Abstract
Despite the significant advances in neurological medicine, it remains difficult to treat ailments directly involving the brain. The blood brain barrier (BBB) is a tightly regulated, selectively permeable barrier that restricts access from the blood into the brain extracellular fluid (BEF). Many conditions such as tumors or infections in the brain are difficult to treat due to the fact that drugs and other therapeutic agents are unable to easily pass through this relatively impermeable barrier. Human Immunodeficiency Virus (HIV) presents a particular problem as it is able to remain dormant in the brain for years protected from antiretroviral drugs by the BBB. The development of nanoscale carriers over the past few decades has made possible the delivery of therapies with the potential to overcome membrane barriers and provide specific, targeted delivery. This review seeks to provide a comprehensive overview of the various aspects of nanoparticle formulation and their applications in improving the delivery efficiency of drugs, specifically antiretroviral therapeutics to the brain to treat HIV.
Keywords: HIV, nanoparticles, exosomes, CNS, drug delivery
1. INTRODUCTION
1.1 Blood brain barrier: Physiology and Transport Regulation
While numerous advances have been made in the treatment of neurological medicine, specifically in the treatment of disorders of the brain, it remains difficult to treat ailments directly involving the brain. The blood brain barrier (BBB) is a tightly regulated, selectively permeable barrier that restricts access from the blood into the brain extracellular fluid (BEF). Many conditions such as tumors or infections in the brain are difficult to treat due to the fact that drugs and other therapeutic agents are unable to easily pass through this barrier due to the relative impermeability of the blood brain barrier.
The BBB is an entity made up of capillary endothelium, astrocyte, pericytes and extracellular matrix. It functions as a diffusion barrier that is vital to the function of the central nervous system (Ballah et al. 2004). The permeability of the BBB is controlled via the cerebral capillary endothelium which has a junction complex that restricts permeability and separates circulating blood from brain extracellular fluid (Lawther et al. 2011). This selective permeability prevents potentially harmful molecules and bacteria from crossing into the brain with possibly deleterious effects to the Central Nervous System (CNS). The BBB does allow some compounds such, as water, lipid soluble molecules and gasses to diffuse through this membrane, while other molecules must enter via membrane transport.
The barrier function of the BBB is due in large part to the tight junctions that are between endothelial cells. These tight junctions present high selectivity that limits the passage of solutes from the blood into the BEF. These junctions are made of transmembrane proteins such as occludins, claudins, and junctional adhesion molecule (JAM) (Stamatovic et al. 2008). Astrocytes make up a key feature of the BBB in that, astrocyte cell projections help to maintain the expression of enzymatic activity, tight junctions and protein transporters to ensure normal functioning of the blood brain barrier (Watkins et al. 2014). These astrocytic feet provide biochemical support to the endothelial cells that they surround (Abbott et al. 2006). While transport across the BBB is tightly regulated, there are mechanisms that allow the passage of some chemical and biological agents to cross this membrane. Some small molecules, such as hormones, O2, or CO2 are able to diffuse through the BBB, while other larger molecules, such as glucose, can transverse the BBB via transport receptors.
1.2 Blood Brain Barrier Transport Techniques
Currently, there are several strategies for transporting drugs across the BBB. These strategies include physical, chemical and biological means to circumvent the obstacles involved in delivering therapeutic treatment directly to the brain. Transcranial drug delivery is a neurosurgical method for delivering drugs directly to the central nervous system (Pardridge 2005). Transcranial drug delivery can either be performed by one of three methods: intracerebral implantation, intracerebralventricular (ICV) infusion, or convection enhanced diffusion (CED) (Pardridge 2005). While intracerebral infusion and intracerebral implantation is able to bypass the blood brain barrier, a key limitation is its reliance on diffusion. As the drug penetrates into the brain tissue, the concentration of the drug decreases significantly with each millimeter of tissue it travels away from the injection site (Pardridge 2005). Passing through the BBB can be also accomplished by the use of osmotic disruption of the BBB. This disruption can be performed by the use of vasoactive substances, such as bradykinins, or by exposure to high-intensity focused ultrasound (HIFU) (McDonnaold et al. 2008). It has also been suggested that the permeability of the blood brain barrier may be increased upon exposure to electromagnetic pulses (Yang et al. 2015).
Another method of passing therapeutics through the BBB involves the use of BBB transporters. A “Trojan horse” method can be employed to bypass endothelial transport proteins and allow the passage of drugs across the BBB. A targeting ligand, or antibody can bind to an endocytotic receptor. Once bound, the drug is then associated with this ligand and transport is facilitated (Meairs 2015). Water-soluble molecules that would not ordinarily be capable of bypassing the blood brain barrier have been modified into lipophilic analogs that can more easily penetrate the barrier (Meairs 2015). These prodrugs are typically used to treat neuronal diseases and can augment the efficacy of therapeutics (Upadhyay and Upadhyay 2014).
Despite these advances in delivering therapeutics past the BBB, there remain several distinct limitations. In the case of directly diffusing drugs, as in the case of infusion, diffusion, or neurosurgically placing the drug behind the BBB, there is a significant loss of concentration of the therapeutic for every millimeter of brain tissue through which it passes. This could result in a loss of effectiveness of the therapeutic prior to it reaching its intended target. Current methods for passing drugs through the BBB have the limitation of drug molecules becoming entrapped in BBB endothelial cells rather than passing through to their intended targets. A promising new method for this problem is the use of nanotechnology to ferry drugs across the BBB. This novel and exciting technology, more specifically use of nanoparticles, has the potential to perform multiple therapeutic functions at once, and perhaps to carry out multiple functions in a predetermined sequence. This function is of particular interest in the treatment illnesses associated with the CNS and would result in significant benefit to patient care and treatment (Silva 2008).
2. NANOPARTICLES
Nanoparticles are small structures that may be utilized to carry and deliver therapeutic agents to complex biological systems (Kreuter 2014, Patel et al. 2012). Therapeutic nanocarriers can range in size from 1 to 1000 nm and be generated from a wide variety of substances, both biodegradable and non-biodegradable, including polymers, lipids, carbon nanotubes and ceramics (Kreuter 2014, Patel et al. 2012, Gastaldi et al. 2014). Nanoparticles are especially important for therapeutic delivery to areas that are otherwise difficult to access, such as the central nervous system and areas beyond the blood-brain barrier.
Biodegradable nanoparticles are becoming increasingly popular in the creation of therapeutics to increase bioavailability, retention, and solubility. Nanoparticles tend to be more stable than liposomes (Zensi et al. 2009). Drugs can be encapsulated by different types of nanoparticles to protect the drug from chemical and physical degradation, to allow slow release, to target specific tissue or cell types, and to cross barriers that are otherwise impassable (Gelperina et al 2010). Medicinal drugs can be encapsulated into nanomedicines to improve drug efficacy, tolerability, specificity, therapeutic index (Nagpal et al. 2010) and also to decrease the dosage, toxicity, side effects (Kreuter 2001). Nanomedicines are being tested for use in treating cancers, diabetes, malaria, acquired immunodeficiency syndrome (AIDS), tuberculosis, and prion diseases (Nagpal et al 2010).
Polymers, either artificially- or naturally-derived, have been the material of choice for nanoparticles for several reasons; they are highly stable, allowing for a high amount of agent to be loaded, they are readily able to be customized to present a wide variety of surface-attached ligands depending on the recipient cell type or tissue of choice, they are rapidly biodegradable and have been demonstrated to be safe for in vivo use (Kreuter 2014), and they allow for control over drug release kinetics (Patel et al. 2012). There are many considerations in determining which nanomedicine will be most effective (Nagpal et al. 2010). Many kinds of polymers such as proteins, polysaccharides, and polyesters, have been used for nanoparticles designed to deliver drugs to the CNS, but poly(alkyl cyanoacrylates) such as poly(butyl cyanoacrylate) (PBCA) or poly(isohexyl cyanoacrylate) (PIHCA) and polyesters such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA) or their copolymer poly(lactide-co-glycolide) (PLGA) are especially popular because of their rapid biodegradation and history of safe use (Kreuter 2014; Patel et al. 2012). Human serum albumin (HSA) and chitosan may also be useful polymers as they have low toxicity and are biodegradable (Nagpal et al. 2010).
2.1 Delivery and Mechanism
Different modes of nanoparticle delivery are also being studied, including brain (Rao et al. 2008), nasal, oral, transdermal, cardio-vascular methods (Ham et al. 2009), and intravenous (Gastaldi et al. 2014). However, the specific type of nanoparticle is dependent on both the drug and the drug target. Some considerations when choosing a nanoparticle are size, surface charge, hydrophobicity (Gastaldi et al. 2014), molecular weight, and persistence in tissue or circulation (Kumari et al. 2010). Circulation time can be increased by using a smaller sized nanoparticle and/or coating the surface of the nanoparticle with hydrophobic polymers so they will more easily move between membranes (Gastaldi et al. 2014).
Internally, it is thought that the most likely mechanism of nanoparticle uptake is receptor mediated transcytosis by brain capillary endothelial cells after intravenous injection (Kreuter 2001, Kreuter 2013, Wohlfast et al. 2011). It has been experimentally demonstrated in many cell lines of various species, including rodent, human, bovine, and porcine primary endothelial, neuronal, and glioblastoma cell lines, that nanoparticles coated with polysorbate 80 or poloxamer 188 were taken up by an endocytotic mechanism (Kreuter 2014). Demonstration of apolipoprotein interaction with several brain capillary endothelial cell lipoprotein receptors has also been shown in numerous studies (Zensi et al. 2009, Zensi et al. 2010). Apo A-I has been shown to interact with the scavenger receptor class B type I (SR-BI) (Balazs et al. 2004, Panzenboeck et al. 2002) and Apo E and B were demonstrated to interact with the LDL receptor (LRP1) (Wagner et al. 2012). Interestingly, the transcytosis of nanoparticles across the blood-brain barrier also appears to be dependent on circadian rhythms, although the specifics may vary based on species (Ramge et al. 1999). Additionally, nanoparticles can be endocytosed by endothelial cells, can open endothelial tight junctions, and can increase endothelial cell membrane solubility in order to increase drug transport efficiency (Gastaldi et al. 2014).
2.2 Tailoring Nanoparticles
Alterations of the surface ligands based on the desired target tissue can also serve to customize the nanoparticles. Different surfactants and targeting ligands can be modified in order to optimize the ability of the nanoparticles to enter and deliver drugs to specific locations in the brain. Surfactants such as polysorbates 80, 20, 40, and 60, as well as poloxamer 188 are some of the most frequently utilized in order to anchor various targeting ligands such as apolipoproteins (A-I, B, or E, most commonly), which interact directly with receptors on the brain to allow access (Kreuter 2014, Gelperina et al. 2010, Kreuter 2001, Kreuter 2013, Petri et al. 2007, Wohlfart et al. 2011, Wohlfart et al. 2012). Nanoparticles can also be conjugated to targeting components like cell-penetrating peptides (CPP), such as transactivator of transcription (Tat)-peptide, to increase uptake of the nanoparticle by cells (Borgmann et al. 2011; Rao et al. 2008).
In many studies, it has been shown that nanoparticles of various compositions were able to successfully transport drugs across the blood-brain barrier and induce therapeutic effects in the target tissues, whereas drugs without the accompaniment of nanoparticles were significantly less effective. Delivery of anticancer drugs such as DOXorubicin (DOX) for treatment of brain tumors has been challenging, as it cannot cross the blood-brain barrier in its intact form to reach peritumoral areas within the brain. However, some studies have shown that transport of DOX across the BBB and into the brain parenchyma is possible when bound to PBCA nanoparticles coated with polysorbate 80 (Wohlfart et al. 2011, Gulyaev et al. 1999) and that injections of these DOX nanoparticles has been shown to significantly inhibit tumor growth and increase the survival time of rats with glioblastomas (Steiniger et al. 2004). In addition, DOX bound to PLGA nanoparticles coated with poloxamer 188 was able to show similar or better anti-tumor effects in the same model system (Gelperina et al. 2010, Wohlfast et al. 2011). Treatment with these nanoparticles in rats has shown dramatic reduction in angiogenesis and necrosis in a dose-dependent manner (Wohlfart et al. 2012, Hekmatara et al. 2009, Wohlfart et al. 2009). Not only is it advantageous to use nanoparticles for drug delivery in the case of cancer, but they have also shown promise in Alzheimer’s disease treatment. As with brain tumors, the delivery of drugs past the blood-brain barrier is inhibited in Alzheimer’s patient treatment. Nanoparticles can offer a similar solution for this restriction (Andrieux and Couvreur 2013, Roney et al. 2005). Several drugs such as rivastigmine, tacrine, quinolone, piperine, and curcumin have been bound to PBCA, chitosan, and PLGA nanoparticles for this purpose (Kreuter 2014), resulting in a significant increase in the drugs’ concentration in the brain. Additionally, Alzheimer’s drugs bound to nanoparticles have been demonstrated in animal models to interact with neurons, reduce plaques and tangles, and positively affect memory (Matthew et al. 2012, Yusuf et al. 2013).
2.3 Nanoparticle Limitations
While the therapeutic potential of nanoparticles in drug delivery to the CNS is promising, there are some limitations that exist as well. Toxicological effects are still of significant concern and have been investigated in-depth by many experts. DOX alone, empty nanoparticles, and DOX-PBCA nanoparticles were examined in glioma rat model systems. Nanoparticles showed little to no toxicity in doses of up to 400 mg/kg within 30 days whereas DOX alone caused dose-dependent mortality and weight loss (Steiniger et al. 2004, Gelperina et al. 2003, Pereverzeva et al. 2007, Pereverzeva 2008). Additional studies demonstrated considerably less toxicity in animals treated with DOX-bound nanoparticles compared to those treated with a DOX solution (Coureur et al. 1982, Soppimath et al. 2001), likely due to the altered biodistribution of nanoparticle-bound drug (Wohlfart et al. 2012). These studies are promising in terms of the safety of nanoparticle use clinically, however, many more studies of possible toxicological effects especially for long-term use is required. In the case of treating chronic illnesses such as Alzheimer’s disease, permanent, frequent injections would be required. It is essential to determine the safety of nanoparticle treatment in this setting, where the polymer material may be capable of accumulating. It may be possible that the clinical use of nanoparticles for drug delivery may be feasible only for treatment of acute diseases, such as stroke, brain tumors, and enzyme replacement therapies that require only infrequent drug administration (Kreuter 2014).
3. BIODEGRADABLE NANOPARTICLES
3.1 Polymeric Nanoparticles
3.1.1 Poly-D,L-lactide-co-glycolide nanoparticles (PLGA)
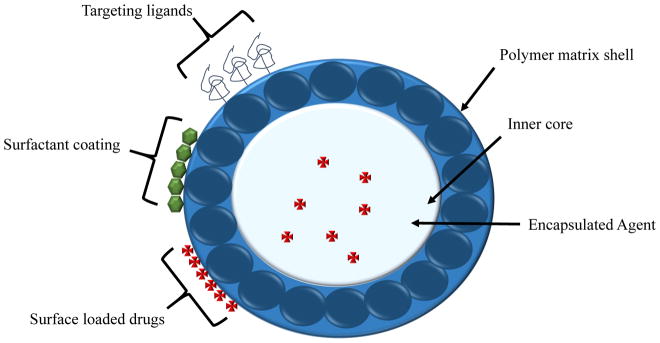
Nanoparticles can deliver drugs by incorporating them on the surface of nanospheres or by releasing the encapsulated drugs from within nanocapsules (Kumari et al. 2010). Figure 1 shows the basic structure of polymeric nanoparticles. The most widely used nanosystem to date is poly-D,L-lactide-co-glycolide (PLGA) due to the fact that it can undergo hydrolysis within the body to make biodegradable and biocompatible metabolites (Ham et al. 2009; Kumari et al. 2010). PLGAs are small enough to diffuse through the interstitial space to the targeted cells (Ham et al. 2009). When they are smaller than 250 μm, they remain in circulation for up to two weeks, but when larger than 250 μm, they cleared from circulation in just two days (Lai et al. 2014).
Figure 1. Basic structure of polymeric nanoparticles.
Polymeric nanoparticles are the most widely used nanosystem to date, specifically poly-D,L-lactide-co-glycolide (PLGA), due to the fact that it can undergo hydrolysis within the body to make biodegradable and biocompatible metabolites (Ham et al. 2009; Kumari et al. 2010). Polymeric nanoparticles are small enough to diffuse through the interstitial space and into target cells (Ham et al. 2009). Diameter size dictates circulating levels. However, when polymeric nanoparticles are smaller than 250 μm, they remain in circulation for up to two weeks, and when larger than 250 μm, they are cleared from circulation in only two days (Lai et al. 2014).
These nanoparticles can be made by one process, called emulsification-diffusion method, which involves dissolving PLGA polymers in an organic solvent such as acetone or methanol, adding a stabilizer in an aqueous solution, and lastly adding water with constant stirring (Kumari et al. 2010; Lai et al. 2014). Another way to make PLGA nanoparticles, solvent evaporation method, is by dissolving the polymers in volatile organic solvents, adding a stabilizer in an aqueous solution, sonicating to break apart the emulsion droplets, then stirring to remove the organic solvent (Kumari et al. 2010; Lai et al. 2014). These nanoparticles can also be made by interfacial deposition method in an interfacial layer of water and organic solvent, then separated by centrifugation (Kumari et al. 2010). However, most often, they are made by nanoprecipitation by dissolving the polymers in a polar solvent like acetone or methanol, drop-wise added to aqueous phase with a stabilizer, and the organic phase is evaporated (Kumari et al. 2010; Lai et al. 2014).
FDA has approved use of PLGA in many human anticancer and anti-viral therapeutics (Kumari et al. 2010). 9-Nitrocamptothecin (9-NC) is an antitumor therapeutic that can be encapsulated by PLGA via nanoprecipitation to prevent pH dependent instability (Derakhshandeh et al. 2007, Kumari et al. 2010). Encapsulated 9-NC has been shown to work well in vitro and has demonstrated a sustained release up to 160 hours (Derakhshandeh et al. 2007, Kumari et al. 2010). Paclitaxel, a drug which causes cell death by polymerizing tubulin, is used to treat ovarian and colon cancers, and can be encapsulated by PLGA via interfacial deposition method to increase solubility (Fonseca et al. 2002, Kumari et al. 2010). One group found that encapsulating Paclitaxel increased efficiency of the drug in vitro via enhancing the drug’s cytotoxic effect. Furthermore they found that these PLGA nanoparticles release Paclitaxel rapidly in the first 24 hours, then more gradually over the next few days (Fonseca et al. 2002). Triptorelin, a drug which can decrease luteinizing hormone to decrease testosterone production can be loaded onto the surface of nanospheres by double emulsion solvent evaporation method. The encapsulation of this drug was found to have improved that release profile of the drug (Nicoli et al. 2001). However, this drug-nanoparticle complex has yet to be tested in vivo (Nicoli et al. 2001, Kumari et al. 2010).
PLGA nanoparticles were found to be highly effective in increasing the half-life and stability of several drugs. Cisplatin, a drug which interferes with cell division by triggering DNA damage response, can be encapsulated by PLGA via double emulsion to increase the half-life in the bloodstream, demonstrated in mice (Avgoustakis et al. 2002, Kumari et al. 2010). Xanthones is an anticancer agent that can be loaded onto the surface of nanospheres by solvent displacement and was found to increase stability up to 4 months (Teixeira et al. 2005, Kumari et al. 2010). A rose bengal formulation, which can make singlet oxygen to treat melanoma, can be encapsulated by PLGA via interfacial deposition to increase the half-life in the bloodstream, demonstrated in rats (Redhead et al. 2001, Kumari et al. 2010).
3.1.2 poly(alkyl cyanoacrylate) nanoparticles (PACA)
Poly(alkyl cyanoacrylate) polymer-based nanoparticles (PACA) are made by emulsion polymerization and can incorporate drugs with van-der-Waals interactions (Wohlfart et al. 2011). PACA nanoparticles have become popular do to the fact that they can be easily tailored to target specific tissues. In order to bypass the BBB, they are typically coated with surfactants such as Tween 80 (Ćurić et al. 2015; Wohlfart et al. 2011). Most PACAs are safe to use since they are biodegradable and can be easily made in large quantities (Ćurić et al. 2015). Modified PACA nanoparticles have been used in studies involving neurodegenerative diseases such as Alzheimer’s since it has the ability to cross the BBB and cause minimal inflammation (Orlando et al. 2013). A 2013 study used several types of nanoparticles including PACA to deliver phosphatidic acid or cardiolipin to the brain (Orlando et al. 2013). These nanoparticles were found to be a potential therapeutic for Alzheimer’s due to their limited effect on vascular homeostasis (Orlando et al. 2013). However, they may cause an increase in nitric oxide production in target cells when using high doses (Orlando et al. 2013)
3.1.2.1 poly(butyl cyanoacrylate)-PBCA
The poly(butylcyanoacrylate) nanoparticles (PBCA) are a member of PACA family. In general, PBCA are BBB-penetrable, non-toxic, and have a wide variety of applications. They cross the BBB by transcytosis (Åslund et al. 2015). PBCAs have been found to be non- toxic by themselves, although, the loaded drug cargo could potentially be toxic to cells (Melguizo et al. 2015). Additionally, PBCAs can be used to transfer fluorescent dyes across the BBB which can be useful in imaging studies (Åslund et al. 2015). Although PBCAs show promise as drug delivery systems, they can only last a few days inside the body due to the fact that the polymers become polymeric acids that dissolve in water (Kuo, 2005). Furthermore, these nanoparticles are hydrophobic, as a result they are resisted to certain types of therapeutics and they do not work well with hydrophilic drugs (Kuo, 2005).
PBCAs can be made by drop wise adding an acetonic monomer solution to a solution with HCl and dextran (Kuo, 2005; Melguizo et al. 2015). After a few hours, NaOH is added, then acetone is removed by evaporation, and the nanoparticles ae purified by centrifugation (Melguizo et al. 2015). If a drug, such as the anti-tumor drug DOX, was to be incorporated into the PBCAs, it would be added at the first step with the monomer (Kuo and Chen, 2006; Melguizo et al. 2015). PBCA stability can be improved using a lyophilization method involving freeze-drying (Kuo, 2005). Ćurić et al were able to make PBCA nanoparticles smaller than 80 nm with a high concentration of itraconazole, which has previously been difficult to work with since it is not very soluble (Ćurić et al. 2015). They have greatly increased the shelf-life and decreased the leakage rate of itraconazole-loaded PBCA nanoparticles by using the freeze-drying method (Ćurić et al. 2015).
These types of nanoparticles have also shown promise in the use of antitumor drugs (Melguizo et al. 2015). DOX, a commonly used chemotherapy for solid tumors, has been loaded into poly(butylcyanoacrylate) nanoparticles, a type of PACA nanoparticles in order to increase delivery efficacy of the drug to previously inaccessible lung adenocarcinomas (Melguizo et al. 2015). The drug-nanoparticle complex significantly increased cellular uptake of DOX thereby reducing tumor volumes and increasing survival rates (Melguizo et al. 2015).
3.1.2.2 poly(isohexyl cyanoacrylate) PIHCA
Poly(isohexyl cyanoacrylate) nanoparticles (PIHCA) are another type of PACA. PIHCA are being studied to aid in cancer treatment, specifically liver and brain cancer, incorporating the drug DOX (Wohlfart et al. 2011). Surfactant-loaded PIHCA are made by adding isohexylcyanoacrylate to a stabilizer (poloxamer 188 or dextran 70,000) in HCl (Wohlfart et al. 2011). After polymerization, NaOH is added to the solution, which is then filtered then resuspended in polysorbate 80 (Wohlfart et al. 2011). When a drug is incorporated into the nanoparticles, it is added during polymerization step (Wohlfart et al. 2011). DOX, an anti-tumor drug, loaded into nanoparticles in this way has been shown to have higher potency in rat glioblastoma cells compared to free DOX, as measured by several outcomes such as tumor size, proliferation activity, vessel density, necrotic areas, and expression of glial fibrillary acidic protein (Wohlfart et al 2011). Furthermore PIHCA-DOX nanoparticle complexes may have increased efficiency compared to DOX-loaded PBCAs (Wohlfart et al. 2011).
3.1.3 Methacrylate Nanoparticles
Polymethylmethacrylate (PMMA), methylmethacrylate (MMA), poly(glycidyl methacrylate) (PGMA) and sulfopropylmethacrylate (SPM) are all biocompatible polymers used to create nanoparticles with low to no toxicity (Kuo and Lee, 2012). These polymers can be altered to carry several types of therapeutic agents. For example, when MMA and SPM are copolymerized, incorporated drugs like anti-retrovirals can be transported across the BBB (Kuo and Chen, 2006; Kuo and Lee, 2012). When MMA-SPM are charged, they are able to carry hydrophilic drugs, an ability absent from the PBCA nanoparticles (Kuo, 2005). SPM is normally negatively charged since it is a strong acid (Kuo and Chen, 2006), and therefore in order to make these nanoparticles, SPM is mixed with MMA in deionized water, then an initiator (APS) is added and the solution is incubated overnight (Kuo, 2005). The solution is then filtered, frozen, and lyophilized with mannitol (Kuo, 2005). A 2015 study utilized a hybrid nanoparticle consisting of a network of poly(glycidyl methacrylate) chains secured to a silica nanoparticle (Li et al. 2015). Li et al. found this type of hybrid nanoparticle to be an ideal anti-cancer drug carrier, specifically of DOX (Li et al. 2015). The DOX-loaded nanoparticles were effective in inhibiting the growth of the cancer cell line, A549 (Li et al. 2015).
3.1.4 Human serum albumin (HSA)
Serum albumin, a protein present in blood, has been used to construct nanoparticles. Albumin has been studied as a drug delivery agent since the 1970s, and has been found to require cross-linking or stabilization before it can be effective as a drug carrier (Shton et al. 2015). The nanoparticles are made by first dissolving HSA in deionized water while a lipophilic drug and cholesterol are dissolved each in a chloroform-ethanol solution (Thao et al. 2016). The solutions were then mixed together followed by homogenization (Thao et al. 2016). Finally, the chloroform was evaporated, nanoparticles centrifuged, and further purified by lyophilization (Thao et al. 2016). These biodegradable nanoparticles are used in targeting of tumor cells because many tumor cells have a large number of albumin receptors on their surface (Shton et al. 2015; Thao et al. 2016). Such nanoparticles have also been effective in targeting autoimmune disorders such as rheumatoid arthritis (RA) (Thao et al. 2016). A 2016 study used albumin- based nanoparticles to increase the delivery of tacrolimus (TAC), an immunosuppressive drug used to treat RA (Thao et al. 2016). The TAC- nanoparticle complex was successful in significantly reducing the incidence of rheumatoid arthritis compared to an intravenously delivered free TAC solution and an orally administered TAC solution (Thao et al. 2016).
3.1.5 PCL (poly-ε-caprolactone)
Poly-ε-caprolactone (PCL) is an FDA-approved biodegradable nanoparticle composed of biodegradable polyester that can easily attach to cells (Daňková et al. 2015). These nanoparticles are found to be relatively safe due to the fact that the degradation process of PCL does not cause acidification (Daňková et al. 2015). These nanoparticles are typically created by dissolving PLGA, PCL, and the drug of choice in dimethylformamide/acetonitrile, then adding the dissolved solution to deionized water, followed by dialysis of the solution against deionized water. After dialysis, the solution is centrifuged and filtered to purify the nanoparticles. PLGA is sometimes used as the outer matrix of the nanoparticles, and PCL is typically used to actually encapsulate the drug (Song et al. 2015). The several drugs, including Disulfiram (DSF), a drug used to treat alcoholism that has been repurposed to target tumors, have recently been incorporated into poly-ε-caprolactone nanoparticles to increase the stability of the drug and also control release (Song et al. 2015). In a 2015 study, Song et al. used ethoxy poly(ethylene glycol)-b-poly(lactide-co-glycolide)/poly(ε-caprolactone) or mPEG-PLGA/PCL nanoparticles to incorporate DSF (Song et al 2015). The results showed that mPEG-PLGA/PCL nanoparticles successfully inhibited tumor growth by 43.2% in 24 days compared to a 0% inhibition of tumor growth exhibited by free DSF (Song et al. 2015).
3.1.6 Hydrogels
Hydrogels are becoming increasingly popular in the field of nanomedicine because of their high water content. They are composed of a 3D network of hydrophilic polymers. Hydrogels promote drug stability, but they also tend to have high drug diffusivity (Wischke et al. 2013). Since hydrogels have such a high diffusivity, methods must be taken to prevent the drug from diffusing too quickly, selecting proper polymeric mesh size, utilizing specific types of bonding, and creating hybrid nanoparticles via selection of an outer coating (Wischke et al. 2013). Hydrogels are easily modified by taking advantages of protein-ligand interactions and antibody-antigen interactions (Wischke et al. 2013). These modifications can be used to decrease mesh size or to trap the drug until the nanoparticle receives a specific release signal (Wischke et al. 2013). Hydrogels are of particular interest to the field of cancer treatments because they have shown to be a tractable, biodegradable, and biocompatible drug delivery system of chemotherapy drugs (Liu et al. 2016). Liu et al. loaded DOX into an injectable hydrogel created with sericin, a natural photoluminescent and dextran, a branch polysaccharide (liu et al. 2016). These injectable hydrogels inhibited melanoma tumor growth by 50% compared to free DOX administration in male C57BL/6 mice models (Liu et al. 2016). Additionally, the hydrogels increased survival rate of the mice by 33% compared to those mice treated with free DOX (Liu et al. 2016).
3.1.6.1 Hybrid hydrogel-glassy nanoparticles
Hydrogel based nanoparticles have been shown to have substantial promise for drug delivery of many therapeutics. A significant limitation associated with a pure hydrogel based nanoparticle platform is that many of the polymeric structures comprising the hydrogel are permeated with channels. These channels can make the resulting nanoparticles porous with respect to the solvent and the deliverable. As a result drug release may be much more rapid than is therapeutically desirable. A solution to this problem has emerged that not only allows for controlled release of deliverables but allows for a solid state reDOX reaction that generates nitric oxide (NO) in situ within the nanoparticles. The idea for this technology emerged from molecular biophysics studies using both silane derived hydrogels to restrict large amplitude protein dynamics (Samuni et al. 2008, Samuni et al. 2006, Samuni et al. 2002, Khan et al. 2000) and sugar derived glassy matrices (Dantsker et al. 2002, Gottfried et al. 1996) to limit small side chain fluctuations in proteins. In the course of these studies it was found that sugar derived glassy matrices can support long range reDOX reactions (Ray et al. 2002, Navati and Friedman 2010, Navati and Friedman 2009, Navati and Friedman 2006) and that these property could be used to generate NO from nitrite. The production of NO within the glassy matrices was highly efficient but NO release occurred in a burst when the glass was exposed to an aqueous environment. In an attempt to slow the release, the glass concept was combined with the hydrogel concept with the idea being that strong hydrogen binding between a glass-forming sugar or polysaccharide with the hydrogel back side chains would create a glassy interior within the hydrogel. It was found that chitosan in combination with tetramethoxysilane derived hydrogel formulation not worked but spontaneously formed nanoparticles (Friedman and Friedman 2009, Friedman et al. 2008). The resulting nanoparticles, which also included a small amount of PEG, allowed for the solid state reduction of nitrite into NO. The resulting nanoparticles have the following important properties: They are stable when dry and only begin to release NO when exposed to water. This effect is attributable to water induced disruption of the hydrogen bonding network responsible for the glassy interior. The release rates for NO can be modulated through several minor modifications of the preparative process including the use of different sized PEG chains within the nanoparticles as well as doping the with different trimethoxy silanes as means of tuning the internal hydrophobicity of the nanoparticles. The mechanism of production of NO within these nanoparticles can be either (or a combination) the protonation of nitrite under high nitrite levels to generate first nitrous acid and then N2O3 or a direct reduction of nitrite in the presence of protons to yield NO and water.
The NO releasing nanoparticles (NOnp) have been extensively tested in several in vivo settings including both topical applications and systemic applications. The results indicate that this platform is highly effective when administered either topically or IV. The topical studies show that not only are these nanoparticles extremely efficient as broad spectrum antimicrobials (including ESKAPE organisms and fungi) (Qin et al. 2015, Friedman et al. 2014, Schairer et al. 2012, Friedman et al. 2012, Friedman et al. 2011, Mihu et al. 2010, Martinez et al. 2009, Han et al. 2009) but also accelerate wound healing (Han et al. 2012, Blecher et al. 2012). These nanoparticles have the capacity to penetrate the top layers of skin and deliver their payload. This property is dramatically evidenced in studies using NOnp as a topical treatment of erectile dysfunction (ED) in rat models (Han et al. 2010, Tar et al. 2014) and in treatment of MRSA infected abscesses (Han et al. 2009, Schairer et al. 2012). Most recently, NOnp has been shown (Qin et al. 2015) to have a direct impact on the inflammasome response in a preclinical acne study, indicating that the combination of NO effects including anti-inflammatory and antimicrobial perform well for treatment of a wide range of topical indication. Therapeutic implications for systemic indications are evident from the IV infusion of NOnp, which has been shown to produce a sustained vascular response (Cabrales et al. 2010) that includes anti-inflammatory properties as well as vascular influences, the most significant of which is enhanced tissue perfusion, as reflected in several physiological parameters, the most important being functional capillary density. These properties have been manifest in the positive consequences of using IV infused NOnp in animal models evaluating efficacy in response to acellular hemoglobin induced toxicity (Cabrales et al. 2011) and to hemorrhagic shock (Nachuraju et al. 2011).
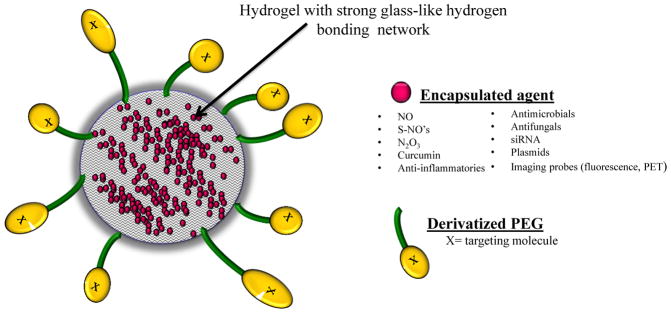
The nanoparticle platform used for NOnp is also suitable to the delivery of a very wide range of therapeutics. To date, the platform has been used both for topical delivery of curcumin (Krausz et al. 2015), antifungals (Sanchez et al. 2014 ), S-nitrosothiols ( Nacharaju et al. 2012, Mordorski et al. 2015), siRNA (Charafeddine et al. 2015), peptides (Han et al. 2010) and PDE5 inhibitors (Han et al. 2010) and for systemic delivery of S-nitrosothiols derivatives of N-acetylcysteine (Nacharaju et al. 2012). The platform can be surface modified to tune surface charge as well as introducing large PEG chains. Preliminary studies (Friedman, Navati and Cabrales) show that PEG2K coated nanoparticles (NOnp) have greatly extended circulation half-life (from several hours to days). A schematic of this platform that includes potential deliverables as well as surface modifications is shown in Figure 2.
Figure 2. Hybrid hydrogel-glassy nanoparticle structure, potential encapsulated agents, and surface modifications.
The hydrogel-glassy nanoparticle platform are used for the delivery of many therapeutics. Specifically, the platform has been used both for topical delivery of curcumin (Krausz et al. 2015), antifungals (Sanchez et al. 2014 ), S-nitrosothiols (Mordorski et al. 2015, Nacharaju et al. 2012), siRNA (Charafeddine et al. 2015), peptides (Han et al. 2010), PDE5 inhibitors (Han et al. 2010) and for systemic delivery of S-nitrosothiols derivatives of N-acetylcysteine (Nacharaju et al. 2012). To further tailor the nanoparticle, the surface can be modified to tune surface charge as well as introducing large PEG chains. Preliminary studies (Friedman, Navati and Cabrales) show that PEG2K coated nanoparticles (NOnp) have greatly increased circulation half-life from several hours to days.
3.2 Lipid Based Nanoparticles
3.2.1 Solid lipid nanoparticles (SLN)
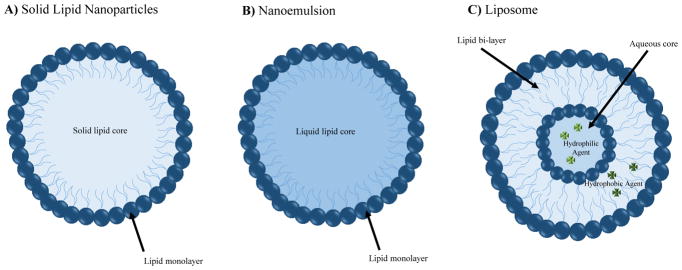
Solid lipid nanoparticles (SLN) are made of glycerides, waxes, or fatty acids, all solid lipids, and stabilized with emulsifiers like phospholipids, Tween, or bile salts (Gastaldi et al. 2014). These nanoparticles are typically spherical in shape and contain a solid lipid core, which makes them ideal carriers of lipophilic and hydrophobic drugs, as seen in Figure 3A. SLNs are made by high pressure homogenization, specifically called hot homogenization, that utilizes high pressure and high temperatures (Gastaldi et al. 2014). Currently, additional methods which do not involve high pressure and temperature are being designed in order to provide a safer creation method. These alternative methods include microemulsion templates, coacervation, solvent-based, membrane contactor, melt- emulsification and supercritical fluid technology methods (Gastaldi et al. 2014). For example, the melt-emulsification method, a technique generally thought of as a safer alternative to high pressure homogenization, melts lipids, then a stabilizer is added, then the solution is diluted and slowly cooled so the nanoparticles form with a solid lipid center with phospholipid and a stabilizer on the outside (Orlando et al. 2013).
Figure 3. Various Nanoparticle, Nanoemulsion and Nanocarriers utilized for delivery of active molecules.
A) Solid Lipid Nanoparticle Schematic. Solid lipid nanoparticles (SLN) are made of glycerides, waxes, or fatty acids, all solid lipids. Furthermore these nanoparticles are stabilized with emulsifiers like phospholipids, Tween, or bile salts (Gastaldi et al. 2014). They are typically spherical in shape and contain a solid lipid core, which makes them ideal carriers of lipophilic and hydrophobic drugs. B) Nanoemulsion Schematic. Nanoemulsions are made by adding oil to drug solution diluted in ethanol and stirring so the ethanol evaporates (Vyas et al. 2008). Nanoemulsions are ideal carriers of lipophilic and hydrophobic drugs. C) Liposome Nanocarrier Schematic. Liposomes consist of an aqueous core surrounded by a phospholipid bilayer, have been shown to be effective in encapsulating both hydrophobic and hydrophilic drugs. Liposomes are often a sphilglomyelin-cholesterol nontoxic structure, about 100 nm, that can contain nucleic acid, protein, or even some types of drugs (Kooijmans et al. 2012; Orlando et al. 2013).
Solid Lipid nanoparticles have a variety of applications, but research shows these nanoparticles work best when delivered orally, parenterally, and topically (Gastaldi et al. 2014). Due to the fact that SLNs are lipid based, these nanoparticles are biocompatible, extremely stable, are not made using organic solvents which makes them a safe means of drug delivery (Gastaldi et al. 2014). Additionally, SLNs are easily mass produced, easily sterilized (Gastaldi et al. 2014). As these nanoparticles are generally 120–200 nm, they are able to bypass filtration in the liver and spleen, can be modified to target specific tissue or cell types that were previously unreachable via the attachment of ligands to their surface (Gastaldi et al. 2014). For example, solid lipid nanoparticles show major promise in the treatment of neurological issues. Battaglia et al. utilized DOX loaded SLNs to bypass the BBB for the treatment of glioblastoma (Battaglia et al. 2014). The results showed that DOX maintained its cytotoxic effect towards tumor cells despite being encapsulated in the SLN (Battaglia et al. 2014). These results are promising as most pharmaceuticals have a difficult time maintaining potency as they cross the BBB.
3.2.2 Nanoemulsions
Nanoemulsions are made by adding oil to drug solution diluted in ethanol and stirring so the ethanol evaporates (Vyas et al. 2008). The aqueous phase was made with deionized distilled water and egg phosphatidylcholine (Lipoid E80®) and deoxycholic acid (Vyas et al. 2008). Both oil and aqueous phase are heated on a hot plate before stirring together and sonicating (Vyas et al. 2008). Nanoemulsions are heated again on hot plate, cooled to room temperature, filtered through 0.45 μm filter, and stored at 4°C in the dark (Vyas et al. 2008). Using a similar technique, a 2014 group effectively encapsulated a chemically modified DALDA (a mu-opioid peptide analogue) (Shah et al. 2014). The encapsulated analgesic peptide was administered to rodents, and functional magnetic resonance imaging (fMRI) was performed. The fMRI study showed that the administered nanoemulsion was both well tolerated and provided analgesic effects upon a painful stimulus (Shah et al. 2014). A schematic diagram of nanoemulsions can be seen in Figure 3B.
3.3 Cell derived Nanoparticles
3.3.1 Liposomes
Liposomes, a nano-delivery system that consists of an aqueous core surrounded by a phospholipid bilayer, have been shown to be effective in encapsulating both hydrophobic and hydrophilic drugs, as shown in Figure 3C. Liposomes are often a sphilglomyelin-cholesterol nontoxic structure, about 100 nm, that can contain nucleic acid, protein, or even some types of drugs (Kooijmans et al. 2012; Orlando et al. 2013). Liposomes do not contain nearly as many proteins and genetic material as extracellular vesicles such as exosomes, which allows for easier elucidation of the effects of each individual component (Kooijmans et al. 2012). Despite this, many researchers are trying to modify liposomes to look like exosomes when delivered in vivo (Kooijmans et al. 2012). They can be made by dissolving lipids in chloroform and methanol, drying them in a vacuum, rehydrating in saline, and filtering them to achieve the end product (Orlando et al. 2013). Another production method is to dissolve the lipids in chloroform, evaporate the solvent with nitrogen gas, vacuum desiccation, dilute the lipids with a saline solution containing fentanyl, and finally sonicate the solution (Hoekman et al. 2014).
Several drugs encapsulated by liposomes have reached the clinical trial stage (Kooijmans et al. 2012; Puri et al. 2009). For example, Marqibo, a vincristine sulfate liposome injection formulated for non-Hodgkin lymphoma, was able to deliver twice the dose intensity compared to standard vincristine in a phase II study (Rodriguez et al. 2009). A phase III clinical trial used a cytarabine liposome injection, DepoCyt, to improve the treatment of neoplastic meningitis (NM) (Phuphanich et al. 2007). The results showed an increase in the concentration of cytarabine in both the ventricular and lumbar cerebrospinal fluid that was detectable for up to 14 days post-administration resulting in longer exposure of the target tumor to therapeutic concentrations of cytarabine (Phuphanich et al. 2007). This is a drastic increase compared to free, non-liposome encapsulated cytarabine which could only occasionally be detected (Phuphanich et al. 2007).
Despite the promise showed by liposomes as drug delivery vehicles, they remain expensive, difficult to sterilize, and possess a short shelf life (Jeong et al. 2007). Another potential drawback to using liposomes to deliver drugs is their ability to release contents to a localized target. For this to occur a trigger is required for the liposome membrane to disrupt and expel the contents and a ligand or receptor is required for the liposome to target to specific cells or areas of the body (Puri et al. 2009). This required mechanism makes the application of liposome drug delivery systems more complex leading to other possible complications.
3.3.2 Exosomes
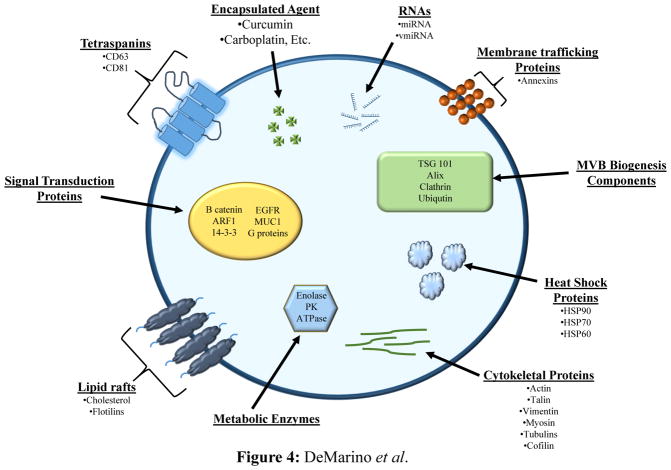
Exosomes, Figure 4, are small secreted vesicles about 40–100 nm released from all cell types (Sun et al. 2010, Kooijmans et al. 2012, Batrakova and Kim, 2015) and have so far been found in several bodily fluids including plasma, lymph, breast milk, semen, saliva, amniotic fluid, and urine (Aryani and Denecke, 2014). They can attach to target cell membranes because of the numerous surface proteins and ligands, such as tetraspanins and integrins, and transfer material into the recipient cells (Batrakova and Kim, 2015). Exosomes can carry proteins present in all cell types or proteins specific for the cell of origin (Batrakova and Kim, 2015). Recipient cells can be a great distance away, yet acquire information from exosomes (Aryani and Denecke, 2014). Since exosomes are made by the host, they can be considered immune privileged vesicles that can carry foreign material across otherwise impassable barriers. Exosome formation occurs within a cell when the endosomal membrane buds inward to create multivesicular bodies (MVB) (Batrakova and Kim, 2015). MVB may be sent to the lysosome to be degraded, or can be sent to the surface membrane and released outside the cell (Aryani and Denecke, 2014; Batrakova and Kim, 2015). It is important to note there are other types of extracellular vesicles. Microvesicles are 100–500 nm, formed by outward budding of the cell membrane, and can be difficult to separate from exosomes ( Kooijmans et al. 2012, Batrakova and Kim, 2015). There are a few databases available internationally online called Vesiclepedia (http://microvesicles.org/) and ExoCarta (http://www.exocarta.org/) (Kooijmans et al. 2012) that contain information on the types of extracellular vesicles and the different proteins, lipids, and nucleic acids found within (Aryani and Denecke, 2014).
Figure 4. Exosomes as nanocarriers.
Exosomes, small secreted vesicles about 40–100 nm, are released from all cell types (Batrakova and Kim, 2015; Kooijmans et al. 2012; Sun et al. 2010) and have been found in several bodily fluids (Aryani and Denecke, 2014). Exosomes possess the ability to attach to target cell membranes due to numerous surface proteins and ligands, such as tetraspanins and integrins, and therefore are able to transfer material into the target recipient cells (Batrakova and Kim, 2015).
There are several methods to analyze exosomes such as flow cytometry, mass spectrometry (MS), and western blotting (Batrakova and Kim, 2015). Using these methods, several protein markers have been found to distinguish exosomes from other extracellular vesicles. These include TGS101, CD9, CD81, CD63, and Alix (Aryani and Denecke, 2014; Batrakova and Kim, 2015; Kooijmans et al. 2012). The most common method of exosome purification is multiple ultracentrifugation and filtration steps (Aryani and Denecke, 2014; Batrakova and Kim, 2015). Other purification methods use chromatography and polymer-based precipitation (Batrakova and Kim, 2015).
Recently, exosomes have been studied for possible use in therapeutics. This requires loading a drug into the exosomes, which can be accomplished by these different methods: purify the exosomes from donor cell media then load the drug; add drug to donor cells then isolate exosomes from the media; or transfect donor cells with DNA that encodes for the drug, then isolate the exosomes from the media (Batrakova and Kim, 2015).
The first method using naïve exosomes works by incubating the exosomes with a lipophilic drug that will passively load itself into the exosomes (Sun et al. 2010; Zhuang et al. 2011, Batrakova and Kim, 2015). This same technique has been modified using electroporation in order to load short interfering RNA (siRNA) into exosomes (Alvarez-Erviti et al. 2011, Kooijmans et al. 2012, Batrakova and Kim 2015). Additionally, permabilization agents, such as saponin, can be used to increase loading efficiency (Batrakova and Kim, 2015). The second method of loading exosomes via adding drug to donor cells then isolating exosomes from the donor media, has been successfully used with anticancer drugs Etoposide, Irinotecan, Carboplatin, and Mitoxantrone (Batrakova and Kim, 2015). The last method of genetically modifying the donor cells by transfecting them with DNA that encodes for the desired drug was shown to work when a plasmid was transfected into macrophages (Batrakova and Kim, 2015).
These techniques have been shown to increase the delivery of pharmaceuticals to their target location. For example, Sun et al. used curcumin, an anti-inflammatory agent, loaded into exosomes to started monocyte-derive myeloid cells in order to reduce inflammation due to autoimmune and inflammatory diseases (Sun et al. 2010). They found that the exosome delivery of curcumin increased both stability and concentration in the blood without creating numerous off-target effects (Sun et al. 2010). Beyond pharmaceutical drugs, exosomes have been successful in the delivery of siRNA specific for BACE1, a target of Alzheimer’s disease, to the brain of mice (Alvarez-Erviti et al. 2011). The siRNA- exosome complexes were able to produce both mRNA and protein knockdown of BACE1, 60% and 62% knockdown respectively (Alvarez-Erviti et al. 2011).
3.4 Natural polymers
Natural polymers have become a topic of interest due to their biocompatibility and non-toxic nature. They also offer a diverse range of therapeutic applications. Some natural polymers include: chitosan, gelatin, alginate, pectin, xanthan gum, guar gum, gellan gum, and carrageenan (Jana et al. 2011).
3.4.1 Chitosan
Chitosan is a polysaccharide that acts as an antiviral, antimicrobial, and anti-tumor agent that will bind to mucus, such as in the intestines (Lai et al. 2014). Chitosan is isolated from chitin by hydration and heating the basic solution for several hours (Lai et al. 2014). Ionotropic gelation method of making chitosan nanoparticles begins with chitosan dissolved in an acidic solution diluted with water (Lai et al. 2014). Tripolyphosphate is slowly added, causing the nanoparticles to form by changing the pH of the solution (Lai et al. 2014). These nanoparticles are usually 100 nm (Lai et al. 2014). Ionotropic gelation method uses highly diluted solutions with a small yield (Grabowski et al. 2015). Chitosan nanoparticles can also be made by coacervation or nanoprecipitation methods (Lai et al. 2014). These nanoparticles are of particular interest because they possess the capability to cross mucus membranes, specifically the nasal cavity and intestinal tract, as they are able open tight junctions of epithelial cells (Vila et al. 2002, Grabowski et al. 2015). Due to chitosan’s unique ability to cross mucus membranes, a 2016 study utilized the nasal administration of chitosan nanoparticles (Hanfy et al. 2016). The chitosan complexes were used to encapsulate galantamine, a drug used to manage mild to moderate Alzheimer’s disease. This study showed no toxicity or histopathological signs and decreased the level of AChE protein and activity in the brains of rats (Hanafy et al. 2016). The efficacy and safety of these gelatin nanoparticles make them a promising candidate in future research of nasally delivered drugs.
3.4.2 Gelatin
Gelatin can be isolated from the collagen in bones before being used to make biodegradable nanoparticles by double desolvation method (Kaur et al. 2008; Lai et al. 2014). Gelatin is first dissolved in water, then acetone is added, the supernatant removed, the gelatin is redissolved in distilled water, the drug is added to the upper phase and the pH changed to <4.0, acetone is added drop wise, then the nanoparticles are cross linked with a glutaraldehyde solution, then filtered or sonicated and centrifuged to purify (Kaur et al. 2008; Lai et al. 2014). Water-in-oil emulsification method is another technique of making these nanoparticles (Lai et al. 2014). The side chains of the proteins can be adapted to allow hydrophobic drugs to be incorporated into the nanoparticles (Lai et al. 2014). Gelatin nanoparticles are frequently tailored to target specific sites with mannan, with this method they tend to be about 100–150 nm, an ideal size for allowing uptake in the lymph nodes (Kaur et al. 2008). A 2008 study showed that mannan-coated gelatin nanoparticles are an effective means of increasing the cellular uptake in macrophages. Didanosine encapsulated in mannan-coated gelatin nanoparticles showed a 5 fold increase in cellular uptake compared to the same drug in solution (Kaur et al. 2008). The same study showed an in vivo increase in biodistribution of didanosine in several locations specifically the lymph nodes, the spleen, and the brain (Kaur et al. 2008). The same nanoparticles were found to have the greatest impact on the brain, increasing the localization of dianosine 12.4 fold compared to the injection of a PBS-didanosine solution (Kaur et al. 2008).
4. NANOPARTICLES IN CNS HIV INFECTION
4.1 HIV Neuropathology
There are 34 million people living with Human Immunodeficiency Virus (HIV) as of 2014 worldwide (WHO Fact Sheet No.360, 2015). Despite years of research, HIV treatment still remains a major issue due in part to anatomical viral reservoirs within the body that accumulate latently infected cells and are unreachable by even the most effective drugs (Siliciano et al 2003). The Central Nervous System (CNS) represents one of these viral reservoirs for several reasons. It is usually infected during HIV infection; it houses an integrated provirus which can be reactived to produce new virus; and finally CNS cells have long half-lives and have the ability to establish viral latency (Coiras et al. 2009, Churchill and Nath 2013, Zayyad and Spudich 2015, Gray et al 2015).
The Trojan horse hypothesis is the predominate mechanism of HIV infection of the CNS. This hypothesis is characterized by the entry of infected peripheral blood mononuclear cells (PBMCs), mostly monocytes, across the blood brain barrier (BBB) (Peluso et al. 1985). Once infected monocytes have gained access to the CNS, they cause an increase in the expression of specific molecules to disrupt normal functioning of the BBB. The increased permeability of the BBB during HIV infection occurs as a result of aberrant levels of chemokines, chemokine receptors, adhesion molecules and matrix metalloproteinases. Additionally, there are other mechanisms which involve direct infection of blood brain barrier cells. (Hazelton et al. 2010).
Chemokine dysregulation is a major player in the increased migration of leukocytes across the blood brain barrier during HIV infection. Chemokine (C-C motif) ligand 2 (CCL2) is a chemoattractant chemokine that is found in increased levels in the cerebrospinal fluid and brain tissues of patients with HIV- associated dementia. Increased CCL2 causes an increase in the migration of PBMCs, mostly monocytes, which causes increased BBB permeability. Additionally, HIV entry into the CNS causes an upregulation of chemokine receptors such as chemokine (C-C motif) receptor 2 (CCR2), CXCR4 and CCR5 (Seilhean et al. 1997, Eugenin et al. 2006, Hazelton et al. 2010).
HIV infection in the CNS has different consequences for each type of cell. The virus can infect astrocytes, endothelial cells, microglia, and monocytes/macrophages. However, the virus has no known direct effect on neurons and oligodendrocytes (Bilgrami and O’Keefe 2014). For example, astrocytes play various roles in the uninfected, normal-functioning of the brain such as synaptic homeostasis and maintenance of the blood brain barrier. Infection causes an increase in the migration of infected astrocytes across the blood brain barrier via an increase in astrocyte adhesion molecules. In 2007, a team of colleagues proposed a model by which infected astrocytes in the CNS cause apoptosis in adjacent uninfected astrocytes and neighboring neurons via gap junctions (Eugenin and Berman, 2007). Apoptosis of uninfected astrocytes and neurons, along with increases in chemokines and inflammation, causes damage to the white matter.
HIV infection causes an increase in the expression of the chemokine (C-C motif) ligand 2 (CCL2), which in healthy individuals is responsible for recruitment of monocytes, macrophages, T-cells, and dendritic cells to the site of injury or infection. However, in an HIV-infected individual, the increase of CCL2 expression causes an increase in the migration of infected leukocytes, which have a high rate of viral replication, across the blood brain barrier. HIV infected monocytes in the CNS secrete substances such as chemokines, cytokines, and viral proteins (Seilhean et al 1997, Yi et al 2004, Eugenin and Berman 2007). Eugenin et al showed HIV infection to decrease gap junction communication in astrocytes by 40% (Eugenin and Berman 2007). The remaining functional gap junctions, however, were still able communicate important signals between the astrocytes (Eugenin and Berman 2007).
The numerous HIV viral proteins including gp120, Nef, Vpr, and Tat cause a disruption of the blood brain barrier via various sites of action and mechanisms. An envelope glycoprotein, gp120, causes an increase in permeability of the endothelial monolayer and increases monocyte migration across the BBB (Cioni and Annunziata 2002, Kanmogne et al. 2007, Alturi et al. 2015). Additionally, gp120 selectively down-regulates the tight junction proteins, ZO-1, ZO-2, and occludin, which further deteriorates BBB integrity (Kanmongne et al. 2005). Gp120 decreases BBB integrity by at most 20%, and is restored in the absence of gp120 (Kanmogne et al. 2007). Others reported up to 47% disruption of the BBB by gp120 with restoration of when anti-gp120 antibodies are added (Cioni and Annunziata 2002). Another viral protein, Nef, negative regulatory factor, has been shown to cause a rupturing of the BBB via matrix metalloproteinase-9 activity (Sporer et al. 2000, Alturi et al. 2015). Furthermore, Nef has demonstrated the ability to induce apoptosis in primary human brain microvascular endothelial cells via activation of several caspases, a couple tumor proteins, tumor necrosis factor receptor 12, MAPK, and MAPK 7 (Acheampong et al 2005). A pleiotrophic protein essential to HIV replication, Viral Protein R (Vpr), disrupts astrocyte metabolism and decreases neuronal survival via an increase in caspases, chemoattractants, and prokinflammatory cytokines (Ferrucci et al. 2013, Alturi et al. 2015). Moreover, Vpr has caused disruption of sodium and calcium levels in both neurons and astrocytes, respectively (Ferrucci et al 2013). Finally, Tat, a trans-activator of transcription, increases the number of lysosomes, philopodia, and vesicular Golgi complexes in monocytes, which results in an increased migration of moncytes across the BBB (Persidsky et al. 1997, Alturi et al. 2015). Tat also has shown to cause Rho activation, which in turn causes a decrease in tight junction integrity (Persidsky et al. 2006). HIV infected patients with severe encephalitis had an observed 54% disruption of occludin and claudin-5 in the brain (Persidsky 2006).
Although HIV disrupts the BBB, the disruption is not necessarily permanent nor is it a complete disruption. The majority of the BBB remains undisturbed. Furthermore, the percentage of BBB disruption could be improved in the subject was treated with ART drugs, however, the studies cited above include only ART naïve samples. Some ART drugs, Zidovudine, Nevirapine, and Indinavir, have above average CNS penetration whereas some ART drugs, Nelfinavr, Ritonavir, and Tenofovir, have below average CNS penetration (Letendre et al. 2010). Adequate CNS penetration of anti-retroviral drugs allows for repair of the damage caused by HIV infection whereas low CNS penetration increases the probability that the CNS will not repair itself.
4.2 Current HIV treatments
There are currently more than 20 anti-retroviral drugs used in highly active anti-retroviral therapy (HAART) for the treatment of HIV-1 in the United States. There are several classes of drugs used in combined anti-retroviral regimen typically consisting of two nucleoside reverse transcriptase inhibitors (NRTI) and one additional drug from one of three classes including non-nucleoside reverse transcriptase inhibitor (NNRTI), integrase strand transfer inhibitor (INSTI), or a protease inhibitor (PI). These regiments are sometimes supplemented with other drugs such as CCR5 antagonists in ART treated patients (NIH AIDSinfo, 2016). While HAART therapeutics has increased survival in patients, latent HIV continues to reside in reservoirs throughout the body. A major reservoir of focus is the central nervous system, which is protected by the blood brain barrier, which gives way to poor drug delivery and availability in the brain. The low permeability of ART across the blood brain barrier leads to high levels of HIV in the cerebrospinal fluid, which gives way to HIV-associated neurocognitive disorders (HAND) (Rao et al 2009, Hazelton et al 2010, Bilgrami and O’Keefe, 2014, Grey et al. 2014, Zayyad and Spudich, 2015, Gelman, 2015). Recently research has been directed towards utilizing existing transport systems via various types of nanoparticles and surface modifications.
5. USE OF BIODEGRADABLE NANOPARTICLES IN THE TREATMENT OF HIV
5.1 Polymeric Nanoparticles
Many types of polymeric nanoparticles have been successful in creating a more effective anti-retroviral therapy. Destache et al. utilized PLGA nanoparticles to encapsulate ritonavir, lopinavir, and efavirenz. Their results show that the use of polymeric nanoparticles increased the length of time the drugs were found in detectable concentrations in comparison to freely injected drugs (Destache et al. 2010). Additionally, a modified PSC-RANTES protein with a biotin tag encapsulated by PLGA has shown potential as a CCR5 receptor inhibitor (Ham et al. 2009).
Kuo and Su showed that the polymeric nanoparticles are an effective means of increasing the permability of anti-retroviral drugs across the blood brain barrier (2007). MMA-SPM nanoparticles increased BBB permeability of three anti-retroviral drugs, stavudine (D4T), delavirdine (DLV), and saquinavir (SQV), by 3–7 fold. Whereas PBCA increased by BBB of these three anti-retrovirals by 12 to 16 fold. Both MMA-SPMs and PBCAs were found to have similar loading efficiencies (LE) with regards to the same anti-retrovirals. The trend demonstrated that loading efficiency decreased with an increase in particle size (Kuo and Su, 2007). The same group later experimented with MMA-SPM modifications such as RMP-7, a pseudopeptide of bradykinin analog, in order to increase D4T, DLV, and SQV loading capabilities (Kuo and Lee 2012).
Tat-conjugated nanoparticles have been effective in the transport of the protease inhibitor, ritonavir into the central nervous system. This PLA nanoparticle works by bypassing P-glycoprotein’s efflux capabilities and enters the endothelium of the brain vasculature via transcytosis. The protease inhibitor levels with use of the conjugated nanoparticles is 800- fold higher in comparison to levels associated with the drug in solution (Rao et al 2008). Others have found that tat-conjugated nanoparticles do not cause neurotoxic side effects (Borgmann et al. 2011). HIV p24 levels were significantly decreased in the brain of the treated macrophages, showing this to be a potential anti-viral method (Borgmann et al. 2011). Further research will show if this method is as effective in other organs (Borgmann et al. 2011).
Polymeric nanoparticles show great promise in the treatment of HIV, specifically the previously inaccessible neurological components. However, further research is needed to explore the use of other types of polymeric nanoparticles such as human serum albumin, PCL, and hydrogels.
5.2 Lipid Based Nanoparticles
Kuo and Su showed that solid lipid nanoparticles are an effective means of increasing the permeability of anti-retroviral drugs across the blood brain barrier. SLNs increased BBB permeability of three anti-retroviral drugs, D4T, DLV, and SQV, by 4–11 fold. Additionally, they demonstrated that SLNs were most efficient carriers of hydrophobic/ lipophilic drugs (Kuo and Su, 2007). More specifically, SQV is a protease inhibitor that is used to treat HIV patients; it inhibits cleavage of pol and gag polyproteins and thus preventing viral maturation (Vyas et al. 2008). It is a lipophilic drug with low bioavailability and can be inhibited by the P-glycoprotein (P-gp) pathway since it is a P-gp substrate (Kuo and Chen, 2009). Entrapment of SQV within SLN increases the efficiency of drug delivery (Kuo and Chen, 2009).
Nanoemulsion techniques are used to overcome the limited drug delivery due to the efflux of P-gp. SQV, a protease inhibitor, has been shown to have enhanced oral bioavailability and brain distribution when used in a nanoemulsion made with various edible oils such as flax-seed oil, and safflower oil, which are rich in poly-unsaturated fatty acids (PUFA). Additionally, PUFA nanoemulsions caused an increased uptake of paclitaxel (P-gp substrate and anticancer agent) by the gastrointestinal tract (Vyas et al. 2008). Indinavir, another protease inhibitor, has demonstrated increase oral bioavilabity when used in transferrin-coupled solid lipid nanoemulsion. Transferrin is an endogenous glycoprotein involved in the movement of iron. In this case the naturally occurring transferrin receptors, a receptor which is overexpressed on brain cells, are utilized as an additional means of penetrating the blood brain barrier via receptor mediated transcytosis (Prabhakar et al. 2011)
5.3 Cell Derived Nanoparticles
Liposomes have shown great potential as drug carriers in the treatment of HIV. However, there is still more research needed to further develop this technology. Many techniques are used to sustain the liposome drug delivery in HIV infected systems including, prodrugs and drug polymer conjugates. In 2005, a study was conducted that showed zidovudine (AZT) entrapment in liposomes was low and leaky. In order to overcome this challenge, the AZT was modified to form a prodrug, zidovudine myristate. The prodrug was then loaded into the liposomes and injected into the subjects and circulating levels were compared to that of freely injected zidovudine myristate. The results showed a dramatic increase in circulating levels due to liposome encapsulation (Jin et al. 2005). Additionally, Garg et al. showed an increase in tissue distribution of the anti-retroviral drug D4T when encapsulated in liposomes that were mannosylated. The group compared free circulating D4T to liposome encapsulated D4T to mannosylated liposome encapsulated stavudine. The study showed the drug polymer conjugated liposomes are a promising method of site-specific and ligand-directed delivery of anti-retrovirals in HIV patients. Although liposomes show potential as drug carriers in the treatment of HIV, they are found to be most effective in the liver spleen and lungs (Garg et al 2006). While exosomes have shown to be effective drug delivery systems, further research is needed to determine if exosomes are a suitable nanocarrier for the treatment of HIV.
5.4 Natural polymers
Biological tissue engineering has led to increased interest in natural polymers especially due to their relatively safe, non-toxic nature. A 2015 study involving ursodeoxycholic acid conjugated zidovudine (UDCA-AZT) demonstrated that this conjugated form of AZT is able to escape active efflux transporters, the transporters in the brain responsible for removing drugs. The conjugated form of AZT was able to enter the brain with twenty times more efficiency compared to AZT alone. Additionally, this group used the nasal administration of UDCA-AZT encapsulated within chitosan chloride-based microparticles to increase the dissolution rate (Dalpiaz et al. 2015).
Gelatin nanoparticles have shown to successfully accumulate in the target organs via intravenous injection. Vinogradov et al. demonstrated that gelatin nanoparticles were found to produce a 15 fold increase in contents delivered to the brain compared to free circulating levels and demonstrated reduced mitochondrial toxicity. This increase in circulating levels was due to charge alternations and surface additions of brain- specific ligands (Vinogradov et al 2004). Furthermore, the same group demonstrated a reduction of neurotoxicity by modifying the same gelatin based nanoparticles with the peptide binding brain-specific apolipoprotein E receptor. The reduction of neurotoxicity was due to a decrease in the formation of reactive oxygen species and a decreased level of apoptosis compared to that of free circulating drug (Gerson et al. 2014).
5.5 siRNA ENCAPSULATION
RNA interference is a regulatory mechanism found in most eukaryotic cells which utilizes short interfering double stranded RNA (siRNA), which is 21 to 25 nucleotides in length to moderate gene activity (Aagaard and Rossi 2007). The siRNA, which is produced by cleaving longer dsRNA, then targets a short nucleotide sequence on the genome of interest (Asgaard and Rossi 2007). Consequently, researchers have recognized the potential of this system as a therapeutic. This technique is desirable because siRNA is able to silence a specific target gene even at very low concentrations (Date and Destache 2014).
HIV targets for siRNA include HIV genome targets such as nef, vpr, env, gag, pol, tat, vif, rev, and TAR in the hopes of inhibiting HIV replication and CCR5 and CXCR4 for immunization potential via down regulating HIV receptors. There is great interest in the use of siRNA as a HIV therapeutic because of its potential to bypass issues with current HIV treatment such as toxicity and drug resistance. However, due to the fact that siRNA is both extremely hydrophilic and carries a negative charge, drug delivery and cellular uptake have become an issue (Date and Destache 2014). As a response to this hurdle, researchers have begun encapsulating various siRNA molecules inside assorted nanoparticles. Furthermore, this technique is being explored as a potential therapy and/or prophylactic for patients with HIV-1.
RNA interference as a treatment for HIV offers a wide array of target sites to inhibit viral replication. A 2012 study investigated the use of pegylated poly-(ethylene imine) nanoparticles as a means of delivery of siRNA that targeted the HIV gene nef. They found that 2–3 day treatment with siRNA encapsulated in nanoparticles inhibited viral replication at a comparable level to a 15 day azidothymidine treatment (Weber et al. 2012). A similar study used nef siRNA encapsulated in nanoparticles to target HIV-1 infected primary astrocytes. The nanoparticles were found to successfully transverse the BBB and subsequently were effective in silencing the nef gene without causing cell death (Serramia et al. 2015). Mahajan et al. used siRNA specific for HIV-1 poly A/TAR RNA of the LTR region, the binding site of the Tat protien, in order to achieve viral inhibition (Mahajan et al. 2011). They found a significant decrease in both the levels of p24 and HIV-1 LTR gene expression. Furthermore, the team saw a 90% reduction of viral replication that persisted to 1 week post-intervention with greater than 95% viability (Mahajan et al. 2011).
In addition to viral replication inhibition, siRNA targeted towards the HIV-1 receptors CCR5 and CXCR4 show great promise in the field of HIV-1 vaccines. In 2002, a group of researchers used siRNA to target the HIV co-receptors. They found that within 48 hours of treatment there was a marked reduction in the expression of CXCR4 and CCR5 in 63 and 48% respectively (Martinez et al. 2002). More recently, researchers have used various nanoparticles to enhance delivery and increase effectiveness of receptor targeted siRNAs. Kim et al utilized lymphocyte function associated antigen-1 (LFA-1)-targeted immunoliposome to deliver anti-CCR5 siRNA. Their results showed that in vivo treatment with the nanoparticles resulted in gene silencing for 10 days. Additionally, mice treated with HIV post-siRNA therapy demonstrated an increased resistance to infection (Kim et al 2010). A study in 2012 showed delivery of anti-CCR5 siRNA via polymeric nanoparticles resulted in a reduction of CCR5 expression to below 15% (Yan et al 2012).
Although siRNA therapeutics shows great potential for the treatment and prevention of HIV-1, there are still limitations that need to be addressed. These limitations include; off-target silencing, the activation of the innate immune system, and competition with other RNAs (Aagaard and Rossi 2007). Further research is needed to address these shortcomings and improve the use of siRNA in the treatment of HIV-1 and other viruses.
6. CONCLUSION
The anatomy and physiology of the blood brain barrier presents a major obstacle in the treatment of neurological conditions. Currently, there are several techniques for transporting drugs across the BBB; however, there are still several limitations. The use of nanotechnology for CNS drug delivery has the potential to address the limitations of current techniques. Biodegradable nanoparticles have become an increasingly popular solution to treatment of many diseases such as cancer, diabetes, malaria, AIDS, tuberculosis, and several prion diseases due to their ability to increase bioavilability, retention and drug solubility. Additionally, they offer a variety of customizable options such as primary nanoparticle composition (polymeric, lipid-based, cell-derived, and natural materials), targeting ligands, and surface coatings to target tissues of choice. However, further research is needed to address limitations such as toxicity and dosing.
CNS HIV infection presents a complex obstacle to treatment due to the fact that the virus itself has direct impacts on the structural integrity of the BBB via several different mechanisms. Nanoparticle based approaches show promise in the field of central nervous system drug delivery of viral therapeutics, specifically in the treatment of HIV and the treatment of latent viral reservoirs. Research with several types of nanoparticles show that there is a several fold increase in anti-retroviral drug contents delivered across the BBB to the brain and also a sustained increase is circulating levels compared to drugs in solution (Garg et al 2006; Kuo and Su, 2007; Kuo and Chem, 2009). Beyond the use of anti-retrovirals, nanoparticles have been implicated in the delivery of HIV siRNAs to target sites as a potential technique to increase delivery efficiency of the siRNAs to target sites. Collectively, nanoparticles offer a potential solution to drug delivery difficulties associated with CNS diseases and infections.
Acknowledgments
We would like to thank the members of the FK lab for assistance and proof reading with the manuscript. This work was supported by National Institutes of Health grant AI070740, AI043894, AI11340, and AI114490 to FK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
8. CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Aagaard L, Rossi J. RNAi Therapeutics: Principles, Prospects and Challenges. Adv Drug Deliv Rev. 2007;59(2–3):75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott N, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Acheampong E, Parveen Z, Muthoga L, Kalayeh M, Mukhtar M, Pomerants R. Human Immunodeficiency virus type 1 Nef potently induces apoptosis in primary human brain microvascular endothelial cells via the activation of caspases. J Virol. 2005;79(7):4257–69. doi: 10.1128/JVI.79.7.4257-4269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood M. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Andrieux K, Couvreur P. Nanomedicine as a promising approach for the treatment and diagnosis of brain diseases: The example of Alzheimer’s disease. Ann Pharm Fr. 2013;71(4):225–33. doi: 10.1016/j.pharma.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Aryani A, Denecke B. Exosomes as a Nanodelivery System: a Key to the Future of Neuromedicine? Mol Neurobiol. 2014 doi: 10.1007/s12035-014-9054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åslund A, Berg S, Hak S, Mørch Ý, Torp S, Sandvig A, Widerøe M, Hansen R, de Lange Davies C. Nanoparticle delivery to the brain — By focused ultrasound and self-assembled nanoparticle-stabilized microbubbles. J Controlled Release. 2015;220:287–294. doi: 10.1016/j.jconrel.2015.10.047. [DOI] [PubMed] [Google Scholar]
- Atluri V, Hidalgo M, Samikkannu T, Kurapati KRV, Jayant RD, Sagar V, Nair MPN. Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front Cell Neurosci. 2015;9:212. doi: 10.3389/fncel.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas A, Ithakissios D. PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J Control Release. 2002;79(1–3):123–35. doi: 10.1016/s0168-3659(01)00530-2. [DOI] [PubMed] [Google Scholar]
- Balazs Z, Panzenboeck U, Hammer A, Sovic A, Quehenberger O, Malle E, Sattler W. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated α-tocopherol by an in vitro blood brain barrier model. J Neurochem. 2004;89(4):939–50. doi: 10.1111/j.1471-4159.2004.02373.x. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood brain barrier: an overview: Structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Batrakova E, Kim M. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release Off J Control Release Soc. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgrami M, O’Keefe P. Neurologic diseases in HIV-infected patients. Handb Clin Neurol. 2014;121:1321–1344. doi: 10.1016/B978-0-7020-4088-7.00090-0. [DOI] [PubMed] [Google Scholar]
- Blecher K, Martinez LR, Tuckman-Vernon C, Nacharaju P, Schairer D, Chouake J, Friedman JM, Alfieri A, Guha C, Nosanchuk JD, Friedman AJ. Nitric oxide-releasing nanoparticles accelerate wound healing in NOD-SCID mice. Nanomedicine. 2012 doi: 10.1016/j.nano.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Borgmann K, Rao K, Labhasetwar V, Ghorpade A. Efficacy of Tat-conjugated ritonavir-loaded nanoparticles in reducing HIV-1 replication in monocyte-derived macrophages and cytocompatibility with macrophages and human neurons. AIDS Res Hum Retroviruses. 2011;27:853–862. doi: 10.1089/aid.2010.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrales P, Han G, Nacharaju P, Friedman AJ, Friedman JM. Reversal of hemoglobin-induced vasoconstriction with sustained release of nitric oxide. Am J Physiol Heart Circ Physiol. 2011;300:H49–56. doi: 10.1152/ajpheart.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrales P, Han G, Roche C, Nacharaju P, Friedman AJ, Friedman JM. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med. 2010;49:530–538. doi: 10.1016/j.freeradbiomed.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charafeddine RA, Makdisi J, Schairer D, O’Rourke BP, Diaz-Valencia JD, Chouake J, Kutner A, Krausz A, Adler B, Nacharaju P, Liang H, Mukherjee S, Friedman JM, Friedman A, Nosanchuk JD, Sharp DJ. Fidgetin-Like 2: A Microtubule-Based Regulator of Wound Healing. J Invest Dermatol. 2015 doi: 10.1038/jid.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill M, Nath A. Where does HIV hide? A focus on the central nervous system. Curr Opin HIV AIDS. 2013;8(3):165–9. doi: 10.1097/COH.0b013e32835fc601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioni C, Annunziata P. Circulating gp120 alters the blood-brain barrier permeability in HIV-1 gp120 transgenic mice. Neurosci Lett. 2002;330:299–301. doi: 10.1016/s0304-3940(02)00814-5. [DOI] [PubMed] [Google Scholar]
- Coiras M, Lopez-Huertas M, Peres-Olmeda M, Alcami J. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat Rev Microbiol. 2009;7(11):798–812. doi: 10.1038/nrmicro2223. [DOI] [PubMed] [Google Scholar]
- Couvreur P, Kante B, Grislain L, Roland M, Speiser P. Toxicity of polyalkylcyanoacrylate nanoparticles II: DOXorubicin-loaded nanoparticles. J Pharm Sci. 1982;71(7):790–2. doi: 10.1002/jps.2600710717. [DOI] [PubMed] [Google Scholar]
- Ćurić A, Keller B, Reul R, Möschwitzer J, Fricker G. Development and lyophilization of itraconazole loaded poly(butyl cyanoacrylate) nanospheres as a drug delivery system. Eur J Pharm Sci. 2015;78:121–131. doi: 10.1016/j.ejps.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Dalpiaz A, Fogagnolo M, Ferraro L, Capuzzo A, Pavan B, Rassu G, Salis A, Giunchedi P, Gavini E. Nasal Chitosan microparticles target a AIDS prodrug to brain HIV sanctuaries. Antiviral Res. 2015;123:146–57. doi: 10.1016/j.antiviral.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Daňková J, Buzgo M, Vejpravová J, Kubíčková S, Sovková V, Vysloužilová L, Mantlíková A, Nečas A, Amler E. Highly efficient mesenchymal stem cell proliferation on poly-ε-caprolactone nanofibers with embedded magnetic nanoparticles. Int J Nanomedicine. 2015;10:7307–7317. doi: 10.2147/IJN.S93670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantsker D, Samuni U, Friedman AJ, Yang M, Ray A, Friedman JM. Geminate rebinding in trehalose-glass embedded myoglobins reveals residue-specific control of intramolecular trajectories. J Mol Biol. 2002;315:239–251. doi: 10.1006/jmbi.2001.5218. [DOI] [PubMed] [Google Scholar]
- Date A, Destache C. A review of nanotechnological approaches for the prophylaxis of HIV/AIDS. Biomaterials. 2014;34(26):6202–28. doi: 10.1016/j.biomaterials.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshandeh K, Erfan M, Dadashzadeh S. Encapsulation of 9-nitrocamptothecin, a novel anticare drug, in biodegradable nanoparticles: factorial design, characterization, and release kinetics. Eur J Pharm Biopharm. 2007;66:34–41. doi: 10.1016/j.ejpb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Destache C, Belgum T, Goede M, Shibata A, Belshan M. Antiretroviral release from poly(DL-lactide-co-glycolide) nanoparticles in mice. J Antimicrob Chemother. 2010;65:2183–2187. doi: 10.1093/jac/dkq318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin E, Berman J. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci. 2007;27:12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin E, Osiecki K, Lopez L, Goldstein H, Calderon T, Berman J. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci A, Nonnemacher M, Wigdahl B. Extracellular HIV-1 viral protein R astrocytic glyceraldehyde 3-phosphate dehydrogenase activity and neuronal survival. J Neurovirol. 2013;19:239–253. doi: 10.1007/s13365-013-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca C, Simoes S, Gaspar R. Paclitatexel-loaded PLGA nanoparticles:preparation, physiochemical characterization and in vitro anti-tumoral activity. J Control Release. 2002;83(2):273–286. doi: 10.1016/s0168-3659(02)00212-2. [DOI] [PubMed] [Google Scholar]
- Friedman A, Blecher K, Sanchez D, Tuckman-Vernon C, Gialanella P, Friedman JM, Martinez LR, Nosanchuk JD. Susceptibility of Gram-positive and -negative bacteria to novel nitric oxide-releasing nanoparticle technology. Virulence. 2012;2:217–221. doi: 10.4161/viru.2.3.16161. [DOI] [PubMed] [Google Scholar]
- Friedman A, Friedman J. New biomaterials for the sustained release of nitric oxide: past, present and future. Expert Opin Drug Deliv. 2009;6:1113–1122. doi: 10.1517/17425240903196743. [DOI] [PubMed] [Google Scholar]
- Friedman AJ, Blecher K, Schairer D, Tuckman-Vernon C, Nacharaju P, Sanchez D, Gialanella P, Martinez LR, Friedman JM, Nosanchuk JD. Improved antimicrobial efficacy with nitric oxide releasing nanoparticle generated S-nitrosoglutathione. Nitric Oxide. 2011;25:381–386. doi: 10.1016/j.niox.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, Friedman JM. Sustained release nitric oxide releasing nanoparticles: characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide. 2008;19:12–20. doi: 10.1016/j.niox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Friedman J, Friedman A, Cabrales P, Navati M, Nachuraju P, Davies K. Harnessing the topical and systemic therapeutic applications of nitric oxide with nitrite based nitric oxide releasing nanoparticles. Nitric Oxide. 2014;42C:121–122. [Google Scholar]
- Garg M, Asthana A, Agashe HB, Agrawal GP, Jain NK. Stavudine-loaded mannosylated liposomes: in-vitro anti-HIV-I activity, tissue distribution and pharmacokinetics. J Pharm Pharmacol. 2006;58:605–616. doi: 10.1211/jpp.58.5.0005. [DOI] [PubMed] [Google Scholar]
- Gastaldi L, Battaglia L, Peira E, Chirio D, Muntoni E, Solazzi I, Gallarate M, Dosio F. Solid lipid nanoparticles as vehicles of drugs to the brain: current state of the art. Eur J Pharm Biopharm Of J Arbeitsgemeinschaft Für Pharm Verfahrenstechnik EV. 2014;87:433–444. doi: 10.1016/j.ejpb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Gelman B. Neupathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegenertaion Reconsidered. Curr HIV/AIDS Rep. 2015;12(2):272–9. doi: 10.1007/s11904-015-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelperina S, Khalansky A, Skidan I, Smirnova Z, Bobruskin A, Severin S, Turowski B, Zanella F, Kreuter J. Toxicological studies of DOXorubicin bound to polysorbate 80-coated poly(butyl cyanoacrylate) nanoparticles in healthy rats and rats with intracranial glioblastoma. Toxicol Lett. 2002;126(2):131–41. doi: 10.1016/s0378-4274(01)00456-8. [DOI] [PubMed] [Google Scholar]
- Gelperina S, Maksimenko O, Khalansky A, Vanchugova L, Shipulo E, Abbasova K, Berdiev R, Wohlfart S. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: Influence of the formulation parameters. Eur J Pharm Biopharm. 2010;74(2):157–63. doi: 10.1016/j.ejpb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Gerson T, Makarov E, Senanayake T, Gorantla S, Poluektova L, Vinogradov S. Nano-NRTIs demonstrate low neurotoxicity and high antiviral activity against HIV infection in the brain. Nanomedicine. 2014;10:177–185. doi: 10.1016/j.nano.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried D, Peterson E, Sheikh A, Yang M, Wang J, Friedman J. Evidence for Damped Hemoglobin Dynamics in a Room Temperature Trehalose Glass. J Phys Chem. 1996;100:12034–12042. [Google Scholar]
- Grabowski N, Hillaireau H, Vergnaud J, Tsapis N, Pallardy M, Kerdine-Romer S, Fattal E. Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. Int J Pharm. 2015;482(1–2):75–82. doi: 10.1016/j.ijpharm.2014.11.042. [DOI] [PubMed] [Google Scholar]
- Gray L, Roche M, Flynn J, Wesselingh S, Gorry P, Churchill M. Is the central nervous system a reservoir of HIV-1? Curr Opin HIV AIDS. 2014;9:552–558. doi: 10.1097/COH.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidelines for the Use of Antiretroviral Agents in HIV-1 Infected Adults and Adolescents. 2016 https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0.
- Gulyaev A, Gelperina S, Skidan I, Antropov A, Kivman G, Kreuter J. Significant Transport of DOXorubicin into the Brain with Polysorbate 80-Coated Nanoparticles. Pharm Res. 1999;16(10):1564–9. doi: 10.1023/a:1018983904537. [DOI] [PubMed] [Google Scholar]
- Ham A, Cost M, Sassi A, Dezzutti C, Rohan L. Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharm Res. 2009;26:502–511. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Martinez LR, Mihu MR, Friedman AJ, Friedman JM, Nosanchuk JD. Nitric oxide releasing nanoparticles are therapeutic for Staphylococcus aureus abscesses in a murine model of infection. PLoS One. 2009;4:e7804. doi: 10.1371/journal.pone.0007804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Nguyen LN, Macherla C, Chi Y, Friedman JM, Nosanchuk JD, Martinez LR. Nitric oxide-releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition. Am J Pathol. 2012;180:1465–1473. doi: 10.1016/j.ajpath.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Han G, Tar M, Kuppam DS, Friedman A, Melman A, Friedman J, Davies KP. Nanoparticles as a novel delivery vehicle for therapeutics targeting erectile dysfunction. J Sex Med. 2010;7:224–233. doi: 10.1111/j.1743-6109.2009.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafy A, Farid R, Helmy M, ElGamal E. Pharmacological, toxicological and neuronal localization assessment of galatamine/chitosan complex nanoparticles in rats: future potential contribution in Alzheimer’s disease management. Drug Deliv. 2016;4:1–12. doi: 10.3109/10717544.2016.1153748. [DOI] [PubMed] [Google Scholar]
- Hazleton J, Berman J, Eugenin E. Novel mechanisms of central nervous system damage in HIV infection. HIV AIDS (Auckl) 2010;2:39–49. doi: 10.2147/hiv.s9186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekmatara T, Bernreuther C, Khalansky A, Theisen A, Weissenberger J, Matschke J, Gelperina S, Kreuter J, Glatzel M. Efficient systemic therapy of rat glioblastoma by nanoparticle-bound DOXorubicin is due to antiangiogenic effects. Clin Neuropathol. 2009;28(3):153–64. doi: 10.5414/npp28153. [DOI] [PubMed] [Google Scholar]
- HIV/AIDS Fact sheet No. 360. 2015 http://www.who.int/mediacentre/factsheets/fs360/en.
- Hoekman J, Srivastava P, Ho R. Aerosol Stable Peptide-Coated Liposome Nanoparticles: A Proof-of-Concept Study with Opioid Fentanyl in Enhancing Analgesic Effects and Reducing Plasma Drug Exposure. J Pharm Sci. 2014;103:2231–2239. doi: 10.1002/jps.24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana S, Gandhi A, Sen K, Basu S. Natural Polymers and their Application in Drug Delivery and Biomedical Field. J PharmaSciTech. 2011;1(1):16–27. [Google Scholar]
- Jeong J, Sugii Y, Minamiyama M, Takeuchi H, Okamoto K. Interaction between liposomes and RBC in microvessels in vivo. Microvasc Res. 2007;73(1):39–47. doi: 10.1016/j.mvr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Jin S, Bi D, Wang J, Wang Y, Hu H, Deng Y. Pharmacokinetics and tissue distribution of zidovudine in rats following intravenous administration of zidovudine myristate loaded liposomes. Pharmazie. 2005;60:840–843. [PubMed] [Google Scholar]
- Kanmogne G, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implication for pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol. 2005;64(6):498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- Kanmogne G, Schall K, Leibhart J, Knipe B, Gendelman H, Persidsky Y. HIV-1 gp120 compromises blood brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogensis. J Cereb Blood Flow Metab. 2007;27(1):123–34. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Jain S, Tiwary A. Mannan-coated gelatin nanoparticles for sustained and targeted delivery of didanosine: in vitro and in vivo evaluation. Acta Pharm Zagreb Croat. 2008;58:61–74. doi: 10.2478/v10007-007-0045-1. [DOI] [PubMed] [Google Scholar]
- Khan I, Shannon CF, Dantsker D, Friedman AJ, Perez-Gonzalez-de-Apodaca J, Friedman JM. Sol-gel trapping of functional intermediates of hemoglobin: geminate and bimolecular recombination studies. Biochemistry. 2000;39:16099–16109. doi: 10.1021/bi000536x. [DOI] [PubMed] [Google Scholar]
- Kim S, Peer D, Kumar P, Subramanya S, Wu H, Asthana D, Habiro K, Yang Y, Manjunath N, Shimaoka M, Shankar P. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol Ther. 2010;18:370–376. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijmans S, Vader P, van Dommelen S, van Solinge W, Schiffelers R. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausz AE, Adler BL, Cabral V, Navati M, Doerner J, Charafeddine RA, Chandra D, Liang H, Gunther L, Clendaniel A, Harper S, Friedman JM, Nosanchuk JD, Friedman AJ. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine. 2015;11:195–206. doi: 10.1016/j.nano.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47(1):65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB) J Microencapsul. 2013;30(1):49–54. doi: 10.3109/02652048.2012.692491. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev. 2014;71:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Krol S, Macrez R, Docagne F, Deefre G, Laurent S, Rahman M, Hajipour M, Kehoe P, Mahmoudi M. Therapeutic benefits from nanoparticles: the potential significance of nanoscience in diseases with compromise to the blood brain barrier. Chem Rev. 2013;113:1877–1903. doi: 10.1021/cr200472g. [DOI] [PubMed] [Google Scholar]
- Kumari A, Yadav S, Yadav S. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Kuo Y. Loading efficiency of stavudine on polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate copolymer nanoparticles. Int J Pharm. 2005;290:161–172. doi: 10.1016/j.ijpharm.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Kuo Y, Su F. Transport of stavudine, delavirdine, and saquinavir across the blood-brain barrier by polybutylcyanoacrylate, methylmethacrylate-sulfopropylmethacrylate, and solid lipid nanoparticles. Int J Pharm. 2007;340:143–152. doi: 10.1016/j.ijpharm.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Kuo Y, Chen H. Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate sulfopropylmethacrylate on the permeability of zidovudine and lamivudine across the in vitro blood brain barrier. Int J Pharm. 2006;327:160–169. doi: 10.1016/j.ijpharm.2006.07.044. [DOI] [PubMed] [Google Scholar]
- Kuo Y, Chen H. Entrapment and release of saquinavir using novel cationic solid lipid nanoparticles. Int J Pharm. 2009;365:206–213. doi: 10.1016/j.ijpharm.2008.08.050. [DOI] [PubMed] [Google Scholar]
- Kuo Y, Lee C. Methylmethacrylate-sulfopropylmethacrylate nanoparticles with surface RMP-7 for targeting delivery of antiretroviral drugs across the blood-brain barrier. Colloids Surf B Biointerfaces. 2012;90:75–82. doi: 10.1016/j.colsurfb.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Kuo Y, Lee C. Methylmethacrylate sulfopropylmethacrylate nanoparticles with surface RMP-7 for targeting delivery of antiretroviral drugs across the blood brain barrier. Colloids Surf B Biointerfaces. 2012;90:75–82. doi: 10.1016/j.colsurfb.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Lai P, Daear W, Löbenberg R, Prenner E. Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly(d,l-lactide-co-glycolic acid) and polyalkylcyanoacrylate. Colloids Surf B Biointerfaces. 2014;118:154–163. doi: 10.1016/j.colsurfb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Lawther B, Kumar S, Krovvidi H. Blood brain barrier. Contin Educ Anaesth Crit Care Pain. 2011;11:128–132. [Google Scholar]
- Letendre S, Ellis R, Ances B, McCutchan J. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18(2):45–55. [PMC free article] [PubMed] [Google Scholar]
- Li Q, Xu S, Zhou H, Wang X, Dong B, Gao H, Tang J, Yang Y. pH and Glutathione Dul-Responsive Dynamic Cross-Linked Supramolecular Network on Mesoporous Silica Nanoparticles for Controlled Anticancer Drug Release. ACS Appl Mater Interfaces. 2015;7(51):28656–64. doi: 10.1021/acsami.5b10534. [DOI] [PubMed] [Google Scholar]
- Liu J, Qi C, Tao K, Chang J, Xu L, Giang X, Zhang Y, Huang L, Li Q, Xie H, Gao J, Shuai X, Wang G, Wang Z, Wang L. Sericin/Dextran Injectable Hydrogel as an Optically Trackable Drug Delivery System for Malignant Melanoma Treatment. ACS Appl Mater Interfaces. 2016 doi: 10.1021/acsami.6b00959. [DOI] [PubMed] [Google Scholar]
- Mahajan S, Aalinkeel R, Reynolds J, Bindukumar N, Sykes D, Wing-Cheng L, Ding H, Bergey E, Prasad P, Schwartz S. Nanotherapeutics Using an HIV-1 Poly A and Transactivator of HIV-1 LTR-(TAR) Specific siRNA. Patholog Res Int. 2011;2011:719139. doi: 10.4061/2011/719139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, Friedman AJ, Nosanchuk JD, Friedman JM. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Invest Dermatol. 2009;129:2463–2469. doi: 10.1038/jid.2009.95. [DOI] [PubMed] [Google Scholar]
- Martinez M, Gutierrez A, Armand-Ugon M, Parera M, Gomez J, Clotet B, Este J. Suppression of chemokine receptor expression by RNA interference allows for inhibition of HIV-1 replication. AIDS. 2002;16(18):2385–90. doi: 10.1097/00002030-200212060-00002. [DOI] [PubMed] [Google Scholar]
- Mathew A, Fukuda T, Nagaoka Y, Hasumura T, Morimoto H, Yoshida Y, Maekawa T, Venugopal K, Kumar D. Curcumin Loaded-PLGA Nanoparticles Conjugated with Tet-1 Peptide for Potential Use in Alzheimer’s Disease. PLoS ONE. 2012;7(3):e32616. doi: 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med Bio. 2008;34:834–840. doi: 10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meairs S. Facilitation of Drug Transport across the Blood Brain Barrier with Ultrasound and Microbubbles. Pharmaceutics. 2015;7:275–293. doi: 10.3390/pharmaceutics7030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melguizo C, Cabeza L, Prados J, Ortiz R, Caba O, Rama A, Delgado Á, Arias J. Enhanced antitumoral activity of DOXorubicin against lung cancer cells using biodegradable poly(butylcyanoacrylate) nanoparticles. Drug Des Devel Ther. 2015;9:6433–6444. doi: 10.2147/DDDT.S92273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence. 2010;1:62–67. doi: 10.4161/viru.1.2.10038. [DOI] [PubMed] [Google Scholar]
- Mordorski B, Pelgrift R, Adler B, Krausz A, da Costa Neto AB, Liang H, Gunther L, Clendaniel A, Harper S, Friedman JM, Nosanchuk JD, Nacharaju P, Friedman AJ. S-nitrosocaptopril nanoparticles as nitric oxide-liberating and transnitrosylating anti-infective technology. Nanomedicine. 2015;11:283–291. doi: 10.1016/j.nano.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacharaju P, Tuckman-Vernon C, Maier KE, Chouake J, Friedman A, Cabrales P, Friedman JM. A nanoparticle delivery vehicle for S-nitroso-N-acetyl cysteine: Sustained vascular response. Nitric Oxide. 2012;27:150–160. doi: 10.1016/j.niox.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachuraju P, Friedman AJ, Friedman JM, Cabrales P. Exogenous nitric oxide prevents cardiovascular collapse during hemorrhagic shock. Resuscitation. 2011;82:607–613. doi: 10.1016/j.resuscitation.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal K, Singh S, Mishra D. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem Pharm Bull (Tokyo) 2010;58(11):1423–30. doi: 10.1248/cpb.58.1423. [DOI] [PubMed] [Google Scholar]
- Navati MS, Friedman JM. Sugar-derived glasses support thermal and photo-initiated electron transfer processes over macroscopic distances. J Biol Chem. 2006;281:36021–36028. doi: 10.1074/jbc.M606866200. [DOI] [PubMed] [Google Scholar]
- Navati MS, Friedman JM. Reactivity of glass-embedded met hemoglobin derivatives toward external NO: implications for nitrite-mediated production of bioactive NO. J Am Chem Soc. 2009;131:12273–12279. doi: 10.1021/ja903364h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navati MS, Friedman JM. Glass matrix-facilitated thermal reduction: a tool for probing reactions of met hemoglobin with nitrite and nitric oxide. J Phys Chem B. 2010;114:2938–2943. doi: 10.1021/jp909425z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoli S, Santi P, Couvreur P, Couarraze G, Colombo P, Fattal E. Design of triptorelin loaded nanosphere for transdermal iontophoretic administration. Int J Pharm. 2001;214(1–2):31–5. doi: 10.1016/s0378-5173(00)00632-3. [DOI] [PubMed] [Google Scholar]
- Orlando A, Re F, Sesana S, Rivolta I, Panariti A, Brambilla D, Nicolas J, Couvreur P, Andrieux K, Masserini M, Cazzaniga E. Effect of nanoparticles binding β-amyloid peptide on nitric oxide production by cultured endothelial cells and macrophages. Int J Nanomedicine. 2013;8:1335–1347. doi: 10.2147/IJN.S40297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzenboeck U, Balazs Z, Sovic A, Hrzenjak A, Levak-Frank S, Wintersperger A, Malle E, Satter W. ABCA1 and Scavenger Receptor Class B, Type I, Are Modulators of Reverse Sterol Transport at an in Vitro Blood-Brain Barrier Constituted of Porcine Brain Capillary Endothelial Cells. J Biol Chem. 2002;277(45):42781–9. doi: 10.1074/jbc.M207601200. [DOI] [PubMed] [Google Scholar]
- Pardridge W. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T, Zhou J, Piepmeier J, Saltzman W. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):701–5. doi: 10.1016/j.addr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985;147:231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Pereverzeva E, Treschalin I, Bodyagin D, Maksimenko O, Kreuter J, Gelperina S. Intravenous tolerance of a nanoparticle-based formulation of DOXorubicin in healthy rats. Toxicol Lett. 2008;178(1):9–19. doi: 10.1016/j.toxlet.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Pereverzeva E, Treschalin I, Bodyagin D, Maksimenko O, Langer K, Dreis S, Asmussen B, Kreuter J, Gelperina S. Influence of the formulation on the tolerance profile of nanoparticle-bound DOXorubicin in healthy rats: Focus on cardio- and testicular toxicity. Int J Pharm. 2007;337(1–2):346–56. doi: 10.1016/j.ijpharm.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber G, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107(12):4770–80. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Stins W, Way D, Witte M, Weinand M, Kim K, Bock P, Gendelman H, Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158:3499–510. [PubMed] [Google Scholar]
- Petri B, Bootz A, Khalansky A, Hekmatara T, Müller R, Uhl R, Kreuter J, Gelperina S. Chemotherapy of brain tumour using DOXorubicin bound to surfactant-coated poly(butyl cyanoacrylate) nanoparticles: Revisiting the role of surfactants. J Controlled Release. 2007;117(1):51–8. doi: 10.1016/j.jconrel.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Phuphanich S, Maria B, Braeckman R, Chamberlain M. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepotCyt) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J Neurooncol. 2007;81(2):201–8. doi: 10.1007/s11060-006-9218-x. [DOI] [PubMed] [Google Scholar]
- Prabhakar K, Afzal SM, Kumar PU, Rajanna A, Kishan V. Brain delivery of transferrin coupled indinavir submicron lipid emulsions--pharmacokinetics and tissue distribution. Colloids Surf B Biointerfaces. 2011;86:305–313. doi: 10.1016/j.colsurfb.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Puri A, Loomis K, Smith B, Lee J, Yavlovich A, Heldman E, Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26(6):523–80. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Landriscina A, Rosen JM, Wei G, Kao S, Olcott W, Agak GW, Paz KB, Bonventre J, Clendaniel A, Harper S, Adler BL, Krausz AE, Friedman JM, Nosanchuk JD, Kim J, Friedman AJ. Nitric Oxide-Releasing Nanoparticles Prevent Propionibacterium acnes-Induced Inflammation by Both Clearing the Organism and Inhibiting Microbial Stimulation of the Innate Immune Response. J Invest Dermatol. 2015;135:2723–2731. doi: 10.1038/jid.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramge P, Kreuter J, Lemmer B. Circadian Phase-Dependent Antinociceptive Reaction in Mice Determined by the Hot-Plate test and the Tail-Flick Test After Intravenous Injection of Dalargin-Loaded Nanoparticles. Chronobiol Int. 1999;16(6):767–77. doi: 10.3109/07420529909016944. [DOI] [PubMed] [Google Scholar]
- Rao K, Ghorpade A, Labhasetwar V. Targeting anti-HIV drugs to the CNS. Expert Opin Drug Deliv. 2009;6(8):771–84. doi: 10.1517/17425240903081705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K, Reddy M, Horning J, Labhasetwar V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29:4429–4438. doi: 10.1016/j.biomaterials.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Friedman BA, Friedman JM. Trehalose glass-facilitated thermal reduction of metmyoglobin and methemoglobin. J Am Chem Soc. 2002;124:7270–7271. doi: 10.1021/ja0201348. [DOI] [PubMed] [Google Scholar]
- Redhead H, Davis S, Illum L. Drug delivery in poly(lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterization and in vivo evaluation. J Control Release. 2001;70(3):353. doi: 10.1016/s0168-3659(00)00367-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Pytlik R, Kozak T, Chhanabhai M, Gascoyne R, Lu B, Deitcher S, Winter J. Vincristine sulfate liposomes injection (Marqibo) in heavily pretreated patients with refractory agggressive non-Hodgkin lymphoma: report of the pivotal phase 2 study. Cancer. 2009;115(15):3475–82. doi: 10.1002/cncr.24359. [DOI] [PubMed] [Google Scholar]
- Roney C, Kulkarni P, Arora V, Antich P, Bonte F, Wu A, Mallikarjuana N, Manohar S, Liang H, Kulkarni A, Sung H, Sairam M, Aminabhavi T. Targeted nanoparticles for drug delivery through the blood brain barrier for Alzheimer’s disease. J Controlled Release. 2005;108(2–3):193–214. doi: 10.1016/j.jconrel.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Samuni U, Dantsker D, Khan I, Friedman AJ, Peterson E, Friedman JM. Spectroscopically and kinetically distinct conformational populations of sol-gel-encapsulated carbonmonoxy myoglobin. A comparison with hemoglobin. J Biol Chem. 2002;277:25783–25790. doi: 10.1074/jbc.M200301200. [DOI] [PubMed] [Google Scholar]
- Samuni U, Roche C, Dantsker D, Friedman J. T- and R-state Tertiary Relaxations in Sol-gel Encapsulated Haemoglobin. In: Bolognesi MdPG, Verde C., editors. Dioxygen Binding and Sensing Proteins. A Tribute to Beatrice and Jonathan Wittenberg. Springer; Springer Berlin Heidelberg New York: 2008. pp. 133–159. [Google Scholar]
- Samuni U, Roche CJ, Dantsker D, Juszczak LJ, Friedman JM. Modulation of reactivity and conformation within the T-quaternary state of human hemoglobin: the combined use of mutagenesis and sol-gel encapsulation. Biochemistry. 2006;45:2820–2835. doi: 10.1021/bi050010i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez DA, Schairer D, Tuckman-Vernon C, Chouake J, Kutner A, Makdisi J, Friedman JM, Nosanchuk JD, Friedman AJ. Amphotericin B releasing nanoparticle topical treatment of Candida spp. in the setting of a burn wound. Nanomedicine. 2014;10:269–277. doi: 10.1016/j.nano.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Schairer D, Martinez LR, Blecher K, Chouake J, Nacharaju P, Gialanella P, Friedman JM, Nosanchuk JD, Friedman A. Nitric oxide nanoparticles: pre-clinical utility as a therapeutic for intramuscular abscesses. Virulence. 2012;3:62–67. doi: 10.4161/viru.3.1.18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilhean D, Dzia-Lepfoundzou A, Sazdovitch V, Cannella B, Raine C, Katlama C, Bricaire F, Duyckaerts C, Hauw J. Astrocytic adhesion molecules are increased in HIV-1-associated cognitive/motor complex. Neuropathol Appl Neurobiol. 1997;23:83–92. [PubMed] [Google Scholar]
- Serramia M, Alvarez S, Fuentes-Paniagua E, Clemente M, Sanchez-Nieves J, Gomez R, de la Mata J, Munoz-Fernandez A. In vivo delivery of siRNA to the brain by carbosilane dendrimer. J Control Release. 2015;200:60–70. doi: 10.1016/j.jconrel.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Shah L, Kulkarni P, Ferris C, Amiji M. Analgesic Efficacy and Safety of DALDA Peptide Analog Delivery to the Brain using Oil-in-Water Nanoemulsion Formulation. Pharm Res. 2014;31(10):2724–2734. doi: 10.1007/s11095-014-1370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shton I, Sarnatskaya V, Prokopenko I, Gamaleia N. Chlorin e6 combined with albumin nanoparticles as a potential composite photosensitizer for photodynamic therapy of tumors. Exp Oncol. 2015;37:250–254. [PubMed] [Google Scholar]
- Siliciano J, Kajdas J, Finzi D, Quinn T, Chadwick K, Margolick J, Kovacs C, Gange S, Siliciano R. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- Silva GA. Nanotechnology approaches to crossing the blood-brain barrier and drug delivery to the CNS. BMC Neurosci. 2008;9:S4. doi: 10.1186/1471-2202-9-S3-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Tang Z, Lei T, Wen X, Wang G, Zhang D, Deng M, Tang X, Chen X. Stable loading and delivery of disulfiram with mPEG-PLGA/PCL mixed nanoparticles for tumor therapy. Nanomedicine Nanotechnol Biol Med. 2015 doi: 10.1016/j.nano.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Soppimath K, Aminabhavi T, Kulkarni A, Rudzinski W. Biodegradable polymeric nanoparticles as drug delivery devices. J Controlled Release. 2001;70(1–2):1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- Sporer B, Koedel U, Paul r, Kohleisen B, Erfle V, Fontana A, Pfister H. Human immunodeficiency virus type-1 Nef protein induces blood-brain barrier disruption in the rat: role of matrix metalloproteinase-9. J Neuroimmunol. 2000;102(2):125–30. doi: 10.1016/s0165-5728(99)00170-8. [DOI] [PubMed] [Google Scholar]
- Stamatovic S, Keep R, Andjelkovic A. Brain Endothelial Cell-Cell Junctions: How to ‘Open’ the Blood Brain Barrier. Curr Neuropharmacol. 2008;6:179–192. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiniger S, Kreuter J, Khalansky A, Skidan I, Bobruskin A, Smirnova Z, Severin S, Uhl R, Kock M, Geirger K, Gelperina S. Chemotherapy of glioblastoma in rats using DOXorubicin-loaded nanoparticles. Int J Cancer. 2004;109(5):759–67. doi: 10.1002/ijc.20048. [DOI] [PubMed] [Google Scholar]
- Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang H. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther J Am Soc Gene Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tar M, Cabrales P, Navati M, Adler B, Nacharaju P, Friedman AJ, Friedman J, Davies KP. Topically Applied NO-Releasing Nanoparticles Can Increase Intracorporal Pressure and Elicit Spontaneous Erections in a Rat Model of Radical Prostatectomy. J Sex Med. 2014;11:2903–2914. doi: 10.1111/jsm.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixerira M, Alonso M, Pinto M, Barbosa C. Development and characterization of PLGA nanospheres and nanocapsules containing xanthone and 3-methoxyxanthone. Eur J Pharm Biopharm. 2005;59(3):491–500. doi: 10.1016/j.ejpb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Thao L, Byeon H, Lee C, Lee S, Lee E, Choi H, Park E, Youn Y. Pharmaceutical potential of tacrolimus-loaded albumin nanoparticles having targetability to rheumatoid arthritis tissues. Int J Pharm. 2016;497:268–276. doi: 10.1016/j.ijpharm.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Upadhyay R, Upadhyay R. Drug Delivery Systems, CNS Protection, and the Blood Brain Barrier, Drug Delivery Systems, CNS Protection, and the Blood Brain Barrier. BioMed Res Int BioMed Res In. 2014:e869269. doi: 10.1155/2014/869269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila A, Sanchez A, Tobio M, Calvo P, Alonso M. Design of Biodegradable particles for protein delivery. J Control Release. 2002;78(1–3):15–24. doi: 10.1016/s0168-3659(01)00486-2. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Batrakova E, Kabanov A. Nanogels for oligonucleotide delivery to the brain. Bioconjug Chem. 2004;15:50–60. doi: 10.1021/bc034164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas T, Shahiwala A, Amiji M. Improved oral bioavailability and brain transport of Saquinavir upon administration in novel nanoemulsion formulations. Int J Pharm. 2008;347:93–101. doi: 10.1016/j.ijpharm.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Zensi A, Wien S, Tschickardt S, Maier W, Vogel T, Worek F, Pietrzik C, Kreuter J, Von Briesen H. Uptake Mechanism of ApoE-Modified Nanoparticles on Brain Capillary Endothelial Cells as a Blood-Brain Barrier Model. PLoS ONE. 2012;7(3):e32568. doi: 10.1371/journal.pone.0032568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S, Robel S, Kimbrough I, Robert S, Ellis-Davies G, Sontheimer H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014;5:4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber N, Merkel O, Kissel T, Muñoz-Fernández M. PEGylated poly(ethylene imine) copolymer-delivered siRNA inhibits HIV replication in vitro. J Control Release. 2012;157:55–63. doi: 10.1016/j.jconrel.2011.09.059. [DOI] [PubMed] [Google Scholar]
- Wischke C, Schneider C, Neffe AT, Lendlein A. Polyalkylcyanoacrylates as in situ formed diffusion barriers in multimaterial drug carriers. J Control Release Off J Control Release Soc. 2013;169:321–328. doi: 10.1016/j.jconrel.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Wohlfart S, Bernreuther C, Khalansky A, Theisen A, Weissenberger J, Gelperina S, Glatzel M, Kreuter J. Increased Numbers of Injections of DOXorubicin Bound to Nanoparticles Lead to Enhanced Efficacy Against Rat Glioblastoma 101/8. J Nanoneurosci. 2009;1(2):144–51. [Google Scholar]
- Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood brain barrier by nanoparticles. J Controlled Release. 2012;161(2):264–73. doi: 10.1016/j.jconrel.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Wohlfart S, Khalansky A, Bernreuther C, Michaelis M, Cinatl J, Jr, Glatzel M, Kreuter J. Treatment of glioblastoma with poly(isohexyl cyanoacrylate) nanoparticles. Int J Pharm. 2011;415:244–251. doi: 10.1016/j.ijpharm.2011.05.046. [DOI] [PubMed] [Google Scholar]
- Wohlfart S, Khalansky A, Gelperina S, Begley D, Kreuter J. Kinetics of transport of DOXorubicin bound to nanoparticles across the blood brain barrier. J Controlled Release. 2011;154(1):103–7. doi: 10.1016/j.jconrel.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Wohlfart S, Khalansky A, Gelperina S, Maksimenko O, Bernreuther C, Glatzel M, Kreuter J. Efficient Chemotherapy of Rat Glioblastoma Using DOXorubicin-Loaded PLGA Nanoparticles with Different Stabilizers. PLoS ONE. 2011;6(5):e19121. doi: 10.1371/journal.pone.0019121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Liang M, Wen J, Liu Y, Lu Y, Chen I. Single siRNA nanocapsules for enhanced RNAi delivery. J Am Chem Soc. 2012;134:13542–13545. doi: 10.1021/ja304649a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhou Y, Tian W, Li H, Kang-Chu-Li, Miao X, An G, Wang X, Guo G, Ding G. Electromagnetic pulse activated brain microglia via the p38 MAPK pathway. Neurotoxicology. 2015;52:144–149. doi: 10.1016/j.neuro.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Yi Y, Lee C, Liu Q, Freedman B, Collman R. Chemokine receptor utilization and macrophage signaling by human immunodeficiency virus type 1 gp120: Implications for neuropathogenesis. J Neurovirol. 2004;10(Suppl 1):91–96. doi: 10.1080/753312758. [DOI] [PubMed] [Google Scholar]
- Yusuf M, Khan M, Khan R, Ahmed B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted Piperine solid lipid nanoparticles in an experimentally induced Alzheimer’s disease model. J Drug Target. 2013;21(3):300–11. doi: 10.3109/1061186X.2012.747529. [DOI] [PubMed] [Google Scholar]
- Zayyad Z, Spudich S. Neuropathogensis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND) Curr HIV/AIDS Rep. 2015;12(1):16–24. doi: 10.1007/s11904-014-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Büchel C, Kreuter J. Human serum albumin nanoparticles modified with apolipoprotein A-I cross the blood-brain barrier and enter the rodent brain. J Drug Target. 2010;18(10):842–8. doi: 10.3109/1061186X.2010.513712. [DOI] [PubMed] [Google Scholar]
- Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Wagner S, Buchel C, von Briesen H, Kreuter J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurons. J Controlled Release. 2009;137(1):78–86. doi: 10.1016/j.jconrel.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell R, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang H. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther J Am Soc Gene Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]