Abstract
Purpose
Many cellular and molecular studies in experimental animals and early retinal function tests in patients with diabetic retinopathy (DR) have shown that retinal neurodegeneration is an early event in the pathogenesis of the disease. Somatostatin (SST) is one of the most important neuroprotective factors synthesized by the retina: SST levels are decreased in parallel to retinal neurodegeneration in early stages of DR. In this study, we characterized the induction of apoptosis (programmed cell death) in a 661W photoreceptor-like cell line cultured under high glucose (HG) conditions and the effect of SST.
Methods
A 661W photoreceptor-like cell line and retinal explants from 10-week-old male C57BL/6 mice were cultured under HG conditions and treated with SST.
Results
Hyperglycemia significantly reduced the cellular viability by increasing the percentage of apoptotic cells, and this effect was ameliorated by SST (p˂0.05). Activation of caspase-8 by hyperglycemia was found in the 661W cells and retinal explants and decreased in the presence of SST (p˂0.05). Moreover, we detected activation of calpain-2 associated with hyperglycemia-induced cell death, as well as increased protein tyrosine phosphatase 1B (PTP1B) protein levels; both had a pattern of cleavage that was absent in the presence of SST (p˂0.05). Treatment of the 661W cells and retinal explants with SST for 24 h increased the phosphorylation of type 1 insulin-like growth factor receptor (IGF-IR; tyrosine 1165/1166) and protein kinase B (Akt; serine 473), suggesting this survival signaling is activated in the neuroretina by SST (p˂0.05).
Conclusions
This study has provided new mechanistic insights first into the involvement of calpain-2 and PTP1B in the loss of cell survival and increased caspase-8-dependent apoptosis induced by hyperglycemia in photoreceptor cells and second, on the protective effect of SST against apoptosis by the enhancement of IGF-IR-mediated Akt phosphorylation.
Introduction
The global incidence of diabetes is set to rise dramatically from an estimated 382 million people in 2013 to 592 million by 2030. Diabetic retinopathy (DR) is one of the most frequent complications of diabetes and the leading cause of blindness among working-age individuals [1]. In the past, DR was recognized solely as a vascular disease; however, a large number of cellular and molecular preclinical studies and retinal function tests in patients with DR have shown that retinal neurodegeneration (diabetic retinal neuropathy) is an early event in the pathogenesis of DR that predates and participates in diabetic retinal vasculopathy [2,3].
Increasing evidence shows that in the retina apoptosis of neural cells and reactive gliosis are basic pathological features of early DR [4]. Among all neuronal cell types in the retina, retinal ganglion cells (RGCs) are highly susceptible to hyperglycemia-mediated apoptosis, but photoreceptors are also one of the primary cell types affected in diabetic retinal neuropathy. Increased levels of photoreceptor apoptosis have been found in histological sections of animal models of diabetes [5], and thinning of the photoreceptor layer was noted on optical coherence tomography (OCT) scanning in patients with diabetes [6]. Importantly, injury in RCGs and photoreceptors, which was reflected by the results of electroretinogram (ERG) examination, was not associated with DR-specific vascular injury [7].
At the molecular level, the mitochondria-dependent (intrinsic) pathway has been demonstrated to be closely related to diabetes-induced retinal cell apoptosis [8]. This pathway, activated by oxidative and endoplasmic reticulum stress, is controlled through the balance in the expression of the Bcl-2 family proteins, including the antiapoptotic members Bcl-2 and Bcl-xL and the proapoptotic protein Bax, and determines the survival or death of the retinal cells following diabetic stimuli [9]. Activation of the death receptor-mediated (extrinsic) apoptotic pathway is also involved in retinal apoptosis during DR. We have recently reported that several proapoptotic molecules of both classical pathways (FasL, active caspase-8, truncated Bid, Bim, and active caspase-3) are significantly increased in the neuroretina of diabetic patients with diagnosed DR [10]. Based on all these and other studies, it is reasonable to hypothesize that the identification of novel molecular regulatory mechanisms of apoptosis in the neural cells of the retina will be useful for the development of therapeutic strategies against DR based on neuroprotection.
Somatostatin (SST) is one of the most important neuroprotective factors synthesized by the retina, and the RPE is the main source of SST in the human eye [11]. The human retina produces significant amounts of SST, as deduced by the strikingly high levels reported within the vitreous fluid [12]. Among the five types of SST receptors, SST1 and SST2 are the most widely expressed in the retina [13,14]. The production of SST and SST1 receptor simultaneously suggests a relevant autocrine action in the retina. SST1 receptor immunoreactivity has been reported in SST-expressing amacrine cells of the rat [15]. Moreover, the SST1 receptor has been shown to modulate the release of SST in the rat retina and acts as SST’s autoreceptor [16]. In human ocular tissue, SST1 and SST2 receptor immunostaining was reported in the outer and inner segments of rods and cones [13], whereas in the rat retina the two isoforms of the SST2 receptor, SST2A and SST2B, have been localized in the outer retina to cone photoreceptors [17] and to rod and cone photoreceptors [18], respectively. Importantly, during early DR, lower SST expression parallels retinal neurodegeneration [11]. Moreover, the lower production of SST in the retina is associated with a dramatic decrease in SST levels in the vitreous in diabetic patients with proliferative DR [12,19] and with diabetic macular edema [20].
We recently analyzed the effect of topical treatment with SST in the apoptotic cascade of an experimental model of DR in rats [21]. In this previous study, we found a significant rate of apoptosis in the retinas from streptozotocin (STZ)-induced diabetic rats in comparison with non-diabetic control rats, which correlated with a misbalance between the apoptotic and survival signaling pathways. This balance was favored toward survival by treatment with SST in parallel with an improvement of the visual function. On that basis, the aim of the present work is to provide in a cellular-specific context using 661W photoreceptor-like cells new molecular insights into the effect of SST in the altered balance between apoptosis and cell survival under diabetic conditions.
Methods
Reagents and drugs
Fetal bovine serum (FBS) and culture media were obtained from Invitrogen (Grand Island, NY). Bovine serum albumin (BSA) was purchased from Sigma-Aldrich (St. Louis, MO). Bradford reagent, acrylamide, immunoblot polyvinylidene fluoride (PVDF) membranes, and chemoluminescent horseradish peroxidase (HRP) substrate were purchased from Bio-Rad (Madrid, Spain). Somatostatin was obtained from BCN peptides S.A. (Barcelona, Spain).
Retinal explants
Ex vivo assays were performed in retinas from 10-week-old male C57BL/6 mice purchased from Charles River Laboratories (Barcelona, Spain). Mice were maintained in light/dark (12 h:12 h light-dark) and temperature- (22 °C), and humidity-controlled rooms, and fed ad libitum with free access to water. All animal experimentation was conducted in accordance with the regulations of the Association for Research in Vision and Ophthalmology (ARVO) and approved by the Animal Care and Use Committees of Spanish National Research Council (CSIC). Animals were killed by cervical dislocation, eyes were enucleated, and then the lens, anterior segment, vitreous body, RPE, and sclera were removed. For experiments, the retinas were cultured in R16 medium (provided by PA Ekstrom, Lund University, Sweden) containing 19 mM glucose (basal condition; B) or in the same medium supplemented with glucose up to a concentration of 55 mM (high glucose, HG) in the absence or presence of SST (10−6 M) during 24–48 h.
Cell culture
The 661W cell line, derived from immortalized cone photoreceptors (provided by Muayyad Al-Ubaidi, University of Oklahoma), was maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, ThermoFisher Scientific, Waltham, MA) containing 4.5 g/l (24.5 mM) glucose and supplemented with 10% (vol/vol) heat-inactivated FBS as described [22]. For mouse cell line authentication, genomic DNA was isolated from the 661W cells and analyzed with PCR as described [23]. PCR conditions were as follows: 10 min 95 ºC; 45 s 94 ºC, 2 min 59 ºC, 1 min 72 ºC (30 cycles) and 10 min 60 ºC. Amplified products were analyzed in a 2% agarose gel. The following primer sequences were used for amplification of the mouse short tandem repeat (STR) loci named 6-7 and 15-3: forward (6–7), 5′-AGT CCA CCC AGT GCA TTC TC-3′; reverse (6–7), 5′-CAT GTG GCT GGT ATG CTG TT-3′; forward (15–3), 5′-TCT GGG CGT GTC TGT CAT AA-3′; and reverse (15–3), 5′-TTC TCA GGG AGG AGT GTG CT-3′. Appropriate positive (mouse) and negative (human) controls were run and confirmed for each sample submitted. The STR analyses are presented in Appendix 1. In this study we have used a cell line from mouse origin and we did not analyze different STR locus as in human cell line authentication. For mouse (661W) cell line analysis we just amplify two mouse specific STR named 6-7 and 15-3. For the experiments, confluent cells were maintained in 24.5 mM glucose (basal condition) or cultured in 55 mM glucose (HG) during 24–48 h in the presence or absence of SST (10−6 M).
Western blot
For western blot analysis, the proteins were resolved using denaturing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to PVDF membranes (Bio-Rad, Madrid, Spain). Levels of phosphorylated proteins (type 1 insulin-like growth factor receptor [pIGF-IR] and protein kinase B [pAkt]) were normalized by the levels of their corresponding total (IGF-IR and Akt) proteins that were used as loading controls. Levels of protein tyrosine phosphatase 1B (PTP1B), caspase-8, and calpain-2 were normalized with β-actin. Anti-IGF-IR (sc-713), anti-phospho-Akt1/2/3 (Ser473; sc-7985-R), anti-Akt1/2/3 (sc-8312), and anti-caspase-8 (sc-7890) antibodies were purchased from Santa Cruz Biotechnology (Palo Alto, CA). Anti-PTP1B (05–1087, recognizes cleavaged PTP1B) antibody was purchased from Merk-Millipore (Merck KGaA, Darmstadt, Germany). Anti-calpain-2 (C3989) and anti-β-actin (A-2547) antibodies were from Sigma-Aldrich (Madrid, Spain). Anti-phospho-IGF-IRβ (Tyr1135/1136; #3024) was from Cell Signaling Technology (Danvers, MA).
Analysis of cellular viability
Quantification of cellular viability was performed using crystal violet staining. Cells were seeded in six-well tissue culture plates and allowed to stabilize overnight in a 5% CO2 incubator at 37 °C. The cells were then cultured in 24.5 mM (basal) or 55 mM (HG) glucose-containing medium in the absence or presence of SST (10−6 M) for 24–48 h, and cellular viability was measured with crystal violet staining as described [24].
Flow cytometry
After apoptosis was induced, adherent and non-adherent cells were collected by centrifugation, washed with PBS (1X; 136 mM NaCl, 2,67 mM KCl, 10 mM NaPO4, 1,76 mM KPO4, pH 7), and fixed with cold ethanol (70% vol/vol). The cells were then washed, resuspended in PBS, and incubated with RNase (25 µg/106 cells) for 30 min at 37 °C. After 0.05% propidium iodide was added, the cells were analyzed with flow cytometry on a FACSCalibur using CellQuest software (BD Biosciences, San Jose, CA).
Caspase-8 activity assay
Caspase-8 activity was measured in retinal explants with a fluorometric method. After treatment, the retinal explants were lysed at 4 °C in 5 mM Tris/HCl pH 8, 20 mM EDTA, and 0.5% Triton X-100. Lysates were clarified by centrifugation at 13,000 ×g for 10 min. Caspase 8-activity was measured with the ApoAlert caspase-8 fluorescent assay kit (Clontech, Ref. K2028) accordingly with the manufacturer’s instructions using IETD-AFC as a fluorometric substrate. Protein concentration of cell lysates was determined with the Bradford method, and results are expressed as caspase-8 activity per µg of total protein.
Statistics
Densitometry of the western blots was performed using the Image J program. Values in all graphs represented the mean ± standard error of the mean (SEM). Statistical tests were performed using SPSS 21.0 for Windows (SPSS Inc. IBM, Armonk, NY) with data corresponding to three independent experiments performed in duplicate and/or n = 5 independent retinas per condition. Data were analyzed with one-way ANOVA followed by the Bonferroni t test or by the paired t test when comparisons were among two groups. Differences were considered statistically significant at a p value of less than 0.05.
Results
SST increased survival and decreased hyperglycemia-induced apoptosis and caspase-8 activation in 661W photoreceptors and retinal explants
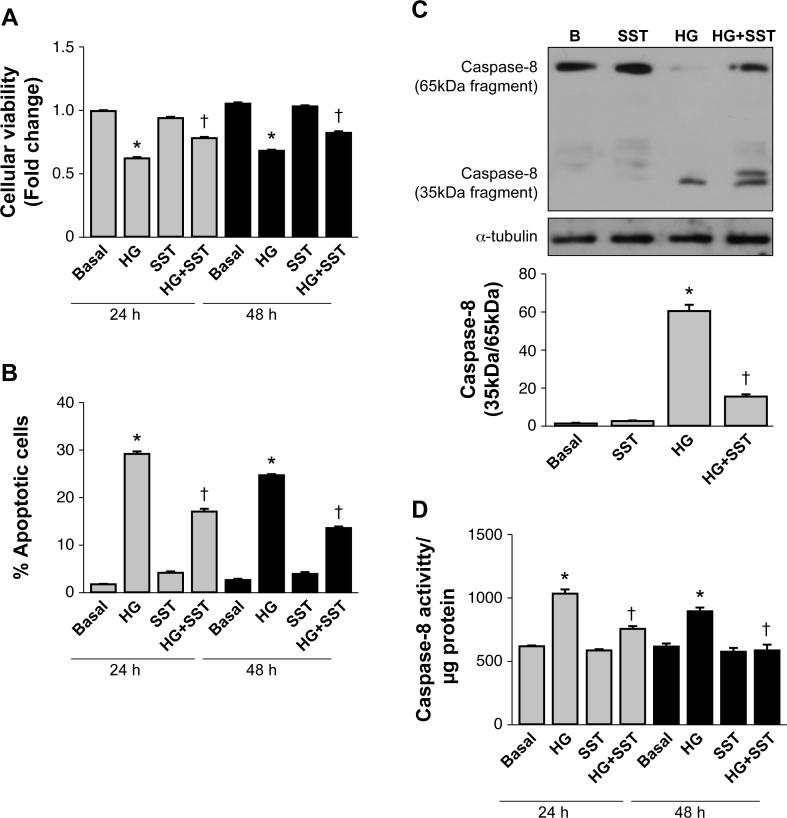
It is known that during DR, hyperglycemia is present in neuroretinal tissue and plays a key role in neurodegeneration [25]. We first characterized the induction of programmed cell death in the 661W photoreceptor-like cell line cultured under HG for 24 and 48 h compared with cells cultured under basal conditions (B). As shown in Figure 1A, hyperglycemia statistically significantly reduced the cellular viability of the 661W cells as reflected by quantification of the crystal violet staining (p<0.05 HG versus B); this effect was reversed in cells cultured in HG plus SST (10−6 M; p<0.05 versus HG).
Figure 1.
SST increases survival and decreases apoptosis and caspase-8 activation induced by hyperglycemia in 661W photoreceptors and retinal explants. The 661W cells were cultured under basal conditions (B) or in medium supplemented with 55 mM glucose (HG) during 24 or 48 h in the absence or presence of 10−6 M somatostatin (SST). A: Analysis of cellular survival measured with crystal violet staining. B: Analysis of the percentage of apoptotic (hypodiploid) cells with iodine propidium staining with flow cytometry. Results are means ± standard error of the mean (SEM; n = 5 independent experiments performed in triplicate). C: 661W cells were cultured under basal conditions (B) or with medium supplemented with 55 mM glucose (HG) during 24 h in the absence or presence of 10−6 M SST. The activation of caspase-8 was expressed by the ratio of the 35-kDa active fragment/65-kDa proform that was analyzed with western blot. β-actin was used as a loading control. A representative experiment out of three is shown. The graph corresponds to the quantification and statistical analysis of data corresponding to three independent experiments performed in duplicate. D: Retinal explants were prepared from the C57BL/6 mice and treated with basal medium (B) or medium supplemented with 55 mM glucose (HG) during 24 or 48 h in the presence or absence of SST (10−6 M). Caspase-8 enzymatic activity was analyzed with the fluorometric method. Results are means ± SEM (n=5 independent retinas per condition); *p<0.05 versus basal or †p<0.05 versus HG condition at each time.
Next, we measured the percentage of hypodiploid (apoptotic) cells in these experimental conditions. As depicted in Figure 1B, the percentage of apoptotic cells significantly increased in the 661W cells cultured in HG-supplemented medium for 24 and 48 h. However, in cells cultured with HG plus SST, a statistically significant decrease was found in the percentage of apoptotic cells (p<0.05 versus HG). These results are in agreement with our recent in vivo study in which topical administration of SST decreased apoptotic (terminal deoxynucleotidyl transferase dUTP nick end labeling [TUNEL] positive) cells in the retina and improved visual function in diabetic rats [21].
The molecular analysis of the retina in the SST-treated diabetic rats revealed that hyperglycemia positively modulated the death receptor (extrinsic) apoptotic pathway [21]. To examine the effect of SST on this apoptotic pathway in a cell autonomous-specific manner, we treated the 661W photoreceptor cells with HG-supplemented medium in the absence or presence of SST for 24 h and analyzed the cleavage of caspase-8 proform (65 kDa) in its 35-kDa active fragment as a hallmark of activation. As shown in Figure 1C, in the 661W cells treated for 24 h with HG-supplemented medium the activation of caspase-8, monitored by the increased ratio of the 35-kDa active fragment/65-kDa proform, was detected. Treatment with SST prevented the activation of caspase-8 as assessed by the reduced levels of the 35-kDa/65-kDa ratio. To evaluate the relevance of the effect of SST on the modulation of caspase-8 activity in the whole retina, retinas from the C57BL/6 mice were cultured under hyperglycemic conditions with or without SST for 24 and 48 h. As the active fragment of caspase-8 was not detected with western blot in the retinal explants (results not shown), we measured caspase-8 enzymatic activity with a fluorometric method. The results shown in Figure 1D revealed a statistically significant increase in caspase-8 enzymatic activity in the retinal explants cultured in the HG-supplemented medium (p<0.05 versus basal) that was statistically significantly decreased in the explants cultured in HG plus SST (p<0.05 versus HG).
SST reduces calpain-2 activation and PTP1B expression and cleavage induced by high glucose and directly activates IGF-IR/Akt phosphorylation in 661W cells and retinal explants
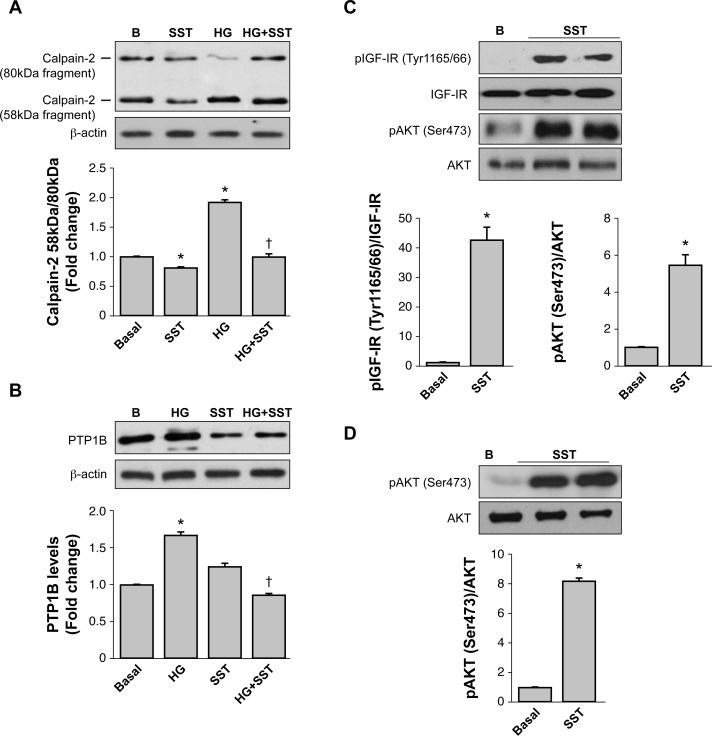
To decipher new molecular mechanisms involved in the loss of cellular viability in 661W cells cultured under HG and the beneficial effect of SST, we analyzed the activation of calpain-2. This experimental approach was based on our previous work demonstrating that pharmacological inhibition of calpain-2 reduced apoptosis during retinal degeneration that occurs under increased Ca2+ flux [26]. As demonstrated by Saido et al. [27], calpain-2 activation can be measured by the autolysis of the 80-kDa catalytic subunit (proform) into the 58-kDa fragment [28]. Based on that, we analyzed the ratio of the 58-kDa fragment/80-kDa proform of calpain-2 in 661W cells cultured in HG-supplemented medium for 24 h. Figure 2A shows that this ratio was increased in the 661W cells treated with HG-supplemented medium (p<0.05 versus basal), but the ratio was decreased in cells treated with HG plus SST (p<0.05 versus HG), reaching levels that were comparable to the basal condition. Interestingly, SST also reduced the ratio of the 58-kDa fragment/80-kDa proform of calpain-2 under basal conditions, suggesting that 661W cells are highly sensitive to the modulation of calpain-2 activity.
Figure 2.
SST reduces calpain-2 activation and PTP1B expression and cleavage induced by high glucose and directly activates IGF-IR/Akt phosphorylation in 661W cells and retinal explants. The 661W cells were cultured under basal conditions (B) or with medium supplemented with 55 mM glucose (high glucose, HG) during 24 h in the absence or presence of 10−6 M somatostatin (SST). Levels of the calpain-2 proform (80-kDa) and the 58-kDa fragment (A) and the levels of protein tyrosine phosphatase 1B (PTP1B) (B) were analyzed with western blot with β-actin as a loading control. The graphs correspond to the quantification and statistical analysis of data obtained in three independent experiments performed in duplicate. *p<0.05 versus basal or †p<0.05 versus HG condition. The 661W cells (C) and the retinal explants (D) were treated with SST (10−6 M) for 24 h or left untreated. At the end of the culture time, the phosphorylation levels of type 1 insulin-like growth factor receptor (IGF-IR; tyrosine 1165/66) and protein kinase B (Akt; serine 473) were analyzed with western blot. Results were normalized with respect to the total IGF-IR or Akt levels. The graphs correspond to the quantification and statistical analysis of data obtained in three independent experiments performed in duplicate (C) or five retinas per condition (D). *p<0.05 versus Basal.
It has been shown that in platelets treated with the calcium ionophore A23187 the activation of calpain-2 correlated with the cleavage of PTP1B and the increase in its tyrosine phosphatase activity [29]. Likewise, we recently reported a similar pattern of cleavage and an increase in PTP1B protein expression and enzymatic activity in 661W photoreceptors cultured with a mixture of proinflammatory cytokines [30]. These previous results and the present findings regarding the activation of calpain-2 in HG-treated 661W photoreceptors prompted us to analyze the cleavage of PTP1B in our experimental settings. As depicted in Figure 2B, culture of 661W cells under HG for 24 h increased the total PTP1B protein levels and induced a pattern of cleavage that was not detected in cells cotreated with HG and SST (p<0.05 versus basal; p<0.05 versus HG).
Previous results of different groups including ours using experimental systems mimicking cellular damage of the retina during DR have demonstrated that the IGF-IR/Akt pathway mediates survival and protects against the diabetic milieu [30-32]. Based on that, we addressed whether SST was able to activate this pathway in 661W cells. As shown in Figure 2C, treatment with SST for 24 h increased tyrosine phosphorylation of the IGF-IR at tyrosines 1165/1166 of the catalytic domain, as well as Akt phosphorylation at serine 473. The phosphorylation of Akt by SST was confirmed in retinal explants (Figure 2D) suggesting the physiologic relevance of SST in activating the survival signaling in the whole retina.
Discussion
Increasing evidence shows that retinal cell apoptosis and reactive gliosis are basic pathological features of neurodegeneration during early DR [33]. Consequently, it is important to unravel the molecular mechanisms involved in these processes to develop neuroprotective treatments aimed at avoiding cellular damage in the neural retina at an early stage of the disease.
In this study, we used the 661W photoreceptor-derived cell line to demonstrate that hyperglycemia triggered caspase-8-dependent apoptosis. This was manifested by a statistically significant increase in the 35-kDa active fragment/65-kDa proform ratio, in parallel with an increase in the percentage of hypodiploid (apoptotic) cells and with a decrease in the total cellular viability. Although this is the first evidence of the involvement of caspase-8 in apoptosis of 661W photoreceptors under diabetic-like conditions, caspase-8 has been previously linked to apoptosis in neural cells cultured under HG conditions. For instance, caspase-8 was activated by hyperglycemia during apoptotic cell death of neuroblastoma PC12 cells [34]. Regarding the impact of caspase-8 activation in photoreceptors, a previous study by Chang and coworkers showed that light-induced cell death of 661W cells was also mediated by caspase-8 [35]. A step further, our results demonstrated the activation of caspase-8 in retinal explants cultured under HG, suggesting that in addition to photoreceptors, other cell types in the neuroretina, likely RGCs, are also involved in caspase-8 activation as previously suggested by Fuchs and coworkers in a model of tumor necrosis factor-alpha (TNF-α) stimulation that mimics inflammation during DR [36].
Based on the beneficial effect of the topical administration of SST by improving visual function and reducing neurodegeneration, caspase-8 activity and apoptosis in the retina of hyperglycemic rats [21], in the present study we assessed SST effects in a cellular-specific context in photoreceptors. In total agreement with our previous in vivo findings, the in vitro and the ex vivo data presented for the 661W cells and retinal explants, respectively, demonstrated that SST significantly reduced HG-induced caspase-8 activity and the percentage of apoptotic (hypodiploid) cells. These findings shed light on the mechanisms by which SST could be a useful neuroprotective agent for DR.
Various physiologic and molecular abnormalities that are consistent with inflammation have been found to be increased in the retinas or vitreous humor of diabetic animals and patients [37]. Moreover, persistent and unresolved inflammation triggers the activation of proinflammatory signaling cascades that ultimately lead to apoptotic cell death [38,39]. Among the critical molecules that link the inflammatory and cell death processes, PTP1B has been shown to play a pivotal role. PTP1B expression is increased in peripheral tissues during obesity, and this effect is due to elevated proinflammatory signaling [40]. Regarding photoreceptors, we have recently reported that treatment of 661W cells with proinflammatory cytokines increased PTP1B mRNA and protein levels, and this effect was also found in the whole retina. Interestingly, cytokines induced a cleavage of PTP1B with a similar pattern to that reported in platelets, resulting in increased enzymatic activity [29]. Conversely, deficiency in the Ptpn1 gene enhanced the IGF-IR/Akt survival signaling in the retina [31] in agreement with another study showing that intravitreal administration of a PTP1B inhibitor prevented the cleavage of caspase 3 during light stress [41]. Altogether, these studies have shown the key role of PTP1B in modulating the balance between death and survival processes in the retina. In this regard, the results presented here have revealed that PTP1B expression and cleavage are markedly enhanced by hyperglycemia in 661W photoreceptor-like cells. This is the first evidence for hyperglycemia-mediated induction of PTP1B expression in retinal cells, as occurs in hepatocytes exposed to HG [42], suggesting that increased PTP1B expression may partly explain glucose toxicity in diabetes. In a previous work, Rajala and coworkers did not found elevations in PTP1B expression by hyperglycemia in the retinas from STZ-treated mice [43]. However, our recent data in the retinas from db/db or Irs2−/− diabetic mice models clearly showed higher PTP1B expression compared to that of their age-matched db+ or Irs2+/+ non-diabetic controls, respectively [30,31]. The fact that db/db mice are diabetic and obese and display elevations in various inflammatory markers in the retina [44] suggests that inflammation might boost the effect of hyperglycemia in the enhancement of PTP1B expression in the retina.
One potential candidate responsible for the PTP1B cleavage by hyperglycemia is calcium-dependent protease calpain-2. It has been demonstrated that PTP1B is a substrate of calpain-2 and that calpain-2-mediated PTP1B cleavage increases its enzymatic activity [45]. Regarding the retina, we have previously reported that inhibition of calpain-2 reduced apoptosis induced by increased calcium influx and prevented retinal degeneration [26]. These studies support our results in 661W cells cultured in HG-supplemented medium showing an increase in the ratio of the 58-kDa fragment/80-kDa proform of calpain-2, strongly suggesting that hyperglycemia increases its protease activity [27,28]. Interestingly, treatment with SST prevented calpain-2 activation and PTP1B cleavage. These results reinforce the link between hyperglycemia and the activation of calpain-2 and PTP1B in photoreceptors.
PTP1B has emerged as a negative modulator of IR/IGF-IR tyrosine phosphorylation [46]. However, IGF-IR-mediated signaling was impaired in the retinas of patients with DR [10], and as a result, the balance between apoptosis and survival favored cell death. Similarly, in db/db mice that recapitulate DR progression, phosphorylation of the IGF-IR and its downstream mediator Akt were lower than that of the db/+ controls, and importantly, these responses were recovered by topical administration of the glucagon-like peptide 1 agonist liraglutide [32]. Regarding the effect of SST in prosurvival signaling in the retina, our results show for the first time that treatment of 661W photoreceptor cells with SST markedly induced the tyrosine phosphorylation of the IGF-IR at the 1165/1166 residues within the catalytic domain, and as a result, it also induced Akt phosphorylation at serine 473. Therefore, our data strongly suggest that in 661W cells, SST might counteract the deleterious effects of hyperglycemia by enhancing IGF-IR-mediated Akt phosphorylation. Whether SST is able to enhance the local production of IGF-I in photoreceptors that, in turn, triggers the activation of the IGF-IR in an autocrine manner deserves further investigation. Moreover, the activation of Akt phosphorylation in retinal explants by SST opens up the possibility of the effect on prosurvival signaling in other retinal cells.
In conclusion, this study has provided new mechanistic insights first into the involvement of calpain-2 and PTP1B in the loss of cell survival and increased caspase-8-dependent apoptosis induced by hyperglycemia in 661W photoreceptor cells and second, on the protective effect against apoptosis and the enhancement of IGF-IR-mediated Akt phosphorylation by SST. These results support previous findings on the beneficial effect of SST in neurodegeneration in the retina and pinpoint the therapeutic use of SST against DR progression.
Acknowledgments
This work was supported by grants from European Union: (EUROCONDOR project grant agreement number 278,040) and FP7-People-2012-CIG (grant agreement number 333,594), Spanish Ministry of Economy and Competitivity: SAF2012-35562 and SAF2015-65267R cofunded by the Fondos Europeos de Desarrollo Regional de la Unión Europea [FEDER] and Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERdem). BCN Peptides S.A. kindly provide SST used on this work. We acknowledge Dr. Patricia Vázquez (CIBERdem, Madrid) for her assistance with 661W cell line authentication. Ángela M. Valverde (avalverde@iib.uam.es) and Ana I. Arroba (aarroba@iib.uam.es) are co-corresponding authors for this paper. CONTRIBUTORS: AIA researched data and wrote and reviewed the manuscript. AMV provided funding, wrote and reviewed the manuscript. AM researched data. DC researched data. EB reviewed the manuscript. CH reviewed the manuscript.MP reviewed the manuscript. RS reviewed the manuscript and provided funding. AMV and AIA are responsible for the integrity of the work as a whole.
Appendix 1. STR analysis.
To access the table, click or select the words “Appendix 1.”
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Abcouwer SF, Gardner TW. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann N Y Acad Sci. 2014;1311:174–90. doi: 10.1111/nyas.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jindal V. Neurodegeneration as a primary change and role of neuroprotection in diabetic retinopathy. Mol Neurobiol. 2015;51:878–84. doi: 10.1007/s12035-014-8732-7. [DOI] [PubMed] [Google Scholar]
- 4.Simo R, Hernandez C. European Consortium for the Early Treatment of Diabetic R. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends in endocrinology and metabolism. TEM. 2014;25:23–33. doi: 10.1016/j.tem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Park SH, Park JW, Park SJ, Kim KY, Chung JW, Chun MH, Oh SJ. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46:1260–8. doi: 10.1007/s00125-003-1177-6. [DOI] [PubMed] [Google Scholar]
- 6.Boynton GE, Stem MS, Kwark L, Jackson GR, Farsiu S, Gardner TW. Multimodal characterization of proliferative diabetic retinopathy reveals alterations in outer retinal function and structure. Ophthalmology. 2015;122:957–67. doi: 10.1016/j.ophtha.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordeiro MF, Migdal C, Bloom P, Fitzke FW, Moss SE. Imaging apoptosis in the eye. Eye (Lond) 2011;25:545–53. doi: 10.1038/eye.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marigo V. Programmed cell death in retinal degeneration: targeting apoptosis in photoreceptors as potential therapy for retinal degeneration. Cell Cycle. 2007;6:652–5. doi: 10.4161/cc.6.6.4029. [DOI] [PubMed] [Google Scholar]
- 9.Khalfaoui T, Basora N, Ouertani-Meddeb A. Apoptotic factors (Bcl-2 and Bax) and diabetic retinopathy in type 2 diabetes. J Mol Histol. 2010;41:143–52. doi: 10.1007/s10735-010-9271-9. [DOI] [PubMed] [Google Scholar]
- 10.Valverde AM, Miranda S, Garcia-Ramirez M, Gonzalez-Rodriguez A, Hernandez C, Simo R. Proapoptotic and survival signaling in the neuroretina at early stages of diabetic retinopathy. Mol Vis. 2013;19:47–53. [PMC free article] [PubMed] [Google Scholar]
- 11.Carrasco E, Hernandez C, Miralles A, Huguet P, Farres J, Simo R. Lower somatostatin expression is an early event in diabetic retinopathy and is associated with retinal neurodegeneration. Diabetes Care. 2007;30:2902–8. doi: 10.2337/dc07-0332. [DOI] [PubMed] [Google Scholar]
- 12.Simo R, Lecube A, Sararols L, Garcia-Arumi J, Segura RM, Casamitjana R, Hernandez C. Deficit of somatostatin-like immunoreactivity in the vitreous fluid of diabetic patients: possible role in the development of proliferative diabetic retinopathy. Diabetes Care. 2002;25:2282–6. doi: 10.2337/diacare.25.12.2282. [DOI] [PubMed] [Google Scholar]
- 13.Klisovic DD, O’Dorisio MS, Katz SE, Sall JW, Balster D, O’Dorisio TM, Craig E, Lubow M. Somatostatin receptor gene expression in human ocular tissues: RT-PCR and immunohistochemical study. Invest Ophthalmol Vis Sci. 2001;42:2193–201. [PubMed] [Google Scholar]
- 14.Cervia D, Casini G, Bagnoli P. Physiology and pathology of somatostatin in the mammalian retina: a current view. Mol Cell Endocrinol. 2008;286:112–22. doi: 10.1016/j.mce.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Helboe L, Moller M, Norregaard L, Schiodt M, Stidsen CE. Development of selective antibodies against the human somatostatin receptor subtypes sst1-sst5. Brain Res Mol Brain Res. 1997;49:82–8. doi: 10.1016/s0169-328x(97)00127-7. [DOI] [PubMed] [Google Scholar]
- 16.Mastrodimou N, Thermos K. The somatostatin receptor (sst1) modulates the release of somatostatin in rat retina. Neurosci Lett. 2004;356:13–6. doi: 10.1016/j.neulet.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Johnson J, Wu V, Wong H, Walsh JH, Brecha NC. Somatostatin receptor subtype 2A expression in the rat retina. Neuroscience. 1999;94:675–83. doi: 10.1016/s0306-4522(99)00170-0. [DOI] [PubMed] [Google Scholar]
- 18.Vasilaki A, Gardette R, Epelbaum J, Thermos K. NADPH-diaphorase colocalization with somatostatin receptor subtypes sst2A and sst2B in the retina. Invest Ophthalmol Vis Sci. 2001;42:1600–9. [PubMed] [Google Scholar]
- 19.Hernandez C, Carrasco E, Casamitjana R, Deulofeu R, Garcia-Arumi J, Simo R. Somatostatin molecular variants in the vitreous fluid: a comparative study between diabetic patients with proliferative diabetic retinopathy and nondiabetic control subjects. Diabetes Care. 2005;28:1941–7. doi: 10.2337/diacare.28.8.1941. [DOI] [PubMed] [Google Scholar]
- 20.Simo R, Carrasco E, Fonollosa A, Garcia-Arumi J, Casamitjana R, Hernandez C. Deficit of somatostatin in the vitreous fluid of patients with diabetic macular edema. Diabetes Care. 2007;30:725–7. doi: 10.2337/dc06-1345. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez C, Garcia-Ramirez M, Corraliza L, Fernandez-Carneado J, Farrera-Sinfreu J, Ponsati B, Gonzalez-Rodriguez A, Valverde AM, Simo R. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2013;62:2569–78. doi: 10.2337/db12-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanan Y, Kasus-Jacobi A, Moiseyev G, Sawyer K, Ma JX, Al-Ubaidi MR. Retinoid processing in cone and Muller cell lines. Exp Eye Res. 2008;86:344–54. doi: 10.1016/j.exer.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida JL, Hill CR, Cole KD. Mouse cell line authentication. Cytotechnology. 2014;66:133–47. doi: 10.1007/s10616-013-9545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badisa RB, Tzakou O, Couladis M, Pilarinou E. Cytotoxic activities of some Greek Labiatae herbs. Phytotherapy research. PTR. 2003;17:472–6. doi: 10.1002/ptr.1175. [DOI] [PubMed] [Google Scholar]
- 25.Simo R, Hernandez C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res. 2015;48:160–80. doi: 10.1016/j.preteyeres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Arroba AI, Wallace D, Mackey A, de la Rosa EJ, Cotter TG. IGF-I maintains calpastatin expression and attenuates apoptosis in several models of photoreceptor cell death. Eur J Neurosci. 2009;30:975–86. doi: 10.1111/j.1460-9568.2009.06902.x. [DOI] [PubMed] [Google Scholar]
- 27.Saido TC, Sorimachi H, Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 1994;8:814–22. [PubMed] [Google Scholar]
- 28.Campbell RL, Davies PL. Structure-function relationships in calpains. Biochem J. 2012;447:335–51. doi: 10.1042/BJ20120921. [DOI] [PubMed] [Google Scholar]
- 29.Pasquet JM, Dachary-Prigent J, Nurden AT. Microvesicle release is associated with extensive protein tyrosine dephosphorylation in platelets stimulated by A23187 or a mixture of thrombin and collagen. Biochem J. 1998;333:591–9. doi: 10.1042/bj3330591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arroba AI, Valverde AM. Inhibition of Protein Tyrosine Phosphatase 1B Improves IGF-I Receptor Signaling and Protects Against Inflammation-Induced Gliosis in the Retina. Invest Ophthalmol Vis Sci. 2015;56:8031–44. doi: 10.1167/iovs.15-17234. [DOI] [PubMed] [Google Scholar]
- 31.Arroba AI, Revuelta-Cervantes J, Menes L, Gonzalez-Rodriguez A, Pardo V, de la Villa P, Burks DJ, Valverde AM. Loss of protein tyrosine phosphatase 1B increases IGF-I receptor tyrosine phosphorylation but does not rescue retinal defects in IRS2-deficient mice. Invest Ophthalmol Vis Sci. 2013;54:4215–25. doi: 10.1167/iovs.12-11438. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez C, Bogdanov P, Corraliza L, Garcia-Ramirez M, Sola-Adell C, Arranz JA, Arroba AI, Valverde AM, Simo R. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes. 2016;65:172–87. doi: 10.2337/db15-0443. [DOI] [PubMed] [Google Scholar]
- 33.Villarroel M, Ciudin A, Hernandez C, Simo R. Neurodegeneration: An early event of diabetic retinopathy. World J Diabetes. 2010;1:57–64. doi: 10.4239/wjd.v1.i2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharifi AM, Eslami H, Larijani B, Davoodi J. Involvement of caspase-8, −9, and −3 in high glucose-induced apoptosis in PC12 cells. Neurosci Lett. 2009;459:47–51. doi: 10.1016/j.neulet.2009.03.100. [DOI] [PubMed] [Google Scholar]
- 35.Chang Q, Peter ME, Grassi MA. Fas ligand-Fas signaling participates in light-induced apoptotic death in photoreceptor cells. Invest Ophthalmol Vis Sci. 2012;53:3703–16. doi: 10.1167/iovs.11-8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchs C, Forster V, Balse E, Sahel JA, Picaud S, Tessier LH. Retinal-cell-conditioned medium prevents TNF-alpha-induced apoptosis of purified ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2983–91. doi: 10.1167/iovs.04-1177. [DOI] [PubMed] [Google Scholar]
- 37.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–58. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang LP, Zhu XA, Tso MO. Role of NF-kappaB and MAPKs in light-induced photoreceptor apoptosis. Invest Ophthalmol Vis Sci. 2007;48:4766–76. doi: 10.1167/iovs.06-0871. [DOI] [PubMed] [Google Scholar]
- 39.Agrawal NK, Kant S. Targeting inflammation in diabetes: Newer therapeutic options. World J Diabetes. 2014;5:697–710. doi: 10.4239/wjd.v5.i5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283:14230–41. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajala RV, Tanito M, Neel BG, Rajala A. Enhanced retinal insulin receptor-activated neuroprotective survival signal in mice lacking the protein-tyrosine phosphatase-1B gene. J Biol Chem. 2010;285:8894–904. doi: 10.1074/jbc.M109.070854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inada S, Ikeda Y, Suehiro T, Takata H, Osaki F, Arii K, Kumon Y, Hashimoto K. Glucose enhances protein tyrosine phosphatase 1B gene transcription in hepatocytes. Mol Cell Endocrinol. 2007;271:64–70. doi: 10.1016/j.mce.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Rajala RV, Wiskur B, Tanito M, Callegan M, Rajala A. Diabetes reduces autophosphorylation of retinal insulin receptor and increases protein-tyrosine phosphatase-1B activity. Invest Ophthalmol Vis Sci. 2009;50:1033–40. doi: 10.1167/iovs.08-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Wang JJ, Chen D, Mott R, Yu Q, Ma JX, Zhang SX. Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res. 2009;89:71–8. doi: 10.1016/j.exer.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cortesio CL, Chan KT, Perrin BJ, Burton NO, Zhang S, Zhang ZY, Huttenlocher A. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J Cell Biol. 2008;180:957–71. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bakke J, Haj FG. Protein-tyrosine phosphatase 1B substrates and metabolic regulation. Semin Cell Dev Biol. 2015;37:58–65. doi: 10.1016/j.semcdb.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]