Abstract
This study investigated the protective effects of diallyl disulfide (DADS) against acetaminophen (AAP)-induced acute renal injury in male rats. We also investigated the effects of DADS on kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL), which are novel biomarkers of nephrotoxicity in renal tissues, in response to AAP treatment. The following four experimental groups were evaluated: (1) vehicle control, (2) AAP (1,000 mg/kg), (3) AAP&DADS, and (4) DADS (50 mg/kg/day). AAP treatment caused acute kidney injury evidenced by increased serum blood urea nitrogen (BUN) levels and histopathological alterations. Additionally, Western blot and immunohistochemistry analysis showed increased expression of KIM-1 and NGAL proteins in renal tissues of AAP-treated rats. In contrast, DADS pretreatment significantly attenuated the AAP-induced nephrotoxic effects, including serum BUN level and expression of KIM-1 and NGAL proteins. Histopathological studies confirmed the renoprotective effect of DADS. The results suggest that DADS prevents AAP-induced acute nephrotoxicity, and that KIM-1 and NGAL may be useful biomarkers for the detection and monitoring of acute kidney injury associated with AAP exposure.
Keywords: Acetaminophen, nephrotoxicity, KIM-1, NGAL, diallyl disulfide, protective effect
Acetaminophen (N-acetyl-p-aminophenol; AAP), also known as paracetamol, is a popular analgesic and antipyretic medication that is safe at therapeutic dosages [1]. However, its overdose can result in renal tubular damage and acute renal failure in both humans and animals [2,3]. The kidney is one of the primary sites of xenobiotic-induced toxicity [4]. Nephrotoxicity has been traditionally evaluated by the elevated serum biochemical markers, blood urea nitrogen (BUN) and creatinine. However, these biomarkers are rather insensitive and only indicate damage on 70-80% loss of the renal epithelial mass [5]. Therefore, there is an urgent demand for identification of more sensitive and reliable biomarkers, which may better predict minor kidney injury during drug development and chemical safety testing. Recently, it has been reported that tests for measuring levels of new biomarkers of acute kidney injury, such as kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), cystatin-C, and interleukin-18, are more favorable than tests for BUN and creatinine levels in several experimental and clinical systems [6,7].
KIM-1, a type 1 membrane protein, is below detectable levels in a healthy kidney, but is strongly up-regulated in dedifferentiated proximal tubule epithelial cells in kidney after a toxic injury [8,9]. NGAL, also known as Lipocalin-2, is a protein belonging to the lipocalin superfamily that is initially found in activated neutrophils, in accordance with its role as an innate antibacterial factor [10]. However, other cells, like kidney tubular cells, may also produce NGAL in response to various insults [11,12]. It has been demonstrated that KIM-1 and NGAL may be the most promising new biomarkers, and are specifically induced at the target site of toxicity in both animal and human studies involving acute injury of the proximal tubule epithelium [8,11,13,14,15].
Diallyl disulfide (DADS), which is an organosulfur component of garlic, is a potent compound that prevents toxicant-induced oxidative injury [16,17]. Recently, we have reported anti-oxidative, anti-inflammatory, and anti-apoptotic effects of DADS in various experimental systems [18,19]. There are also some reports on the ameliorating effect of DADS against nephrotoxicant-induced renal injury [16,17]. However, the protective capacity of DADS against the nephrotoxicity of AAP has not been explored. The aim of the present study was to evaluate the protective effects of DADS on AAP-induced nephrotoxicity and the possible application of KIM-1 and NGAL as newly identified biomarkers of AAP-induced acute kidney injury.
Materials and Methods
Animals handling and environmental conditions
Male Sprague-Dawley rats (aged 9 weeks) were obtained from a specific pathogen-free colony at Samtako Co. (Osan, Korea) and were subjected to 1 week of quarantine and acclimatization before the experiments. Two rats per stainless wire mesh cage were housed in a room maintained under the following conditions: temperature of 23±3℃, relative humidity of 50±10%, artificial lighting from 08:00 to 20:00, and 13 to 18 air changes per hour. Rats were provided with tap water that had been sterilized by ultraviolet irradiation, and commercial rodent chow (Samyang Feed, Wonju, Korea) ad libitum. The Institutional Animal Care and Use Committee of Chonnam National University approved the protocols for animal study, and the animals were cared for in accordance with the Guidelines for Animal Experiments of Chonnam National University.
Test chemicals and treatment
AAP was purchased from Sigma Aldrich Co. (St. Louis, MO, USA). DADS was purchased from Tokyo Kasei Chemical Co. (Tokyo, Japan). All other chemicals were of the highest grade that is commercially available. DADS was dissolved in corn oil. AAP was dissolved in a saline solution kept in warm boiling water bath, and was used after cooling to 37℃. These chemicals were prepared freshly before treatment. The daily application volumes of AAP (20 mL/kg body weight) and DADS (5 mL/kg body weight) were calculated in advance, based on the most recently recorded body weight of the individual animal. DADS (50 mg/kg/day) was gavaged to rats once daily for a period of 5 days. One hour after the final DADS treatment, the rats were injected with a single intraperitoneal dose of AAP (1,000 mg/kg).
Experimental groups and dose selection
Twenty-four healthy male rats were randomly divided into four experimental groups (n=6 per group): (1) vehicle control, (2) AAP, (3) AAP&DADS, and (4) DADS. The selected AAP dose was based on a previous study that demonstrated significant acute renal injury in rats [3]. The effective dose of DADS was also based on a previous study [19].
Body weight changes and clinical signs
All animals were observed daily for any clinical signs of toxicity and mortality throughout the test period. Abnormal signs were recorded individually for type, observation day and time, and duration. The body weight of each rat was measured on test days 0 and 1.
Necropsy, organ weight, and serum biochemistry
After 24 h of acute kidney injury induction, all male rats were euthanized by carbon dioxide for blood serum collection and exsanguination from the aorta. Serum samples were collected by centrifugation at 3,000 rpm for 10 min and stored in the −80℃ freezer before they were analyzed. Serum creatinine and BUN were evaluated using a serum biochemical autoanalyzer (Dri-chem 4000i, Fujifilm Co., Japan). The absolute and relative (organ-to-body weight ratio) weights of the kidneys of all rats were also measured.
Histopathological examination
The left kidney was fixed in 10% neutral buffered formalin solution for 1 week. The tissues were routinely processed, embedded in paraffin, sectioned at 4 µm thickness, deparaffinized, and rehydrated using standard techniques. The sections were stained with a hematoxylin-eosin (H&E) stain for microscopic examination. All sections were examined with a light microscope by a pathologist, who was blinded to the sample treatments.
Immunohistochemistry (IHC)
The paraffin-embedded sections were deparaffinized and rehydrated. After incubation with a protein block (Rabbit Specific HRP/DAB Detection IHC Kit; Abcam, Cambridge, MA, USA), the sections were incubated overnight with anti-KIM-1antibodies (1:200; LifeSpan Biosciences, Seattle, WA, USA) and anti-NGAL antibodies (1:500; Abcam) at 4℃. The expression of KIM-1 and NGAL was visualized using Rabbit Specific HRP/DAB Detection IHC Kit (Abcam), according to the manufacturer's protocol. The sections were counterstained with Harris's hematoxylin before mounting. The number of KIM-1 and NGAL positive cells was counted in 10 different fields in each section under 100× magnification.
Western blot analysis
The frozen right kidney tissues were lysed in a RIPA lysis buffer (Cell Signaling Technology, Lexington, KY, USA), and centrifuged at 12,000×g at 4℃ for 10 min to isolate the cellular proteins in the supernatant. The kidney tissues supernatants were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA), and blocked in blocking buffer (150 mM NaCl in 10 mM Tris, pH 7.5 containing 5% non-fat dry milk) for 1 h at room temperature. The membranes were incubated with primary antibodies against KIM-1 (1:1,000; LifeSpan Biosciences) and NGAL (1:1,000; Abcam) for 18 h at 4℃, washed three times (20 mM Tris-HCl, pH 7.5, 137 mM NaCl, and 0.1% Tween 20), incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000, Thermo Scientific, Rockford, IL, USA) for 1 h at room temperature, washed three times, and then detected with an enhanced chemiluminescence method (Supersignal West Pico, Pierce, IL, USA). The protein concentration was determined using the BCA Protein Assay Kit (Pierce).
Statistical analyses
The data are expressed as means±SD, and all statistical comparisons were made by means of one-way analysis of variance, followed by Tukey's multiple comparison test. Statistical analyses were performed by comparisons of the treatment groups with the control group, using the GraphPad InStat v. 3.0 (GraphPad Software, La Jolla, CA, USA). Differences with a P-value of 0.05 or lower were considered to be statistically significant.
Results
Effects of DADS on clinical sign, body weight, and kidney weight
No treatment-related mortality was observed in rats that were treated with AAP and/or DADS during the study period. However, the incidence and severity of clinical signs, such as decreased locomotor activity (n=4) and dull fur (n=3), increased in the AAP group, as compared with those in the control group. There were no significant differences in the body weights and kidney weights between the groups (data not shown).
Effects of DADS on renal function
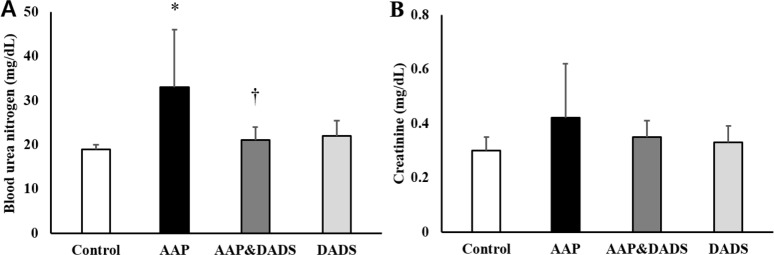
As shown in Figure 1A, the rats treated with 1,000 mg/kg of AAP showed significantly increased serum BUN level, as compared with that in the control group. In contrast, the serum BUN level in the AAP&DADS group decreased significantly, as compared with that in the AAP group. There were no statistically significant differences in the serum creatinine levels between the groups (Figure 1B).
Figure 1. Serum blood urea nitrogen (A) and creatinine (B) in male rats treated with AAP and/or DADS. Values are presented as means±SD (n=6). *P<0.05 compared with the control group. †P<0.05 compared with the AAP group.
Effects of DADS on renal histopathology
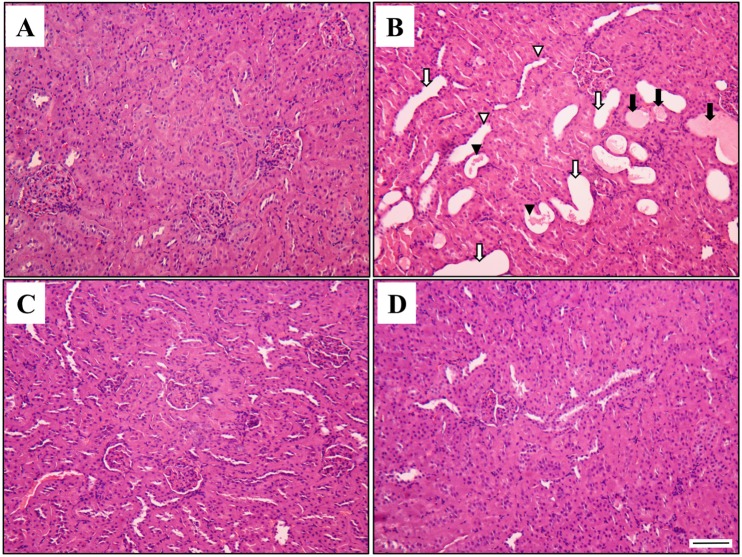
The results of the histopathological examination are presented in Figure 2. The control (Figure 2A) and DADS (Figure 2D) groups showed kidneys with normal morphology. However, kidney tissues from rats treated with AAP showed extensive injuries, characterized by cast, tubular dilation, tubular necrosis, and tubular degeneration (Figure 2B). Although these changes were also observed in the AAP&DADS group (Figure 2C), the incidence and severity of histopathologic lesions were significantly lower than those in the AAP group.
Figure 2. Representative photographs of kidney sections treated AAP and/or DADS. Kidney from vehicle control (A) and DADS (D) treated rats showed a normal morphology. However, kidney from an AAP-treated rat (B) showed cast (black arrow), tubular dilation (white arrow), tubular necrosis (black arrow head), and tubular degeneration (white arrow head). Kidney from an AAP&DADS-treated rat (C) showed nearly normal appearance. H&E stain. Bar=50 µm.
Effects of DADS on KIM-1 and NGAL expression
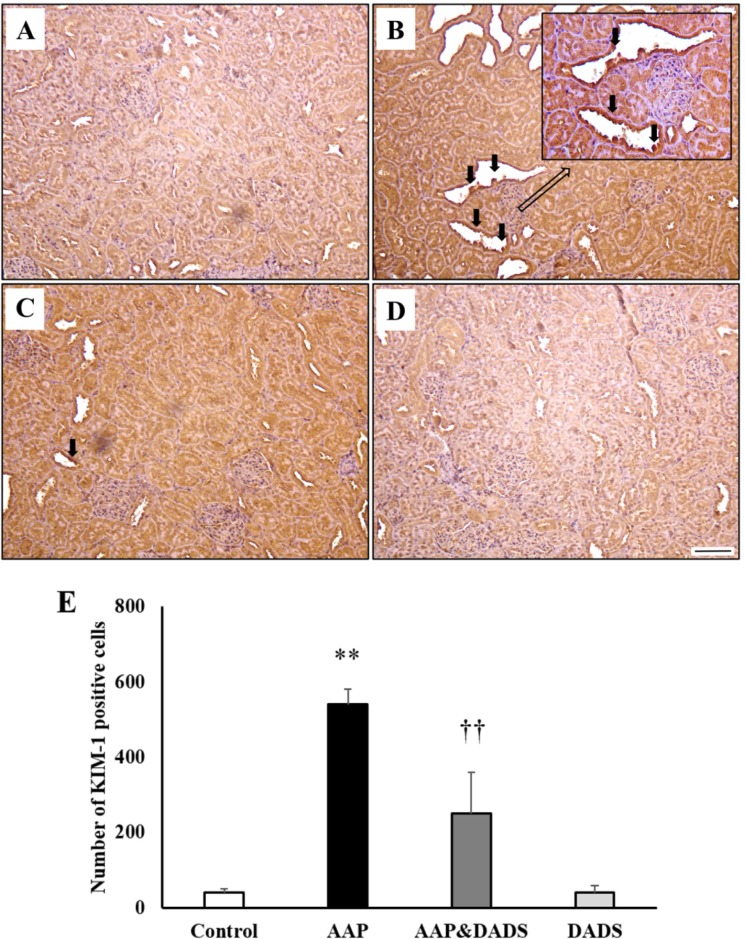
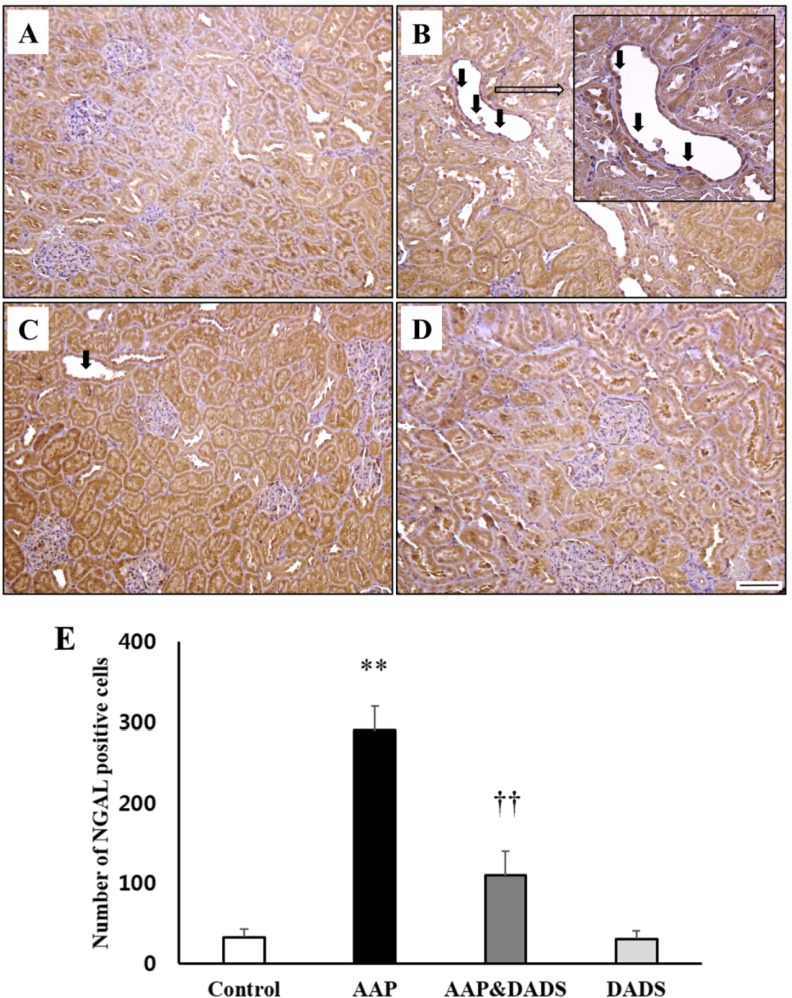
To assess a possible contribution of KIM-1 (Figure 3) and NGAL (Figure 4) in the kidneys, IHC for KIM-1 and NGAL was conducted. KIM-1 and NGAL positive cells were seldom observed in the control and DADS groups (Figures 3A, 3D, 4A, and 4D). However, rats treated with AAP manifested many KIM-1 and NGAL positive cells, which were mainly distributed in proximal tubular epithelial cells (Figures 3B and 4B). This increase was attenuated in rats treated with DADS (Figures 3C and 4C). Accordingly, the number of KIM-1 and NGAL positive cells in the AAP group was significantly higher than that in the control group (Figures 3E and 4E). The number of KIM-1 and NGAL positive cells in the AAP&DADS group decreased significantly compared with that in the AAP group.
Figure 3. Representative photographs of immunohistochemical analysis of KIM-1 in kidney sections of (A) controls and rats treated with (B) AAP, (C) AAP&DADS, and (D) DADS. Arrows indicate KIM-1 positive cells (brownish-stained cells). Bar=50 µm. (E) The number of KIM-1 positive cells was counted in ten different fields in each section under 100× magnification. Results are presented as means±SD (n=6). **P<0.01 compared with the control group. ††P<0.01 compared with the AAP group.
Figure 4. Representative photographs of immunohistochemical analysis of NGAL in kidney sections of (A) controls and rats treated with (B) AAP, (C) AAP&DADS, and (D) DADS. Arrows indicate NGAL positive cells (brownish-stained cells). Bar=50 µm. (E) The number of KIM-1 positive cells was counted in ten different fields in each section under 100× magnification. Results are presented as means±SD (n=6). **P<0.01 compared with the control group. ††P<0.01 compared with the AAP group.
The protein expression levels of KIM-1 and NGAL are shown in Figure 5. The expression levels of KIM-1 and NGAL in the AAP group increased significantly, as compared with those in the control group. In contrast, the expression levels of KIM-1 and NGAL in the AAP&DADS group decreased significantly, as compared with those in the AAP group.
Figure 5. Western blot analysis of KIM-1 and NGAL expressions in rats treated with AAP and/or DADS. Detection of β-actin expression was used as a loading control. The bar graphs show quantitative relative levels of KIM-1 (B) and NGAL (C) protein expressions for vehicle, AAP, AAP&DADS, and DADS-treated rats. Values are presented as means±SD (n=6). **P<0.01 compared with the control group. ††P< 0.01 compared with the AAP group.
Discussion
It has been demonstrated that DADS has renoprotective effects in models of renal injury induced by gentamicin and cisplatin [16,17]. The results of the present study showed that DADS has a protective effect against acute kidney injury induced by AAP treatment in male rats.
Several investigators have reported that AAP treatment leads to nephrotoxicity, characterized by poor renal function and increased serum creatinine and BUN levels [3,20]. In the present study, the nephrotoxicity observed in the AAP group included elevated BUN levels. These alterations correlated well with histopathological findings: increased incidence of cast, tubular dilation, tubular necrosis, and tubular degeneration. These findings observed in the AAP group may represent impaired renal function. However, DADS pretreatment effectively prevented the AAP-induced elevation in serum BUN levels, indicating the renoprotective activity of DADS against acute renal injury of AAP. This phenomenon was also confirmed by histopathological examination, which showed a decrease in the incidence and severity of renal histopathological lesions.
Animal models are commonly used for toxicity evaluation of new therapeutics and assessment of potential chemical hazards in industry and environment. Therefore, KIM-1 and NGAL may serve as useful biomarkers for drug safety and chemical hazard-related renal injury tests. Previous studies have shown that KIM-1 may function as an extracellular sensor or a receptor for adhesion/signaling in a variety cell-cell or cell-pathogen interactions [21,22]. The protein structure of this molecule suggests that KIM-1 may be an adhesion and/or protective molecule for the cell surface [23]. Therefore, it is considered that KIM-1 might alter cellular adhesion and/or modulate interactions between the injured epithelial cell and the luminal contents, and enhance mobility and proliferation of surviving epithelial cells [24]. The NGAL protein is expressed by neutrophils and various epithelial cells, and is found at very low concentrations in various human tissues: kidney, trachea, lungs, stomach, and colon [12,25]. It has also been found to play a role in kidney development and tubular regeneration and repair after injury [12]. Many factors leading to tubular epithelial cell injury result in an increased expression of KIM-1 and NGAL proteins. Thus, reduction of KIM-1 and NGAL expression is a major target for renoprotective therapy.
Although it is clear that expression of KIM-1 and NGAL protein is up-regulated by renal injury of ischemia-reperfusion, ochratoxin A, and gentamicin in animal models [26,27,28], the response to AAP-induced nephrotoxicity was not clear. Therefore, we investigated the role of DADS on the expression of KIM-1 and NGAL induced by AAP treatment. In the present study, AAP caused a significant increase in KIM-1 and NGAL protein expression without a significant increase in serum creatinine level. Ichimura et al. [8] also reported that nephrotoxicants treatment increased the expression of KIM-1 and NGAL, before causing a measurable increase in serum creatinine level. Although the specific functions of KIM-1 and NGAL are still unknown, up-regulation of these proteins is associated with proliferation/regeneration and repair in response to toxicity or disease [9,12,14]. Changes in the expression of marker proteins were confirmed by immunohistochemistry. In accordance with other studies [8,13,14,15], KIM-1 and NGAL occurred in proximal tubular epithelial cells. However, DADS pretreatment effectively inhibited AAP-induced KIM-1 and NGAL protein expression, suggesting that DADS-mediated improvement in nephrotoxicity might be mediated, at least in part, by its ability to reduce the KIM-1 and NGAL expression in the kidney. These results are in accordance with the decreased histopathological changes in the kidney.
In conclusion, DADS has protective effects against AAP-induced nephrotoxicity in male rats. KIM-1 and NGAL could be useful in preclinical and clinical studies vital to drug development and evaluation. They may also serve in the monitoring of disease states that manifest as injury to the proximal tubule and be useful in guiding interventional strategies.
Acknowledgments
This study was financially supported by Chonnam National University, 2014.
Footnotes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
References
- 1.Kanno S, Tomizawa A, Hiura T, Osanai Y, Kakuta M, Kitajima Y, Koiwai K, Ohtake T, Ujibe M, Ishikawa M. Melatonin protects on toxicity by acetaminophen but not on pharmacological effects in mice. Biol Pharm Bull. 2006;29(3):472–476. doi: 10.1248/bpb.29.472. [DOI] [PubMed] [Google Scholar]
- 2.Hengy B, Hayi-Slayman D, Page M, Christin F, Baillon JJ, Ber CE, Allaouchiche B, Rimmele T. Acute renal failure after acetaminophen poisoning: report of three cases. Can J Anaesth. 2009;56(10):770–774. doi: 10.1007/s12630-009-9155-1. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh J, Das J, Manna P, Sil PC. Acetaminophen induced renal injury via oxidative stress and TNF-alpha production: therapeutic potential of arjunolic acid. Toxicology. 2010;268(1-2):8–18. doi: 10.1016/j.tox.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Singh VP, Singh N, Jaggi AS. A review on renal toxicity profile of common abusive drugs. Korean J Physiol Pharmacol. 2013;17(4):347–357. doi: 10.4196/kjpp.2013.17.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnellman RG. Toxic responses of the kidney. In: Klassen CD, editor. Casarett & Doull's Toxicology: The Basic Science of Poisons. 6th ed. New York: McGraw-Hill Medical Publishing Division; 2001. pp. 491–514. [Google Scholar]
- 6.Mediæ B, Rovcanin B, Vujovic KS, Obradovic D, Duric D, Prostran M. Evaluation of Novel Biomarkers of Acute Kidney Injury: The Possibilities and Limitations. Curr Med Chem. 2016;23(19):1981–1997. doi: 10.2174/0929867323666160210130256. [DOI] [PubMed] [Google Scholar]
- 7.Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury - pathophysiological basis and clinical performance. Acta Physiol (Oxf) 2016;30:12764. doi: 10.1111/apha.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286(3):F552–F563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 9.Huo W, Zhang K, Nie Z, Li Q, Jin F. Kidney injury molecule-1 (KIM-1): a novel kidney-specific injury molecule playing potential double-edged functions in kidney injury. Transplant Rev (Orlando) 2010;24(3):143–146. doi: 10.1016/j.trre.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52(3):595–605. doi: 10.1053/j.ajkd.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, Devarajan P. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21(6):856–863. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 12.Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, de Cal M, Corradi V, Virzi G, Ronco C. NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010;42(1):141–150. doi: 10.1007/s11255-009-9608-z. [DOI] [PubMed] [Google Scholar]
- 13.Han WK, Bonventre JV. Biologic markers for the early detection of acute kidney injury. Curr Opin Crit Care. 2004;10(6):476–482. doi: 10.1097/01.ccx.0000145095.90327.f2. [DOI] [PubMed] [Google Scholar]
- 14.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 15.Ding H, He Y, Li K, Yang J, Li X, Lu R, Gao W. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin Immunol. 2007;123(2):227–234. doi: 10.1016/j.clim.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Pedraza-Chaverrí J, González-Orozco AE, Maldonado PD, Barrera D, Medina-Campos ON, Hernández-Pando R. Diallyl disulfide ameliorates gentamicin-induced oxidative stress and nephropathy in rats. Eur J Pharmacol. 2003;473(1):71–78. doi: 10.1016/s0014-2999(03)01948-4. [DOI] [PubMed] [Google Scholar]
- 17.Chiarandini Fiore JP, Fanelli SL, de Ferreyra EC, Castro JA. Diallyl disulfide prevention of cis-Diamminedichloroplatinum-induced nephrotoxicity and leukopenia in rats: potential adjuvant effects. Nutr Cancer. 2008;60(6):784–791. doi: 10.1080/01635580802100869. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Lee IC, Baek HS, Shin IS, Moon C, Bae CS, Kim SH, Kim JC, Kim HC. Mechanism for the protective effect of diallyl disulfide against cyclophosphamide acute urotoxicity in rats. Food Chem Toxicol. 2014;64:110–118. doi: 10.1016/j.fct.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Lee IC, Kim SH, Baek HS, Moon C, Kang SS, Kim SH, Kim YB, Shin IS, Kim JC. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem Toxicol. 2014;63:174–185. doi: 10.1016/j.fct.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Das J, Ghosh J, Manna P, Sil PC. Taurine protects acetaminophen-induced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation. Toxicology. 2010;269(1):24–34. doi: 10.1016/j.tox.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, Kikutani H. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419(6907):629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- 22.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 23.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 24.Van Klinken BJ, Dekker J, Büller HA, Einerhand AW. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol. 1995;269:G613–G627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- 25.Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochim Biophys Acta. 2000;1482:298–307. doi: 10.1016/s0167-4838(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 26.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63(5):1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 27.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290(2):F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann D, Adler M, Vaidya VS, Rached E, Mulrane L, Gallagher WM, Callanan JJ, Gautier JC, Matheis K, Staedtler F, Dieterle F, Brandenburg A, Sposny A, Hewitt P, Ellinger-Ziegelbauer H, Bonventre JV, Dekant W, Mally A. Performance of novel kidney biomarkers in preclinical toxicity studies. Toxicol Sci. 2010;116(1):8–22. doi: 10.1093/toxsci/kfq029. [DOI] [PMC free article] [PubMed] [Google Scholar]