Abstract
Background
Previous event-related potentials (ERPs) studies of response inhibition in children with fetal alcohol spectrum disorders (FASD) have used a visual Go/NoGo task to study the impact of prenatal alcohol exposure on response inhibition. No studies exist using auditory versions of the task; thus, it is unclear how the deficits observed in visual tasks translate into the auditory domain.
Methods
This study examined ERPs using an auditory Go/NoGo paradigm in a sample of 35 school-age children—18 with heavy prenatal alcohol exposure and 17 normally developing controls.
Results
Alcohol-exposed children performed as well as controls in terms of inhibiting their responses; however, their reaction times were significantly slower under the Go condition. As in the ERP visual Go/NoGo task previously administered to these children, group differences were seen in early perceptual processing, specifically related to stimulus discrimination, with a decrease in P2 amplitude in the alcohol-exposed group. Alcohol-exposed children also exhibited greater N2 amplitude in the NoGo compared to the Go condition, suggesting a group difference in the neural substrates underlying conflict monitoring. The alcohol-exposed group demonstrated longer latency P3 with reduced amplitude, suggesting poorer allocation of attention. The alcohol-exposed group also exhibited a late positive component (LPC) similar to the one observed in the previous visual ERP study. This LPC may indicate compensatory neurophysiological function related to resetting of attentional control networks in preparation for the next trial. None of the ERP outcomes in this study were related to potential confounders which included cognitive and socioeconomic measures as well as ADHD diagnosis.
Conclusions
The observed ERP group differences point to elements of perceptual and attentional processing likely to be involved in the performance-deficits often observed in children with FASD. We also observed changes in ERPs related to conflict-monitoring/response-inhibition, highlighting fetal alcohol-related effects on how the brain responds when there is need to identify and respond to environmental cues by switching away from a prepotent motor response to an inhibited state.
Keywords: Event-related potentials (ERP), response inhibition, fetal alcohol syndrome, fetal alcohol spectrum disorders, prenatal alcohol exposure, Go/NoGo
1. Introduction
Fetal alcohol spectrum disorders (FASD) is an umbrella term used to describe the range of disorders that have been linked to maternal alcohol consumption during pregnancy (Chudley et al., 2005; Hoyme et al., 2005). Symptoms include, but are not limited to facial dysmorphologies, pre- and postnatal growth retardation as well as cognitive and behavioural deficits (Fryer et al., 2012; Jones and Smith, 1973). Heavy alcohol exposure during pregnancy, with or without overt craniofacial dysmorphologies, is also associated with a wide range of neuropsychological deficits (Mattson and Riley, 1998).
Response inhibition, which is often assessed using a Go/NoGo paradigm, refers to the ability to inhibit/suppress a prepotent motor response: a motor response (button press) is cued during a large proportion of the trials (Go condition) interspersed with a limited number of cues to withhold the response (NoGo condition). Behavioural performance is assessed in terms of the proportion of correctly withheld responses. Although high-functioning children with fetal alcohol syndrome (FAS) performed as well as controls on a simple Go/NoGo task (Kodituwakku et al., 1995), previous research has linked prenatal alcohol exposure to impairment of response inhibition on more challenging measures of inhibitory control, such as the Stroop Color-Word Test (Connor et al., 2000; Mattson et al., 1999). Prenatal alcohol-related changes in patterns of regional brain activation have also been found during a response inhibition n-back task using functional magnetic resonance imaging (Diwadkar et al., 2013).
An event-related potential (ERP) involves a spatially widespread phase reset of the on-going neocortical activity, which is time-locked to stimulus onset (Luck, 2012). The phase reset is driven by afferent fibers projecting from the thalamus up to primary sensory areas within the cerebral cortex (primary visual, auditory, and tactile areas). By averaging repeated trials, the phase locked oscillatory activity is enhanced, while the on-going “noise” is attenuated. The resultant waveform/feature is known as an evoked-potential (EP) or an event-related potential (Pfurtscheller and Lopes da Silva, 1999; Pfurtscheller, 2006). EPs and ERPs are useful indices used to assess neurophysiological function in healthy and clinical populations.
Two ERP components elicited under the Go/NoGo experimental paradigm are the N2 and P3. The N2 is linked to response inhibition and conflict monitoring; the P3, to attention and working memory processes. The P3 has been shown to be a sensitive biomarker for alcoholism (Bauer, 2001). When the Go/NoGo paradigm is administered to normally developing samples, the amplitude of the N2 is larger for the NoGo compared to the Go condition, and greater amplitude is observed for the P3 in the NoGo compared to the Go condition (Davis et al., 2003; Johnstone et al., 2005). Kaneko et al. (1996), using an auditory oddball-plus-noise task, reported that decreased P3 amplitude discriminated participants with developmental disorders (FAS and Down syndrome) from each other: the FAS group showed decreased amplitude over the frontal regions. The FAS group also differentiated itself from controls in that they showed longer P3 latencies over the parietal regions—this task is similar in terms of stimulus presentation to our auditory Go/NoGo task.
Burden et al. (2009) conducted the only previous study to examine ERPs using a Go/NoGo paradigm in children with heavy prenatal alcohol exposure. This study, conducted in Cape Town, South Africa, used a simple visual Go/NoGo task. Despite similar behavioural performance during the task, several differences were found in the ERPs. When participants were required to inhibit a response, the control group showed an increased amplitude N2 within the NoGo compared with the Go condition, a response that is observed in normally developing samples across both the visual and auditory stimulus modalities (Davis et al., 2003; Johnstone et al., 2005). By contrast, the alcohol-exposed group exhibited a reduction in N2 amplitude, which has been shown to be indicative impairment in conflict monitoring processes.
In the Burden et al. (2009) visual ERP study, under the Go condition, the controls exhibited a larger P2 component than in the NoGo condition, an amplitude difference that was not observed within the alcohol-exposed group. In addition, the alcohol-exposed group exhibited longer P2 latencies in both conditions. Using the same paradigm, these P2 latency effects were seen in a less heavily alcohol-exposed group of Inuit children in Arctic Quebec (Burden et al., 2011). Based on the limited data available using the Go/NoGo task within normally developing samples, the P2 amplitude effects seen within the control group on the visual task (larger P2 in the Go compared to the NoGo conditions) are also seen in an auditory version of the task (Johnstone et al., 2005). Thus, the increase in amplitude of the P2 in the Go condition within normally developing samples occurs independent of stimulus modality. The P2 component has been linked to early perceptual processes involving initial interactions between bottom-up perceptual and top-down executive control driven processes.
In Burden et al. (2009), a late positive component (LPC) was observed under the NoGo condition within the alcohol-exposed group. This response was attributed to “increased cognitive effort”, but is more likely to signify resetting of frontal-parietal attentional networks in preparation for the next trial; namely, regions of the anterior cingulate cortex, the dorsolateral prefrontal cortex and the parietal lobules that need to be integrated to implement attentional control (Wang et al., 2010).
We conducted the first study to examine effects of prenatal alcohol exposure on response inhibition during a Go/NoGo task in the auditory domain. The aim of this study was to examine the degree to which the ERP waveforms, elicited during aurally-cued response inhibition, resemble those elicited in Burden et al.’s (2009) visual ERP study. Based on previous studies in normally developing and alcohol-exposed samples, we hypothesised that: (1) increased P2 amplitude would be observed in the Go compared to the NoGo-condition within the control group, but not the alcohol-exposed group; (2) slower P2 latency would be observed in the alcohol-exposed compared to the control group across both conditions; (3) the NoGo condition would elicit greater N2 amplitude when compared with the Go condition for the control group, but not in the alcohol-exposed group; (4) increased P3 amplitude would be observed in the NoGo compared to the Go condition within the control group but not in the alcohol-exposure group; (5) an LPC would be manifest in the NoGo condition only for the alcohol-exposed group.
2. Methods
2.1 Subjects
Thirty-five Cape Coloured children from our Cape Town FASD cohort (Jacobson et al., 2011) took part in this study. Fourteen of these children had previously participated in the Burden et al. (2009) visual ERP study. The Cape Coloured are descendants from a mixed ancestral lineage including European colonists, Malaysian slaves, Khoisan aboriginals, and the African Nguni ethnic group. Poor socioeconomic circumstances and historical practices of compensating farm labour in part with wine have contributed to a tradition of heavy recreational weekend binge drinking in a portion of this population. This pattern of consumption persists amongst pregnant women within the population. As a result, the Cape Coloured community experience one of the highest levels of FASD in the world (May et al., 2007, 2013). Twenty-three children participating in this study were the older siblings of participants in our Cape Town Longitudinal Cohort study (Jacobson et al., 2008). The others were identified by screening all of the 8–12-year-old children from an elementary school in a rural section of Cape Town where there is a very high incidence of alcohol abuse among local farm workers (Jacobson et al., 2011; Meintjes et al., 2010). All of the children were right-handed as they were originally recruited to participate in an FASD neuroimaging study (Dodge et al., 2009; Meintjes et al., 2010).
Each mother was interviewed in her primary language (Afrikaans or English) regarding her alcohol consumption during pregnancy using a timeline follow-back approach (Jacobson et al., 2002). Volume was recorded for each type of beverage consumed on a daily basis, converted to absolute alcohol (AA) using multipliers proposed by Bowman et al. (1975), and averaged to provide a summary measure of alcohol consumption during pregnancy (AA/day). Two groups were recruited: (1) heavy drinkers who consumed at least 14 standard drinks per week (1.0 oz AA/day) or engaged in binge drinking (5 or more drinks/occasion); (2) controls who abstained or drank no more than minimally during pregnancy. Number of cigarettes smoked on a daily basis during pregnancy was also recorded, as was use of illicit drugs. Mothers were also interviewed regarding their education and occupational status and that of their spouse/partner; these data were used to assess socioeconomic status (SES) on the Hollingshead Four Factor Index of Social Status (Hollingshead, 2011).
2.2 IQ, FASD and Attention Deficit Hyperactivity Disorder (ADHD) Diagnoses
IQ Assessment
The children were administered 7 of the 10 subtests from the Wechsler Intelligence Scale for Children, 3rd edition (WISC-III)—Similarities, Arithmetic, Digit Span, Symbol Search, Coding, Block Design, and Picture Completion—and Matrix Reasoning from the WISC-IV at the first 11-year visit. IQ was estimated from these subtests using Sattler’s (1992) formula for computing Short Form IQ; validity coefficients for Sattler Short Form IQ based on 5 or more subtests consistently exceed r = 0.90. The cognitive and Go/NoGo assessments were administered by an advanced graduate research assistant (MP) who was blind regarding FASD diagnosis and prenatal alcohol history.
FASD Diagnosis
The children were independently examined by two U.S. expert dysmorphologists (H.E. Hoyme, M.D., and, L.K. Robinson, M.D.) using a standard protocol based on the IOM-revised criteria (Hoyme et al. 2005) at a dysmorphology clinic held in 2005 (Jacobson et al., 2011). Children who could not attend the clinic were examined by a South African expert FASD dysmorphologist (N Khaole, M.D.), whose assessments of key anomalies were highly correlated with those provided by HEH and LKR (Jacobson et al. 2011) and whose diagnoses were all confirmed by examinations conducted in follow-up clinics we held with the same dysmorphologists in 2009 and with HEH in 2013. HEH, LKR, SWJ, JLJ, and CDM subsequently conducted case conferences to reach consensus regarding which chldren met criteria for FAS or PfAS diagnoses.
ADHD Diagnosis
CDM administered the Schedule for Affective Disorders and Schizophrenia for School Aged Children (K-SADS) to each mother to assess ADHD, and each child’s classroom teacher completed the Disruptive Behavior Disorders (DBD) Scale (Pelham et al. 1992). Participants were assigned a DSM-IV ADHD diagnosis following criteria developed in consultation with Joel Nigg, Ph.D., an expert in ADHD research. An ADHD classification was assigned if (a) at least 6 of the 9 inattention and/or 6 of the 9 hyperactivity /impulsivity symptoms were endorsed (“pretty much” or “very much true”) by one or more informants, and (b) some impairment was reported by 7 years of age and in two or more settings.
In addition to the above-mentioned scales, blood samples were obtained from the children at 10.4 years (SD=1.2) and checked for lead concentrations (pb; ug/dl).
2.3 Go/NoGo Task
In the Go/NoGo task, the Go condition was represented by a 1000Hz tone; the NoGo, by a 2000Hz tone. In the Go condition the child was instructed to press a button with his/her right index finger, whereas in the NoGo condition s/he was told to inhibit the motor response. The inter-trial stimulus interval (ITI) was constant throughout the experiment. In each trial the auditory stimulus was presented for 500ms, followed by 4000ms of silence. The Go and NoGo conditions were randomised with the only constraint being that there were 35 Go-trials and 15 NoGo-trials equating to 50 trials per block. Seven 50-trial blocks were presented to each participant. Each block was interleaved with a 1-minute rest period. Subjects were instructed to execute a response as quickly and accurately as possible.
2.4 Procedure
All protocols were approved by Wayne State University and the University of Cape Town institutional review boards. The mothers provided written informed consent, and the children provided oral assent. The mothers and children were given breakfast, a snack, and lunch and at the end of the visit, each mother received a small monetary compensation for her participation; the child was given a small gift.
The children were seated in-front of the computer screen in a dimly lit room approximately 70 cm away from the monitor and were fitted with a 128-channel Electrical Geodesics net (Electrial Geodesics Inc., Eugene, OR), which had been soaked in an electrolytic solution. The net was adjusted to fit the participant, and an impedance check was conducted. The testing took approximately 20 minutes. The presentation of the Go/NoGo task was controlled by E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA).
2.5 Data Acquisition
EEG signals were acquired using NetStation 4, a Netamps amplifier, and a high-resolution 128-channel Geodesic net (EGI System 200 Technical Manual). Impedance was kept below 50kΩ according to the manufacturers specifications. Data were recorded using a sampling rate of 250Hz and a 0.1Hz – 80Hz on-line band-pass filter. A vertex reference (central midline) was used during data acquisition. Data were stored and analysed off-line.
2.6 ERP Analysis
All processing was performed within the MATLAB computing environment using custom scripts built upon functions provided by the EEGLAB toolbox (Delrome and Makieg, 2004). Data were imported into MATLAB and filtered using a band-pass filter from 0.5Hz – 30Hz. Recordings were scanned for gross-movement artefacts, and those portions of data were excluded from the data set. The 1-minute rest periods between blocks were also excluded from further analysis. Noisy channels were identified, removed, and replaced by an average of surrounding electrodes. In addition, the perimeter ring of electrodes was removed from further analysis due to gross-movement artefact. Data were then segmented relative to stimulus onset: 200ms prior to stimulus onset and 1500ms post-stimulus onset. Segments had their mean baseline amplitude removed from the entire segment to yield a set of baseline corrected trials. The segments were then scanned for behavioural response data using custom software written within the MATLAB computing environment. The software classified and stored information associated with the following responses: correct Go, correct NoGo, incorrect Go, and incorrect NoGo. The response time data were used for further analysis of participant behaviour under the experimental conditions. The data tables containing the behavioural response data formed the basis for automated segment rejection whereby error-trials (incorrect Go’s and incorrect NoGo’s) were removed from further analysis—we did not look at error-related ERPs as the number of incorrect trials in this relatively simple task was too few to perform a meaningful analysis.
The segmented, error response-free-data were submitted to a blind source separation (BSS) algorithm in order to identify and remove unwanted artefacts from the data: eye-movement, eye-blinks, and electrical activity arising from muscles of the jaw and face (Delrome and Makieg, 2004). Artefact free data were then re-referenced to the average of all electrodes (an average reference). Any segment with extreme values exceeding 50μV was excluded from further analysis as such extreme values are not cortical in origin and introduce bias into the ERP waveforms. The remaining segments were averaged by condition for each participant as well as for each group. Automated peak/trough detection within predefined latency windows was used to identify peaks/troughs in the average ERP waveforms for each participant. We were interested in the P2, N2, P3, and LPC as these were the ERP features that were analysed in the visual study—“P” indicating a maximal amplitude peak in the waveform, while “N” is a minimal amplitude trough; a series of these peaks/troughs constitute and ERP waveform. The algorithm searched for maximal-peaks and minimal-troughs across all recording sites within each participant’s data using the following latency windows: P2 (150–300 ms); N2 (175–300 ms); P3 (350–500 ms); and LPC (500–1050 ms). The data segments were then visually checked to ensure that the algorithm identified ERP peaks and troughs, rather than boundary markers at the start and end of each latency window. Initially, we used latency windows defined in the visual study as a guideline, adjusting these incrementally to accommodate all observed peaks and troughs within the artefact free segments.
To calculate the averages of amplitude and latency measures for the ERP waveform features, the identified recording site (electrode) with the maximal-peak/minimal-trough as well as the corresponding peak/trough from adjacent surroundings electrodes were included in the averages; these averages were computed for each participant within the sample. For each peak or trough within the ERP waveform, maximal amplitude and latency effects were consistently observed within the same scalp region across participants; for example, the N2 was maximal over the frontal regions for all participants and thus the amplitude and latency averages were formed by taking into account a subset of electrodes from within the frontal region (F7-FC5-F3-AF3-FC1-Fz-FC2-AF4-F4-FC6-F8). This was true for each of the ERP features we analysed in terms of the respective regional origins. Amplitude and latency averages were stored for statistical analysis. For the LPC, an average was taken in a predefined latency window since clear peaks were not well-defined.
2.7 Behavioural Analysis
Response times were checked for outliers using an iterative Grubbs algorithm—extreme studentized deviate method (Snedecor and Cochran, 1989). In its simplest form, the Grubbs test is calculated as the difference between the sample mean and the most extreme value in the data divided by the sample standard deviation (z-test). The resultant statistic is then assessed for significance, and if significant, the data point is excluded from analysis. Extreme outliers in the current experiment appear to represent trials in which there were lapses in attention leading to extreme response times; their exclusion resulted in normally distributed variables which otherwise deviated from normality. The corresponding EEG data segments were excluded from further analysis, this resulted in 5% of trials being rejected.
2.8 Statistical Analysis
Statistical analyses were conducted in MATLAB using the Statistics toolbox and customised scripts. Independent samples t-tests were used to compare response times in the Go Condition between the alcohol-exposed and control groups. For the ERP data, separate repeated measures ANOVAs were used to test for significant differences in amplitude and latency for each ERP component (P2, N2, P3 and LPC), with two experimental groups (alcohol-exposed vs. controls) under two experimental conditions (Go vs. NoGo).
Nine covariates were collected and are listed in Table 1. Eight of the nine were considered as potential confounders of any observed effects of prenatal alcohol exposure on the ERP component amplitudes and latencies: (1) child’s sex, (2) age at EEG recording, (3) postnatal lead exposure, (4) ADHD, (5) mother’s age at delivery, (6) socioeconomic status, (7) maternal education, and (8) cigarettes smoked per day during pregnancy. Because none of the covariates were even weakly related to any of the ERP components (all p’s>0.20), there was no need to adjust statistically for potential confounders in any of the analyses. We also examined the relation of IQ to each of the ERP component amplitues and latencies. Although child IQ was related to prenatal alcohol exposure, it was not related to any of these ERP outcomes (all p’s>0.15) and, therefore, cannot be a mediator of any observed effects of alcohol exposure.
Table 1.
Sample characteristics
|
Alcohol-Exposed (n
= 13) M (SE) |
Controls (n
= 12) M (SE) |
F or χ2 | |
|---|---|---|---|
| Child characteristics | |||
| Sex | M – 7; F – 6 | M – 7; F – 5 | <1 |
| Age at testing (years) | 11.8 (1.2) | 12.1 (1.1) | <1 |
| Postnatal lead (pb; ug/dl) | 5.2 (2.3) | 7.2 (3.4) | 4.43* |
| WISC IQa | 66.5 (11.7) | 77.8 (11.6) | 5.85* |
| ADHD (yes/no)b | 3 / 10 | 1 / 11 | 1.01 |
| Maternal characteristics | |||
| Age at delivery (years) | 25.3 (5.2) | 26.7 (6.5) | 1.28 |
| Socioeconomic statusc | 17.4 (6.7) | 19.3 (9.0) | <1 |
| Education (years) | 7. 6(2.8) | 8.2 (1.5) | <1 |
| Cigarettes during pregnancy (per day) | 10.8 (8.5) | 9.7 (6.0) | <1 |
| Maternal alcohol consumption | |||
| AA/day (oz) | 2.9 (2.7) | 0.1 (0.02) | 6.40* |
| AA/drinking day (oz) | 6.3 (2.8) | 0.3 (0.5) | 32.73*** |
| Frequency (days/week) | 2.8 (2.1) | 0.07 (0.14) | 16.39** |
Note. AA refers to absolute alcohol; 1 oz AA ≈ 2 standard drinks.
Wechsler Intelligence Scale for Children.
Attention deficit/hyperactivity disorder.
Hollingshead (2011) Four Factor Index of Social Status Scale.
p <0. 05;
p < 0.01;
p < 0.0001;
3. Results
3.1 Exclusions and Sample Characteristics
Accuracy and reaction time on the Go trials were used to identify subjects with performance that did not align with the rest of the sample. Reaction time paradigms characteristically will include some data validation processes and filtering of results prior to final analyses as reaction times are affected by lapses in attention, compulsive responding, or simply button pressing unrelated to the goal of the task. Thus, it was important to address this to avoid a single outlying subject biasing the results. 10 children (4 exposed and 6 controls) had accuracy scores < 80% and/or reaction times < 420ms or > 750 ms; their EEG data were also contaminated with heavy movement-related artifact. These children’s data were, therefore, excluded from further analysis, leaving a sample of 25 subjects: 13 alcohol-exposed and 12 controls. We compared the excluded participants with those retained in the sample to see if they were more likely to have low IQ scores or meet criteria for ADHD. There were no IQ differences, t(33)=0.13, ns, and the excluded group did not have a higher proportion of children with ADHD: 2 of 10 (20.0%) of the excluded children compared to 4 of the 25 (16%) retained were diagnosed with ADHD, χ2(1)= 0.08, ns. In addition, there was no bias created by excluding participants from either of the groups in our analysis, χ2(1)= 0.412, ns—proportionally a similar number of participants were excluded from both groups.
Table 1 provides background information from the children whose data were included in the analysis. As planned, the mothers of the children in the alcohol-exposed group drank substantially higher quantities of alcohol during pregnancy, averaging 12 drinks per occasion. Ten (83.3%) of the control mothers abstained during pregnancy; the other two drank minimal amounts: one reported drinking 2 drinks on 1 occasion, and the other, 2 drinks/occasion monthly. In addition to prenatal alcohol exposure, the control children had slightly higher lead body burdens (pb; ug/dl) than the alcohol-exposed group in this sample. Among the 13 children in the exposed group, 3 (23.1%) met criteria for fetal alcohol syndrome (FAS) and 2 (15.4%) for PFAS; 8 (61.5%) were heavily exposed but nonsyndromal (HE). As expected, children in the alcohol-exposed group had lower IQ scores than the nonexposed. By contrast, ADHD diagnosis did not differ between the groups.
3.2 Behavioural Go/NoGo Data
Overall, subjects were more accurate when executing a Go response than a NoGo response F(1,23)=7.89, p<0.01. No group differences were seen in terms of accuracy of Go and NoGo responses (Table 2); both groups performed well on the task. Response times were significantly longer in the alcohol-exposed group.
Table 2.
Behavioural data for the control and alcohol-exposed group
| Group | Statistics | ||
|---|---|---|---|
|
| |||
| Alcohol-Exposed (n = 13) | Controls (n = 12) | t-tests | |
| Accuracy Correct Go (%) | 97.2 (0.0) | 98.1 (0.0) | <1 |
| Accuracy Correct NoGo (%) | 90.5 (0.1) | 89.2 (0.1) | <1 |
| RT Correct Go (ms) | 601.5 (77.1) | 533.7 (133.4) | 16.29* |
p<0.0001
3.3 Event-related Potentials
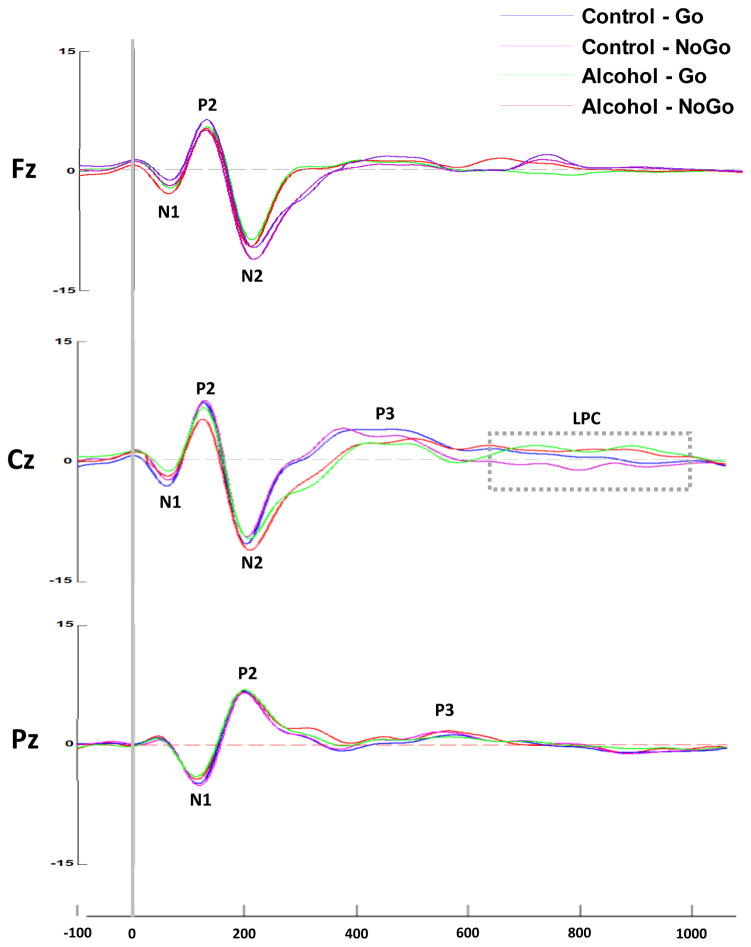
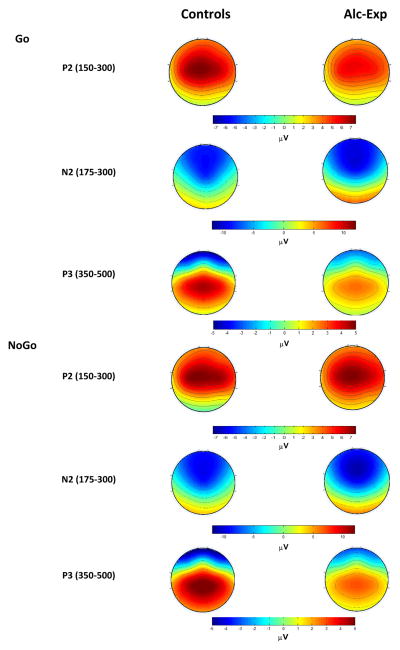
The midline (Fz, Cz, Pz) ERPs for each group under each condition are presented in Figure 1, followed by the topographical distribution of the P2, N2 and P3 component amplitudes in Figure 2. The ERP component latency and amplitude data are presented in Table 3.
Figure 1.
Grand average ERPs for each group under each condition along the midline (Frontal-Fz, Central-Cz, Parietal-Pz). Controls Go-condition light blue, controls NoGo-condition purple, alcohol exposed Go-condition green, and alcohol exposed NoGo-condition red. The P1-N1-P2-N2-P3-LPC waveform complex is visible along the mid-line electrodes.
Figure 2.
Topographical maps illustrating the N2 and the P3 ERP components. Colors on heatmaps represent amplitude changes relative to pre-stimulus baseline; the values are in microvolts (μV).
Table 3.
ERP quantification: mean and standard errors
| Alcohol-Exposed | Control | |||
|---|---|---|---|---|
| Go | NoGo | Go | NoGo | |
| P2 | ||||
| Latency (ms) | 148.0 (12.1) | 161.3 (10.5) | 150.4 (25.2) | 159.5 (13.2) |
| Amplitude (uV) | 5.1 (3.1) | 6.1 (4.0) | 7.1(3.2) | 7.2(3.5) |
| N2 | ||||
| Latency (ms) | 217.8 (23.4) | 215.3 (22.5) | 208.2 (23.9) | 211.1 (18.2) |
| Amplitude (uV) | −10.8 (3.4) | −11.8 (2.2) | −9.1 (3.2) | −11.0 (5.2) |
| P3 | ||||
| Latency (ms) | 430.5 (30.5) | 418.5 (17.8) | 387.7 (32.4) | 368.1 (13.2) |
| Amplitude (uV) | 3.0 (3.6) | 3.1 (4.2) | 4.3 (3.2) | 4.8 (4.5) |
| LPC | ||||
| Amplitude (uV) | 2.00 (0.9) | 2.2 (0.4) | 1.0 (0.8) | 0.43 (0.5) |
There was a main effect for condition for P2 latency, with longer latencies in the NoGo than the Go condition for both groups, F (1,23)=3.86, p<0.05. There was also a main effect for exposure group, with controls having significantly greater amplitude across both conditions, F(1,23)=4.01, p<0.05. No significant interaction effects were observed. There was a main effect for exposure group for N2 latency, with the alcohol-exposed children having longer latencies than the controls, F(1,23)=3.99, p<0.05. There was a significant condition x exposure group interaction for N2 amplitude, F(1,23)=5.12, p<0.05. In the control group, the N-2 component was of a greater amplitude in the NoGo-condition (M=−11.00, SE=5.2) compared to the Go-condition (M=−9.08, SE=3.2, p < 0.01). This difference was not seen in for the exposed group: NoGo-condition (M=−11.82, SE=2.23); the Go-condition (M=−10.75, SE=3.4). There was also a significant group x condition interaction for P3 latency, F(1,23)=9.86, p<0.01. In the exposed group, P3 latency was slower in the Go-condition (M=430, SE=30.5) than in the NoGo-condition (M=418, SE=17.8, p<0.01) but no latency difference was seen in the control group. In addition, there was a signficant group x condition effect for P3 amplitude, F(1,23)=7.16, p<0.01. In the control group, P3 amplitude was larger in the Go-condition (M=4.25, SE=3.2) than in the No-condition (M=4.75, SE=4.5, p<0.05) but no difference was seen in the FASD group. There was a main effect for exposure group for LPC amplitude, F(1,23)=5.98, p<0.05, with the alcohol-exposed group exhibiting an LPC over the central region in both conditions; this effect was not seen in the control group.
Five ERP measures were affected by maternal alcohol consumption: P2 amplitude, P3 latency, P3 amplitude, N2 latency, and the LPC amplitude. None of the covariates reported in Table 1 were related to any of the ERP components at p<0.20.
4. Discussion
The children with prenatal alcohol exposure whose ERP data were examined in this study performed as accurately as the healthy controls, indicating competent performance on this relatively simple auditory Go/NoGo response inhibition task. In contrast to the visual domain, in which no exposure group differences were seen on reaction time (Burden et al., 2009, 2011), reaction time in the auditory domain was significantly slower in the alcohol-exposed compared to the control group. This finding is consistent with previous research showing that infants and children diagnosed with an FASD demonstrate slower processing speed (e.g., Jacobson et al., 1993, 1994; Coles et al., 2002; Burden et al., 2005).
Despite similar performance accuracy on this task, the alcohol-exposed and control groups differed in latencies and amplitudes on several ERP components. As with the visual Go/NoGo paradigm, group differences were seen in early perceptual processing related to stimulus discrimination: lower P2 amplitude in the alcohol-exposed compared to the control group. However, in our study, these differences were seen as a difference across groups and not a group x condition effect—as in Burden et al., 2009—wherein an enhanced P2 was only observed in the control group within the Go condition (Go>NoGo). P2 latency was observed as a significant main effect across conditions in this study: NoGo were slower versus Go. The P2 component is believed to reflect discrimination between stimuli through perceptual facilitation, i.e. differentiating stimuli based on meaning as opposed to the conditions they represent (‘X’ vs all consonants other than ‘X’ or 1000Hz vs 2000Hz sinusoidal tone). This component may have been affected by functional differences between groups within higher brain regions or by delivery of degraded representations of sensory inputs to higher brain regions in the alcohol-exposed group. Data from animal studies demonstrate dysfunction in auditory and visual pre-cortical pathways that is attributable to ethanol exposure (Church et al., 1996; Pettigrew and Hutchinson, 2008; Rössig et al., 1994; Scher et al., 1998). Prenatal alcohol exposure has also been linked to outer-ear disorders as late as early adulthood (Church and Gerkin, 1988). Using magnetoencephalography (MEG), Stephen et al. (2012) reported a functional deficit in younger preschool-aged children with FASD, i.e. a sluggish response from within the primary auditory regions in response to a repetitive tone. Stephen et al.’s observations were attributed to changes within peripheral auditory pathways. In our study, no main effect of alcohol-exposure was observed on P2 latency, the first major component downstream from the auditory cortices; the data from animal models of ethanol exposure indicate group differences in early auditory responses decrease as a function of cortical maturation—our participants were older than those of Stephen et al. So, while Stephen et al highlight speed of nerve conduction at the periphery as an important biomarker for FASD at younger ages, we argue that these deficits translate, at a later stage of development, into issues around the quality of mental of representations, i.e. the delivery of degraded representations to higher brain regions for perceptual processing. Degraded representations may also account, in part, for the observed slower reaction times, which may be attribu0 to the increased time needed to execute compensatory top-down processing to aid in perceptual discrimination between simple stimuli.
The P2 differences across modalities may also be attributable to differences in experimental design: the visual task of Burden et al. (2009, 2011) had multiple Go stimuli (any consonant other than ‘X’), while the auditory task used a single tone at a set frequency (1000Hz). Differences in stimulus-response sets maintained in working memory—particularly related to memory load—lead to a different allocation of processing resources during task performance over-and-above modality-specific effects. Top-down mediated effects have been shown to modulate early ERP components in the visual and auditory domains (Alain et al., 2001; Gazzaley et al, 2005). Thus, the effects of top-down modulations arising for stimulus-response set complexity and associated differential allocation of processing resources might also modulate early components, leading to differences in ERP responses across tasks.
In-line with our hypothesis and the finding in the Burden et al. (2009) visual Go/NoGo study, N2 amplitude was greater in the NoGo compared to the Go condition for the control group but not for the alcohol-exposed group. In addition, alcohol-exposed participants showed significantly longer latencies under the NoGo condition. The N2 effects related to alcohol exposure may reflect compensatory neurophysiological mechanisms—due to reduced cellular populations in key cortical regions—that drive performance in this paradigm, or dysregulation of neurotransmitter systems as demonstrated in some rodent models of response inhibition (Archibald et al., 2001; Eagle and Baunez, 2010; Sowell et al., 2001; 2002).
Alterations in the N2 may also reflect effects of prenatal alcohol exposure on connectivity among the pre-supplementary motor area (preSMA), anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC) and projections into the striatum. The N2 source generators have been shown to be frontally-oriented, both in human studies and animal models. Given that the DLPFC, a key area in decision-making processes, is known to be involved in Go/NoGo performance, the N2 component may reflect decision-making prompted by conflict between the on-line behavioural response (the prepotent motor response) and the demands of the environment (the cue to inhibit). Studies with the macaca fuscata by Sasaki et al. (1989) have demonstrated a functional homologue of the human N2 ERP component, elicited in frontal regions on inhibition of a prepotent motor-response. Electrical stimulation of the area in subsequent testing had the effect of cancelling the pre-potent motor-response, while electrical stimulation of surrounding tissue had the effect of delaying the response. The interaction of the preSMA with frontally-oriented regions is key in conflict monitoring in humans during motor task performance (Chikazoe, 2010; Simmonds, Pekar, and Mostofsky, 2008). Thus, changes in neurophysiological function in frontally-oriented processes may explain the changes in the N2 component observed in the heavy-exposed group.
In contrast to the visual ERP studies, in which both the exposure and control groups exhibited the increased P3 amplitude in the NoGo condition that is characteristic of normally developing samples, in the auditory task only the alcohol-exposed subjects exhibited longer latency and reduced amplitude of the P3 ERP component. The P3 is elicited in tasks involving working memory and attention allocation, the latter of which is crucial to response inhibition. Kaneko et al. (1996) also observed a reduced P3 in an auditory odd-ball paradigm in alcohol-exposed children relative to their Down syndrome group. Thus, contrasting our data with Burden et al. (2009) suggests that an alcohol-related deficit in attention allocation may influence response inhibition in the auditory but not in the visual domain—a modality specific effect.
The LPC that was observed in the Burden et al. (2009) visual study under the NoGo condition within the alcohol-exposed group was also observed in the current study. However, it was elicited within the alcohol-exposed group under both the Go and NoGo conditions in this auditory study and had a different scalp topography, i.e. midline as opposed to left-hemisphere. Burden et al. interpreted the LPC as reflecting an increase in the need for cognitive effort to inhibit against prepotency within the heavy-exposed group. However, that explanation would not account for why the LPC is seen in both the Go and NoGo conditions in our auditory ERP task. Instead, we suggest that the increased LPC may be a manifestation of compensatory neurophysiological function related to resetting of attentional control networks—after a behavioural response—in preparation for the next trial. The only remaining demand placed upon participants later on in the trial is to prepare for the next stimulus; this invariably involves reorientation of attentional resources.
The manifestation of the LPC only under the NoGo condition during the visual ERP task may be related to the different size stimulus-response sets, which in that task consist of a variable number of Go stimuli. The different stimulus-response sets will place different demands on higher cognitive functioning during task performance, both in terms of memory load and attentional resources. The LPC may be manifest only under the NoGo condition in the visual task due to increased attentional demands required to differentiate a number of Go stimuli relative to the NoGo cue. Relatively similar attention demands are required in the auditory task across conditions owing to one-on-one stimulus-response-correspondence under each condition, which may account for the elicitation of the LPC in the heavy-exposed group under both conditions.
Attentional control is believed to involve functional integration of frontal and parietal regions including the ACC, DLPFC, and regions around the inferior parietal sulcus (Wang et al., 2010). The ACC is thought to be involved in sudden shifts in attention, while the DLPFC is known to be more involved in slower shifts (Onton et al., 2005). Parietal hypoplasias as well as structural changes in the superior parietal lobules have been reported in FASD (Meintjes et al., 2014; Sowell et al., 2002). In heavily alcohol-exposed subjects, malformations of the orbital extending into the ventrolateral prefrontal regions have been observed (Sowell et al., 2002). Due to morphological changes in cerebral structure, the LPC may reflect changes in functional and anatomical connectivity between the frontal and parietal regions, which is believed to be important in integrating information while attempting to achieve attentional control. If so, compensatory activations in the alcohol-exposed children might well alter the location and orientation of underlying dipole sources within the cerebral cortex, leading to the manifestation of an unconventional scalp potential in the presence of an FASD. The extended reaction times observed in this study for the alcohol-exposed group under the Go condition are also consistent with less efficient function in relation to the resetting of attentional networks.
Our ERP findings point to fetal alcohol-related effects in how the brain responds when there is a need to switch away from a prepotent response to an inhibited state. As in all human correlational studies, there are always unmeasured potential confounders. However, the observed effects remained significant after adjustment for the most likely potential confounding variables, including the Hollingshead Scale, which we have found in this and other cohorts from this community to be valid in relation to a large number of maternal and child measures, including maternal (r=0.47; p=0.003), and child IQ (r=0.43, p<0.001), at levels similar to those found in the U.S. These findings are, for the most part, also consistent across both the visual and auditory domains. Our data indicate fetal alcohol-related deficits in attention processing in response to stimulus onset and resetting of the attentional network in preparation for the next trial as well as impairment of conflict monitoring. It is important to note that these differences in processing were not due to differences in IQ or in ADHD diagnosis. Although the alcohol-exposed and control groups did not differ in behavioural performance on this simple response inhibition task, the differences in neural processing revealed by the ERP analysis point to specific deficits in attentional processing. These deficits are likely to contribute to the poorer performance commonly seen in more complex response-inhibition tasks in children with FASD.
Acknowledgments
The research was supported by a Fogarty International Research Collaboration Award (R03TW007030); a Focus Area grant (FA2005040800024) from the National Research Foundation of South Africa; the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa; a Children’s Bridge grant from the Office of the President of Wayne State University; and seed money grant from the University of Cape Town and the Lycaki-Young Fund from the State of Michigan. The FAS clinical assessments were supported by grants awarded in conjunction with the National Institute on Alcohol Abuse and Alcoholism Collaborative Initiative on Fetal Alcohol Spectrum Disorder (U01AA014790 to S.W. Jacobson and U24AA014815 to K. L. Jones). We thank H. Eugene Hoyme, M.D., Luther K. Robinson, M.D., and Nathaniel Khaole, M.D., who performed the dysmorphology assessments as well as Joel Nigg, Ph.D., who consulted on the ADHD diagnoses. We also thank Maggie September, who coordinated participant recruitment and scheduling; Mariska Pinaar, M.A., who administered the ERP assessments; and Neil Dodge, Ph.D., and Renee Sun, for their work on the alcohol and drug exposure and background data. The authors declare that there are no conflicts of interest.
Contributor Information
Matthew M. Gerhold, MRC/UCT Medical Imaging Research Unit, Department of Human Biology, University of Cape Town
Sandra W. Jacobson, Department of Psychiatry and Behavioural Neurosciences, Wayne State University School of Medicine and Departments of Human Biology and Psychiatry and Mental Health, University of Cape Town
Joseph L. Jacobson, Department of Psychiatry and Behavioural Neurosciences, Wayne State University School of Medicine and Departments of Human Biology and Psychiatry and Mental Health, University of Cape Town
Christopher D. Molteno, Department of Psychiatry and Mental Health, Faculty of Health Sciences, University of Cape Town
Ernesta M. Meintjes, MRC/UCT Medical Imaging Research Unit, Department of Human Biology, University of Cape Town
Colin M. Andrew, MRC/UCT Medical Imaging Research Unit, Department of Human Biology, University of Cape Town
References
- Alain C, Arnott SR, Picton TW. Bottom–up and top–down influences on auditory scene analysis: Evidence from event-related brain potentials. J Exp Psychol Hum Percept Perform. 2001;27:1072–1089. doi: 10.1037//0096-1523.27.5.1072. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Bauer LO. Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology. 2001;25:332–340. doi: 10.1016/S0893-133X(01)00236-6. [DOI] [PubMed] [Google Scholar]
- Bowman RS, Stein LI, Newton JR. Measurement and Interpretation of Drinking Behavior. J Stud Alcohol Drugs Suppl. 1975;36:1154. doi: 10.15288/jsa.1975.36.1154. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Jacobson JL. The relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res. 2005;29:1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Andrew C, Saint-Amour D, Meintjes EM, Molteno CD, Hoyme HE, Robinson LK, et al. The Effects of fetal alcohol syndrome on response execution and inhibition: An event-related potential study. Alcohol Clin Exp Res. 2009;33:1994–2004. doi: 10.1111/j.1530-0277.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Westerlund A, Muckle G, Dodge N, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. The effects of maternal binge drinking during pregnancy on neural correlates of response inhibition and memory in childhood. Alcohol Clin Exp Res. 2011;35:69–82. doi: 10.1111/j.1530-0277.2010.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Curr Opin Psychiatry. 2010;23:267–272. doi: 10.1097/YCO.0b013e3283387a9f. [DOI] [PubMed] [Google Scholar]
- Chudley AE, Conry J, Cook JL, Loock C, Rosales Y, LeBlanc N. Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. Can Med Assoc J. 2005;172:S1–21. doi: 10.1503/cmaj.1040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church MW, Gerkin KP. Hearing disorders in children with fetal alcohol syndrome: Findings from case reports. Paediatrics. 1988;82:147–154. [PubMed] [Google Scholar]
- Church MW, Abel EL, Kaltenbach JA, Overbeck GW. Effects of prenatal alcohol exposure and aging on auditory function in the rat: Preliminary results. Alcohol Clin Exp Res. 1996;20:172–179. doi: 10.1111/j.1530-0277.1996.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol Clin Exp Res. 2002;26:263–271. [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol. 2000;18:331–354. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- Davis EP, Bruce J, Snyder K, Nelson CA. The X-trials: neural correlates of an inhibitory control task in children and adults. J Cogn Neurosci. 2003;15:432–443. doi: 10.1162/089892903321593144. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diwadkar V, Meintjes EM, Goradia D, Dodge NC, Molteno CD, Jacobson SW, Jacobson JL. Differences in cortico-striatal-cerebellar activations during working memory in syndromal and non-syndromal children with prenatal alcohol exposure. Hum Brain Mapp. 2013;34:1931–1945. doi: 10.1002/hbm.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge NC, Jacobson JL, Molteno CD, Meintjes EM, Bangalore S, Diwadkar V, Hoyme EH, Robinson LK, Khaole N, Avison MJ, Jacobson SW. Prenatal alcohol exposure and interhemispheric transfer of tactile information: Detroit and Cape Town findings. Alcohol Clin Exp Res. 2009;33:628–1637. doi: 10.1111/j.1530-0277.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Baunez C. Is there an inhibitory-response control system in the rat? Evidence from anatomical and pharmacological studies of behavioural inhibition. Neurosci Biobehav Rev. 2010;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGI System 200 Technical Manual [Electronic version] Electrical Geodesics Inc; USA: 2001. [Google Scholar]
- Fryer SL, Mattson SN, Jernigan TL, Archibald SL, Jones KL, Riley EP. Caudate volume predicts neurocognitive performance in youth with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2012;36:1932–1941. doi: 10.1111/j.1530-0277.2012.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D’Esposito M. Top-down Enhancement and Suppression of the Magnitude and Speed of Neural Activity. J Cogn Neurosci. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. YJS. 2011;8:21–51. [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW. Prenatal alcohol exposure and infant information processing ability. Child Develop. 1993;64:1706–1721. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ. Effects of fetal alcohol exposure on infant reaction time. Alcohol Clin Exp Res. 1994;18:1125–1132. doi: 10.1111/j.1530-0277.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Didge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired delay and trace eyeblink conditioning in shool-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2011;35:250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SJ, Pleffer CB, Barry RJ, Clarke AR, Smith JL. Development of inhibitory processing during the Go/NoGo task: a behavioral and event-related potential study of children and adults. Int J Psychophysiol. 2005;19:11–23. [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kaneko WM, Ehlers CL, Phillips EL, Riley EP. Auditory event-related potentials in fetal alcohol syndrome and down’s syndrome children. Alcohol Clin Exp Res. 1996;20:35–42. doi: 10.1111/j.1530-0277.1996.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcohol Clin Exp Res. 1995;19:1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ. The Oxford Handbook of Event-Related Potential Components. Oxford University Press; USA: 2012. [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NCO, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen D. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37:818–830. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes EM, Jacobson JL, Molteno CD, Gatenby JC, Warton C, Cannistraci CJ, Hoyme HE, Robinson LK, Khaole N, Gore J, Jacobson SW. An fMRI study of number processing in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2010;34:1450–1464. doi: 10.1111/j.1530-0277.2010.01230.x. [DOI] [PubMed] [Google Scholar]
- Meintjes EM, Narr KL, van der Kouwe AJW, Molteno CD, Pirnia T, Gutman B, Woods RP, Thompson PM, Jacobson JL, Jacobson SW. A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. NeuroImage Clin. 2014;5:152–160. doi: 10.1016/j.nicl.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27:341–56. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Evans SW, Gnagy EM, Greenslade KE. Teacher ratings of DSM–III–R symptoms for the disruptive behavior disorders: prevalence, factor analyses, and conditional probabilities in a special education sample. School Psychol Rev. 1992;21:285–299. [Google Scholar]
- Pfurtscheller G. The cortical activation model (CAM) In: Neuper C, Klimesch W, editors. Progress in Brain Research. Vol. 159. Elsevier; 2006. pp. 19–27. Retrieved from http://www.sciencedirect.com/science/article/pii/S0079612306590028. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, da Silva FHL. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/NoGo tasks demonstrating the fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew AG, Hutchinson J. Effects of alcohol on functional development of the auditory pathway in the brainstem of infants and chick embryos. Ciba Foun Symp. 2008;105:26–46. doi: 10.1002/9780470720868.ch3. [DOI] [PubMed] [Google Scholar]
- Rössig C, Wässer S, Oppermann P. Audiologic manifestations in fetal alcohol syndrome assessed by brainstem auditory-evoked potentials. Neuropaediatrics. 1994;25:245–249. doi: 10.1055/s-2008-1073029. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Gemba H, Tsujimoto T. Supression of visually initiated hand movement by stimulation of the prefrontal cortex in the monkey. Brain Res. 1989;495:100–107. doi: 10.1016/0006-8993(89)91222-5. [DOI] [PubMed] [Google Scholar]
- Scher MS, Richardson GA, Robles N, Geva D, Goldschmidt L, Dahl RE, Sclabassi RJ, et al. Effects of prenatal substance exposure: altered maturation of visual evoked potentials. Pediatr Neurol. 1998;18:236–243. doi: 10.1016/s0887-8994(97)00217-8. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User’s Guide. Psychology Software Tools, Inc; Pittsburgh: 2002. [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. 8. Iowa State University Press; Iowa: 1989. [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001;12:515–23. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002;12:856–65. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Kodituwakku PW, Kodituwakku EL, Romero L, Peters AM, Sharadamma NM, Caprihan A, Coffman BA. Delays in auditory processing identified in preschool children with FASD. Alcohol Clin Exp Res. 2012;36:1720–1727. doi: 10.1111/j.1530-0277.2012.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu X, Guise KG, Knight RT, Ghajar J, Fan J. Effective connectivity of the fronto-parietal network during attention control. J Cogn Neurosci. 2010;22:543–553. doi: 10.1162/jocn.2009.21210. [DOI] [PubMed] [Google Scholar]