Abstract
BACKGROUND
The need to repeat peripheral blood stem cell mobilization and collection in healthy donors arises infrequently but may be required due to insufficient initial collection, graft failure, or relapse of the recipient’s disease. Little data exists on the efficacy of remobilization. Therefore, we retrospectively reviewed 18 years of remobilization records from healthy stem cell donors at our institution.
STUDY DESIGN AND METHODS
We identified 62 healthy donors who underwent remobilization, a cohort of 30 mobilized and remobilized with cytokines and a cohort of 32 mobilized with a CXCR4 antagonist and remobilized with cytokines. For each cohort we compared the peripheral blood CD34+/uL level, the number of CD34+ cells collected per kg (recipient weight), and the number of CD34+ cells per L collected on the first day of leukapheresis during initial mobilization and remobilization.
RESULTS
Initial mobilization with cytokines was associated with reduced remobilization. The mean peripheral blood PB CD34/uL at initial mobilization was 69 compared to 37 at remobilization (p = 0.029). In contrast, initial mobilization with a CXCR4 antagonist was not associated with reduced remobilization. The mean PB CD34/uL at initial mobilization was 15 compared to 68 at remobilization (p < 0.001). In both cohorts, initial mobilization results were positively correlated with remobilization results but the interval between was not.
CONCLUSIONS
This study suggests that poor remobilization yields may be due to decreased efficacy of cytokines after repeat exposure. The underlying mechanism of these findings remains unclear and further studies are needed.
Keywords: peripheral blood stem cells, mobilization, remobilization
INTRODUCTION
Peripheral blood stem cells (PBSCs) are the graft source for more than 80% of allogeneic hematopoietic stem cell transplantations (allo-HSCT) in the U.S.1 The vast majority of healthy donors are able to collect the minimum number of PBSC following mobilization with cytokines or CXCR4 antagonists; therefore, the need to repeat PBSC mobilization and collection arises infrequently, but may be required due to insufficient initial collection, graft failure, or relapse of the recipient’s disease2–3. Little data exists on the efficacy of remobilization of PBSC donors; only a single study of 38 donors undergoing remobilization has been published in a peer reviewed journal4. To address this gap in the literature, we reviewed remobilization success in healthy donors who underwent more than one mobilized PBSC collection at our center.
MATERIALS AND METHODS
We performed a retrospective chart review of 967 consecutive healthy adult (≥18 years old) donors who underwent PBSC mobilization and collection at Washington University School of Medicine from 1995 through 2013. We identified 66 who had undergone more than one mobilization. Two cohorts were identified for analysis. Cohort 1 included 30 donors mobilized initially and again subsequently with G-CSF [filgrastim, Amgen Inc] (10 ug/kg/day), or GM-CSF [sargramostim, Amgen Inc] (5 ug/kg/day) + G-CSF (10 ug/kg/day) and again subsequently with the same regimen. Cohort 2 consisted of 32 donors mobilized with a CXCR4 antagonist [plerixafor, Genzyme Corp or pol6326, Polyphor Ltd] alone and subsequently remobilized with G-CSF (10 ug/kg/day). Following mobilization, all donors underwent leukapheresis (~20L processed) for 1–4 days. Four additional donors who underwent remobilization were excluded from the analysis as they did not fit within one of the two cohorts identified. CD34+ cell determination was performed by flow cytometry per the International Society of Hematotherapy and Graft Engineering (ISHAGE) guidelines.5
STATISTICS
As the number of leukapheresis days varied, mobilization and collection were compared only at the first day of leukapheresis for each mobilization. Spearman correlations were performed to analyze the relationship between peripheral blood (PB) CD34+/uL level; the number of CD34+ cells collected per kg (recipient weight); and the number of CD34+ cells per L of leukapheresis collected during initial mobilization (MOB1) and remobilization (MOB2); and the interval (days) between MOB1 and MOB2. One-way ANOVA with repeated measures analyses were performed to determine the relationship of PB CD34+/uL, CD34+/kg and CD34+/L during MOB1 and MOB2. An inter-cohort analysis was performed to determine the relationship of PB CD34+/uL, CD34+/kg and CD34+/L during MOB1 of Cohort 1 and MOB2 of Cohort 2 using Mann-Whitney tests. The level of significance for all tests was set at p < 0.05.
RESULTS
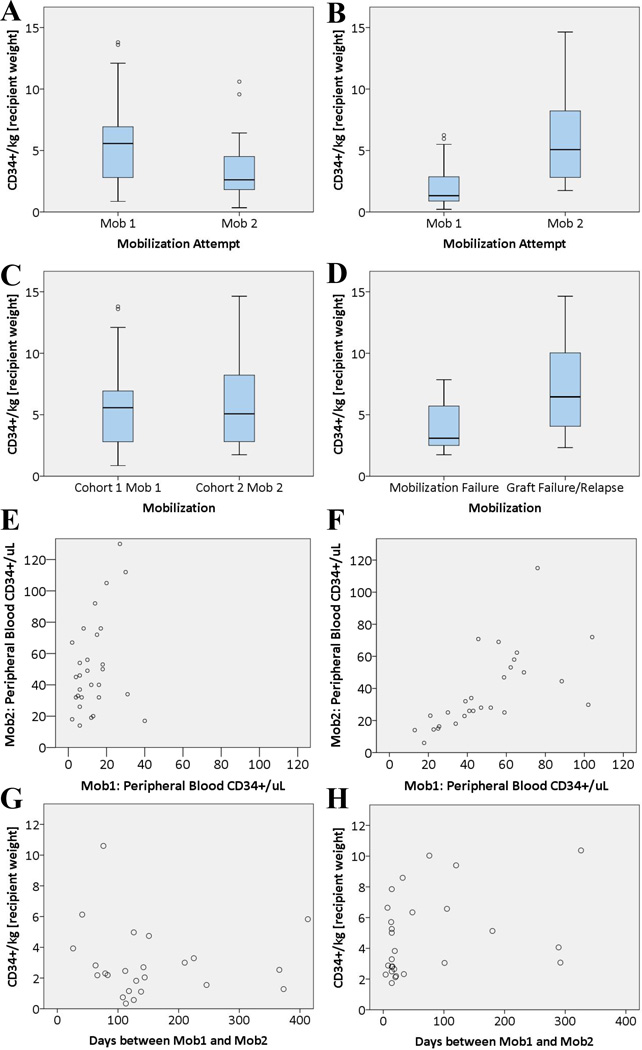
In Cohort 1 [Cytokines (n=30)], the median age was 49 years (range 18–75) and 15 were male. The median number of days between MOB1 and MOB2 was 140 (range 26–2238). All donors were remobilized due to graft failure or relapse of the allo-HSCT recipient’s disease. The mean PB CD34+/uL at MOB1 was 69 compared to 37 at MOB2 (p = 0.029); the mean CD34+/kg collected on the first day of leukapheresis was 5.6x106 compared to 3.3x106 (p = 0.002) [Figure 1A]; and the mean CD34+/L was 24.0x106 compared to 17.6x106 (p = 0.023). PB CD34+/uL, CD34+/kg and CD34+/L at MOB1 were all positively correlated with the measurement at MOB2 [Figure 1E]. The interval between MOB1 and MOB2 did not correlate with any of the MOB2 variables [Figure 1G]. Results from the analysis are summarized in Table 1.
Figure 1.
[A] Remobilization yield was significantly lower than initial mobilization yield for patients mobilized and remobilized with cytokines Cohort 1 (p = 0.002). [B] Remobilization yield was significantly higher than initial mobilization yield for patients initially mobilized with a CXCR4 antagonist and remobilized with cytokines Cohort 2 (p < 0.001). [C] Remobilization yield of Cohort 2 was not significantly different from initial mobilization yield in Cohort 1 (p = 0.838). [D] In Cohort 2, initial mobilization failure predicted lower remobilization yield compared to patients who had successful initial mobilization (p = 0.028). Initial mobilization and remobilization results were correlated in both Cohort 1 (p < 0.001) [E] and Cohort 2 (p = 0.013) [F]. There was no clear relationship between the interval from initial mobilization and remobilization yield in either Cohort 1 [G] or Cohort 2 [H]. Note: all results are from the first day of leukapheresis.
Table 1.
Results of Mobilization and Remobilization of Peripheral Blood Stem Cells in Healthy Donors
| Mobilized and Remobilized with Cytokines (n=30) | ||||
|---|---|---|---|---|
| MOB 1 | MOB 2 | One-way ANOVA | Spearman Correlation | |
| PB CD34/ul | 69 (13–417) | 37 (1–115) | F(1.0, 29.0) = 5.26, p = 0.029 | R = 0.615, p < 0.001 |
| CD34/kg (x106) | 5.6 (0.8–13.8) | 3.3 (0.3–10.6) | F(1.0, 29.0) = 11.77, p = 0.002 | R = 0.483, p = 0.007 |
| CD34/L (x106) | 24.0 (4.5–72.0) | 17.6 (2.8–41.3) | F(1.0, 29.0) = 5.74, p = 0.023 | R = 0.566, p < 0.001 |
| Mobilized with CXCR4 Antagonist; Remobilized with Cytokines (n=32) | ||||
| MOB 1 | MOB 2 | One-way ANOVA | Spearman Correlation | |
| PB CD34/ul | 15 (2–54) | 68 (14–358) | F(1.0, 31.0) = 23.16, p < 0.001 | R = 0.433, p = 0.013 |
| CD34/kg (x106) | 2.5 (0.2–19.7) | 7.1 (1.7–42.4) | F(1.0, 31.0) = 33.84, p < 0.001 | R = 0.769, p < 0.001 |
| CD34/L (x106) | 10.6 (1.4–67.1) | 30.1 (6.0–165.0) | F(1.0, 31.0) = 34.70, p < 0.001 | R = 0.774, p < 0.001 |
Note: all results are from the first day of leukapheresis.
In Cohort 2 [CXCR4 Antagonists (n=32)], the median age was 51 years (range 21–67) and 18 were male. The median number of days between MOB1 and MOB2 was 20 (range 4–1123). Eighteen donors were remobilized due to mobilization failure, while 14 were due to graft failure or relapse of the allo-HSCT recipient’s disease. The mean PB CD34+/uL at MOB1 was 15 compared to 68 at MOB2 (p < 0.001); the mean CD34+/kg collected on the first day of leukapheresis was 2.5x106 compared to 7.1x106 (p < 0.001) [Figure 1B]; and the mean CD34+/L collected was 10.6x106 compared to 30.1x106 (p < 0.001). PB CD34+/uL, CD34+/kg and CD34+/L at MOB1 were all positively correlated with the measurement at MOB2[Figure 1F]. The interval between MOB1 and MOB2 did not correlate with any of the MOB2 variables [Figure 1H]. The donors remobilized due to mobilization failure had reduced remobilization compared to those remobilized due to graft failure or relapse (p = 0.028) [Figure 1D]. Results from the analysis are summarized in Table 1.
In the inter-cohort analysis, the mean PB CD34+/uL of Cohort at 1 at MOB1 was 69 compared to 68 for Cohort 2 at MOB2 (p = 0.855); the mean CD34+/kg collected on the first day of leukapheresis was 5.6x106 compared to 7.1x106 (p = 0.838) [Figure 1C]; and the mean CD34+/L collected was 24.0x106 compared to 30.1x106 (p = 0.627).
DISCUSSION
Most of the data currently available on remobilization is from patients undergoing autologous hematopoietic stem cell transplantation. Early studies suggested that high dose G-CSF (32 mcg/kg) was the preferred remobilization regimen, but subsequent studies found that standard dose (10 mcg/kg) yielded similar rates of success6–7. Alternatives to remobilization, such as bone marrow collection, have been found to be inferior to remobilization8. The combination of other mobilization agents with G-CSF for remobilization has recently been an area of intense interest. In particular, the addition of the CXCR4 antagonist plerixafor has been found to greatly improve rates of successful remobilization9–11.
In the current study of healthy allogeneic transplant donors, we found that initial mobilization with cytokines was associated with reduced CD34+ mobilization and thus lower PBSC yields upon remobilization with the same regimen. This is consistent with observations by Chang et al who found poorer cell yields in 38 healthy donors undergoing remobilization compared to a cohort undergoing initial mobilization, and data from animal models4, 12.
In contrast, initial mobilization with a CXCR4 antagonist was not associated with reduced CD34+ mobilization or lower PBSC yields upon remobilization with G-CSF. These patients had similar yields on remobilization compared to patients undergoing primary mobilization with cytokines. It is important to note that mobilization with a CXCR4 antagonist alone is not common practice; typically CXCR4 antagonists are administered in addition to cytokines. All of the donors reported herein who received single-agent CXCR4 antagonist participated on clinical trials at our institution (clinicaltrials.gov NCT00241358, NCT00914849, NCT01158118, NCT01413568). There is sparse data regarding remobilization success following initial mobilization with cytokines and a CXCR4 antagonist.
Flomenberg, et al reported poor CD34+ remobilization with G-CSF in 4 autologous transplant patients who previously underwent successful mobilization with G-CSF + plerixafor13. This could reflect a decrease in efficacy after repeated exposure to G-CSF, the effect of chemotherapy exposure on stem cell reserve, or indicate these patients were innately poor mobilizers with G-CSF but the coadministration of plerixafor during the initial mobilization was able to overcome this. The current study implies that initial mobilization attempts with CXCR4 antagonists do not adversely affect later mobilization with cytokine regimens. However, failure to mobilize with CXCR4 antagonists did predict poor remobilization with cytokines. Moreover, there was a significant correlation in PBSC yield during initial mobilization and remobilization among individual patients, implying that some patients are simply poor mobilizers, regardless of the regimen used or prior mobilizations.
The optimal interval between mobilization and remobilization has not been well characterized. A prior study in healthy donors by Chang et al noted a moderate positive correlation between stem cell yield and time elapsed between first and second mobilization4. Consequently, they suggested that allowing nine or more months to elapse between mobilizations may lead to improved stem cell yields. In the autologous setting, Pusic et al found that patients undergoing remobilization in the lowest interquartile (<16 days) mobilized significantly better than patients in the highest interquartile (>25 days)9. In contrast, we found that remobilization success was independent of time between collections; this was true of both patients initially mobilized with cytokines and CXCR4 antagonists. While more research is needed, it is reasonable to conclude that there is no indication to delay remobilization of healthy donors in hope of improving stem cell yields. However, given substantial differences between healthy donors and patients undergoing autologous transplantation, generalizing these findings to the autologous setting contrary to Pusic et al would be unwarranted.
The limitations of this study include the heterogeneity within the study cohorts, and the lack of patients failing initial mobilization in the cohort 1. While we grouped the cohorts by broad categories (i.e. cytokines vs CXCR4 antagonists), each cohort contained multiple regimens, which theoretically could impact the outcomes of interest. Due to the limited sample size, it was not possible to perform sub-group analyses. Lastly, no patients in the cytokine-mobilization cohort had failed initial mobilization, while 56.3% of patients (18/32) in the CXCR4 antagonist cohort had. In the context of this study, the impact of this difference is difficult to discern but should not be discounted given the positive correlation between PBSC yield from initial mobilization and remobilization we observed.
In conclusion, remobilization with G-CSF or GM-CSF and G-CSF after initial successful mobilization with the same regimen resulted in decreased mobilization. Repeat mobilization with G-CSF after initial mobilization with a CXCR4 antagonist did not result in decreased mobilization. Initial mobilization success correlated with remobilization success; however, the interval between collections did not. This study suggests that poor remobilization yields may be due to decreased efficacy of cytokines after repeat exposure. The underlying mechanism of these findings remains unclear and further studies are needed.
Acknowledgments
Michael Slade’s research is supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, sub-award TL1TR000449, from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Funding Support: None.
Abbreviations
- PBSCs
peripheral blood stem cells
- allo-HSCT
allogeneic hematopoietic stem cell transplantation
- ISHAGE
International Society of Hematotherapy and Graft Engineering
- PB
peripheral blood
Footnotes
Conflicts of Interest: Dr. DiPersio has received research support from AnorMED Inc, the developer of plerixafor, and Genzyme Corporation who subsequently acquired them. The author authors of no relevant disclosures.
REFERENCES
- 1.Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR Summary Slides. Available at: http://www.cibmtr.org.
- 2.Holig K, Kramer M, Kroschinsky F, et al. Safety and efficacy of hematopoietic stem cell collection from mobilized peripheral blood in unrelated volunteers, 12 years of single-center experience in 3928 donors. Blood. 2009;114(18):3757–3763. doi: 10.1182/blood-2009-04-218651. [DOI] [PubMed] [Google Scholar]
- 3.Devine SM, Vij R, Rettig M, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112(4):990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 4.Chang YJ, Huo MR, Zhao XY, et al. [Determination of optimal time to second allogeneic peripheral blood stem cell harvest from healthy donors] Zhonghua Xue Ye Xue Za Zhi. 2009;30(8):509–513. [PubMed] [Google Scholar]
- 5.Sutherland DR, Anderson L, Keeney M, et al. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Socitery of Hematohterpy and Engineering. J Hematother. 1996;5(3):213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 6.Gazitt Y, Freytes CO, Callander N, et al. Successful PBSC mobilization with high-dose G-CSF for patients failing a first round of mobilization. J Hematother. 1999;8(2):173–183. doi: 10.1089/106161299320442. [DOI] [PubMed] [Google Scholar]
- 7.Boeve S, Strupeck J, Creech S, et al. Analysis of remobilization success in patients undergoing autologous stem cell transplants who fail an initial mobilization: risk factors, cytokine use and cost. Bone Marrow Transplant. 2004;33(10):997–1003. doi: 10.1038/sj.bmt.1704486. [DOI] [PubMed] [Google Scholar]
- 8.Goterris R, Hernandez-Boluda JC, Teruel A, et al. Impact of different strategies of second-line stem cell harvest on the outcome of autologous transplantation in poor peripheral blood stem cell mobilizers. Bone Marrow Transplant. 2005;36(10):847–853. doi: 10.1038/sj.bmt.1705147. [DOI] [PubMed] [Google Scholar]
- 9.Pusic I, Jiang SY, Landua S, et al. Impact of Mobilization and Remobilization Strategies on Achieving Sufficient Stem Cell Yields for Autologous Transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045–1056. doi: 10.1016/j.bbmt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Yuan S, Nademanee A, Krishnan A, et al. Second time a charm? Remobilization of peripheral blood stem cells with plerixafor in patients who previously mobilized poorly despite using plerixafor as a salvage agent. Transfusion. 2013;53(12):3244–3250. doi: 10.1111/trf.12198. [DOI] [PubMed] [Google Scholar]
- 11.Calandra G, McCarty J, McGuirk J, et al. AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin’s lymphoma, Hodgkin's disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplant. 2008;41(4):331–338. doi: 10.1038/sj.bmt.1705908. [DOI] [PubMed] [Google Scholar]
- 12.Shi PA, Pomper GJ, Metzger ME, et al. Assessment of rapid remobilization intervals with G-CSF and SCF in murine and rhesus macaque models. Transfusion. 2001;41(11):1438–1444. doi: 10.1046/j.1537-2995.2001.41111438.x. [DOI] [PubMed] [Google Scholar]
- 13.Flomenberg N, Devine SM, DiPersio JF, et al. The use of AMD31000 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106(5):1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]