Abstract
Nociceptor sensory neurons protect organisms from dangers by eliciting pain and driving avoidance. Pain also accompanies many types of inflammation and injury. It is increasingly clear that active crosstalk occurs between nociceptor neurons and the immune system to regulate pain, host defense, and inflammatory diseases. Immune cells at peripheral nerve terminals and within the spinal cord release mediators that modulate mechanical and thermal sensitivity. In turn, nociceptor neurons release neuropeptides and neurotransmitters from nerve terminals that regulate vascular, innate, and adaptive immune cell responses. Therefore, the dialogue between nociceptor neurons and the immune system is a fundamental aspect of inflammation, both acute and chronic. A better understanding of these interactions could produce approaches to treat chronic pain and inflammatory diseases.
Keywords: neuro-immunology, pain, sensory neuron, nociceptor, inflammation
Neuronal pathways of pain sensation
Pain is one of four cardinal signs of inflammation defined by Celsus in the 1st century AD (De Medicina). Nociceptors are a specialized subset of sensory neurons that mediate pain and densely innervate peripheral tissues including the skin, joints, respiratory, and gastrointestinal tract. Various subsets of nociceptors exist, and can respond to mechanical, chemical or thermal noxious stimuli (Box 1). Nociceptor nerve terminals express ligand-gated and voltage-gated ion channels including TRPV1, TRPA1, Nav1.7, Nav1.8, and Nav1.9, which are key molecular transducers of these noxious stimuli (Box 2). Given the ability of the nervous system to propagate signals within milliseconds, nociceptors are ideally positioned to be first responders to pathogens and tissue injury. While pain is critical to induce behavioral changes that lead to avoidance of noxious stimuli, it is also increasingly clear that pain sensation is closely linked to molecular and cellular interactions between the nervous and immune systems. Immune cells release mediators that modulate nociceptor neuron activity and pain sensitivity. Nociceptors in turn release neuropeptides and neurotransmitters that act on innate and adaptive immune cells to modulate their function. Thus, neural signaling can define the pattern of immune responses and, consequently, contribute to the development of local and systemic inflammatory diseases. In this review, we discuss recent advances in understanding this bidirectional neuro-immune crosstalk in pain and inflammation.
Text Box 1.
Nociceptive Neuron Subsets and Pain Sensitization
Nociceptor neurons show remarkable diversity with various cellular subsets mediating heat, cold, and mechanical pain [103]. Specific coupling of nociceptor subtypes with distinct immune cell-types at the molecular level could be a mechanism by which neuro-immune signaling is finely tuned. Nociceptor neuron subtypes innervate different epithelial layers in the skin, lung, and gastrointestinal tract, allowing them to interface with environmental stimuli. C-fiber nociceptor neurons are non-myelinated, slow-conducting neurons that are mostly capsaicin-sensitive and often mediate thermal pain sensitivity. Aβ and Aδ nociceptor neurons are faster conducting and myelinated neurons, often mediating mechanosensation and mechanical pain sensitivity. However, these classic groupings are broad and overly simplistic. Recent work has shown that nociceptors are highly diverse with distinct molecular expression patterns of ion channels, growth factor receptors, G-protein coupled receptors, and neuropeptides. Therefore, the same cell may be able to respond to multiple sensory stimuli and mediate distinct functional outcomes.
Pain sensitization is defined as increased responsiveness of nociceptor neurons to their normal or subthreshold afferent imput by the International Association for the Study of Pain (IASP). Pain sensitization can be further categorized as hyperalgesia or allodynia. Hyperalgesia is increased pain due to a normally noxious stimuli. Allodynia is a painful response to normally innocuous mechanical or thermal stimuli.
Pain sensitization is mediated by multiple mechanisms at both the biophysical and transcriptional levels. Inflammatory stimuli including cytokines can induce phosphorylation of ligand-gated channels (e.g. TRPV1, TRPA1) or modification of voltage-gated sodium channels (e.g. Nav1.7, Nav1.8, Nav1.9), producing changes in membrane properties, increased action potential firing, and heightened sensitivity to thermal or mechanical stimuli. Ligand-gated G-protein coupled receptors are often coupled with TRP channel signaling. For example, bradykinin is released during inflammation to activate the bradykinin receptor on nociceptors, inducing phospholipase C and protein kinase A signaling, which potentiates TRPA1 opening and pain signaling. Inflammation and injury can also lead to changes in the transcriptional profiles of DRG sensory neurons with upregulation of TRP channels or other noxious molecular transducers, allowing previously unresponsive neurons to gain the ability to respond to noxious stimuli. Furthermore, inflammatory responses can act through growth factor regulation to modulate both the quantity and quality of tissue innervation by nociceptor nerve endings. Therefore, the mechanisms of pain sensitization are complex, and involve changes in nociceptor neurons at both the molecular and cellular level.
Text Box 2.
Nociceptive Ion Channels as Molecular Transducers of Pain
Nav1.7, Nav1.8, and Nav1.9 are voltage-gated sodium channels enriched in nociceptor neurons compared to other neuronal subtypes [104]. These channels shaping action potential generation and are critical for nociceptor neuron depolarization, thus mediating the initiation of pain signaling. Nav1.7 loss-of-function mutations have been linked to inability to feel pain in humans [104]. Nav1.7, Nav1.8 and Nav1.9 gain-of-function mutations have been linked to increased pain in inherited erythromelalgia and painful neuropathy [104]. Inflammatory signaling pathways in nociceptors can lead to phosphorylation or modification of cytoplasmic residues in Nav1.7, Nav1.8, or Nav1.9, which induce more-ready action potential generation and pain sensitivity [105].
Transient receptor potential (TRP) ion channels, a protein family consisting of 30 distinct subtypes in mammals are key mediators of thermal and mechanical sensation [106]. TRPV1 is the founding member of a group of TRP channels gated by temperature. TRPV1 is critical for induction of heat pain hypersensitivity and it is also activated by capsaicin, the pungent ingredient in chili peppers. TRPM8, by contrast, is gated by cold temperatures and mediates cold pain hypersensitivity. TRPV1 and TRPM8 are expressed by mostly distinct neuronal subsets in adult animals.
TRPA1 is another nociceptive ion channel that is thought to play a role in chemical and mechanical pain sensitivity. TRPA1 was first identified to mediate noxious responses to allyl isothiocyanates (from mustard oils) and allicin (garlic). These electrophilic reactive chemicals covalently modify intracellular cysteine residues of TRPA1, which leads to its gating and subsequent pain production. Mechanosensation in DRG neurons and other cell-types is mediated by the newly identified ion channel Piezo2 [107]. It remains to be determined how TRPA1 and Piezo2 synergize to mediate mechanical hypersensitivity and pain.
Modulation of Pain Sensitivity by Immune Cells
The immune system plays a critical role in pain by releasing molecular mediators that sensitize nociceptor neurons. Tissue injury and inflammation are intimately coupled to increases in pain sensation. Nociceptor peripheral nerve terminals possess receptors and ion channels that detect molecular mediators released during inflammation (Figure 1). Upon activation, action potentials are transduced to nociceptor cell bodies within the dorsal root ganglia (DRG), and relayed to the spinal cord and brain to be processed as pain. During inflammation, the threshold for nociceptor neurons to fire action potentials is reduced, leading to pain sensitivity or “hyperalgesia”. Chronic pain accompanies inflammatory conditions including rheumatoid arthritis and inflammatory bowel disease. Recent studies have aimed to define the specific immune cells and mediators involved in chronic pain. Pain tends to reduce with resolution of the tissue immune response, highlighting the importance of the immune system in neuronal sensitization. A deeper understanding of how neuro-immune mechanisms produce neuronal sensitization could lead to treatments for chronic pain.
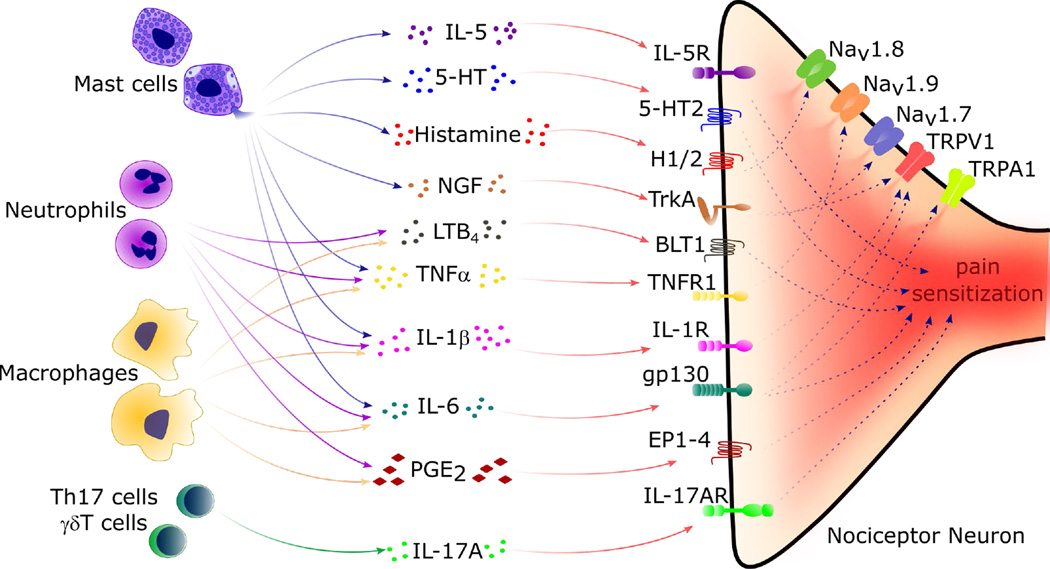
Figure 1. Immune Cells Release Mediators that Produce Peripheral Sensitization of Nociceptor Sensory Neurons and Pain.
During inflammation, tissue resident and recruited immune cells secrete molecular mediators that act on the peripheral nerve terminals of nociceptor neurons to produce pain sensitization. In these neurons, specific cytokine, lipid, and growth factor receptor intracellular signaling pathways lead to phosphorylation and/or gating of ion channels Nav1.7, Nav1.8, Nav1.9, TRPV1 and TRPA1, leading to increased action potential generation and pain sensitivity. Upon degranulation, mast cells release Interleukin 5 (IL-5), serotonin (5-HT), histamine and nerve growth factor (NGF) that act on IL-5R, 5-HT2, histamine receptor 2 (H2), TrkA, on nociceptor neurons, respectively, to produce pain sensitization. Nociceptor neurons are also sensitized by tumor necrosis factor alpha (TNFα), IL-1β and IL-6 produced by mast cells, macrophages, and neutrophils. TNFα receptor 1 (TNFR1) activation leads to phosphorylation of Nav1.9 channels. Activation of IL-1 receptor 1 (IL-1R1) increases TRPV1 expression by nociceptors, while IL-6 binds gp130 on nociceptors and this increases expression of both TRPV1 and TRPA1, enhancing responsiveness to heat and reactive chemicals. Prostaglandin E2 (PGE2) released by macrophages and other innate immune cells also sensitize nociceptor neurons through PGE2 receptors 1–4 (EP1-4). Th17 cells and γδT cells can also sensitize nociceptor neurons through IL-17A release and neuronal IL-17RA signaling.
Immune Regulation of Peripheral Pain Sensitization
Nociceptor neurons express receptors for immune cell-derived cytokines, lipids, proteases, and growth factors (Figure 1). Upon activation, these receptors mediate signaling cascades that modify the gating properties of ion channels including TRPV1, TRPA1, Nav1.7, Nav1.8, and Nav1.9 through phosphorylation or other mechanisms, leading to increased neuronal firing (For a comprehensive review of this topic, see [1]). Emerging studies have begun to elucidate the role of specific immune mediators in mediating pain sensitivity in different disease contexts. In mouse models of carrageenan-induced inflammatory pain [2] and neuropathic pain [3], neutrophils migrate to tissues where they sustain pain through production of cytokines and prostaglandin E2 (PGE2). In incisional wound injury, non-neutrophilic CD11b+ myeloid cells (likely macrophages) are responsible for pain sensitization [4]. Mast cells also play key roles in sensitizing nociceptors. Electron microscopy indicates close association of nociceptor nerve terminals with mast cells in mucosal tissues. Upon activation, mast cells degranulate and release cytokines (IL-5, TNFα, IL-6, IL-1β), 5-HT, histamine, and nerve growth factor (NGF), which act through receptors on nociceptors leading to pain sensitization [5–7]. Besides their contribution to pain during acute inflammation [8], mast cells accumulate in chronic inflammatory conditions and contribute to the chronicity of pain [9]. Macrophages are sentinel myeloid cells present throughout the body, and monocytes are blood-borne myeloid cells prominently recruited to inflammatory sites during tissue injury. A role for macrophages and monocytes in chronic painful disease conditions has been extensively demonstrated [10–14]. These cells produce many inflammatory cytokines, growth factors, and lipids that can act directly on nociceptor neurons to increase pain (Figure 1). T cells also play a role in neuropathic pain by releasing IL-17A and IFN-γ, which can act at nerve terminals to sensitize nociceptors.
In addition to neuro-immune interactions that occur at the site of injury, studies suggest that immune cells may also interact with the cell bodies (somas) of nociceptor neurons within DRGs to produce pain. The soma represents an area that controls neuroplasticity and long-term sensitization through protein synthesis. A small number of innate and adaptive immune cells reside in DRGs and there is evidence that their numbers increase in chronic pain conditions. In chemotherapy- and sciatic nerve ligation-induced neuropathic pain, there are increases in the number of macrophages, monocytes, neutrophils, and T cells in the DRG [15, 16]. T cells also release leukocyte elastase in the DRG to contribute to pain after nerve injury [17]. The role of activated mast cells in the DRG has also been related to pain in sickle cell disease [18]. Despite the work presented above, targeted analysis of how specific innate and adaptive immune cell-types mediate pain sensitization is lacking in multiple disease states. However, recent work is beginning to define the critical role of immune mediators and their activation of neuronal signaling in chronic neuropathic and inflammatory pain. We summarize below some major classes of immune mediators that have been found to directly mediate nociceptor neuron sensitization and activation.
Lipid Mediators in Pain
Non-steroidal anti-inflammatory drugs (NSAIDs) are potentially the most widely used pharmacological inhibitors of inflammatory pain. Their main mechanism of action is inhibition of cyclooxygenases (COX), which produce prostanoids (prostaglandins, prostacyclins and thromboxanes). PGE2 is a potent booster of inflammatory pain. It activates neuronal EP1-EP4 receptors and sensitizes nociceptor neurons to other painful stimuli. PGE2 acts as a sensitizer of nociceptor activity by acting on proximal ion channels, and not as a direct activator of nociceptive neurons, an essential finding in understanding the analgesic effect of NSAIDs [19]. On a longer timescale, PGE2 also induces persistent hyperalgesia via PKA and PKC-mediated activation of NFκB in DRG neurons [20]. Other than prostaglandins, it is now clear that many classes of pro-inflammatory and anti-inflammatory lipids are involved in activation and silencing of nociceptor activity (Figure 1). Lysophosphatidic acid and sphingosine-1-phosphate, for example, are produced during inflammation and act directly on nociceptor neurons to increase TRPV1 activity [21, 22]. Leukotriene B4 (LTB4) injection induces hyperalgesia in humans [23], activating C-fibers and Aδ-fibers [24]. A subset of TRPV1+ DRG neurons express BLT1, the receptor for LTB4, which mediates calcium flux in response to ligand activation [25]. Recent work also highlights a role for pro-resolving lipids in the silencing of pain (see Box 3) during the resolution phase of inflammation. Therefore, inflammatory lipids play a key role in the modulation of pain signaling.
Text Box 3.
Anti-inflammatory Lipids, Pro-resolving Lipids and Pain Blockade
It has been found that certain anti-inflammatory and pro-resolving lipids have significant abilities to silence pain. Anti-inflammatory PGJ2 signals to block pain by activating PPARy and indirect activation of K+ATP channels in nociceptors [108]. Pro-resolving lipids include lipoxins, resolvins, protectins and maresins, have generally been shown to have analgesic effects [109,110]. For instance, spinal cord astrocytes express the lipoxin receptor ALXR/FPR2, and lipoxin A4 reduces inflammatory pain by inhibiting ERK and JNK activation in astrocytes. Resolvin E1 (RvE1) receptor (ChemR23) is expressed by TRPV1 positive neurons. RvE1 inhibits TNFα and capsaicin-induced ERK activation in these DRG and spinal cord neuron. As a result, there was reduced release of excitatory glutamate, leading to diminished pain sensitivity [110]. Protectin D1 inhibits the neuronal plasticity induced by TRVP1 activation and TNFα [111], and maresin 1 inhibits TRPV1 currents in DRG neurons and inflammatory pain [110]. Both Protectin D1 and maresin 1 do not affect nociceptive mechanisms downstream of TRPA1 [110,111]. Taken together, these results are the flip side of the role of pro-inflammatory lipids such as PGE2 and LTB4 in activation of pain. Therefore, lipid mediators have an important role in both inducing and silencing nociceptor neuron sensitization and activation. Future treatment of pain may involve the induction or administration of PGJ2 and pro-resolving lipids.
Cytokines in Pain
Inflammatory cytokines derived from immune cells are critical mediators of nociceptor activity and pain sensitization. A historical perspective on the role of cytokines in pain has been reviewed elsewhere [26]. Nevertheless, it is important to mention that the first cytokine described to be hyperalgesic was IL-1β [27]. This was a seminal finding in the field of neuro-immunology demonstrating that a molecule considered as part of the immune system could induce neuronal sensitization and that a receptor antagonist could inhibit its endogenous hyperalgesic effect [27]. Overtime, a role for cytokines in pain modulation has been demonstrated in virtually all types of painful disease conditions including arthritis, neuropathic pain and cancer-related pain [26]. In particular, IL-1β, IL-6, TNFα, IL-17A, and IL-5 have been shown to act directly on nociceptor neurons (Figure 1). IL-1β sensitizes nociceptor neurons via p38 MAPK phosphorylation of Nav1.8 sodium channels, leading to increased action potential generation and resulting in mechanical and thermal hyperalgesia [28]. IL-1β also activates IL-1R1 on nociceptor neurons to increase TRPV1 expression and, consequently, pain sensitivity to thermal stimuli [29]. IL-6 also contributes to inflammatory pain by inducing prostaglandin production [30] and by binding to its signal transducer gp130 expressed by nociceptors, leading to increased TRPV1 and TRPA1 expression [31,32]. TNFα-induced neuronal sensitization and hyperalgesia is dependent on TRPV1 and TRPA1 [33,34]. This sensitization is also linked to neuronal production of prostaglandins, as TNFα-induced capsaicin responsiveness in cultured nociceptors could be blocked with COX-2 inhibitors [35]. In agreement, TNFα induced inflammatory pain in vivo is dependent on both TNFR1 and prostaglandins [30,36]. TNFα also induces rapid modulation of nociceptor sensitivity by p38MAPK mediated phosphorylation of Nav1.8 and Nav1.9 sodium channels to alter neuron excitability [33,37,38]. In a model of cancer-related pain, TNFα acted via TNFR2 to increase TRPV1 expression resulting in thermal hyperalgesia [39]. Therefore, accumulating evidence shows that IL-1β, IL-6 and TNFα act via signaling mechanisms to induce prostaglandin synthesis and/or to potentiate TRP and Nav channel activation, leading to rapid sensitization of nociceptor neurons. Autoimmune diseases such as arthritis and psoriasis are painful. It is interesting to note that those diseases are associated with Th17 responses, and that IL-17A receptors are broadly expressed by nociceptor neurons. IL-17 induces a fast increase in neuronal excitability, suggesting a functional role of IL-17A in pain during autoimmune diseases [40]. In addition, IL-17A induces hyperalgesia dependent on amplification of TNFα, IL-1β, CXCL1, endothelin-1 and prostaglandins in antigen-induced arthritis [41]. Mast cells, Th2 cells and ILC2s produce IL-5, a cytokine that mediates type 2 immunity. IL-5 can also sensitize nociceptor neurons expressing IL-5 receptors [29]. An interesting remaining question is whether specific subsets of T cells (e.g. Th1/17/2) induce distinct modalities of pain, whether heat, cold, or mechanical, due to their action of specific types of nociceptor neurons (see Outstanding Questions Box).
Outstanding Questions.
How do nociceptor neurons intersect with other branches of the nervous system (autonomic, enteric) during the regulation of peripheral immune responses.
Do distinct innate and adaptive immune cell-types communicate with nociceptor neuron subsets as determined by their phenotypic or anatomical categorization?
Do certain immune mediators, cytokines or lipids potentiate specific pain modalities? For example, is there an “immunological code” for heat, cold, or mechanical pain? Is there a similar “immunological code” for nociceptor neuron neuropeptide release?
How do immune mediators regulate epigenetic and transcriptional changes in nociceptor neurons to make the transition from acute to chronic pain?
Do nociceptor neurons regulate the immune response during the transition of acute inflammation to its resolution phase?
How do neurotransmitters and neuropeptides signal to change the functional phenotypes of innate and adaptive immune cells?
What role do nociceptor neurons play in modulating immune responses to cancer?
Do central nervous system (CNS) circuits activated by pain signaling play a role in regulating peripheral inflammation through neuro-endocrine or autonomic reflexes?
Does pain blockade by current analgesic approaches (e.g. opioids) lead to defects in host-pathogen defense or immune-mediated disease outcomes?
Can blockade of pain signaling produce new treatments for chronic inflammatory diseases including rheumatoid arthritis, psoriasis, asthma, and colitis?
Immune-Derived Growth Factors and Neurotransmitters in Pain
Tissues that are highly innervated by nociceptor neurons are more responsive to noxious stimuli and this explains why different parts of the body present differential pain sensitivities. Innervation is, however, a dynamic process modulated by neurotrophic factors that are often up-regulated during tissue injury and inflammation. These neurotrophic factors are important to restore the nerve density of an injured area but also contribute to increased pain sensitivity. Nerve Growth Factor (NGF) is produced by innate immune cells during inflammation and activates its receptor TrkA in nociceptor neurons (Figure 1). Activation of TrkA activates PI3K/Src kinase signaling which leads to phosphorylation of TRPV1 that in turn is rapidly inserted into membrane explaining the rapid sensitizing actions of NGF [43,44]. Further corroborating the NGF-induced translocation of TRPV1 to membrane, NGF induces p38 MAPK activation in DRG neurons increasing the membrane positivity to TRPV1 in peripheral terminals independently of transcription [45]. Additionally, the neurotrophic activity of these molecules causes sprouting of axon terminals and contributes to increased local pain sensitivity and autotomy [46,47]. Neurotransmitters including histamine and serotonin (5-HT) are also released by immune cells to modulate pain signaling. Mast cells contain histamine- and 5-HT-rich granules that are released upon activation. Histamine binds to H1 and H2 receptors in nociceptor neurons to increase the expression of Nav1.8 channels and cause increased sensitivity to mechanical and thermal stimuli. A critical role for histamine in pain is not restricted to allergic inflammatory conditions but is also an important mediator of neuropathic pain induced by sciatic nerve ligation [48] and inflammatory pain induced by AITC, capsaicin, and formalin [49–51]. 5-HT binding to 5-HT2 receptor and PKC activation increases the expression of neuronal acid-sensing ion channels (ASICs), which sense extracellular protons and mediate increased pain signaling [52].
Microglia Regulation of Central Pain Sensitization
Pain experience depends on efficient transmission of nociceptive information from peripheral nociceptor neurons to second order interneurons in the spinal cord. The spinal cord dorsal horn is the site where synapses between these neurons occur and critically controls pain intensity [53]. It is increasingly clear that the action of immune cells and their mediators within the dorsal horn at both pre-synaptic sites (DRG neuron central terminals) and post-synaptic sites (interneurons) play an important role in pain sensitivity. While peripheral sensitization increases nociceptive inputs to the spinal cord, spinal events triggered by these inputs lead to “central sensitization”, a process critical for the persistence of pain and contributes to its chronicity [54]. Upon nerve injury or in chronic pain conditions, nociceptor neurons express and release inflammatory mediators into the spinal cord including neurotransmitters (e.g. CGRP), cytokines (e.g. CCL2, CX3CL1, TNFα), growth factors (e.g. CSF-1), ATP, and enzymes (e.g. Caspase-6) via their central nerve terminals. These mediators communicate with and activate microglia, a key immune cell in central pain sensitization mechanisms [55–58] (Figure 2). Microglia are resident innate immune cells of the spinal cord and brain that act as sentinels of neuronal activity. Microglia are of myeloid lineage and share similarities with peripheral macrophages including production of TNFα, IL −1β, and PGE2, but also generate neurotrophins including brain-derived neurotrophic factor (BDNF) that sensitize primary nociceptor neurons and second order pain-mediating interneurons [59]. T cells also infiltrate the spinal cord during chronic pain, and play a role in neuronal sensitization (Figure 2). Curiously, the role of T cells and microglia in spinal sensitization may be gender-specific. In male mice, p38 activation in microglia following peripheral inflammation induces their expression of BDNF, which activates and sensitizes post-synaptic neurons to induce mechanical hyperalgesia, while in female mice this process is primarily mediated by infiltrating T cells [60,61]. Microglia signaling also activates resident astrocytes and oligodendrocytes, two glial cell-types that are sources of inflammatory mediators. Peripherally injured primary afferent nociceptor neurons release CX3CL1 into the spinal cord that activates microglial production of TNFα in a p38 MAPK-dependent manner. In turn, TNFα activates spinal cord astrocytes to produce CCL2 in a JNK MAPK-dependent mechanism. CCL2 activates central neurons through CCR2, culminating in neuropathic pain [58]. Astrocytes also secrete CXCL1, which activates spinal cord dorsal horn neurons expressing CXCR2 in cancer pain models [62]. Recently, it was demonstrated that spinal cord oligodendrocytes contribute to neuropathic pain by producing IL-33, which is able to activate microglia and astrocytes in mice [63]. Therefore, crosstalk between T cells, microglia, astrocytes and oligodendrocytes in the spinal cord mediate central sensitization of neuronal circuits to produce chronic pain.
Figure 2. Microglia and T Cells Mediate Central Sensitization of Pain in the Spinal Cord.
Microglia are the resident immune cells of the central nervous system, and play a key role in mediating central pain sensitization. Primary afferent nociceptor neurons transduce action potentials from the periphery to the dorsal horn of spinal cord, where synapses between first order and second order neurons occur. In chronic inflammatory or neuropathic pain, nociceptors release of mediators including caspase 6 (Casp 6), adenosine triphosphate (ATP), chemokine ligand 2 (CCL2), tumor necrosis factor alpha (TNFα), colony-stimulating factor-1 (CSF-1), and calcitonin gene-related peptide (CGRP) that activate microglia. Microglia produce inflammatory mediators including interleukin 1 beta (IL-1β), TNFα, brain-derived neurotropic factor (BDNF), and prostaglandin E2 (PGE2) which sensitize first and second order neurons. This process is called spinal sensitization and contributes to chronic pain. T cells also infiltrate the spinal cord and cross-talk with microglia cells and neurons to amplify pain sensitivity. Upon peripheral nerve injury, primary afferent nociceptor neurons release CX3CL1 into the spinal cord which induce dorsal horn microglia to produce TNFα, which activates astrocytes to produce CCL2 and CXCL1 that induce changes in spinal cord neurons leading to central sensitization. Oligodendrocytes produce IL-33 and cross-talk with microglia and astrocytes to increase pain sensitivity.
Nociceptor Neuron Regulation of Inflammation and Immunity
Emerging research is showing that nociceptor neurons play an active and significant role in regulating the immune response and inflammation. Thus, pain is not just a symptom of inflammation, but an active participant in regulating immunity. Upon activation by noxious/harmful stimuli, nociceptors release neuropeptides and neurotransmitters from their peripheral terminals that have potent effects on the vasculature and on the function of innate and adaptive immune cells (Figure 3). Dendritic cells, neutrophils, macrophages, mast cells, and T cells express receptors for these neuronal mediators, allowing them to respond directly to nociceptors. Nociceptors also participate in neural reflex circuits through other neuronal subtypes that dampen inflammation (For a review on this topic, see [64]). Here we highlight how nociceptor neurons play an active role in modulating vascular function, immune cells function, and inflammation in health and disease conditions (Figure 4).
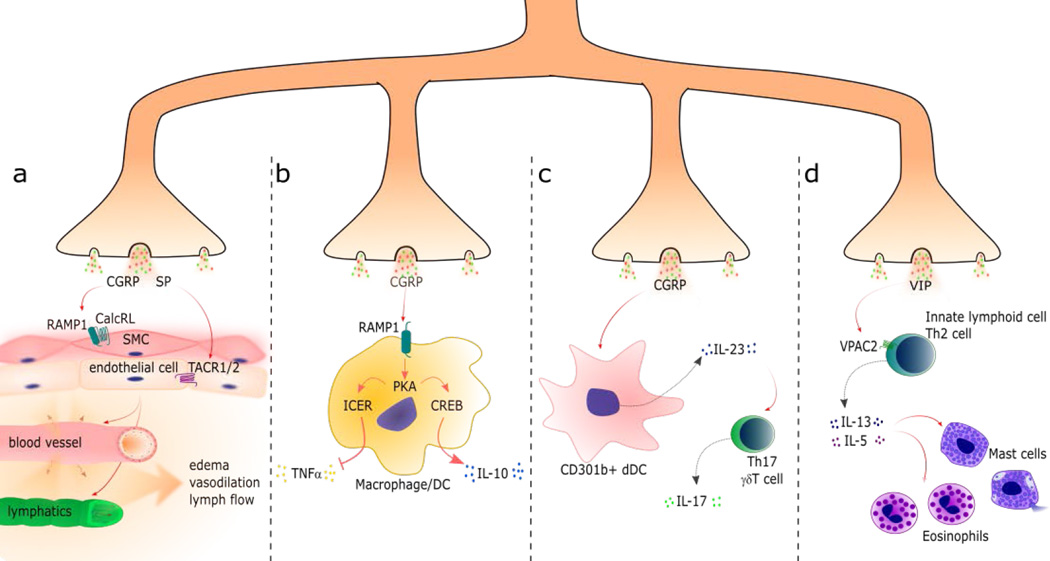
Figure 3. Nociceptor Neurons Release Neuropeptides that Regulate Vascular, Innate and Adaptive Immune Cell Function.
While noxious stimuli generates pain through afferent signals to the central nervous system, calcium influx in the peripheral nerve terminals also causes local release of dense-core vesicles containing neuropeptides. These neuropeptides have potent effects on the vasculature and immune cells to regulate tissue inflammation: (a) The neuropeptide calcitonin gene-related peptide (CGRP) activates the RAMP1/CalcRL receptor complex in vascular smooth muscle cells (SMC) to promote muscle relaxation and vasodilation. Substance P (SP), another nociceptive neuropeptide, activates tachykinin receptor 1 and 2 (TACR1/2) in vascular endothelial cells to increase vascular permeability, which results in edema formation. CGRP and SP also act on lymphatic endothelial cells and SMC to regulate lymph flow. (b) CGRP binds RAMP1 in macrophages and dendritic cells (DC), leading to downstream PKA activity, which affects cytokine production by two different pathways. The first pathway (left side) occurs by induction of the transcriptional inducible cAMP early repressor (ICER) and inhibition of tumor necrosis factor alpha (TNFα) expression. The second pathway (right side) occurs by induction of the transcription factor cAMP response element binding (CREB) and induction of interleukin 10 (IL-10) expression. (c) CGRP increases IL-23 production by dermal dendritic cells (CD301b+ dDCs) that, in turn, promotes IL-17 production by Th17 cells and γδT cells. (d) Vasoactive intestinal peptide (VIP) activates its receptor VPAC2 expressed by innate lymphoid cells and Th2 cells and stimulates these cells to produce IL-5 and IL-13, important mediators of allergic reactions that cause degranulation of eosinophils and mast cells.
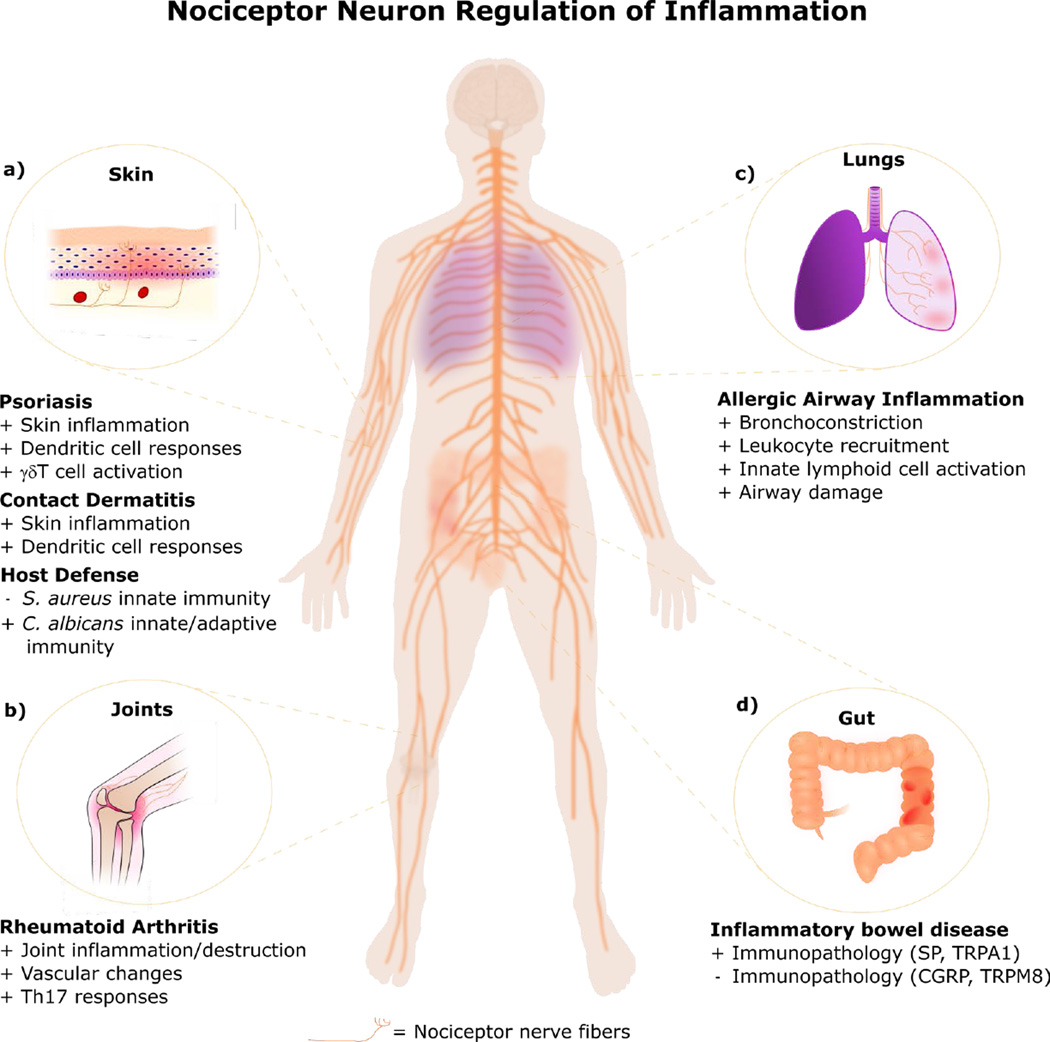
Figure 4. Nociceptor Neurons Actively Contribute to Inflammatory Disease Conditions.
Nociceptor neurons actively modulate the immune response and disease progression in inflammatory conditions. (a) In the skin, nociceptor neurons play a role in driving dendritic cell activation and γδT cell IL-17 production in psoriasis-like inflammation. They also play a role in mediating oxazalone and FITC driven mouse models of contact dermatitis. Nociceptor neurons reduce skin host protection to Staphylococcus aureus infection, but promote skin immunity against Candida albicans (b) In joints, neurons regulate the severity of rheumatoid arthritis due to their effects in promoting Th17 cell responses and changes in vascular endothelial cells. (c) In the lungs, nociceptor neurons contribute to asthmatic airway inflammation and its deleterious effects by driving type 2 innate lymphoid cells and mediating bronchoconstriction. (d) In the gastrointestinal tract, nociceptor neurons regulate the progression of mouse models of colitis. While nociceptor neurons drive immunopathology (cytokine production and weight loss) through mechanisms related to substance P (SP) release and activation of the nociceptive ion channel TRPA1, they also reduce immunopathology through release of calcitonin gene-related peptide (CGRP) and activation of the cold-sensing ion channel TRPM8.
Neuronal Regulation of Vasculature and Lymphatic Vessels
Neuronal regulation of inflammation was first demonstrated by experiments showing that chemical irritants produced redness, heat, and swelling dependent on sensory nerve supply [65]. Electrical nerve stimulation directly produced vasodilation and permeability, a process termed “neurogenic inflammation” [66]. This process is mediated by axon-axon reflexes, whereby calcium influx and antidromic signaling (back-propagation of action potentials) leads to signal transduction in neighboring axons and the release of mediators stored within dense-core vesicles located at the axon terminals in the periphery. Nociceptor mediators are diverse (Box 4), including the neuropeptides calcitonin-gene related peptide (CGRP) and substance P (SP), which are some of the most potent mediators of vasodilation and tissue edema known (Figure 3a) [67]. They act directly on smooth muscle cells and vascular endothelial cells to mediate neurogenic inflammation. Nociceptor nerve fibers are also closely associated with lymphatic vessels. Substance P stimulates lymphatic vessel contractility and increases pump efficiency through its receptors TACR1 and TACR3 expressed by lymphatic smooth muscle cells [68,69]. CGRP increases constriction frequency of perfused lymphatic vessels, a phenomenon that is dependent on nitric oxide [70]. The CGRP receptor RAMP1 is necessary for proper angiogenesis and lymphangiogenesis during skin wound healing, and regulates VEGF production [71]. In spite of this evidence for interactions between nociceptor neurons with blood and lymph vessels, it remains to be determined whether this crosstalk shapes antigen drainage and adaptive immunity. An interesting question would be to determine whether nociceptor neurons regulate initiation or priming of T or B cell responses in draining lymph nodes.
Text Box 4.
Neuropeptides and a Role in Migraine
Nociceptive neurons express a diverse set of neuropeptides, which are stored in dense core granules and released both at central and peripheral terminals during activation. Some well-studied nociceptive neuropeptides include calcitonin-gene related peptide (CGRP), substance P (SP), vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating peptide (PACAP), Galanin (Gal), and Somatostatin (SST). These neuropeptides have distinct expression patterns within nociceptive neurons, and also bind to different G-protein coupled receptors on their target tissues. Neuropeptides also mark distinct innervation patterns by nociceptors. For example, in the skin, CGRP+ “peptidergic” nociceptors innervate the stratum spinosum of the epidermis and the dermal/epidermal interface, whereas Mrgprd+ neurons innervate the superficial stratum granulosum layer [112]. Therefore, neuropeptide release could be a key mechanism by which nociceptors regulate CNS and peripheral responses.
CGRP in particular, also plays a critical in migraine. Migraine is a neurological disorder with complex mechanisms involving alterations of sensory perception and processing. A common feature of most migraine headaches is the involvement of the trigeminovascular structure, which encompasses an anatomical unit including cortex, hypothalamus, brainstem, trigeminal nerve and meninges. The release of neuropeptides such as CGRP may be an underlying basis of increased pressure and pain of migraine. In fact, CGRP is a promising target in clinical trials to treat the chronic pain of migraine. Initial use of small molecules targeting CGRP in migraine has shown promise but is accompanied by side effects. These side effects are not produced by the recent antibody blockade strategies developed to target CGRP. CGRP levels are elevated in spontaneous or induced experimental migraine; CGRP infusion induces migraine, and targeting CGRP in phase II and III reduced migraine symptoms including photophobia. Mechanistically, it is thought that trigeminal afferent nerve fibers release CGRP in the dura mater and subarachnoid space during migraine, which binds to and activates RAMP1 causing vasodilatation and induces dura mater mast cells degranulation and neuroinflammation. As a consequence, CGRP induces peripheral and central sensitization observed as pain, photophobia and phonophobia. The animal data [113], advances in targeting CGRP in migraine [114], and migraine mechanisms and CGRP systemic functions [115] have been reviewed elsewhere.
Neuronal Regulation of Host-Pathogen Defense
Bacterial, viral, and fungal infections are often accompanied by pain. Recent studies show that nociceptor neurons actively participate in mammalian host responses against pathogens. Nociceptor neurons are able to directly sense bacteria and fungal-derived molecules including lipopolysaccharide (LPS), flagellin, bacterial toxins, and zymosan to produce pain during infection (reviewed in [72,73]). Nociceptor neurons then release mediators that modulate the function of macrophages, dendritic cells, T cells and innate lymphoid cells. The effects on the outcome of host defense can be protective or harmful depending on the type of pathogenic infection.
Nociceptor neurons drive protective skin immunity against the fungal pathogen Candida albicans [74]. In a mouse model of C. albicans infection, nociceptor neuron deficiency led to significantly reduced IL-23 production by CD301b+ dermal dendritic cells, which was required for IL-17A production by skin γδT cells. The result was significantly reduced host clearance of C. albicans. These defenses were restored by repeated injections of CGRP during infection in nociceptor-deficient mice. C. albicans and its cell-wall component zymosan activated nociceptor neuron calcium flux [74]. Therefore, nociceptor neurons mediate dendritic cell and T cell responses against cutaneous C. albicans invasion.
By contrast, nociceptor neurons downmodulate immune responses against the gram-positive bacterial pathogen Staphylococcus aureus. In S. aureus skin infection, pain is produced by neuronal sensing of bacterial N-formylated peptides and the pore-forming toxin α-hemolysin [75]. Nociceptor neuron deficiency led to increased neutrophil and monocyte infiltration at the infection site and draining lymph node hypertrophy. Nociceptor-derived neuropeptides CGRP, galanin and somatostatin decreased macrophage TNFα production in response to S. aureus, and CGRP administration restored lymph node hypertrophy, including the recruitment of T cells and B cells during infection, though the effects on subsequent adaptive immunity has not yet been determined [75]. CGRP potently upregulates IL-10 and downregulates TNFα expression in macrophages and dendritic cells via two pathways: CGRP induces IL-10 through PKA-dependent activation of cAMP response element binding (CREB), while TNFα inhibition is mediated by PKA-dependent signaling through inducible cAMP early repressor (ICER), which silences ATF-2 binding to the TNFα promoter [76–78] (Figure 3b).
While nociceptor suppression of innate immunity may not be beneficial during localized bacterial infections, it may be important in suppressing systemic immunopathology during bacterial sepsis. In LPS-induced shock, nociceptor-derived neuropeptides CGRP, vasoactive intestinal peptide (VIP), and pituitary adenylate cyclase-activating polypeptide (PACAP) decrease cytokine levels and mortality in mice with exogenous administration [79,80]. In a model of polymicrobial septic peritonitis, deficiency in RAMP1 led to increased innate immune cell recruitment and decreased IL-10 levels [77]. Thus, nociceptor neurons-derived neuropeptides limit the innate inflammatory response. In the cecal ligation and puncture (CLP) model of sepsis, absence of TRPV1 leads to increased cytokine levels, decreased macrophage phagocytosis, and bacterial clearance, resulting in an exacerbated inflammatory response [81]. LPS potently induces inflammatory pain, a mechanism dependent on TLR4/MyD88 but not TLR4/TRIF signaling [82]. LPS also directly activates nociceptor neurons by gating the nociceptive ion channel TRPA1, a process mediated by the Lipid A moiety [83]
In addition to a role in sepsis, the nervous system also regulates gastrointestinal defenses against bacterial pathogens. Blockade of TACR1 leads to increased IgA and Th2 cytokine production, a mechanism that is protective against Salmonella enterica infection [84]. Gut extrinsic sympathetic neurons are activated downstream of Salmonella infections in the gut lamina propria and trigger a site-restricted anti-inflammatory polarization in macrophages in the myenteric plexus without affecting the response of macrophages in superficial layers [85]. A better understanding of the molecular interactions between nociceptor neurons, pathogens, and immune cells may lead to novel approaches to treating infectious diseases.
Neuronal Regulation of Inflammatory Diseases
Pain is characteristic of many chronic inflammatory diseases. Recent work has shown that nociceptors actively regulate joint, skin, lung, and gastrointestinal diseases and targeting pain could treat inflammation (Figure 4).
Nociceptor neurons drive inflammation in psoriasis and contact dermatitis. It was found that chemical denervation of TRPV1+ nociceptors or genetic ablation of Nav1.8+ neurons led to decreased skin pathology in the Imiquimod-based mouse model of psoriasis [86]. This study found that nociceptors drive dendritic cell production of IL-23, which mediates γδT cell expression of IL-17 (Figure 3c) [86]. Nociceptor neurons also directly respond to the haptens oxazolone and urushiol, the contact allergen of poison ivy, a response dependent on the ion channel TRPA1 [87]. TRPA1−/− mice showed significantly less inflammation in acute and chronic models of oxazolone-contact dermatitis [87]. CGRP and its receptor RAMP1 is multifaceted, playing an inhibitory role in Th1 driven 2,4,6-trinitrochlorobenzene (TCNB) contact dermatitis, while promoting Th2 driven fluorescein isothiocyanate (FITC) contact dermatitis [88]. CGRP activates RAMP1 to inhibit dendritic cell production of IL-12, IL-6, TNFα and expression of CCR7, leading to downregulation of Th1 cell differentiation [88,89]. The inhibitory effects of CGRP on macrophages differs from DCs, in that IL-10-producing macrophages still migrate to the lymph node where they contribute to antigen presentation and induction of Th2 responses [88]. Therefore, nociceptor neurons drive or suppress inflammation depending on crosstalk with specific innate and adaptive cell-types (Figure 4a).
Rheumatoid arthritis is characterized by joint swelling and intense pain. Interestingly, denervation decreases joint pathology in both human and animal models, a process which may be due to nerve-vascular interactions. In the K/BxN model of serum-transfer arthritis, it was found that denervation led to significant changes in the transcriptome of vascular endothelial cells [90]. CGRP stimulates endothelial cells to produce IL-6, which generates Th17 cells following antigen presentation and, consequently, IL-17A production [91], a process which could contribute to autoimmune pathology. In mBSA antigen-induced arthritis (AIA), the initial steps of joint inflammation are abrogated when IL-6 signal transducer is knocked out of Nav1.8-positive nociceptor neurons [92]. By contrast, removal of TRPV1-positive nociceptor neurons by pre-treatment with resiniferatoxin (RTX) exacerbates joint inflammation in the K/BxN arthritis model [93] (Figure 4b). This could be due to differences in animal models as well as the role of specific nociceptor subtypes targeted in these studies.
Nociceptors may also play a critical role in driving lung hyperreactivity and airway inflammation in asthma [42,94,95] (Figure 4c). The respiratory tract is innervated by nociceptor neurons that can induce cough, mucus production, and bronchoconstriction. These neurons detect noxious stimuli including chemical irritants and allergens. Blockade of the nociceptive ion channel TRPA1 or treatment of mice with the TRPA1 antagonist HC-030031 led to reduced allergic airway inflammation and immune cell recruitment in a mouse model of asthma [94]. Targeted ablation of TRPV1+ nociceptors or Tetanus toxin mediated silencing of these neurons led to decreased bronchoconstriction [95]. Silencing of nociceptor neuron activity using a charged form of lidocaine (QX-314) and targeted ablation of Nav1.8-lineage neurons led to reduced allergic airway inflammation and immune cell influx [42]. Nociceptor neurons may drive lung inflammation through their release of the neuropeptide VIP, which acts on the VPAC2 receptor expressed by Type 2 innate lymphoid cells (ILC2) to induce IL-5 production [42] (Figure 3d). Neuropeptides also potentiate degranulation of mast cells, key mediators of allergic inflammation [5,96]. Thus, silencing neurons may be a strategy to treat asthmatic lung inflammation.
Nociceptor neurons actively regulate inflammation in gastrointestinal diseases (Figure 4d). Pain affects the quality of life of patients with irritable bowel syndrome (IBS), Crohn’s disease, and ulcerative colitis. In dextran-sulfate-sodium (DSS) and 2,4,6-trinitrobenzene-sulfonic-acid (TNBS) models of colitis, the nociceptive ion channel TRPA1 is activated, resulting in neuronal SP release, which drives colonic inflammation [97]. By contrast, TRPM8, a nociceptive ion channel mediating cold sensation, attenuates cytokine levels and immunopathology in DSS and TNBS colitis models [98]. TRPM8+ mucosal sensory neurons release CGRP, which suppress colitogenic myeloid cell signaling [99]. Therefore, nociceptor neurons differentially regulate colonic inflammation depending on the type of nociceptive ion channel or neuropeptide involved.
Besides direct communication with immune cells, nociceptor neurons also have an active dialog with epithelial cells during inflammation. CGRP activates MAPK pathways to induce keratinocyte proliferation and production of TNFα, IL-1β, IL-6, and NGF [100]. CGRP and SP induce NLRP1/caspase-1 inflammasome signaling in keratinocytes, which mediates IL-1β-dependent mechanical hyperalgesia [101]. In addition to the classic free nerve endings of nociceptor neurons in peripheral tissues, nociceptors innervate specialized structures where they play neuro-endocrine roles that can be immunomodulatory. In the lungs, pulmonary neuroendocrine cells (PNECs) are sensory organoids composed by innervated epithelial cells that produce CGRP. Dysfunctional PNECs result in increased CGRP release and immune cell recruitment in lung disease [102]. Future work will determine and define the importance of specific nociceptor neuron-immune and neuron-endocrine interactions in different inflammatory disease conditions.
Concluding Remarks
It is increasingly clear that nociceptor neuron-immune interactions play a critical role in pain and inflammation. The immune and nociceptive systems are specialized to recognize damaging/harmful stimuli, and their functional interactions may play important roles in driving responses to prevent tissue damage and restore homeostasis. Dysregulation of these interactions could underlie the pathogenesis of inflammatory diseases in the skin, joint, respiratory and gastrointestinal tracts. These neuro-immune interactions occur within peripheral sites of injury, as well as in the central nervous system. Targeting specific immune cells, cytokines or lipid mediators may lead to novel approaches to treat chronic pain. Conversely, modulation of nociceptor neuron activity or mediators could lead to new approaches to treat infection and chronic inflammatory diseases. Altogether, it is clear that the sensory nervous system is a key modulator of host protective responses and understanding its interactions with immune cells could reveal new mechanisms that could be targeted in order to treat and prevent diseases.
Trends Box.
A bidirectional crosstalk between nociceptor sensory neurons and immune cells actively regulates pain and inflammation.
Immune cells release lipids, cytokines, and growth factors that play a key role in sensitizing nociceptor sensory neurons by acting in peripheral tissues and the spinal cord to produce neuronal plasticity and chronic pain.
Nociceptor neurons release neuropeptides that drive changes in the vasculature, lymphatics, and polarization of innate and adaptive immune cell function.
Nociceptor neurons modulate host defenses against bacterial and fungal pathogens, and in some cases neural activity benefits the host while in others the pathogen.
Interactions between nociceptor neurons and immune cells contribute to pathology in chronic inflammatory diseases including rheumatoid arthritis, psoriasis, asthmatic lung disease, and colitis.
Acknowledgments
F.A.P-R. acknowledges the PhD fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil). W.A.V.J. acknowledges support from the Center for Research in Inflammatory Disease (CRID, Brazil), and Senior Researcher fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil). I.M.C acknowledges funding and support from NIH/NCCIH DP2AT009499, NIH/NIAID K22AI114810, and the Harvard Digestive Disease Center, NIH grant P30 DK348345.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wood JN, et al. Voltage-gated sodium channels and pain pathways. J. Neurobiol. 2004;61:55–71. doi: 10.1002/neu.20094. [DOI] [PubMed] [Google Scholar]
- 2.Cunha TM, et al. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J. Leukoc. Biol. 2008;83:824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- 3.Kiguchi N, et al. Epigenetic Augmentation of the Macrophage Inflammatory Protein 2/C-X-C Chemokine Receptor Type 2 Axis through Histone H3 Acetylation in Injured Peripheral Nerves Elicits Neuropathic Pain. J. Pharmacol. Exp. Ther. 2012;340:577–587. doi: 10.1124/jpet.111.187724. [DOI] [PubMed] [Google Scholar]
- 4.Ghasemlou N, et al. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E6808–E6817. doi: 10.1073/pnas.1501372112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aich A, et al. Mast Cell-Mediated Mechanisms of Nociception. Int. J. Mol. Sci. 2015;16:29069–29092. doi: 10.3390/ijms161226151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjea D, Martinov T. Mast cells: versatile gatekeepers of pain. Mol. Immunol. 2015;63:38–44. doi: 10.1016/j.molimm.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolf CJ, et al. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br. J. Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira SM, et al. Involvement of mast cells in a mouse model of postoperative pain. Eur. J. Pharmacol. 2011;672:88–95. doi: 10.1016/j.ejphar.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Li W-W, et al. Substance P Signaling Controls Mast Cell Activation, Degranulation, and Nociceptive Sensitization in a Rat Fracture Model of Complex Regional Pain Syndrome. Anesthesiology. 2012;116:882–895. doi: 10.1097/ALN.0b013e31824bb303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi Y, et al. Macrophage-T cell interactions mediate neuropathic pain through the glucocorticoid-induced tumor necrosis factor ligand system. J. Biol. Chem. 2015;290:12603–12613. doi: 10.1074/jbc.M115.636506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuh CD, et al. Prostacyclin mediates neuropathic pain through interleukin 1β-expressing resident macrophages. Pain. 2014;155:545–555. doi: 10.1016/j.pain.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Trevisan G, et al. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain. 2016;139:1361–1377. doi: 10.1093/brain/aww038. [DOI] [PubMed] [Google Scholar]
- 13.Old EA, et al. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J. Clin. Invest. 2014;124:2023–2036. doi: 10.1172/JCI71389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shutov LP, et al. The Complement System Component C5a Produces Thermal Hyperalgesia via Macrophage-to-Nociceptor Signaling That Requires NGF and TRPV1. J. Neurosci. 2016;36:5055–5070. doi: 10.1523/JNEUROSCI.3249-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim CF, Moalem-Taylor G. Interleukin-17 Contributes to Neuroinflammation and Neuropathic Pain Following Peripheral Nerve Injury in Mice. J. Pain. 2011;12:370–383. doi: 10.1016/j.jpain.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu X-J, et al. Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 2014;24:1374–1377. doi: 10.1038/cr.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vicuña L, et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat. Med. 2015;21:518–523. doi: 10.1038/nm.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent L, et al. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122:1853–1862. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira SH. Prostaglandins, aspirin-like drugs and analgesia. Nat. New Biol. 1972;240:200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- 20.Souza GR, et al. Involvement of nuclear factor kappa B in the maintenance of persistent inflammatory hypernociception. Pharmacol. Biochem. Behav. 2015;134:49–56. doi: 10.1016/j.pbb.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Nieto-Posadas A, et al. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat. Chem. Biol. 2012;8:78–85. doi: 10.1038/nchembio.712. [DOI] [PubMed] [Google Scholar]
- 22.Langeslag M, et al. Sphingosine 1-phosphate to p38 signaling via S1P1 receptor and Gαi/o evokes augmentation of capsaicin-induced ionic currents in mouse sensory neurons. Mol. Pain. 2014;10:74. doi: 10.1186/1744-8069-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bisgaard H, Kristensen JK. Effects of synthetic leukotriene D-4 on the local regulation of blood flow in human subcutaneous tissue. Prostaglandins. 1985;29:155–159. doi: 10.1016/0090-6980(85)90198-4. [DOI] [PubMed] [Google Scholar]
- 24.Martin HA, et al. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience. 1987;22:651–659. doi: 10.1016/0306-4522(87)90360-5. [DOI] [PubMed] [Google Scholar]
- 25.Andoh T, Kuraishi Y. Expression of BLT1 leukotriene B4 receptor on the dorsal root ganglion neurons in mice. Mol. Brain Res. 2005;137:263–266. doi: 10.1016/j.molbrainres.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Verri WA, et al. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol. Ther. 2006;112:116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira SH, et al. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 28.Binshtok AM, et al. Nociceptors are interleukin-1beta sensors. J. Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebbinghaus M, et al. The role of interleukin-1β in arthritic pain: main involvement in thermal, but not mechanical, hyperalgesia in rat antigen-induced arthritis. Arthritis Rheum. 2012;64:3897–3907. doi: 10.1002/art.34675. [DOI] [PubMed] [Google Scholar]
- 30.Cunha FQ, et al. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malsch P, et al. Deletion of interleukin-6 signal transducer gp130 in small sensory neurons attenuates mechanonociception and down-regulates TRPA1 expression. J. Neurosci. 2014;34:9845–9856. doi: 10.1523/JNEUROSCI.5161-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang D, et al. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. Pain. 2015;156:1124–1144. doi: 10.1097/j.pain.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 33.Jin X, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J. Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes ES, et al. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor alpha-induced inflammatory hyperalgesia and Freund’s complete adjuvant-induced monarthritis. Arthritis Rheum. 2011;63:819–829. doi: 10.1002/art.30150. [DOI] [PubMed] [Google Scholar]
- 35.Nicol GD, et al. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J. Neurosci. 1997;17:975–982. doi: 10.1523/JNEUROSCI.17-03-00975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunha TM, et al. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gudes S, et al. The role of slow and persistent TTX-resistant sodium currents in acute tumor necrosis factor-α-mediated increase in nociceptors excitability. J. Neurophysiol. 2015;113:601–619. doi: 10.1152/jn.00652.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorkin LS, Doom CM. Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J. Peripher. Nerv. Syst. 2000;5:96–100. doi: 10.1046/j.1529-8027.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 39.Constantin CE, et al. Endogenous tumor necrosis factor α (TNFα) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J. Neurosci. 2008;28:5072–5081. doi: 10.1523/JNEUROSCI.4476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richter F, et al. Interleukin-17 sensitizes joint nociceptors to mechanical stimuli and contributes to arthritic pain through neuronal interleukin-17 receptors in rodents. Arthritis Rheum. 2012;64:4125–4134. doi: 10.1002/art.37695. [DOI] [PubMed] [Google Scholar]
- 41.Pinto LG, et al. IL-17 mediates articular hypernociception in antigen-induced arthritis in mice. Pain. 2010;148:247–256. doi: 10.1016/j.pain.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Talbot S, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron. 2015;87:341–354. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eskander MA, et al. Persistent Nociception Triggered by Nerve Growth Factor (NGF) Is Mediated by TRPV1 and Oxidative Mechanisms. J. Neurosci. 2015;35:8593–8603. doi: 10.1523/JNEUROSCI.3993-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, et al. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji RR, et al. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 46.Ro LS, et al. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain. 1999;79:265–274. doi: 10.1016/s0304-3959(98)00164-x. [DOI] [PubMed] [Google Scholar]
- 47.Tang X-Q, et al. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J. Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yue J-X, et al. Histamine upregulates Nav1.8 expression in primary afferent neurons via H2 receptors: involvement in neuropathic pain. CNS Neurosci. Ther. 2014;20:883–892. doi: 10.1111/cns.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perin-Martins A, et al. Mechanisms underlying transient receptor potential ankyrin 1 (TRPA1)-mediated hyperalgesia and edema. J. Peripher. Nerv. Syst. 2013;18:62–74. doi: 10.1111/jns5.12010. [DOI] [PubMed] [Google Scholar]
- 50.Massaad CA, et al. Involvement of substance P, CGRP and histamine in the hyperalgesia and cytokine upregulation induced by intraplantar injection of capsaicin in rats. J. Neuroimmunol. 2004;153:171–182. doi: 10.1016/j.jneuroim.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Parada CA, et al. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience. 2001;102:937–944. doi: 10.1016/s0306-4522(00)00523-6. [DOI] [PubMed] [Google Scholar]
- 52.Qiu F, et al. Potentiation of acid-sensing ion channel activity by the activation of 5-HT2 receptors in rat dorsal root ganglion neurons. Neuropharmacology. 2012;63:494–500. doi: 10.1016/j.neuropharm.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 53.Foster E, et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron. 2015;85:1289–1304. doi: 10.1016/j.neuron.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daou I, et al. Optogenetic Silencing of Nav1.8-Positive Afferents Alleviates Inflammatory and Neuropathic Pain. eNeuro. 2016;3:1–12. doi: 10.1523/ENEURO.0140-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shubayev VI, Myers RR. Axonal transport of TNF-alpha in painful neuropathy: distribution of ligand tracer and TNF receptors. J. Neuroimmunol. 2001;114:48–56. doi: 10.1016/s0165-5728(00)00453-7. [DOI] [PubMed] [Google Scholar]
- 56.Guan Z, et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 2015;19:1–10. doi: 10.1038/nn.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berta T, et al. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-α secretion. J. Clin. Invest. 2014;124:1173–1186. doi: 10.1172/JCI72230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol. Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji RR, et al. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154:S10–S28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sorge RE, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015;18:1–5. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taves S, et al. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain. Behav. Immun. 2016;55:70–81. doi: 10.1016/j.bbi.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu J, et al. NFκB-mediated CXCL1 production in spinal cord astrocytes contributes to the maintenance of bone cancer pain in mice. J. Neuroinflammation. 2014;11:38. doi: 10.1186/1742-2094-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zarpelon AC, et al. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB J. 2016;30:54–65. doi: 10.1096/fj.14-267146. [DOI] [PubMed] [Google Scholar]
- 64.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 2012;30:313–335. doi: 10.1146/annurev-immunol-020711-075015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bruce AN. Vaso-dilator axon-reflexes. Quat. J. Exp. Physiol. 1913;6:339–354. [Google Scholar]
- 66.Jancsó N, et al. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br. J. Pharmacol. Chemother. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brain SD, Williams TJ. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br. J. Pharmacol. 1985;86:855–860. doi: 10.1111/j.1476-5381.1985.tb11107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis MJ, et al. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H587–H597. doi: 10.1152/ajpheart.01029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chakraborty S, et al. Substance P activates both contractile and inflammatory pathways in lymphatics through the neurokinin receptors NK1R and NK3R. Microcirculation. 2011;18:24–35. doi: 10.1111/j.1549-8719.2010.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hosaka K, et al. Calcitonin gene-related peptide activates different signaling pathways in mesenteric lymphatics of guinea pigs. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H813–H822. doi: 10.1152/ajpheart.00543.2005. [DOI] [PubMed] [Google Scholar]
- 71.Kurashige C, et al. Roles of receptor activity-modifying protein 1 in angiogenesis and lymphangiogenesis during skin wound healing in mice. FASEB J. 2014;28:1237–1247. doi: 10.1096/fj.13-238998. [DOI] [PubMed] [Google Scholar]
- 72.Liu T, et al. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baral P, et al. Pain and Itch: Beneficial or Harmful to Antimicrobial Defense? Cell Host Microbe. 2016;19:755–759. doi: 10.1016/j.chom.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Kashem SW, et al. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity. 2015;43:515–526. doi: 10.1016/j.immuni.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiu IM, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Altmayr F, et al. The neuropeptide calcitonin gene-related peptide causes repression of tumor necrosis factor-alpha transcription and suppression of ATF-2 promoter recruitment in Toll-like receptor-stimulated dendritic cells. J. Biol. Chem. 2010;285:3525–3531. doi: 10.1074/jbc.M109.066787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jusek G, et al. Deficiency of the CGRP receptor component RAMP1 attenuates immunosuppression during the early phase of septic peritonitis. Immunobiology. 2012;217:761–767. doi: 10.1016/j.imbio.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 78.Baliu-Piqué M, et al. Neuroimmunological communication via CGRP promotes the development of a regulatory phenotype in TLR4-stimulated macrophages. Eur. J. Immunol. 2014;44:3708–3716. doi: 10.1002/eji.201444553. [DOI] [PubMed] [Google Scholar]
- 79.Martinez C, et al. Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1053–1058. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomes RN, et al. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock. 2005;24:590–594. doi: 10.1097/01.shk.0000183395.29014.7c. [DOI] [PubMed] [Google Scholar]
- 81.Fernandes ES, et al. TRPV1 Deletion Enhances Local Inflammation and Accelerates the Onset of Systemic Inflammatory Response Syndrome. J. Immunol. 2012;188:5741–5751. doi: 10.4049/jimmunol.1102147. [DOI] [PubMed] [Google Scholar]
- 82.Calil IL, et al. Lipopolysaccharide induces inflammatory hyperalgesia triggering a TLR4/MyD88-dependent cytokine cascade in the mice paw. PLoS One. 2014;9:e90013. doi: 10.1371/journal.pone.0090013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meseguer V, et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014;5:3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walters N, et al. Enhanced immunoglobulin A response and protection against Salmonella enterica serovar typhimurium in the absence of the substance P receptor. Infect. Immun. 2005;73:317–324. doi: 10.1128/IAI.73.1.317-324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gabanyi I, et al. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riol-Blanco L, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu B, et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013;27:3549–3563. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mikami N, et al. Calcitonin gene-related peptide is an important regulator of cutaneous immunity: effect on dendritic cell and T cell functions. J. Immunol. 2011;186:6886–6893. doi: 10.4049/jimmunol.1100028. [DOI] [PubMed] [Google Scholar]
- 89.Mikami N, et al. Calcitonin gene-related peptide regulates type IV hypersensitivity through dendritic cell functions. PLoS One. 2014;9:e86367. doi: 10.1371/journal.pone.0086367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stangenberg L, et al. Denervation protects limbs from inflammatory arthritis via an impact on the microvasculature. Proc. Natl. Acad. Sci. U.S.A. 2014;111:11419–11424. doi: 10.1073/pnas.1410854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ding W, et al. Calcitonin Gene-Related Peptide-Exposed Endothelial Cells Bias Antigen Presentation to CD4+ T Cells toward a Th17 Response. J. Immunol. 2016;196:2181–2194. doi: 10.4049/jimmunol.1500303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ebbinghaus M, et al. Interleukin-6-dependent influence of nociceptive sensory neurons on antigen-induced arthritis. Arthritis Res. Ther. 2015;17:334. doi: 10.1186/s13075-015-0858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borbély É, et al. Capsaicin-sensitive sensory nerves exert complex regulatory functions in the serum-transfer mouse model of autoimmune arthritis. Brain. Behav. Immun. 2015;45:50–59. doi: 10.1016/j.bbi.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caceres AI, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tränkner D, et al. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc. Natl. Acad. Sci. U.S.A. 2014;111:11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim JH, et al. CGRP, a neurotransmitter of enteric sensory neurons, contributes to the development of food allergy due to the augmentation of microtubule reorganization in mucosal mast cells. Biomed. Res. 2014;35:285–293. doi: 10.2220/biomedres.35.285. [DOI] [PubMed] [Google Scholar]
- 97.Engel MA, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141:1346–1358. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 98.Ramachandran R, et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc. Natl. Acad. Sci. U.S.A. 2013;110:7476–7481. doi: 10.1073/pnas.1217431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Jong PR, et al. TRPM8 on mucosal sensory nerves regulates colitogenic responses by innate immune cells via CGRP. Mucosal Immunol. 2015;8:491–504. doi: 10.1038/mi.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shi X, et al. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul. Pept. 2013;186:92–103. doi: 10.1016/j.regpep.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi X, et al. Neuropeptides contribute to peripheral nociceptive sensitization by regulating interleukin-1β production in keratinocytes. Anesth. Analg. 2011;113:175–183. doi: 10.1213/ANE.0b013e31821a0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Branchfield K, et al. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science. 2016;351:707–710. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Basbaum AI, et al. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dib-Hajj SD, Waxman SG. Translational pain research: Lessons from genetics and genomics. Sci. Transl. Med. 2014;6:249sr4. doi: 10.1126/scitranslmed.3007017. [DOI] [PubMed] [Google Scholar]
- 105.Von Hehn CA, et al. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Julius D. TRP channels and pain. Annu. Rev. Cell. Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 107.Ranade SS, et al. Mechanically activated ion channels. Neuron. 2015;87:1162–1179. doi: 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Napimoga MH, et al. 15d–prostaglandin J2 inhibits inflammatory hypernociception: involvement of peripheral opioid receptor. J. Pharmacol. Exp. Ther. 2008;324:313–321. doi: 10.1124/jpet.107.126045. [DOI] [PubMed] [Google Scholar]
- 109.Ji RR, et al. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34:599–609. doi: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Park C-K, et al. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J. Neurosci. 2011;31:18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hong Wang, Mark Zylka. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J. Neuroscience. 2009;29:13202–13209. doi: 10.1523/JNEUROSCI.3248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Russo AF. CGRP as a neuropeptide in migraine: lessons from mice. Br. J. Clin. Pharmacol. 2015;80:403–414. doi: 10.1111/bcp.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bigal ME, et al. Therapeutic antibodies against CGRP or its receptor. Br. J. Clin. Pharmacol. 2015;79:886–895. doi: 10.1111/bcp.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Russell FA, et al. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 2014;94:1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]