Abstract
NADPH oxidase (Nox)-derived oxyradicals contribute to atherosclerosis by oxidizing low-density lipoproteins (LDL), leading to their phagocytosis by vascular macrophages. Endocannabinoids, such as 2-arachidonoylglycerol (2-AG), might be an important link between oxidative stress and atherosclerosis. We hypothesized that 2-AG biosynthesis in macrophages is enhanced following ligation of oxidized LDL by scavenger receptors via a signal transduction pathway involving Nox-derived ROS that activates diacylglycerol lipase-β (DAGL-β), the 2-AG biosynthetic enzyme. To test this idea, we challenged macrophage cell lines and murine primary macrophages with a xanthine oxidase system or with nonphysiological and physiological Nox stimulants [phorbol 12-myristate 13-acetate (PMA) and arachidonic acid (AA)]. Each stressor increased cellular superoxide levels and enhanced 2-AG biosynthetic activity in a Nox-dependent manner. Levels of cytosolic phospholipase A2-dependent AA metabolites (eicosanoids) in primary macrophages were also dependent on Nox-mediated ROS. In addition, 2-AG levels in DAGL-β-overexpressing COS7 cells were attenuated by inhibitors of Nox and DAGL-β. Furthermore, ROS induced by menadione (a redox cycling agent) or PMA could be partially attenuated by the cannabinoid 1/2 receptor agonist (WIN 55,212-2). Finally, cells that overexpress Nox2 components (Phox-COS7) synthesized larger amounts of 2-AG compared with the parental COS7 cells. Together, the results suggest a positive correlation between heightened oxygen radical flux and 2-AG biosynthesis in macrophage cell lines and primary macrophages. Because of the antioxidant and anti-inflammatory effects associated with 2-AG, the increased levels of this bioactive lipid might be an adaptive response to oxidative stress. Thus oxyradical stress may be counteracted by the enhanced endocannabinoid tone.
Keywords: 2-arachidonoylglycerol, macrophages, NADPH oxidase, atherosclerosis, endocannabinoids
development of cardiovascular disease (CVD) is caused by several environmental, genetic, and lifestyle factors (27). Atherosclerosis is a primary cause of CVD and is due to altered homeostatic processes that regulate lipid metabolism and inflammation (48). Initiating mechanisms that contribute to the development of atherosclerosis include endothelial dysfunction, hypertension, hyperlipidemia, and inflammation (48). These factors are typically interrelated. The resulting pathology leads to the release of inflammatory cytokines and chemokines that recruit immune cells to the site of injury, leading to the overproduction of reactive oxygen species (ROS) that chemically modify entrapped low-density lipoproteins (LDL) yielding toxic oxidized LDL (oxLDL). Danger-associated molecular patterns revealed on oxLDL are recognized by scavenger receptors on intimal macrophages in the vessel wall, enabling phagocytosis of these cholesterol-enriched particles and development of macrophage foam cells (36). Thus an imbalance in ROS biosynthesis and detoxication by vascular wall cells, including the immune cells that are recruited there, results in significant oxidative stress. This is an important contributing factor in disease etiology.
ROS, such as superoxide and hydrogen peroxide (H2O2), are produced in most cell types within the vessel wall in response to external stimuli, including oxLDL, lipid mediators, shear stress, and angiotensin II. These stimuli can activate several ROS-generating enzymes, including NADPH oxidase (Nox), cyclooxygenase, lipoxygenase, xanthine oxidase, (uncoupled) nitric oxide synthase, and myeloperoxidase, resulting in significant oxidative stress (8, 14, 48). The elevated ROS also leads to a buildup of oxLDL levels in the vessel wall and the subsequent influx of monocytes and neutrophils from the bloodstream, resulting in an inflammatory feedback loop. The increased flux of oxyradicals and peroxidation of extracellular LDL are hallmarks of atherosclerosis.
OxLDL can stimulate CD36 scavenger receptor-evoked signal transduction pathways in macrophages and platelets, leading to the activation of Nox and the synthesis of superoxide (31, 35, 43, 46). Nox is a multisubunit holoenzyme formed at lipid membranes that catalyzes the transfer of electrons from NADPH to molecular oxygen (23). This results in the production of superoxide anion, which has a limited diffusion radius in the cell but can cross lipid membranes via anion channels. Superoxide also rapidly dismutates to H2O2, which has a more tempered reactivity and larger diffusion radius compared with that of superoxide. Because H2O2 is not charged, it is also capable of moving through lipid membranes via passive diffusion. Overactive Nox activity is countered by several compensatory mechanisms, including the detoxification of excess superoxide by superoxide dismutase (48), the activation of endoplasmic reticulum stress-induced apoptotic pathways (13), and, possibly, by the endocannabinoid (eCB) system (41).
The eCB system is composed of several components. These include two G protein-coupled receptors, CB1 and CB2, which are mainly expressed in the central nervous system and hematopoietic system, respectively. The CB receptors are activated by endogenous arachidonoyl-containing ligands, the best studied being 2-arachidonoylglycerol (2-AG) and anandamide (AEA). Diacylglycerol lipase (DAGL)-α and -β are the rate-limiting enzymes responsible for the biosynthesis of 2-AG in the brain and macrophages, respectively (18), while monoacylglycerol lipase (MAGL) is the primary enzyme that degrades 2-AG (7). Other candidate enzymes that hydrolyze or oxidize 2-AG have also been identified, contributing to 2-AG catabolism (50).
The CB1 receptor has important roles in neurotransmission in the central nervous system, whereas the CB2 receptor promotes immunomodulatory functions in peripheral immune responses. During cell/tissue injury, rapid increases in eCB levels and their cognate receptors are observed, which might modulate critical context-dependent functions (41). For example, CB2 activation attenuates the following immune and biochemical processes in macrophages: inflammatory cell migration, endotoxin-induced oxidative stress, Nox activity, p38-MAPK activation, and TNF-α production (16, 17, 42, 46). Furthermore, increased biosynthesis of 2-AG and eCB-evoked CB2 receptor-dependent signal transduction pathways are associated with the amelioration of atherosclerotic lesions in a mouse model (60). Cultured macrophages exposed to oxLDL also produce greater amounts of 2-AG and AEA compared with untreated macrophages (21). Thus it seems clear that the eCB system plays an important role in immune cell function. However, a gap in knowledge exists regarding the specific signaling pathways that are evoked by oxLDL binding to scavenger receptors and the resulting increases in eCB levels.
Because it is already known that exposure to oxLDL results in increased ROS production by macrophages, the purpose of this study was to explore whether (and how) the eCB system becomes activated in macrophages in response to acutely elevated superoxide levels (and heightened Nox activity). Specifically, the levels of 2-AG and AEA were determined following treatments of cultured macrophage cell lines and primary murine macrophages with an extracellular superoxide generating system (xanthine oxidase) or with Nox stimulators [phorbol 12-myristate 13-acetate (PMA) and arachidonic acid (AA)]. Although PMA is a nonphysiological research tool, esterified AA is found in high concentrations in LDL particles, and nonesterified (free) AA can be released by lipases during enzymatic modifications of LDL (34). Thus AA has an important physiological role in inflammation and foam cell development (9, 25). A large body of evidence also suggests that activation of the CB2 receptor renders cardioprotective effects (16, 17, 42, 46). Therefore, localized increased 2-AG levels may be part of a compensatory/adaptive mechanism to activate anti-inflammatory and anti-oxidative pathways within vascular cells. We addressed this issue by inducing oxidative stress in cells by using a redox cycling agent [menadione (MD)] or by activating Nox activity in the absence or presence of the nonselective cannabinoid receptor agonist (WIN 55,212-2). Our results indicate that oxyradical stress increased the biosynthesis rate of 2-AG in human and mouse macrophage cell lines, cell lines engineered to overexpress either DAGL-β or Nox2, and in primary murine macrophages. It is suggested that the increased 2-AG biosynthesis is part of an adaptive response of cells to attenuate oxidative stress.
MATERIALS AND METHODS
Chemicals and Reagents
Authentic 2-AG, 2-AG-d8, AA, 1-stearoyl-2-arachidonoylglycerol (SAG), WIN 55,212-2, AM630, and rimonabant were from Cayman Chemicals (Ann Arbor, MI). O,O′-diethyl p-nitrophenyl phosphate (paraoxon, PO) was a kind gift from Dr. Howard Chambers (Mississippi State University). Avidin-horseradish peroxidase, dimethyl sulfoxide (DMSO), lactate dehydrogenase (LDH), xanthine and xanthine oxidase, trypan blue solution (0.4% wt/vol), β-mercaptoethanol, PMA, fatty-acid free bovine serum albumin, penicillin, streptomycin, gentamicin, MD, hydroethidine (HE), ionomycin, apocynin, U-73122, orlistat (tetrahydrolipstatin), and all buffer components were purchased from Sigma (St. Louis, MO). VAS-2870 (VAS), a selective Nox inhibitor, was purchased from Enzo Life Sciences (Farmingdale, NY). Opti-MEM medium, bicinchoninic acid (BCA) reagent, and HPLC grade solvents were purchased from Thermo-Fisher. FuGene 6 Transfection Reagent was purchased from Promega Chemicals (Madison, WI). Plasmid Midi Kits were purchased from QIAGEN. Human DAGL-β plasmid was purchased from Origene (Bethesda, MD). Anti-human DAGL-β (ab103100) antibody was from Abcam (Cambridge, MA). Goat anti-rabbit IgG and goat anti-mouse IgG antibodies were from Cayman Chemicals. Acetylated LDL (acLDL) and oxLDL were from Intracel (Bethesda, MD).
Cells and Culture Conditions
Human THP-1 monocytes, murine J774A.1 macrophages, human HL-60 cells, COS7 cells, RPMI-1640 medium with and without phenol red (containing 2 mM l-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 4500 mg/l glucose, and 1,500 mg/l sodium bicarbonate), Dulbecco's modified Eagle's medium (DMEM) with and without phenol red (containing 4 mM l-glutamine, 4,500 mg/l glucose, 1 mM sodium pyruvate, and 1,500 mg/l sodium bicarbonate), Opti-MEM medium, and gentamicin sulfate solution (50 mg/ml) were purchased from the American Type Culture Collection (Manassas, VA). Phox-COS7 cells that overexpress Nox2 and its regulatory subunits (45) were kindly provided by Dr. Mary Dinauer (Washington University, St. Louis, MO). Fetal bovine serum (FBS) and One-Shot TOP10 chemically competent E. coli were purchased from Invitrogen (Grand Island, NY).
THP-1 monocytes were passaged in RPMI-1640 containing 10% vol/vol FBS and 50 μg/ml gentamicin (complete medium) (62); J774A.1 macrophages and COS7 cells were passaged in DMEM medium containing 10% vol/vol FBS and 10 U/ml penicillin and 10 μg/ml streptomycin (complete medium) (62); HL-60 cells were passaged in RPMI-1640 containing 10% vol/vol FBS and penicillin and streptomycin. All cells were cultured at 37°C in an atmosphere of 95% air/5% CO2. THP-1 cells and HL-60 cells were differentiated into macrophage-like cells by adding PMA to the culture medium (final concentration 100 nM), and the cells were cultured for 72 h.
Resident peritoneal macrophages (RPMs) were isolated from 10- to 12-wk-old female C57BL/6 mice in cold PBS containing 3% vol/vol FBS. Following centrifugation, cells were washed with PBS, counted, and plated in individual 60-mm dishes at a density of 5 × 106 cells per dish in complete DMEM medium. After overnight incubation at 37°C in 5% CO2, the culture medium was removed, and the adherent cells were washed twice with PBS before experimental manipulations. Animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 8th edition, 2011). All experimental procedures were approved in advance by the Institutional Animal Care and Use Committee of Mississippi State University (protocol no. 15-090).
Stable Expression of Human DAGL-β in COS7 Cells
A human DAGL-β expression plasmid was transformed into One-Shot Top10 chemically competent E. coli, and the cloned plasmid was purified using QIAGEN Plasmid Midi Kit following the manufacturer's instructions. COS7 cells were transfected with either human DAGL-β cDNA or empty plasmid (control) using the FuGene 6 Transfection Reagent in Opti-MEM medium, and the transfected cells incubated overnight. The transfection medium was removed and replaced with fresh (complete) DMEM medium. After an additional 48-h incubation, the medium was replaced again with (complete) DMEM medium supplemented with G418 (500 μg/ml), and the cells were maintained in this medium for 3 wk. The culture medium was replaced every 3 days with fresh medium containing G418 to select for positive clones. After 3 wk, total RNA was isolated from the DAGL-β-transfected and mock-transfected cells, and the level of DAGL-β mRNA was determined by quantitative real-time PCR. In addition, the transformed cells were washed with PBS and harvested in 50 mM Tris·HCl (pH 7.4) buffer and sonicated with three short bursts on ice. The SAG hydrolysis activity of the cell lysates was determined (see below), and human DAGL-β protein was detected by immunoblot.
Preparation of Cell Lysates
THP-1 monocytes were collected by centrifugation (1,000 g for 5 min), washed with cold phosphate-buffered saline (PBS), resuspended in ice-cold 50 mM Tris·HCl (pH 7.4) buffer, and lysed by sonication (three 15-s bursts on ice at 30% maximum power). THP-1 and J774A.1 macrophages (∼80–90% confluent) were washed with cold PBS, scraped into ice-cold 50 mM Tris·HCl (pH 7.4) buffer, and sonicated. COS7 cells transfected with DAGL-β and control COS7 cells were harvested with Accutase (2 ml, 5 min). Fresh DMEM containing 10% vol/vol FBS was added to stop the Accutase reaction, and the detached cells were pelleted at 1,000 g (5 min), washed three times with sterile PBS, resuspended in ice-cold 50 mM Tris·HCl (pH 7.4) buffer and sonicated. Protein concentrations of the cell lysates were determined using the BCA reagent, according to the manufacturer's instructions (Thermo-Fisher). Cell lysates were used fresh or flash frozen in liquid nitrogen and stored at −80°C before use.
Quantitative Real-Time PCR Analysis and Western Blot
Total RNA was isolated from HL-60 cells that had been treated with PMA, all-trans retinoic acid, or oxLDL using the Rneasy Plus Mini Kit (Qiagen) according to the manufacturer's protocol. Recovered RNA was quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA) and cDNA was synthesized with an iScript Select cDNA Synthesis Kit (BioRad) using oligo(dT) primers, according to the manufacturer's protocol. Real-time PCR of cDNA products was performed on a Stratagene Mx3005P thermal cycler with Quantifast SYBR Green PCR master mix (Qiagen) using primers specific for DAGLβ, p47phox (NCF1), Nox2 (CYBB), and GAPDH. Differential expression of target genes was assessed by the ΔΔCt method using GAPDH as the reference gene.
Total proteins were isolated from vehicle and AA-treated cells, and phopho-p47phox and total p47phox protein levels were detected by the approach described in Ref. 33.
2-AG Biosynthesis by THP-1 Macrophages
THP-1 macrophage lysates (0.5 mg/ml protein concentration) were treated with CaCl2 (10 μM final concentration) in 200 μl of 50 mM Tris·HCl (pH 7.4) in the absence or presence of 1 mM ethylene glycol tetraacetic acid (EGTA), 10 μM U-73122, or 10 μM orlistat. Incubations went for 5 min at 37°C with shaking (550 rpm). The reactions were quenched by the addition of 300 μl of ethyl acetate (containing 0.1% acetic acid) doped with 2-AG-d8 (internal standard). After centrifugation of samples (1,500 g, 5 min, 4°C), the organic layer was collected in a clean tube and dried under N2. Residues were reconstituted in 100 μl methanol-water (1:1 vol/vol), and the levels of 2-AG were quantified by liquid chromatography tandem mass spectrometry (LC-MS/MS).
SAG Hydrolysis Assay
Cell lysate protein was diluted to 0.3 mg/ml lysate in DAGL buffer (5 mM CaCl2, 100 mM NaCl, 50 mM HEPES buffer, pH 7.4) (final volume 70 μl). SAG (20 μM final concentration) was added, and the reaction duration was 30 min (37°C). Reactions were stopped by adding ethyl acetate (containing 0.1% acetic acid) doped with 2-AG-d8. After drying the organic extracts, the residues were reconstituted in methanol-water (1:1 vol/vol) for LC-MS/MS analysis.
2-AG Biosynthesis by Human DAGL-β-Transfected COS7 Cells
To determine the amount of 2-AG produced by DAGL-β-transfected COS7 cells compared with parental COS7 cells, the two types of cells were seeded in two six-well plates and cultured until they reached 80% confluency. The cells were then incubated with ionomycin (3 μM, 30 min) in serum-free medium. The culture medium was collected and extracted with ethyl acetate doped with 2-AG-d8, and the organic extract was dried under nitrogen and reconstituted in methanol-water (1:1 vol/vol) for LC-MS/MS analysis. In a separate experiment, cells were pretreated with 10 μM orlistat (DAGL-β inhibitor) for 30 min, followed by treatment with 3 μM ionomycin for an additional 30 min. The culture medium was extracted to quantify 2-AG levels, and cell lysates used for the SAG hydrolysis assay.
To determine the role of Nox in the DAGL-β-transfected COS7 cells, the cells were pretreated with a Nox inhibitor (10 μM VAS, 30 min) followed by an additional 30 min incubation with 10 μM AA to stimulate Nox activity (33). The culture medium was collected and extracted to quantify 2-AG levels, whereas cell lysates were used for the SAG hydrolysis assay.
Treatment of Macrophages with Either Extracellular Superoxide or Nox Stimulants
LDH cytotoxicity assay.
THP-1 monocytes (2 × 105 cells/well) were differentiated into macrophages (PMA, 100 nM, 72 h) in a 96-well plate. The macrophages were treated with increasing concentrations of xanthine (0–250 μM) and a fixed amount of xanthine oxidase (0.1 mU/ml) in 200 μl of PBS for 15 min at 37°C in an atmosphere of 95% air/5% CO2. Cells were monitored for overt cytotoxicity by mixing 100 μl of the culture supernatant with two volumes of LDH reagent solution, followed by incubation at 37°C for 30 min to determine LDH activity (at 490 nm). Alternatively, cell lysates were prepared in 50 mM Tris·HCl (pH 7.4) buffer, and the intracellular LDH activity was determined (at 690 nm).
Quantitation of ROS in macrophages.
J774A.1 macrophages, THP-1 macrophages, or mouse RPMs in phenol red-free and serum-free culture medium (1 × 105 cells/well in a black-walled clear bottom 96-well plate) were treated with either PMA (0.32 μM) or DMSO for 30 min, followed by addition of the oxyradical probe HE (20 μM final concentration). The fluorescence signal was monitored for up to 90 min in a Molecular Devices SpectraMax M5 plate reader (excitation: 485 nm; emmission: 595 nm). Fluorescence data were normalized against cell protein content to control for variations in cell number in each well.
Nox activity of macrophage cell lysates was determined by incubating lysate protein (0.3–0.5 mg/ml) with lucigenin (5 μM final concentration) and NADPH (100 μM final concentration) in 50 mM Tris·HCl (pH 7.4) buffer containing 1 mM EGTA. The resulting luminescence was monitored for 15 min in a Molecular Devices SpectraMax M5 plate reader. Generation of superoxide by a xanthine oxidase system was also determined using the lucigenin probe.
The superoxide-specific product 2-hydroxyethidium (2-OH-Et+) (65) in cells was quantified, as previously reported (33). Briefly, cells were treated with stimulant (PMA, AA, or oxLDL) in phenol red-free and FBS-free culture medium for 30 min at 37°C, followed by the addition of the HE probe (20 μM final concentration). After a 20-min incubation with the HE probe, the cells were harvested and washed with PBS. The cells were then sonicated in 300 μl methanol-water (1:1 vol/vol), and the lysates were centrifuged (16,100 g, 5 min). The supernatant was loaded into LC vials and analyzed by LC-MS/MS (33).
2-AG levels in macrophages following treatment with various stimulants.
THP-1 macrophages (in 60-mm dishes) were pretreated with 1 μM PO in serum-free medium for 30 min, to inhibit 2-AG degradation by hydrolytic enzymes, followed by treatments with either 150 μM xanthine/0.1-mU/ml xanthine oxidase (10 min) or acLDL (20 μg/ml, 24 h). When THP-1 macrophages were treated with AA (10 μM, 60 min), the cells were not pretreated with PO. Following each treatment, the culture medium was harvested, fortified with 2-AG-d8, and extracted with two volumes ethyl acetate containing 0.1% acetic acid. The organic layer was collected and dried under N2, and samples were reconstituted with 100 μl methanol-water (1:1 vol/vol). Extracts were analyzed by LC-MS/MS to quantify 2-AG levels.
J774A.1 macrophages were treated with increasing concentrations of PMA (0, 0.32, 3.2, or 32 μM, 10 min), AA (10 μM, 60 min), or acLDL (20 μg/ml, 24 h) in serum-free medium. Following each treatment, the culture medium was collected, fortified with 2-AG-d8, and extracted with ethyl acetate to quantify 2-AG levels. Because 2-AG is not degraded as rapidly in J774A.1 cells compared with THP-1 cells (64), the J774 cells were not pretreated with a serine hydrolase inhibitor (i.e., PO) before adding stimulants. To determine whether Nox has a role in 2-AG biosynthesis, macrophages were pretreated with a Nox inhibitor (10 μM apocynin, 30 min) before adding stimulants (PMA or AA).
Mouse RPMs were pretreated with VAS (10 μM, 30 min) or vehicle (DMSO) at 37°C in serum-free DMEM medium, followed by addition of AA (100 μM). After 60-min incubation at 37°C, the medium was removed, fortified with 2-AG-d8 (336 pmol), and extracted using C18 SepPak columns. The cells were harvested in 1.1-ml PBS and a 0.1-ml aliquot removed for protein determination. The remaining 1 ml of cell suspension was extracted with ethyl acetate (2 ml) and methanol (1 ml) by vortex mixing (1 min), followed by centrifugation to separate the aqueous and organic layers. The organic extracts were evaporated to dryness, and the residues redissolved in 100-μl methanol for 2-AG analysis using LC-MS/MS.
Role of cannabinoid receptors in MD-derived superoxide levels in macrophages.
Macrophages (murine J774A.1 or human THP-1) were overlaid with FBS-free and phenol red-free culture medium containing either vehicle (DMSO), CB1 receptor antagonist rimonabant (1 μM), or CB2 receptor antagonist AM630 (1 μM) for 30 min, followed by addition of the nonspecific cannabinoid receptor agonist WIN 55,212-2 (10 or 25 μM, 4 h) or vehicle (DMSO). The cells were then challenged with the redox cycling agent MD (40 μM, 30 min) in the presence of the agonists and antagonists. After 30 min, the culture medium was removed, and fresh medium containing the oxyradical probe HE (10 μM) was overlaid on the cells. After 20 min, the macrophages were washed with PBS and subsequently harvested in 300 μl of 1:1 methanol-water and sonicated, and the lysate centrifuged (16,100 g, 10 min, 4°C). The supernatant was collected for LC-MS/MS analysis of 2-OH-Et+ to quantify intracellular superoxide levels (33). A 10-μl aliquot of the supernatant was saved back to assay protein content using the BCA reagent.
In a separate experiment, freshly isolated mouse RPMs were plated for 4 h at which point the nonadherent cells were removed. Attached RPMs were washed with PBS and pretreated with either WIN 55,212-2 (10 μM) or vehicle (DMSO). After a 4-h incubation with agonist, RPMs were stimulated with PMA (3.2 μM, 30 min) with the agonist still present in medium. After 30 min, medium was removed, and fresh medium containing HE (10 μM) was placed on the cells and incubated for a further 20 min. RPMs were harvested and processed as described above.
LC-MS/MS Analysis
2-AG analysis was performed on a UPLC-MS/MS system (Waters Acquity UPLC interfaced with a Thermo Quantum Access Max triple quadrupole mass spectrometer) using the method described by Wang et al. (62). Extracts were injected (10 μl) onto an Acquity UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm) equipped with a VanGuard precolumn (2.1 mm × 5 mm, 1.7 μm). For 2-AG and 2-AG-d8, the mobile phases were either a blend of solvent A (2 mM ammonium acetate and 0.1% acetic acid in water)/solvent B (2 mM ammonium acetate and 0.1% acetic acid in methanol) or solvent A (0.1% acetic acid in water)/solvent B (0.1% acetic acid in methanol). The elution program was described previously (62), and the flow rate was 0.4 ml/min (ammonium acetate in mobile phase) or 0.2 ml/min (no ammonium acetate in mobile phase). The column eluate was directed into the mass spectrometer using heated electrospray ionization in the positive ion mode. Single-reaction monitoring of analytes were as follows: (ammonium acetate in mobile phase) 2-AG, [M+NH4]+ mass-to-charge ratio (m/z) 396 > 287; 2-AG-d8, [M+NH4]+ m/z 404 > 294; (no ammonium acetate in mobile phase) 2-AG, [M+H]+ m/z 379.2 > 287.1; 2-AG-d8, [M+H]+ m/z 387.2 > 292.3. Scan times were 0.2 s per single-reaction monitoring, and the scan width was m/z 0.01. The internal standard 2-AG-d8 was used for quantification.
LC-MS/MS conditions for analysis of 2-OH-Et+, the superoxide-specific product, is described in Ref. 33.
Statistical Analyses
Data are expressed as means ± SD. Statistical significance between two groups was determined by the Student's t-test. Statistical significance between more than two groups of data were compared by either one-way or two-way ANOVA (Student-Newman-Keuls method). Values of P ≤ 0.05 were considered to be significant.
RESULTS
Treatment of Human Macrophage-like Cells with Either oxLDL or AA Enhances Superoxide Levels
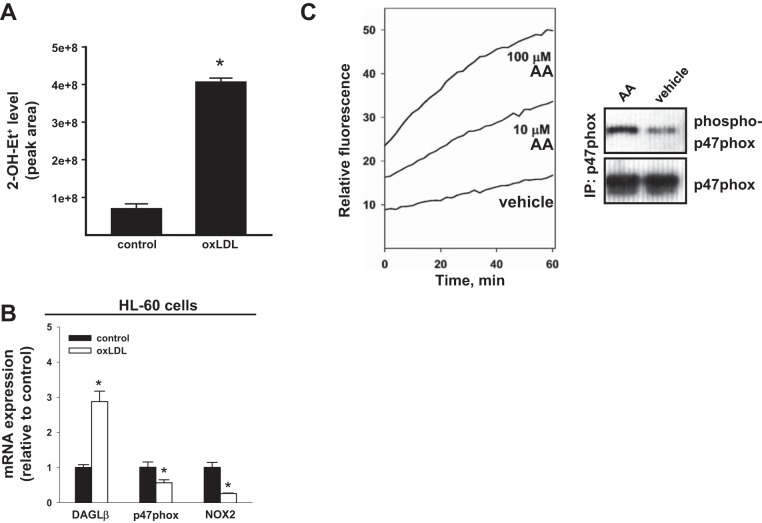
It was previously reported that modified LDLs could stimulate Nox activity and elevate ROS levels in macrophages in a CD36-dependent (24, 43, 44) or TLR4-dependent (3) manner. Differentiated HL-60 macrophage-like cells express both CD36 (28) and Nox2 (also called gp91phox) (51). We verified that PMA-primed human HL-60 macrophage-like cells, treated with oxLDL for 24 h, exhibited significantly higher superoxide levels than untreated controls (Fig. 1A). It should be noted that the previous reports (3, 24, 43, 44) had measured ROS using nonspecific fluorescent probes, such as carboxy-2′,7′-dichlorodihydrofluorescein diacetate, that can react with multiple species of ROS. In contrast, we assessed the levels of superoxide (the reactive product generated by Nox) by measuring the content of 2-OH-Et+, which is the superoxide-specific product formed by the reaction of superoxide with the oxyradical HE probe, using LC-MS/MS (33, 65). This is the first time this approach, to our knowledge, has been used to assess superoxide levels in cells that had been stimulated with oxLDL.
Fig. 1.
mRNA of DAGL-β and Nox components and Nox-derived reactive oxygen species are induced in human HL-60 macrophages by various stimulants. A: human HL-60 cells were differentiated into macrophages with PMA (100 nM, 72 h) and then treated with oxLDL (50 μg/ml, 24 h). The level of superoxide in cells was determined by adding the HE probe (20 μM, 20 min). After removal of the medium, the cells were lysed in methanol-water (1:1 vol/vol, 300 μl), and the amount of 2-OH-Et+ (surrogate measure of superoxide) was quantified by LC-MS/MS by measuring the area under the chromatographic peak. B: expression of DAGLβ, p47phox (NCF1), and Nox2 (CYBB) mRNA in HL-60 macrophages treated with oxLDL (50 μg/ml, 24 h). Values in bar graphs are means ± SD (n = 3). *P < 0.05, Student's t-test. C, left: PMA-primed HL-60 cells were treated with the indicated concentrations of arachidonic acid (AA), followed by addition of the oxyradical probe HE (20 μM). The rate of HE oxidation was monitored by fluorescence for 60 min. Right: immunoprecipitation (IP) of p47phox (33) and subsequent immunoblot analysis indicated that 10 μM AA treatment increased the level of phosphorylated p47phox compared with that following vehicle treatment.
Human leukemia cell lines, such as HL60 and THP-1, are differentiated into macrophage-like cells in the presence of PMA, resulting in the induction of Nox2 and p47phox gene expression (32, 37, 51). We verified that Nox2 and p47phox mRNA was induced in THP-1 cells when treated with PMA (∼5-fold each, data not shown). Furthermore, oxLDL treatment of HL-60 macrophage-like cells for 24 h induced the transcription of DAGLβ, an enzyme that catalyzes 2-AG biosynthesis, nearly threefold compared with that in control cells (Fig. 1B). On the other hand, p47phox and Nox2 mRNA were downregulated by oxLDL, which is consistent with repressed Nox2 expression previously reported in oxLDL-treated macrophages compared with untreated macrophages (19, 56). Furthermore, Nox activity in PMA-primed human macrophage cell lines could be stimulated by exogenous AA, as assessed by 1) the increased rate of oxidation of the HE probe (Fig. 1C, left); and 2) the increased level of p47phox phosphorylation (Fig. 1C, right) compared with that in vehicle controls. These data are consistent with the notion that oxLDL and lipid mediators, such as AA present in oxLDL or released during inflammation, can stimulate Nox activity and increase superoxide levels in macrophages (31, 54, 55).
Human THP-1 and Murine J774 Cells Have the Biosynthetic Machinery to Produce 2-AG
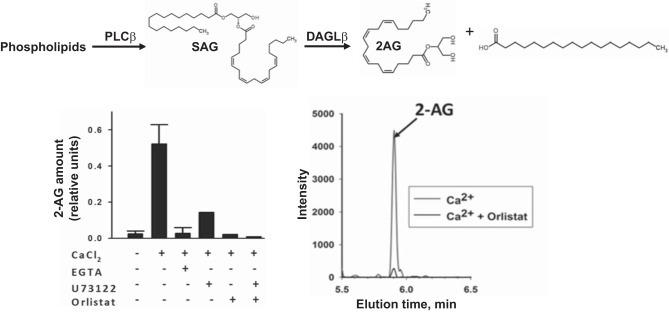
Macrophages exposed to oxLDL are also known to make the eCB 2-AG (21, 64); however, the mechanism behind this response has not been clarified. Phospholipase C (PLC)-β and DAGL-β are calcium-dependent enzymes that have important roles in signal transduction pathways and are also involved in the biosynthesis of 2-AG. PLC-β cleaves phosphatidylinositol 4,5-bisphosphate lipids releasing diacylglycerol, then DAGL-β specifically hydrolyzes the diacylglycerol 1-acyl-2-arachiondoylglycerol at the sn-1 position to provide 2-AG. To better understand the connection between oxLDL-derived ROS and 2-AG biosynthesis, we examined whether 2-AG biosynthesis is increased in human and murine macrophage models under various states of oxidative stress. Human THP-1 cells and murine J774A.1 cells were initially used because they are common macrophage models to study molecular and cellular mechanisms of atherosclerosis and inflammation. First, we verified that macrophage lysate (THP-1) could be activated by calcium ions to yield 2-AG (Fig. 2). Addition of PLC-β and DAGL-β inhibitors (U-73122 and orlistat, respectively) blocked the effect of calcium on 2-AG production. Furthermore, addition of the calcium chelator EGTA also prevented 2-AG synthesis. Second, incubation of SAG, a DAGL-β substrate, with macrophage lysate (J774A.1) resulted in a marked increase in 2-AG (∼14-fold; data not shown). Together, these data are consistent with literature indicating that elevated calcium levels are vital for the production of 2-AG (39, 58, 59, 61). The data also demonstrate that THP-1 and J774 cells have the biochemical machinery to produce 2-AG.
Fig. 2.
Human and mouse macrophage cell lines have the biochemical machinery to produce 2-AG. Human THP-1 macrophage lysates make 2-AG when stimulated with Ca2+. Left: calcium activates both PLC-β and DAGL activities, which can be blocked with the chelator EGTA. U-73122 and orlistat inhibit PLC-β and DAGL, respectively. In the presence of one or both of these inhibitors, 2-AG biosynthesis by the THP-1 macrophage lysate was blocked. Values are means ± SD (n = 3). Right: LC-MS/MS mass chromatograms indicated that the structural isomers of arachidonoylglycerol [2-AG and 1(3)-AG] elute as one peak, and that its biosynthesis was blocked by a DAGL inhibitor (orlistat). Rearrangement of 2-AG to 1(3)-AG due to acyl migration is a well-characterized phenomenon (49).
Macrophage Cell Lines Challenged by Extracellular Superoxide Flux
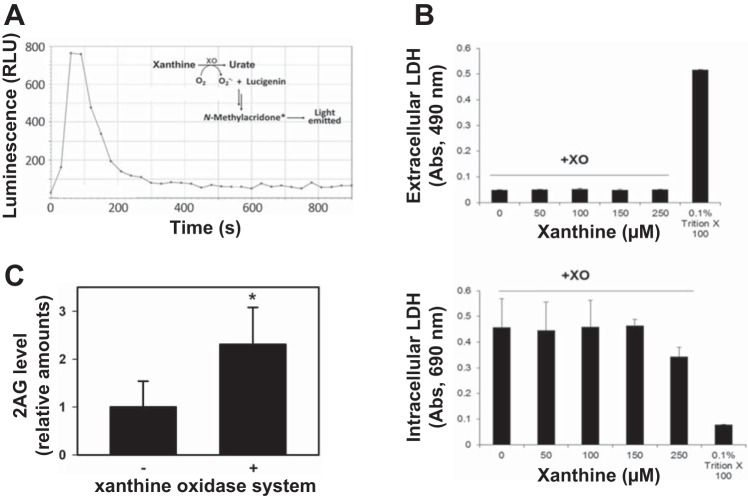
Next, intact THP-1 macrophages were subjected to oxyradical stress by treatment with an extracellular superoxide generating system (xanthine oxidase; 10 min) (Fig. 3). Superoxide production by the xanthine oxidase system was verified by the detection of both the lucigenin-derived product (Fig. 3A) and the HE-derived 2-OH-Et+ product (data not shown). A cell-free incubation of the xanthine oxidase system with cytochrome c for 3 min enabled the superoxide flux to be quantified (271 ± 2 pmol/min or 1.36 ± 0.01 μM/min). The xanthine oxidase-derived superoxide flux was not overtly cytotoxic to THP-1 cells (Fig. 3B). Moreover, intact THP-1 macrophages treated with the xanthine oxidase system produced increased amounts of 2-AG (2.3-fold) compared with those in untreated cells (Fig. 3C). Increased levels of 2-AG were also observed when another cell line (COS7 cells) was treated with the xanthine oxidase system (data not shown). Thus extracellular superoxide flux can elicit increases in 2-AG biosynthesis within intact cells.
Fig. 3.
Human THP-1 macrophages treated with extracellular superoxide via a xanthine oxidase system enhanced the amount of 2-AG made by the cells. A: the xanthine oxidase system liberates superoxide, which reacts with lucigenin to form the chemiluminescent product N-methylacridone*. The area under the curve is a measure of the amount of superoxide produced. LC-MS/MS analysis confirmed that the superoxide-specific oxidation product 2-OH-Et+ was produced when the oxyradical probe HE was added to the xanthine oxidase system (data not shown). B: on the basis of the LDH leakage assay, xanthine oxidase (XO)-derived superoxide was not cytotoxic to THP-1 macrophages following a 15-min incubation and can be used as a model of paracrine superoxide signaling (i.e., superoxide derived from the extracellular space). 0.1% Triton X-100 was used as positive control for cell death. Top: extracellular LDH. Bottom: intracellular LDH. C: THP-1 macrophages (pretreated with 1 μM PO for 30 min to inhibit 2-AG hydrolytic enzymes) were challenged with an extracellular xanthine oxidase system (10 min), which enhanced the production of 2-AG compared with the negative control. Values are means ± SD. *P < 0.05, Student's t-test.
Activation of Macrophage Nox Is Associated with Increases in 2-AG Levels
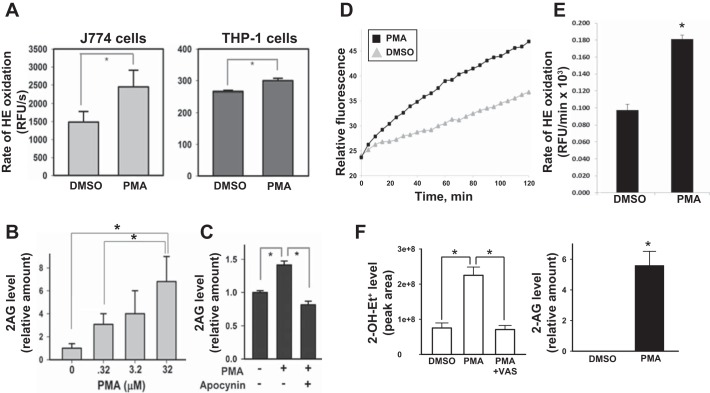
We next showed that short-term (<120 min) treatment of either J774A.1 macrophages or THP-1 macrophages with PMA (a Nox stimulant that activates protein kinase C), in the presence of the oxyradical probe HE, caused a significant increase in the rate of HE oxidation relative to that in vehicle-treated cells (Fig. 4A). Furthermore, PMA caused a concentration-dependent increase in 2-AG levels in J774A.1 macrophages (Fig. 4B). Importantly, the PMA-induced 2-AG production by macrophages was abrogated by the Nox inhibitor apocynin (Fig. 4C). Similar findings were observed in PMA-primed HL-60 cells treated acutely with PMA (3.2 μM; Fig. 4, D–F). Taken together, these results suggest that the 2-AG biosynthesis rate in cells correlates with Nox activity and oxyradical stress. These data were obtained in three different macrophage cell lines, further strengthening the evidence of this observation. All three cell lines express Nox2 and DAGL-β and make 2-AG (www.humanproteinatlas.org) (2, 3, 32, 51). The fact that similar findings were obtained in three different macrophage cell lines suggests that this phenomenon might be a generalized response to oxidative stress in cells.
Fig. 4.
PMA-activated macrophages produce elevated amounts of superoxide and 2-AG. A: murine J774A.1 macrophages (left) and human THP-1 macrophages (right) were treated with either DMSO or PMA (0.32 μM) for 30 min and then loaded with the oxyradical probe HE (20 μM). The resulting fluorescence signal was monitored over 90 min at 37°C. PMA-treated J774A.1 macrophages and THP-1 macrophages exhibited a 65% and 13% increase in HE oxidation rates, respectively, compared with vehicle-treated control. RFU, relative fluorescence units. B: murine J774A.1 macrophages treated with the indicated amounts of PMA for 10 min exhibited a concentration-dependent increase in 2-AG production, suggesting that 2-AG biosynthesis is correlated with increased oxidative stress. C: pretreatment of J774A.1 macrophages with the Nox inhibitor apocynin abrogated PMA-stimulated production of 2-AG. D: human HL-60 cells were differentiated into macrophages (PMA, 100 nM, 72 h) and then treated with either PMA (3.2 μM) or DMSO, followed by the addition of HE (20 μM). The rate of HE oxidation was monitored in a fluorescent plate reader for 120 min. E: quantitative analysis of data shown in D is provided as a bar graph. F, left: levels of the superoxide-specific product 2-OH-Et+ were determined in HL-60 macrophages treated with either DMSO, PMA (3.2 μM), or PMA (3.2 μM) plus VAS (Nox inhibitor, 10 μM) for 30 min. The culture medium was removed, and cells overlaid with fresh medium containing 20 μM HE, followed by an additional incubation period of 20 min. Right: 2-AG levels were determined in HL-60 macrophages treated with either DMSO or PMA (3.2 μM, 30 min). Values are means ± SD (n = 3). *P < 0.05, Student's t-test when two groups are compared, and one-way ANOVA (Student-Newman-Keuls method) when more than two groups are compared.
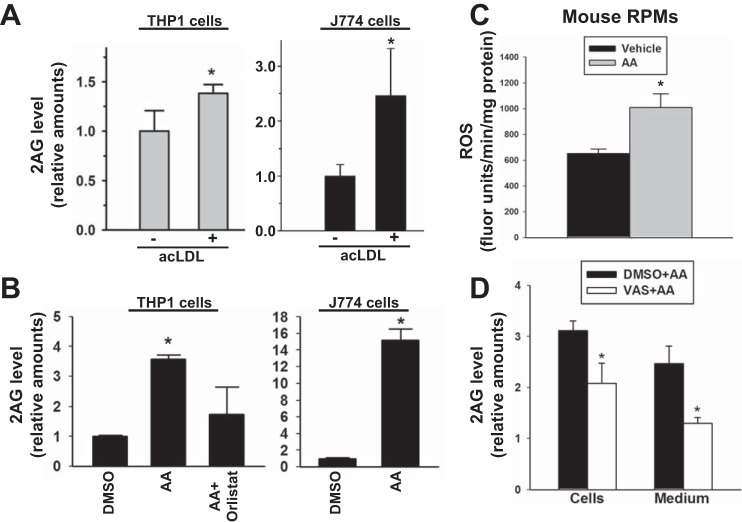
Because modified LDLs are known to stimulate Nox activity in a CD36-dependent manner (24, 38, 43, 44, 54, 55), human and murine macrophage cell lines (THP-1 and J774A.1, respectively) were treated with acLDL, a ligand that strongly binds to the CD36 scavenger receptor (1, 10, 64). acLDL-treated cells exhibited an increased level of 2-AG compared with that in untreated cells (Fig. 5A). Furthermore, when both macrophage cell lines were stimulated with AA (Nox stimulant), significantly more 2-AG was produced compared with that in DMSO-treated cells (Fig. 5B). This effect could be attenuated by a DAGL-β inhibitor. The levels of AA-stimulated 2-AG were greater in the murine cell line compared with that in the human cell line, which might reflect the relative differences in Nox2 expression in mouse macrophages compared with human macrophages or the greater lability of 2-AG in THP-1 cells relative to J774 cells (64). Furthermore, preincubation of mouse RPMs with VAS (Nox inhibitor) resulted in the significant reduction of Nox-derived 2-AG levels in both cells and extracellular medium (Fig. 5, C and D). This result indicated that these effects were recapitulated in primary mouse macrophages.
Fig. 5.
Human and murine macrophage cells challenged with acetylated low-density lipoprotein, arachidonic acid, and H2O2 produced elevated amounts of 2-AG. A: human THP-1 macrophages (left) and murine J774 macrophages (right) challenged with acLDL (20 μg/ml, 24 h) exhibited an increase in 2-AG levels. B: exogenous arachidonic acid (AA; 10 μM, 60 min) stimulated DAGL-dependent biosynthesis of 2-AG by murine and human macrophage cell lines, which was inhibited by orlistat. C and D: mouse RPMs were plated ex vivo and pretreated with vehicle (DMSO) or VAS-2870 (10 μM) for 30 min, followed by stimulation with AA (100 μM, 60 min). C: ROS levels were quantified using HE. D: cells and culture supernatants (medium) were separately extracted and 2-AG levels quantified by LC-MS/MS. Values are means ± SD (n = 3). *P < 0.05 (relative to control or vehicle), Student's t-test when two groups are compared, and one-way ANOVA (Student-Newman-Keuls method) when more than two groups are compared.
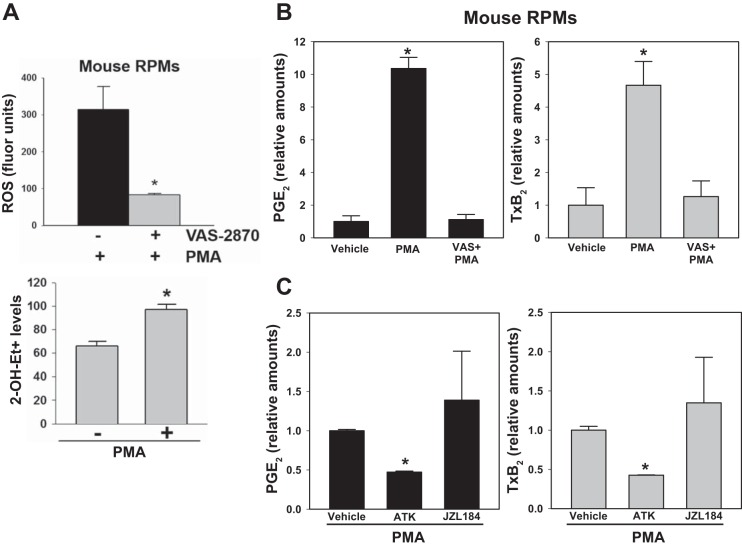
To determine whether Nox-derived ROS causes AA release and eicosanoid production, mouse RPMs were pretreated with VAS followed by stimulation with PMA. (Exogenous AA could not be used to stimulate Nox, because it would out compete the smaller endogenous pools of AA liberated from phospholipids and 2AG in terms of substrate binding to the COX enzyme.) As shown in Fig. 6, PMA could stimulate the production of Nox2-derived ROS from resident RPMs (Fig. 6A), while also inducing the biosynthesis of large quantities of the AA metabolites PGE2 and thromboxane (Tx) B2 (Fig. 6B). Production of eicosanoids was dependent on Nox2, because pretreatment with VAS significantly attenuated their levels (Fig. 6B). Eicosanoid production by mouse RPMs was also blocked by the antioxidant N-acetyl-cysteine (data not shown), further suggesting that their production was dependent on elevated levels of ROS. Furthermore, on the basis of experiments using inhibitors for cytosolic phospholipase A2 (cPLA2) (ATK) and MAGL (JZL184), a significant pool of AA in phospholipids was liberated by Ca2+-dependent cPLA2 and was subsequently oxygenated by COX. This is supported by the fact that pretreatment of cells with ATK significantly attenuated eicosanoid production (∼50% reduction), whereas JZL184 pretreatment did not affect eicosanoid levels (Fig. 6C). It should be noted that MAGL is not the only 2AG hydrolase in murine macrophages; α/β-hydrolase domain containing 6 (ABHD6) is also expressed in murine macrophages and can degrade 2AG (2). However, ABHD6 is not inhibited by JZL184 (29). Therefore, release of AA by ABHD6-mediated 2AG hydrolysis, which is subsequently oxygenated by COX to give eicosanoids, cannot be ruled out completely.
Fig. 6.
Mouse RPMs challenged with PMA produce elevated levels of eicosanoids by a Nox-dependent mechanism. A, top: nonspecific ROS levels were quantified in mouse RPMs following stimulation with PMA (3.2 μM, 30 min) using a DCFDA probe in the absence and presence of VAS-2870 (10 μM). Bottom: in a separate experiment, superoxide levels were determined in mouse RPMs using the HE probe. *P < 0.05, +VAS-2870+PMA relative to −VAS-2870+PMA (top), +PMA relative to −PMA (bottom). B: following pretreatment of RPMs with VAS-2870 (10 μM, 30 min), eicosanoids (PGE2 and TxB2) were quantified after stimulating the cells with PMA (3.2 μM, 30 min). *P < 0.05, PMA relative to vehicle. C: following pretreatment of RPMs with ATK or JZL184 (10 μM each, 30 min), eicosanoids (PGE2 and TxB2) were quantified after stimulating the cells with PMA (3.2 μM, 30 min). *P < 0.05, ATK+PMA relative to PMA alone. One-way ANOVA (Student-Newman-Keuls method) was used when more than two groups are compared.
In cells, superoxide rapidly dismutates to H2O2, an important second messenger in signal transduction pathways (14, 48). The addition of H2O2 to either intact living THP-1 macrophages or THP-1 cell lysates also increased the levels of 2-AG compared with vehicle controls (Fig. 7). This suggested that superoxide/H2O2 might stimulate DAGL-β activity. However, when COS7 cell lysate containing overexpressed human DAGL-β was pretreated with H2O2, the activity of the lysate toward the substrate SAG was unchanged (data not shown). This suggested that any effects of H2O2 on DAGL-β are probably indirect and not the result of chemical modification of a cysteine residue important for regulating enzyme activity, as was recently reported for MAGL and H2O2 (11).
Fig. 7.
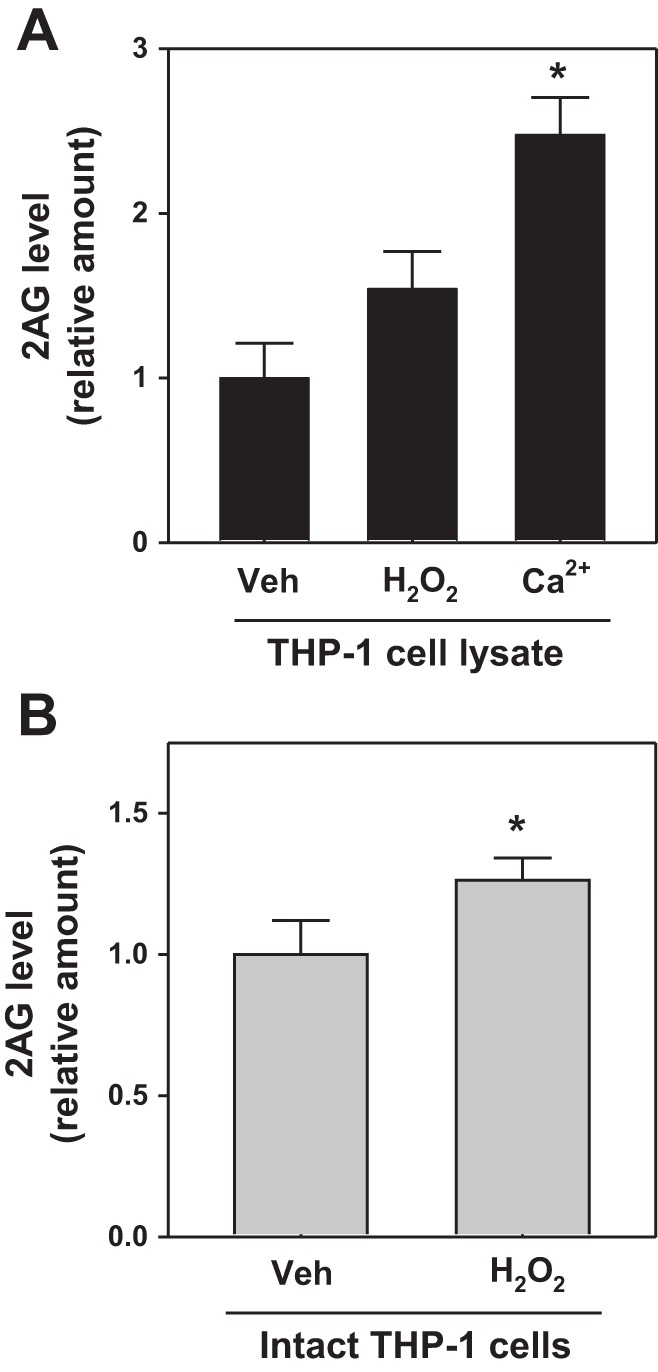
Hydrogen peroxide (H2O2) induces 2-AG levels in THP-1 cells. THP-1 cell lysates (A) or intact living THP-1 cells (B) were treated with H2O2 (1 mM, 60 min), followed by 2-AG quantitation. Cell lysate was also treated with Ca2+ to stimulate 2-AG synthesis (as a positive control). The vehicle (Veh) for H2O2 was water. Values are means ± SD (n = 2–3). *P < 0.05 (relative to vehicle).
We also found that the levels of DAGLβ, p47phox, and Nox2 mRNA levels in THP-1 macrophages, which had been incubated with an inhibitor of 2-AG hydrolysis (JZL184, 1 μM, 24 h), were not significantly different from those in control cells (data not shown). This implied that increasing the concentration of cellular 2-AG (by preventing its degradation) does not impact the expression of these genes in THP-1 macrophages.
MD-Derived and Nox-Derived Superoxide Levels Are Attenuated by the Cannabinoid Receptor Agonist WIN 55,212-2
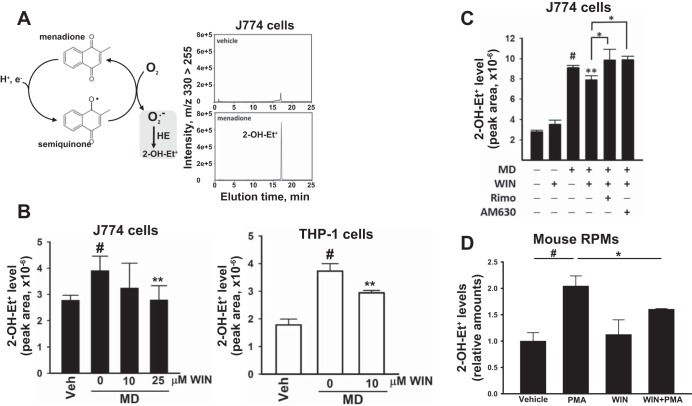
Addition of the redox cycling agent MD (40 μM) to either J774A.1 or THP-1 macrophages resulted in a significant increase in superoxide levels compared with those in vehicle-treated cells, as measured by the superoxide surrogate 2-OH-Et+ (Fig. 8, A and B). A nonselective cannabinoid receptor agonist, WIN 55,212-2, attenuated the levels of MD-derived superoxide in J774 macrophages (Fig. 8B, left). Similar effects of WIN 55,212-2 on superoxide levels were also observed in THP-1 macrophages (Fig. 8B, right). It should be noted that WIN 55,212-2 on its own did not stimulate superoxide production in cells (Fig. 8C). In addition, the CB1 and CB2 receptor antagonists (rimonabant and AM630, respectively) both abrogated the anti-oxidative effect of WIN 55,212-2 in J774A.1 macrophages treated with MD (Fig. 8C). Furthermore, pretreatment of mouse RPMs with WIN 55,212-2 also attenuated PMA-stimulated Nox-derived superoxide levels (Fig. 8D). Collectively, these results, obtained in different cell types, including primary cells, indicated that activation of CB receptors with an agonist could reduce superoxide levels, whereas the agonist alone did not affect superoxide levels relative to that of controls.
Fig. 8.
Pretreatment of murine and human macrophage cell lines with the nonselective CB agonist WIN 55,212-2 (WIN) attenuated superoxide (2-OH-Et+) levels following treatment with menadione (MD). A: MD is a redox cycling agent that produces superoxide in cells. Addition of MD (40 μM) to J774A.1 macrophages for 30 min increased superoxide (2-OH-Et+) levels relative to vehicle (LC-MS/MS chromatograms are shown). B: WIN (10 or 25 μM) attenuated the levels of MD (40 μM, 30 min)-derived superoxide (2-OH-Et+) in J774A.1 macrophages and THP-1 macrophages. C: the attenuating effect of WIN (25 μM) on MD (40 μM, 30 min)-derived superoxide (2-OH-Et+) levels in J774A.1 macrophages was abrogated by CB1 and CB2 receptor antagonists [rimonabant (Rimo) and AM630, respectively]. Values in B and C are means ± SD (n = 4–9). #P < 0.05, MD only relative to vehicle (Veh) control; **P = 0.02 WIN+MD relative to MD only; *P < 0.05 antagonists (Rimo or AM630) relative to WIN+MD, one-way ANOVA (Student-Newman-Keuls method). D: mouse RPMs were treated with PMA (3.2 μM, 30 min) in the presence and absence of WIN (25 μM), and superoxide (2-OH-Et+) levels were determined. #P = 0.002, PMA alone relative to vehicle; *P < 0.05, WIN+PMA relative to PMA alone, one-way ANOVA (Student-Newman-Keuls method).
DAGL-β-Dependent 2-AG Biosynthesis Following Nox Activation by AA
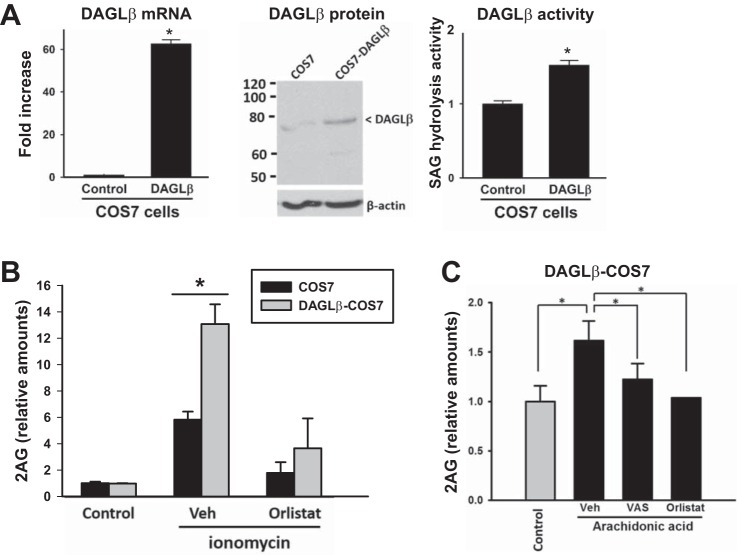
To mechanistically establish the relationship between Nox-derived ROS and DAGL-β-dependent 2-AG biosynthesis, experiments were performed using cells that ectopically express DAGL-β and Nox2 components. Stable overexpression of human DAGL-β expression in COS7 cells was verified by measuring the DAGL-β mRNA level, DAGL-β protein l evel, and SAG hydrolysis activity (Fig. 9A); each parameter was significantly elevated in the DAGL-β-transfected cells compared with mock-transfected cells. Furthermore, COS7 cells overexpressing DAGL-β could produce more 2-AG than control COS7 cells when each cell type was stimulated with ionomycin, further indicating that DAGL-β was overexpressed and functional (Fig. 9B). Pretreatment of cells with a DAGL inhibitor, orlistat, abrogated the response to ionomycin. Importantly, stimulation of DAGL-β-overexpressing cells with exogenous AA (Nox stimulant) resulted in a marked increase in 2-AG levels compared with control (untreated DAGL-β-overexpressing cells) (Fig. 9C). This effect was significantly attenuated with either a Nox inhibitor or a DAGL inhibitor (Fig. 9C). These data were consistent with the Nox-dependent induction of 2-AG levels obtained with human and mouse macrophage cells treated with AA (Fig. 5, B and D).
Fig. 9.
COS7 cells stably overexpressing human DAGL-β produced more 2-AG than control COS7 cells, and inhibition of Nox activity attenuated 2-AG levels. A: stable overexpression of human DAGL-β in COS7 cells was verified by measuring its mRNA content (left), protein content (middle), and SAG hydrolysis activity (right). B: 2-AG levels were determined in DAGL-β-COS7 cells and control COS7 cells following stimulation with a calcium ionophore (ionomycin, 3 μM, 30 min) in the presence and absence of the DAGL inhibitor orlistat. C: 2-AG levels were determined in DAGL-β-COS7 cells following stimulation with arachidonic acid in the presence and absence of a Nox inhibitor (VAS) or DAGL inhibitor (orlistat). Values are means ± SD (n = 2–7). *P < 0.05, Student's t-test when two groups are compared, and one-way ANOVA (Student-Newman-Keuls method) when more than two groups are compared.
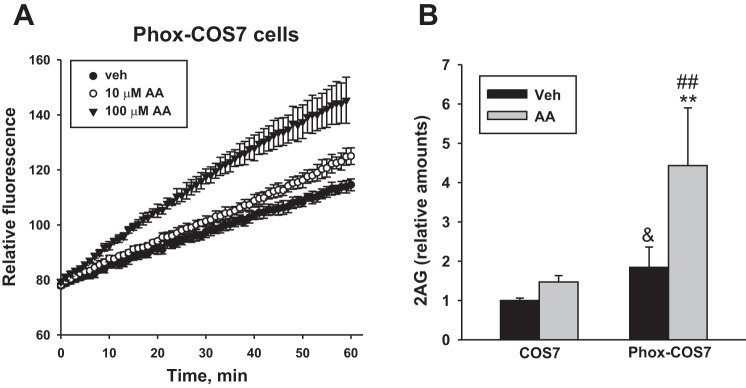
Next, when Phox-COS7 cells that overexpress Nox subunits (45) were stimulated with AA, they produced greater amounts of ROS than vehicle-treated Phox-COS7 cells, as measured using the oxyradical probe HE (Fig. 10A). Importantly, AA-treated Phox-COS7 cells produced substantially more 2-AG than either the vehicle-treated Phox-COS7 cells or AA-treated COS7 did (Fig. 10B). In addition, the basal amounts of 2-AG in vehicle-treated Phox-COS7 cells were higher than those in vehicle-treated parental COS7 cells (Fig. 10B). Together these data lend direct support to the hypothesis that increased Nox activity can activate 2-AG biosynthesis in cells.
Fig. 10.
Phox-COS7 cells exhibit greater levels of ROS and 2-AG than those in parental COS7 cells when stimulated with AA. A: Phox-COS7 cells were preloaded with the oxyradical probe HE, followed by treatment with DMSO, and 10 or 100 μM AA, and the fluorescence was immediately monitored for 60 min. B: parental COS7 cells or Phox-COS7 cells were treated with AA (100 μM, 60 min), and the levels of 2-AG in the medium and cells were determined by LC-MS/MS. Values are means ± SD (n = 6). &P < 0.01 relative to vehicle (Veh)-treated parental COS7 cells; **P < 0.001 relative to vehicle (Veh)-treated Phox-COS7 cells; ##P < 0.001 relative to AA-treated parental COS7 cells, two-way ANOVA (Student-Newman-Keuls method).
DISCUSSION
Macrophages have important roles in lipid metabolism and inflammation. In this study, we extended our laboratory's previous work on Nox in monocytes/macrophages (33) to examine whether stimulation of this enzyme complex modulates the eCB system by activating the 2-AG biosynthetic enzyme DAGL-β. Nox is the only oxidoreductase that produces superoxide as its primary end product (6); however, whether Nox-derived ROS interfaces with bioactive eCB has not been studied. Thus we asked whether Nox-mediated oxidative stress can stimulate downstream 2-AG biosynthesis to reestablish homeostasis. In this study, the levels of 2-AG in macrophage cell lines (THP-1, HL-60, and murine J774A.1) and primary mouse macrophages were significantly elevated following exposures to either extracellular oxyradicals (via the xanthine oxidase system) or intracellular oxyradicals derived from Nox following its activation by the stimulants PMA and AA. The results suggest that enhanced biosynthesis of 2-AG is causally related to oxyradical stress in macrophages. In support of this, the effects of the cellular stressors on 2-AG biosynthetic activity were blunted by the addition of Nox and DAGL inhibitors. Furthermore, engineered COS7 cells that overexpress either DAGL-β or Nox subunits were shown to synthesize higher levels of 2-AG compared with the parental COS7 cells when treated with the Nox stimulant AA. All of these lines of evidence support a model in which activated Nox can signal (directly or indirectly via superoxide-derived ROS) to DAGL-β to increase the biosynthesis of 2-AG.
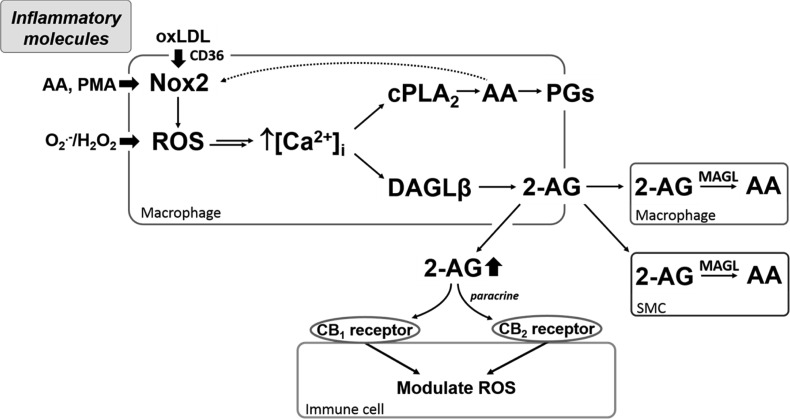
Current therapies for CVD mainly target well-established risk factors, such as high blood pressure and elevated cholesterol levels. However, the eCB system and oxidoreductases that generate ROS are emerging as attractive targets for reducing inflammation and oxidative stress in the cardiovascular wall. Our data suggest that increased rates of superoxide production, induced by either PMA, AA, or xanthine oxidase system, correlated with increased rates of 2-AG biosynthesis. From a mechanistic perspective, PMA is a potent activator of protein kinase C and the small G protein Rac. Once activated, these proteins work in concert to assemble the Nox complex and stimulate the biosynthesis of Nox-derived superoxide (52, 53), which, in turn, cause the release of AA and increased eicosanoid production (20, 22). As part of a positive feedback loop, the transient increase in intracellular AA concentration can further stimulate Nox activity. AA stimulates Nox activity in part by promoting the concerted assembly of regulatory cytosolic Nox subunits with membrane-bound Nox2 (gp91phox)/p22phox subunits via AA-mediated conformational changes (57). Xanthine oxidase, on the other hand, is an oxidoreductase that generates superoxide anion via the sequential oxidation of hypoxanthine to xanthine and xanthine to uric acid (5). All of these stimuli were found to induce 2-AG biosynthesis in cells. These findings were further confirmed in cells that overexpress Nox subunits (Phox-COS7 cells), which exhibited higher levels of 2-AG than the parental control COS7 cells (Fig. 10). One commonality between DAGL-β and cPLA2 is that their activities depend on calcium ions (26, 47), which can be released from intracellular stores by superoxide anions generated by oxidases via ROS activation of MAPKs (e.g., JNK, p38, and ERK1/2) (22). Because H2O2 had not directly affected DAGL-β activity in COS7 cell lysates that overexpress this lipase, it is unlikely that oxidative modification of a cysteine residue in DAGL-β is responsible for its increased activity during oxidative stress. Thus, mechanistically, it is likely that DAGL-β and cPLA2 are activated simultaneously by Ca2+, which is released from intracellular stores by Nox-derived ROS (Fig. 11). The increased amounts of 2-AG, PGE2, and TxB2 released upon cell stimulation, which is dependent on functional Nox activity, is consistent with this (Figs. 5 and 6).
Fig. 11.
Model describing how physiological and nonphysiological stimulants can activate signaling pathways to enhance 2-AG and prostaglandins (PG) biosynthesis via NADPH oxidase (Nox)-derived ROS. Several physiological mediators [e.g., oxLDL, arachidonic acid (AA)] and nonphysiological chemicals [e.g., phorbol 12-myristate 13-acetate (PMA)] can stimulate Nox2. Elevated ROS levels in cells (either via Nox2 activation or exogenous oxidants) cause a transient increase in intracellular calcium concentration ([Ca2+]i) that activates diacylglycerol lipase (DAGL)-β and cytosolic phospholipase A2 (cPLA2), thereby increasing the synthesis of 2-AG and oxygenated AA metabolites (PGs), respectively. 2-AG can be released extracellularly where it acts in a paracrine and autocrine manner to possibly counteract inflammation and oxidative stress via cannabinoid (CB) receptor-evoked signaling in immune cells (15). 2-AG can also be degraded by neighboring cells or by cells that produce it. Double arrow between ROS and [Ca2+]i indicates that oxidants can release intracellular Ca2+ stores by several mechanisms, including the inactivation of MAPK phosphatases, which maintains MAPKs in their active state (22). Dotted arrow indicates that cPLA2-derived AA can stimulate Nox2 (33). H2O2, hydrogen peroxide; MAGL, monoacylglycerol lipase; O2−, superoxide.
The increased rates of 2-AG biosynthesis caused by elevated superoxide levels might be a compensatory mechanism evoked by oxidative stress. For example, eCB have well-known homeostatic regulatory functions that help to dampen the effects of noxious stimuli (41). Consistent with this idea, we demonstrated that the (nonselective) cannabinoid receptor agonist WIN 55,212-2 attenuated the levels of superoxide induced by either a redox cycling chemical (MD) or a Nox stimulant (PMA) in macrophages. Furthermore, either selective CB1 or selective CB2 receptor antagonists could abrogate the antioxidative effect of WIN 55,212-2 (Fig. 8C). These data indicate that the eCB system, including the CB1 and CB2 receptors and their ligands, might be an important therapeutic target to modulate oxidative stress. The specific signaling pathways evoked by CB1 and CB2 receptor activation are undefined in our study; however, CB1- and CB2-dependent signaling is known to modulate p38 and ERK1/2 stress kinase activity (15), which might account in part for the WIN 55,212-2-dependent decrease in superoxide levels seen here. More work is needed to characterize the complex integration of signals emanating from the two cannabinoid receptors and how they balance each other to regulate the intracellular redox state.
Our results are also consistent with previous in vivo and in vitro findings. For example, Batkai et al. (4) demonstrated that ischemia-reperfusion injury in mouse liver induced the production of inflammatory mediators that increase oxidative stress; the levels of these inflammatory molecules positively correlated with hepatic eCB levels. Furthermore, increased production of eCB in isolated mouse hepatocytes was observed when peroxynitrite or H2O2 was added directly to the cells (4). Our results also showed that increased levels of 2-AG could be produced in macrophages exposed to acLDL (Fig. 5A). Unregulated uptake of modified LDLs by macrophages leads to foam cell formation; however, CB1 receptor blockade or CB2 receptor activation was previously shown to reduce foam cell formation (60) and atherosclerosis development (8). In addition, when high-fat diet-fed ApoE−/− mice were treated with WIN 55,212-2, the number of macrophages found in plaques were decreased compared with those in plaques from control ApoE−/− mice, as was the expression of TNF-α, IL-6, and monocyte chemotactic protein-1 (63). The beneficial effects of WIN 55,212-2 were blocked with a CB2 antagonist. It is known that modified LDL can activate Nox activity and increase ROS in macrophages in a CD36-dependent manner (44). Thus a compensatory response of cells to Nox activation under these conditions might involve an increase in 2-AG levels to help dampen oxidative stress and inflammatory cytokine production via CB2 signaling by autocrine/paracrine feedback loops.
The relationship between oxidative stress and eCB is complex and still poorly understood. Nevertheless, Rajesh et al. (46) demonstrated that Nox-derived superoxide levels in cultured human coronary artery endothelial cells were attenuated by a synthetic CB2-selective agonist. This effect is consistent with the known beneficial properties of CB2 agonists in several contexts. In support of this, treatment of endothelial cells and smooth muscle cells with a synthetic CB2 agonist attenuated TNF-α production and evoked other anti-inflammatory responses (46). On the other hand, CB1 receptor activation induced the production of proinflammatory mediators and substantial oxidative stress (42). Because eCB ligands bind to CB1 and CB2 receptors with roughly equal affinity, an increased eCB concentration in the vessel wall might elicit both pro- and anti-inflammatory and oxidative effects, depending on the relative strength of the signal transduction pathways evoked by CB1 and CB2 receptor signaling. Although there is conflicting literature concerning the role of eCB in the pathogenesis of atherogenesis (30), it is generally accepted that an imbalance in the eCB system and the overproduction of Nox-derived superoxide and H2O2 will disrupt redox circuits and induce inflammatory mediators (12, 40, 42).
In conclusion, this study provides important insight into how Nox-derived ROS in cells may interface with the activation of the rate-limiting enzyme of 2-AG biosynthesis, DAGL-β. We demonstrated that heightened oxyradical flux caused by extracellular (paracrine)-superoxide and intracellular (Nox)-derived superoxide can lead to increased biosynthesis of 2-AG, thereby enhancing the macrophage “endocannabinoid tone.” The reason for the rapid increase in local eCB concentrations during injury is still a matter of debate (41). However, it is suggested that it is part of a compensatory mechanism to counteract inflammation and oxidative stress (Fig. 11), perhaps by a CB2-receptor-dependent signaling mechanism and downstream signaling cascades in inflammatory cells (15, 41).
GRANTS
This study was supported by grants from the National Institutes of Health (1R15-ES-015348-02 and F31-HL-122082-01A1).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.T.M., J.H.L., A.B., L.C.M., and X.H. performed experiments; A.T.M., J.H.L., and M.K.R. analyzed data; A.T.M., J.H.L., A.B., and M.K.R. interpreted results of experiments; A.T.M., J.H.L., and M.K.R. prepared figures; A.T.M. drafted manuscript; A.T.M., J.H.L., A.B., L.C.M., X.H., and M.K.R. approved final version of manuscript; J.H.L. and M.K.R. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Stephen Pruett (Mississippi State University) and Dr. Ran Wang (Jiangsu Academy for Agricultural Sciences, Nanjing, China) for research advice. We are grateful to Dr. Mary Dinauer (Washington University, St. Louis, MO) for providing the Phox-COS7 cells.
Present addresses: A. T. Matthews, Morehouse University School of Medicine, 720 Westview Dr. SW, Atlanta, GA 30310; L. C. Mangum, US Army Institute of Surgical Research, 3698 Chambers Pass, Ste. B, JBSA, Ft. Sam Houston, TX 78234-7767.
REFERENCES
- 1.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem 269: 21003–21009, 1994. [PubMed] [Google Scholar]
- 2.Alhouayek M, Masquelier J, Cani PD, Lambert DM, Muccioli GG. Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6. Proc Natl Acad Sci U S A 110: 17558–17563, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res 104: 210–218, 221p following 218, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Hasko G, Huffman JW, Gao B, Kunos G, Pacher P. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J 21: 1788–1800, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battelli MG, Polito L, Bortolotti M, Bolognesi A. Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxid Med Cell Longev 2016: 3527579, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacol Rev 65: 849–871, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booz GW. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic Biol Med 51: 1054–1061, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest 107: 1339–1345, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvo D, Gomez-Coronado D, Suarez Y, Lasuncion MA, Vega MA. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J Lipid Res 39: 777–788, 1998. [PubMed] [Google Scholar]
- 11.Dotsey EY, Jung KM, Basit A, Wei D, Daglian J, Vacondio F, Armirotti A, Mor M, Piomelli D. Peroxide-Dependent MGL sulfenylation regulates 2-AG-mediated endocannabinoid signaling in brain neurons. Chem Biol 22: 619–628, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5: 781–792, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Griendling AA. Differential roles of NADPH oxidases in vascular physiology and pathophysiology. Front Biosci 4: 1044–1064, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han KH, Lim S, Ryu J, Lee CW, Kim Y, Kang JH, Kang SS, Ahn YK, Park CS, Kim JJ. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res 84: 378–386, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Hao MX, Jiang LS, Fang NY, Pu J, Hu LH, Shen LH, Song W, He B. The cannabinoid WIN55,212–2 protects against oxidized LDL-induced inflammatory response in murine macrophages. J Lipid Res 51: 2181–2190, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev 93: 1317–1542, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Hsu KL, Tsuboi K, Adibekian A, Pugh H, Masuda K, Cravatt BF. DAGLbeta inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat Chem Biol 8: 999–1007, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung YC, Hong MY, Huang GS. Cholesterol loading augments oxidative stress in macrophages. FEBS Lett 580: 849–861, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Ishii T. Close teamwork between Nrf2 and peroxiredoxins 1 and 6 for the regulation of prostaglandin D2 and E2 production in macrophages in acute inflammation. Free Radic Biol Med 88: 189–198, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Jiang LS, Pu J, Han ZH, Hu LH, He B. Role of activated endocannabinoid system in regulation of cellular cholesterol metabolism in macrophages. Cardiovasc Res 81: 805–813, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol 64: 409–421, 2013. [PubMed] [Google Scholar]
- 23.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med 43: 319–331, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SJ, Thien Quach CH, Jung KH, Paik JY, Lee JH, Park JW, Lee KH. Oxidized low-density lipoprotein stimulates macrophage 18F-FDG uptake via hypoxia-inducible factor-1alpha activation through Nox2-dependent reactive oxygen species generation. J Nucl Med 55: 1699–1705, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Leonarduzzi G, Gamba P, Gargiulo S, Biasi F, Poli G. Inflammation-related gene expression by lipid oxidation-derived products in the progression of atherosclerosis. Free Radic Biol Med 52: 19–34, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Leslie CC. Cytosolic phospholipase A(2): physiological function and role in disease. J Lipid Res 56: 1386–1402, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libby P, Sukhova G, Lee RT, Liao JK. Molecular biology of atherosclerosis. Int J Cardiol 62, Suppl 2: S23–S29, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Malavya S, Wang X, Saavedra JE, Keefer LK, Tokar E, Qu W, Waalkes MP, Shami PJ. Gene expression profiling for nitric oxide prodrug JS-K to kill HL-60 myeloid leukemia cells. Genomics 94: 32–38, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol 5: 37–44, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackie AA. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160: 467–479, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magwenzi S, Woodward C, Wraith KS, Aburima A, Raslan Z, Jones H, McNeil C, Wheatcroft S, Yuldasheva N, Febbriao M, Kearney M, Naseem KM. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 125: 2693–2703, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makino J, Nakanishi R, Kamiya T, Hara H, Ninomiya M, Koketsu M, Adachi T. Luteolin suppresses the differentiation of THP-1 cells through the Inhibition of NOX2 mRNA expression and the membrane translocation of p47phox. J Nat Prod 76: 1285–1290, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Mangum LC, Borazjani A, Stokes JV, Matthews AT, Lee JH, Chambers JE, Ross MK. Organochlorine insecticides induce NADPH oxidase-dependent reactive oxygen species in human monocytic cells via phospholipase A2/arachidonic acid. Chem Res Toxicol 28: 570–584, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mertens A, Holvoet P. Oxidized LDL and HDL: antagonists in atherothrombosis. FASEB J 15: 2073–2084, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Miller TW, Isenberg JS, Shih HB, Wang Y, Roberts DD. Amyloid-beta inhibits No-cGMP signaling in a CD36- and CD47-dependent manner. PLoS One 5: e15686, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 145: 341–355, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nisimoto Y, Ogawa H. Interaction between p21-activated protein kinase and Rac during differentiation of HL-60 human promyelocytic leukemia cell induced by all-trans-retinoic acid. Eur J Biochem 269: 2622–2629, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Noda K, Godo S, Saito H, Tsutsui M, Shimokawa H. Opposing roles of nitric oxide and rho-kinase in lipid metabolism in mice. Tohoku J Exp Med 235: 171–183, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Okada Y, Imendra KG, Miyazaki T, Hotokezaka H, Fujiyama R, Toda K. High extracellular Ca2+ stimulates Ca2+-activated Cl− currents in frog parathyroid cells through the mediation of arachidonic acid cascade. PLoS One 6: e19158, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res 50: 193–211, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pacher P, Steffens S. The emerging role of the endocannabinoid system in cardiovascular disease. Semin Immunopathol 31: 63–77, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park L, Wang G, Zhou P, Zhou J, Pitstick R, Previti ML, Younkin L, Younkin SG, Van Nostrand WE, Cho S, Anrather J, Carlson GA, Iadecola C. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci U S A 108: 5063–5068, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest 119: 136–145, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price MO, McPhail LC, Lambeth JD, Han CH, Knaus UG, Dinauer MC. Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood 99: 2653–2661, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Rajesh M, Mukhopadhyay P, Hasko G, Liaudet L, Mackie K, Pacher P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br J Pharmacol 160: 688–700, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reisenberg M, Singh PK, Williams G, Doherty P. The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci 367: 3264–3275, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross M, Matthews A, Mangum L. Chemical atherogenesis: role of endogenous and exogenous poisons in disease development. Toxics 2: 17–34, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rouzer CA, Ghebreselasie K, Marnett LJ. Chemical stability of 2-arachidonylglycerol under biological conditions. Chem Phys Lipids 119: 69–82, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev 111: 5899–5921, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samuelson DJ, Powell MB, Lluria-Prevatt M, Romagnolo DF. Transcriptional activation of the gp91phox NADPH oxidase subunit by TPA in HL-60 cells. J Leukoc Biol 69: 161–168, 2001. [PubMed] [Google Scholar]
- 52.Schroder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 27: 1736–1743, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 89: 1159–1167, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Shen GX. Mitochondrial dysfunction, oxidative stress and diabetic cardiovascular disorders. Cardiovasc Hematol Disord Drug Targets 12: 106–112, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol 88: 241–248, 2010. [DOI] [PubMed] [Google Scholar]
- 56.Shiffman D, Mikita T, Tai JT, Wade DP, Porter JG, Seilhamer JJ, Somogyi R, Liang S, Lawn RM. Large scale gene expression analysis of cholesterol-loaded macrophages. J Biol Chem 275: 37324–37332, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Shiose A, Sumimoto H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J Biol Chem 275: 13793–13801, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Shonesy BC, Winder DG, Patel S, Colbran RJ. The initiation of synaptic 2-AG mobilization requires both an increased supply of diacylglycerol precursor and increased postsynaptic calcium. Neuropharmacology 91: 57–62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Signorello MG, Giacobbe E, Segantin A, Avigliano L, Sinigaglia F, Maccarrone M, Leoncini G. Activation of human platelets by 2-arachidonoylglycerol: role of PKC in NO/cGMP pathway modulation. Curr Neurovasc Res 8: 200–209, 2011. [DOI] [PubMed] [Google Scholar]
- 60.Sugamura K, Sugiyama S, Fujiwara Y, Matsubara J, Akiyama E, Maeda H, Ohba K, Matsuzawa Y, Konishi M, Nozaki T, Horibata Y, Kaikita K, Sumida H, Takeya M, Ogawa H. Cannabinoid 1 receptor blockade reduces atherosclerosis with enhances reverse cholesterol transport. J Atheroscler Thromb 17: 141–147, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Szekeres M, Nadasy GL, Turu G, Soltesz-Katona E, Benyo Z, Offermanns S, Ruisanchez E, Szabo E, Takats Z, Batkai S, Toth ZE, Hunyady L. Endocannabinoid-mediated modulation of Gq/11 protein-coupled receptor signaling-induced vasoconstriction and hypertension. Mol Cell Endocrinol 403: 46–56, 2015. [DOI] [PubMed] [Google Scholar]
- 62.Wang R, Borazjani A, Matthews AT, Mangum LC, Edelmann MJ, Ross MK. Identification of palmitoyl protein thioesterase 1 in human THP1 monocytes and macrophages and characterization of unique biochemical activities for this enzyme. Biochemistry 52: 7559–7574, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Zhou YT, Zhao Q.. [The effect of the activation of cannabinoid receptor on the proliferation and apoptosis of hepatoma HepG2 cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 26: 344–347, 2010. [PubMed] [Google Scholar]
- 64.Xie S, Borazjani A, Hatfield MJ, Edwards CC, Potter PM, Ross MK. Inactivation of lipid glyceryl ester metabolism in human THP1 monocytes/macrophages by activated organophosphorus insecticides: role of carboxylesterases 1 and 2. Chem Res Toxicol 23: 1890–1904, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zielonka J, Hardy M, Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radic Biol Med 46: 329–338, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]