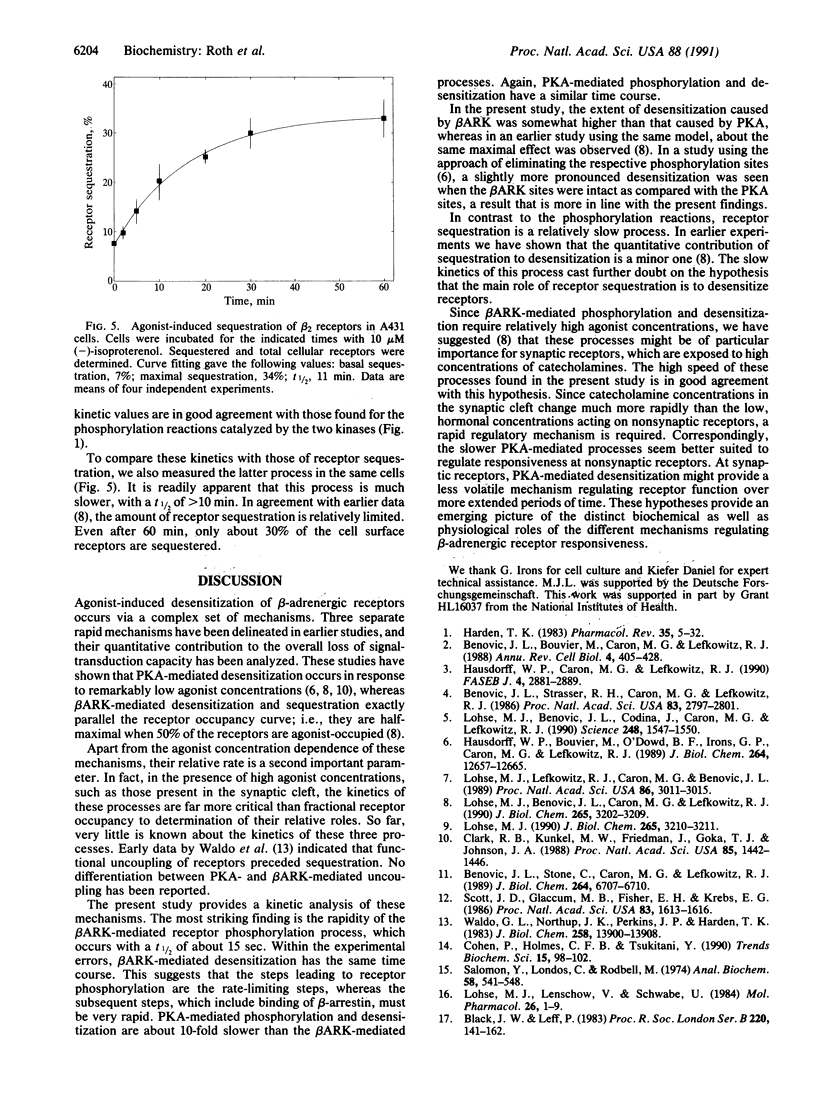
Abstract
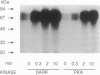
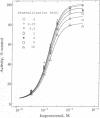
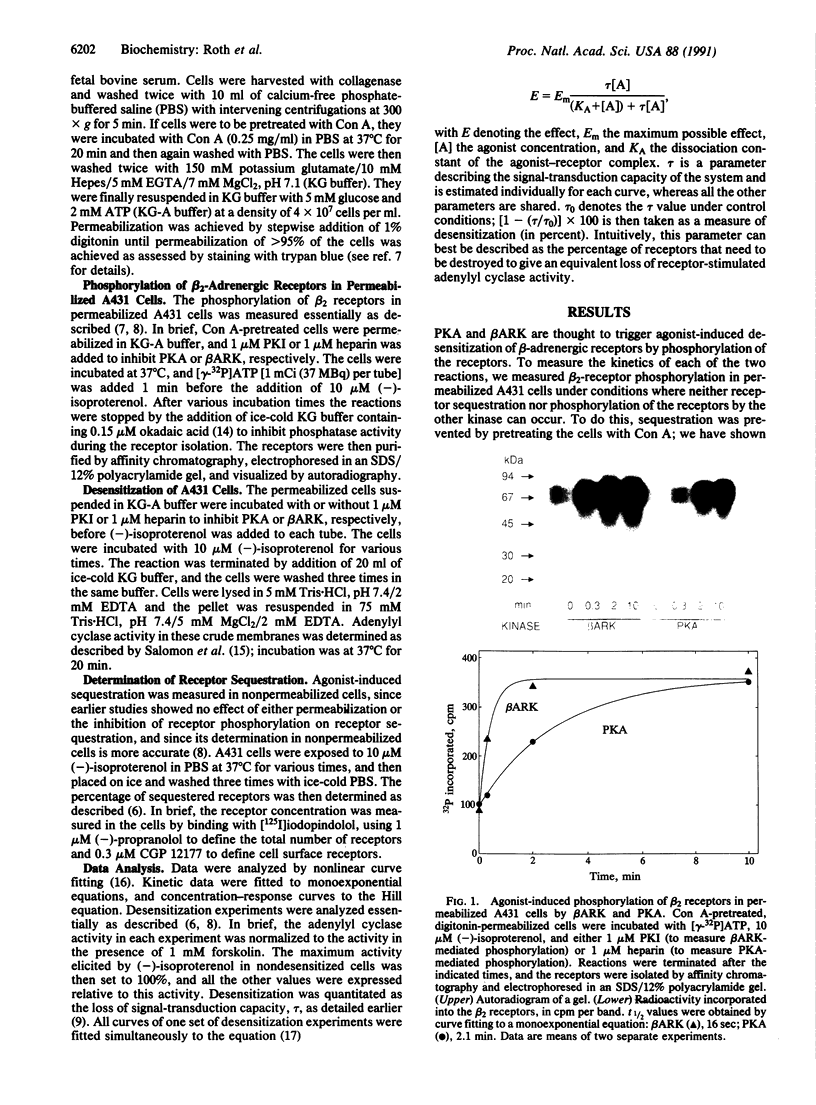
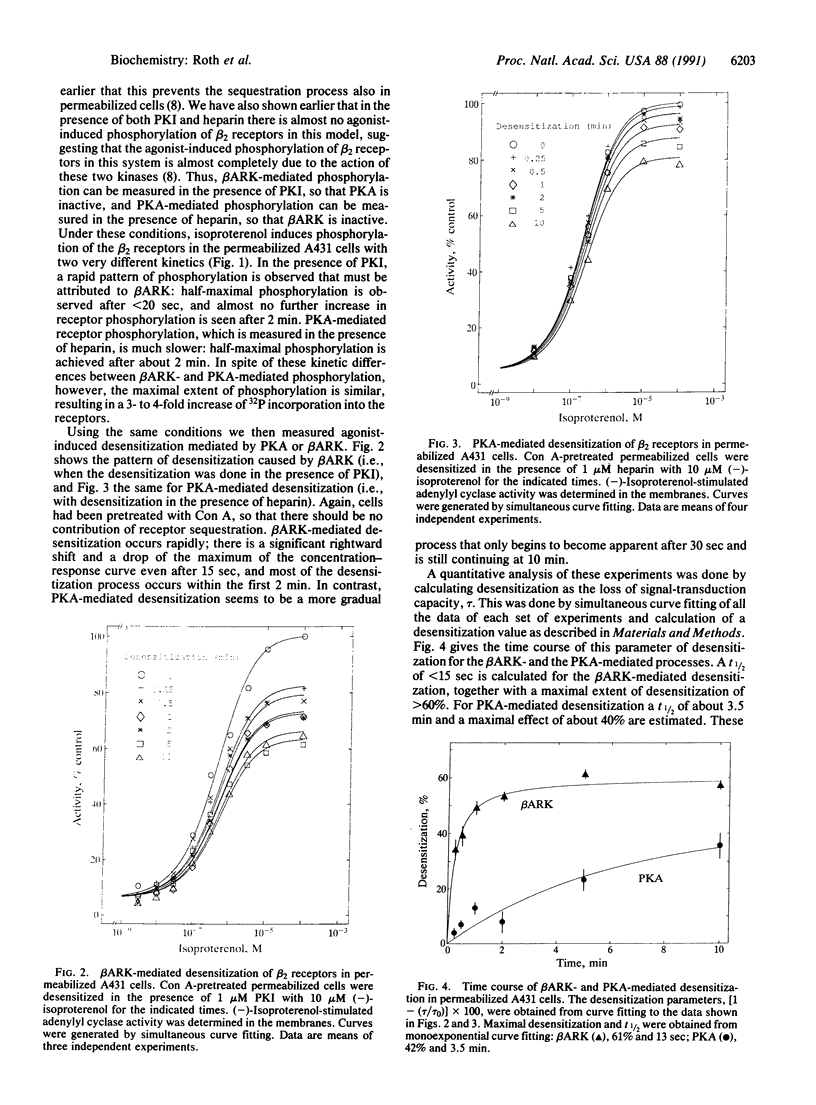
Three separate processes may contribute to rapid beta-adrenergic receptor desensitization: functional uncoupling from the stimulatory guanine nucleotide-binding protein Gs, mediated by phosphorylation of the receptors by two distinct kinases, the specific beta-adrenergic receptor kinase (beta ARK) and the cyclic AMP-dependent protein kinase A (PKA), as well as a spatial uncoupling via sequestration of the receptors away from the cell surface. To evaluate the relative importance and potential role of the various processes in different physiological situations, a kinetic analysis of these three mechanisms was performed in permeabilized A431 epidermoid carcinoma cells. To allow a separate analysis of each mechanism, inhibitors of the various desensitization mechanisms were used: heparin to inhibit beta ARK, the PKA inhibitor peptide PKI to inhibit PKA, and concanavalin A treatment to prevent sequestration. Isoproterenol-induced phosphorylation of beta 2 receptors in these cells by beta ARK occurred with a t1/2 of less than 20 sec, whereas phosphorylation by PKA had a t1/2 of about 2 min. Similarly, beta ARK-mediated desensitization of the receptors proceeded with a t1/2 of less than 15 sec, and PKA-mediated desensitization with a t1/2 of about 3.5 min. Maximal desensitization mediated by the two kinases corresponded to a reduction of the signal-transduction capacity of the receptor/adenylyl cyclase system by about 60% in the case of beta ARK and by about 40% in the case of PKA. Receptor sequestration was much slower (t1/2 of about 10 min) and involved no more than 30% of the cell surface receptors. It is concluded that beta ARK-mediated phosphorylation is the most rapid and quantitatively most important factor contributing to the rapid desensitization. This rapidity of the beta ARK-mediated mechanism makes it particularly well suited to regulate beta-adrenergic receptor function in rapidly changing environments such as the synaptic cleft.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., Bouvier M., Caron M. G., Lefkowitz R. J. Regulation of adenylyl cyclase-coupled beta-adrenergic receptors. Annu Rev Cell Biol. 1988;4:405–428. doi: 10.1146/annurev.cb.04.110188.002201. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Stone W. C., Caron M. G., Lefkowitz R. J. Inhibition of the beta-adrenergic receptor kinase by polyanions. J Biol Chem. 1989 Apr 25;264(12):6707–6710. [PubMed] [Google Scholar]
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983 Dec 22;220(1219):141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Kunkel M. W., Friedman J., Goka T. J., Johnson J. A. Activation of cAMP-dependent protein kinase is required for heterologous desensitization of adenylyl cyclase in S49 wild-type lymphoma cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1442–1446. doi: 10.1073/pnas.85.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Harden T. K. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983 Mar;35(1):5–32. [PubMed] [Google Scholar]
- Hausdorff W. P., Bouvier M., O'Dowd B. F., Irons G. P., Caron M. G., Lefkowitz R. J. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J Biol Chem. 1989 Jul 25;264(21):12657–12665. [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Caron M. G., Lefkowitz R. J. Multiple pathways of rapid beta 2-adrenergic receptor desensitization. Delineation with specific inhibitors. J Biol Chem. 1990 Feb 25;265(6):3202–3211. [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990 Jun 22;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Lefkowitz R. J., Caron M. G., Benovic J. L. Inhibition of beta-adrenergic receptor kinase prevents rapid homologous desensitization of beta 2-adrenergic receptors. Proc Natl Acad Sci U S A. 1989 May;86(9):3011–3015. doi: 10.1073/pnas.86.9.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M. J., Lenschow V., Schwabe U. Two affinity states of Ri adenosine receptors in brain membranes. Analysis of guanine nucleotide and temperature effects on radioligand binding. Mol Pharmacol. 1984 Jul;26(1):1–9. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Scott J. D., Glaccum M. B., Fischer E. H., Krebs E. G. Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1613–1616. doi: 10.1073/pnas.83.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo G. L., Northup J. K., Perkins J. P., Harden T. K. Characterization of an altered membrane form of the beta-adrenergic receptor produced during agonist-induced desensitization. J Biol Chem. 1983 Nov 25;258(22):13900–13908. [PubMed] [Google Scholar]