, 16.8% energy from protein, and 33.7% energy from fat. No significant dietary differences were observed between normal and overweight/obese participants (normal: BMI 18–24.9 kg/m2, overweight/obese: ≥ 25kg/m2) in terms of daily intake. Compliance to the study diets was calculated by dividing the total number of completed eating occasions by the total number of assigned eating occasions in each phase for each individual. For instance, in the low-EF phase, a participant who consumed 58 of the 63 assigned eating occasions would have 92% compliance. Excellent rates of compliance were achieved in both low-EF and high-EF conditions (96% and 100%, respectively) and there was no significant difference detected between the conditions (p=0.07; data not shown).
Table 1.
Baseline characteristics of participants in a randomized cross-over trial testing eating frequency (n=15).1,2
| Characteristics | Males (n=4) | Females (n=11) | Total (n=15) |
|---|---|---|---|
| Age (y) | 25.0 (4.7) | 28.2 (8.4) | 28.5 (8.7) |
| Body Mass Index (kg/m2) | 22.4 (1.2) | 24.3 (4.2) | 23.3 (3.4) |
| Percent body fat from DXA3 | 25.5 (2.8) | 35.2 (6.2) | 31.8 (6.5) |
| Waist/hip ratio | 0.84 (0.05) | 0.80 (0.06) | 0.80 (0.07) |
| Systolic blood pressure (mm Hg) | 118.5 (9.1) | 112.5 (8.5) | 112.1 (10.1) |
| Diastolic blood pressure (mm Hg) | 76.3(3.2) | 72.5 (4.2) | 72.2 (5.1) |
All measures collected during participant’s first study session.
Values are means (Standard Deviation; SD).
Dual X-ray absorptiometry.
Table 2.
Mean Daily Energy, Macronutrient and Fiber Intake of Participants in Randomized Cross-Over Trial Testing Eating Frequency by Body Mass Index (kg/m2) (n=15).1,2,3
| Nutrients (per day) | Normal Weight (BMI 18–24.9) (n=13) | Overweight (BMI ≥ 25)(n=2) | Total (n=15) |
|---|---|---|---|
| Energy (kcals) | 2219.0 (425) | 2538 (560) | 2262 (436) |
| Carbohydrate (g) | 274.9 (50.1) | 269.2 (75.2) | 274.1 (50.6) |
| Carbohydrate (% energy) | 49.0 (4.7) | 41.0 (1.4) | 47.9 (5.2) |
| Protein (g) | 95.9 (26.8) | 102.3 (30.1) | 96.8 (26.2) |
| Protein (% energy) | 17.0 (3.54) | 15.5 (0.7) | 16.8 (3.3) |
| Fat (g) | 83.0 (26.5) | 113.8 (15.0) | 87.1 (27.1) |
| Fat (% energy) | 33.0 (0.06) | 40.0 (0.04) | 33.7 (5.9) |
| Fiber (g) | 25.46 (6.9) | 35.4 (24.1) | 26.8 (9.8) |
Dietary intake data obtained from baseline 7-day food record. Body Mass Index calculated at participant’s first clinic visit.
Values are means (Standard Deviation; SD).
No significant differences were observed between the two categories BMI 18–24.9 and BMI ≥25 kg/m2.
Participant body weight, BMI, waist to hip ratio, and systolic and diastolic blood pressure are shown in Table 3. Using generalized estimating equations with adjustment for baseline values (study baseline and the end of washout), we found no significant differences in mean body weight, BMI, waist to hip ratio, or diastolic blood pressure between the low-EF and high-EF conditions. Systolic blood pressure was significantly lower in the low-EF condition than the high-EF condition (p<0.001).
Table 3.
Anthropometric and Blood Pressure Measures of Participants in Randomized Cross-over Trial Testing Low and High Eating Frequency (n=15).1
| Study conditions | Study timepoints | Weight (kg) | BMI (kg/m2) | Waist/hip ratio | Systolic BP (mm Hg) | Diastolic BP (mm Hg) |
|---|---|---|---|---|---|---|
| Low-EF2 | Baseline | 68.2 (13.8) | 23.3 (3.2) | 0.82 (0.06) | 114.3 (9.5) | 73.2 (6.6) |
| Endpoint | 68.3 (14.4) | 23.3 (3.5) | 0.79 (0.07) | 105.3 (7.6)* | 70.9 (6.0) | |
| High-EF2 | Baseline | 68.5 (14.3) | 23.4 (3.5) | 0.79 (0.07) | 107.5 (7.5) | 69.9 (4.4) |
| Endpoint | 68.2 (14.1) | 23.3 (3.4) | 0.79 (0.06) | 110.8 (8.0)* | 71.1 (4.6) |
Values are means (Standard Deviation; SD).
Low-EF= low eating frequency (eating 3 times per day); High EF= high eating frequency (eating 8 times per day).
p<0.001
Plasma IGF-1 and leptin values are shown in Table 4. We found lower mean IGF-1 in the high-EF condition compared to the low-EF condition (p<0.001). This relationship was not modified by order of conditions, gender, or body fat percentage. Between baseline and endpoint, average plasma IGF-1 increased by 6.8% in the low-EF condition and decreased by 11.6% in the high-EF condition (p<0.001 between low-EF and high-EF conditions).
Table 4.
Effect of Low-Eating Frequency (Low-EF) vs. High-Eating Frequency (High-EF)1 on Plasma IGF-I and Plasma Leptin in a Randomized Cross-Over Trial (n=15) 2
| Biomarkers | Study Conditions | Baseline | Endpoint |
|---|---|---|---|
| IGF-I (ng/ml) | Low-EF | 123.4 (7.1) | 131.8 (7.4)* |
| High-EF | 133.6 (8.7) | 118.2 (6.7)* | |
| Leptin (ng/ml) | Low-EF | 7.0 (1.3) | 6.0 (1.2) |
| High-EF | 7.0(1.0) | 6.2 (1.2) |
Low-EF= low eating frequency (eating 3 times per day); High EF= high eating frequency (eating 8 times per day).
Values are geometric means (Standard Error of the Mean; SEM).
p<0.001.
Regression models showed no significant association between EF and plasma leptin concentrations (p=0.83; Table 4). Plasma leptin decreased in the low-EF condition by 14.2% and decreased in the high-EF condition by 11.5% (p>0.05 between low-EF and high-EF conditions).
Discussion
In this randomized controlled crossover intervention we examined the effect of eating frequency on plasma concentrations of IGF-1 and leptin. Over two three-week phases, participants consumed equal energy and macronutrients, and intake was divided into either three eating occasions/day (low-EF) or eight eating occasions/day (high-EF). In linear regression models adjusted for baseline biomarkers, we found significantly lower mean IGF-1 in the high-EF condition (p<0.001). The importance of these findings is that we have identified a simple lifestyle intervention that can reduce circulating IGF-1, which has been implicated as a cancer risk factor. Any discovery (or validation) of approaches to mitigate cancer susceptibility biomarkers is noteworthy. Despite the interesting result for circulating IGF-1, there was no significant difference between low-EF and high-EF conditions in mean plasma leptin concentrations adjusted for baseline values (p=0.83).
Of particular interest is that we found that mean IGF-1 was lower after completion of the high-EF condition than the low-EF condition after adjusting for baseline values (p<0.001). One possible explanation for this finding is that diurnal insulin concentrations may have been lower in the high-EF condition due to reduced nighttime intake, leading to decreased hepatic production of IGF-1. Our finding that IGF-1 can be decreased by manipulating eating frequency is consistent with the idea that eating smaller meals more frequently may positively impact cancer risk.
In past studies, eating frequency has not consistently influenced fasting plasma leptin concentrations, regardless of small changes in body composition. In one cross-over investigation, no difference in fasting morning plasma leptin concentrations was observed when participants consumed all daily calories within a four-hour window each day (one meal/day) vs. spread over three eating occasions/day for a period of eight weeks (27). Although body weight was intentionally maintained within 2 kg during both intervention periods, body weight and body fat percentage were significantly decreased (p<0.01 and p<0.001, respectively) in the one meal/day condition, making it hard to interpret the leptin data in that study as they relate to EF. In another trial, a significant increase in leptin concentrations measured at their circadian peak (between 00:00 and 01:00) was observed in participants who regularly consumed food at four occasions/day and switched to eating three meals/day (ad libitum food consumption) for a period of 28 days (23). This omission of one eating occasion per day was associated with a significant decrease in the total daily energy consumed (p<0.05), an increase in the percentage of dietary fat consumed (p<0.05), and a significant increase in fat mass (p<0.05), but no significant change in body weight. In our study, Meal Plan Checklists were designed to ensure equal macronutrient intake and eucaloric, and we did not detect a significant difference in plasma leptin concentration or body weight between conditions. It is possible that body fat percentage was affected by eating frequency in our trial, but we detected no differences between conditions in body weight or plasma leptin concentrations to suggest that this was the case. In future studies, measurement of ad-libitum intake at each eating occasion and measurement of body composition may help to clarify whether any observed effects of eating frequency on leptin concentrations are driven by differences in dietary intake and body composition. Nonetheless, it appears that the totality of evidence to date, including our study here, is not consistent regarding the effect of eating frequency on serum leptin concentrations.
We detected a 7.46% decrease in systolic blood pressure in the low-EF condition and a 3.37% increase in systolic blood pressure in the high-EF condition. Between conditions, the difference in systolic blood pressure was significant (p<0.001). In contrast, Stote and colleagues found that participants had significantly higher blood pressure in the lower-EF condition (p<0.05) (21). Further research is needed to determine whether these changes are related to differences between conditions in eating frequency or can be attributed to chance.
Strengths and limitations
The current study had several strengths. This is the first study known to authors that measured eating frequency and IGF-1, a hormone that promotes tumor development via mitogenic and anti-apopototic actions. Our study used a randomized crossover design to eliminate random sources of variation from comparisons of plasma IGF-1 and leptin between conditions. Participants consumed diets equal in energy and macronutrient content during both intervention phases, and no differences were detected in anthropometric measurements between conditions. Excellent compliance to both study diets (low-EF and high-EF) was reported. Therefore, we are confident that the trends observed can be solely attributed to differences in eating frequency between conditions.
Limitations also existed. Participants purchased, prepared, and consumed their own foods in portions designated by the study dietitian during both intervention phases. Despite excellent rates of compliance reported by participants, it is possible that intake was not as precisely controlled as intended. Because participants were assigned eucaloric diets equal in macronutrient content across study interventions, we were not able to assess the role of diet quality. Further, we were not able to determine whether participants would naturally consume different total amounts of food when the number of eating occasions was increased or decreased. Findings only apply to the limited study population of healthy participants with normal BMI who met all inclusion criteria. A more diverse sample including more overweight and obese individuals and a greater percentage of males may have resulted in different findings. We recognize that the relatively small study sample was not a population-based sample and therefore the findings are less generalizable. However, intensive studies such as the present one that require a great deal of commitment and compliance may not be population-based but they provide extremely useful models for understanding diet and dietary patterns and mechanisms related to cancer risk. In the present study, the crossover design and control of participant intake in both conditions were employed to limit confounding based on heterogeneity in participant characteristics, but it remains possible that residual confounding existed. Further, a larger sample size may have been necessary to detect significant differences between conditions in our outcome measures; however, our sample was comparable in size to other similar investigations (5, 20, 21, 24, 33–36).
Conclusion
Eating frequency is an important yet unexplored dimension of dietary behavior that could have a major impact on future cancer risk. We recognize that much remains to be learned with respect to the influence of IGF-1 and leptin. Consumption of small, frequent meals, regardless of nutritional content, may reduce circulating IGF-1, which could have a meaningful impact on cancer risk. The impact of eating frequency on leptin may depend on changes in body weight or body fat content driven by energy or macronutrient intake. Future studies should employ a large, diverse sample and provide study meals to participants (however, such studies are quite expensive and have their own challenges with regards to logistics). The effects of eating frequency on health and in particular on cancer risk has not yet been sufficiently tested and confirmed to the point that specific dietary recommendations can be offered to the general public for cancer prevention. Nonetheless, the results from the present study provide am intriguing suggestion that meal frequency may be another dimension to explore further with regards to nutrition and cancer prevention.
Supplementary Material
1Example Meal Plan Checklist for a participant in the low-EF “Meals” condition, in which three eating occasions were assigned at 8:00 am, 2:00 pm, and 8:00 pm. Completed Meal Plan Checklists were submitted daily to the study coordinator.
Acknowledgments
We thank the Meals and Grazing Study participants and the FHCRC Prevention Center staff for their help completing this project. We also thank Pamela Yang for providing technical assistance on laboratory assays.
FUNDING
This work was supported by the Fred Hutchinson Cancer Research Center and grant R25CA094880 from the National Cancer Institute (NCI).
List of abbreviations
- EF
Eating frequency
- LDL
Low-density lipoprotein
- HDL
High-density lipoprotein
- IGF-1
Insulin-like growth factor-1
- GH
Growth hormone
- mRNA
Messenger RNA (ribonucleic acid)
- FHCRC
Fred Hutchinson Cancer Research Center
- BMI
Body Mass Index; measured in kg/m2
- NSAIDS
Non-steroidal anti-inflammatory drugs
- DEXA
Dual-energy X-ray absorptiometry
- ELISA
Enzyme-linked immunosorbent assay
- GEE
Generalized estimating equation
- NCI
National Cancer Institute
- SD
Standard deviation
Footnotes
DISCLOSURE STATEMENT
The authors declare that they have no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
MLN and MP conceived of the study, carried out study design and coordination and drafted the manuscript. AD and MK provided guidance for study design and manuscript preparation. CW provided oversight for data analysis. XS carried out sample analysis. MLN oversaw all aspects of study design, coordination, data analysis, drafted the manuscript and secured study funding. All authors read and approved the final manuscript.
References
- 1.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body mass indx and incidence of cancer; a systemaitc review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 2.Eheman C, Henly SJ, Ballard-Barbash R, Jacobs E, Schymura MJ, Noone AM, Pan L, Anderson RN, Fulton JE, Kohler BA, Jemel A, Ward E, Plescia M, Ries LAG, Edwards BK. Annual report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weigth and lack of sufficient physical activity. Cancer. 2012;118(9):2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund/AICR. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 4.Mattson MP, Allison DB, Fontana L, Harvis M, Longo VD, Maiaisse WJ, Mosley M, Notterpek L, Ravussin R, Scheer FAJL, Seyfried TN, Varady KA, Panda S. Meal frequency and timing in health and disease. PNAS. 2014;111(47):16647–16653. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kant A. Evidence for efficacy and effectiveness of changes in eating frequency for body weight management. Advances in Nutrition. 2014;5:822–828. doi: 10.3945/an.114.007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zargaran ZH, Salehi M, Heydari ST, Babajafart S. The effects of 6 isocaloric meals on body weight, lipid profiles, leptin and adiponectin in overweight subjects (BMI>25) Int Cardiovas Res J. 2014;8(2):52–56. [PMC free article] [PubMed] [Google Scholar]
- 7.Perrigue MM, Kantor ED, Hastert TA, Patterson RE, Potter JD, et al. Eating frequency and risk of colorectal cancer. Cancer causes & control : CCC. 2013;24 doi: 10.1007/s10552-013-0288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potter JD, McMichael AJ. Diet and cancer of the colon and rectum: a case-control study. Journal of the National Cancer Institute. 1986;76:557–69. doi: 10.1093/jnci/76.4.557. [DOI] [PubMed] [Google Scholar]
- 9.Benito E, Obrador A, Stiggelbout A, Bosch FX, Mulet M, et al. A population-based case-control study of colorectal cancer in Majorca. I. Dietary factors. International Journal of Cancer. 1990;45:69–76. doi: 10.1002/ijc.2910450114. [DOI] [PubMed] [Google Scholar]
- 10.Young TB, Wolf DA. Case-control study of proximal and distal colon cancer and diet in Wisconsin. International Journal of Cancer Journal. 1988;42:167–75. doi: 10.1002/ijc.2910420205. [DOI] [PubMed] [Google Scholar]
- 11.Coates AO, Potter JD, Caan BJ, Edwards SL, Slattery ML. Eating frequency and the risk of colon cancer. Nutrition and Cancer. 2002;43:121–6. doi: 10.1207/S15327914NC432_1. [DOI] [PubMed] [Google Scholar]
- 12.Favero A, Franceschi S, La Vecchia C, Negri E, Conti E, et al. Meal frequency and coffee intake in colon cancer. Nutrition and Cancer. 1998;30:182–5. doi: 10.1080/01635589809514661. [DOI] [PubMed] [Google Scholar]
- 13.Franceschi S, La Vecchia C, Bidoli E, Negri E, Talamini R. Meal frequency and risk of colorectal cancer. Cancer research. 1992;52:3589–92. [PubMed] [Google Scholar]
- 14.de Verdier MG, Longnecker MP. Eating Frequency: A Neglected Risk Factor for Colon Cancer? Cancer Causes & Control. 1992;3:77–81. doi: 10.1007/BF00051916. [DOI] [PubMed] [Google Scholar]
- 15.La Vecchia C, Ferraroni M, Mezzetti M, Enard L, Negri E, et al. Attributable risks for colorectal cancer in northern Italy. International Journal of Cancer. 1996;66:60–4. doi: 10.1002/(SICI)1097-0215(19960328)66:1<60::AID-IJC11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 16.Shoff SM, Newcomb PA, Longnecker MP. Frequency of eating and risk of colorectal cancer in women. Nutrition and Cancer. 1997;27:22–5. doi: 10.1080/01635589709514496. [DOI] [PubMed] [Google Scholar]
- 17.Wei JT, Connelly AE, Satia JA, Martin CF, Sandler RS. Eating frequency and colon cancer risk. Nutrition and Cancer. 2004;50:16–22. doi: 10.1207/s15327914nc5001_3. [DOI] [PubMed] [Google Scholar]
- 18.Tseng M, Ingram DD, Darden R, Ziegler RG, Longnecker MP. Eating frequency and risk of colorectal cancer. Nutrition and Cancer. 2000;36:170–6. doi: 10.1207/S15327914NC3602_5. [DOI] [PubMed] [Google Scholar]
- 19.Mekary RA, Hu FB, Willett WC, Chiuve S, Wu K, et al. The Joint Association of Eating Frequency and Diet Quality With Colorectal Cancer Risk in the Health Professionals Follow-up Study. American Journal of Epidemiology. 2012;175:664–672. doi: 10.1093/aje/kwr363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins DJ, Wolever TM, Vuksan V, Brighenti F, Cunnane SC, et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. The New England Journal of Medicine. 1989;321:929–34. doi: 10.1056/NEJM198910053211403. [DOI] [PubMed] [Google Scholar]
- 21.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85:981–988. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maislos M, Abou-Rabiah Y, Zuili I, Iordash S, Shany S. Gorging and plasma HDL-cholesterol--The Ramadan model. European Journal of Clinical Nutrition. 1998;52:127–130. doi: 10.1038/sj.ejcn.1600526. [DOI] [PubMed] [Google Scholar]
- 23.Chapelot D, Marmonier C, Aubert R, Allègre C, Gausseres N, et al. Consequence of omitting or adding a meal in man on body composition, food intake, and metabolism. Obesity (Silver Spring, Md) 2006;14:215–27. doi: 10.1038/oby.2006.28. [DOI] [PubMed] [Google Scholar]
- 24.Arnold L, Ball M, Mann J. Metabolic effects of alterations in meal frequency in hypercholesterolaemic individuals. Atherosclerosis. 1994;108:167–74. doi: 10.1016/0021-9150(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 25.Gwinup G, Byron RC, Roush W, Kruger F, Hamwi GJ. Effect of nibbling versus gorging on glucose tolerance. Lancet. 1963;2:165–7. doi: 10.1016/s0140-6736(63)92801-0. [DOI] [PubMed] [Google Scholar]
- 26.Arnold LM, Ball MJ, Duncan AW, Mann J. Effect of isoenergetic intake of three or nine meals on plasma lipoproteins and glucose metabolism. Am J Clin Nutr. 1993;57:446–51. doi: 10.1093/ajcn/57.3.446. [DOI] [PubMed] [Google Scholar]
- 27.Carlson O, Martin B, Stote KS, Golden E, Maudsley S, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007;56:1729–1734. doi: 10.1016/j.metabol.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koutkia P, Canavan B, Johnson ML, DePaoli A, Grinspoon S. Characterization of leptin pulse dynamics and relationship to fat mass, growth hormone, cortisol, and insulin. American Journal of Physiology. 2003;285:E372–E379. doi: 10.1152/ajpendo.00097.2003. [DOI] [PubMed] [Google Scholar]
- 29.Houston MS, Holly JMP, Feldman EL. IGF and Nutrition in Health and Disease. Totowa, N.J: Humana Press; 2005. [Google Scholar]
- 30.Maya-Monteiro CM, Bozza PT. Leptin and mTOR: partners in metabolism and inflammation. Cell Cycle (Georgetown, Tex) 2008;7:1713–7. doi: 10.4161/cc.7.12.6157. [DOI] [PubMed] [Google Scholar]
- 31.Amitani M, Asakawa A, Amitani H, Inui A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. 2013;8(7):51. doi: 10.3389/fnins.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris RBS. Leptin-much more than a satiety signal. Annual Review of Nutrition. 2000;20:45–75. doi: 10.1146/annurev.nutr.20.1.45. [DOI] [PubMed] [Google Scholar]
- 33.Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Rumpler WV, Baer DJ, Egan J, Matttson MP. Metabolism. 2007;56(12):1729–1734. doi: 10.1016/j.metabol.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachman JL, Raynor HA. Effects of manipulating eatng frequency during a behavioral weight loss intervention: a pilot randomized controlled trial. Obesity. 2012;20:985–992. doi: 10.1038/oby.2011.360. [DOI] [PubMed] [Google Scholar]
- 35.Heden TD, Liu Y, Sims LJ, Whaley-Connell AT, Chockalingam A, Dellsperger KC, Kanaley JA. Meal frequency differentially alters postprandila triacylglycerol and insulin concentrations in obese women. Obesity Biology and Integrated Physiology. 2013;21:123–129. doi: 10.1002/oby.20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Little TJ, Luscombe-Marsh ND, Gentilcore D, Brook EJ, Feinle-Bisset C. Effects of varying the inter-meal interval on relationships between antral area, gut hormones and energy intake following a nutrient drink in healthy lean humans. Physiology & Behavior. 2014;135:34–43. doi: 10.1016/j.physbeh.2014.05.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1Example Meal Plan Checklist for a participant in the low-EF “Meals” condition, in which three eating occasions were assigned at 8:00 am, 2:00 pm, and 8:00 pm. Completed Meal Plan Checklists were submitted daily to the study coordinator.


