Abstract
Interstitial cells of Cajal (ICC) generate electrical slow waves by coordinated openings of ANO1 channels, a Ca2+-activated Cl− (CaCC) conductance. Efflux of Cl− during slow waves must be significant, as there is high current density during slow-wave currents and slow waves are of sufficient magnitude to depolarize the syncytium of smooth muscle cells and PDGFRα+ cells to which they are electrically coupled. We investigated how the driving force for Cl− current is maintained in ICC. We found robust expression of Slc12a2 (which encodes an Na+-K+-Cl− cotransporter, NKCC1) and immunohistochemical confirmation that NKCC1 is expressed in ICC. With the use of the gramicidin permeabilized-patch technique, which is reported to not disturb [Cl−]i, the reversal potential for spontaneous transient inward currents (ESTICs) was −10.5 mV. This value corresponds to the peak of slow waves when they are recorded directly from ICC in situ. Inhibition of NKCC1 with bumetanide shifted ESTICs to more negative potentials within a few minutes and reduced pacemaker activity. Bumetanide had no direct effects on ANO1 or CaV3.2 channels expressed in HEK293 cells or L-type Ca2+ currents. Reducing extracellular Cl− to 10 mM shifted ESTICs to positive potentials as predicted by the Nernst equation. The relatively rapid shift in ESTICs when NKCC1 was blocked suggests that significant changes in the transmembrane Cl− gradient occur during the slow-wave cycle, possibly within microdomains formed between endoplasmic reticulum and the plasma membrane in ICC. Recovery of Cl− via NKCC1 might have additional consequences on shaping the waveforms of slow waves via Na+ entry into microdomains.
Keywords: electrical slow waves, gastrointestinal motility, Ca2+-activated Cl− current, ANO1, smooth muscle
electrical slow-wave activity drives phasic contractions in the gastrointestinal (GI) tract that are responsible for peristalsis in the stomach and segmental contractions in the small bowel and colon. Electrical slow waves originate in specialized pacemaker cells, known as interstitial cells of Cajal (ICC) (16, 22, 34, 39, 43). A strain of mice with constitutive expression of a reporter molecule, copGFP, in ICC has allowed studies of these cells soon after enzymatic liberation from muscles and has obviated the need for cell culture (30, 47). These studies have begun to piece together the ionic mechanisms responsible for generation and propagation of slow waves (35, 46–48). Studies on intact muscle utilizing electrophysiology and Ca2+ imaging techniques have developed a detailed hypothesis about the propagation of slow waves in ICC networks and how these events interact with electrically coupled smooth muscle cells (SMCs) (2, 7, 23–25, 28, 36).
At the present time, the developing concept of the pacemaker activity GI muscles includes the following (35): 1) ongoing discharge of spontaneous transient inward currents (STICs) is attributable to localized Ca2+ release from intracellular stores via IP3 receptors (37, 40); 2) STICs cause spontaneous transient depolarizations (STDs) in ICC (9, 15, 19, 20, 40, 48); 3) depolarization activates low-threshold, T-type Ca2+ currents (46); however, some investigators have also suggested that depolarization may enhance IP3 production (15, 41); 4) Ca2+ entry (or enhanced IP3 production) coordinates release of Ca2+ from stores, activating a whole-cell slow-wave current (46, 47); 5) cells depolarize to about −10 mV (20); and 6) repolarization resets the slow-wave cycle. STICs and slow-wave currents are due to Cl− efflux via Ca2+-activated Cl− channels (CaCC), and slow waves are absent in mice with genetic deactivation of Ano1 (17, 36, 47). Slow-wave currents in ICC are of large current density, reaching 80 pA/pF (47), which must result in significant efflux of Cl−. Most of the Cl− channels in ICC appear to be localized within microdomains formed by the close apposition between the plasma membrane and the endoplasmic reticulum (49). How the driving force for slow-wave currents (i.e., the difference between the resting membrane potential and ECl; EM-ECl) is maintained from event to event in these constantly active pacemaker cells has not been clarified.
A previous study using suppression-subtractive hybridization demonstrated that the tunica muscularis of the murine jejunum displays high expression of Slc12a2, which encodes the Na+-K+-Cl− cotransporter (NKCC1) (44). Immunohistochemistry revealed that NKCC1 is expressed by the pacemaker class of ICC in the myenteric region of the small intestine (ICC-MY), and block of NKCC1 with bumetanide reduced or blocked slow waves (18, 44). It was also found that slow waves were of much smaller amplitude in mice with constitutive genetic deactivation of Slc12a2, and bumetanide had no effect on the activity remaining in these mice. Thus studies of whole muscles suggest that NKCC1 is involved, in some manner, in the slow-wave cycle. Realization that ANO1 is a fundamental conductance in slow waves suggests the hypothesis that the role of NKCC1 may be to restore the Cl− gradient after loss of Cl− during slow-wave currents. In the present study, we have measured the reversal potential of STICs (ESTICs), which is likely, based on analysis of reversal potentials (48), to approximate ECl in freshly isolated and identified ICC from the murine small intestine. We determined how STICs and slow-wave currents are affected by NKCC1 inhibitor bumetanide. Our results confirm that ESTICs lies positive to the resting potentials of ICC and that the driving force for STICs must be maintained by NKCC1 to sustain the pacemaker activity of these cells.
MATERIALS AND METHODS
Animals.
Kit+/copGFP mice (7–12 days old) were used for the electrophysiological experiments on ICC in this study (30). ICC can be identified unequivocally by the expression of copGFP in these mice. Kit+/copGFP mice and smooth muscle myosin heavy chain (smMHC)/Cre/enhanced green fluorescent protein (eGFP) mice (both 5–8 wk old; the latter strain was donated by Dr. Michael Kotlikoff, Cornell University) were used for gene expression studies. Colon SMCs, used for control studies of L-type Ca2+ channels, were obtained from C57BL/6 mice (2–3 mo old; Jackson Laboratory). The mice were anesthetized with isofluorane and killed by cervical dislocation. The institutional Animal Use and Care Committee at the University of Nevada approved all procedures.
Preparation of dispersed cells.
Small strips of intestinal and colonic muscles were cut and equilibrated in Ca2+-free Hanks solution consisting of the following (in mM): 125.00 NaCl, 5.60 KCl, 15.00 NaHCO3, 0.36 Na2HPO4, 0.40 KH2PO4, 10.00 glucose, 2.00 sucrose, and 10.00 N-2-hydroxyethylpiperazine-N′-ethanesulfonic acid (HEPES) adjusted to pH 7.2 with Tris. ICC were isolated from small intestinal muscles of Kit+/copGFP mice, using an enzyme solution containing the following: collagenase (Worthington type II, 1.3 mg/ml; Worthington Biochemical), BSA (2 mg/ml; Sigma), trypsin inhibitor (2 mg/ml; Sigma), and ATP (0.27 mg/ml). Cells were plated onto sterile glass coverslips coated with murine collagen (2.5 mg/ml; Falcon/BD) in 35-mm culture dishes. The cells were allowed to settle for 10 min before we added smooth muscle growth medium (Clonetics) supplemented with 2% antibiotic/antimycotic (Gibco) and murine stem cell factor (5 ng/ml; Sigma). The cells were placed into a 95% O2-5% CO2 incubator for 2 h at 37°C (47).
Freshly isolated SMCs from small intestine were used in expression studies for comparisons with ICC, and SMCs from colon were used as a source of L-type Ca2+ currents for control studies to evaluate nonspecific effects of bumetanide on this conductance. SMCs were dispersed from small intestinal strips of smMHC/Cre/eGFP mice, as described previously (46). Small pieces of muscle were exposed for 30 ± 2 min at 37°C to a solution containing (per ml) 3.5 mg collagenase (Worthington type II; Worthington Biochemical), 8.0 mg BSA (Sigma), and 8.0 mg trypsin inhibitor (Sigma). SMCs were also dispersed from proximal colons of C57BL/6 mice using the enzyme solution used for small intestinal muscles but with the addition of 1 mg/ml papain and 1 mg/ml dithiothreitol (both from Sigma).
Gene expression.
Total RNA was isolated from copGFP+ cells, and intestinal SMCs were purified by fluorescence-activated cell sorting (FACS) and from samples of dispersed cells before being sorted with the use of an Illustra RNAspin Mini RNA Isolation Kit (GE Healthcare). qScript cDNA SuperMix (Quanta Biosciences) was used to synthesize first-strand cDNA by the manufacturer's instructions. Quantitative PCR (qPCR) was performed with gene-specific primers (Table 1) using Fast Sybr Green chemistry on the 7900HT Fast Real-Time PCR System (Applied Biosystems). Regression analysis was used to produce standard curves from the mean values of technical triplicate qPCRs of log10 diluted cDNA samples. Unknown amounts of messenger RNA (mRNA) were plotted relative to the standard curve for each set of primers using Microsoft Excel. This gave transcriptional quantification of each gene relative to the endogenous Gapdh standard after log transformation of the corresponding raw data. Evaluation of gene expression in ICC was compared with expression in the unsorted cell population cells from small intestinal muscles of Kit+/copGFP mice, and gene expression in sorted SMCs was compared with the unsorted population of cells from small intestinal muscles of smMHC/Cre/eGFP mice.
Table 1.
Primers used for qPCR
| Gene | Primer Sequence | Accession No. |
|---|---|---|
| mGapdh-F | TGAACGGATTTGGCCGTATTG | NM_001289726 |
| mGapdh-R | GATGGGCTTCCCGTTGATGA | |
| mSlc12a1-F all isoforms | CGGCAAGCTGAACATTACCA | NM_183354 |
| mSlc12a1-R all isoforms | TTAAACTGAGCGCTGGCTTC | |
| mSlc12a1-F isoform A | AGTCATGCTCTTCATTCGCC | NM_183354 |
| mSlc12a1-R isoform A | CTCCACCTCCACGAACAAACC | |
| mSlc12a1-F isoform B | GGGAATTGGTCTCGGTGTGA | U20974 |
| mSlc12a1-R isoform B | GGCTCCACCTCCTCTGACAT | |
| mSlc12a1-F isoform F | ATCATTGGCCTGAGCGTAGT | NM_001079690 |
| mSlc12a1-R isoform F | AAGCCTATTGACCCACCGAA | |
| mSlc12a2-F | CTTGCGAGAAGGTGCACAATA | NM_009194 |
| mSlc12a2-R | TCAGGCGGATAACCACAACT | |
| mSlc12a3-F | CCCTTGTGGACTTTGTGAGC | NM_001205311 |
| mSlc12a3-R | TTGTTCAGCCACTTGGTGTG | |
| mSlc4a1-F | ACCTAACCATCCCTGTGACC | NM_011403 |
| mSlc4a1-R | CCACATAGACCTGACCGGAG | |
| mSlc4a2-F | CTGGAGGTGGATAGAGAGCG | NM_009207 |
| mSlc4a2-R | CTCCGCATTCTCAGGGATCT | |
| mSlc4a3-F | CTTTGAGTTTCACCGGCACA | NM_009208 |
| mSlc4a3-R | GATAGGGGGTGTCCCTTCTG | |
| mSlc4a4-F | CAACGACGATTCTGACTGCC | NM_053424 |
| mSlc4a4-R | TGTGTGGCGTTCAAGGAATG | |
| mSlc4a5-F | ACACAGACCAGCGGAAAAAC | NM_001166067 |
| mSlc4a5-R | GCAGACTGGACAAGACGAAC | |
| mSlc4a7-F | CAGGAGAGTGGGATCCTTCC | NM_001033270 |
| mSlc4a7-R | CAAAAAGCCGTCCAGTCCTC |
F, forward; R, reverse.
Immunohistochemistry.
For whole-mount immunohistochemistry, the tunica muscularis of small intestine of C57BL/6 mice was fixed in acetone (10 min at 4°C) and then washed with PBS and immersed in PBS with 1% BSA (1 h) to reduce nonspecific binding of antibodies. Muscles were then incubated with anti-c-KIT antibody (ACK2; eBioscience) diluted (1:500) in PBS with 0.5% Triton X-100 for 48 h at 4°C, washed briefly with PBS, and then incubated with anti-NKCC1 antibody (SC-21547; Santa Cruz Biotechnology) diluted (1:100) in PBS with 0.5% Triton X-100 for 48 h at 4°C. After being washed in PBS, the muscles were incubated with secondary antibodies diluted (1:500) in PBS for 1 h at room temperature. The secondary antibodies used for the anti-c-KIT antibody and anti-NKCC1 antibody were Alexa Fluor 594 donkey anti-rat IgG and Alexa Fluor 488 donkey anti-goat IgG (Molecular Probe, Life Technologies), respectively. Controls using no primary antibody confirmed that labeling was specific.
For immunohistochemistry, sections of small intestines of C57BL/6 mice were fixed in 4% paraformaldehyde (15 min at 4°C) and were then washed with PBS. Frozen tissue blocks were made with optimum cutting temperature compound (Sakura Finetek) by standard methods, and 10-μm sections were cut with a cryostat (Carl Zeiss). Tissue sections were incubated in 1% BSA (Sigma) in PBS for 1 h at room temperature to block nonspecific antibody binding. After being blocked, tissue sections were incubated with the same primary antibodies overnight at 4°C and then the same secondary antibodies for 1 h at room temperature, as used for whole mounts.
After immunolabeling, the samples were examined with a confocal microscope (Olympus FluoView 1000; Olympus), and images were obtained using an ×100 objective (UPlanSApo 100x, NA1.40; Olympus) for whole-mount tissues and an ×60 objective (PlanApo N 60x, NA1.42; Olympus) for sectioned tissues. The micrographs shown are composites of Z-series scans (0.5-μm optical section) through a depth of 6 μm constructed by Olympus Fluoview Ver.2.1c Viewer (FV10-ASW).
Electrophysiological experiments.
Whole-cell voltage- and current-clamp experiments were performed on ICC identified by the expression of GFP, using the permeabilized patch technique or conventional dialyzed cell techniques. For experiments with permeabilized patches, gramicidin (50 mg/ml, Sigma) was dissolved in ethanol with sonication and diluted with the pipette solution to a final concentration of 100 μg/ml. Membrane currents or potentials were amplified with an Axopatch 200B patch-clamp amplifier (Axon Instruments), digitized with a 16-bit analog-to-digital converter (Digidata 1322A; Axon Instruments), and stored digitally using pCLAMP software (version 9.2; Axon Instruments). Data were sampled at 4 kHz and filtered at 2 kHz using an eight-pole Bessel filter. Changes in holding currents (basal currents) were continuously monitored by miniDigi (Axon Instruments) using Axoscope 9.2 software (Axon Instruments). All data were analyzed using clampfit (pCLAMP version, 9.2; Axon Instruments) and GraphPad Prism (version 3.0; GraphPad Software) software. Pipette tip resistances ranged between 3 and 5 MΩ, and all experiments on ICC were conducted at 30°C using a CL-100 bath heater (Warner Instruments).
Solutions and chemicals for patch-clamp experiments.
The external bath solution for whole-cell recordings from ICC was a Ca2+-containing physiological salt solution (CaPSS, Solution I; Table 2). The pipette solution used for these experiments was Solution II (Table 2) and Solution III for experiments with reduced external Cl−. In studies of Cl− currents, cells were dialyzed with NMDG-Cl (Solution V) and the external solution to achieve a symmetrical Cl− gradient was Solution VI. The effects of bumetanide (Sigma) and various concentrations of external Cl− were tested using a fast bath perfusion system (AutoMate Scientific). Changes of the bath solution were completed within 1 min.
Table 2.
The composition of solutions
| Solutions (mM) | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| KCl | 5 | 135 | 5 | 0 | 0 | 0 |
| NaCl | 135 | 0 | 0 | 0 | 0 | 0 |
| Na isethionate | 0 | 0 | 135 | 0 | 0 | 0 |
| CsCl | 0 | 0 | 0 | 135 | 0 | 0 |
| NMDGCl | 0 | 0 | 0 | 0 | 147.7 | 150 |
| CaCl2 | 2 | 0 | 2 | 0 | 4.14 | 2 |
| MgCl2 | 1.2 | 0 | 1.2 | 0 | 0 | 1 |
| MgATP | 0 | 3 | 0 | 3 | 0 | 0 |
| NaGTP | 0 | 0.1 | 0 | 0.1 | 0 | 0 |
| CPD | 0 | 2.5 | 0 | 2.5 | 0 | 0 |
| Glucose | 10 | 0 | 10 | 0 | 0 | 0 |
| EGTA | 0 | 0.1 | 0 | 0.1 | 10 | 0 |
| HEPES | 10 | 10 | 10 | 10 | 10 | 10 |
| pH | 7.4 | 7.2 | 7.4 | 7.2 | 7.2 | 7.4 |
All solutions were adjusted for pH with Tris. CPD, creatinine phosphate disodium.
Expression of ANO1 and Cav3.2 in HEK293.
We expressed Ano1 in HEK293 cells (American Type Culture Collection, ATCC) to test whether the effects of bumetanide noted in this study could be due to direct blocking effects on ANO1 currents. An expressed sequence tag (IMAGE Consortium cDNA clone no. 30547439) homologous to the A isoform of Ano1 from mouse was subcloned into pcDNA3.1 (Invitrogen) using standard molecular biological techniques. For expression in mammalian cells, Ano1 was subcloned in frame into pAcGFP1-N1 vector (Clontech Laboratories), resulting in a plasmid coding for a COOH-terminal GFP-tagged ANO1 fusion protein. To generate the Ano1-AC variant, containing the alternative exon 13, we inserted the 12-nucleotide C segment into Ano1-coding region using the QuickChange XL site-directed mutagenesis kit (Agilent Technologies). The plasmids were sequenced at the Nevada Genomics Centre. HEK293 cells expressing Ano1 were seeded in 12-well plates for transient transfection. The next day, the pAcGFP1-N1 vector, containing the AC variant of mouse Ano1 tagged with eGFP, was transfected into cells using FuGENE 6 transfection reagent (Promega). Cells were used for patch-clamp recordings 24–48 h after transfection.
HEK293 cells with stable expression of Cav3.2, the dominant T-channel paralog in ICC (4), were donated by Dr. E. Perez-Reyes (University of Virginia). Generation of the cell lines expressing human Cav3.2 and the electrophysiological properties of the currents expressed were described previously (12). Briefly, HEK293 cells (A293; ATCC) were transfected with linearized plasmid (pcDNA3, Invitrogen) containing the human heart Cav3.2 isoform CACNA1H. Cells were grown in DMEM/F-12 (Thermo Fisher Scientific/Gibco), and the media was supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% sodium pyruvate (all media supplements were from Thermo Fisher Scientific/Gibco).
Experiments to test the effects of bumetanide on ANO1, CaV3.2, and L-type Ca2+ currents.
The dialyzed whole-cell patch-clamp configuration was used to record ANO1 currents from HEK293 cells. These experiments were performed at room temperature using an Axopatch 200B amplifier and pClamp 9.0 software (Axon Instruments). ANO1 currents were recorded in response to step depolarizations from −80 mV to +70 mV using Solution V for the pipette solution and Solution VI as the external solution.
Cav3.2 currents were recorded from HEK293 cells using dialyzed whole-cell patch-clamp conditions. L-type Ca2+ currents of colonic SMCs, the dominant voltage-dependent inward current in these cells, were studied using amphotericin-permeabilized patches to reduce rundown of the current (21). Amphotericin B (90 mg/ml; Sigma) was dissolved with DMSO with sonication and was diluted in the pipette solution to give a final concentration of 250 μg/ml. The external and pipette solutions used for measurement of Ca2+ currents were Solutions I and Solution IV (Table 2), respectively. Voltage-dependent Ca2+ currents were evoked by depolarizing steps from −80 mV to +60 mV from a holding potential of −80 mV. The voltage dependence of activation was determined from a Bolzmann fit of the data normalized to maximal conductance (G/Gmax). Time constants of activation and inactivation were calculated from fits of currents with single or double exponentials.
Statistical analysis.
Data were tabulated and presented as means ± SE. The n values given represent the number of animals from which cells or tissues were obtained for the specific protocols performed. Differences between data sets were determined with Student's paired t-test and considered significant when P < 0.05.
RESULTS
Expression of NKCC1 in ICC.
Expression of NKCC (Slc12a family genes) and other Cl− and HCO3− transporters (i.e., Slc4a family genes) in ICC was determined by qPCR and compared with expression in SMCs and unsorted cells from enzymatically dispersed small intestinal muscles. Sorting protocols for these cells and purification of the specific classes of cells obtained from enzymatic dispersion of murine GI muscles were described previously (29). In the present study, we also monitored the sorted cells microscopically to verify that cells enriched by FACS contained the GFPs upon which their selection was based. Expression of Slc12a1 (NKCC2), Slc12a2 (NKCC1), Slc12a3 (NCC), Slc4a1 (anion exchanger 1, AE1), Slc4a2 (AE2), Slc4a3 (AE3), Slc4a4 (Na+/HCO3− cotransporter 4, NBC1), Slc4a5 (NBC4 in human), and Slc4a7 (NBC3) was evaluated and normalized to levels of Gapdh.
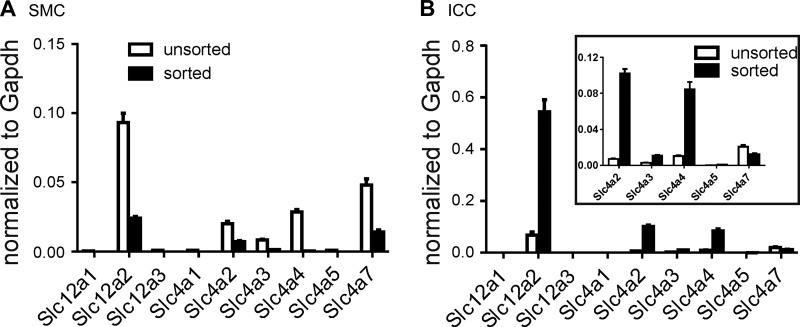
Expression of genes encoding NKCC2, NCC, and AE1 was not detected in SMCs, ICC, or the unsorted cells. In the case of Slc12a1, primers were designed to detect all splice variants including the A, B, and F forms. Transcripts of Slc12a1 were not detected by any of the splice variant-specific primer sets. SMCs and ICC also showed only minimal expression of AE3, NBC4, and NBC3. In contrast, anion exchanger 2 (Slc4a2) was elevated in ICC (0.1019 ± 0.0050; n = 3) compared with SMCs and unsorted cells (0.0072 ± 0.0006 and 0.0071 ± 0.0009, respectively; n = 3 each), and Na+/HCO3− cotransporter 1 (Slc4a4) expression was also elevated in ICC (0.0844 ± 0.0083; n = 3) vs. SMCs and unsorted cells (0.0003 ± 0.00001 and 0.0103 ± 0.0011, respectively; n = 3 each). However, NKCC1 was the most highly expressed of the transporters evaluated in ICC (0.5441 ± 0.0467; n = 3) compared with SMCs and unsorted cells (0.0239 ± 0.0014 and 0.0683 ± 0.0118, respectively; n = 3). Expression data are summarized in Fig. 1.
Fig. 1.
Expression of Cl− and HCO3− transporters. qPCR was performed on extracts of unsorted cells and interstitial cells of Cajal (ICC) (B) and smooth muscle cells (SMC) (A) purified by fluorescence-activated cell sorting. Primers used are provided in Table 1. Inset: portion of the main graph outlined to show an expanded scale for several transcripts. Slc12a2 (Na+K+Cl− cotransporter, NKCC1) transcripts were highly enriched in ICC. The data also showed somewhat enhanced expression of Slc4a2 and Slc4a4 in ICC. Relative expressions of all transcripts were normalized to Gapdh.
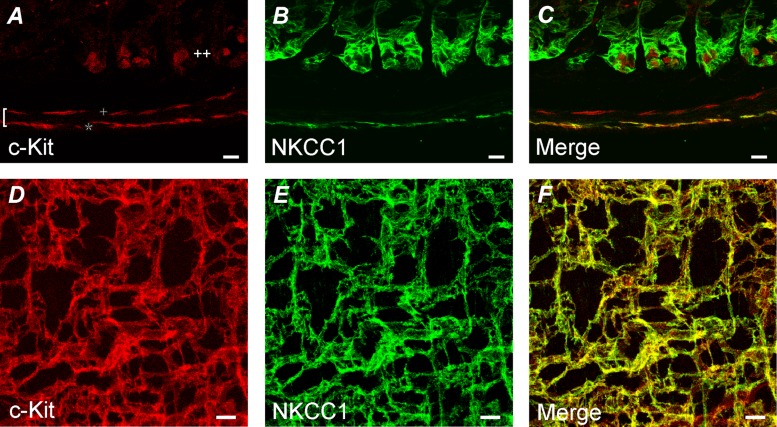
Double immunolabeling was performed to verify the expression of NKCC1 protein in ICC of small intestine. Previous studies have shown that c-KIT-like immunoreactivity (c-KIT-LI) labels ICC in the murine intestine, but it was not resolved in ICC of the deep muscular plexus region (ICC-DMP) (43). We found that NKCC1-like immunoreactivity (NKCC1-LI) colocalized with c-KIT in the ICC-MY, but this protein was not resolved in cells in ICC-DMP (Fig. 2; n = 4).
Fig. 2.
Expression of NKCC1-like immunoreactivity in ICC. A–C show double immunolabeling of c-KIT (A, red), NKCC1 (B, green), and merged files (C) from cryo-cross sections of the intestinal wall. Colocalization is represented in yellow in C. The left bracket in A denotes the width of the tunica muscularis. Double plus sign denotes region of the mucosa, and + and * denote bands of ICC of the deep muscular plexus region (ICC-DMP) and ICC in the myenteric region of the small intestine (ICC-MY), respectively. Note that ICC-MY were labeled by NKCC1 antibody, but no cells in the ICC-DMP were labeled. D–F show whole mounts highlighting the myenteric plexus region of the small intestine. c-KIT immunoreactivity (D, red) colocalized with NKCC1-like immunoreactivity (E, green) in the network of ICC-MY, as shown in the merged file (F, yellow). The scale bars represent 10 μm in all panels.
Effects of bumetanide and low chloride on STICs and the holding current in ICC.
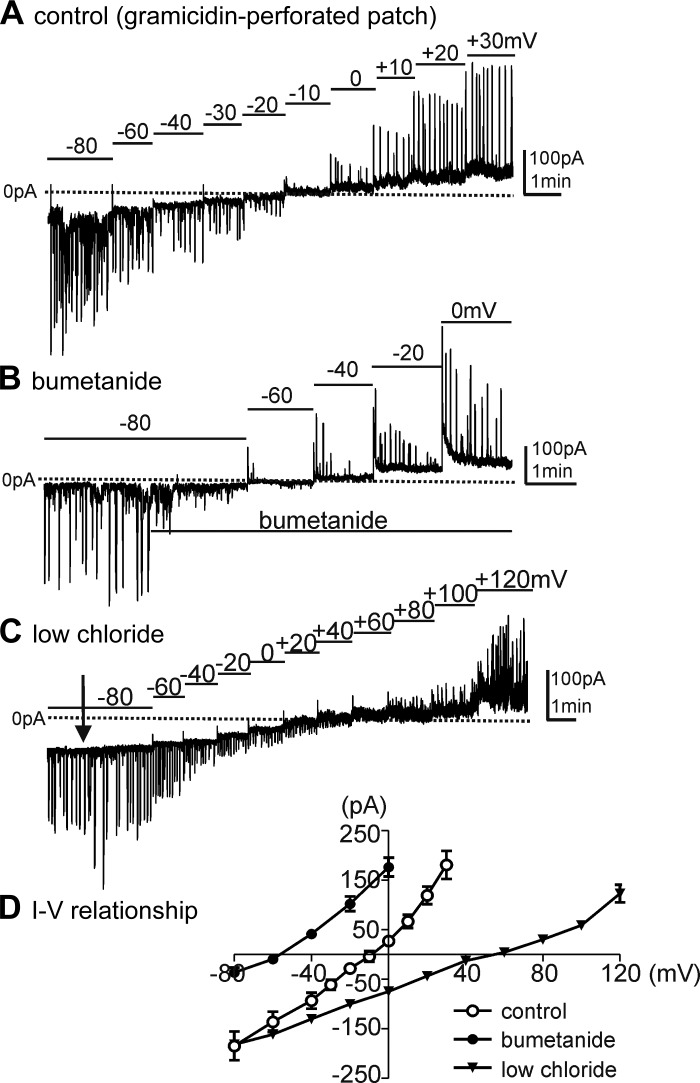
Gramicidin, added to pipette solutions, was used for permeabilized-patch recordings of STICs and slow-wave currents in ICC under voltage-clamp conditions. K+-rich pipette solution (Solution II), CaPSS (Solution I), and an external solution with reduced chloride (Solution III) were used for these experiments (see Table 2). ICC were held at −80 mV and stepped to potentials from −80 to +30 mV to measure steady-state responses. We observed STICs at the holding potential, and the STICs reversed at −9.0 ± 2.6 mV when cells were perfused with CaPSS solution (Fig. 3, A and D; n = 6). Reversal potential was determined by interpolating the voltage intercept from linear regression. Bumetanide (40 μM) shifted the reversal potential such that STICs reversed at −56.0 ± 2.9 mV within 5 min after addition of bumetanide (Fig. 3, B and D; n = 5; reversal potential given without junction potential correction of 5 mV). When extracellular Cl− was reduced to 10 mM (replaced with isethionate, Solution III), the reversal potential of STICs shifted to +55.0 ± 4.5 mV (Fig. 3C; n = 5; reversal potential given without junction potential correction of −2.3 mV), as would be predicted by the Nernst equation for the Cl− gradient. Figure 3D summarizes I–V relationships obtained in the presence of CaPSS (Solution I), after addition of bumetanide, and low-chloride conditions (Solution III).
Fig. 3.
Current-voltage (I–V) relationship of spontaneous transient inward currents (STICs). Cells were studied using gramicidin-perforated, whole-cell voltage-clamp conditions. A: ICC generated STICs at a holding potential of −80 mV. Cells were stepped to various potentials from −80 to +30 mV. STICs reversed at −10 mV using Ca2+-containing physiological salt solution (CaPSS) as the external solution (Solution I) and K+-rich solution (Solution II, see Table 2) as the pipette solution. B: bumetanide (40 μM) was added at the black bar beneath current trace. Bumetanide reduced the amplitude of STICs at −80 mV and shifted reversal of STICs to −56.0 ± 2.9 mV. C: reducing extracellular Cl− to 10 mM (low chloride, Solution III; Table 2) shifted the reversal potential of STICs to +55.0 ± 4.5 mV. D: summary I-V relationship for STICs in control (n = 6) and during bumetanide (n = 5) and low-chloride (n = 5) conditions.
Effects of bumetanide on STICs and slow-wave currents in ICC.
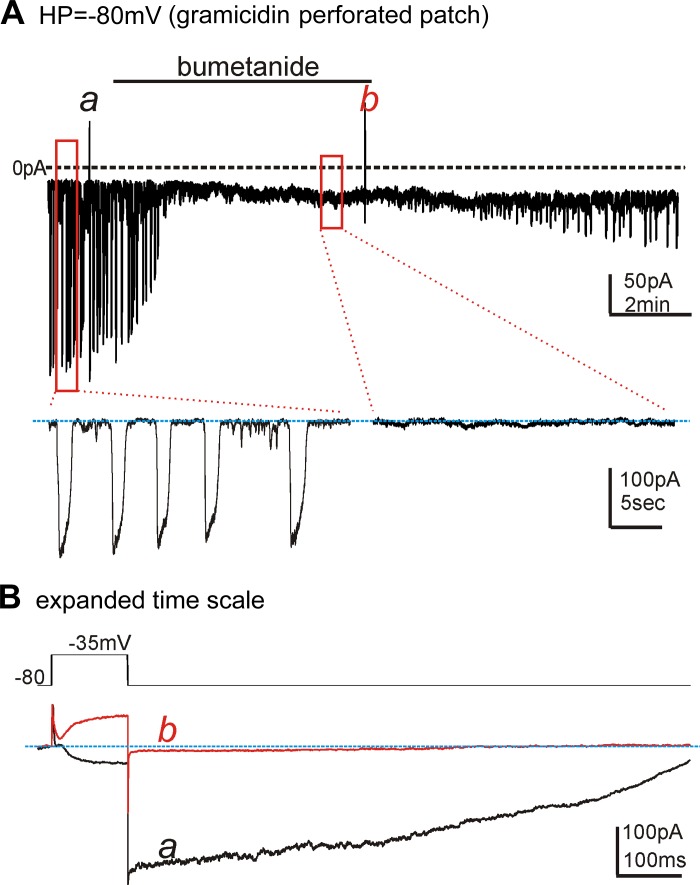
Whole-cell patch-clamp experiments with gramicidin-permeabilized patches were performed to determine whether STICs and slow-wave currents run down after the blocking of NKCC1. CaPSS (Solution I) and the K+-rich pipette solution (Solution II) were used for these experiments. When cells were held at −80 mV, STIC amplitude was −153.6 ± 39.3 pA (n = 5). Bumetanide (40 μM) reduced STICs to −24.4 ± 4.8 pA (Fig. 4A; n = 5, P < 0.01). These experiments suggest that, when Cl− recovery was reduced by bumetanide, ESTICs shifted to more negative potentials. Step depolarizations (−80 mV to −35 mV) were used to elicit slow-wave currents in ICC (47). Stepping to −35 mV induced an inward current that was autonomous in nature, resulting in a long-duration tail current even after repolarization to −80 mV (Fig. 4Ba). Note that the current was inward at −35 mV and increased in amplitude to −326 ± 32 pA upon stepping back to −80 mV. After addition of bumetanide, current responses shifted such that the current response within a few minutes was characterized by development of outward current upon steps to −35 mV and small inward tail currents averaging −46 ± 7 pA (n = 5, P <0.001) upon returning the potential to −80 mV (Fig. 4Bb).
Fig. 4.
Effects of bumetanide on STICs and slow-wave currents. Cells were studied using gramicidin-perforated, whole-cell voltage-clamp conditions. CaPSS was used as the external solution (Solution I), and K+-rich solution (Solution II, see Table 2) was used as the pipette solution. A: STICs occurred in cells held at −80 mV. HP = holding potential. Addition of bumetanide (40 μM) caused rapid reduction in STICs within a few minutes. Insets: STICs at an expanded time scale before and in the presence of bumetanide during the portions of the record designated by the red boxes. Slow-wave currents were evoked at a and b in the trace shown in A by stepping the cell from −80 mV to −35 mV. B: step protocol and the superimposed slow-wave current responses evoked by the step depolarizations, as indicated in A, at an expanded time scale. Under control conditions (black trace, a), depolarization evoked an inward current, and then a long-lasting tail current was observed when the cell was returned to the holding potential. Bumetanide (40 μM) changed characteristics of the slow-wave currents. At b (red trace), the initial current evoked by depolarization was outward, and the tail current was minimal. These observations indicate a progressive shift in ECl toward the holding potential in response to bumetanide. Blue lines denote the baseline of holding current at −80 mV.
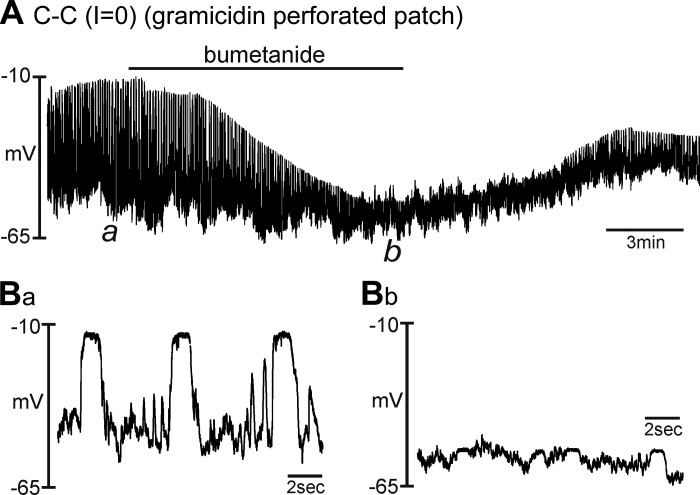
Effects of bumetanide on STDs in ICC.
As reported previously, isolated ICC generate STDs under current-clamp conditions (47). We have previously associated STDs with the large-amplitude slow-wave currents (47, 48). Others have tested bumetanide on slow waves recorded from intact small intestinal muscles (presumably via impalement of SMCs) and found that bumetanide decreased the frequency and amplitude of slow waves and blocked these events in three of nine muscles tested (44). Slow waves recorded directly from ICC in the rabbit small intestine were also reduced by bumetanide (18). Therefore, we tested the effects bumetanide on STDs in single ICC under current-clamp conditions (I = 0) using gramicidin-permeabilized, patch-clamp, whole-cell recording. CaPSS (Solution I) and a K+-rich pipette solution (Solution II) were used for these experiments. STDs averaged 53.0 ± 5.4 mV (n = 6) under control conditions (Fig. 5, A and Ba). Bumetanide (40 μM) decreased the amplitude of the STDs to 10.0 ± 1.8 mV within 9 min (n = 6, P < 0.001; Fig. 5, A and Bb). Resting membrane potential was −58.0 ± 1.7 mV before bumetanide and not significantly changed by the drug (i.e., −59.9 ± 2.6 mV; n = 6; P = 0.49). Expanded time scales show the differences in STDs after bumetanide treatment (Fig. 5B).
Fig. 5.
Effects of bumetanide on spontaneous transient depolarizations (STDs) in ICC. Cells were studied under current-clamp conditions (I = 0) using perforated whole-cell patch-recording conditions. CaPSS was used as the external solution (Solution I), and the K+-rich solution (Solution II) was used as the pipette solution. A shows STDs recorded under control conditions and the effects of bumetanide (40 μM). Bumetanide progressively decreased the maximal level of depolarization that occurred during STDs. Ba and Bb show portions of the extended trace in A at expanded time scales. These excerpts are taken at the points denoted by a and b in A.
Effects of bumetanide on ANO1 and voltage-dependent Ca2+ currents.
Nonspecific effects of bumetanide on the ionic currents responsible for STICs, slow-wave currents, and STDs might explain some of the observations here. Previous studies have demonstrated the role of ANO1 current in STICs and slow waves in ICC (17, 36, 47, 48), and regeneration of slow waves may depend on T- and L-type Ca2+ currents (35, 46). Therefore, we tested the effects of bumetanide on these conductances in a series of control experiments. We used HEK293 with heterologous expression of ANO1 or CaV3.2 channels to test the effects of bumetanide on these conductances, and murine colonic SMCs were used to test effects on L-type currents because these cells have robust expression of this conductance (21).
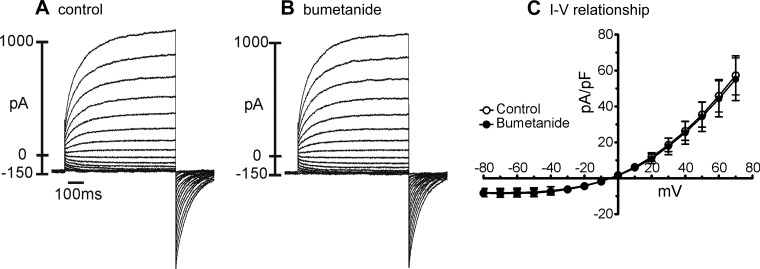
ANO1 is highly expressed in ICC, and these channels mediate the slow-wave currents in ICC and, therefore, the pacemaker activity of GI muscles (17, 47). Because our data show that bumetanide reduced holding current and blocked STICs and slow-wave currents in ICC, we also tested whether this drug has direct effects on ANO1 currents. We used the AC splice variant of ANO1 channels for these tests because this is a prominent isoform expressed by ICC (17, 36). HEK293 cells expressing ANO1 channels (as indicated by eGFP expression in these cells) were voltage clamped in symmetrical N-methyl-d-glucamine+ Cl− (NMDGCl) solutions with free [Ca2+]i set at 100 nM. The external solution was Solution VI, and the internal solution was Solution V (Table 2) for these experiments. Stepping to potentials from −80 mV to +70 mV revealed large-amplitude, time-dependent outwardly rectifying Cl− currents in these cells. Nontransfected cells did not display such a conductance. Bumetanide (50 μM) had no direct effects on ANO1 currents (Fig. 6, A and B). Summarized data from five cells show no change in the current-voltage relationship for ANO1 currents after addition of bumetanide (Fig. 6C, n = 5).
Fig. 6.
Bumetanide did not inhibit STICs and slow-wave currents by blocking ANO1 currents. A: representative currents evoked in HEK293 cells expressing the AC isoform of Ano1. Currents were elicited by stepping potential from −80 mV to +70 mV in 10-mV increments using symmetrical N-methyl-d-glucamine+ Cl− (NMDGCl) solutions with free [Ca2+]i set at 100 nM (Solutions V and VI). B: current-voltage protocols were repeated 10 min after application of bumetanide (50 μM). C: I-V relationships for ANO1 currents from 5 cells evoked by stepping to different potentials before and in the presence of bumetanide. Currents plotted are the maximum currents elicited at each potential. No indication of any direct effects of bumetanide on ANO1 currents was observed.
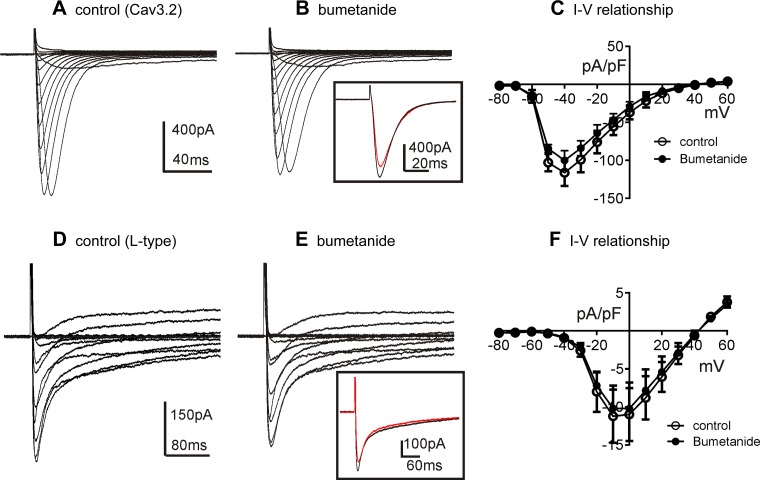
Experiments were also performed to test the effects of bumetanide on T- and L-type Ca2+ currents. CaV3.2 currents were recorded in whole-cell voltage-clamp experiments from HEK293 cells transfected with CACNA1H (12). Cells were exposed to CaPSS (Solution I) and dialyzed with Ca+-rich solution (Solution IV; Table 2) and stepped from −80 to +60 mV. Responses were evoked before and in the presence of bumetanide (50 μM). Bumetanide had no significant effect on the amplitude of CaV3.2 currents or on the activation and inactivation kinetics (e.g., voltage dependence and time constants; Fig. 7, A and B). For example, the peak current at −40 mV (evoked from a holding potential of −80 mV) had a current density of −113 ± 8 pA/pF in control and −100 ± 5 pA/pF in the presence of bumetanide (n = 5; P = 0.061). The half-activation voltage was −54.0 ± 0.5 mV in control and −54.0 ± 0.7 mV in the presence of bumetanide. The activation and inactivation time constants at −40 mV were 3.70 ± 0.22 ms and 11.70 ± 0.60 ms under control conditions, respectively. The time constant of activation was 3.50 ± 0.23 ms (P = 0.45 vs. control), and inactivation was 12.20 ± 0.74 ms (P = 0.25 vs. control) in the presence of bumetanide (n = 5). These values were not significantly different before and after bumetanide, and the control time constants were similar to those reported previously for this conductance (42). Summarized data show no significant effect on the current-voltage relationship before and after bumetanide (Fig. 7C).
Fig. 7.
Effects of bumetanide on Cav3.2 and L-type Ca2+ currents. A: representative traces showing CaV3.2 currents in HEK293 cells. Currents were evoked by stepping potential from −80 mV to +60 mV in 10-mV increments using external CaPSS (Solution I) and a Ca+-rich solution (Solution IV; Table 2) as the pipette solution. B: current-voltage protocols were repeated 10 min after application of bumetanide (50 μM). Inset: superimposed current traces at −40 mV in control (black line) and after bumetanide (red line). C: I-V relationships for CaV3.2 currents from 5 cells evoked by stepping to different potentials before and in the presence of bumetanide. D: representative traces showing L-type Ca2+ currents of colonic SMCs using amphotericin-perforated whole-cell configuration. Currents were evoked by stepping potential from −80 mV to +60 mV in 10-mV increments using external CaPSS (Solution I) and a Ca+-rich solution (Solution IV; Table 2) as the pipette solution. E: current-voltage protocols were repeated 10 min after application of bumetanide (50 μM). Inset: superimposed current traces at −10 mV in control (black line) and after bumetanide (red line). F: I-V relationships for L-type Ca2+ currents from 4 cells evoked by stepping to different potentials before and in the presence of bumetanide.
The effects of bumetanide were also tested on L-type Ca2+ currents, using colonic SMC and the amphotericin-permeabilized whole-cell configuration. Pipette solutions were Ca+ rich (Solution IV) for these studies. Voltage-dependent inward current, previously shown to be attributable to dihydropyridine-sensitive, L-type Ca2+ channels in these cells (21), was evoked by step depolarizations from −80 to +60 mV (Fig. 7D). Bumetanide (50 μM) had no significant effect on the L-type Ca2+ currents (Fig. 7E). Peak amplitude of L-type Ca2+ currents was evoked at −10 mV. Current densities at this potential were not significantly different before (11.2 ± 1.7 pA/pF) and in the presence of bumetanide (10.2 ± 1.5 pA/pF) at 0 mV (n = 4; P = 0.06). The half-activation voltage was −19.3 ± 0.4 mV in control and −19.5 ± 0.6 mV in the presence of bumetanide. The activation time constants at −10 mV were 1.3 ± 0.1 ms and 1.4 ± 0.1 ms in the presence of bumetanide (P = 0.54). Inactivation time constants were calculated from double exponential fit of the current traces. The fast time constant (τf) averaged 13.5 ± 1.3 ms in control and 12.4 ± 1.2 ms after bumetanide (P = 0.29), and the slower time constant (τs) was 260.0 ± 11.8 ms in control and 245.0 ± 31.0 ms in the presence of bumetanide (P = 0.51). Average current-voltage curves show no significant effects of bumetanide over the range of voltages tested (Fig. 7F).
DISCUSSION
In the present study, we confirmed the expression of Slc12a2 transcripts and NKCC1 protein in ICC of the murine small intestine, as suggested previously by comparing expression in intestinal muscles of wild-type and WLacZ/WV and Sl/Sld mice (44). There are two paralogs of Slc12a (Slc12a2 and Slc12a1) that encode NKCC1 and NKCC2, respectively. Slc12a2 is expressed ubiquitously and is involved in volume regulation in many cells and Cl− secretion in epithelial cells (26, 45). Slc12a1 is expressed predominantly in the thick ascending limb of Henle (13). Consistent with these expression patterns, we found that Slc12a2 is the dominant paralog expressed in ICC, and it is far more highly expressed in ICC than in other cells of the tunica muscularis in the small intestine. Immunohistochemistry confirmed that gene expression was matched by protein expression in ICC-MY, and we did not resolve NKCC1-LI in cells other than ICC-MY in the tunica muscularis. These data are consistent with a previous screen of gene expression in ICC-MY and ICC-DMP of the murine small intestine in which Slc12a2 was found to be highly expressed in ICC-MY [i.e., nearly 16-fold higher than in whole muscle (4)].
Although not an exhaustive study of all genes encoding Cl− and bicarbonate (HCO3−) transporters, our demonstration of Slc4a2 and Slc4a4 expression in ICC may provide additional insights into Cl− recovery and homeostasis of transmembrane ion gradients. Slc4a2 encodes an electroneutral Cl−/HCO3− anion exchanger typically involved in regulation of cell pH (3). ICC have been characterized routinely as having an abundance of mitochondria (11, 31, 32, 38), and the presence and activity of these organelles may be necessary for pacemaker activity and active maintenance of ionic gradients. A major byproduct of mitochondrial respiration and production of ATP is CO2, which causes acidification via the reaction: CO2 + H2O ⇄ H+ + HCO3−. Acidification has inhibitory effects on electrical rhythmicity in intact GI muscle strips (5). Through the coupled actions of Na+/H+ exchange [Slc9a1 is expressed in ICC (4)], Na+ and Cl− influx can occur in exchange for removal of H+ and HCO3− (3). Another mechanism of Cl− influx might occur through the expression of Slc4a4. This is an electrogenic Na+-HCO3− symporter that can result in the influx of Na+ and HCO3− using the electrochemical gradient for Na+ (6). Transporting HCO3− into cells promotes Cl− uptake by subsequent actions of Cl−/HCO3− exchange. Thus the proteins encoded by both Slc4a2 and Slc4a4, found to be relatively elevated in ICC, might result in enhancing Na+ and Cl− influx into ICC and could possibly aid in the restoration of the Cl− gradient and the modified slow waves that occur in animals lacking Slc12a2 expression (44). However, under the conditions of our experiments and in the presence of HEPES buffering, mechanisms requiring HCO3− would have been excluded, and the bumetanide-sensitive exchange mechanism was important for maintaining ESTICs in ICC. It should be noted that, although parallel pathways for Cl− recovery may exist in vivo and in tissues in vitro perfused with physiological buffers, a study performed under these conditions showed that genetic deactivation of Slc12a2 had significant effects on slow waves (44). Thus, if other transporters contribute to maintenance of [Cl−]i, they are not capable of compensating for the loss of NKCC1. Another class of Cl−/HCO3− exchangers, encoded by the Slc26a family of genes, showed no appreciable expression in ICC of the small intestine in a previous gene array study (4), and these genes were not further characterized in the present study.
Reversal of STICs has been shown to follow equilibrium potential for Cl−, and STICs are inhibited by ANO1 blockers (48). Thus the reversal potential for STICs is likely to approximate ECl. Previous reports, based on measurements of the activity of [Cl−]i (a[Cl− ]i) with ion-selective electrodes, set ECl of SMCs at ∼−25 mV (1), and some investigators have assumed similar levels for ECl in ICC (14). Such a negative value for ECl in ICC would be problematic to the idea that current carried by ANO1 channels is responsible for slow waves because intracellular recordings from ICC have shown the cells to depolarize to about −10 mV during slow waves (19, 20). The present study, using gramicidin-perforated patch recording to leave a[Cl−]i undisturbed (8), suggests a level for ECl in ICC of ∼−9 mV based on the interpolation of the reversal potential of STICs. Gramicidin-perforated patch recording was also used to measure the shift in ESTICs when NKCC1 was inhibited or when extracellular Cl− was reduced.
We hypothesized that loss of Cl− during pacemaker activity would need to be restored by a transporter that is capable of concentrating Cl− to maintain ECl at a level that can support inward current carried by Cl−. NKCC transporters are electroneutral and move Na+ and K+ in concert with 2 Cl− ions (26, 33). Thus the stoichiometry of NKCC is 1 Na:1 K:2 Cl. The transporter uses energy stored in the Na+ gradient to concentrate Cl− into ICC, creating an ECl that is far less negative than the resting membrane potentials [i.e., ∼−60 mV; (17)]. This creates an ∼50-mV driving force for Cl− efflux when ANO1 is activated during STICs and slow-wave currents. NKCC1 is selectively and reversibly blocked by loop diuretics, such as bumetanide (33). In the present study, after ICC were treated with bumetanide, ESTICs rapidly shifted toward more negative levels, and STICs and slow-wave currents were reduced in amplitude at normal physiological membrane potentials (i.e., −60 mV).
Results from the present study help to explain observations from ion replacement studies on intact GI muscles. Studies in which extracellular Cl− was replaced with less permeant (isethionate) or more permeant (iodide) anions resulted in reduction and/or inhibition of slow waves (10, 14). On the surface, this observation would appear contradictory to the role of a Cl− conductance (ANO1) in generating slow-wave currents because reducing [Cl−]o should increase the gradient for Cl− efflux. As we demonstrated, reducing [Cl−]o to 10 mM caused a substantial positive shift in ECl. This issue was recognized in the study of El-Sharkawy and Daniel (10), and indeed the initial effect they observed in response to reduced [Cl−]o was an increase in slow-wave amplitude. Without cycle-to-cycle restoration of [Cl−]i, however, the Cl− gradient runs down, shifting ECl to more negative levels. Such a shift in ECl decreases the driving force for Cl− current. The permeability of anions through Cl− channels (e.g., iodide) was found to be unimportant in the responses to reduced extracellular Cl− (14). Our data explain this result; if I− could be concentrated into cells in place of Cl−, it would carry charge through ANO1 channels, but I− is a poor substitute for Cl− in NKCC1 (27). Thus substitution of [Cl−]o with [I−]o would have similar consequences as the addition of bumetanide in terms of reducing Cl− recovery into ICC.
Despite the important physiological role played by NKCC1 in many cells (26) and in the pacemaker activity of ICC, global knockouts of Slc12a2 survive. A previous report in which the electrical activity was recorded from small intestinal muscles of Slc12a2−/− mice showed that slow waves were reduced in amplitude and frequency and were insensitive to bumetanide treatment (44). These data seem to contrast with the rapid shift in ECl and cessation of spontaneous activity that we noted after treatment of isolated ICC with bumetanide. It is possible that some form of compensation occurs in global knockouts and/or that another Cl− transporter is capable of providing maintenance of ECl in the absence of NKCC1.
In summary, our experiments have demonstrated the dynamic nature of ESTICs (which is likely to be nearly equivalent to ECl) in ICC. The large Cl− currents generated during slow waves through activation of ANO1 channels result in loss of Cl− from the microdomains of pacemaker units. Loss of Cl− must be compensated for to sustain pacemaker activity, and NKCC1 appears to have a significant role in maintenance of ECl. Blocking NKCC1 caused a negative shift in ESTICs, collapse of the driving force for efflux of Cl− through ANO1 channels, and inhibition of pacemaker activity.
GRANTS
This work was supported by a Program Project Grant from NIDDK, P01 DK41325.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.H.Z., T.S.S., S.D.K., and K.M.S. conception and design of research; M.H.Z., T.S.S., M.K., L.E.O., and K.O. performed experiments; M.H.Z., T.S.S., M.K., L.E.O., K.O., and S.D.K. analyzed data; M.H.Z., T.S.S., M.K., L.E.O., K.O., S.D.K., and K.M.S. interpreted results of experiments; M.H.Z., T.S.S., M.K., L.E.O., S.D.K., and K.M.S. prepared figures; M.H.Z., S.D.K., and K.M.S. drafted manuscript; M.H.Z., S.D.K., and K.M.S. edited and revised manuscript; M.H.Z., T.S.S., L.E.O., K.O., S.D.K., and K.M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Yasuko Nakano for performing immunohistochemistry for NKCC1.
REFERENCES
- 1.Aickin CC, Brading AF. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. J Physiol 326: 139–154, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayguinov O, Ward SM, Kenyon JL, Sanders KM. Voltage-gated Ca2+ currents are necessary for slow-wave propagation in the canine gastric antrum. Am J Physiol Cell Physiol 293: C1645–C1659, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bonar PT, Casey JR. Plasma membrane Cl(−)/HCO(3)(−) exchangers: Structure, mechanism and physiology. Channels 2: 337–345, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Ordog T, Chen J, Young DL, Bardsley MR, Redelman D, Ward SM, Sanders KM. Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics 31: 492–509, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Cho SY, Beckett EA, Baker SA, Han I, Park KJ, Monaghan K, Ward SM, Sanders KM, Koh SD. A pH-sensitive potassium conductance (TASK) and its function in the murine gastrointestinal tract. J Physiol 565: 243–259, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordat E, Casey JR. Bicarbonate transport in cell physiology and disease. Biochem J 417: 423–439, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol 550: 829–844, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebihara S, Shirato K, Harata N, Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J Physiol 484: 77–86, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards FR, Hirst GD, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. J Physiol 519: 235–250, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Sharkaway TY, Daniel EE. Ionic mechanisms of intestinal electrical control activity. Am J Physiol 229: 1287–1298, 1975. [DOI] [PubMed] [Google Scholar]
- 11.Faussone Pellegrini MS, Cortesini C, Romagnoli P. [Ultrastructure of the tunica muscularis of the cardial portion of the human esophagus and stomach, with special reference to the so-called Cajal's interstitial cells]. Ital J Anat Embryol 82: 157–177, 1977. [PubMed] [Google Scholar]
- 12.Gomora JC, Murbartian J, Arias JM, Lee JH, Perez-Reyes E. Cloning and expression of the human T-type channel Ca(v)3.3: Insights into prepulse facilitation. Biophys J 83: 229–241, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greger R, Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflügers Arch 392: 92–94, 1981. [DOI] [PubMed] [Google Scholar]
- 14.Hirst GD, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig gastric antrum involves a delayed increase in [Ca(2+)](i) and Cl(−) channels. J Physiol 540: 907–919, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirst GD, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach–a stochastic process. J Physiol 535: 165–180, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373: 347–349, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587: 4887–4904, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kito Y, Mitsui R, Ward SM, Sanders KM. Characterization of slow waves generated by myenteric interstitial cells of Cajal of the rabbit small intestine. Am J Physiol Gastrointest Liver Physiol 308: G378–G388, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol 553: 803–818, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Physiol Cell Physiol 288: C710–C720, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Koh SD, Sanders KM. Modulation of Ca2+ current in canine colonic myocytes by cyclic nucleotide-dependent mechanisms. Am J Physiol Cell Physiol 271: C794–C803, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci USA 86: 7280–7284, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology 132: 1852–1865, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterology 133: 907–917, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HT, Hennig GW, Park KJ, Bayguinov PO, Ward SM, Sanders KM, Smith TK. Heterogeneities in ICC Ca2+ activity within canine large intestine. Gastroenterology 136: 2226–2236, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markadieu N, Delpire E. Physiology and pathophysiology of SLC12A1/2 transporters. Pflügers Arch 466: 91–105, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owen NE, Prastein ML. Na/K/Cl cotransport in cultured human fibroblasts. J Biol Chem 260: 1445–1451, 1985. [PubMed] [Google Scholar]
- 28.Park KJ, Baker SA, Cho SY, Sanders KM, Koh SD. Sulfur-containing amino acids block stretch-dependent K+ channels and nitrergic responses in the murine colon. Br J Pharmacol 144: 1126–1137, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peri LE, Sanders KM, Mutafova-Yambolieva VN. Differential expression of genes related to purinergic signaling in smooth muscle cells, PDGFRalpha-positive cells, and interstitial cells of Cajal in the murine colon. Neurogastroenterol Motil 25: e609–e620, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ro S, Park C, Jin J, Zheng H, Blair PJ, Redelman D, Ward SM, Yan W, Sanders KM. A model to study the phenotypic changes of interstitial cells of Cajal in gastrointestinal diseases. Gastroenterology 138: 1068–1078 e1061–e1062, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rumessen JJ, Mikkelsen HB, Thuneberg L. Ultrastructure of interstitial cells of Cajal associated with deep muscular plexus of human small intestine. Gastroenterology 102: 56–68, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Rumessen JJ, Thuneberg L. Interstitial cells of Cajal in human small intestine. Ultrastructural identification and organization between the main smooth muscle layers. Gastroenterology 100: 1417–1431, 1991. [PubMed] [Google Scholar]
- 33.Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev 80: 211–276, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 111: 492–515, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Sanders KM, Ward SM, Koh SD. Interstitial cells: Regulators of smooth muscle function. Physiol Rev 94: 859–907, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh RD, Gibbons SJ, Saravanaperumal SA, Du P, Hennig GW, Eisenman ST, Mazzone A, Hayashi Y, Cao C, Stoltz GJ, Ordog T, Rock JR, Harfe BD, Szurszewski JH, Farrugia G. Ano1, a Ca2+-activated Cl− channel, coordinates contractility in mouse intestine by Ca2+ transient coordination between interstitial cells of Cajal. J Physiol 592: 4051–4068, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol 525: 105–111, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torihashi S, Kobayashi S, Gerthoffer WT, Sanders KM. Interstitial cells in deep muscular plexus of canine small intestine may be specialized smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 265: G638–G645, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res 280: 97–111, 1995. [DOI] [PubMed] [Google Scholar]
- 40.van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol 524: 245–265, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Helden DF, Laver DR, Holdsworth J, Imtiaz MS. Generation and propagation of gastric slow waves. Clin Exp Pharmcol Physiol 37: 516–524, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Vitko I, Chen Y, Arias JM, Shen Y, Wu XR, Perez-Reyes E. Functional characterization and neuronal modeling of the effects of childhood absence epilepsy variants of CACNA1H, a T-type calcium channel. J Neurosci 25: 4844–4855, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 480: 91–97, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wouters M, De Laet A, Donck LV, Delpire E, van Bogaert PP, Timmermans JP, de Kerchove d'Exaerde A, Smans K, Vanderwinden JM. Subtractive hybridization unravels a role for the ion cotransporter NKCC1 in the murine intestinal pacemaker. Am J Physiol Gastrointest Liver Physiol 290: G1219–G1227, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Yang T, Huang YG, Singh I, Schnermann J, Briggs JP. Localization of bumetanide- and thiazide-sensitive Na-K-Cl cotransporters along the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 271: F931–F939, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Zheng H, Park KS, Koh SD, Sanders KM. Expression and function of a T-type Ca2+ conductance in interstitial cells of Cajal of the murine small intestine. Am J Physiol Cell Physiol 306: C705–C713, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca(2+)-activated Cl(−) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol 587: 4905–4918, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu MH, Sung IK, Zheng H, Sung TS, Britton FC, O'Driscoll K, Koh SD, Sanders KM. Muscarinic activation of Ca2+-activated Cl− current in interstitial cells of Cajal. J Physiol 589: 4565–4582, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu MH, Sung TS, O'Driscoll K, Koh SD, Sanders KM. Intracellular Ca2+ release from endoplasmic reticulum regulates slow wave currents and pacemaker activity of interstitial cells of Cajal. Am J Physiol Cell Physiol 308: C608–C620, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]