Abstract
Background
Growing evidence supports a central role for the circadian system in alcohol use disorders, but few studies have examined this relationship during adolescence. In mammals, circadian rhythms are regulated by the suprachiasmatic nucleus (SCN), a biological clock whose timing is synchronized (reset) to the environment primarily by light (photic) input. Alcohol (ethanol) disrupts circadian timing in part by attenuating photic phase-resetting responses in adult rodents. However, circadian rhythms change throughout life and it is not yet known whether ethanol has similar effects on circadian regulation during adolescence.
Methods
General circadian locomotor activity was monitored in male C57BL6/J mice beginning in adolescence (P27) or adulthood (P61) in a 12 h light, 12 h dark photocycle for ~2 weeks to establish baseline circadian activity measures. On the day of the experiment, mice received an acute injection of ethanol (1.5 g/kg, i.p.) or equal volume saline 15 min prior to a 30-min light pulse at Zeitgeber Time 14 (2 h into the dark phase), then were released into constant darkness (DD) for ~2 weeks to assess phase-resetting responses. Control mice of each age group received injections but no light pulse prior to DD.
Results
While adults showed the expected decrease in photic phase-delays induced by acute ethanol, this effect was absent in adolescent mice. Adolescents also showed baseline differences in circadian rhythmicity compared to adults, including advanced photocycle entrainment, larger photic phase-delays, a shorter free-running (endogenous) circadian period, and greater circadian rhythm amplitude.
Conclusions
Collectively, our results indicate that adolescent mice are less sensitive to the effect of ethanol on circadian photic phase-resetting and that their daily activity rhythms are markedly different than those of adults.
Keywords: alcohol, circadian, adolescent, photic phase-resetting
INTRODUCTION
Alcohol (ethanol) is highly disruptive to circadian behavioral, physiological, and gene expression rhythms, and a large body of research supports a central role for chronodisruption in the pathophysiology of alcohol use disorders (Rosenwasser, 2010). In mammals, circadian rhythms are regulated by the suprachiasmatic nucleus (SCN) of the hypothalamus, a clock whose timing is regulated by oscillatory gene expression and synchronized to the environment by photic (light), and nonphotic (behavioral) stimuli (Pickard, 1982, Webb et al., 2014). These stimuli elicit either advances or delays of the circadian rhythm based upon the time of day at which they are presented. Investigations into the mechanisms by which ethanol disrupts circadian timing have revealed that it potently inhibits both photic and nonphotic phase-resetting in several adult rodent species (Brager et al., 2010, Brager et al., 2011, Ruby et al., 2009a, Ruby et al., 2009b, Seggio et al., 2009). Thus, ethanol effectively disables synchronization of the circadian clock to the environment, which is required daily to maintain 24-hour physiological and behavioral functioning. The resulting misalignment of internal time and external demands may reinforce use of alcohol for sedation in a misguided attempt to correct the problem. In line with this notion, shiftwork has long been known to increase risk of heavy alcohol drinking (Smart, 1979), and sleep disturbance in abstinent alcoholics is highly predictive of relapse (Roehrs and Roth, 2001).
In humans, circadian activity and sleep patterns change throughout life and are generally delayed during adolescence (Hagenauer et al., 2009). The basis for this delayed chronotype is unknown, though it has been speculated that adolescents may exhibit enhanced sensitivity to phase-delays and/or reduced sensitivity to phase-advances compared to adults (Hagenauer et al., 2009). Many studies correlate a high “eveningness” / low “morningness” profile (peak alertness occurring in the evening rather than the morning hours) with use of alcohol and other drugs (Kanerva et al., 2012, Robinson et al., 2013, Tavernier and Willoughby, 2014, Taylor et al., 2011, Urban et al., 2011, Watson et al., 2013, Whittier et al., 2014) as well as addiction (Kervran et al., 2015, Lemoine et al., 2013). This correlation is further accentuated within adolescent and college-age populations (Tavernier and Willoughby, 2014, Taylor et al., 2011). Moreover, behavioral traits commonly associated with addiction and typified by adolescents, such as reward dependence, impulsivity, and novelty-seeking, are also linked with eveningness (Hsu et al., 2012).
Very little is known about developmental changes in rodents’ circadian rhythmicity and how they compare to humans. One study reported that pubertal mice showed accelerated re-entrainment to a delayed photocycle (Weinert et al., 1994). Another study found that female mice show larger photic phase-delays, but not phase-advances, during puberty (P49) compared to adult females (P140) (Weinert and Kompauerova, 1998), consistent with the suggested mechanism underlying eveningness in human adolescents. Adolescent rats submitted to chronic, 6-hour shifts of the photocycle (similar to photocycle changes associated with shiftwork or repeated jetlag) were resistant to the circadian misalignment seen in their adult counterparts (Albert et al., 2013). Adolescent C57BL/6J mice (the same strain used in the present study) showed robust endogenous and light-entrained circadian rhythms in social investigation time compared to BALB/cJ mice, though there were no adult control groups in this study (Panksepp et al., 2008).
Animal models have revealed marked differences in alcohol consumption and sensitivity in adolescents compared to adults. Pre-adolescent and adolescent rats show less severe alcohol deprivation effect than adults (Garcia-Burgos et al., 2009). Compared to adult rats, adolescents are less sensitive to ethanol-induced sedation (Little et al., 1996) and more sensitive to ethanol-induced spatial memory impairment (Markwiese et al., 1998). Results from studies employing C57BL/6J mice are somewhat more variable, although generally comparable to those in rats. During adolescence, mice of this strain consume more alcohol than adults, particularly in models of limited access, intermittent access, and binge-drinking (Holstein et al., 2011, Melendez, 2011, Moore et al., 2010, Quoilin and Boehm, 2016; but see also Hefner and Holmes, 2007). Adolescent C57BL/6J mice also show differential sensitivity to acute ethanol, including increased sensitivity to ethanol-induced locomotor stimulation (Hefner and Holmes, 2007, Melon and Boehm, 2011), anxiolysis (Hefner and Holmes, 2007), and certain memory impairments (Lacaille et al., 2015, Spanos et al., 2012), and decreased sensitivity to ethanol-induced conditioned taste aversion (CTA; (Holstein et al., 2011, Moore et al., 2013), sedation (Hefner and Holmes, 2007, Linsenbardt et al., 2009), hypothermia and locomotor suppression (Lopez et al., 2003).
Little is known about circadian rhythms in adolescent animal models, and no studies to date have included alcohol-related endpoints. However, as many of the age-dependent behavioral changes discussed above, including alcohol drinking, ethanol-induced loss of righting reflex (LORR) and ataxia, and memory functions vary diurnally and are regulated in part by the circadian clock (Perreau-Lenz et al., 2009, Ruby et al., 2013, Ruby et al., 2008, Ruby et al., 2016), there is a clear need to understand the role of the circadian system in alcohol-related behavior during adolescence. To begin addressing this gap in our knowledge, we compared the effect of acute ethanol on photic phase-resetting, the primary mechanism by which circadian rhythms are synchronized to the environment, in adolescent and adult mice. Because a previous study suggests that adolescent mice are more resilient than adults to chronodisruption (Albert et al., 2013), we hypothesized that they would also be less sensitive to ethanol-induced inhibition of photic phase-resetting. We also report several baseline differences in circadian rhythms between adolescent and adult mice.
MATERIALS AND METHODS
Animals
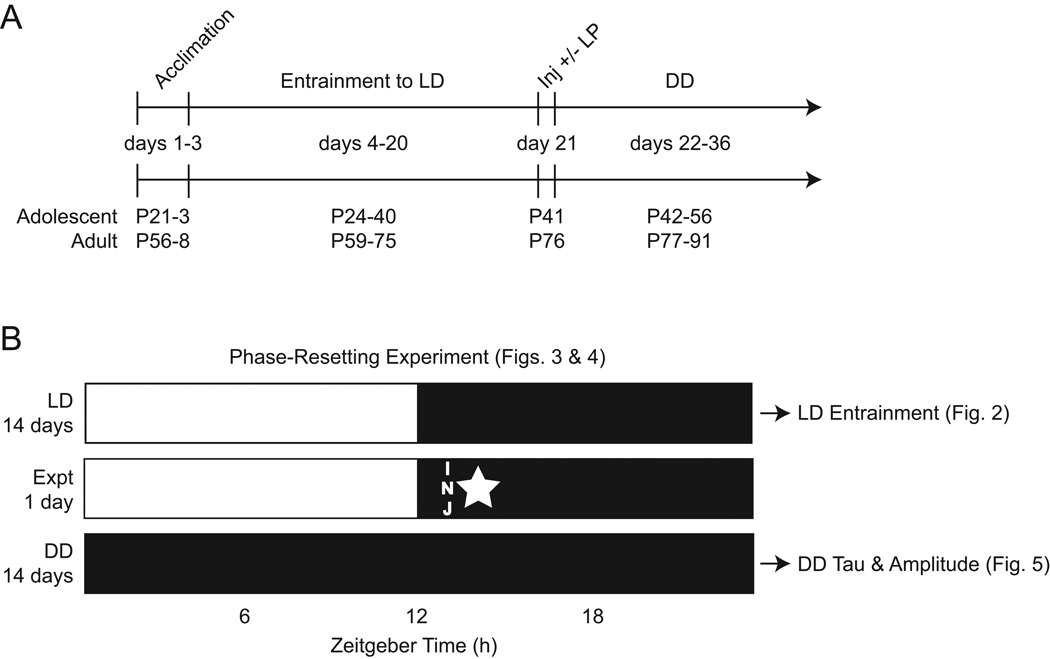
Male C57BL6/J mice were purchased from The Jackson Laboratory and arrived at adolescent (P21 ± 3) and adult (P56 ± 3) age. Upon arrival, mice were allowed to acclimate in the testing room for 3 days in group-housed cages within their own age group to minimize stress. After the 3-day acclimation period, mice were individually caged and placed under infrared sensors to monitor their circadian activity for the entire ~40 day experimental procedure (Figure 1A). Four cohorts of approximately 20 mice each were used in serial experiments for a total of 41 adolescents and 43 adults (n ≈ 10/cohort). All mice were tested in the same testing room under the same environmental conditions (except the experimental variables, described in the following sections). For the first 20 days, the testing room was maintained on a 12-hour light, 12-hour dark (LD) photocycle, with lights-on at 2:00 AM, and lights-off at 2:00 PM. This photocycle allowed the phase-resetting experiments, which take place during the dark phase, to occur at a convenient time for the investigators. LD entrainment measures were taken from the 14 day period prior to the phase-resetting experiments. Following the phase-resetting experiments on day 21, the testing room was then maintained in constant darkness (DD) for an additional 14 days to assess phase-resetting responses and endogenous circadian period and amplitude (described below). The entire experimental procedure and ages of mice during each segment are delineated in Figure 1A. Figure 1B shows the phase-resetting experiment design relative to the photocycles and which segments of the procedure are represented by the data in each subsequent Figure. Food and water was available ad libitum throughout the experimental procedure. Animal care/handling and experimental procedures were approved by the Indiana University of Pennsylvania IACUC according to NIH guidelines.
Fig. 1.
Experimental procedure. (A) Timeline showing the experimental procedure relative to the ages of mice in each age group (P = postnatal day, LD = light-dark photocycle, Inj = injection, LP = light pulse, DD = constant darkness). (B) Design for the phase-resetting experiments showing the duration of time in each photocycle used in the analyses, the timing of injection (INJ), and timing of the light-pulse (star; control mice received injections at the same time, but no light pulse). Also shown are the periods of time represented by the data in each subsequent figure.
LD Entrainment
General circadian locomotor activity rhythms for each individually-housed mouse were monitored using infrared motion detectors interfaced with a computerized data acquisition system (Clocklab; Coulbourn Instruments, Whitehall, PA). All data was analyzed using the ClockLab circadian toolbox (Actimetrics, Wilmette, IL) for MATLAB (Mathworks, Natick, MA). The following baseline entrainment measures were taken for all mice (n = 41 adolescents, n = 43 adults) after they had time to acclimate to the testing room and photocycle (e.g. during the last 14 days in LD). Circadian period (tau) was determined using ClockLab’s chi-squared periodogram analysis. The peak magnitude (amplitude) of the periodogram was used to estimate the robustness of the rhythm. Activity onset was defined as the first 10 min activity that 1) exceeded 10% maximum daily rate, 2) was preceded by ≥4 h inactivity, and 3) was followed by ≥30 min sustained activity. Onsets were then calculated relative Zeitgeber Time 12 (ZT12), the beginning of the dark phase (active phase for nocturnal rodents). Activity offset was defined as the final 10 min that was preceded by ≥60 min sustained activity and followed by ≥4 h inactivity and was calculated in relation to ZT0, the time of lights-on (inactive/rest phase for nocturnal rodents). Alpha (the duration of the active phase) was calculated as the period between activity onset and activity offset (h).
Photic Phase-Resetting
Two of the 4 cohorts of mice were used to determine whether there were age-related changes in the effect of ethanol on photic phase-delays. Once mice showed stable entrainment to the LD photocycle for at least 14 days (as described above), they were divided into treatment groups. On the day of the experiment, mice received an i.p. injection of either ethanol (1.5 g/kg, n = 11 adolescents, n = 13 adults) or saline (n = 10 adolescents, n = 12 adults) 20 min preceding a 30-min phase-delaying light pulse (25 lux) at ZT14 (2 hours into the dark phase). Injections were undertaken in the dark using dim red light. Immediately after the light pulse, mice were released into DD for 14 days to assess phase shifting using an Aschoff Type II procedure (Aschoff, 1965). Phase-shifts were calculated as the difference between the projected times of activity onset (defined as above) on the day after stimulation as determined by 1) back extrapolation of the least-squares line through activity onsets on days 2–10 after treatment and 2) extrapolation of the least-squares line calculated from activity onset data collected during the 10 days before treatment.
Phase-Resetting Control
The other two of the 4 cohorts of mice were used to rule out the possibility that ethanol has a phase-resetting action on its own at ZT14. Once mice showed stable entrainment to the LD photocycle for at least 14 days (as described above), they were divided into treatment groups. On the day of the experiment, mice received an i.p. injection of either ethanol (1.5 g/kg, n = 10 adolescents, n = 8 adults) or saline (n = 10 adolescents, n = 10 adults) 20 min preceding ZT14, but no light pulse was given. Injections were undertaken in the dark using dim red light. Following the injection, mice remained in DD for 14 days to assess phase shifts (as described above).
DD Tau and Amplitude
To determine whether there were age-related differences in endogenous circadian period and amplitude, tau and amplitude were determined for each mouse during the 14 day period in DD using the chi-squared periodogram analysis as described above. Although the single dose of ethanol administered would not be expected to alter free-running period, we ruled out this possibility statistically (below) prior to reporting the data for all adolescents (n = 41) compared to all adults (n = 43) used in this study.
Statistical Analysis
LD entrainment measures were analyzed using Student’s t-tests to find any differences between age groups. Phase-resetting data were analyzed using a two-way ANOVA with factors of age (adolescent vs. adult) and treatment (ethanol vs. saline). Separate ANOVAs were performed for the photic phase-resetting experiment and the phase-resetting control experiment because each experiment addressed a separate question, as posed above (e.g. the presence or absence of a light pulse was not an experimental condition per se). Tau and amplitude in DD were analyzed in two ways: 1) using a two-way ANOVA with factors of age (adolescent vs. adult) and treatment (ethanol vs. saline) to rule out the unlikely possibility that acute ethanol affected these measures, and 2) using Student’s t-test to compare age groups. ANOVA were followed by Sidak’s multiple comparisons post-hoc tests where interactions between variables were found. Results were considered significant when p < 0.05.
RESULTS
Adolescent Mice Display Higher Circadian Amplitude and Advanced Photocycle Entrainment Compared to Adults
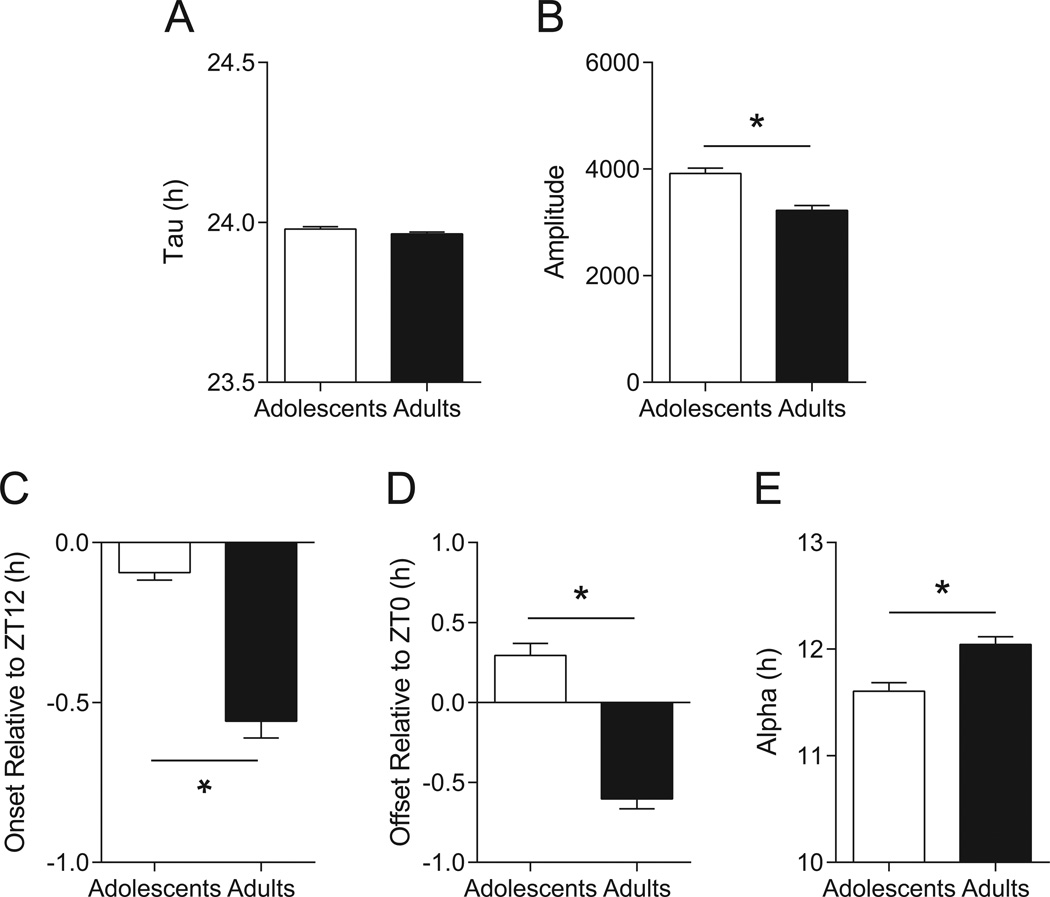
Both adolescent (n = 41) and adult (n = 43) mice entrained to the LD photocycle with a period of nearly 24 hours; tau values averaged 23.98 ± 0.01 h and 23.96 ± 0.01 h, respectively, and there was no difference between groups (t = 1.652, p = 0.1024, Figure 2A). Adolescent mice showed higher amplitude rhythmicity than adults (t = 4.968, p < 0.0001), with respective means of 3916 ± 103 and 3222 ± 95 (Figure 2B). Adolescent mice became active earlier than adults (t = 7.612, p < 0.0001), averaging −0.09 ± 0.02 h and −0.54 ± 0.05 h relative to ZT12 (lights-off; Figure 2C), respectively. Note that negative values indicate that mice became active after ZT12, referred to as a negative phase-angle of entrainment, which is typical for C57BL/6J mice (e.g. (Valentinuzzi et al., 1997). Activity offset was also advanced in adolescent mice compared to adults (t = 8.986, p < 0.0001), with the former averaging 0.29 ± 0.07 h and the latter −0.58 ± 0.06 h relative to ZT0 (lights on; Figure 2D). Again, negative values indicate that activity offset occurred after ZT0, while positive values reflect offset occurring before ZT0. Alpha, the duration of the active phase, was shorter in adolescent than adult mice (t = 3.981, p = 0.0001; Figure 2E), averaging 11.61 ± 0.08 h and 12.04 ± 0.08 h, respectively.
Fig. 2.
Age-related differences in photocycle entrainment. (A) Period length did not differ between adolescents (n = 41) and adults (n = 43) in LD, indicating that both were able to entrain normally to a 24-hour photocycle. (B) Circadian rhythm amplitude was higher in adolescents compared to adults. (C) Adolescent mice became active before adults, much closer to the time of lights-off (ZT12). (D) Adolescent mice also became restful before adults, ceasing activity prior to the time of lights-on (ZT0), while adults became inactive after ZT0. (E) The duration of nightly activity (alpha) in adolescents was slightly but significantly shorter than in adults. Data are mean ± SEM; *p < 0.05.
Adolescent Mice Are Insensitive to Ethanol-Induced Inhibition of Circadian Photic Phase-Delays
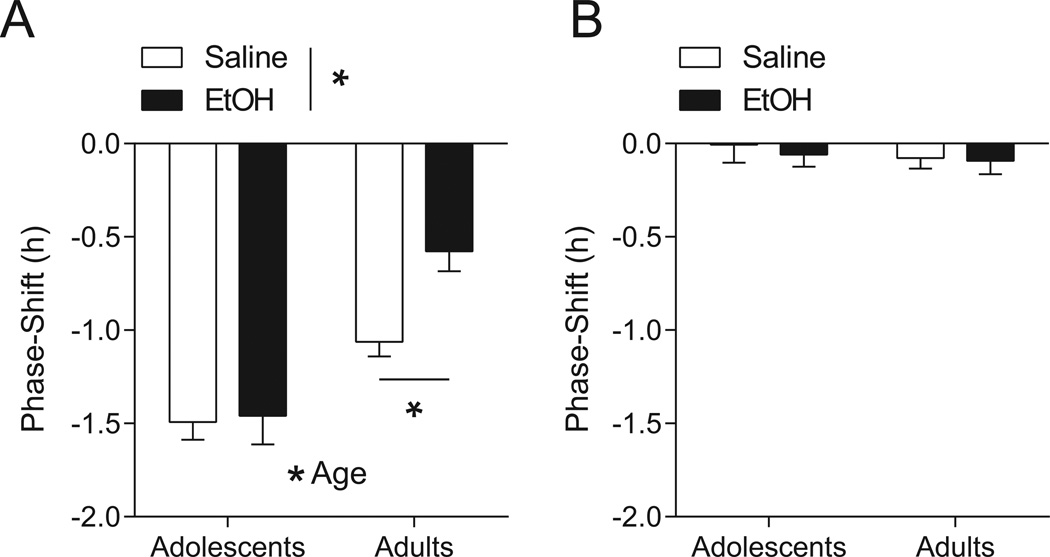
Two-way ANOVA testing of photic phase-delays in adolescent and adult mice treated with either ethanol (1.5 g/kg, i.p.) or saline revealed main effects of age (F1,42 = 34.48, p < 0.0001) and treatment (F1,42 = 5.377, p = 0.0253), and an interaction between the two variables (F1,42 = 4.097, p = 0.0494). Subsequent post-hoc testing confirmed that photic phase-delays were attenuated in ethanol-treated (n = 13) compared to saline-treated (n = 12) adult mice (p < 0.05; Figure 3A), with average phase-shifts of −0.58 ± 0.11 h and −1.06 ± 0.08 h, respectively. In contrast, ethanol-treated adolescent mice (n = 11) showed no such impairment relative to saline-treated adolescents (n = 10); average phase-shifts for the former were −1.46 ± 0.15 h, and for the latter, −1.49 ± 0.10. Representative actograms from mice in each group are shown in Figure 4A.
Fig. 3.
Differential sensitivity to ethanol and photic input in adolescent versus adult mice. (A) Adolescent mice (n = 10–11/tx) had larger phase-delays to light input at ZT14 than did adults (n = 12–13/tx) regardless of treatment and were unimpaired by acute ethanol (EtOH; 1.5 g/kg) compared to age-matched, saline-treated controls. Adult mice receiving ethanol showed the expected attenuation in photic phase-delays compared to saline-treated adult controls. (B) Neither ethanol (n = 8–10/age group) nor saline (n = 10/age group) had a phase-delaying effect at ZT14 in the absence of a light pulse. Data are mean ± SEM; *p < 0.05.
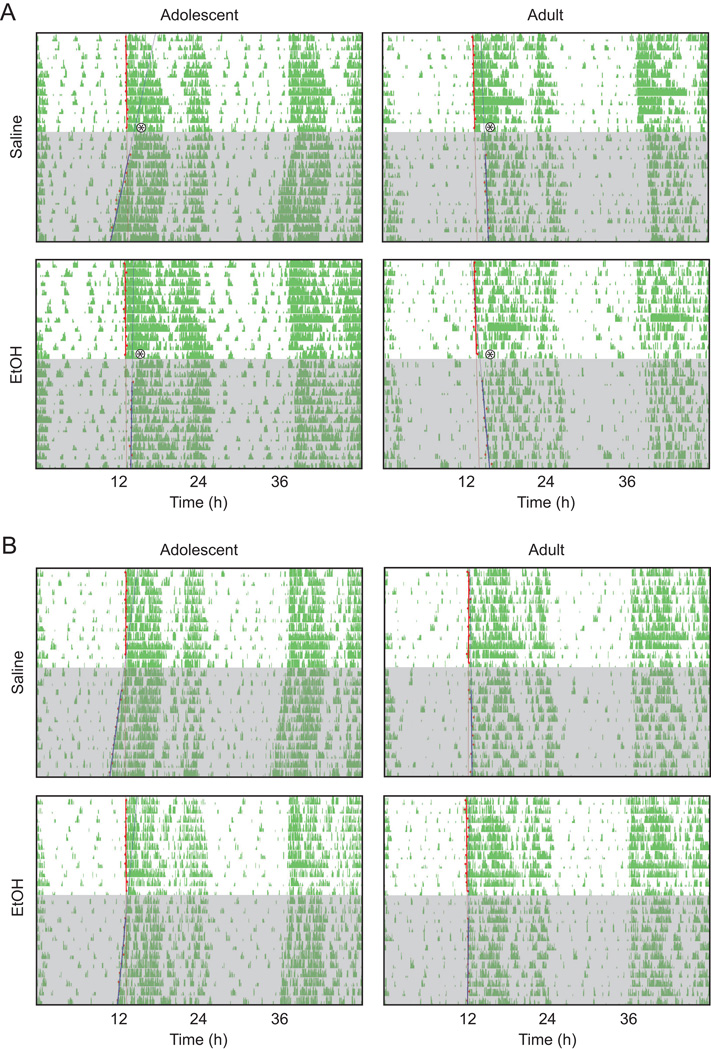
Fig. 4.
Representative, double-plotted actograms of a mouse from each of the four age × treatment groups in the photic phase-resetting experiment (A) and the control phase-resetting experiment (B). The asterisk denotes the time of the light pulse and shading denotes the days spent in DD.
Neither ethanol nor saline had phase-resetting effects in either age group in the no light-pulse control experiment (F1,34 = 0.4870, p = 0.49 for treatment; F1,34 = 0.2092, p = 0.6503 for age; F1,34 = 0.06958, p = 0.7935 for the interaction; Figure 3B). Average phase-shifts for adolescent mice were −0.01 ± 0.10 h for the saline-treated group (n = 10) and −0.06 ± 0.06 h for the ethanol-treated group (n = 10). Average phase-shifts for adults were −0.08 ± 0.06 h and −0.09 ± 0.07 h for saline-treated (n = 10) and ethanol-treated (n = 8) mice, respectively. Representative actograms from mice in each group are shown in Figure 4B.
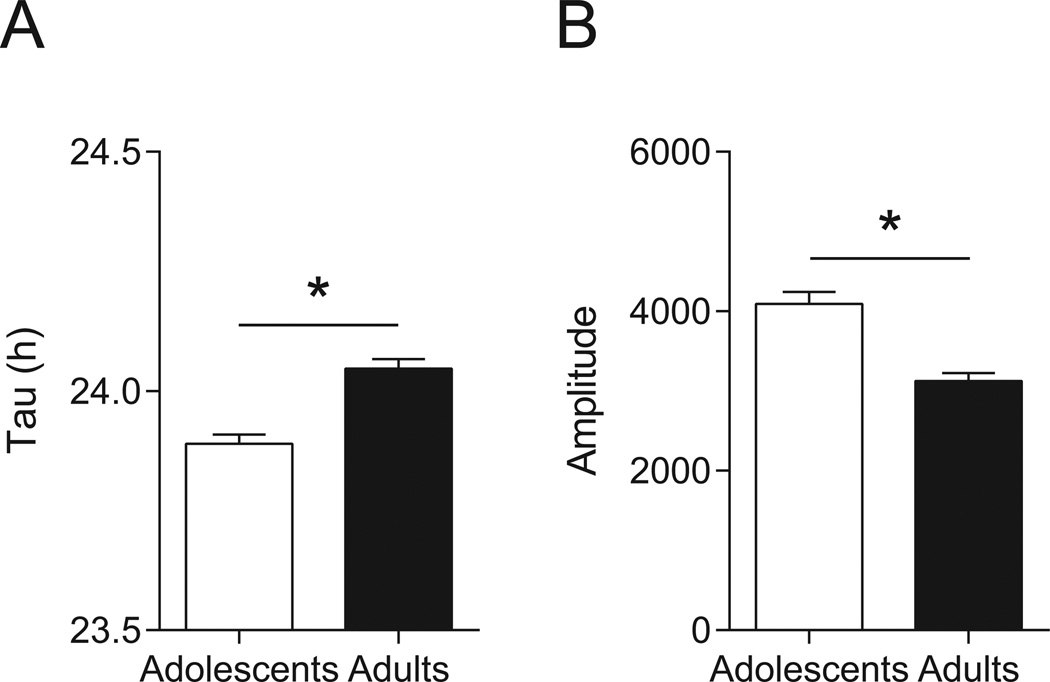
Adolescent Mice Have a Shorter Endogenous Period and Greater Amplitude in Constant Darkness Than Adults
Two-way repeated measures ANOVA testing of endogenous circadian period (tau in DD) in each age × treatment group revealed a main effect of age (F1,80 = 29.55, p < 0.0001), with no effect of treatment (F1,80 = 0.1542, p = 0.6956) and no age × treatment interaction (F1,80 = 1.348, p = 0.2491). Mean tau measurements for the adolescent-saline (n = 20), adolescent-ethanol (n = 21), adult-saline (n = 22), and adult-ethanol (n = 21) were 23.87 ± 0.03 h, 23.91 ± 0.02 h, 24.05 ± 0.03 h, and 24.03 ± 0.03 h, respectively. To report these findings more simply, we conducted a Student’s t-test comparing the age groups, and found that adolescents had an average free-running period of 23.89 ± 0.02 h and adults had an average of 24.04 ± 0.02 h (t = 5.445, p < 0.0001; Figure 5A).
Fig. 5.
Age-related changes in endogenous circadian period. (A) Endogenous, or free-running, period (tau in DD) was shorter in adolescents (n = 41) than adults (n = 43). Notably, free-running period in adolescents was shorter than 24 h, whereas it was longer than 24 h in adults. (B) Circadian rhythm amplitude remained higher in adolescents compared to adults in DD. The possibility that acute ethanol affected endogenous period or amplitude in mice from either age group was ruled out prior to this analysis. Data are mean ± SEM; *p < 0.05.
Two-way repeated measures ANOVA testing of rhythm amplitude in DD in each age × treatment group revealed a main effect of age (F1,80 = 29.69, p < 0.0001), with no effect of treatment (F1,80 = 0.05354, p = 0.8176) and no age × treatment interaction (F1,80 = 0.4586, p = 0.5003). Mean amplitudes for the adolescent-saline (n = 20), adolescent-ethanol (n = 21), adult-saline (n = 22), and adult-ethanol (n = 21) were 4177 ± 194, 4013 ± 228, 3071 ± 104, and 3152 ± 180, respectively. Again, to report these findings more simply, we conducted a Student’s t-test comparing the age groups, and found that adolescents had an average amplitude of 4093 ± 149 and adults had an average of 3110 ± 102 (t = 5.495, p < 0.0001; Figure 5B).
DISCUSSION
The present study adds to the growing body of evidence that adolescents have differential sensitivity to the effects of acute ethanol. In this case, we show a lack of sensitivity to ethanol-induced inhibition of photic phase-delays among adolescent mice. As photic phase-resetting is the primary mechanism by which circadian rhythms are synchronized to the environment, this suggests that adolescent mice may be relatively resilient to the chronodisruptive effects of acute ethanol. This insensitivity is consistent with the only other study of which we are aware that examines the effect of developmental ethanol exposure on circadian endpoints, which reports changes in slow-wave sleep, but not circadian activity or temperature rhythms, in mice exposed to ethanol on postnatal day 7 (Wilson et al., 2016). It is also noteworthy that adolescent rats are initially less sensitive to ethanol-induced sleep disruption (Ehlers et al., 2013). Thus, it is conceivable that the initial lack of circadian- and sleep-related consequences adolescent rodents experience with acute ethanol may contribute to their higher alcohol consumption (Holstein et al., 2011, Melendez, 2011, Moore et al., 2010, Quoilin and Boehm, 2016). However, given that adolescent rats also show greater slow wave sleep suppression the day following acute ethanol compared to adults (Ehlers et al., 2013), it is also tempting to speculate that heavy drinking in adolescents may be reinforced during hangover to induce sleep.
Photic phase-resetting is mediated by glutamate release from retinal afferents and subsequent activation of NMDA receptors in the SCN (Abe et al., 1991, Colwell et al., 1991, Ding et al., 1994, Mintz and Albers, 1997, Mintz et al., 1999). It is well-established that ethanol inhibits glutamatergic signaling at many levels and potentiates GABA signaling through the GABAA receptor; both mechanisms have been implicated in the effects of ethanol on photic phase-resetting (Prosser et al., 2008, McElroy et al., 2009). Whether there are age-related changes in these transmitter systems that might explain our observations is unknown. Given that adolescent mice had larger photic phase-delays than adults in general, and that ethanol-induced attenuation of photic phase-resetting can be overcome by a brighter light pulse (i.e., more glutamate release) (Ruby et al., 2009a), it is possible that adolescent mice have enhanced glutamate release and/or responsiveness in the SCN sufficient to overcome the moderate ethanol dose tested. We are planning future studies to address this possible mechanism.
The enhancement of photic phase-delays we observed among adolescent mice is consistent with a previous study in which female mice had exaggerated photic responsiveness during puberty (Weinert and Kompauerova, 1998), and another in which pubertal mice showed accelerated re-entrainment to a new photocycle (Weinert et al., 1994). Taken together with our observations that adolescent mice have a shorter free-running period than adults, these findings suggest that enhanced sensitivity to the phase-delaying effects of light may function to maintain robust entrainment to a 24 h photocycle, as we also observed (discussed below). Another possibility that has been suggested is that adolescent mice may be less sensitive to the phase-advancing effects of light, which was not tested in the present study. The phase-response curve produced by Weinert and Kompauerova (1998) supports this notion, but the data points are only representative of female mice during the time of puberty and are too few to be conclusive (Hagenauer et al., 2009). If photic phase-delays are augmented while advances are attenuated during adolescence, more complex developmental changes in glutamatergic neurotransmission or other modulatory systems in the SCN may be at play. Given the advanced photocycle entrainment in adolescent mice (discussed below), another possible explanation for larger phase-delays is that the timing of the light pulse at ZT14 (2 hours into the dark phase) occurred during different phases of the underlying pacemaker in adolescents versus adults. In other words, the light pulse occurred later in the active phase for adolescents because they were active earlier than adults. While the magnitude of the difference in phase-shifting between saline-treated adolescents and saline-treated adults (~30 min) corresponds well to the 30 min difference between relative onsets in LD, this would not explain the differential sensitivity to photic inhibition in the ethanol-treated groups.
Also important are the age-related differences in photocycle entrainment and other circadian measures we observed in this study. In LD, onsets in adolescent mice were closer to ZT12 (less than 10 min after lights-off) and earlier than in adults by approximately a half-hour. Similarly, offsets in adolescents occurred ~30 min prior to ZT0 (lights-on), while adults remained active about 45 min into the daytime. These data indicate that rather than delayed entrainment, adolescent mice have relatively advanced circadian activity rhythms in LD. It is also interesting that adolescent mice had a shorter tau in DD (endogenous, free-running period length) compared to adults. This is in contrast to studies in humans, wherein adolescents show slight (~10 min) but significant lengthening of free-running circadian period versus adults (Carskadon et al., 1999). Rodent studies are inconclusive with regard to tau; male pubertal rats showed a longer period than adults (McGinnis et al., 2007), while tau was unaltered during puberty in degus (Hummer et al., 2007). While our results do not resolve this discrepancy, the observation that adolescent mice showed larger photic phase-delays suggests that adolescents may have increased sensitivity to light during the delay portion of the phase-response curve (Hagenauer et al., 2009), and that this mechanism may be translationally relevant. The fact that adult mice in our study had an endogenous period exceeding 24 hours was somewhat surprising for this strain, but the direction of age-related change (e.g. period lengthening) has been observed before (Valentinuzzi et al., 1997). Finally, alpha, the duration of the active phase, was about half an hour longer in adults compared to adolescents, and adolescents showed more robust circadian activity rhythms as evidenced by their high amplitude rhythms in both LD and DD. These results are not surprising in light of the well-known dampening of circadian rhythm amplitude that occurs with advancing age.
There are a few limitations to the present study. First, we used only one relatively moderate dose of ethanol. As ethanol-induced attenuation of photic phase-resetting is dose-dependent (Brager et al., 2011, Ruby et al., 2009b), it is likely that higher doses of ethanol would indeed affect adolescent mice. However, we anticipate that there would be a rightward shift of the dose-response curve for adolescents, as is seen with ethanol-induced LORR (Hefner and Holmes, 2007, Little et al., 1996). Second, we did not measure blood ethanol concentrations (BEC) in the present study. However, two other studies failed to detect age-related differences in ethanol clearance in this strain at 20–30 min after ethanol administration (the interval between injection and the light pulse in our study; (Hefner and Holmes, 2007, Linsenbardt et al., 2009), while a third study reported no age difference in BEC at all (Lacaille et al., 2015). A third limitation is that only photic phase-delays were examined in this report, because adult mice typically show only small advances to light. Since adolescent mice may be even less responsive than adults to the phase-advance portion of the phase-response curve (Hagenauer et al., 2009), further decrements induced by ethanol would be difficult to detect reliably. In this regard, ethanol has been shown to inhibit photic phase-advances but not delays in adult hamsters due to the fact that delays did not occur in the saline-treated controls (Ruby et al., 2009b), and are known to be small in this species. Thus, it may be informative to examine whether age modulates ethanol impairment of photic advances in a species like hamsters, which show large photic advances. The fourth limitation relates to the possibility that shipping stress may have influenced the results of our study. However, very few studies have addressed shipping as a stressor in mice of any age, and none have examined endpoints relevant to the present study. It is also extremely unlikely that a transient stressor like shipping would affect responses to a single, moderate dose of ethanol given 3 weeks after shipping had occurred. The final limitation of our study is the fact that only male mice were used. While there are several reports suggesting sex × age interactions in alcohol-related endpoints in C57BL/6 mice (Gallego et al., 2015, Melon et al., 2013, Roger-Sanchez et al., 2012, Strong et al., 2010), there is no such literature on sex differences and developmental changes in murine circadian timing. This represents an important future direction of inquiry for both chronobiology and alcohol research.
In summary, this study reveals that sensitivity to ethanol-mediated inhibition of circadian photic phase-resetting represents another way in which adolescent sensitivity to acute ethanol differs from the adult. We also confirmed that adolescent mice have larger light-induced phase-delays than adults and further described other age-related differences in photocycle entrainment, free-running period, and amplitude. Future studies to elucidate the neurochemical and molecular basis for increased sensitivity to light and reduced sensitivity to ethanol in adolescent mice will be informative. Given that adolescent rats were more sensitive than adults to hangover-related disruption of slow wave sleep (Ehlers et al., 2013), it will also be important to examine circadian measures during ethanol withdrawal. Finally, chronic alcohol exposure during adolescence leads to long-term changes in ethanol sensitivity and drinking (Spear and Swartzwelder, 2014). Thus, it will be critical to study the long-term effects of adolescent ethanol exposure on circadian rhythm and sleep endpoints. Such studies may illuminate new ways to interfere with the development of alcohol use disorders and prevent drinking relapses triggered by chronodisruption and sleep loss.
Acknowledgments
This work was supported by the College of Natural Sciences and Mathematics at Indiana University of Pennsylvania (CLR), NIH grant AA2U01AA019925 (NADIA; HSS), and VA Senior Research Career Scientist Award (HSS).
Footnotes
The authors declare no conflict of interest.
REFERENCES
- Abe H, Rusak B, Robertson HA. Photic induction of Fos protein in the suprachiasmatic nucleus is inhibited by the NMDA receptor antagonist MK-801. Neurosci Lett. 1991;127:9–12. doi: 10.1016/0304-3940(91)90881-s. [DOI] [PubMed] [Google Scholar]
- Albert N, da Silva C, Diez-Noguera A, Cambras T. Different adaptation of the motor activity rhythm to chronic phase shifts between adolescent and adult rats. Behav Brain Res. 2013;252:347–355. doi: 10.1016/j.bbr.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Response curves in circadian periodicity. In: Aschoff J, editor. Circadian Clocks. Amsterdam: North-Holland; 1965. pp. 95–111. [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcohol Clin Exp Res. 2010;34:1266–1273. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Acute ethanol disrupts photic and serotonergic circadian clock phase-resetting in the mouse. Alcohol Clin Exp Res. 2011;35:1467–1474. doi: 10.1111/j.1530-0277.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci Lett. 1999;260:129–132. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Foster RG, Menaker M. NMDA receptor antagonists block the effects of light on circadian behavior in the mouse. Brain Res. 1991;554:105–110. doi: 10.1016/0006-8993(91)90177-w. [DOI] [PubMed] [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Desikan A, Wills DN. Developmental differences in EEG and sleep responses to acute ethanol administration and its withdrawal (hangover) in adolescent and adult Wistar rats. Alcohol. 2013;47:601–610. doi: 10.1016/j.alcohol.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego X, Cox RJ, Funk E, Foster RA, Ehringer MA. Voluntary exercise decreases ethanol preference and consumption in C57BL/6 adolescent mice: sex differences and hippocampal BDNF expression. Physiol Behav. 2015;138:28–36. doi: 10.1016/j.physbeh.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Burgos D, Gonzalez F, Manrique T, Gallo M. Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse-like conditions. Alcohol Clin Exp Res. 2009;33:722–728. doi: 10.1111/j.1530-0277.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology (Berl) 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcohol Clin Exp Res. 2011;35:1842–1851. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY, Gau SS, Shang CY, Chiu YN, Lee MB. Associations between chronotypes, psychopathology, and personality among incoming college students. Chronobiol Int. 2012;29:491–501. doi: 10.3109/07420528.2012.668995. [DOI] [PubMed] [Google Scholar]
- Hummer DL, Jechura TJ, Mahoney MM, Lee TM. Gonadal hormone effects on entrained and free-running circadian activity rhythms in the developing diurnal rodent Octodon degus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R586–R597. doi: 10.1152/ajpregu.00043.2006. [DOI] [PubMed] [Google Scholar]
- Kanerva N, Kronholm E, Partonen T, Ovaskainen ML, Kaartinen NE, Konttinen H, Broms U, Mannisto S. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol Int. 2012;29:920–927. doi: 10.3109/07420528.2012.699128. [DOI] [PubMed] [Google Scholar]
- Kervran C, Fatseas M, Serre F, Taillard J, Beltran V, Leboucher J, Debrabant R, Alexandre JM, Daulouede JP, Philip P, Auriacombe M. Association between morningness/eveningness, addiction severity and psychiatric disorders among individuals with addictions. Psychiatry Res. 2015;229:1024–1030. doi: 10.1016/j.psychres.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Lacaille H, Duterte-Boucher D, Liot D, Vaudry H, Naassila M, Vaudry D. Comparison of the deleterious effects of binge drinking-like alcohol exposure in adolescent and adult mice. J Neurochem. 2015;132:629–641. doi: 10.1111/jnc.13020. [DOI] [PubMed] [Google Scholar]
- Lemoine P, Zawieja P, Ohayon MM. Associations between morningness/eveningness and psychopathology: an epidemiological survey in three in-patient psychiatric clinics. J Psychiatr Res. 2013;47:1095–1098. doi: 10.1016/j.jpsychires.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., 2nd Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Lopez M, Simpson D, White N, Randall C. Age- and sex-related differences in alcohol and nicotine effects in C57BL/6J mice. Addict Biol. 2003;8:419–427. doi: 10.1080/13556210310001648176. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- McElroy B, Zakaria A, Glass JD, Prosser RA. Ethanol modulates mammalian circadian clock phase resetting through extrasynaptic GABA receptor activation. Neuroscience. 2009;164:842–848. doi: 10.1016/j.neuroscience.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol Behav. 2007;92:1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melon LC, Boehm SL., 2nd Role of genotype in the development of locomotor sensitization to alcohol in adult and adolescent mice: comparison of the DBA/2J and C57BL/6J inbred mouse strains. Alcohol Clin Exp Res. 2011;35:1351–1360. doi: 10.1111/j.1530-0277.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melon LC, Wray KN, Moore EM, Boehm SL., 2nd Sex and age differences in heavy binge drinking and its effects on alcohol responsivity following abstinence. Pharmacol Biochem Behav. 2013;104:177–187. doi: 10.1016/j.pbb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz EM, Albers HE. Microinjection of NMDA into the SCN region mimics the phase shifting effect of light in hamsters. Brain Res. 1997;758:245–249. doi: 10.1016/s0006-8993(97)00022-x. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci. 1999;19:5124–5130. doi: 10.1523/JNEUROSCI.19-12-05124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Forrest RDt, Boehm SL., 2nd Genotype modulates age-related alterations in sensitivity to the aversive effects of ethanol: an eight inbred strain analysis of conditioned taste aversion. Genes Brain Behav. 2013;12:70–77. doi: 10.1111/gbb.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melon LC, Boehm SL., 2nd Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol Clin Exp Res. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Wong JC, Kennedy BC, Lahvis GP. Differential entrainment of a social rhythm in adolescent mice. Behav Brain Res. 2008;195:239–245. doi: 10.1016/j.bbr.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau-Lenz S, Zghoul T, de Fonseca FR, Spanagel R, Bilbao A. Circadian regulation of central ethanol sensitivity by the mPer2 gene. Addict Biol. 2009;14:253–259. doi: 10.1111/j.1369-1600.2009.00165.x. [DOI] [PubMed] [Google Scholar]
- Pickard GE. The afferent connections of the suprachiasmatic nucleus of the golden hamster with emphasis on the retinohypothalamic projection. J Comp Neurol. 1982;211:65–83. doi: 10.1002/cne.902110107. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience. 2008;152:837–848. doi: 10.1016/j.neuroscience.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoilin C, Boehm SL., 2nd Involvement of the GABAA Receptor in Age-Dependent Differences in Binge-Like Ethanol Intake. Alcohol Clin Exp Res. 2016;40:408–417. doi: 10.1111/acer.12953. [DOI] [PubMed] [Google Scholar]
- Robinson D, Gelaye B, Tadesse MG, Williams MA, Lemma S, Berhane Y. Daytime Sleepiness, Circadian Preference, Caffeine Consumption and Khat Use among College Students in Ethiopia. J Sleep Disord Treat Care. 2013;3 doi: 10.4172/2325-9639.1000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5:287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- Roger-Sanchez C, Aguilar MA, Rodriguez-Arias M, Aragon CM, Minarro J. Age- and sex-related differences in the acquisition and reinstatement of ethanol CPP in mice. Neurotoxicol Teratol. 2012;34:108–115. doi: 10.1016/j.ntt.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM. Circadian clock genes: non-circadian roles in sleep, addiction, and psychiatric disorders? Neurosci Biobehav Rev. 2010;34:1249–1255. doi: 10.1016/j.neubiorev.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol Regul Integr Comp Physiol. 2009a;297:R729–R737. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Marinos LN, Zisk CF, Zhang J, Palmer KN, Bunion DJ, Dietzel JD, Verbanes NM. Caffeine alters diurnal variation in ethanol-induced ataxia in mice. J Alcohol Drug Depend. 2016;4:1–5. [Google Scholar]
- Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol Regul Integr Comp Physiol. 2009b;296:R411–R418. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Fernandez F, Garrett A, Klima J, Zhang P, Sapolsky R, Heller HC. Spatial memory and long-term object recognition are impaired by circadian arrhythmia and restored by the GABAA antagonist pentylenetetrazole. PLoS One. 2013;8:e72433. doi: 10.1371/journal.pone.0072433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci U S A. 2008;105:15593–15598. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J Biol Rhythms. 2009;24:304–312. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart RG. Drinking problems among employed, unemployed and shift workers. J Occup Med. 1979;21:731–736. doi: 10.1097/00043764-197911000-00005. [DOI] [PubMed] [Google Scholar]
- Spanos M, Besheer J, Hodge CW. Increased sensitivity to alcohol induced changes in ERK Map kinase phosphorylation and memory disruption in adolescent as compared to adult C57BL/6J mice. Behav Brain Res. 2012;230:158–166. doi: 10.1016/j.bbr.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. "Binge" drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm Behav. 2010;58:82–90. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernier R, Willoughby T. Are all evening-types doomed? Latent class analyses of perceived morningness-eveningness, sleep and psychosocial functioning among emerging adults. Chronobiol Int. 2014;31:232–242. doi: 10.3109/07420528.2013.843541. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Clay KC, Bramoweth AD, Sethi K, Roane BM. Circadian phase preference in college students: relationships with psychological functioning and academics. Chronobiol Int. 2011;28:541–547. doi: 10.3109/07420528.2011.580870. [DOI] [PubMed] [Google Scholar]
- Urban R, Magyarodi T, Rigo A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiol Int. 2011;28:238–247. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273:R1957–R1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- Watson NF, Buchwald D, Harden KP. A twin study of genetic influences on diurnal preference and risk for alcohol use outcomes. J Clin Sleep Med. 2013;9:1333–1339. doi: 10.5664/jcsm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb IC, Antle MC, Mistlberger RE. Regulation of circadian rhythms in mammals by behavioral arousal. Behav Neurosci. 2014;128:304–325. doi: 10.1037/a0035885. [DOI] [PubMed] [Google Scholar]
- Weinert D, Eimert H, Erkert HG, Schneyer U. Resynchronization of the circadian corticosterone rhythm after a light/dark shift in juvenile and adult mice. Chronobiol Int. 1994;11:222–231. doi: 10.3109/07420529409067791. [DOI] [PubMed] [Google Scholar]
- Weinert D, Kompauerova V. Light induced phase and period responses of circadian activity rhythms in laboratory mice of different age. Zoology. 1998;101:45–52. [Google Scholar]
- Whittier A, Sanchez S, Castaneda B, Sanchez E, Gelaye B, Yanez D, Williams MA. Eveningness chronotype, daytime sleepiness, caffeine consumption, and use of other stimulants among Peruvian university students. J Caffeine Res. 2014;4:21–27. doi: 10.1089/jcr.2013.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Masiello K, Lewin MP, Hui M, Smiley JF, Saito M. Developmental ethanol exposure-induced sleep fragmentation predicts adult cognitive impairment. Neuroscience. 2016;322:18–27. doi: 10.1016/j.neuroscience.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]