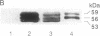Abstract
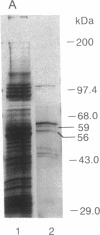
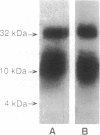
The lymphocyte-specific protein-tyrosine kinase p56lck has been purified 90-fold to approximately 30% purity in 30% yield from a baculovirus expression system by a two-column purification procedure. At least two forms of p56lck were isolated, differing in the extent of phosphorylation and migrating as 56- and 59-kDa species on SDS/PAGE but as a single 56-kDa band after treatment with potato acid phosphatase. Autophosphorylation of purified p56lck occurred at a rate of 25 fmol/min to a maximum incorporation of approximately 2 mol of phosphate per mol of p56lck with tyrosine-394 (but not tyrosine-505) and other, unidentified tyrosine residue(s) being the major sites of phosphorylation in vitro. Phosphorylation of tyrosine-containing peptides was monitored using an automated HPLC system. Although peptide substrate Km values were in the 1-5 mM range, the Vmax for the 13-amino acid peptide RRLIEDAEYAARG (modified p60src autophosphorylation site) was 120 min-1 (350 min-1 when adjusted for p56lck purity), suggesting that the enzyme purified from recombinant baculovirus-infected Sf9 cells has a high catalytic turnover compared with other tyrosine kinases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R., Cantrell D. A. Kinases and phosphatases in T-cell activation. Immunol Today. 1989 Jun;10(6):200–205. doi: 10.1016/0167-5699(89)90325-3. [DOI] [PubMed] [Google Scholar]
- Amrein K. E., Sefton B. M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniyash M., Garcia-Morales P., Luong E., Samelson L. E., Klausner R. D. The T cell antigen receptor zeta chain is tyrosine phosphorylated upon activation. J Biol Chem. 1988 Dec 5;263(34):18225–18230. [PubMed] [Google Scholar]
- Barber E. K., Dasgupta J. D., Schlossman S. F., Trevillyan J. M., Rudd C. E. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci U S A. 1989 May;86(9):3277–3281. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blithe D. L., Richert N. D., Pastan I. H. Purification of a tyrosine-specific protein kinase from Rous sarcoma virus-induced rat tumor. J Biol Chem. 1982 Jun 25;257(12):7135–7142. [PubMed] [Google Scholar]
- Bolen J. B., Veillette A. A function for the lck proto-oncogene. Trends Biochem Sci. 1989 Oct;14(10):404–407. doi: 10.1016/0968-0004(89)90288-0. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Gould K., Sefton B. M. The absence of myristic acid decreases membrane binding of p60src but does not affect tyrosine protein kinase activity. J Virol. 1986 May;58(2):468–474. doi: 10.1128/jvi.58.2.468-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Myristic acid, a rare fatty acid, is the lipid attached to the transforming protein of Rous sarcoma virus and its cellular homolog. J Virol. 1985 Jan;53(1):7–12. doi: 10.1128/jvi.53.1.7-12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Hellstrom K. E., Krebs E. G. A lymphoma protein with an in vitro site of tyrosine phosphorylation homologous to that in pp60src. J Biol Chem. 1982 Dec 10;257(23):13877–13879. [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Pike L. J., Hellström K. E., Krebs E. G. Phosphorylation of synthetic peptides by a tyrosine protein kinase from the particulate fraction of a lymphoma cell line. Proc Natl Acad Sci U S A. 1982 Jan;79(2):282–286. doi: 10.1073/pnas.79.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner P., Bunte T., Owada M. K., Moelling K. Biochemical characterization of pp60src-associated protein kinase from avian sarcoma virus Schmidt-Ruppin strain. J Biol Chem. 1981 Aug 25;256(16):8786–8794. [PubMed] [Google Scholar]
- Feder D., Bishop J. M. Purification and enzymatic characterization of pp60c-src from human platelets. J Biol Chem. 1990 May 15;265(14):8205–8211. [PubMed] [Google Scholar]
- Fukami Y., Lipmann F. Purification of the Rous sarcoma virus src kinase by casein-agarose and tyrosine-agarose affinity chromatography. Proc Natl Acad Sci U S A. 1985 Jan;82(2):321–324. doi: 10.1073/pnas.82.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R. B., Cambier J. C. Signal transmission pathways and lymphocyte function. Immunol Today. 1990 Jun;11(6):187–189. doi: 10.1016/0167-5699(90)90078-n. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Masaracchia R. A., Feramisco J. R., Kemp B. E. Isolation of phosphorylated peptides and proteins on ion exchange papers. Anal Biochem. 1978 Jul 1;87(2):566–575. doi: 10.1016/0003-2697(78)90707-8. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Presek P., Eigenbrodt E. Association of the src-gene product of Rous sarcoma virus with a pyruvate-kinase inactivation factor. Mol Cell Endocrinol. 1981 Jul;23(1):49–63. doi: 10.1016/0303-7207(81)90116-7. [DOI] [PubMed] [Google Scholar]
- Hsi E. D., Siegel J. N., Minami Y., Luong E. T., Klausner R. D., Samelson L. E. T cell activation induces rapid tyrosine phosphorylation of a limited number of cellular substrates. J Biol Chem. 1989 Jun 25;264(18):10836–10842. [PubMed] [Google Scholar]
- Hunter T. Synthetic peptide substrates for a tyrosine protein kinase. J Biol Chem. 1982 May 10;257(9):4843–4848. [PubMed] [Google Scholar]
- Hurley T. R., Sefton B. M. Analysis of the activity and phosphorylation of the lck protein in lymphoid cells. Oncogene. 1989 Mar;4(3):265–272. [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Varmus H. E., Bishop J. M. The purified product of the transforming gene of avian sarcoma virus phosphorylates tyrosine. J Biol Chem. 1980 Dec 25;255(24):11973–11980. [PubMed] [Google Scholar]
- Luo K. X., Sefton B. M. Analysis of the sites in p56lck whose phosphorylation is induced by tetradecanoyl phorbol acetate. Oncogene. 1990 Jun;5(6):803–808. [PubMed] [Google Scholar]
- Luo K., Hurley T. R., Sefton B. M. Transfer of proteins to membranes facilitates both cyanogen bromide cleavage and two-dimensional proteolytic mapping. Oncogene. 1990 Jun;5(6):921–923. [PubMed] [Google Scholar]
- Maeda S. Expression of foreign genes in insects using baculovirus vectors. Annu Rev Entomol. 1989;34:351–372. doi: 10.1146/annurev.en.34.010189.002031. [DOI] [PubMed] [Google Scholar]
- Marchildon G. A., Casnellie J. E., Walsh K. A., Krebs E. G. Covalently bound myristate in a lymphoma tyrosine protein kinase. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7679–7682. doi: 10.1073/pnas.81.24.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Cooper J. A., King C. S., Ziegler S. F., Tinker D. A., Overell R. W., Krebs E. G., Perlmutter R. M. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol Cell Biol. 1988 Feb;8(2):540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Miller L. K. Baculoviruses as gene expression vectors. Annu Rev Microbiol. 1988;42:177–199. doi: 10.1146/annurev.mi.42.100188.001141. [DOI] [PubMed] [Google Scholar]
- Miller L. K. Insect baculoviruses: powerful gene expression vectors. Bioessays. 1989 Oct;11(4):91–95. doi: 10.1002/bies.950110404. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Marth J. D., Ziegler S. F., Garvin A. M., Pawar S., Cooke M. P., Abraham K. M. Specialized protein tyrosine kinase proto-oncogenes in hematopoietic cells. Biochim Biophys Acta. 1989 Feb;948(3):245–262. doi: 10.1016/0304-419x(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Pike L. J. Assay of growth factor-stimulated tyrosine kinases using synthetic peptide substrates. Methods Enzymol. 1987;146:353–362. doi: 10.1016/s0076-6879(87)46036-9. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Marquardt H., Todaro G. J., Gallis B., Casnellie J. E., Bornstein P., Krebs E. G. Transforming growth factor and epidermal growth factor stimulate the phosphorylation of a synthetic, tyrosine-containing peptide in a similar manner. J Biol Chem. 1982 Dec 25;257(24):14628–14631. [PubMed] [Google Scholar]
- Piwnica-Worms H., Williams N. G., Cheng S. H., Roberts T. M. Regulation of pp60c-src and its interaction with polyomavirus middle T antigen in insect cells. J Virol. 1990 Jan;64(1):61–68. doi: 10.1128/jvi.64.1.61-68.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presek P., Reuter C., Findik D., Bette P. High-yield purification of a pp60c-src related protein-tyrosine kinase from human platelets. Biochim Biophys Acta. 1988 May 13;969(3):271–280. doi: 10.1016/0167-4889(88)90062-6. [DOI] [PubMed] [Google Scholar]
- Rudd C. E., Anderson P., Morimoto C., Streuli M., Schlossman S. F. Molecular interactions, T-cell subsets and a role of the CD4/CD8:p56lck complex in human T-cell activation. Immunol Rev. 1989 Oct;111:225–266. doi: 10.1111/j.1600-065x.1989.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Shaw A. S., Chalupny J., Whitney J. A., Hammond C., Amrein K. E., Kavathas P., Sefton B. M., Rose J. K. Short related sequences in the cytoplasmic domains of CD4 and CD8 mediate binding to the amino-terminal domain of the p56lck tyrosine protein kinase. Mol Cell Biol. 1990 May;10(5):1853–1862. doi: 10.1128/mcb.10.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Erikson E., Graziani Y., Erikson R. L. Inter- and intramolecular interactions of highly purified Rous sarcoma virus-transforming protein, pp60v-src. J Biol Chem. 1985 Nov 5;260(25):13838–13843. [PubMed] [Google Scholar]
- Tinker D. A., Krebs E. A., Feltham I. C., Attah-Poku S. K., Ananthanarayanan V. S. Synthetic beta-turn peptides as substrates for a tyrosine protein kinase. J Biol Chem. 1988 Apr 15;263(11):5024–5026. [PubMed] [Google Scholar]
- Veillette A., Bolen J. B., Bookman M. A. Alterations in tyrosine protein phosphorylation induced by antibody-mediated cross-linking of the CD4 receptor of T lymphocytes. Mol Cell Biol. 1989 Oct;9(10):4441–4446. doi: 10.1128/mcb.9.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Horak I. D., Bolen J. B. Post-translational alterations of the tyrosine kinase p56lck in response to activators of protein kinase C. Oncogene Res. 1988 May;2(4):385–401. [PubMed] [Google Scholar]
- Veillette A., Horak I. D., Horak E. M., Bookman M. A., Bolen J. B. Alterations of the lymphocyte-specific protein tyrosine kinase (p56lck) during T-cell activation. Mol Cell Biol. 1988 Oct;8(10):4353–4361. doi: 10.1128/mcb.8.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Sefton B. M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986 Feb 20;319(6055):682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. In vitro phosphorylation of angiotensin analogs by tyrosyl protein kinases. J Biol Chem. 1983 Jan 25;258(2):1022–1025. [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. Kinetics and mechanism of angiotensin phosphorylation by the transforming gene product of Rous sarcoma virus. J Biol Chem. 1984 Mar 10;259(5):3127–3131. [PubMed] [Google Scholar]
- Yu G., Glazer R. I. Purification and characterization of p93fes- and p60src-related tyrosine protein kinase activities in differentiated HL-60 leukemia cells. J Biol Chem. 1987 Dec 25;262(36):17543–17548. [PubMed] [Google Scholar]