Abstract
Aims
We aimed to determine alcohol use’s effect on HIV treatment success and whether alcohol use mediates the relation between male sex and treatment failure.
Design
Longitudinal cohort.
Setting
Clinics in Botswana.
Participants
938 HIV-infected treatment naïve adults initiating regimens containing efavirenz between June 2009 and February 2013, including 478 (51%) males, median age 38 years, and plasma HIV RNA 4·9 log10 copies/ml.
Measurements
Primary outcome was a composite of treatment failure over 6 months including death, loss to care, or plasma HIV RNA >25 copies/ml. Exposures included alcohol use and gender.
Findings
Failure in 339 (36%) participants included 40 (4%) deaths, 194 (21%) lost to care, and 105 (11%) with HIV RNA>25 copies/ml. Both hazardous alcohol use in the past year [aOR 1·4 (95% CI: 1·0, 1·9)] and male sex [aOR 2·1 (95% CI: 1·5, 2·9)] were associated with failure. Alcohol use was more common among men (51%) than women (19%), p<0·001. There was no difference in alcohol use’s effect on failure between sexes (p for interaction>0.5). Controlling for hazardous alcohol use did not change the relation between sex and failure.
Conclusion
Hazardous alcohol users had worse HIV treatment outcomes. Males were more commonly hazardous drinkers, but hazardous alcohol use had neither a mediating nor moderating effect on the relation between sex and failure. We found no evidence that alcohol reduction interventions for improving HIV outcomes should preferentially target males. Identification of other characteristics in males causing worse outcomes warrant investigation.
Introduction
The introduction of antiretroviral therapy for HIV infection in Africa has been remarkable in the sheer numbers of patients enrolled and initiated on antiretroviral therapy (1, 2). The successes are measurable by the high proportion of individuals needing treatment receiving it(3), the number in care who achieve virologic suppression(4, 5), and in the increases in life expectancy observed(6, 7). The potential benefit of the rollout lies not only in the lives saved, but also in decreasing the spread of HIV if a high proportion of the HIV infected population have undetectable viral loads(8).
Treatment success is a multicomponent phenomenon defined along all stages of the “treatment continuum”(9). Once initiated on therapy, particularly with very low CD4 counts, individuals have to survive early opportunistic infections (10, 11) and adhere to therapy at sufficiently high levels to achieve virologic suppression(12). Then, they need to remain in contact with the healthcare system over time to continue to receive and adhere to their antiretrovirals(13, 14). When treatment failure occurs, it is important to determine how the failure occurred since interventions to improve outcomes might be different for different components of the cascade(15).
Unfortunately, challenges in sustaining the impact of antiretroviral therapy treatment programs remain(16). It has been well recognized that particular risk factors predispose individuals to having worse treatment outcomes in many resource limited settings. Two factors related to failure and to each other are hazardous alcohol use (17, 18)and male sex(19, 20). Hazardous alcohol use is very common in sub-Saharan Africa and contributes to the high incidence of HIV(21, 22). In Botswana, a country with one of the highest HIV prevalence rates in the world (23), approximately 40% of the population drinks alcohol, with a high average annual volume per drinker of 25.3 L of pure alcohol for men and 9.5 L for women (24); men are more likely to be heavy alcohol users than women (25).
Health behaviors, such as medication adherence and retention in care are thought to be cognitively regulated through people’s expectations about the outcomes they are likely to experience if the behavior is implemented as well as their confidence in their ability to take the recommended action (26–28). Alcohol plausibly could affect expectations and/or confidence. Alcohol myopia, for example, is a phenomenon whereby drinking alcohol leads to a narrowing of perception and cognitive functioning, which could lead to episodic or general disregard for pill-taking (29). Potential specific mechanisms whereby alcohol might affect treatment outcome include intoxication leading to forgetting doses (30), intentional avoidance of doses because of fears of alcohol-medication interactions (31), post-intoxication adverse effects (e.g., hangover) affecting motivation to cope with nausea or other antiretroviral adverse effects (32), or hazardous alcohol use simply being a marker for dysfunctional coping with negative affect (33), itself a barrier to adherence (34).
The etiology of this disparity in outcomes between the sexes remains unknown, but is likely multifactorial. Men typically present later in the disease course with more opportunistic infections, lower CD4 counts(35), and lower rates of retention in care than women, particularly in western and southern Africa(36, 37). It has been postulated that men are less likely to seek help for health related problems (38), and that their adherence to therapy is in general lower than for women in African settings(39). However, alcohol use may also contribute.
While further behavioral research into the effects of alcohol on HIV outcomes is needed, given the high prevalence of alcohol use and the increased risk of poor treatment outcomes among HIV-infected African males than females, outcomes data evaluating alcohol use and sex as interrelated risk factors for treatment failure in HIV-infected cohorts in African settings are urgently needed.
Specifically, if alcohol use mediates the relation between male sex and failure, then focusing intervention efforts on individuals who drink alcohol might reduce the gender disparity in HIV outcomes. If alcohol does not mediate the effect of male sex on treatment failure, further investigation into the determinants of treatment failure in men would be warranted. Moreover, if the effect of alcohol use on treatment failure differs between the sexes, alcohol reduction interventions might be more efficiently allocated if targeted and tailored to the group in which alcohol use is more strongly associated with failure. Therefore, we aimed to assess the association between alcohol use and sex on suboptimal outcomes and their potential mediating and moderating effects on each other in patients initiating antiretroviral therapy in Botswana.
Methods
Study Design and Population
We conducted a prospective cohort study of HIV infected individuals initiating antiretroviral therapy at eight HIV clinics in and around Gaborone, Botswana. Eligibility criteria included being an HIV-infected Botswana citizen of black African origin, age≥21 years, HIV treatment naïve, initiating antiretroviral therapy with efavirenz 600 mg once daily in combination with either tenofovir disoproxil fumarate 300 mg once daily or zidovudine 300 mg twice daily plus either emtricitabine 200 mg once daily or lamivudine 150 mg twice daily. Exclusion criteria were having plans to transfer care to a distant treatment site, prior use of single dose nevirapine to prevent mother to child transmission of HIV, and current pregnancy. We recruited participants by first presenting the study to the group of patients in the waiting area of the HIV clinics. Potential participants who were to initiate antiretroviral therapy at that visit were invited to meet with the study nurse immediately or, if preferred, to return within two weeks. Consent and questionnaires were administered in a private room in the clinic in either English or Setswana depending on the participant’s preference. Follow-up visits occurred at one month and six months after enrollment. If participants did not show for their study visit at month 6, an intensified protocol was implemented to obtain 6 month outcomes including phone calls to the participants and their contacts, review of clinic records for follow-up visits or death, and review of Botswana’s centralized computer laboratory records.
Outcomes
The primary outcome was composite treatment failure defined as death from any cause, detectable plasma HIV viral load (>25 copies/ml, NucliSENS Easy Q HIV-1, Biomerieux), or loss to follow-up by 6 months. Death was obtained from medical records or verbal report by the participant’s family or friends. Loss to follow-up was defined as no clinical visit within 2 weeks of the scheduled month 6, and no evidence of contact with the health system from the 6 month timepoint up until 9 months after enrollment (i.e., 3 months late for the 6 month visit). The secondary outcomes were the individual components of the composite primary outcome.
Exposures
Demographic data and alcohol use were obtained by one-on-one interview with the study nurse. Alcohol use in the prior month and prior year were assessed using the National Epidemiological Survey on Alcohol and Related Conditions (NESARC) version of the Alcohol Use Disorders Identification Test (AUDIT-C) (40) at baseline, month 1 and month 6. Alcohol use at last contact was included as the exposure of interest to account for newly incident hazardous use as a determinant of outcome. Binge drinking was defined as having episodes of >5 drinks per sitting. Hazardous alcohol use was defined as binge drinking or ≥14 drinks per week for men or ≥7 drinks per week for women. Plasma HIV RNA and CD4 counts were measured at enrollment.
Statistical Analysis
The primary analysis assessed the association between the exposure variables of interest (alcohol use, sex, age, baseline HIV RNA, baseline CD4 count) and composite treatment failure. Secondary analyses included the association between these exposure variables and the individual components of treatment failure, namely, death, lost to care, and detectable viral load. For the analyses of death, only those known to have died were compared to the rest of the cohort. For the analyses of lost to care, individuals who died prior to month 6 were excluded. We compared baseline characteristics by sex using Wilcoxon rank sum tests for continuous variables and chi-squared tests for dichotomous variables. We calculated odds ratios with 95% confidence intervals (CI) for the magnitude of the relation between each of the variables and the outcomes, individually. In adjusted analyses, we used logistic regression in which each of the variables associated with the outcomes in the unadjusted analyses became the focus of individual models. In each of these models, the other variables that were associated with the outcome with p<0.1 were included as potential confounders. If the association between the variable of interest and outcome changed by >15%, the secondary variables were retained in the models. We tested for effect modification of the key exposure variables (hazardous alcohol use and male sex) on each other by including an interaction term (alcohol use by sex) in the logistic regression model of composite treatment failure. We also assess for mediation of the relation between male sex and failure using the approach suggested by Baron and Kenny (41). We assessed the relation between alcohol use and male sex using the Wilcoxon rank sum test. If both hazardous alcohol use and male sex were associated with failure and alcohol use and male sex were associated with each other, we would include both hazardous alcohol use and male sex in the logistic regression models. We interpreted the presence of mediation of the relation between male sex and failure by alcohol use if the relation between male sex and outcome was attenuated in the models that simultaneously included both alcohol use and male sex. Analyses were performed using SAS (SAS Institute, Cary, NC).
Sample Size Considerations
The sample size was selected based on considerations of another aim of the study, namely, to determine the association between slow efavirenz metabolism and treatment outcome (42). We targeted enrollment of 940 individuals for 80% power to determine if slow metabolism genotype increased the risk of treatment failure by 20% at an alpha level of 0.05. We assumed a prevalence of slow efavirenz metabolism of 10% (43) and a treatment failure rate of 50%. We inflated the resulting sample size by 50% to allow for potential differences in the association of genotype and treatment outcome by HIV treatment adherence. Since hazardous alcohol use was more common than 10% and the treatment failure rate was lower than 50%, we had substantially more than 80% power to detect an effect of alcohol use.
Ethical Considerations
All participants provided written informed consent after having the consent form read to them in either English or Setswana, whichever they preferred. The study was approved by the Health Research and Development Committee Ethics Board of the Botswana Ministry of Health and the University of Pennsylvania Committee on Human Subjects Research.
Role of the Funding Source
The NIH had no role in the interpretation of the results. The corresponding author had full access to the data and final responsibility for submitting the work for publication.
Results
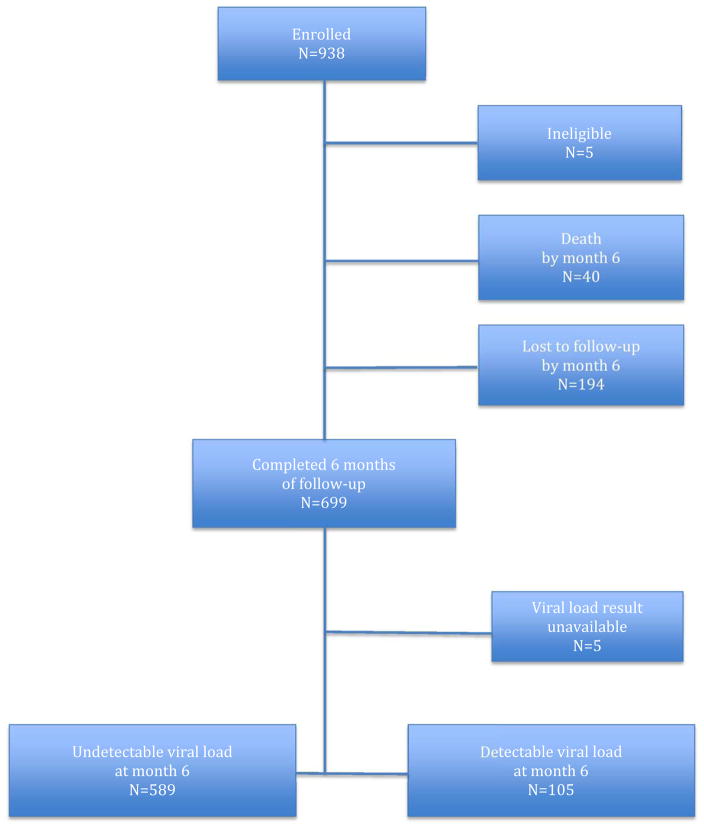
We enrolled 938 individuals between June 2009 and February 2013 with follow-up through November 2013 to allow the last enrollees an opportunity to be seen within 9 months of enrollment to avoid being incorrectly declared lost to follow-up. The 5 individuals with plasma viral loads unavailable were excluded from the failure and detectable viral load analyses, but not the loss to care analyses. Figure 1 depicts the disposition of the patients during follow-up. The population was nearly evenly divided by sex with Table 1 comparing the baseline characteristics between men and women. Overall, men had statistically significant, although clinically marginally lower CD4 counts and higher viral loads than women. Men were substantially more likely to be hazardous alcohol users. All of the hazardous alcohol use was in the form of binge drinking.
Figure 1.
Disposition of Study Participants
Table 1.
Characteristics at Treatment Initiation Overall and by Sex
| Characteristic | Overall N=938 |
Males N=478 |
Females N=460 |
p value |
|---|---|---|---|---|
| Median age, years (range) | 37 (21, 67) | 38 (21, 66) | 37 (21, 67) | >0.5 |
| CD4 count cells/mm3 (IQR) | 194 (110, 252) | 173 (90, 237) | 211 (141, 272) | <0.001 |
| HIV RNA log10 copies/ml (IQR) | 4.9 (4.2, 5.4) | 5.0 (4.5, 5.5) | 4.6 (4.0, 5.3) | <0.001 |
| History of Confirmed or Suspected Tuberculosis | 40 (4%) | 25 (5%) | 15 (3%) | 0.15 |
| Hazardous Alcohol Use in Prior Year at Baseline | 385 (41%) | 274 (57%) | 111 (24%) | <0.001 |
| Hazardous Alcohol Use in Prior Month at Baseline | 183 (20%) | 143 (30%) | 40 (9%) | <0.001 |
| Hazardous Alcohol Use in Prior Year at Last Contact | 335 (36%) | 246 (51) | 89 (19%) | <0.001 |
| Hazardous Alcohol Use in Prior Month at Last Contact | 115 (12%) | 89 (19%) | 26 (6%) | <0.001 |
Combined Treatment Failure
Treatment failure occurred in 338 (36%) of participants overall. The associations between various risk factors and combined treatment failure are shown in Table 2. In unadjusted analyses, men were slightly less than twice as likely as females to fail and hazardous alcohol use in the past year increased the risk for failure by 40%. Other risk factors for failure included older age and higher baseline plasma HIV RNA. There was no evidence of effect modification by alcohol use and sex in their associations with failure (p value for alcohol use by sex interaction term>0.5). After adjustment for potential confounders the risk of failure for hazardous alcohol use in the past year was unchanged while the risk of failure in males increased to more than double that of females.
Table 2.
Relation between Exposures and Composite Failure by Month 6
| Variable of Interest | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Male sex | 1.7 (1.3, 2.2) | 2.1 (1.5, 2.9) |
| Age (per 10 years) | 1.2 (1.1, 1.4) | 1.2 (1.1, 1.5) |
| Baseline HIV RNA (per log10 copies/ml) | 1.4 (1.2, 1.6) | 1.2 (1.0, 1.4) |
| Baseline CD4 Count (per 50 cells/mm3) | 0.86 (0.81, 0.93) | 0.92 (0.85, 0.99) |
| Hazardous alcohol use in prior month | 1.2 (0.8, 1.8) | - |
| Hazardous alcohol use in prior year | 1.4 (1.1, 1.9) | 1.4 (1.0, 1.9) |
Loss to Care
Loss to care was the most common reason for participants to be declared treatment failures, occurring in 21%. The associations between various risk factors and loss to follow-up are shown in Table 3. In unadjusted analyses, hazardous alcohol use was associated with a more than 50% increase in the risk of loss to care and men were slightly less than twice as likely to be lost to care than women. In adjusted analyses, the magnitude of the risk of hazardous alcohol use in the past year was similar to the unadjusted estimate, but the association was no longer statistically significant. The association between male sex and loss to care was only minimally attenuated by adjustment and remained significantly elevated at slightly less than twice the rate of women.
Table 3.
Relation between Exposures and Loss to Care by Month 6
| Variable of Interest | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Male sex | 2.1 (1.5, 3.0) | 1.9 (1.3, 2.7) |
| Age (per 10 years) | 1.2 (0.98, 1.4) | - |
| Baseline HIV RNA (per log10 copies/ml) | 1.1 (0.9, 1.3) | - |
| Baseline CD4 Count (per 50 cells/mm3) | 0.98 (0.96, 1.00) | - |
| Hazardous alcohol use in prior month | 1.4 (0.93, 2.0) | - |
| Hazardous alcohol use in prior year | 1.6 (1.1, 2.2) | 1.4 (0.98, 2.0) |
Detectable Viral Load
Detectable viral load was the second most common cause of treatment failure, occurring in 11% of individuals. The associations between various risk factors and detectable viral load are shown in Table 4. Only hazardous alcohol use in the past month, but not hazardous alcohol use in the past year was associated with virologic failure. There was no difference between men and women with respect to virologic failure. For every log10 higher baseline HIV RNA, the probability of detectable viral load at month 6 was slightly less than doubled.
Table 4.
Relation between Exposures and Detectable HIV RNA at Month 6
| Variable of Interest | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Male sex | 1.2 (0.8, 1.8) | - |
| Age (per 10 years) | 1.0 (0.8, 1.3) | - |
| Baseline HIV RNA (per log10 copies/ml) | 1.9 (1.4, 2.5) | 1.9 (1.4, 2.5) |
| Baseline CD4 Count (per 50 cells/mm3) | 0.98 (0.96, 1.0) | - |
| Hazardous alcohol use in prior month | 1.8 (1.0, 3.1) | 1.7 (1.0, 3.0) |
| Hazardous alcohol use in prior year | 1.0 (0.7, 1.6) | - |
Death
Death was relatively uncommon in both groups with 22 (5%) men and 18 (4%) women dying. The associations between various risk factors and survival are shown in Table 5. Neither hazardous alcohol use nor male sex had a statistically significant association with death. Greater age and lower baseline CD4 count were associated with a higher rate of death in both unadjusted and adjusted analyses.
Table 5.
Relation between Exposures and Survival
| Variable of Interest | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Male sex | 0.85 (0.45, 1.6) | - |
| Age (per 10 years) | 0.56 (0.42, 0.78) | 0.59 (0.49, 0.82) |
| Baseline HIV RNA (per log10 copies/ml) | 0.78 (0.48. 1.1) | - |
| Baseline CD4 Count (per 50 cells/mm3) | 1.6 (1.3, 1.9) | 1.6 (1.3, 1.9) |
| Hazardous alcohol use in prior month | 3.1 (0.94, 10.2) | 3.0 (0.4, 23) |
| Hazardous alcohol use in prior year | 0.94 (0.50, 1.8) | - |
Discussion
We evaluated antiretroviral therapy outcomes during the initial 6 months of treatment overall and by type of suboptimal outcome. We found that hazardous alcohol users and men had substantially worse outcomes than non-hazardous users and women, respectively. The most common cause of suboptimal outcome was loss to care which and hazardous alcohol users were much more commonly lost to care than non-hazardous drinkers.. Males were also substantially more likely to consume alcohol hazardously and also be lost to care. However, controlling for hazardous alcohol use in the models of the relation between sex and loss to care only minimally attenuated the association. If hazardous alcohol use was the mediator of males being lost to care, controlling for this variable in the logistic regression models should have resulted in a substantial decrease in the association between male sex and outcome (41).
Hazardous alcohol use in the past year remained associated with overall failure and was marginally associated with loss to care. Our findings are consistent with prior studies, including more recent findings that demonstrate that this phenomenon is not limited to sub-Saharan Africa (44, 45). In our setting, virtually all the hazardous alcohol use was binge drinking. Why binge drinking causes suboptimal outcomes is potentially complex and the timing of the drinking may help untangle the relation between drinking and the various outcomes. Distant binge drinking was associated with failure overall and loss to care, but only recent binge drinking (in the past month), was associated with virologic failure. A possible biological explanation for this finding is that binge drinking may interfere with metabolism of antiretrovirals(46), although recent evidence suggests a lack of association (47). There are many potential behavioral explanations for the effect. Acute intoxication is associated with lack of attention to important, but potentially stressful actions like remembering to take medications (29). However, most of the hazardous drinking in our study was episodic and therefore, this mechanism would only have operated intermittently. Binge drinking may reflect or cause depression, a known risk factor for suboptimal adherence and retention in care(34). Or, it may be that individuals who binge drink have a weaker commitment to healthy lifestyle choices (46). Further investigation into whether alcohol use itself causes the non-adherence or whether it is simply a marker of dysfunctional coping with the disease is needed to determine how interventions to reduce binge drinking in this setting might impact treatment outcomes.
Prior research has demonstrated that men have worse outcomes than women in subSaharan Africa (19, 20). Men may be more likely than women to drop out of care when they feel well and only seek care and adhere to therapy when they feel their health is threatened (48). Although they may have noted the health threat at enrollment into the study, that feeling may have waned with the return to health that typically occurs within months of initiating antiretroviral therapy. Yet, for those who stayed in care, there was no difference in outcome by gender, demonstrating the importance of focusing on retention in care for men.
The group that dropped out of care are particularly concerning for continuing the spread of HIV since virologic rebound would be expected within weeks of stopping antiretroviral therapy(49). Remien et al. demonstrated that non-adherence and unsafe sex behavior were associated, particularly in heterosexual men(50). This phenomenon may in part be responsible for the sustained levels of HIV transmission in Botswana despite the high rates of success of those in care and on therapy. Hazardous alcohol use in these individuals may contribute to higher risk sex and transmission (51).
This study has several strengths including the relatively large cohort size and assessment of potential etiologies of treatment failure. The extensive attempts at follow-up are also a strength, although the lack of proof of survival or virologic failure prevents us from making definitive conclusions on the secondary outcomes assessed. It is possible that some of those lost to care received care elsewhere(14). Yet, based on data from other settings, it is most likely that these individuals are either dead or alive and off treatment(52). While alcohol is clearly the most common psychoactive substance used in this setting, we are unable to comment on whether other recreational drugs may have played a role in treatment failure (53). Finally, the contact with study personnel over and above clinical care could have positively influenced the behavior of the study participants and thereby reduce generalizability. If so, the rates of composite treatment failure may actually be even higher than our study found, which would be even more troubling for the sustainability of the gains in life expectancy accrued to date.
In conclusion, HIV treatment outcomes in Botswana differ greatly between those who do and do not use alcohol at hazardous levels and between the sexes. The absence of a moderating effect between hazardous alcohol use and sex on treatment failure, argues against alcohol reduction interventions preferentially targeting one sex or the other. The absence of evidence of a mediating effect of hazardous alcohol use on the higher failure rate in males argues for the exploration of other factors present in males as targets for future interventions to improve HIV treatment outcomes.
Acknowledgments
The authors wish to acknowledge the contribution of the participants and of the staff of the Health Research and Development Committee of Botswana Ministry of Health and of the Botswana-UPenn Partnership for facilitating this work.
Footnotes
Declaration of Interests
The authors declare no conflicts of interest.
References
- 1.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006;296(7):782–93. doi: 10.1001/jama.296.7.782. Epub 2006/08/15. [DOI] [PubMed] [Google Scholar]
- 2.Grimsrud A, Balkan S, Casas EC, Lujan J, Van Cutsem G, Poulet E, et al. Outcomes of antiretroviral therapy over a 10-year period of expansion: a multicohort analysis of African and Asian HIV programs. J Acquir Immune Defic Syndr. 2014;67(2):e55–66. doi: 10.1097/QAI.0000000000000268. Epub 2014/07/01. [DOI] [PubMed] [Google Scholar]
- 3.Wools-Kaloustian K, Kimaiyo S, Musick B, Sidle J, Siika A, Nyandiko W, et al. The impact of the President’s Emergency Plan for AIDS Relief on expansion of HIV care services for adult patients in western Kenya. Aids. 2009;23(2):195–201. doi: 10.1097/QAD.0b013e32831cc0e6. Epub 2008/12/23. [DOI] [PubMed] [Google Scholar]
- 4.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. The Lancet infectious diseases. 2010;10(3):155–66. doi: 10.1016/S1473-3099(09)70328-7. Epub 2010/02/27. [DOI] [PubMed] [Google Scholar]
- 5.Bussmann H, Wester CW, Ndwapi N, Grundmann N, Gaolathe T, Puvimanasinghe J, et al. Five-year outcomes of initial patients treated in Botswana’s National Antiretroviral Treatment Program. Aids. 2008;22(17):2303–11. doi: 10.1097/QAD.0b013e3283129db0. Epub 2008/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Annals of internal medicine. 2011;155(4):209–16. doi: 10.7326/0003-4819-155-4-201108160-00358. Epub 2011/07/20. [DOI] [PubMed] [Google Scholar]
- 7.May M, Boulle A, Phiri S, Messou E, Myer L, Wood R, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376(9739):449–57. doi: 10.1016/S0140-6736(10)60666-6. Epub 2010/07/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. Epub 2011/07/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. Epub 2011/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Nadkarni G, Yang WT, Chandrasekhar A, Gupte N, Bisson GP, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PloS one. 2011;6(12):e28691. doi: 10.1371/journal.pone.0028691. Epub 2012/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steele KT, Steenhoff AP, Newcomb CW, Rantleru T, Nthobatsang R, Lesetedi G, et al. Early mortality and AIDS progression despite high initial antiretroviral therapy adherence and virologic suppression in Botswana. PloS one. 2011;6(6):e20010. doi: 10.1371/journal.pone.0020010. Epub 2011/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisson GP, Rowh A, Weinstein R, Gaolathe T, Frank I, Gross R. Antiretroviral failure despite high levels of adherence: discordant adherence-response relationship in Botswana. J Acquir Immune Defic Syndr. 2008;49(1):107–10. doi: 10.1097/QAI.0b013e3181820141. Epub 2008/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan AT, Maskew M, Sanne I, Fox MP. The importance of clinic attendance in the first six months on antiretroviral treatment: a retrospective analysis at a large public sector HIV clinic in South Africa. Journal of the International AIDS Society. 2010;13:49. doi: 10.1186/1758-2652-13-49. Epub 2010/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng EH, Glidden DV, Bwana MB, Musinguzi N, Emenyonu N, Muyindike W, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PloS one. 2011;6(7):e21797. doi: 10.1371/journal.pone.0021797. Epub 2011/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahonkhai AA, Noubary F, Munro A, Stark R, Wilke M, Freedberg KA, et al. Not all are lost: interrupted laboratory monitoring, early death, and loss to follow-up (LTFU) in a large South African treatment program. PloS one. 2012;7(3):e32993. doi: 10.1371/journal.pone.0032993. Epub 2012/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox MP, Shearer K, Maskew M, Macleod W, Majuba P, Macphail P, et al. Treatment outcomes after 7 years of public-sector HIV treatment. Aids. 2012;26(14):1823–8. doi: 10.1097/QAD.0b013e328357058a. Epub 2012/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug and alcohol dependence. 2010;112(3):178–93. doi: 10.1016/j.drugalcdep.2010.06.014. Epub 2010/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. doi: 10.1097/QAI.0b013e3181b18b6e. Epub 2009/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Druyts E, Dybul M, Kanters S, Nachega J, Birungi J, Ford N, et al. Male sex and the risk of mortality among individuals enrolled in antiretroviral therapy programs in Africa: a systematic review and meta-analysis. Aids. 2013;27(3):417–25. doi: 10.1097/QAD.0b013e328359b89b. Epub 2012/09/06. [DOI] [PubMed] [Google Scholar]
- 20.Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med. 2012;9(9):e1001304. doi: 10.1371/journal.pmed.1001304. Epub 2012/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott-Sheldon LA, Carey KB, Carey MP, Cain D, Simbayi LC, Kalichman SC. Alcohol use disorder, contexts of alcohol use, and the risk of HIV transmission among South African male patrons of shebeens. Drug and alcohol dependence. 2014;140:198–204. doi: 10.1016/j.drugalcdep.2014.04.022. Epub 2014/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medley A, Seth P, Pathak S, Howard AA, DeLuca N, Matiko E, et al. Alcohol use and its association with HIV risk behaviors among a cohort of patients attending HIV clinical care in Tanzania, Kenya, and Namibia. AIDS Care. 2014;26(10):1288–97. doi: 10.1080/09540121.2014.911809. Epub 2014/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UNAIDS: Botswana. 2014 [cited 2016 25 February]; Available from: http://www.unaids.org/en/regionscountries/countries/botswana.
- 24.Global Alcohol Report: Botswana. WHO; 2014. [cited 2016 25 February]; Available from: http://www.who.int/substance_abuse/publications/global_alcohol_report/profiles/bwa.pdf. [Google Scholar]
- 25.Peltzer K, Davids A, Njuho P. Alcohol use and problem drinking in South Africa: findings from a national population-based survey. African journal of psychiatry. 2011;14(1):30–7. doi: 10.4314/ajpsy.v14i1.65466. Epub 2011/04/22. [DOI] [PubMed] [Google Scholar]
- 26.Ajzen I. The Theory of Planned Behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–211. [Google Scholar]
- 27.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentiss Hall; 1986. [Google Scholar]
- 28.Fishbein M, editor. Nebraska Symposium on Motivation. Lincoln: University of Nebraska Press; 1979. A Theory of Reasoned Action: Some Applications and Implications. [PubMed] [Google Scholar]
- 29.Steele CM, Josephs RA. Alcohol myopia. Its prized and dangerous effects. The American psychologist. 1990;45(8):921–33. doi: 10.1037//0003-066x.45.8.921. Epub 1990/08/01. [DOI] [PubMed] [Google Scholar]
- 30.Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. Journal of general internal medicine. 2001;16(2):83–8. doi: 10.1111/j.1525-1497.2001.00122.x. Epub 2001/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalichman SC, Grebler T, Amaral CM, McNerey M, White D, Kalichman MO, et al. Intentional non-adherence to medications among HIV positive alcohol drinkers: prospective study of interactive toxicity beliefs. Journal of general internal medicine. 2013;28(3):399–405. doi: 10.1007/s11606-012-2231-1. Epub 2012/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ammassari A, Murri R, Pezzotti P, Trotta MP, Ravasio L, De Longis P, et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr. 2001;28(5):445–9. doi: 10.1097/00042560-200112150-00006. Epub 2001/12/18. [DOI] [PubMed] [Google Scholar]
- 33.Elliott JC, Aharonovich E, O’Leary A, Wainberg M, Hasin DS. Drinking motives as prospective predictors of outcome in an intervention trial with heavily drinking HIV patients. Drug and alcohol dependence. 2014;134:290–5. doi: 10.1016/j.drugalcdep.2013.10.026. Epub 2013/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–7. doi: 10.1097/QAI.0b013e31822d490a. Epub 2011/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills EJ, Bakanda C, Birungi J, Chan K, Hogg RS, Ford N, et al. Male gender predicts mortality in a large cohort of patients receiving antiretroviral therapy in Uganda. Journal of the International AIDS Society. 2011;14:52. doi: 10.1186/1758-2652-14-52. Epub 2011/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Differences between HIV-Infected men and women in antiretroviral therapy outcomes - six African countries, 2004–2012. MMWR Morbidity and mortality weekly report. 2013;62(47):945–52. Epub 2013/11/28. [PMC free article] [PubMed] [Google Scholar]
- 37.Wandeler G, Keiser O, Pfeiffer K, Pestilli S, Fritz C, Labhardt ND, et al. Outcomes of antiretroviral treatment programs in rural Southern Africa. J Acquir Immune Defic Syndr. 2012;59(2):e9–16. doi: 10.1097/QAI.0b013e31823edb6a. Epub 2011/11/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chikovore J, Hart G, Kumwenda M, Chipungu GA, Desmond N, Corbett L. Control, struggle, and emergent masculinities: a qualitative study of men’s care-seeking determinants for chronic cough and tuberculosis symptoms in Blantyre, Malawi. BMC public health. 2014;14:1053. doi: 10.1186/1471-2458-14-1053. Epub 2014/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, Regensberg L, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43(1):78–84. doi: 10.1097/01.qai.0000225015.43266.46. Epub 2006/08/01. [DOI] [PubMed] [Google Scholar]
- 40.Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcoholism, clinical and experimental research. 2005;29(5):844–54. doi: 10.1097/01.alc.0000164374.32229.a2. Epub 2005/05/18. [DOI] [PubMed] [Google Scholar]
- 41.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51(6):1173–82. doi: 10.1037//0022-3514.51.6.1173. Epub 1986/12/01. [DOI] [PubMed] [Google Scholar]
- 42.Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18(18):2391–400. Epub 2004/12/29. [PubMed] [Google Scholar]
- 43.Gross R, Aplenc R, Tenhave T, Foulkes AS, Thakur R, Mosepele M, et al. Slow efavirenz metabolism genotype is common in Botswana. J Acquir Immune Defic Syndr. 2008;49(3):336–7. doi: 10.1097/QAI.0b013e31817c1ed0. Epub 2008/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran BX, Nguyen LT, Do CD, Nguyen QL, Maher RM. Associations between alcohol use disorders and adherence to antiretroviral treatment and quality of life amongst people living with HIV/AIDS. BMC public health. 2014;14:27. doi: 10.1186/1471-2458-14-27. Epub 2014/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woolf-King SE, Neilands TB, Dilworth SE, Carrico AW, Johnson MO. Alcohol use and HIV disease management: the impact of individual and partner-level alcohol use among HIV-positive men who have sex with men. AIDS care. 2014;26(6):702–8. doi: 10.1080/09540121.2013.855302. Epub 2013/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otwombe KN, Variava E, Holmes CB, Chaisson RE, Martinson N. Predictors of delay in the diagnosis and treatment of suspected tuberculosis in HIV co-infected patients in South Africa. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2013;17(9):1199–205. doi: 10.5588/ijtld.12.0891. Epub 2013/08/10. [DOI] [PubMed] [Google Scholar]
- 47.McCance-Katz EF, Gruber VA, Beatty G, Lum PJ, Rainey PM. Interactions between alcohol and the antiretroviral medications ritonavir or efavirenz. Journal of addiction medicine. 2013;7(4):264–70. doi: 10.1097/ADM.0b013e318293655a. Epub 2013/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Addis M, Mahalik J. Men, masculinity, and the contexts of help seeking. American Psychologist. 2003;58(1):5–14. doi: 10.1037/0003-066x.58.1.5. [DOI] [PubMed] [Google Scholar]
- 49.Papasavvas E, Kostman JR, Mounzer K, Grant RM, Gross R, Gallo C, et al. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med. 2004;1(3):e64. doi: 10.1371/journal.pmed.0010064. Epub 2005/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Remien RH, Dolezal C, Wagner GJ, Goggin K, Wilson IB, Gross R, et al. The association between poor antiretroviral adherence and unsafe sex: differences by gender and sexual orientation and implications for scale-up of treatment as prevention. AIDS Behav. 2014;18(8):1541–7. doi: 10.1007/s10461-013-0656-0. Epub 2013/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiser SD, Leiter K, Heisler M, McFarland W, Percy-de Korte F, DeMonner SM, et al. A population-based study on alcohol and high-risk sexual behaviors in Botswana. PLoS medicine. 2006;3(10):e392. doi: 10.1371/journal.pmed.0030392. Epub 2006/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geng EH, Glidden DV, Emenyonu N, Musinguzi N, Bwana MB, Neilands TB, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Tropical medicine & international health: TM & IH. 2010;15(Suppl 1):63–9. doi: 10.1111/j.1365-3156.2010.02507.x. Epub 2010/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS and behavior. 2007;11(2):185–94. doi: 10.1007/s10461-006-9152-0. Epub 2006/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]