Abstract
The widely-distributed North American species Peromyscus leucopus and P. maniculatus of cricetine rodents are, between them, important natural reservoirs for several zoonotic diseases of humans: Lyme disease, human granulocytic anaplasmosis, babesiosis, erhlichiosis, hard tickborne relapsing fever, Powassan virus encephalitis, hantavirus pulmonary syndrome, and plague. While these infections are frequently disabling and sometimes fatal for humans, the peromyscines display little pathology and apparently suffer few consequences, even when prevalence of persistent infection in a population is high. While these Peromyscus spp. are unable to clear some of the infections, they appear to have partial resistance, which limits the burden of the pathogen. In addition, they display traits of infection tolerance, which reduces the damage of the infection. Research on these complementary resistance and tolerance phenomena in Peromyscus has relevance both for disease control measures targeting natural reservoirs and for understanding the mechanisms of the comparatively greater sickness of many humans with these and other infections.
Keywords: vectorborne disease, Borrelia, zoonoses, spirochete, ecoimmunology, ecological immunology
1. Introduction
In September 2014 the United States’ Institute of Medicine held a forum on “Vector-Borne Diseases: Exploring the Environment, Ecological, and Health Connections” [1]. The workshop highlighted the importance of animal reservoirs and arthropod vectors of pathogens for public health. Changes in climate, landscapes, transportation, and global commerce have brought the animal reservoirs of infectious diseases in ever closer contact with human populations. The majority of the emerging infectious diseases on the National Institute of Allergy and Infectious Diseases’ high priority list, are zoonoses [2], which are infections acquired from a vertebrate reservoir, either by direct exposure to, or through an arthropod vector, and not another human. The reservoirs are usually mammals, and the vectors are usually either ticks or insects. For the following five high-priority zoonoses a Peromyscus species, the white-footed mouse, P. leucopus, is a major reservoir: Lyme disease, babesiosis, Powassan virus encephalitis, human granulocytic anaplasmosis, and hard tick-borne relapsing fever (Table 1). In North America another species, P. maniculatus, the deer mouse, is an important reservoir for a hantavirus [3] and Yersinia pestis, the agent of plague [4].
Table 1.
Zoonotic pathogens, their human diseases, vectors, and Peromyscus spp. Hosts
| Microbe/parasite | Human disease | Vector/intermediate host | P. leucopus [references] | P. maniculatus [references] |
|---|---|---|---|---|
| Borrelia burgdorferi | Lyme disease (Lyme borreliosis) | Ixodes scapularis, I. pacificus ticks | + a [21, 22, 77] |
+ [28, 47] |
| Borrelia miyamotoi | Hard tick-borne relapsing fever | I. scapularis, I. pacificus | + [34, 78] |
? |
| Anaplasma phagocytophilum | Human granuclocytic anaplasmosis | I. scapularis, I. pacificus, I. spinipalpis | + [79, 80] |
+ [81] |
| Babesia microti | Babesiosis | I. scapularis | + [82, 83] |
– |
| Powassan/deer tick virus | Viral encephalitis | I. scapularis | + [84] |
+ [85] |
| Ehrlichia muris subsp. euclairensis | Ehrlichiosis | I. scapularis | + [9, 12] |
– |
| Sin Nombre-like hantaviruses | Hantavirus pulmonary syndrome | None | + [86] |
+ [87] |
| Yersinia pestis | Plague | Fleas | – | + [4, 88] |
| Bartonella spp. | Bartonellosis | Fleas | + [89] |
+ [54, 55] |
| Borrelia bissettii | None known | I. pacificus, I. spinipalpis | – | + [90, 91] |
| “Borrelia davisi” | None known | ? | + [92] |
? |
| Trypanosoma sp.a | None known | ? | + [58] |
+ [57] |
| Schistosomatium douthitti | None known | Snail | ? | + [56, 93] |
+, the listed Peromyscus species is a reservoir; –, not a reservoir; ?, reservoir status not known
Exclusive of agents of Chagas disease (T. cruzi) and African trypanosomiasis (T. brucei)
The familiar names of Peromyscus spp. include “mouse”, but the genus belongs to the taxon Cricetidae, together with hamsters, voles, and wood rats [5], not to the more distant clade Muridae, which includes the house mouse Mus musculus and, the black rat, Rattus rattus, by wide margins the most frequently used animal models in biomedical research. Peromyscines are distributed across North America and in a variety of habitats [6]. The Peromyscus species in an area is usually its most abundant mammal [7].
In North America the distribution of the P. leucopus largely overlaps with that of the blacklegged tick, Ixodes scapularis, which is commonly called the “deer tick”, because of the association of its adult stage with white-tailed deer [8]. I. scapularis is the vector for the agents of Lyme disease (Borrelia burgdorferi), babesiosis (Babesia microti), Powassan virus encephalitis, human granulocytic anaplasmosis (Anaplasma phagocytophilum), and hard tick-borne relapsing fever (Borrelia miyamotoi) in their various distributions in the northeastern and north-central United States and adjoining regions of Canada. Two newly-described tick-borne agents, Erhlichia muris subsp. auclairensis and Borrelia mayonii are also carried by I. scapularis [9–11], and the ehrlichiosis agent uses P. leucopus as reservoir [12]. In the far-western U.S., the vector for B. burgdorferi, A. phagocytophilum, B. miyamotoi, and another Babesia species is the western blacklegged tick, I. pacificus.
Table 1 lists these tick-borne infections, as well as those transmitted by insects, like fleas, through an intermediate host, or by direct exposure to the rodents or their excrement, as for hantaviruses. The table also includes two other borrelias, a trypanosome species, and a schistosome that infect P. leucopus and/or P. maniculatus in nature, but for which there are no documented human infections. This list is undoubtedly incomplete. A variety of metazoan parasites, including cestodes (tapeworms), trematodes (flukes), nematodes (round worms), and other exoparasites, besides ticks and fleas, including mites, chiggers, lice, and botfly larvae are known to infect or infest Peromyscus spp. [13]. Botfly parasitism of Peromyscus spp. is not a subject of this article but has been studied by others, e.g. [14].
Here, I review natural and experimental infections of Peromyscus spp. with vector-borne zoonotic pathogens, and highlight the comparative health of these animals during infections with microbes that otherwise cause extensive disease in humans. The emphasis is on B. burgdorferi and P. leucopus, because that host-pathogen relationship has been most studied. But investigations of the Peromyscus model are far out-numbered by literature reports on experiments with B. burgdorferi in the house mouse, which is not the natural reservoir of a Lyme disease agent, nor any of the other infections listed in the table. So, there is scope in the review for considering other infections in the white-footed mouse and the deer mouse. There is also literature on Peromyscus spp. and hantaviruses, a pathogen-host relationship that is subject of the article by Vandegrift et al. of this issue [3].
2. Resistance to and tolerance of infection in disease reservoirs
Four key terms for what follows are “reservoir”, “competence”, “resistance”, and “tolerance”. These terms, each of which has multiple meanings in wider usage, are defined here for the present context. A reservoir in a life-cycle of a vector-borne virus, bacterium, or parasite is a definitive host, upon which the pathogen depends for its long-term maintainence in an environment and which also serves as a blood-meal host for the arthropod vector for the pathogen. For human zoonoses, the reservoir is almost always a vertebrate, usually a mammal. The competence of a reservoir in the life cycle is the capacity of that host for transmission of the pathogen to another vertebrate host, either directly or through a vector. In the example of B. burgdorferi and its transmission by ticks, reservoir competence of the host mammal can be defined as the proportion of the nymphs bearing infectious levels of the microbe after having fed as larvae upon an infected animal. So, it is not just the capacity of the animal to become infected and to house the pathogen transiently or persistently. The reservoir would also need to be sufficiently infectious for the feeding tick for that tick to subsequently pass through to the next stage, e.g. from larva to nymph, and then retain enough the organism for the tick to transmit the microbe to another vertebrate host at its next blood meal, as a nymph in the example.
In a simple unrestrained system, which does not take into account about the host population’s aggregate fitness, a pathogen proliferates in the vertebrate to numbers high enough for successful transmission before it kills or otherwise disables the host. The resistance to the pathogen’s exploitation is the degree to which the host reduces the frequency of infection, increases the rate of pathogen clearance among the infected, or both. Resistance mechanisms act on the invading pathogen. When resistance measures fail or are only partially successful, a fall-back position for the host is to minimize the amount of damage or disease caused by the infection. The mechanisms serving this trait of tolerance reduce the net detrimental effects of the microbe. They act on the host and mitigate effects of the infection without altering its development [15]. (This is different from a definition in the field of immunity for “tolerance”, i.e. lack of responsiveness to an antigen.) The damage to be limited could be the consequence of unmoderated responses of the host, e.g. excessive inflammation, or of virulence properties of the microbe, e.g. a toxin.
For long-term sustainable maintenance of a tick-borne pathogen in an environment, a pathogen’s main reservoir presumably should remain reproductively fit—better to provide for a new generation of competent hosts–and active enough in its home range to be exposed to ticks bearing the pathogen. What this effectively means is usually a combination of resistance to and tolerance of infection in reservoir hosts of long-standing [16]. As for the implications of the tolerance phenomenon for human health, Ayres and Schneider put it this way, “What mechanisms keep us healthy while we are fighting infections?” [15]. Experimental and field studies of natural reservoirs of infection may provide insights not only about the pathogenesis of disease but processes that keep up us comparatively healthy in the face of infection.
3. B. burgdorferi infections of Peromyscus spp. in nature
B. burgdorferi’s life-cycle comprises its tick vector, small mammals and birds that serve as both reservoirs for the pathogen and blood-meal hosts for larval and nymphal stages, and a larger mammal, usually a deer, that provides the final blood meal of the adult female for reproduction [17, 18]. This life cycle in North America has existed since at least the Last Glacial Maximum [19, 20]. In most regions of the northeastern and north-central U.S., as well as adjoining regions of Canada, P. leucopus is usually the predominant one, in terms of its cumulative contribution to infection of larval I. scapularis ticks with B. burgdorferi [21, 22]. This status is attributable to both its reservoir competency for B. burgdorferi and its greater numbers over other small mammals in most endemic locations [23]. Figure 1 illustrates the white-footed mouse’s greater competency in comparison with some other mammals existing in the same environment. The figure shows the ranges for B. burgdorferi numbers in nymphal ticks that had been feeding as larvae on captured P. leucopus or different medium-sized mammals [24, 25].
Figure 1.
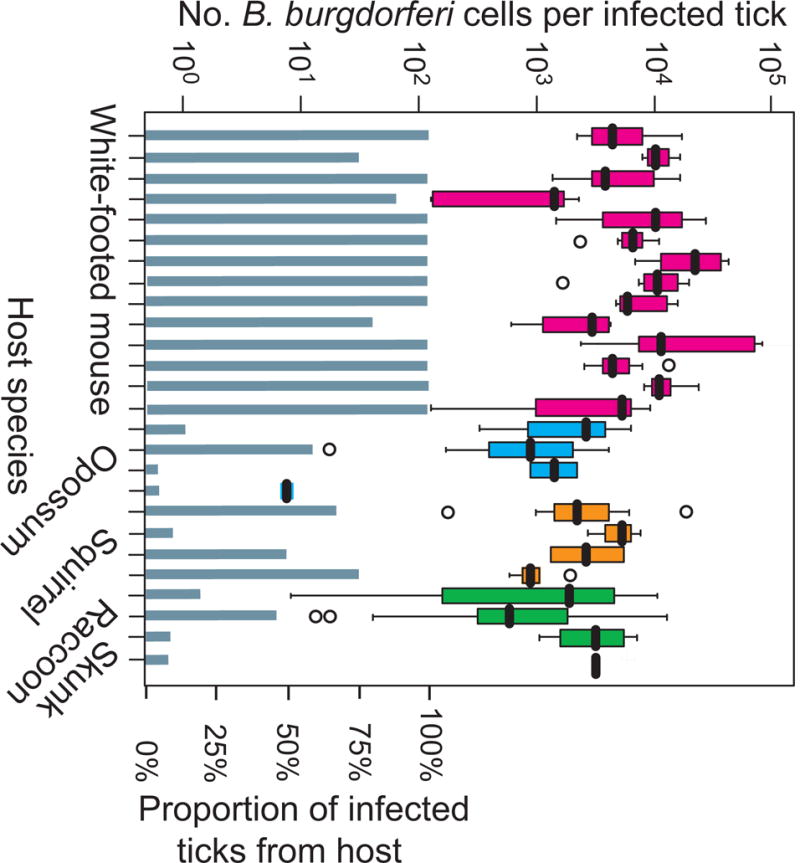
Greater reservoir competence of the white-footed mouse P. leucopus in comparison to medium-sized small mammals in a Connecticut forest.
Larval Ixodes scapularis ticks were collected after they had been found feeding on different mammalian species, which had been captured and temporarily held before release. The engorged larvae that fell off were allowed to molt to nymphs, and then the numbers of B. burgdorferi cells per individual tick were determined by quantitative PCR. For this analysis the results for 14 white-footed mice (red), 4 Virginia opossum (royal blue), 4 gray squirrel (orange), 3 common raccoon (green), and 1 striped skunk (black) are shown. Box-whisker plots summarize the distributions of bacterial counts per individual tick, with the values indicated on the left y-axis. Each horizontal box indicates the first and third quartiles, and the black horizontal line inside the box is the median. The 1.5× interquartile range is indicated by the whiskers bisecting the box, and a value outside this range is indicated by an open circle. The lower half of the graph shows the proportions (right y-axis) of the collected ticks from each animal that were infected with B. burgdorferi. Methods and data are from the studies of references 25 and 94.
In the northeastern and north-central U.S. white-footed mice become infected during the Spring and Summer, as ticks which had become infected as larvae feed again as nymphs on naïve animals (reviewed in [26]). Since the majority of P. leucopus born in a calendar year do not survive to the following Spring, there are few immune animals in the population as it expands with the new year. When antibody-mediated immunity is elicited, it is strain-specific, leaving an animal at risk of infection by other strains [26], as tick infestations continue throughout the season. In areas as small as a few hectares, there may be 10–15 different strains of B. burgdorferi present in the ticks [27]. Although white-tailed deer, as providers of the last blood meal for adult female ticks, are important for reproduction of I. scapularis, deer are not competent as reservoirs and, as is true for humans, are dead-ends for the pathogen.
(P. maniculatus in the laboratory is also a competent reservoir for B. burgdorferi [28, 29]. But with the exception of a few areas, such as Ontario, Canada, where both P. leucopus and P. maniculatus exist and contribute to B. burgdorferi’s maintenance [30], it has been difficult to assess their relative competencies under natural conditions.)
In a study at a forested field site in Connecticut, a New England state, wild P. leucopus, were captured, tagged, released, and re-captured [31]. The prevalence of B. burgdorferi in nymphal I. scapularis ticks ranged between 25–50% at this location. The incidence of infection was 0.2 infections per mouse each week during the period of tick activity in this study. About 10% of the mice had B. burgdorferi bacteria circulating in their blood, as determined by PCR, at the time of capture. Prevalence of antibodies to B. burgdorferi increased with the age of the animal and, overall, from 57% in late May-early June to 95% by the end of August (Figure 2). Most of the mice that seroconverted between first and second captures had antibody to the strain-specific OspC proteins, which is a commonly recognized antigen among patients with Lyme disease [32].
Figure 2.
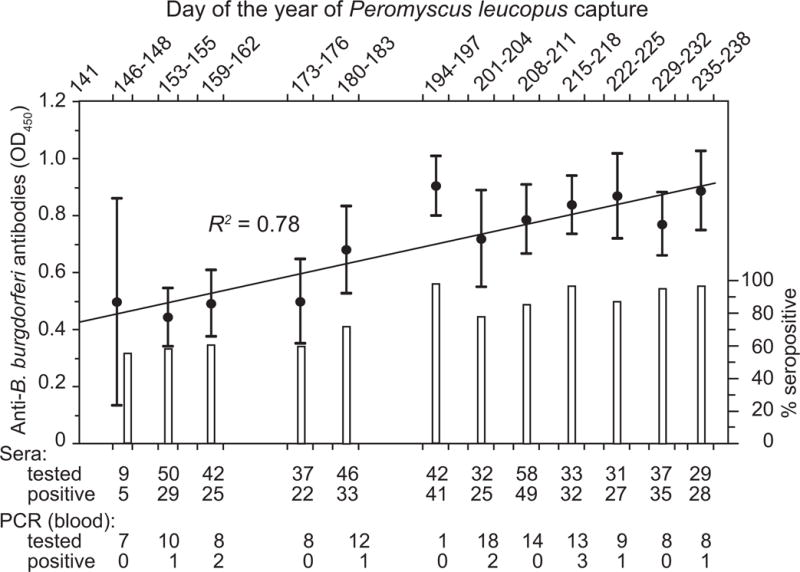
Highly prevalent Borrelia burgdorferi infection of Peromyscus leucopus in a Connecticut forest.
Infection in capture-release rodents was monitored over a late Spring-Summer tick transmission season with enzyme-linked immunoassay (EIA) of serum samples for antibodies to B. burgdorferi and polymerase chain reaction (PCR) assay of whole blood samples for B. burgdorferi. The colorimetric EIA OD405 value intervals and the percentage of positive serum samples for a sampling period are shown on the left and the right y-axis, respectively. Below the graph are figures on the total number of blood samples subjected to EIA and PCR analysis, from different collection periods, as well as the number of samples that were found to be positive by each of these assays. The coefficient of determination (R2) value between the collection dates and the values by EIA is shown. Methods and data are from reference 31.
Additional sera collected from captured P. leucopus at the Connecticut site were compared with sera from Lyme disease patients for their antibody reactivities on an microarray of 1292 proteins representing the majority of open reading frames (ORF) of the genome of B. burgdorferi [33]. The naturally infected white-footed mice generally recognized as antigens the same subset of proteins as the infected humans. Of the 103 most commonly recognized ORFs with the human sera, 70 were also high-ranking immunogens for the naturally-infected P. leucopus. This included the OspC proteins, which elicit antibodies that protect M. musculus against re-infection with the same strain (reviewed in [32]). This was further evidence that white-footed mice are capable of responding to infection with a variety of antibodies.
In a subsequent investigation of samples collected from P. leucopus at the Connecticut site, both B. burgdorferi and B. miyamotoi were studied [34]. Of 556 blood specimens, 69 (12%) contained B. burgdorferi DNA and 36 (6%) had B. miyamotoi DNA at the time of the rodent’s capture. On average there were ~100 B. burgdorferi and ~1000 B. miyamotoi cells per milliliter of the blood of infected animals. A quarter of the bacteremic mice were still bacteremic with B. burgdorferi upon recapture a few weeks later. The prevalence of B. burgdorferi in ear biopsies from the captured white-footed mice was even higher: 65 (76%) of 86. The ear tissue tends to remain infected long after the bacteria have been cleared from the blood [35].
At a study site in Maryland, a mid-Atlantic state, captured and re-captured P. leucopus were studied for active infection with PCR and culture of ear tissue and for previous exposure by immunoassays for antibodies to B. burgdorferi [36]. The overall prevalence of B. burgdorferi in the biopsies of the captured rodents was ~30%, the prevalence rising with age of the animals. Of 77 animals who were infected on first capture, all remained infected on follow-up sampling an average of 160 days later. The authors reported “no measurable effect on the survival of infected mice.”
Voordouw et al. carried out a capture-mark-capture study over a 4 year period on an island off the Connecticut coast [37]. On the basis of cultures of ear biopsies, the prevalence of active B. burgdorferi infection in the captured white-footed mice at the different trapping sites on the island ranged from 40–70%. As found in other studies, the prevalence of infection increased with age. The authors modeled mouse survival as a function of B. burgdorferi prevalence. Overall, monthly survival rates for the 4 year duration ranged from 0.55 to 0.88. For this variable, stratified by month of the year, there was no difference between infected and non-infected animals. While individual survival is an important component of fitness, neither this study nor the other longitudinal field studies to date reported on the effects of B. burgdorferi infection on another aspect of fitness: reproduction.
4. B. burgdorferi infections of Peromyscus spp. in the laboratory
The first experimental animal model for the newly-discovered Lyme disease agent was the golden or Syrian hamster, Mesocricetus auratus, a cricetine rodent like Peromyscus [38]. The golden hamster may remain persistently infected after inoculation [39]. Histopathologic study of chronically infected animals showed mild follicular lymphoid hyperplasia in the spleen but no signs of inflamation of kidney, eye, liver, or heart [40].
Given the minimal pathology and disability in the hamster, investigators interested in disease models turned to the laboratory mouse, M. musculus, which not only manifested more pathology than hamsters but came in a variety of inbred strains (reviewed in [35]). Most of these studies with inbred mouse strains focused on pathogenesis and immune response to infection, so investigators often chose strains and experimental conditions that provided for the most noticeable pathologic changes in the mice. However, while inbred strains differed in the severity of pathologic changes in tissues like the joints and heart, there was not a strain that was free of inflammation [41].
Reports of experimental infections of a Peromyscus sp. generally confirm the conclusions from the field studies: white-footed mice are highly susceptible to being infected with B. burgdorferi. In response to that infection, they produce many kinds of antibodies, which is the arm of the immune system that is most important for controlling Borrelia infection [32]. Yet, in spite of that immune response, P. leucopus remain persistently infected, sufficiently so for transmission to ticks to be possible for weeks to months after infection’s onset. This stand-off between pathogen and host is not accompanied by much if any pathologic change or disability. The white-footed mouse has little or no defense against infection to begin with, unless it has become specifically immune to particular strain. But failing to eliminate it entirely, these rodents appear to limit the amount of growth of the organism, i.e. they are resistant to infection to some degree. At the same time, P. leucopus, to a greater extent than M. musculus, also limits or suppresses the amount of consequent damage, i.e. they are tolerant of the infection. What follows is a largely chronological summary of the studies that provided the evidence in support of this view.
Using both needle inoculation and tick bite routes, Schwan et al. infected laboratory-reared P. leucopus with B. burgdorferi and then examined the animals after 2 or 3 weeks [42]. Similarly to what has been observed with samples collected from the field, ~10% of the animals had B. burgdorferi in the blood at the time of sampling. Recovery of the organisms was highest from the bladders (94%), kidneys (75%), and spleens (61%) of the animals. Despite its presence in the kidney and bladder wall, the pathogen was not recovered in culture from the urine. In a study the subsequent year, Schwan et al. noted that P. leucopus developed persistent infections in spite of IgM and IgG antibody responses to several different antigens [43].
Wright and Nielson infected P. leucopus with B. burgdorferi by needle and tick transmission routes [44]. They observed the presence of the spirochetes in the kidney, liver, and spleen of the rodents, and the elicitation of anti-B. burgdorferi antibodies and their persistence in the blood over 4 months of observation. They reported that “regardless of the source of infection, no mice developed clinical signs or had any pathologic change resulting from infection.” There was also no evidence of an effect on reproduction or of transplacental transmission of B. burgdorferi in their limited study. Mather et al. also concluded that transplacental transmission of B. burgdorferi to the offspring of P. leucopus does not occur [45].
Moody et al. reported that adult mice who became infected from needle injections of large inocula showed no gross or microscopic lesions on pathological examination [46]. On the other hand, white-footed mice inoculated as infants became infected and also developed carditis and multifocal arthritis, similar to what was observed in adult M. musculus.
There have been few studies of P. maniculatus with experimental infections by B. burgdorferi, but in a small study Brown et al. found that while dusky-footed woodrats (Neotoma fuscipes) with infection had inflammatory infiltrates in the joints, muscles, and heart, the infected deer mice showed no pathologic changes [47]. Over an observation period of 40 days, the authors did not observe clinical signs of disease, such as reduced appetite, lethargy, lameness, or neurologic disorder, in the deer mice.
Other evidence about the duration of the transmissibility of B. burgdorferi from P. leucopus comes from a xenodiagnosis procedure, that is, the placement of unfed, uninfected larval ticks on the rodents and then assessing the proportion of the ticks on each animal that become infected. Linsday et al. reported that transmission to ticks continued out to at least 49 days after inoculation, albeit at a lower frequency than after 7 days [48]. The pathogen was isolated from tissues of most of the mice 2 months after inoculation.
The functional effects of tick-transmitted B. burgdorferi infection on P. leucopus were studied by Schwanz et al. [49]. Infection was confirmed by the serology. After 5 weeks, infected rodents did not significantly differ from uninfected controls in blood cell counts of erythrocytes, neutrophils, eosinophils, basophils, lymphocytes, or monocytes. By the parameter of wheel running behavior, there was no measureable effect of infection after 1, 2, 3, or 6 weeks. On the basis of these findings, the authors suggested that “infection with the spirochete B. burgdorferi has little impact on the field activity of white-footed mice.”
In our own studies of P. leucopus with laboratory infections, we confirmed the findings from sera from field studies of robust antibody responses to the pathogen. The immunodominant antigens for white-footed mice largely corresponded to those that elicited antibodies during Lyme disease [31]. The specificities of the anti-OspC antibody responses among sera from experimentally-infected P. leucopus were similar to those exhibited by antibodies of patients with Lyme disease [32]. White-footed mice infected with different strains of B. burgdorferi had quantitatively similar antibody responses against whole cells, as well as purified antigens, though some strains achieved higher densities in ear, joint, tail, and heart tissues than other strains [50].
The latter study also revealed differences of an order of magnitude or greater between the indivual outbred P. leucopus in their burdens of the bacteria [50]. Diversity in the capacities of the white-footed mice to limit pathogen burdens during infection was similarly noted in our study of experimental infections with the relapsing fever agent B. hermsii [51]. White-footed mice did not differ between sexes matched for age in any of the examined parameters, including change in weight over 3 weeks and burdens of bacteria in the blood or spleen. But among 30 adult male P. leucopus, there was a wide variation between animals in the counts of spirochetes in blood and spleen and in their spleen masses after 7 days of infection. There was also no discernible association between spirochete burdens in blood or spleen and either pathology in the liver or change in weight.
Figure 3 shows the result of a similar experiment of infection with a single strain of B. burgdorferi of 16 laboratory-reared P. leucopus and measurements of body weight before infection and then after 3 and 5 weeks; controls were 8 animals injected with PBS alone (V.J. Cook and A.G. Barbour, unpublished study). At sacrifice at 5 weeks, ear and ankle joint tissue were subjected to quantitative PCR, as previously described [50, 51]. The infected animals tended to gain less weight over the 5 weeks than uninfected animals, but the association was weak (Mann Whitney U; p = 0.12). While the burdens of B. burgdorferi in the two tissues varied between animals over 2–3 orders of magnitude, there was no discernible association between pathogen burden and weight change over the 5 week course of the study (Figure 3).
Figure 3.
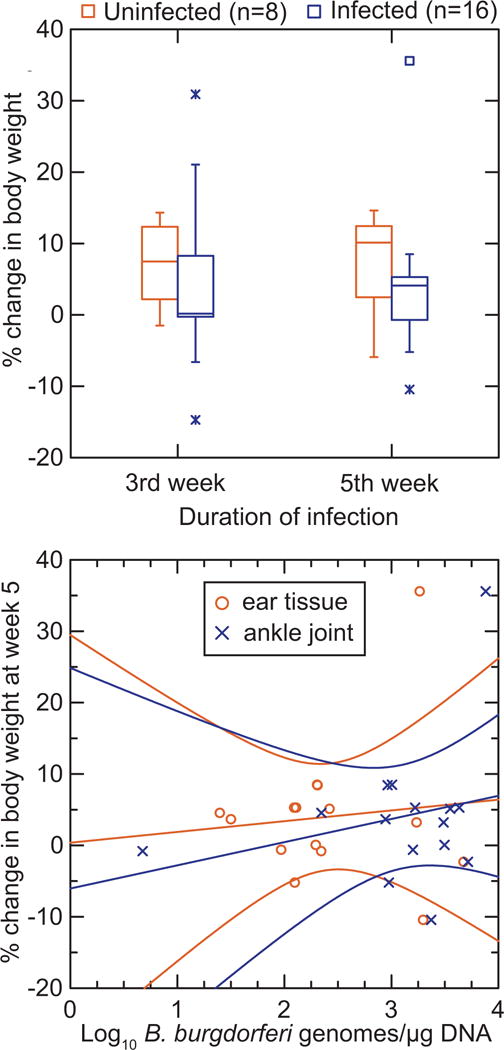
Lack of effect on body weight over 5 weeks during an experimental infection of laboratory-reared P. leucopus with B. burgdorferi.
Adult male P. leucopus were infected by needle injection with the HB19 strain of B. burgdorferi or with a buffer control. The animals were weighed just before injection and then at 3 weeks and 5 weeks, at which point they were euthanized. DNA was extracted from ear tissue and ankle joints and subjected to quantitive PCR. The sources of animals and bacteria, methods, and conditions of the experiment are described in references 50 and 51. The upper panel compares in box-whisker plots the % change in weight from day of injection at weeks 3 and 5 for uninfected (left plots in each pair) and infected mice (right plots in each pair). The lower panel is a scatter plot of % change in body weight on counts of bacteria in either ear tissue or ankle joints. The linear regression lines with 95% confidence intervals are shown.
5. Other infectious agents in Peromyscus in the field or laboratory
In a study of the agents of human granulocytic anaplasmosis and Lyme disease in I. scapularis and mammalian hosts in the north-central state of Minnesota, Johnson et al. found that prevalences of infection with A. phagocytophilum and B. burgdorferi were 20% and 42%, respectively, in white-footed mice [52]. These values corresponded to prevalences of antibodies to each agent of 29% and 48%, respectively, in the animals. By examining recaptured mice that had been infected on a previous capture, the authors noted that the rodents were more effective at eliminating A. phagocytophilum (~50% of the animals cleared) than B. burgdorferi (only 20% of the animals cleared).
The Sin Nombre hantavirus of Peromyscus spp. is considered at length in an accompanying article of this issue [3]. But for the present context, I note that in an experimental model with P. maniculatus, Botten et al. detected viral RNA throughout many of the organs and tissues of the rodents, but noted “no consistent histopathologic changes associated with infection, even when RNA load was high” [53].
Bartonella spp. in North America are usually transmitted by fleas and not ticks, but these blood-borne bacteria are common in many mammalian species in the wild. Bai et al. observed a high prevalence of persistent infection with B. vinsonii subsp. arupensis among in P. maniculatus in Colorado, but also no correlation between Bartonella prevalence and deer mouse weight [54]. This sub-species of Bartonella was isolated from a case of human illness [55].
While metazoan parasites of Peromyscus spp. do not appear to pose the same cross-over threat to humans as the foregoing microbes do, there is a considerable literature on parasitic worms in wildlife, either in the field or as an experimental model. Of relevance here is a report of laboratory infection of P. maniculatus with its natural parasite, the schistosome trematode, Schistosomatium douthitti [56]. While the parasitized deer mice had increased liver and spleen masses, the animals did not consume more food, decrease in body weight, or reduce their activity. The authors noted reduced thermoregulation during short-term cold exposure, which might be a fitness cost of the parasitism, but otherwise no changes in basal metabolic rate and cold-induced maximal metabolic rate.
On the other hand, Peromyscus is not resistant and/or tolerant of all potential pathogens, especially if it is not one for which it serves as a natural reservoir. Although Peromyscus spp. host their own vector-borne trypanosomes [57, 58], the agent of African trypanosomiasis, Trypanosoma brucei, and the closely-related apicomplexan parasite T. equiperdum are not among these. Following the Packchanian’s description of the T. brucei-P. maniculatus model, in which average survival of the animals after inoculation of the parasite was 80 days [59], Moulton and colleagues carried out a series of studies [60]. They observed predictable mortality between 60–100 days after inoculation, pathologic changes in a number of organs, including the brain, a proliferative glomerulonephritis with antigen-antibody complexes, and suppression of cell-mediated immunity. While this certainly is a worse outcome for this rodent than we have seen up to now, the deer mouse was attractive as an experimental model because it did not undergo an “acute parasitemic death” [60], unlike the usual laboratory models of mice and rats.
6. Peromyscus spp. as target for disease control measures
P. leucopus and P. maniculatus are of importance for translational research or disease prevention applications, in particular for strategies to lower risk of human disease by targeting the infection reservoirs. This includes attracting the rodents with baits for application of pesticides to kill the attached ticks [61], and administering antibiotics in the field to kill bacterial pathogens the animals may be carrying [62]. There is also the controversial concept of increasing the proportion of animals that are less competent as reservoirs than P. leucopus to eventually “dilute out” B. burgdorferi in the population of ticks [63–65].
But more relevant to this review is the candidacy of P. leucopus as targets for vaccines to interrupt the life cycle for B. burgdorferi. The feasibility of this was demonstrated by Tsao et al. in the laboratory with an antigen, the OspA protein, that is expressed in the tick but not in mammals with natural infections [66]. A proof-of-principle for such a vaccine was provided by a field trial [67]. Subsequent studies of an orally-delivered OspA protein or a recombinant viral vector for OspA confirmed the efficacy of this approach [68–70]. P. leucopus would also be the prime target for an anti-tick vaccine [71], which would aim to reduce transmission to this reservoir of not only B. burgdorferi but also other Ixodes tick-transmitted pathogens. A transmission-blocking vaccine is the thinking behind a Sin Nombre hantavirus vaccine directed at P. maniculatus [72].
7. Conclusions
As some of the cited articles indicate, there is increasing interest in traits that allow an organism–be it a plant, nematode, fruitfly, mouse, or human—to minimize the fitness or health consequences of an infection, even if it fails to eliminate it [73–75]. This tolerance of infection is usually in partnership with resistance to infection in a mixed strategy of defense. While none of the field and laboratory studies of Peromyscus spp. discussed here were intentionally designed to tease out the relative contributions of resistance and tolerance, as they are defined here, one can see in the cumulative results different phenotypes that might align with either the resistance or tolerance trait. The status of these Peromyscus spp. as widely-distributed, abundant, major reservoirs for several disease agents for humans in North America (Table 1) suggests a more-or-less stable, probably long-standing and mutual accommodation between parasitic microbes and mammalian species that appear to prosper in many parts of their distributions.
Further Peromyscus spp. studies should have testable hypotheses about resistance and tolerance and specify quantitative phenotypes. Whether they will provide insights of eventual benefit for human health remains to be seen [15, 76]. But I can think of no other vertebrate that would as well fit the bill for a model organism for these sorts of questions, including the causes of the disabilities of Lyme disease.
Acknowledgments
My laboratory’s research that was summarized here was supported by National Institutes of Health grants AI065359 and AI100236. I thank Vanessa Cook for her contributions to the research for this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Institute of Medicine, Meeting. Vector-Borne Diseases: Exploring the Environmental, Ecological, and Health Connections. 2014 < http://www.iom.edu/activities/publichealth/microbialthreats/2014-sep-16.aspx>. (accessed April 7, 2015)
- 2.NIAID. NIAID Emerging Infectious Diseases/Pathogens. 2015 < http://www.niaid.nih.gov/topics/BiodefenseRelated/Biodefense/Pages/CatA.aspx>. (accessed April 7, 2015)
- 3.Vandegrift KJ, et al. Semin Cell Develop Biol. 2016 [hantavirus article] In press. [Google Scholar]
- 4.Walsh M, Haseeb MA. Modeling the ecologic niche of plague in sylvan and domestic animal hosts to delineate sources of human exposure in the western United States. PeerJ. 2015;3:e1493. doi: 10.7717/peerj.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steppan S, Adkins R, Anderson J. Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst Biol. 2004;53(4):533–53. doi: 10.1080/10635150490468701. [DOI] [PubMed] [Google Scholar]
- 6.Dewey MJ, Dawson WD. Deer mice: “The Drosophila of North American mammalogy”. Genesis. 2001;29(3):105–9. doi: 10.1002/gene.1011. [DOI] [PubMed] [Google Scholar]
- 7.Hall ER. Mammals of North America. John Wiley and Sons; New York, NY: 1979. [Google Scholar]
- 8.Mead PS. Epidemiology of Lyme Disease. Infect Dis Clin North Am. 2015;29(2):187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Pritt BS, Sloan LM, Johnson DK, Munderloh UG, Paskewitz SM, McElroy KM, McFadden JD, Binnicker MJ, Neitzel DF, Liu G, Nicholson WL, Nelson CM, Franson JJ, Martin SA, Cunningham SA, Steward CR, Bogumill K, Bjorgaard ME, Davis JP, McQuiston JH, Warshauer DM, Wilhelm MP, Patel R, Trivedi VA, Eremeeva ME. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med. 2011;365(5):422–9. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolan MC, Hojgaard A, Hoxmeier JC, Replogle AJ, Respicio-Kingry LB, Sexton C, Williams MA, Pritt BS, Schriefer ME, Eisen L. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick Borne Dis. 2016 Feb 12; doi: 10.1016/j.ttbdis.2016.02.012. S1877-959X(16)30026-7. [DOI] [PubMed] [Google Scholar]
- 11.Pritt BS, Mead PS, Johnson DK, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, Paskewitz SM, Ray JA, Bjork J, Steward CR, Deedon A, Lee X, Kingry LC, Miller TK, Feist MA, Theel ES, Patel R, Irish CL, Petersen JM. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5; doi: 10.1016/S1473-3099(15)00464-8. S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo CG, Eremeeva ME, Paskewitz SM, Sloan LM, Lee X, Irwin WE, Tonsberg S, Pritt BS. Detection of human pathogenic Ehrlichia muris-like agent in Peromyscus leucopus. Ticks Tick Borne Dis. 2015;6(2):155–7. doi: 10.1016/j.ttbdis.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Whitaker JO. Parasites. In: King JA, editor. Biology of Peromyscus (Rodentia) American Society of Mammalogists; Stillwater, OK: 1968. pp. 254–311. [Google Scholar]
- 14.Jaffe G, Zegers DA, Steele MA, Merritt JF. Long-term patterns of botfly parasitism in Peromyscus maniculatus, P. leucopus, and Tamias striatus. J Mammal. 2005;86(1):39–45. [Google Scholar]
- 15.Ayres JS, Schneider DS. Tolerance of infections. Ann Rev Immunol. 2012;30:271–294. doi: 10.1146/annurev-immunol-020711-075030. [DOI] [PubMed] [Google Scholar]
- 16.Mandl JN, Ahmed R, Barreiro LB, Daszak P, Epstein JH, Virgin HW, Feinberg MB. Reservoir host immune responses to emerging zoonotic viruses. Cell. 2015;160(1–2):20–35. doi: 10.1016/j.cell.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsao JI. Reviewing molecular adaptations of Lyme borreliosis spirochetes in the context of reproductive fitness in natural transmission cycles. Vet Res. 2009;40(2):36. doi: 10.1051/vetres/2009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piesman J, Schwan TG. Ecology of borreliae and their arthropod vectors. In: Samuels DS, Radolf JD, editors. Borrelia. Molecular Biology, Host Interaction, and Pathogensis. Caister Academic Press; Norfolk, UK: 2010. pp. 251–278. [Google Scholar]
- 19.Hoen AG, Margos G, Bent SJ, Diuk-Wasser MA, Barbour A, Kurtenbach K, Fish D. Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc Natl Acad Sci U S A. 2009;106(35):15013–8. doi: 10.1073/pnas.0903810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margos G, Tsao JI, Castillo-Ramirez S, Girard YA, Hamer SA, Hoen AG, Lane RS, Raper SL, Ogden NH. Two boundaries separate Borrelia burgdorferi populations in North America. Appl Environ Microbiol. 2012;78(17):6059–67. doi: 10.1128/AEM.00231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donahue JG, Piesman J, Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg. 1987;36(1):92–6. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- 22.Mather TN, Wilson ML, Moore SI, Ribiero JM, Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am J Epidemiol. 1989;130(1):143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- 23.Ostfeld RS, Levi T, Jolles AE, Martin LB, Hosseini PR, Keesing F. Life history and demographic drivers of reservoir competence for three tick-borne zoonotic pathogens. PLoS One. 2014;9(9):e107387. doi: 10.1371/journal.pone.0107387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanincová K, Kurtenbach K, Diuk-Wasser M, Brei B, Fish D. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg Infect Dis. 2006;12(4):604. doi: 10.3201/eid1204.051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbour AG, Bunikis J, Fish D, Hanincová K. Association between body size and reservoir competence of mammals bearing Borrelia burgdorferi at an endemic site in the northeastern United States. Parasit Vectors. 2015;8:299. doi: 10.1186/s13071-015-0903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtenbach K, Hanincova K, Tsao JI, Margos G, Fish D, Ogden NH. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol. 2006;4(9):660–9. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 27.Bunikis J, Garpmo U, Tsao J, Berglund J, Fish D, Barbour AG. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology. 2004;150(Pt 6):1741–55. doi: 10.1099/mic.0.26944-0. [DOI] [PubMed] [Google Scholar]
- 28.Rand PW, Lacombe EH, Smith RP, Jr, Rich SM, Kilpatrick CW, Dragoni CA, Caporale D. Competence of Peromyscus maniculatus (Rodentia: Cricetidae) as a reservoir host for Borrelia burgdorferi (Spirochaetares: Spirochaetaceae) in the wild. J Med Entomol. 1993;30(3):614–8. doi: 10.1093/jmedent/30.3.614. [DOI] [PubMed] [Google Scholar]
- 29.Peavey CA, Lane RS. Transmission of Borrelia burgdorferi by Ixodes pacificus nymphs and reservoir competence of deer mice (Peromyscus maniculatus) infected by tick-bite. J Parasitol. 1995;81(2):175–178. [PubMed] [Google Scholar]
- 30.Fiset J, Tessier N, Millien V, Lapointe FJ. Phylogeographic structure of the white-footed mouse and the deer mouse, two Lyme disease reservoir hosts in Quebec. PLoS One. 2015;10(12):e0144112. doi: 10.1371/journal.pone.0144112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bunikis J, Tsao J, Luke CJ, Luna MG, Fish D, Barbour AG. Borrelia burgdorferi infection in a natural population of Peromyscus leucopus mice: a longitudinal study in an area where Lyme Borreliosis is highly endemic. J Infect Dis. 2004;189(8):1515–23. doi: 10.1086/382594. [DOI] [PubMed] [Google Scholar]
- 32.Baum E, Randall AZ, Zeller M, Barbour AG. Inferring epitopes of a polymorphic antigen amidst broadly-cross reactive antibodies using protein microarrays: a study of OspC proteins of Borrelia burgdorferi. PLoS One. 2013;8(6):e67445. doi: 10.1371/journal.pone.0067445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbour AG, Jasinskas A, Kayala MA, Davies DH, Steere AC, Baldi P, Felgner PL. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun. 2008;76(8):3374–89. doi: 10.1128/IAI.00048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009;81(6):1120–31. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barthold SW, Cadavid D, Phillip MT. Animal models of borreliosis. In: Radolf JD, Samuels DS, editors. Borrelia: Molecular Biology, Host Interaction, and Pathogenesis. Caister Academic Press; Norfolk, UK: 2010. pp. 359–412. [Google Scholar]
- 36.Hofmeister EK, Ellis BA, Glass GE, Childs JE. Longitudinal study of infection with Borrelia burgdorferi in a population of Peromyscus leucopus at a Lyme disease-enzootic site in Maryland. Am J Trop Med Hyg. 1999;60(4):598–609. doi: 10.4269/ajtmh.1999.60.598. [DOI] [PubMed] [Google Scholar]
- 37.Voordouw MJ, Lachish S, Dolan MC. The Lyme disease pathogen has no effect on the survival of its rodent reservoir host. PLoS One. 2015;10(2):e0118265. doi: 10.1371/journal.pone.0118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson RC, Marek N, Kodner C. Infection of Syrian hamsters with Lyme disease spirochetes. J Clin Microbiol. 1984;20(6):1099–101. doi: 10.1128/jcm.20.6.1099-1101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodman JL, Jurkovich P, Kodner C, Johnson RC. Persistent cardiac and urinary tract infections with Borrelia burgdorferi in experimentally infected Syrian hamsters. J Clin Microbiol. 1991;29(5):894–6. doi: 10.1128/jcm.29.5.894-896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duray PH, Johnson RC. The histopathology of experimentally infected hamsters with the Lyme disease spirochete, Borrelia burgdorferi. Exp Biol Med. 1986;181(2):263–269. doi: 10.3181/00379727-181-42251. [DOI] [PubMed] [Google Scholar]
- 41.Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162(1):133–8. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 42.Schwan TG, Burgdorfer W, Schrumpf ME, Karstens RH. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus) J Clin Microbiol. 1988;26(5):893–5. doi: 10.1128/jcm.26.5.893-895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwan TG, Kime KK, Schrumpf ME, Coe JE, Simpson WJ. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi) Infect Immun. 1989;57(11):3445–51. doi: 10.1128/iai.57.11.3445-3451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright SD, Nielsen SW. Experimental infection of the white-footed mouse with Borrelia burgdorferi. Am J Vet Res. 1990;51(12):1980–7. [PubMed] [Google Scholar]
- 45.Mather TN, Telford SR, 3rd, Adler GH. Absence of transplacental transmission of Lyme disease spirochetes from reservoir mice (Peromyscus leucopus) to their offspring. J Infect Dis. 1991;164(3):564–7. doi: 10.1093/infdis/164.3.564. [DOI] [PubMed] [Google Scholar]
- 46.Moody KD, Terwilliger GA, Hansen GM, Barthold SW. Experimental Borrelia burgdorferi infection in Peromyscus leucopus. J Wildl Dis. 1994;30(2):155–61. doi: 10.7589/0090-3558-30.2.155. [DOI] [PubMed] [Google Scholar]
- 47.Brown RN, Lane RS. Natural and experimental Borrelia burgdorferi infections in woodrats and deer mice from California. J Wildlife Dis. 1994;30(3):389–98. doi: 10.7589/0090-3558-30.3.389. [DOI] [PubMed] [Google Scholar]
- 48.Lindsay LR, Barker IK, Surgeoner GA, McEwen SA, Campbell GD. Duration of Borrelia burgdorferi infectivity in white-footed mice for the tick vector Ixodes scapularis under laboratory and field conditions in Ontario. J Wildl Dis. 1997;33(4):766–75. doi: 10.7589/0090-3558-33.4.766. [DOI] [PubMed] [Google Scholar]
- 49.Schwanz LE, Voordouw MJ, Brisson D, Ostfeld RS. Borrelia burgdorferi has minimal impact on the Lyme disease reservoir host Peromyscus leucopus. Vector Borne Zoonotic Dis. 2011;11(2):117–24. doi: 10.1089/vbz.2009.0215. [DOI] [PubMed] [Google Scholar]
- 50.Baum E, Hue F, Barbour AG. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. MBio. 2012;3(6):e00434–12. doi: 10.1128/mBio.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook V, Barbour AG. Broad diversity of host responses of the white-footed mouse Peromyscus leucopus to Borrelia infection and antigens. Ticks Tick Borne Dis. 2015;6(5):549–58. doi: 10.1016/j.ttbdis.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson RC, Kodner C, Jarnefeld J, Eck DK, Xu Y. Agents of human anaplasmosis and Lyme disease at Camp Ripley, Minnesota. Vector Borne Zoonotic Dis. 2011;11(12):1529–34. doi: 10.1089/vbz.2011.0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Botten J, Mirowsky K, Kusewitt D, Bharadwaj M, Yee J, Ricci R, Feddersen RM, Hjelle B. Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus) Proc Natl Acad Sci U S A. 2000;97(19):10578–83. doi: 10.1073/pnas.180197197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai Y, Calisher CH, Kosoy MY, Root JJ, Doty JB. Persistent infection or successive reinfection of deer mice with Bartonella vinsonii subsp. arupensis. Appl Environ Microbiol. 2011;77(5):1728–31. doi: 10.1128/AEM.02203-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welch DF, Carroll KC, Hofmeister EK, Persing DH, Robison DA, Steigerwalt AG, Brenner DJ. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J Clin Microbiol. 1999;37(8):2598–601. doi: 10.1128/jcm.37.8.2598-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwanz LE. Schistosome infection in deer mice (Peromyscus maniculatus): impacts on host physiology, behavior and energetics. J Exp Biol. 2006;209(Pt 24):5029–37. doi: 10.1242/jeb.02601. [DOI] [PubMed] [Google Scholar]
- 57.Wood SF. New localities for mammal blood parasites from southwestern United States. J Parasitol. 1975;61(5):969–970. [PubMed] [Google Scholar]
- 58.McKown RD, Upton SJ, Klemm RD, Ridley RK. New host and locality record for Trypanosoma peromysci. J Parasitol. 1990;76(2):281–3. [PubMed] [Google Scholar]
- 59.Packchanian A. A method of maintaining laboratory strains of Trypanosoma brucei in a subspecies of Peromyscus maniculatus. J Lab Clin Med. 1935;20(5):510–515. [Google Scholar]
- 60.Moulton J, Stevens D. Animal model of human disease: trypanosomiasis, sleeping sickness. Am J Pathol. 1978;91(3):693. [PMC free article] [PubMed] [Google Scholar]
- 61.Mather TN, Ribeiro JM, Spielman A. Lyme disease and babesiosis: acaricide focused on potentially infected ticks. Am J Trop Med Hyg. 1987;36(3):609–14. doi: 10.4269/ajtmh.1987.36.609. [DOI] [PubMed] [Google Scholar]
- 62.Dolan MC, Schulze TL, Jordan RA, Dietrich G, Schulze CJ, Hojgaard A, Ullmann AJ, Sackal C, Zeidner NS, Piesman J. Elimination of Borrelia burgdorferi and Anaplasma phagocytophilum in rodent reservoirs and Ixodes scapularis ticks using a doxycycline hyclate-laden bait. Am J Trop Med Hyg. 2011;85(6):1114–20. doi: 10.4269/ajtmh.2011.11-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci U S A. 2003;100(2):567–71. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.States S, Brinkerhoff R, Carpi G, Steeves T, Folsom-O’Keefe C, DeVeaux M, Diuk-Wasser M. Lyme disease risk not amplified in a species-poor vertebrate community: similar Borrelia burgdorferi tick infection prevalence and OspC genotype frequencies. Infect Genet Evol. 2014;27:566–75. doi: 10.1016/j.meegid.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bouchard C, Beauchamp G, Leighton PA, Lindsay R, Belanger D, Ogden NH. Does high biodiversity reduce the risk of Lyme disease invasion? Parasit Vectors. 2013;6:195. doi: 10.1186/1756-3305-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsao J, Barbour AG, Luke CJ, Fikrig E, Fish D. OspA immunization decreases transmission of Borrelia burgdorferi spirochetes from infected Peromyscus leucopus mice to larval Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1(1):65–74. doi: 10.1089/153036601750137705. [DOI] [PubMed] [Google Scholar]
- 67.Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A. 2004;101(52):18159–64. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomes-Solecki MJ, Brisson DR, Dattwyler RJ. Oral vaccine that breaks the transmission cycle of the Lyme disease spirochete can be delivered via bait. Vaccine. 2006;24(20):4440–9. doi: 10.1016/j.vaccine.2005.08.089. [DOI] [PubMed] [Google Scholar]
- 69.Meirelles Richer L, Aroso M, Contente-Cuomo T, Ivanova L, Gomes-Solecki M. Reservoir targeted vaccine for Lyme borreliosis induces a yearlong, neutralizing antibody response to OspA in white-footed mice. Clin Vaccine Immunol. 2011;18(11):1809–16. doi: 10.1128/CVI.05226-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhattacharya D, Bensaci M, Luker KE, Luker G, Wisdom S, Telford SR, Hu LT. Development of a baited oral vaccine for use in reservoir-targeted strategies against Lyme disease. Vaccine. 2011;29(44):7818–25. doi: 10.1016/j.vaccine.2011.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bensaci M, Bhattacharya D, Clark R, Hu LT. Oral vaccination with vaccinia virus expressing the tick antigen subolesin inhibits tick feeding and transmission of Borrelia burgdorferi. Vaccine. 2012;30(42):6040–6. doi: 10.1016/j.vaccine.2012.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bharadwaj M, Mirowsky K, Ye C, Botten J, Masten B, Yee J, Lyons CR, Hjelle B. Genetic vaccines protect against Sin Nombre hantavirus challenge in the deer mouse (Peromyscus maniculatus) J Gen Virol. 2002;83(7):1745–1751. doi: 10.1099/0022-1317-83-7-1745. [DOI] [PubMed] [Google Scholar]
- 73.Roy B, Kirchner J. Evolutionary dynamics of pathogen resistance and tolerance. Evolution. 2000;54(1):51–63. doi: 10.1111/j.0014-3820.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 74.Restif O, Koella JC. Concurrent evolution of resistance and tolerance to pathogens. Am Nat. 2004;164(4):E90–E102. doi: 10.1086/423713. [DOI] [PubMed] [Google Scholar]
- 75.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nature Rev Immunol. 2008;8(11):889–95. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Philos Trans R Soc Lond B Biol Sci. 2009;364(1513):37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson JF, Magnarelli LA, Burgdorfer W, Barbour AG. Spirochetes in Ixodes dammini and mammals from Connecticut. Am J Trop Med Hyg. 1983;32(4):818–24. doi: 10.4269/ajtmh.1983.32.818. [DOI] [PubMed] [Google Scholar]
- 78.Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1(1):21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- 79.Telford SR, 3rd, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A. 1996;93(12):6209–14. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levin ML, Nicholson WL, Massung RF, Sumner JW, Fish D. Comparison of the reservoir competence of medium-sized mammals and Peromyscus leucopus for Anaplasma phagocytophilum in Connecticut. Vector Borne Zoonotic Dis. 2002;2(3):125–36. doi: 10.1089/15303660260613693. [DOI] [PubMed] [Google Scholar]
- 81.Zeidner NS, Burkot TR, Massung R, Nicholson WL, Dolan MC, Rutherford JS, Biggerstaff BJ, Maupin GO. Transmission of the agent of human granulocytic ehrlichiosis by Ixodes spinipalpis ticks: evidence of an enzootic cycle of dual infection with Borrelia burgdorferi in Northern Colorado. J Infect Dis. 2000;182(2):616–9. doi: 10.1086/315715. [DOI] [PubMed] [Google Scholar]
- 82.Spielman A, Etkind P, Piesman J, Ruebush TK, 2nd, Juranek DD, Jacobs MS. Reservoir hosts of human babesiosis on Nantucket Island. Am J Trop Med Hyg. 1981;30(3):560–565. doi: 10.4269/ajtmh.1981.30.560. [DOI] [PubMed] [Google Scholar]
- 83.Telford SR, 3rd, Spielman A. Reservoir competence of white-footed mice for Babesia microti. J Med Entomol. 1993;30(1):223–7. doi: 10.1093/jmedent/30.1.223. [DOI] [PubMed] [Google Scholar]
- 84.Ebel GD, Campbell EN, Goethert HK, Spielman A, Telford SR., 3rd Enzootic transmission of deer tick virus in New England and Wisconsin sites. Am J Trop Med Hyg. 2000;63(1–2):36–42. doi: 10.4269/ajtmh.2000.63.36. [DOI] [PubMed] [Google Scholar]
- 85.Deardorff ER, Nofchissey RA, Cook JA, Hope AG, Tsvetkova A, Talbot SL, Ebel GD. Powassan virus in mammals, Alaska and New Mexico, U.S.A., and Russia, 2004–2007. Emerg Infect Dis. 2013;19(12):2012–6. doi: 10.3201/eid1912.130319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morzunov SP, Rowe JE, Ksiazek TG, Peters CJ, St Jeor SC, Nichol ST. Genetic analysis of the diversity and origin of hantaviruses in Peromyscus leucopus mice in North America. J Virol. 1998;72(1):57–64. doi: 10.1128/jvi.72.1.57-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hjelle B, Lee SW, Song W, Torrez-Martinez N, Song JW, Yanagihara R, Gavrilovskaya I, Mackow ER. Molecular linkage of hantavirus pulmonary syndrome to the white-footed mouse, Peromyscus leucopus: genetic characterization of the M genome of New York virus. J Virol. 1995;69(12):8137–41. doi: 10.1128/jvi.69.12.8137-8141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marchette NJ, Bushman JB, Parker DD, Johnson EE. Studies on infectious diseases in wild animals in Utah. IV. A wild rodent (Peromyscus spp.) plague focus in Utah. Zoonoses Res. 1962;1:341–61. [PubMed] [Google Scholar]
- 89.Hofmeister EK, Kolbert CP, Abdulkarim AS, Magera JMH, Hopkins MK, Uhl JR, Ambyaye A, Telford SR, Cockerill FR, Persing DH. Cosegregation of a novel Bartonella species with Borrelia burgdorferi and Babesia microti in Peromyscus leucopus. J Infect Dis. 1998;177(2):409–416. doi: 10.1086/514201. [DOI] [PubMed] [Google Scholar]
- 90.Schneider BS, Zeidner NS, Burkot TR, Maupin GO, Piesman J. Borrelia isolates in Northern Colorado identified as Borrelia bissettii. J Clin Microbiol. 2000;38(8):3103–5. doi: 10.1128/jcm.38.8.3103-3105.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vredevoe LK, Stevens JR, Schneider BS. Detection and characterization of Borrelia bissettii in rodents from the central California coast. J Med Entomol. 2004;41(4):736–45. doi: 10.1603/0022-2585-41.4.736. [DOI] [PubMed] [Google Scholar]
- 92.Bunikis J, Barbour AG. Third Borrelia species in white-footed mice. Emerg Infect Dis. 2005;11(7):1150–1. doi: 10.3201/eid1107.041355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malek EA. Geographical distribution, hosts, and biology of Schistosomatium douthitti (Cort, 1914) Price, 1931. Canad J Zool. 1977;55(4):661–671. doi: 10.1139/z77-087. [DOI] [PubMed] [Google Scholar]
- 94.Hanincová K, Kurtenbach K, Diuk-Wasser M, Brei B, Fish D. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg Infect Dis. 2006;12(4):604–11. doi: 10.3201/eid1204.051016. [DOI] [PMC free article] [PubMed] [Google Scholar]


