Abstract
Understanding how and why affective responses change with age is central to characterizing typical and atypical emotional development. Prior work has emphasized the role of the amygdala and prefrontal cortex (PFC), which show age-related changes in function and connectivity. However, developmental neuroimaging research has only recently begun to unpack whether age effects in the amygdala and PFC are specific to affective stimuli or may be found for neutral stimuli as well, a possibility that would support a general, rather than affect-specific, account of amygdala-PFC development. To examine this, 112 individuals ranging from 6 to 23 years of age viewed aversive and neutral images while undergoing fMRI scanning. Across age, participants reported more negative affect and showed greater amygdala responses for aversive than neutral stimuli. However, children were generally more sensitive to both neutral and aversive stimuli, as indexed by affective reports and amygdala responses. At the same time, the transition from childhood to adolescence was marked by a ventral-to-dorsal shift in medial prefrontal responses to aversive, but not neutral, stimuli. Given the role that dmPFC plays in executive control and higher-level representations of emotion, these results suggest that adolescence is characterized by a shift towards representing emotional events in increasingly cognitive terms.
Keywords: Emotion, Neurodevelopment, Prefrontal cortex, Amygdala, fMRI
1. Introduction
Current neurodevelopmental models posit that changes in amygdala and prefrontal function – or in their connectivity (Casey, 2015) – underlie changes in affective responding in childhood and adolescence (Casey et al., 2008, Ernst et al., 2006). Such models are bolstered by a rich body of animal work demonstrating developmental changes in prefrontal-amygdala dynamics (Bouwmeester et al., 2002a, Bouwmeester et al., 2002b, McCallum et al., 2010, Pattwell et al., 2012), as well as extensive adult neuroimaging research linking the amygdala and prefrontal cortex to a host of emotional processes (Buhle et al., 2014, Costafreda et al., 2008, Kober et al., 2008). However, there is also emerging evidence that the amygdala does not exclusively respond to aversive, or even affective stimuli (Cunningham and Brosch, 2012). As such, it is possible that developmental changes in amygdala and prefrontal function are related not only to emotional development, but also to a broader set of developmental processes (e.g., salience processing, social appraisals) (Pfeifer and Blakemore, 2012, van den Bulk et al., 2013). The present study sought to examine two non-competing possibilities for how amygdala and prefrontal function relate to general and affect-specific changes in development.
The first possibility was that age would predict general changes in the way individuals respond to both negative affective and neutral stimuli. Specifically, it was hypothesized that age would be associated with diminished engagement of subcortical systems like the amygdala which has been broadly implicated in responding to motivationally salient (Cunningham and Brosch, 2012), intense (Anderson et al., 2003), and emotion-eliciting – both positive and negative (Breiter et al., 1996) – stimuli (Costafreda et al., 2008). A sizeable body of neuroimaging work suggests that amygdala responses to aversive stimuli including fearful faces and emotionally evocative scenes are elevated in childhood (Gee et al., 2013, Silvers et al., 2015) and adolescence (Guyer et al., 2008, Hare et al., 2008, Monk et al., 2003, Passarotti et al., 2009) and decrease in adulthood. However, the evidence that age-related changes in amygdala responses are emotion-specific is more mixed (Helfinstein and Casey, 2014). Indeed, neuroimaging studies have revealed age-related decreases in amygdala responding for neutral (Forbes et al., 2011, Thomas et al., 2001), positive (Vasa et al., 2011), or a combination of different types of stimuli (Hare et al., 2008, Swartz et al., 2014, Vink et al., 2014). This suggest that perhaps children interpret a broader variety of affective and neutral stimuli as being salient or personally relevant than do adults and thus show elevated amygdala responses for both aversive and non-aversive stimuli. Among studies that have specifically examined age-related effects in the amygdala for aversive stimuli, most have focused on contrasts between aversive stimuli and fixation (Gee et al., 2013), or, in the case of our own work, on the effects of different regulatory conditions on responses to aversive stimuli (Silvers et al., in press; Silvers et al., 2015). While such approaches are useful for characterizing changes in amygdala function in affective contexts, they do not address whether or not such age-related changes are unique to affective contexts. As such, this prior research leaves open the possibility that the amygdala shows general, rather than negative affect-specific, age-related decreases in responding.
The second possibility we sought to explore was whether age is associated with dynamic changes in how medial prefrontal cortex (mPFC) responds to negative affective stimuli. mPFC presents itself as a strong candidate region for such age-related changes for two reasons. The first is that converging evidence from animal studies and neuroimaging work in adult humans has strongly implicated mPFC in the top-down generation and regulation of emotion, both of which require relatively mature cognitive skills. Within mPFC, dorsal regions (dmPFC) appear to be preferentially involved in generating fear responses (Etkin et al., 2011, Mechias et al., 2010, Sotres-Bayon and Quirk, 2010), though they are also implicated in top-down cognitive regulation of emotion (Buhle et al., 2014) as well as mentalizing processes more generally (Denny et al., 2012, Van Overwalle and Baetens, 2009). Together, this suggests that dmPFC supports abstract and conceptual representations of affective states (Ochsner and Gross, 2014, Satpute et al., 2013). Ventral mPFC (vmPFC) recruitment, by contrast, scales with perceived value (Hare et al., 2009, Kable and Glimcher, 2007), decreases under conditions of stress and threat (Mobbs et al., 2007, Wager et al., 2009), and is strongly implicated in fear extinction (Diekhof et al., 2011, Milad et al., 2006, Quirk et al., 2006), suggesting it may play a key role in regulating the expression of affective responses based on contextual constraints (Ochsner and Gross, 2014, Roy et al., 2012). As such, while dmPFC and vmPFC play complementary roles in shaping affective experiences in adults, each contributes to conceptual and contextual representations of emotion. This ability to consider emotional events in more cognitive terms is central to mature emotion regulatory processes and is therefore likely to be specifically related to changes in affective development.
A second reason to suspect that mPFC underlies negative affect-specific changes in development comes from prior work showing that mPFC responses to aversive stimuli change in striking ways across development (Cohen et al., 2016). With regards to vmPFC, prior neuroimaging studies have revealed that vmPFC responses to aversive stimuli decrease during adolescence (McRae et al., 2012), and that functional connectivity between vmPFC and the amygdala in response to aversive stimuli is initially positive but becomes negative during the transition from childhood to adolescence (Gee et al., 2013; Silvers et al., in press). Such work suggests that vmPFC responses to aversive stimuli generally decrease with age but also that the way in which vmPFC and the amygdala interact changes as well. In contrast to vmPFC, dmPFC responses to aversive stimuli increase steadily from early adolescence to adulthood (Cohen et al., 2016, Williams et al., 2006). As such, we hypothesized that in the present study age would be associated with a simultaneous decrease in vmPFC recruitment and increase in dmPFC recruitment in response to aversive (but not neutral) stimuli.
By characterizing general and negative affect-specific effects of age in the amygdala and, the present work sought to broaden and clarify how prefrontal-amygdala systems give rise to emotional changes during development. Two hypotheses about amygdala and prefrontal development were tested in a large sample (n = 112) of children, adolescents and young adults. The first hypothesis was that the amygdala would show general age-related decreases in activation to both aversive and neutral stimuli. The second hypothesis was that age would predict a ventral-to-dorsal shift in mPFC responses to negative affective stimuli specifically.
2. Methods
2.1. Participants
One hundred and twelve healthy individuals ranging in age from 6 to 23 years participated in the experiment (65 female; mean age = 15.73 years, S.D.=4.36). In addition to the final sample of 112 participants, 17 participants (9 female; mean age = 9.20 years, S.D. = 2.40) were scanned but excluded from analyses due to excessive head motion and one participant (female, 6.34 years) was excluded due to failure to comply with the task (i.e., not making button responses). Participants were screened prior to scanning to ensure that they could read and write in English, had normal or corrected vision, had never been diagnosed with a developmental or psychiatric disorder, had never been prescribed psychotropic medication, and had no medical conditions contraindicated for scanning. Participants were of normal intelligence, as indexed by the Wechsler Abbreviated Scale of Intelligence (mean score = 114.35, S.D. = 15.42), and parents of children under 18 reported lower than average problem behaviors (three parents did not complete the checklist; Mean t-score = 41.67, S.D. = 9.13, t(68) = 7.59, p < 0.001) on the Child Behavioral Checklist (Achenbach, 2001). Participants under 18 provided informed written assent and their parent or guardian provided informed consent while participants 18 and older provided informed written consent. Participants were compensated for their participation. Study procedures were approved by the Institutional Review Boards at Columbia University and Weill Cornell Medical College.
2.2. Experimental procedures
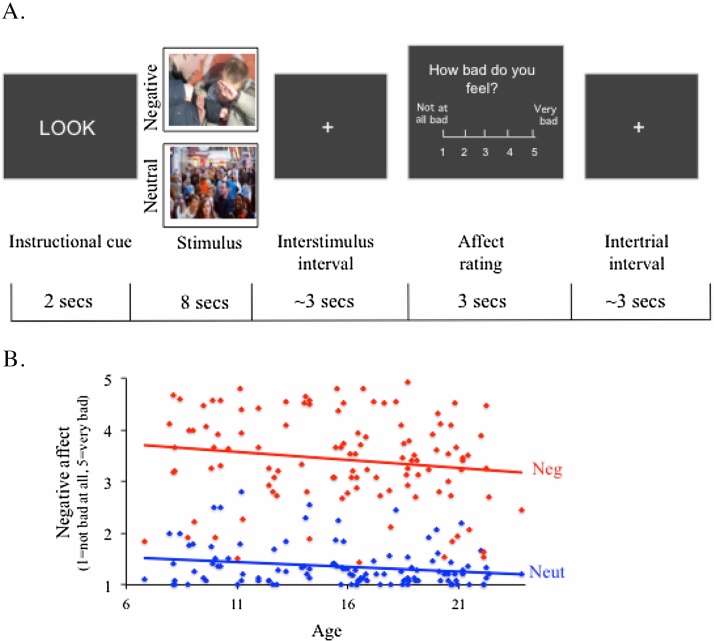
Participants completed a computerized emotional task (programmed in E-prime) while undergoing neuroimaging. The task was projected onto a flat screen mounted in the scanner bore and subjects viewed the screen using a mirror mounted on a 12-channel head coil. Participants made their responses using a five-finger-button response pad. On each trial, participants were presented with a cue (lasting 2 s) instructing them on how to respond to a subsequent photographic image. Participants next viewed either an aversive or neutral social image (i.e., a scene depicting people) for eight seconds and following a jittered interstimulus interval (lasting for an average of 3 s) rated their current negative affect (1 = not at all bad, 5 = very bad). Each trial ended with another jittered interstimulus interval (which lasted on average for 3 s). Stimuli were culled from three sources: (1) the International Affective Picture System (Lang et al., 2001) (IAPS image numbers: 2102, 2104, 2210, 2214, 2235, 2270, 2305, 2372, 2383, 2393, 2394, 2495, 2514, 2515, 2560, 2575, 2579, 2593, 2594, 4621, 6312, 6350, 6838), (2) from a set of similar pictures that had been previously used with adolescents (Silvers et al., 2012), and (3) from online image searches. The aversive images all depicted people and common themes included violence, bullying, rejection, and implied threat (for an example image, see Fig. 1). The neutral images all depicted people engaging in unemotional activities such as walking down the street, working, or sitting in a classroom (for an example image, see Fig. 1). Consistent with prior work (Silvers et al., 2012), parents of participants under the age of 18 prescreened sixty negative photographic stimuli prior to participation and were permitted to exclude up to 10 stimuli (excluded stimuli were replaced with a valence-matched task substitute image).
Fig. 1.
(A) Trial structure for the task. (B) Self-reported affect on negative and neutral trials is plotted as function of age.
As mentioned above, each trial began with an instructional cue. Because the present manuscript is focused on uninstructed emotional responding, only results related to “Look” trials, wherein participants were instructed to “look at the picture like your normally would” are presented here. A separate manuscript reported on results comparing the other two trial types (“Close” and “Far”), wherein participants were instructed to either adopt a more emotionally immersed or distant mindset (Silvers et al., in press), but did not characterize results associated with Look trials. Close and Far trials constrain participant responding so that it is either pushed to be reactive or regulated whereas when individuals are left to their own devices (i.e., on Look trials), they may spontaneously adopt a mindset that is elsewhere on the reactive-regulated continuum. While examining Close and Far trials are useful for unpacking the degree to which reactivity and regulation underlie age-related changes in emotional responding, Look trials grant insight into naturalistic responding that may more closely reflect what happens in everyday life. Put another way, Close and Far trials may be more helpful when examining an individual’s capacity for responding to aversive stimuli in more reactive or regulated ways whereas Look trials may more accurately reflect their natural response tendencies. While both are of clear significance for developmental affective neuroscience, given that results associated with Close and Far trials are described extensively elsewhere (Silvers et al., in press), they will not be discussed further here. The instruction (Look, Close or Far) paired with a given stimulus was counterbalanced across participants and thus, not all participants saw the same stimuli paired with the Look instruction. All participants completed 90 experimental trials, 45 of which contained aversive stimuli and 45 of which contained neutral stimuli. The trials were evenly distributed among the three different instructional conditions. The current manuscript is focused on the 30 Look trials, 15 of which involved aversive stimuli and 15 of which involve neutral stimuli. A diagram of the trial structure used is shown in Fig. 1a.
2.3. Behavioral data analysis
Effects of stimulus valence (negative versus neutral) and mean-centered age were analyzed using a repeated-measures GLM, as implemented in SPSS 19.0. When necessary, follow-up t-tests and correlations were performed, to disambiguate significant F statistics. General age effects were identified using the main effect of age term and emotion-specific effects were identified using the age x valence interaction term.
2.4. fMRI acquisition
All whole-brain fMRI data were collected on a 3 T Siemens Magnetom Trio scanner. A high-resolution, T1-weighted MPRAGE sequence (TR = 2170 ms, TE = 4.33 ms, 120 1.5 mm slices) was used to acquire structural images. Functional images were acquired with a T2*-sensitive EPI BOLD sequence. The functional scans were comprised of thirty-four axial slices, collected with a TR of 2000 ms (TE of 34 ms, flip angle of 90°, field of view of 22.4 cm and 3.5 × 3.5 × 4 mm voxels).
2.5. fMRI analysis
2.5.1. Preprocessing
All preprocessing steps were performed using SPM8 preprocessing tools (Wellcome Department of Cognitive Neurology, UCL) as implemented in NeuroElf (http://neuroelf.net). Preprocessing steps for the functional images included motion correction, slice-time correction and coregistration to the first functional image for each subject. Structural images were normalized (spatially warped using unified segmentation) to a standard template brain (the MNI avg15T1.img) and warping parameters were applied to functional images for each subject. Normalized functional images were interpolated to 3 × 3 × 3 mm voxels and spatially smoothed with a 6-mm Gaussian filter. Volumes with more than 1.5 mm of framewise head motion were removed from the timecourse. Runs were removed if more than 10% of volumes were removed, and participants were removed if more than two out of the five runs were removed. These standards for head motion have been used in prior work in similar age ranges (Somerville et al., 2013).
2.5.2. First-level analyses
First-level GLM analyses were implemented in NeuroElf (http://neuroelf.net). Separate regressors were made for the cue, stimulus-viewing and response portions of each trial and were modeled as boxcar regressors convolved with a canonical hemodynamic response function. Separate regressors were made for the six different trial types so that neural responses associated with strategy (Close, Look, Far) and valence (negative, neutral) could be differentiated. For each subject, a robust regression analysis was performed on the conditions of interest and estimates of global signal in gray matter, white matter, and the ventricles as well as six standard motion parameters and high-pass filters were included as additional regressors of no interest.
2.5.3. Group-level analyses
Group-level analyses were conducted in NeuroElf. A mixed-effects model was performed to examine the effects of mean-centered age, strategy (Close, Look, Far), and stimulus valence (Negative, Neutral) on neural responses. Maps were first thresholded at p < 0.0005 (uncorrected) and significant clusters were subsequently identified using an extent threshold that corresponded to a family wise error corrected p < 0.05, as determined by AlphaSim as implemented in NeuroElf (smoothness estimate: 10.6 mm, extent threshold: 30 voxels). Despite this stringent thresholding, some clusters were still very large and thus subclusters containing 20 or more voxels are also reported. Subclusters were identified using NeuroElf’s “splitclustercoords” function which identifies activation peaks within a cluster that are not connected to the cluster’s central mass in a higher-values-first watershed searching algorithm.
Three sets of analyses were examined in the group results. First, age-independent effects of stimulus valence were examined to identify brain regions that showed differential recruitment for aversive and neutral stimuli irrespective of participant age. Second, the main effect of age term was examined to identify brain regions showing general age-related effects (i.e., age effects irrespective of stimulus type relative to fixation). Changepoint analyses were used to identify specific ages at which activation changed significantly. Specifically, regression analyses were computed with mean-centered age for each year of age and its associated mean-centered age^2 as predictors and amygdala activation as an outcome measure. In this analysis, the beta coefficient for each mean-centered linear age term reflects the instantaneous effect of age on amygdala activation. Third, the age × valence term was examined to identify brain regions with different age effects for aversive and neutral stimuli. Beta values from clusters identified by the age × valence term were extracted and further analyzed in SPSS’s Process Toolbox (Hayes, 2013). Specifically, age was tested as moderator of the relationship between valence and medial prefrontal recruitment (from ROIs defined by the age × valence term) using the Johnson-Neyman technique (Johnson and Fay, 1950).
3. Results
3.1. Behavioral results
3.1.1. Effects of valence and age on self-reported negative affect
As expected, participants reported significantly more negative affect when responding naturally to aversive than neutral stimuli (Mean difference = 2.073, F(1, 110) = 732.403, p < 0.001). A main effect of age was observed (F(1, 110) = 4.506, p < 0.05), suggesting that age is associated with decreased emotional responses to stimuli in general (Fig. 1b). Age and stimulus valence did not interact with one another (F(1, 110) = 0.501, p = 0.481), suggesting that age effects on emotional experience were not specific to aversive or neutral stimuli.
3.2. Imaging results
3.2.1. Main effects of stimulus valence
Aversive and neutral stimuli elicited significantly different patterns of brain activation across the sample. While controlling for age, aversive relative to neutral stimuli elicited greater activation in the amygdala, dmPFC, anterior insula, ventrolateral PFC, thalamus, and midbrain (Table 1). Relative to aversive stimuli, neutral stimuli elicited greater recruitment of vmPFC, dorsal anterior cingulate cortex, sensorimotor cortex, posterior cingulate and cuneus. Given that participants reported significantly more negative affect for aversive than neutral trials, a whole-brain correlational analysis was conducted correlating the difference between self-reported affect for aversive and neutral trials and the aversive > neutral contrast. No brain regions survived correction in this correlational analysis.
Table 1.
Age-independent effects of stimulus valence on brain recruitment.
| MNI Coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | # Voxels | F | x | y | z |
| Aversive > Neutral and Neutral > Aversive* | ||||||
| Aversive > Neutral: | ||||||
| Temporoparietal junction, middle occipital gyri | R | 10401 | 280.64 | 51 | −63 | 6 |
| Inferior frontal gyrus | R | 199 | 45.56 | 42 | 24 | −15 |
| Inferior frontal gyrus | R | 217 | 66.33 | 45 | 3 | 30 |
| Inferior frontal gyrus | R | 205 | 53.35 | 51 | 27 | −3 |
| Inferior and middle frontal gyri | R | 70 | 37.76 | 54 | 15 | 39 |
| Inferior frontal gyrus | R | 199 | 53.58 | 54 | 33 | 6 |
| Inferior frontal gyrus | R | 253 | 79.79 | 57 | 18 | 27 |
| Middle frontal gyrus | R | 234 | 86.96 | 39 | 0 | 42 |
| Superior temporal gyrus | R | 309 | 66.22 | 57 | −45 | 15 |
| Superior temporal gyrus | R | 103 | 56.30 | 45 | −45 | 15 |
| Temporoparietal junction, middle occipital gyri |
L | 1381 | 248.06 | −48 | −72 | 6 |
| Superior parietal lobule | R | 142 | 40.20 | 27 | −72 | 33 |
| Fusiform gyrus | R | 285 | 268.15 | 45 | −54 | −15 |
| Fusiform gyrus | R | 594 | 236.82 | 45 | −66 | −9 |
| Fusiform gyrus | L | 265 | 106.82 | −39 | −51 | −18 |
| Cerebellum | L | 342 | 72.21 | −15 | −78 | −30 |
| Neutral > Aversive: | ||||||
| Anterior insula | R | 133 | 77.29 | 36 | 6 | 9 |
| Mid insula | R | 75 | 29.97 | 42 | 3 | −9 |
| Posterior insula | R | 317 | 100.53 | 51 | −3 | 3 |
| Posterior insula | R | 273 | 56.96 | 39 | −15 | 12 |
| Posterior insula | R | 168 | 51.38 | 42 | −33 | 21 |
| Posterior insula | R | 99 | 32.68 | 36 | −24 | 3 |
| Hippocampus, parahippocampal gyrus |
L | 192 | 73.17 | −30 | −48 | −3 |
| Hippocampus, parahippocampal gyrus |
R | 62 | 49.88 | 33 | −42 | −6 |
| Parahippocampal gyrus | L | 71 | 33.17 | −21 | −33 | −21 |
| Parahippocampal gyrus | R | 89 | 33.07 | 27 | −36 | −18 |
| Parahippocampal gyrus, fusiform gyrus |
R | 195 | 59.87 | 27 | −51 | 0 |
| Precentral gyrus | R | 496 | 70.81 | 57 | −9 | 12 |
| Cuneus | L | 311 | 124.17 | −12 | −69 | 21 |
| Cuneus | R | 323 | 112.79 | 12 | −57 | 6 |
| Cuneus | R | 421 | 106.26 | 12 | −69 | 21 |
| Cuneus | L | 316 | 105.78 | −6 | −78 | 27 |
| Cuneus | L | 347 | 87.14 | −9 | −57 | 3 |
| Cuneus | R | 192 | 81.41 | 9 | −69 | 0 |
| Cuneus | L | 229 | 72.57 | −9 | −81 | 3 |
| Cuneus | L | 230 | 68.63 | −6 | −87 | 18 |
| Cuneus, cerebellum | L | 149 | 103.93 | −6 | −72 | −6 |
| Cerebellum | R | 85 | 62.56 | 9 | −69 | −9 |
| Cerebellum | L | 121 | 26.70 | −21 | −57 | −18 |
| Aversive > Neutral | ||||||
| Inferior frontal gyrus | L | 201 | 29.03 | −54 | 15 | 24 |
| Inferior frontal gyrus | L | 34 | 23.70 | −54 | 15 | 33 |
| Inferior frontal gyrus | L | 37 | 23.53 | −48 | 3 | 33 |
| Inferior frontal gyrus | L | 58 | 26.61 | −45 | 12 | 21 |
| Middle frontal gyrus | L | 113 | 40.52 | −30 | −3 | 45 |
| dmPFC | R | 705 | 68.25 | 3 | 54 | 30 |
| dmPFC | R | 38 | 30.87 | 6 | 18 | 57 |
| dmPFC | R | 132 | 30.66 | 6 | 30 | 51 |
| Anterior cingulate | L | 42 | 25.18 | −9 | 18 | 45 |
| Anterior insula | L | 274 | 41.12 | −30 | 24 | −3 |
| Anterior insula | L | 43 | 33.92 | −36 | 18 | −21 |
| Inferior frontal gyrus | L | 20 | 27.40 | −51 | 36 | −9 |
| Inferior frontal gyrus | L | 61 | 21.98 | −42 | 33 | −6 |
| Hippocampus | R | 260 | 71.03 | 30 | −27 | −3 |
| Hippocampus | R | 29 | 31.65 | 30 | −12 | −15 |
| Amygdala | R | 21 | 26.81 | 33 | −3 | −18 |
| Midbrain | L | 43 | 37.57 | −6 | −33 | −6 |
| Midbrain | L | 22 | 20.49 | −3 | −21 | −15 |
| Midbrain | R | 44 | 34.16 | 6 | −33 | −6 |
| Thalamus | R | 211 | 30.24 | 6 | −9 | 3 |
| Thalamus | L | 30 | 26.88 | −9 | −6 | 0 |
| Thalamus | R | 37 | 26.56 | 6 | −15 | 12 |
| Caudate | R | 34 | 22.78 | 12 | 3 | 15 |
| Caudate | R | 35 | 26.17 | 12 | 9 | 6 |
| Ventral striatum | L | 24 | 24.25 | −12 | 6 | 0 |
| Temporoparietal junction | L | 41 | 25.91 | −60 | −39 | 30 |
| Superior parietal lobule | L | 412 | 70.47 | −27 | −51 | 48 |
| Inferior parietal lobule | L | 108 | 25.90 | −42 | −33 | 39 |
| Superior parietal lobule | R | 362 | 109.43 | 27 | −48 | 45 |
| Superior parietal lobule | R | 208 | 89.85 | 27 | −54 | 54 |
| Cerebellum | R | 247 | 41.82 | 9 | −78 | −39 |
| Cerebellum | M | 83 | 34.95 | 0 | −54 | −36 |
| Cerebellum | R | 92 | 35.01 | 9 | −75 | −30 |
| Neutral > Aversive | ||||||
| Middle frontal gyrus | L | 118 | 29.60 | −24 | 18 | 51 |
| Middle frontal gyrus | L | 43 | 26.68 | −21 | 27 | 33 |
| Middle frontal gyrus | R | 188 | 27.36 | 36 | 36 | 30 |
| Middle frontal gyrus | R | 37 | 23.45 | 30 | 39 | 21 |
| Middle frontal gyrus | R | 83 | 22.23 | 39 | 45 | 21 |
| vmPFC | L | 229 | 34.56 | −9 | 39 | −9 |
| vmPFC | R | 108 | 33.65 | 12 | 36 | −9 |
| Pregenual cingulate | L | 28 | 17.47 | −3 | 30 | 3 |
| Inferior temporal gyrus | R | 52 | 26.56 | 69 | −27 | −21 |
| Superior temporal gyrus | L | 1886 | 71.51 | −57 | −6 | 3 |
| Superior temporal gyrus | L | 220 | 66.60 | −66 | −24 | 6 |
| Superior temporal gyrus | L | 167 | 56.36 | −51 | 3 | −6 |
| Superior temporal gyrus | L | 283 | 54.76 | −54 | −15 | 12 |
| Mid insula | L | 133 | 59.73 | −33 | 3 | 9 |
| Posterior insula | L | 178 | 49.92 | −36 | −36 | 21 |
| Posterior insula | L | 132 | 47.23 | −30 | −21 | 15 |
| Posterior insula | L | 135 | 43.34 | −36 | −21 | 3 |
| Posterior insula | L | 70 | 33.29 | −42 | −21 | −6 |
| Middle temporal gyrus | L | 61 | 26.91 | −66 | −45 | −12 |
| Precentral gyrus | L | 162 | 53.69 | −48 | −12 | 48 |
| Precentral gyrus | L | 60 | 41.02 | −33 | −18 | 42 |
| Inferior temporal gyrus | L | 23 | 17.02 | −57 | −36 | −18 |
| Posterior cingulate | L | 2056 | 70.99 | −6 | −36 | 36 |
| Posterior cingulate | R | 133 | 68.02 | 12 | −36 | 39 |
| Anterior cingulate | R | 168 | 53.93 | 6 | 6 | 36 |
| Mid cingulate | L | 61 | 25.88 | −6 | 0 | 42 |
| Mid cingulate | R | 37 | 21.77 | 12 | −15 | 42 |
| Pre SMA | R | 84 | 53.86 | 6 | 3 | 45 |
| Supplementary Motor Area | M | 168 | 51.40 | 0 | −3 | 60 |
| Supplementary Motor Area | R | 130 | 46.64 | 9 | −9 | 60 |
| Paracentral lobule | R | 155 | 62.21 | 6 | −30 | 48 |
| Paracentral lobule | R | 45 | 18.71 | 9 | −42 | 63 |
| Postcentral gyrus | R | 142 | 46.14 | 51 | −12 | 48 |
| Postcentral gyrus | R | 231 | 37.30 | 21 | −30 | 57 |
| Postcentral gyrus | L | 106 | 36.33 | −21 | −30 | 57 |
| Postcentral gyrus | R | 114 | 20.68 | 42 | −24 | 60 |
| Precentral gyrus | R | 93 | 35.16 | 36 | −18 | 42 |
| Precentral gyrus | L | 69 | 29.17 | −18 | −21 | 57 |
| Precentral gyrus | R | 33 | 20.64 | 60 | 0 | 39 |
| Superior parietal lobule | L | 93 | 39.21 | −18 | −39 | 63 |
| Inferior parietal lobule | L | 396 | 45.32 | −36 | −75 | 39 |
| Inferior parietal lobule | L | 136 | 38.57 | −45 | −66 | 45 |
| Inferior parietal lobule | L | 97 | 34.09 | −42 | −57 | 45 |
| Inferior parietal lobule | L | 24 | 25.60 | −39 | −84 | 36 |
| Inferior parietal lobule | R | 278 | 34.89 | 51 | −66 | 39 |
| Inferior parietal lobule | R | 74 | 28.75 | 39 | −66 | 42 |
| Inferior parietal lobule | R | 114 | 32.15 | 51 | −51 | 45 |
Brain regions identified by the main effect of stimulus valence. For hemisphere, R = right, L = left, M = medial. F = maximum F statistic for a given cluster. *A single large cluster was identified by the main effect of valence term but regions within this cluster varied according to whether they responded more strongly to negative and neutral stimuli. Subclusters that contained 20 or more voxels are reported under their supracluster.
3.2.2. Main effects of age
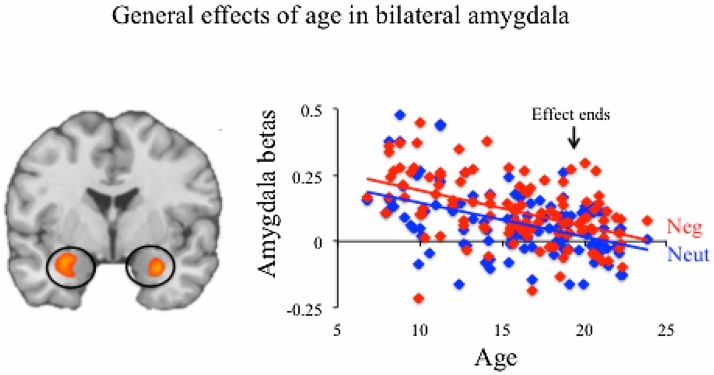
The main effect of age term revealed age-related decreases in activation for vmPFC, bilateral amygdala, temporal cortex, and cerebellum for aversive and neutral stimuli relative to fixation (Table 2; Fig. 2). A change-point analysis revealed that age was associated with significant decreases in amygdala activation starting at the lower bound of our age range (instantaneous slope at 6 years: −0.023, t(109) = 2.41, p < 0.05). Age continued to predict significant decreases for each year of life (p’s < 0.05) until age 20 (instantaneous slope: −0.01, t(109) = 1.52, p = 0.13), after which age no longer predicted significant changes in the amygdala response (p’s > 0.26). Put another way, the amygdala response decreased significantly across childhood and continued to decline until the end of adolescence. Age was associated with general increases in recruitment in dorsolateral prefrontal cortex and posterior parietal cortex. Given that age exerted a main effect on self-reported negative affect, a whole-brain correlational analysis was conducted correlating average self-reported affect (i.e., the mean of self-reported negative affect for aversive and neutral trials) and the aversive + neutral > fixation contrast. No brain regions survived correction in this correlational analysis, both before and after controlling for age.
Table 2.
Brain regions showing a general age effect (main effect of age).
| MNI Coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | # Voxels | F | x | y | z |
| Age predicts less activation | ||||||
| vmPFC | L | 395 | 31.23 | −3 | 45 | −9 |
| Subgenual ACC | L | 38 | 20.62 | −9 | 36 | −9 |
| Subgenual ACC | M | 53 | 21.22 | 0 | 30 | −6 |
| Pregenual ACC | R | 56 | 28.40 | 3 | 30 | 3 |
| Dorsal ACC | M | 36 | 18.49 | 0 | 33 | 18 |
| Caudate | L | 41 | 29.23 | −12 | 15 | 3 |
| Caudate | L | 24 | 24.21 | −6 | 6 | −3 |
| Caudate | R | 39 | 21.23 | 9 | 9 | −6 |
| Putamen | L | 33 | 26.84 | −18 | 12 | −9 |
| Amygdala | L | 118 | 37.70 | −27 | −9 | −15 |
| Hippocampus | L | 24 | 25.16 | −21 | −9 | −27 |
| Anterior insula | L | 29 | 19.41 | −42 | 6 | −18 |
| Amygdala | R | 41 | 24.94 | 30 | −3 | −24 |
| Superior temporal gyrus |
L | 33 | 20.26 | −60 | −24 | 0 |
| Superior temporal gyrus |
L | 20 | 20.23 | −51 | −18 | −3 |
| Cerebellum | R | 114 | 21.86 | 3 | −57 | −21 |
| Cerebellum | R | 32 | 21.82 | 9 | −48 | −18 |
| Cerebellum | R | 37 | 21.82 | 9 | −48 | −18 |
| Age predicts more activation | ||||||
| Inferior and middle frontal gyri | L | 31 | 23.57 | −51 | 12 | 30 |
| Inferior parietal lobule | L | 52 | 28.78 | −36 | −39 | 33 |
Brain regions identified by the main effect of age (i.e., aversive and neutral stimuli compared to fixation). R = right, L = left, M = medial. For hemisphere, F = maximum F statistic for a given cluster. Subclusters that contained 20 or more voxels are reported under their supracluster.
Fig. 2.
Bilateral amygdala responses decreased with age. Average betas from the left and right amygdala clusters (defined by the main effect of age term) are plotted against age. F values are displayed and thus map intensity values correspond to overall significance and not directionality of effects. Arrow indicates end of age effect, as determined by change-point analyses (effect was evident at youngest ages).
3.3. Negative affect-specific effects of age
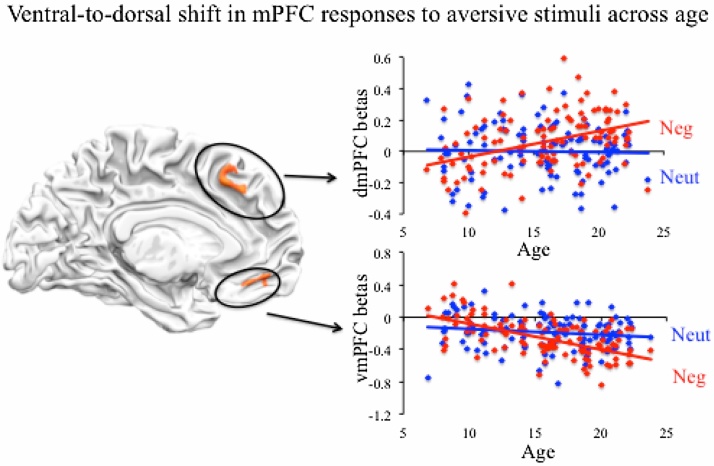
Significant interactions between age and stimulus valence were observed in vmPFC and dmPFC (Table 3; Fig. 3). Brain activation for aversive stimuli decreased with age in vmPFC, but increased with age in dmPFC. An investigation of the regression equations for each cluster revealed that brain responses to aversive and neutral stimuli were equivalent at 12.61 years for vmPFC and 12.47 years for dmPFC. To further examine whether valence is represented differently in medial PFC across age, moderation analyses using the Johnson-Neyman technique were performed with valence as a predictor, age as a moderator and prefrontal responses as an outcome variable. Moderation analyses using age as a predictor and stimulus valence as a moderator revealed identical results, however using age as a moderator allowed us to identify specific ages at which stimulus valence predicted differential recruitment in vmPFC and dmPFC (which the converse analysis would not have allowed). Johnson-Neyman results revealed that in vmPFC aversive stimuli elicited significantly greater recruitment than neutral stimuli at 7.72 years and younger ages (beta coefficient for effect of negative valence on vmPFC at 7.98 years = 0.06, t = 1.97, p = 0.05), whereas aversive stimuli elicited less recruitment than neutral stimuli at 14.96 years and older ages (beta coefficient for effect of negative valence on vmPFC at 15.09 years = −0.03, t = 1.97, p = 0.05). By contrast, neutral and aversive stimuli did elicit statistically significant differences in dmPFC before the age of 14, although neutral stimuli elicited marginally greater recruitment than aversive stimuli in the youngest participants in the sample (beta coefficient for effect of negative valence on dmPFC at 6.84 years = −0.05, t = 1.85, p = 0.06). Starting at 15 years, aversive stimuli elicited significantly greater dmPFC recruitment than neutral stimuli (beta coefficient for effect of negative valence on dmPFC at 15 years = 0.02, t = 1.97, p = 0.05). This trend persisted for each subsequent year of age and older ages (p’s < 0.05).
Table 3.
Brain regions showing differential recruitment as a function of age and stimulus valence.
| Avers | Neut | MNI Coordinates |
||||||
|---|---|---|---|---|---|---|---|---|
| Region | Hemisphere | # Voxels | F | r | r | x | y | z |
| Age predicts less activation for aversive stimuli | ||||||||
| vmPFC | R | 121 | 25.32 | −0.52*** | −0.15 | 6 | 36 | −12 |
| vmPFC | R | 22 | 17.89 | −0.48*** | −0.20* | 3 | 48 | −3 |
| Age predicts more activation for aversive stimuli | ||||||||
| dmPFC | M | 81 | 21.18 | 0.39*** | −0.03 | 0 | 33 | 39 |
| dmPFC | L | 34 | 18.96 | 0.32*** | −0.04 | −3 | 21 | 42 |
Brain regions identified by the interaction term between age and stimulus valence. For hemisphere, R = right, L = left, M = medial. F = maximum F statistic for a given cluster. The correlation between age and each stimulus category is reported under the columns labeled “Avers r” and “Neut r” along with their statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001). Subclusters that contained 20 or more voxels are reported under their supracluster.
Fig. 3.
Age is associated with a ventral-to-dorsal shift in mPFC responses to aversive stimuli. F values are displayed and thus map intensity values correspond to overall significance and not directionality of effects. Top: Age predicted increased dmPFC recruitment for aversive, but not neutral, stimuli. Bottom: Age predicted decreased vmPFC recruitment for aversive, but not neutral, stimuli.
4. Discussion
An individual’s social and emotional landscape changes dramatically during childhood and adolescence. The results of the present study suggest that child and adolescent development are characterized by general decreases in amygdala reactivity and a ventral-to-dorsal shift in medial prefrontal responses to aversive stimuli. Together, these results suggest that maturing affective processes are jointly supported by a reduced tendency to recruit brain regions involved in emotion generation, and an increased tendency to engage brain regions associated with top-down representations of emotion (Ochsner et al., 2009).
4.1. General age-related changes in responding
In looking across age, we found evidence that children, adolescents and adults bore striking similarities as well as differences in how they responded to aversive and neutral stimuli. On the one hand, a main effect of age was observed such that children experienced more negative affect and greater recruitment of the bilateral amygdala, which have been consistently implicated in detecting and responding to motivationally relevant stimuli such as threats (Cunningham and Brosch, 2012), for both aversive and neutral stimuli relative to adolescents and adults. The amygdala develops prior to prefrontal regions that are thought to be important for regulating the amygdala and as such, the amygdala may exert a stronger influence on affective processing during development than during adulthood (Casey et al., 2008). This may lead children to view both affective and non-affective stimuli through a valenced lens. Consistent with this, children tend to interpret ambiguous facial expressions more negatively than do adults (Tottenham et al., 2013), and show robust amygdala recruitment when adults do not (Swartz et al., 2014, Thomas et al., 2001, Todd et al., 2010). At the same time, it should be noted that children’s heightened reactivity ought not to be conflated with indiscernibility. Like their older counterparts, children reported greater negative affect and showed greater amygdala recruitment for aversive than neutral stimuli. This suggests that while children on average report more negative affect for both aversive and neutral stimuli, they are also capable of distinguishing between aversive and neutral stimuli.
Another interpretation of the present findings is that the age-related changes in amygdala responding observed in the present study do not reflect changes in affective processing but rather developmental differences in another domain. For example, both animal and human lesion work suggests that the amygdala plays a critical role in coordinating social processes during development (Prather et al., 2001, Shaw et al., 2004). Given that the present study utilized social (i.e., images containing people) affective and neutral stimuli, it is possible that elevated amygdala responses during childhood reflect heightened attention and orientation towards social cues. Indeed, much prior work has suggested that children and adolescents respond to social stimuli, and in particular, facial expressions (Somerville et al., 2011), in different ways than do adults. While the present study did not utilize facial stimuli, which has been the modal approach in prior studies examining amygdala function across wide age ranges (Gee et al., 2013, Hare et al., 2008, Swartz et al., 2014), it is still possible that different findings would be obtained with non-social stimuli. To further test this possibility, future work might seek to disentangle valence and social content when examining age-related effects in the amygdala as well as in other brain regions involved in evaluating the affective significance of stimuli (e.g., ventral striatum).
4.2. Negative affect-specific age-related changes in emotion responding
The present results suggest that while subcortical structures like the amygdala exhibit general age-related changes in function, medial prefrontal recruitment shows age dissociable age effects for vmPFC and dmPFC that were unique to negative affective stimuli. vmPFC and dmPFC follow dissociable structural developmental trajectories (Markham et al., 2007, Shaw et al., 2008), and are thought to play functionally dissociable but complementary roles in social and emotional processing (Etkin et al., 2011). As such, the significance behind age effects observed in each region is considered in greater depth below.
Consistent with prior work in adults (Lindquist et al., 2015), in the present study, vmPFC showed less activation for aversive versus neutral stimuli in adolescents and adults. By contrast, children’s vmPFC responses were greater for aversive than neutral stimuli. vmPFC is known to support the integration of prior experiences, semantic representations, and the present context in order to update the affective value of stimuli (Roy et al., 2012). In adults, amygdala and vmPFC responses to ambiguous or aversive stimuli are often inversely associated with one another, such that vmPFC responses increase as amygdala responses decrease and vice versa (Kim et al., 2003, Shin et al., 2005, Urry et al., 2006), and with affective experience (Heller et al., 2014). However, recent developmental neuroimaging work suggests that the amygdala and vmPFC are positively associated in childhood when amygdala activation is elevated as well, but negatively associated in adolescence and adulthood when amygdala activation declines (Gee et al., 2013; Silvers et al., in press). When contextualized within broader hierarchical theories of development (Thelen, 2005), these prior results as well as the present findings suggest that heightened amygdala activation in childhood instigates the development of initially immature (i.e., positive) amygdala-vmPFC connections that develop and change during adolescence, and ultimately scaffold the development of connections between vmPFC and other dorsal and lateral portions of PFC (Casey et al., 2016). Such changes in prefrontal development may be related to the profound shifts in social and self-regulatory behavior commonly observed during the transition from childhood to adolescence (Blakemore, 2008, Somerville et al., 2013).
In contrast to the age-related decreases in activation observed in vmPFC for aversive stimuli, we observed striking increases in dmPFC recruitment for aversive stimuli across age. dmPFC supports a variety of domain-general cognitive control processes including inhibition and flexibility (Niendam et al., 2012). In the context of emotional processing dmPFC has been linked to a host of processes that involve appraising emotional stimuli and reflecting on mental states, including learning fear (Mechias et al., 2010), mentalizing (Denny et al., 2012), and cognitively regulating emotion (Buhle et al., 2014). Moreover, while the amygdala supports both bottom-up and top-down generation of emotion, dmPFC is uniquely important for using conceptual and contextual knowledge to generate affective responses (Ochsner et al., 2009). Recent neuroimaging work has revealed that dmPFC activation and dmPFC-vmPFC functional connectivity in response to aversive stimuli strengthens during the transition from adolescence to adulthood at the same time that cognitive performance improves (Cohen et al., 2016). As such, age-related increases in dmPFC engagement may reflect an increased propensity to automatically form higher-level affective appraisals or to engage self-control mechanisms when responding to aversive stimuli.
4.3. Limitations
The present study has several limitations worth nothing when interpreting its results. First, the age-related effects observed were obtained exclusively in the context of uninstructed viewing of aversive and neutral stimuli. In contrast to studies that explicitly instruct participants about how to respond to stimuli (Silvers et al., in press; Silvers et al., 2012, Silvers et al., 2015), this leaves open the possibility that participants interpreted and responded to the stimuli in more varied ways. At the same time, this unconstrained approach may be more ecologically valid when drawing inferences about what individuals do when left to their own devices (i.e., in their everyday lives). Second, the present study speaks to differences in overall activation of the amygdala and prefrontal cortex but not to their connectivity. Finally, while not a limitation in the strictest sense, it is worth noting that individual differences in self-reported negative affect were not significantly correlated with amygdala or medial prefrontal recruitment. One interpretation of this finding is that age predicted more variance in these regions than did between-subject variability in affective experience.
5. Conclusions
By examining age effects across a 17-year range, the present study sought to characterize how development of prefrontal-amygdala circuitry gives rise to general and negative affect-specific changes in responding. While participants of all ages discriminated between aversive and neutral stimuli, children demonstrated generally greater reactivity in terms of self-reported affect and amygdala responses to both aversive and neutral stimuli relative to adolescents and adults. At the same time, age was associated with a ventral-to-dorsal shift in medial prefrontal responses to affective stimuli specifically, suggesting that aversive affective cues are interpreted in increasingly specialized and cognitive ways across development. Together, these results suggest that the transition from childhood to adolescence marks a shift from bottom-up, amygdala-based processing to a tendency to integrate bottom-up and top-down affective cues in adolescence and adulthood.
Conflict of interest
None.
Acknowledgements
The authors would like to thank Danielle Dellarco, Alexa Hubbard, Natasha Mehta, Gloria Pedersen, and Theresa Teslovich Woo for their help in recruiting and testing participants. We thank the families who participated in this study. This work was supported by the National Institutes of Health (R01 NICHD 0691780, F31 NIMH 94056).
Contributor Information
Jennifer A. Silvers, Email: silvers@ucla.edu.
Kevin N. Ochsner, Email: Ochsner@psych.columbia.edu.
References
- Achenbach T.M. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. Manual for the ASEBA School-Age Forms & Profiles. [Google Scholar]
- Anderson A.K., Christoff K., Stappen I., Panitz D., Ghahremani D.G., Glover G., Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Smits K., Van Ree J.M. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. J. Comp. Neurol. 2002;450(3):241–255. doi: 10.1002/cne.10321. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Wolterink G., van Ree J.M. Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J. Comp. Neurol. 2002;442(3):239–249. doi: 10.1002/cne.10084. [DOI] [PubMed] [Google Scholar]
- Breiter H.C., Etcoff N.L., Whalen P.J., Kennedy W.A., Rauch S.L., Buckner R.L., Rosen B.R. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H., Ochsner K.N. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24(11):2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Galván A., Somerville L.H. Beyond simple models of adolescence to an integrated circuit-based account: a commentary. Dev. Cogn. Neurosci. 2016;17:128–130. doi: 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu. Rev. Psychol. 2015;66(1):295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Cohen A.O., Breiner K., Steinberg L., Bonnie R.J., Scott E.S., Taylor-Thompson K.A., Casey B.J. When is an adolescent an adult? Assessing cognitive control in emotional and nonemotional contexts. Psychol. Sci. 2016;27(4):549–562. doi: 10.1177/0956797615627625. [DOI] [PubMed] [Google Scholar]
- Costafreda S.G., Brammer M.J., David A.S., Fu C.H.Y. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res. Rev. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Cunningham W.A., Brosch T. Motivational salience: amygdala tuning from traits, needs, values, and goals. Curr. Dir. Psychol. Sci. 2012;21(1):54–59. [Google Scholar]
- Denny B.T., Kober H., Wager T.D., Ochsner K.N. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J. Cogn. Neurosci. 2012;24(8):1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Geier K., Falkai P., Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58(1):275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. S1364-6613(10)00252-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Phillips M.L., Silk J.S., Ryan N.D., Dahl R.E. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev. Neuropsychol. 2011;36(4):429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Monk C.S., McClure-Tone E.B., Nelson E.E., Roberson-Nay R., Adler A.D., Ernst M. A developmental examination of amygdala response to facial expressions. J. Cogn. Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Camerer C.F., Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. 324/5927/646 [pii] [DOI] [PubMed] [Google Scholar]
- Hayes A.F. The Guilford Press; New York: 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. [Google Scholar]
- Helfinstein S.M., Casey B.J. Commentary on Spielberg at al., Exciting fear in adolescence: does pubertal development alter threat processing? Dev. Cogn. Neurosci. 2014;8:96–97. doi: 10.1016/j.dcn.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., Lapate R.C., Mayer K.E., Davidson R.J. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J. Cogn. Neurosci. 2014;26(9):2102–2110. doi: 10.1162/jocn_a_00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P.O., Fay L.C. The Johnson-Neyman technique, its theory and application. Psychometrika. 1950;15(4):349–367. doi: 10.1007/BF02288864. [DOI] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Somerville L.H., Johnstone T., Alexander A.L., Whalen P.J. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14(18):2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kober H., Barrett L.F., Joseph J., Bliss-Moreau E., Lindquist K., Wager T.D. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. S1053-8119(08)00294-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. The University of Florida; Gainsville, FL: 2001. International Affective Picture System (IAPS) Instruction Manual and Affective Ratings, Technical Report. [Google Scholar]
- Lindquist K.A., Satpute A.B., Wager T.D., Weber J., Barrett L.F. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J.A., Morris J.R., Juraska J.M. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144(3):961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- McCallum J., Kim J.H., Richardson R. Impaired extinction retention in adolescent rats: effects of d-cycloserine. Neuropsychopharmacology. 2010;35(10):2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., Robertson E.R., Sokol-Hessner P., Ray R.D., Ochsner K.N. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc. Cogn. Affect. Neurosci. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias M.L., Etkin A., Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49(2):1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. S1053-8119(09)01019-2 [pii] [DOI] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L., Pitman R.K., Quirk G.J. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol. Psychol. 2006;73(1):61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Petrovic P., Marchant J.L., Hassabis D., Weiskopf N., Seymour B., Frith C.D. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317(5841):1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., McClure E.B., Nelson E.E., Zarahn E., Bilder R.M., Leibenluft E., Pine D.S. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Niendam T.A., Laird A.R., Ray K.L., Dean Y.M., Glahn D.C., Carter C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The neural bases of emotion and emotion regulation: a valuation perspective. In: Gross J.J., Thompson R.A., editors. The Handbook of Emotion Regulation. Guilford Press; New York: 2014. pp. 23–43. [Google Scholar]
- Ochsner K.N., Ray R.R., Hughes B., McRae K., Cooper J.C., Weber J., Gross J.J. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol. Sci. 2009;20(11):1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. PSCI2459 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc. Cogn. Affect. Neurosci. 2009;4(4):387–398. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell S.S., Duhoux S., Hartley C.A., Johnson D.C., Jing D., Elliott M.D., Lee F.S. Altered fear learning across development in both mouse and human. Proc. Natl. Acad. Sci. U. S. A. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Blakemore S.J. Adolescent social cognitive and affective neuroscience: past, present, and future. Soc. Cogn. Affect. Neurosci. 2012;7(1):1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather M.D., Lavenex P., Mauldin-Jourdain M.L., Mason W.A., Capitanio J.P., Mendoza S.P., Amaral D.G. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106(4):653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Garcia R., Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol. Psychiatry. 2006;60(4):337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Roy M., Shohamy D., Wager T.D. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn. Sci. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. S1364-6613(12)00027-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute A.B., Shu J., Weber J., Roy M., Ochsner K.N. The functional neural architecture of self-reports of affective experience. Biol. Psychiatry. 2013;73(7):631–638. doi: 10.1016/j.biopsych.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Shaw P., Lawrence E.J., Radbourne C., Bramham J., Polkey C.E., David A.S. The impact of early and late damage to the human amygdala on ‘theory of mind’ reasoning. Brain. 2004;127(7):1535–1548. doi: 10.1093/brain/awh168. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Wright C.I., Cannistraro P.A., Wedig M.M., McMullin K., Martis B., Rauch S.L. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch. Gen. Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Silvers J.A., McRae K., Gabrieli J.D., Gross J.J., Remy K.A., Ochsner K.N. Age-Related differences in emotional reactivity regulation, and rejection sensitivity in adolescence. Emotion. 2012 doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Shu J., Hubbard A.D., Weber J., Ochsner K.N. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Dev. Sci. 2015;18(5):771–784. doi: 10.1111/desc.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Insel C., Powers A., Franz P., Helion C., Martin R.E., Ochsner K.N. vlPFC-vmPFC-amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cereb. Cortex. 2016 doi: 10.1093/cercor/bhw073. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Fani N., McClure-Tone E.B. Behavioral and neural representation of emotional facial expressions across the lifespan. Dev. Neuropsychol. 2011;36(4):408–428. doi: 10.1080/87565641.2010.549865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Ruberry E.J., Dyke J.P., Glover G., Casey B.J. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol. Sci. 2013;24(8):1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F., Quirk G.J. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20(2):231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz J.R., Carrasco M., Wiggins J.L., Thomason M.E., Monk C.S. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. Neuroimage. 2014;86:212–220. doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E. Dynamic systems theory and the complexity of change. Psychoanal. Dialogues. 2005;15(2):255–283. [Google Scholar]
- Thomas K.M., Drevets W.C., Whalen P.J., Eccard C.H., Dahl R.E., Ryan N.D., Casey B.J. Amygdala response to facial expressions in children and adults. Biol. Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Todd R.M., Evans J.W., Morris D., Lewis M.D., Taylor M.J. The changing face of emotion: age-related patterns of amygdala activation to salient faces. Soc. Cogn. Affect. Neurosci. 2010 doi: 10.1093/scan/nsq007. (nsq007 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Phuong J., Flannery J., Gabard-Durnam L., Goff B. A negativity bias for ambiguous facial-expression valence during childhood: converging evidence from behavior and facial corrugator muscle responses. Emotion. 2013;13(1):92–103. doi: 10.1037/a0029431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., Kalin N.H., Thurow M.E., Schaefer H.S., Davidson R.J. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bulk B.G., Koolschijn P.C.M.P., Meens P.H.F., van Lang N.D.J., van der Wee N.J.A., Rombouts S.A.R.B., Crone E.A. How stable is activation in the amygdala and prefrontal cortex in adolescence? A study of emotional face processing across three measurements. Dev. Cogn. Neurosci. 2013;4:65–76. doi: 10.1016/j.dcn.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48(3):564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vasa R.A., Pine D.S., Thorn J.M., Nelson T.E., Spinelli S., Nelson E., Mostofsky S.H. Enhanced right amygdala activity in adolescents during encoding of positively valenced pictures. Dev. Cogn. Neurosci. 2011;1(1):88–99. doi: 10.1016/j.dcn.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M., Derks J.M., Hoogendam J.M., Hillegers M., Kahn R.S. Functional differences in emotion processing during adolescence and early adulthood. Neuroimage. 2014;91:70–76. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Wager T.D., van Ast V.A., Hughes B.L., Davidson M.L., Lindquist M.A., Ochsner K.N. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47(3):836–851. doi: 10.1016/j.neuroimage.2009.05.044. S1053-8119(09)00556-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M., Brown K.J., Palmer D., Liddell B.J., Kemp A.H., Olivieri G., Gordon E. The mellow years?: neural basis of improving emotional stability over age. J. Neurosci. 2006;26(24):6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]