Abstract
The effects of acute exposure to acidic water on Na+ and Cl− homeostasis, and the mechanisms underlying their compensatory regulation, were investigated in the larval zebrafish Danio rerio. Exposure to acidic water (pH 4.0; control pH 7.6) for 2 h significantly reduced Na+ uptake and whole body Na+ content. Nevertheless, the capacity for Na+ uptake was substantially increased in fish preexposed to acidic water but measured in control water. Based on the accumulation of the Na+-selective dye, Sodium Green, two ionocyte subtypes exhibited intracellular Na+ enrichment after preexposure to acidic water: H+-ATPase rich (HR) cells, which coexpress the Na+/H+ exchanger isoform 3b (NHE3b), and a non-HR cell population. In fish experiencing Na+-Cl− cotransporter (NCC) knockdown, we observed no Sodium Green accumulation in the latter cell type, suggesting the non-HR cells were NCC cells. Elimination of NHE3b-expressing HR cells did not prevent the increased Na+ uptake following acid exposure. On the other hand, the increased Na+ uptake was abolished when the acidic water was enriched with Na+ and Cl−, but not with Na+ only, indicating that the elevated Na+ uptake after acid exposure was associated with the compensatory regulation of Cl−. Further examinations demonstrated that acute acid exposure also reduced whole body Cl− levels and increased the capacity for Cl− uptake. Moreover, knockdown of NCC prevented the increased uptake of both Na+ and Cl− after exposure to acidic water. Together, the results of the present study revealed a novel role of NCC in the compensatory regulation of Na+ and Cl− uptake following acute acidosis.
Keywords: acidosis, ionocyte, chloride, Na+-Cl− cotransporter, Na+/H+ exchanger, sodium, zebrafish
zebrafish (danio rerio) has emerged as an important model for integrative physiological research, including regulation of body fluid homeostasis and acid-base balance (14–16, 21). Zebrafish inhabit a hypoionic environment and are tolerant of a range of environmental stressors, including acidic and ion-poor water conditions (3, 13, 18, 20, 21, 30). In adult zebrafish, the gills are the predominant site for active ion uptake through specific ion-transporting cells, termed ionocytes. During larval stages before the gills are fully developed, regulation of epithelial ion transport is mediated primarily by ionocytes found in the skin of the yolk sac. These ionocytes are thought to be analogous to various renal tubular cells in mammals, in terms of ion transport mechanisms and solute transporter expression (16). Therefore, the zebrafish gill or embryonic/larval skin is emerging as a useful in vivo model for studying the regulation of ion homeostasis in vertebrates.
In mammals, the Na+/H+ exchanger (NHE) and the Na+-Cl− cotransporter (NCC) are the major transporters promoting Na+ reabsorption in the proximal and distal convoluted tubules of the kidney, respectively (29). In zebrafish, a distinct type of NHE isoform, NHE3b (SLC9A3B), is expressed specifically in H+-ATPase-rich cells (HRCs), which are thought to be the major ionocyte responsible for Na+ absorption (8, 17, 20, 26, 30). Acid-sensing ion channels also were recently proposed to mediate the apical uptake of Na+ in HRCs of adult zebrafish exposed to a low Na+ environment (6). In zebrafish, NCC (SLC12A10.2; a NCC-like member distinct from the mammalian orthologous of SLC12A3) is specifically expressed in another subtype of ionocyte, termed the NCC-expressing cell (NCCC) (32). Treatment of larval zebrafish with a NCC-specific inhibitor metolazone was shown to decrease the influx of both Na+ and Cl− in a dose-dependent manner (32). However, whether or not NCC is localized to the apical membrane of NCCCs remains uncertain. Nevertheless, knockdown of NCC significantly reduced Cl− absorption and whole body Cl− levels (32). Additionally, knockdown of NHE3b was found to increase NCC-expressing ionocytes and Na+ uptake, suggesting the role of NCC in the compensatory regulation of Na+ (3). Extrusion of Na+ and Cl− across the basolateral membrane of NCCCs is believed to occur via Na+-K+-ATPase (i.e., ATP1a1a.2) and chloride channels (CLC2), respectively (25, 33).
In natural environments, fish can be challenged by chronic or acute alteration in water pH (as low as pH 3.5–4.5; Refs. 10, 12). Many previous studies have utilized zebrafish as a model organism to understand the chronic effects of low water pH and the possible mechanism of compensation (3, 4, 13, 18, 20, 22, 35). Zebrafish, themselves, are known to occupy natural environments that vary significantly in pH (from 5.9 to 8.1; Ref. 7), and degradation of their habitats owing to local farming is further lowering the pH of the water they inhabit (7). Specifically, zebrafish exhibit a substantial increase in diffusive Na+ loss during prolonged exposure to acidic water (18). In adult zebrafish, mRNA expression of nhe3b was found to decrease after chronic acid exposure (35). Nevertheless, NHE3b, in association with Rhcg1 (a specific apical isoform of Rh protein involved in ammonia excretion), was critically involved in the compensatory uptake of Na+ in larval zebrafish following chronic acid acclimation (20). The number of NCCCs in larval zebrafish also was found to increase after chronic acid exposure (3). Additionally, the whole body Na+ levels in NCC-deficient fish was significantly lower than that in control fish following acid acclimation (3). Recently, it was demonstrated that larval zebrafish, like adults, exhibited an increase in passive Na+ loss during acute acid exposure (22). Moreover, when measured in control water, the capacity for Na+ uptake was significantly increased in larvae exposed to acidic water for only 2 h (19). The mechanisms underlying this rapid adjustment of Na+ uptake have not been investigated and thus are the focus of the present study.
Considering that larval zebrafish HRCs are critically involved in increasing Na+ uptake following chronic acid acclimation (discussed above), we first tested the hypothesis that HRCs are also important during or following acute exposure to acidic water. Because the results demonstrated that fish lacking HRCs were still able to increase Na+ uptake after acute acid exposure, we focused next on the potential role of NCC and evaluated the impacts of acute acidosis on both Na+ and Cl− homeostasis. Overall, the results of the present study demonstrate an important role for NCC in regulating Na+ and Cl− uptake after acute acidosis.
MATERIALS AND METHODS
Fish.
Adult zebrafish (Danio rerio) were bred and raised at the University of Ottawa. Fish were supplied with aerated, dechloraminated City of Ottawa tap water at 28.5°C, and were subjected to a constant 14:10-h light-to-dark photoperiod. Embryos were collected and reared in 50 ml petri dishes supplemented with dechloraminated City of Ottawa tap water (0.8 mM Na+, 0.25 mM Ca2+, 0.4 mM Cl−; pH 7.6) supplemented with 0.05% methylene blue. The petri dishes were kept in incubators at 28°C. All experiments were performed at 4 days postfertilization (dpf), unless mentioned otherwise. The experiments were conducted in compliance with guidelines of the Canadian Council of Animal Care and after the approval of the University of Ottawa Animal Care Committee (protocol BL-1700).
Evaluation of sodium and chloride fluxes.
The uptake of Na+ and Cl− was measured using a radiotracer method. To evaluate the uptake in control or acidic water, the rearing water was replaced with either pH 7.6 (control) or pH 4.0 water (prepared by the addition of H2SO4), and fish were transferred to a 2-ml microfuge tube. Fish were then exposed to 0.25 μCi/ml 22Na+ (Perkin Elmer, Woodbridge, ON, Canada) or 0.2 μCi/ml 36Cl− (American Radiolabeled Chemicals) for 2 h. To examine the effects of acid exposure on the capacity of Na+ or Cl− uptake, in some experiments influx was performed in control water after the 2-h preexposure to acidic water. At the end of the flux period, the fish were killed with an overdose of tricaine methanesulfonate (MS-222) and briefly washed in isotope-free water, and two larvae were pooled as one sample (N = 1). Fish were then digested with a tissue solubilizer (Solvable; Perkin Elmer) overnight at 65°C and later neutralized using glacial acetic acid. The radioactivity of the digest and the water samples was measured using a liquid scintillation counter (LS-6500; Beckman Coulter) following the addition of a scintillation cocktail (BioSafe-II; Research Products International). The Na+ or Cl− influx (Jin; pmol·fish−1·h−1) was determined using the formula: Jin = F/(SA × n × t), where F is the total radioactivity counted in the fish (counts/min), SA is the specific activity of the water (cpm/nmol), n is the number of fish, and t is the duration of the experiment in hours.
To examine whether water Na+ and Cl− levels in acidic water (pH 4.0) affected the capacity of Na+ uptake, the acidic water was supplemented with either 100 mM NaCl or 100 mM Na+ (as 50 mM of Na2SO4). The fish were exposed to this water for 2 h, and influx was subsequently performed in control water (i.e., 0.8 mM Na+, 0.4 mM Cl−; pH 7.6), as described above.
The effects of preexposure to acidic water (pH 4.0) on subsequent Na+ and Cl− uptake were also evaluated in Cl−-free and Na+-free water, respectively. The Na+-free and Cl−-free water were prepared with double deionized water, supplemented with either 0.8 mM choline chloride (Na+ free) or 0.4 mM Na2SO4 (Cl− free). Control water was supplemented with 0.8 mM NaCl. CaSO4·2H2O, MgSO4·7H2O, K2HPO4, and KH2PO4 were added in all experimental waters to obtain 0.25 mM Ca2+, 0.16 mM Mg2+, and 0.3 mM K+. Influx was performed in these waters (pH 7.6) for 2 h, as described above.
Measurement of whole body sodium and chloride content.
To evaluate whole body Na+ and Cl− levels, 20 fish were pooled (N = 1), killed with an overdose of MS-222, and briefly rinsed in deionized water. The fish were then digested with 5 N HNO3 at 65°C for 48 h and diluted appropriately with deionized water. The total Na+ content was measured by flame emission spectrophotometry (Spectra AA 220FS: Varian, Palo Alto, CA). The Cl− concentration was measured colorimetrically following the protocol of Zall et al. (36) adapted for microplate reader.
Real-time PCR.
To evaluate the effects of acute acid exposure on the mRNA abundance of Na+ or Cl− transport-related genes, the mRNA expression of nhe3b (slc9a3b), atp6v1a (encoding for V-ATPase subunit A), and ncc (slc12a10.2) were evaluated. Total RNA was extracted using RNeasy Mini kit (Qiagen), and genomic DNA was removed with DNase I (New England Biolabs), following the manufacturer's guidelines. First-strand cDNA was synthesized using RevertAid H Minus reverse transcriptase (Fermentas, Burlington, ON, Canada) and random hexamer primers. Primer sets used in the present study are summarized in Table 1. RT-quantitative PCR (qPCR) assays were performed using a Bio-Rad CFX96 qPCR system with SsoFast EvaGreen Supermixes (Bio-rad). All RT-qPCR was performed using the following conditions: 95°C for 3 min, 40 cycles of 95°C for 20 s, and 58°C for 20 s, with final extension for 5 min at 72°C. Data were normalized to the expression of 18S and were presented relative to the control group (pH 7.6).
Table 1.
Primers used in the present study
| Gene | Sequence | Ref. No. |
|---|---|---|
| nhe3b (slc9a3b) | FWD: 5′-TGCAGACAGCGCCTCTAGC-3′ | 35 |
| REV: 5′-TGTGGCCTGTCTCTGTTTGC-3′ | ||
| H+-ATPase (atp6v1a) | FWD: 5′-GAG GAA CCA CTG CCA TTC CA-3′ | 4 |
| REV: 5′-CAA CCC ACA TAA ATG ATG ACA TCG-3′ | ||
| NCC (slc12a10.2) | FWD: 5′-GCC CCC AAA GTT TTC CAG TT-3′ | 32 |
| REV: 5′-TAA GCA CGA AGA GGC TCC TTG-3′ | ||
| 18S | FWD: 5′-GGCGGCGTTATTCCCATGACC-3′ | 18 |
| REV: 5′-GGTGGTGCCCTTCCGTCAATTC-3′ |
FWD, forward; REV, reverse.
Assessment of Sodium Green accumulation in ionocytes.
The method used to evaluate Na+ accumulation in ionocytes was similar to that described by Esaki et al. (8). Fish at 4 dpf were exposed to control (pH 7.6) or acidic water (pH 4.0) for 2 h in a petri dish. The water was then replaced with control water, and the fish were transferred to a 2-ml microfuge tube. The fish were first incubated in 10 μM Sodium Green (as sodium green tetra-acetate; Invitrogen) for 1.5 h, and then in both 10 μM Sodium Green and 50 μg/ml concanavalin A (ConA, conjugated with Alexa Fluor 633; Invitrogen) for an additional 0.5 h. ConA is a vital marker for HRCs in zebrafish (26). Subsequently, the fish were anesthetized and gently washed, and images were acquired using a confocal laser scanning microscope (A1R+; Nikon Instruments). For cell density measurement, three areas (100 × 100 μm each) on the yolk sac surface of a larva (N = 1) were randomly chosen for quantification.
Morpholino gene knockdown.
The potential involvement of NHE3b-expressing ionocytes (HRCs) or NCC following acute acid exposure was evaluated using a reverse genetics approach. HRCs and NCC expression were knocked down by microinjecting previously validated morpholinos (GeneTools) against glial cell missing 2 (gcm2) (5′-AAACTGATCTGAGGATTTGGACATG-3′) (9, 11) and NCC (5′-TTGCCAAAATCAGCCTCTCCCATAT-3′) (32), respectively. The gcm2 is a transcription factor regulating differentiation of HRCs (9). The morpholino was diluted in Danieau buffer [58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0 mM HEPES (pH 7.6)] plus 0.05% phenol red and heated at 65°C for 10 min, and 2 ng of each morpholino were injected into one-cell stage embryo. We observed that injection of 2 ng morpholino effectively eliminated HRCs or significantly reduced NCC expression (see results) without inducing developmental abnormalities, and thus this dose was used in all subsequent experiments. A “sham” group was also injected with a standard control morpholino with no known target in zebrafish (5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3′) prepared as the gcm2 or NCC morpholino.
Whole-mount immunohistochemistry.
Methods for NCC immunostaining were performed as described previously (23), with slight modifications. In brief, fish at 4 dpf were fixed at 4°C overnight in a 4% paraformaldehyde solution prepared in phosphate buffered saline (PBS). After fixation, the fish were washed with PBS containing 0.1% Tween 20 (PBST), and then gradually dehydrated with methanol and stored at −20°C until use. Following rehydration with PBST, the fish were treated with 150 mM Tris·HCl (pH 9.0) at 65°C for 15 min, washed with PBST, and then blocked with 3% BSA in PBST plus 0.8% Triton-X at room temperature for 2 h. The fish were then incubated with the custom-made antibody against the zebrafish NCC (Table 2) at 4°C overnight. Subsequently, the fish were washed several times with PBST and incubated with an Alexa Fluor 488-coupled donkey anti-rabbit IgG at 1:500 dilution (Invitrogen) for 1 h in the dark at room temperature. The images were acquired using a confocal laser scanning microscopy (A1R+; Nikon Instruments).
Table 2.
Antibodies used in the present study
| Peptide Target | Antigen Sequence | Name of Antibody | Source | Species Raised | Dilution Used |
|---|---|---|---|---|---|
| NCC (SLC2A10.2) | DMGEADFGKKENQKC | Anti-NCC | Genscript | Rabbit; polyclonal | 1:250 |
| α-Subunit of H+-ATPase | AEMPADSGYPAYLGAR | Anti-H+-ATPase | Dr. M. Uchiyama, University of Toyama | Rabbit; polyclonal | 1:2,000 |
| α-Subunit of Na+-K+-ATPase | N/A | a5 | Developmental Studies Hybridoma Bank, a5 | Mouse; monoclonal | 1:250 |
N/A, not applicable.
To determine whether NCC was expressed in mitochondrion-rich cells, 4 dpf fish were incubated with 1 μM MitoTracker Red CMXRos (Invitrogen) for 10 min before fixation. The staining pattern of MitoTracker Red CMXRos in Na+-K+-ATPase-rich cells (NaRCs) or HRCs also was evaluated by subsequent immunostaining with either H+-ATPase or Na+-K+-ATPase antibody (Table 2), as described previously (23).
Statistical analysis.
All statistical analyses were performed using SigmaPlot (version 12.5; Systat Software). Data were analyzed using Student's t-test, one-way or two-way analysis of variance, followed by a post hoc Holm-Sidak test. Data are reported as means ± SE, and P ≤ 0.05 was taken as the level of significance.
RESULTS
Acute acid exposure disrupts Na+ homeostasis and results in a compensatory increase in Na+ uptake.
The impact of acute acidosis on homeostatic regulation of Na+ was evaluated in 4 dpf larval zebrafish. Influx of Na+ was significantly reduced during exposure to pH 4.0 water for 2 h (Fig. 1A). Whole body levels of Na+ also were decreased after the acid exposure (Fig. 1B). Following 2 h of acid exposure, the influx of Na+ was significantly increased when measured in control water (pH 7.6; Fig. 1C).
Fig. 1.
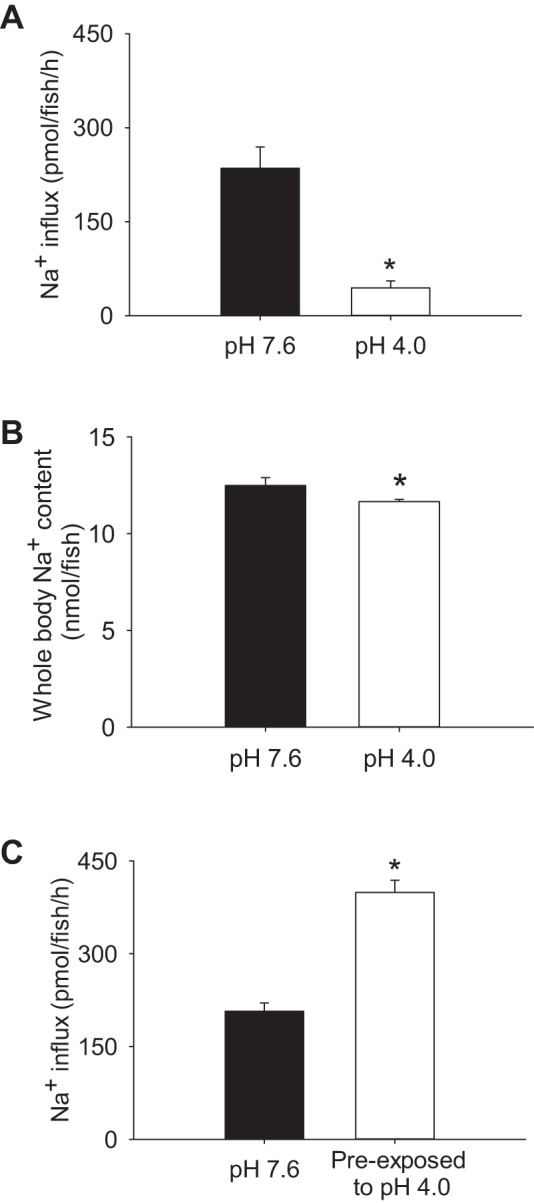
Acute acid exposure disrupts Na+ homeostasis and results in a compensatory increase in Na+ uptake. Larval zebrafish at 4 days postfertilization exhibited a significant reduction in Na+ uptake (A) and whole body Na+ levels (B) during exposure to acidic water (pH 4.0) for 2 h. C: following 2 h preexposure to acidic water, Na+ uptake was increased when influx was performed in control water (pH 7.6). Values are means ± SE. *Statistical difference between groups (Student's t-test, N = 6), P < 0.05.
HRCs do not play an important role in increasing Na+ uptake after acute acid exposure.
To evaluate the potential role of HRCs in the compensatory uptake of Na+ after acute acid exposure, the HRCs were eliminated by knocking down the cell-fate transcription factor gcm2. Compared with sham-injected fish, expression of H+-ATPase (a marker for HRC) was not observed in gcm2 morphants (Fig. 2, A and B). At pH 7.6, influx of Na+ in gcm2 morphants was higher than that of shams (Fig. 2C). In shams, preexposure to acidic water (pH 4.0) significantly increased Na+ uptake. The increased Na+ influx after acid exposure also was observed in gcm2 morphants. Results from real-time PCR demonstrated that mRNA abundance of nhe3b, atp6v1a, and ncc in wild-type fish was unaffected by acute acid exposure (Fig. 2D).
Fig. 2.
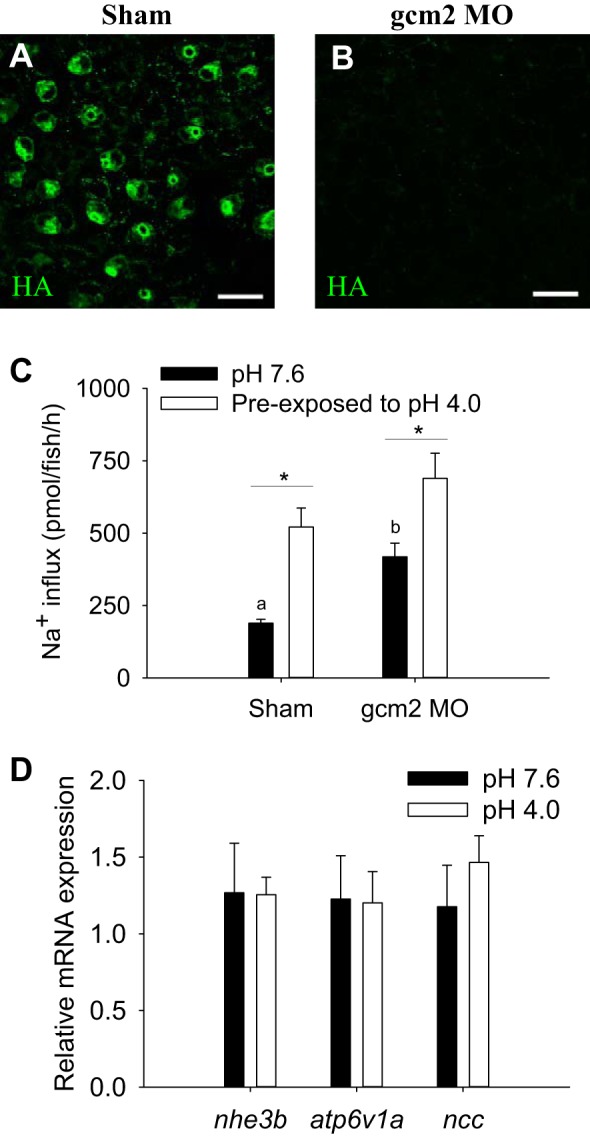
Elimination of H+-ATPase-rich cells (HRCs) does not affect the compensatory increase in Na+ uptake by acute acid exposure. A: in shams, HRCs [labeled with anti-H+-ATPase (HA)] were observed on the skin of yolk sac at 4 days postfertilization. B: HRCs were virtually absent in gcm2 morphants (gcm2 MO). Scale bar = 25 μm. C: preexposure to pH 4.0 water resulted in a significant increase in Na+ uptake in both shams and gcm2 MO. At pH 7.6, uptake of Na+ in gcm2 MO was also higher than that in shams. *Statistical difference between pH 7.6 and pH 4.0 within shams or gcm2 MO, P < 0.05. a,b|Different letters represent a statistical difference between shams and gcm2 MO within the same pH treatment (two-way ANOVA, N = 6), P < 0.05. D: results from real-time PCR revealed that exposure to pH 4.0 water did not affect the mRNA expression of nhe3b, atp6v1a, and ncc (Student's t-test, N = 6). Data are normalized by 18S and are expressed relative to fish at pH 7.6. Values are means ± SE.
The increased Na+ uptake after acute acid exposure is associated with Cl− compensation.
The effects of water Na+ and Cl− levels on the compensatory regulation of Na+ uptake after exposure to acidic water was evaluated at 4 dpf. Preexposure to acidic water resulted in a significant increase in Na+ influx (Fig. 3). Supplementation of acidic water with 100 mM NaCl prevented the increase in Na+ uptake. However, a substantial increase in Na+ uptake was observed when the acidic water was enriched with 100 mM Na+ only.
Fig. 3.
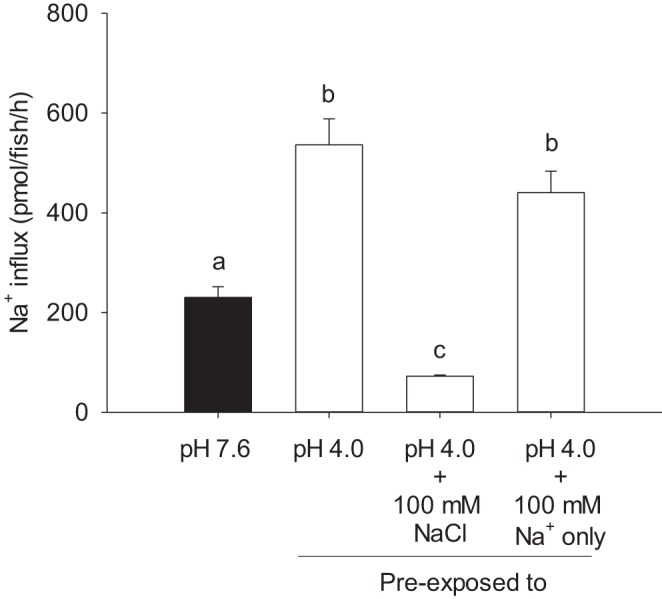
The compensatory increase in Na+ uptake by acute acid exposure is associated with the water Cl− level. Preexposure to pH 4.0 significantly increased Na+ uptake in larval zebrafish at 4 days postfertilization. The increased Na+ uptake was abolished when the acidic water was supplemented with 100 mM NaCl. However, Na+ uptake was still significantly increased when the acidic water was supplemented with 100 mM Na+ only. Note that fish were preexposed to various conditions for 2 h, and subsequently all of the influx experiments were performed in control freshwater (i.e., 0.8 mM Na+, 0.4 mM Cl−; pH 7.6). Values are means ± SE. a,b,c|Different letters represent a statistical difference between treatments (one-way ANOVA, N = 6), P < 0.05.
Acute acid exposure disrupts Cl− homeostasis and results in a compensatory increase in Cl− uptake.
Influx of Cl− was decreased significantly in fish during exposure to acidic water (Fig. 4A). Acid-exposed fish also exhibited a reduction in the whole body Cl− levels (Fig. 4B). When measured in control water (pH 7.6), the influx of Cl− was significantly increased in fish preexposed to acidic water for 2 h (Fig. 4C).
Fig. 4.
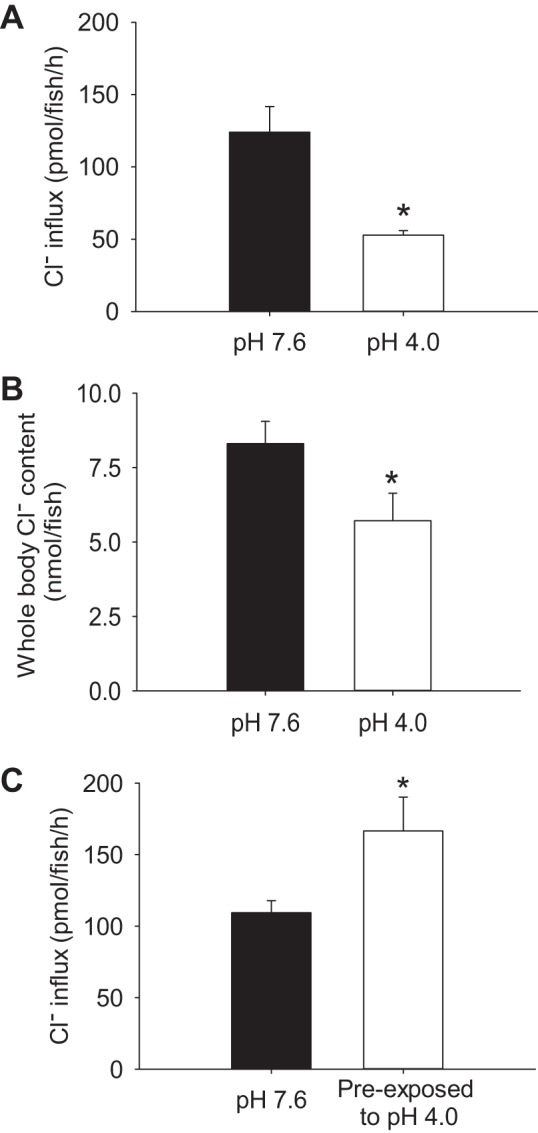
Acute acid exposure disrupts Cl− homeostasis and results in a compensatory increase in Cl− uptake. Larval zebrafish at 4 days postfertilization exhibited a significant reduction in Cl− uptake (A) and whole body Cl− levels (B) during exposure to acidic water (pH 4.0) for 2 h. C: following 2-h preexposure to acidic water, Cl− uptake was increased when influx was performed in control water (pH 7.6). Values are means ± SE. *Statistical difference between groups (Student's t-test, N = 6), P < 0.05.
The increased Sodium Green-positive cells after acute acid exposure are likely NCCCs.
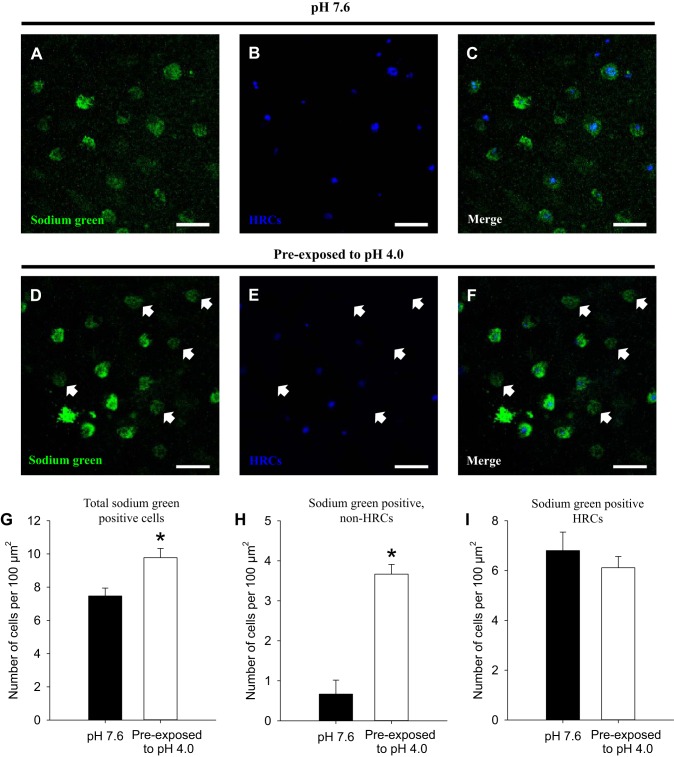
At pH 7.6, Sodium Green was accumulated predominantly in HRCs [i.e., labeled by ConA, a specific marker for HRCs (26); Fig. 5, A–C]. After exposure to acidic water, an increase in the number of Sodium Green-positive cells that were not associated with HRCs was observed (Fig. 5, D–F). Quantitative analysis revealed that the total number of Sodium Green-positive cells was increased significantly after acid exposure (Fig. 5G), owing specifically to an increase in the number of Sodium Green-positive cells that were not HRCs (Fig. 5H). Acid exposure did not affect the number of HRCs labeled with Sodium Green (Fig. 5I).
Fig. 5.
Preexposure to acidic water increases the number of Sodium Green-positive cells that are not HRCs. A–C: at 4 days postfertilization, accumulation of Sodium Green was predominantly confined to HRCs (labeled by concanavalin A) in the skin of larval zebrafish following exposure to control water (pH 7.6). D–F: after preexposure to acidic water (pH 4.0), accumulation of Sodium Green was observed in epithelial cells that were not HRCs. Scale bar = 25 μm. Quantitative analysis revealed that the total number of Sodium Green-positive cells (G) and the numbers of Sodium Green-positive non-HRCs (H) were significantly increased after acid exposure. I: however, acid exposure did not affect the number of Sodium Green-positive HRCs. Values are means ± SE. *Statistical difference between groups (Student's t-test, N = 6), P < 0.05.
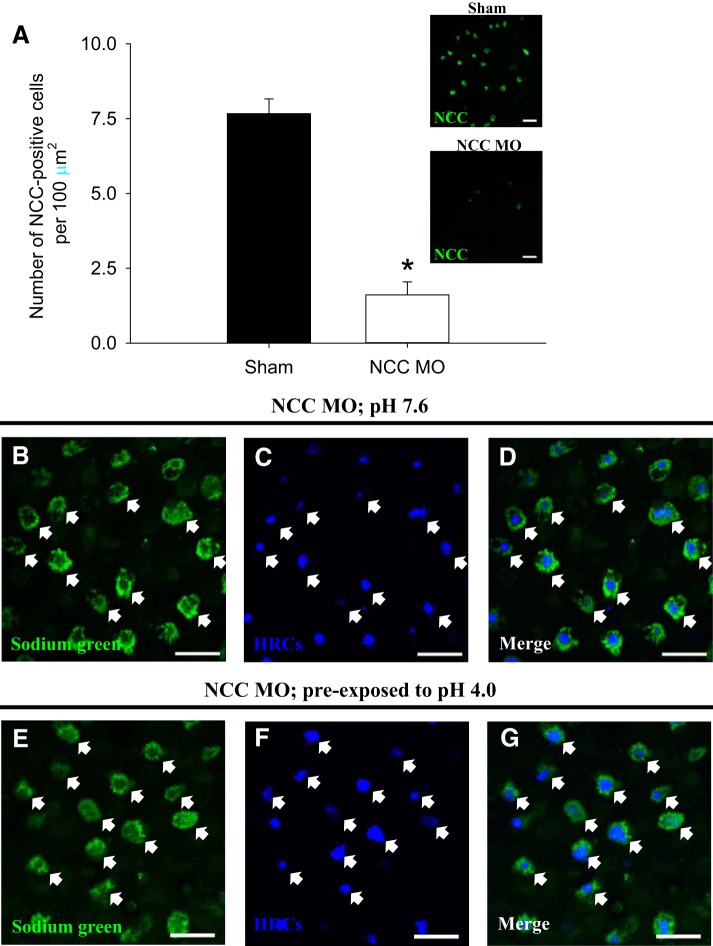
To examine whether the increased number of Sodium Green-positive cells induced by acid exposure was associated with NCCCs, Sodium Green accumulation was assessed in NCC morphants preexposed to acidic water. In NCC morphants, the number of NCC-positive cells was significantly reduced compared with shams (Fig. 6A). At pH 7.6, Sodium Green was predominantly accumulated in HRCs of NCC morphants (Fig. 6, B–D). Preexposure to acidic water did not affect Sodium Green accumulation in HRCs of NCC morphants, and virtually no Sodium Green staining was observed in non-HRCs (Fig. 6, E–G). These findings suggest that, in control/sham fish, the increase in Sodium Green-positive cells after acid exposure (i.e., Fig. 5, D–F) was attributable to enrichment of Na+ in preexisting NCCCs.
Fig. 6.
The increased number of Sodium Green-positive cells by acute acid exposure is not observed in fish experiencing NCC knockdown. A: compared with shams, the number of NCC-positive cells was significantly lowered than that in NCC morphants (NCC MO) at 4 days postfertilization. The inset images showed the immunohistochemistry of NCC in sham and NCC MO. B–D: following exposure to control water (pH 7.6), Sodium Green was predominantly accumulated in HRCs (labeled by concanavalin A) on the skin of NCC MO (depicted by arrows). E–G: similarly, Sodium Green accumulation was confined to HRCs of NCC MO following preexposure to acidic water (pH 4.0). Scale bar = 25 μm.
NCC is critically involved in the compensatory increase in both Na+ and Cl− uptake after acute acidosis.
The effect of acute acid exposure on Na+ uptake was examined under Cl−-free condition or after NCC knockdown. Fish preexposed to acidic water exhibited a significant increase in Na+ uptake when measured in control water (Fig. 7A). However, the increased Na+ uptake after acid exposure was not observed when influx was measured in Cl−-free water. Notably, Na+ uptake at pH 7.6 remained identical in the presence and absence of Cl−. These results suggested that there was no Cl−-dependent Na+ influx under basal conditions, but a significant Cl−-dependent Na+ influx after exposure to acidic water. At pH 7.6, uptake of Na+ in NCC morphants was significantly higher than that in shams (Fig. 7B). However, NCC morphants failed to increase Na+ uptake after acute acid exposure.
Fig. 7.
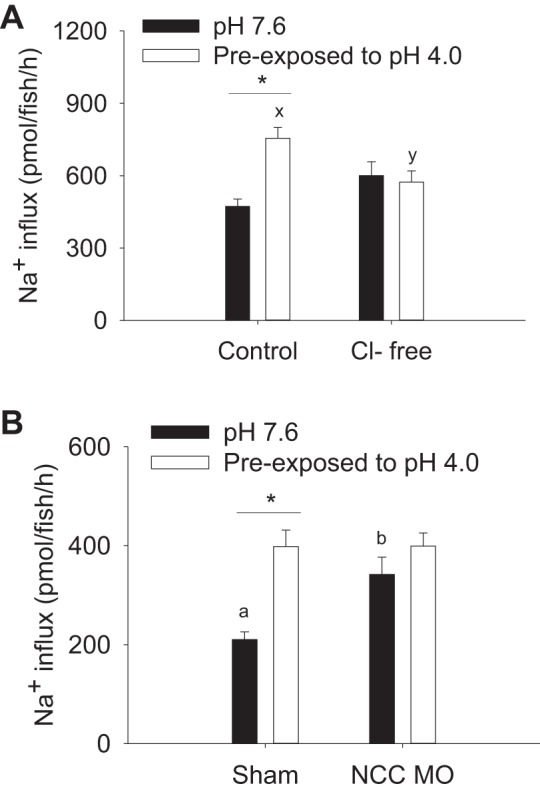
NCC is involved in the compensatory increase in Na+ uptake by acute acid exposure. A: at 4 days postfertilization, preexposure to acidic water (pH 4.0) significantly increased Na+ uptake in control water. Such an increase was not observed when the influx was performed in Cl−-free water. *Statistical difference between pH 7.6 and pH 4.0 within control or Cl−-free condition, P < 0.05. x,y|Different letters represent a statistical difference between control and Cl−-free condition within pH 4.0 treatment (two-way ANOVA, N = 6), P < 0.05. B: preexposure to acidic water increased Na+ influx in sham, but not in NCC morphants (NCC MO). NCC MO also exhibited a higher Na+ influx than sham at pH 7.6. *Statistical difference between pH 7.6 and pH 4.0 within shams, P < 0.05. a,b|Different letters represent a statistical difference between shams and NCC MO at pH 7.6 (two-way ANOVA, N = 6), P < 0.05.
To understand the underlying molecular mechanism for the increased Cl− uptake after acute acid exposure, the uptake of Cl− was examined in fish experiencing gcm2 (fish lacking HRCs) or NCC knockdown. In shams, preexposure to acidic water significantly increased Cl− influx (Fig. 8A); this response was unaffected by knockdown of gcm2. The increase in Cl− influx after acid exposure was not observed in fish experiencing NCC knockdown (Fig. 8B). Knockdown of gcm2 or NCC did not significantly affect Cl− influx at pH 7.6. The increased Cl− uptake by acid exposure was abolished when the influx was performed in Na+-free water (Fig. 8C), suggesting the Na+-dependent uptake of Cl− after acid exposure.
Fig. 8.
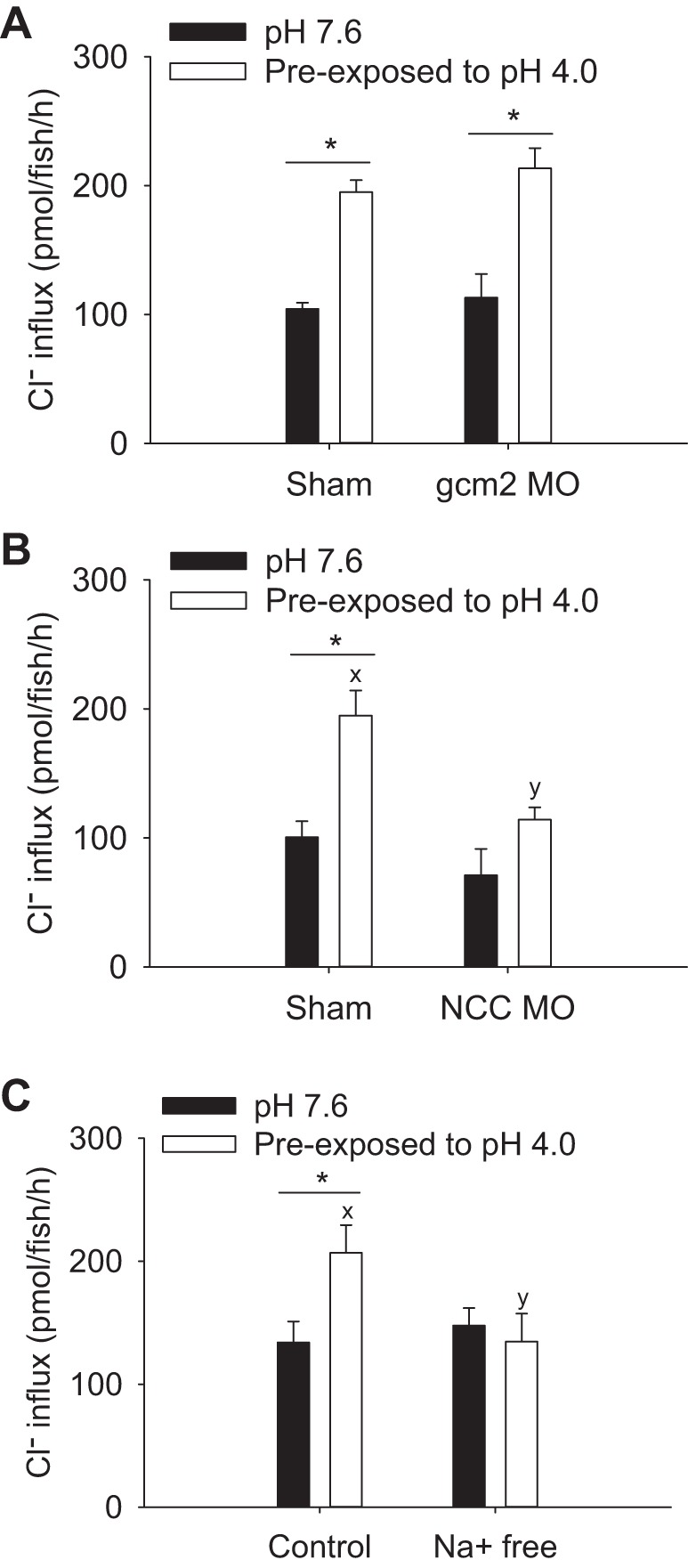
Knockdown of NCC, but not gcm2, prevented the compensatory increase in Cl− uptake by acute acid exposure. A: at 4 days postfertilization, preexposure to acidic water (pH 4.0) significantly increased Cl− influx in both sham and gcm2 morphants (gcm2 MO). B: in contrast, the significant increase in Cl− influx by acid preexposure was not observed in NCC MO. C: the increased Cl− influx also was not observed when the influx was performed in Na+-free water. Values are means ± SE. *Statistical difference between pH 7.6 and pH 4.0, P < 0.05. x,y|Different letters represent a statistical difference between shams and NCC MO, or between control and Na+-free water (two-way ANOVA, N = 6), P < 0.05.
NCC is expressed apically in mitochondrion-rich cells.
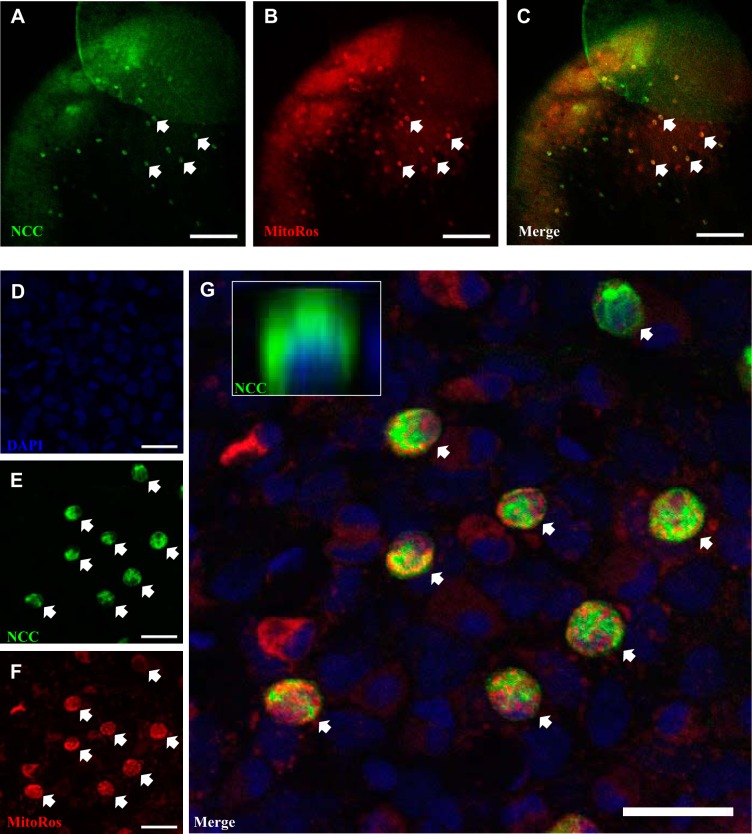
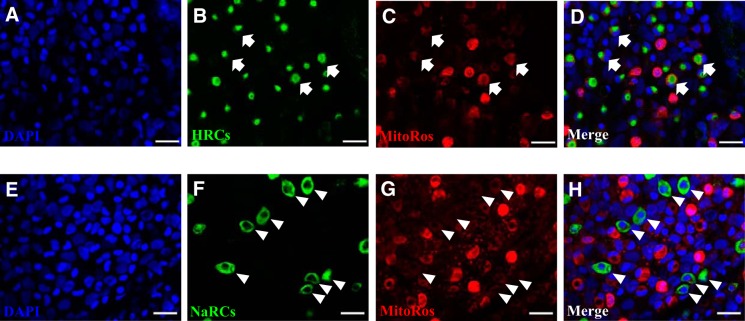
NCC was expressed in the skin covering the yolk sac (Fig. 9). Using MitoTracker Red CMXRos (MitoRos) as a marker for mitochondria, it was demonstrated that the expression of NCC was localized in a subset of mitochondrion-rich cells. All NCCCs appeared to be rich in mitochondria. Z-stack analysis revealed that NCC was expressed in the apical region of the cells. We further evaluated the staining property of MitoRos in other ionocytes and observed that a subset of MitoRos-positive cells were HRCs (labeled by H+-ATPase; Fig. 10, A–D). Interestingly, unlike MitoTracker Green FM, which strongly stained NaRCs (26), MitoRos only weakly stained NaRCs (NKA staining; Fig. 10, E–H).
Fig. 9.
NCCCs are rich in mitochondria. A–C: fluorescent immunohistochemistry and confocal microscopy showing the expression of NCC in the skin of larval zebrafish at 4 days postfertilization. MitoTracker Red CMXRos (MitoRos) staining was observed in all NCCCs. However, not all MitoRos-positive cells were NCCCs. Scale bar = 100 μm. D–G: high magnification of DAPI (i.e., nucleus), NCC, and MitoRos staining in the skin of larval zebrafish. G, inset: z-stack image showing NCC was expressed in the apical region of the cell. The arrows depict NCCCs that were also stained with MitoRos. Scale bar = 20 μm.
Fig. 10.
Differential staining of MitoTracker Red CMXRos in HRCs and NaRCs. A–D: fluorescent immunohistochemistry and confocal microscopy showing the staining of MitoTracker Red CMXRos (MitoRos) in H+-ATPase-rich cells (HRCs; depicted by the arrows). E–H: relatively weak staining of MitoRos in Na+-K+-ATPase-rich cells (NaRCs) was observed (depicted by the arrowheads). Nuclei were labeled by DAPI. HRCs and NaRCs were labeled by H+-ATPase and Na+-K+-ATPase antibody, respectively. Scale bar = 25 μm.
DISCUSSION
It is well documented that acid exposure adversely affects whole body Na+ homeostasis in freshwater fish, likely by a combination of inhibiting active Na+ uptake and increasing paracellular Na+ loss (for a review, see Refs. 21, 27, 34). Unlike other freshwater fish studied, however, zebrafish respond to chronic acid water exposure by increasing their Na+ influx (18, 20), and thus initial decreases in whole body Na+ levels are restored within 1–2 wk of continued exposure to acidic water (18). The increasing Na+ uptake in larvae acclimated to acidic water is probably associated with elevated numbers of HR cells, and increased H+ secretion and NHE3b activity in individual HR cells (13, 20). In addition, NCC may be involved in regulating Na+ balance during chronic acidosis based on increasing numbers of NCC cells during exposure and a greater disruption in whole body Na+ balance in fish experiencing NCC knockdown (2). The significant novel finding of the present study is that acute exposure to acidic water markedly increased Na+ uptake capacity in 4 dpf zebrafish larvae, which, in contrast to chronic acid exposure, resulted exclusively from regulation of Na+ uptake via NCC.
Differentiation of HRCs in zebrafish is regulated by the cell-fate transcription factor gcm2 (4, 31). The results of previous studies demonstrated that elimination of HRCs by gcm2 knockdown caused an increase in the number of NCCCs and an elevated rate of Na+ uptake (3). Similarly, we also found in the present study that Na+ uptake in gcm2 morphants was higher than in control (sham) fish. More importantly, however, our results suggested that gcm2 morphants continued to exhibit a significant increase in Na+ uptake after acute acid exposure. This finding suggested that HRCs were unlikely involved in the rapid regulation of Na+ uptake following acute acidosis. A previous study demonstrated that Sodium Green, a Na+-dependent fluorescent probe, is preferentially accumulated in HRCs (8). Here we also observed that Sodium Green was predominantly confined to HRCs in larvae maintained at pH 7.6. However, following preexposure to acidic water, the number of Sodium Green-positive cells was significantly increased in cells that were not HRCs, suggesting that another ionocyte subtype was involved in the absorption of Na+ after acid exposure. More importantly, virtually no Sodium Green staining was observed in non-HRCs of NCC morphants following the acid exposure, strongly suggesting that the increased accumulation of Sodium Green by acid exposure was NCCCs.
To further elucidate the mechanism(s) by which acute acidosis stimulated Na+ uptake, we evaluated whether enrichment of water Na+ or Cl− levels in acidic water affected the compensatory regulation of Na+ uptake. Presumably, the increased ion levels in the water would reduce the diffusive loss of ions from the zebrafish larvae during acid exposure, thereby diminishing the need for compensatory increases in ion uptake. In agreement with this notion, we observed that the increased Na+ uptake by acute acid exposure was prevented when the acidic water was supplemented with 100 mM NaCl. More interestingly, the increased Na+ uptake still was observed when the acidic water was supplemented with 100 mM Na+ only. Therefore, it is possible that the increased Na+ uptake after acute acid exposure was associated with the compensation of Cl−. Thus we next examined the impact of acute acidosis on Cl− homeostasis, and observed that Cl− uptake and whole body Cl− levels also were decreased in fish exposed to acidic water. Disturbance of Cl− homeostasis by acute acid exposure has been reported in other freshwater fish, including rainbow trout (Oncorhynchus mykiss) (1, 28), angelfish (Pterophyllum scalare), and discus (Symphysodon discus) (5). Two additional novel findings of the present study were that the capacity for Cl− uptake was substantially increased in zebrafish acutely preexposed to acidic water, and that the increased Na+ uptake after acid exposure was abolished when the influx was performed in Cl−-free water. Overall, these findings strongly suggest that the increased uptake of Na+ after acute acid exposure was coupled to the compensation of whole body Cl− levels.
Because the rapid compensatory regulation of Na+ uptake appeared to be associated with that of Cl− and was reliant on external Cl−, we examined the potential involvement of NCC in regulating both Na+ and Cl− uptake after acute acidosis. It was previously shown that zebrafish HRCs and NaRCs are enriched with mitochondria (26). Here, using a MitoTracker Red CMXRos, we observed that NCCCs also are rich in mitochondria. Interestingly, unlike MitoTracker Green FM, which strongly stained NaRCs (26), relatively weak staining of MitoTracker Red CMXRos was observed in NaRCs. Additionally, we demonstrated that NCC is localized in the apical region of the ionocyte, further supporting a role of NCC in apical Na+ and Cl− uptake. Previous studies have suggested that, in zebrafish, NCC could become important under physiological stressors, such as chronic acidosis and other periods of increased paracellular ion losses (3, 24). To further clarify the role of NCC, we assessed the uptake of Na+ and Cl− in NCC morphants preexposed to acidic water. At pH 7.6, Na+ uptake in NCC morphants was significantly higher compared with shams. Similar findings were reported by Wang et al. (32), where NCC morphants exhibited an increase in Na+ uptake, presumably owing to a compensatory increase in nhe3b expression. The present study showed that the increased Na+ and Cl− uptake by acid exposure was not observed in Cl−-free and Na+-free conditions, respectively. Additionally, NCC morphants were unable to increase both Na+ and Cl− uptake after acute acidosis. In contrast, gcm2 morphants lacking HRCs still exhibited a significant increase in Cl− uptake after acid exposure. These results clearly indicated that NCC, but not NHE3b, was involved in increasing Na+ and Cl− uptake following acid preexposure. A previous study showed that knockdown of NCC reduced Cl− influx in larval zebrafish at 5 dpf (32). Interestingly, the results from the present study suggested that, at 4 dpf, NCC plays a limited role in the uptake of Na+ and Cl− at pH 7.6. Therefore, it is likely that, at 4 dpf, other pathways such as NHE3b and Cl−/HCO3− exchanger (slc26 family) are involved in the uptake of Na+ and Cl−, respectively, under basal conditions (2, 3). Preexposure to acidic water appeared to increase the activity of NCC, which would allow rapid compensation for both Na+ and Cl−. Taken together, the results of the present study suggest that NCCCs are critically involved in the compensatory regulation of Na+ and Cl− uptake after acute acidosis. However, how NCC acutely regulates the uptake of Na+ and Cl− remains unclear. It is possible that acid exposure modulates the activity of NCC by posttranslational modification (e.g., phosphorylation) and/or by membrane trafficking. Further research is required to resolve the mechanisms underlying the rapid stimulation of NCC in zebrafish larvae acutely exposed to acidic water.
Conclusions and perspectives.
In summary, the present study demonstrated that NCC is critically involved in the rapid regulation of Na+ and Cl− uptake after acute acidosis. In some environments, fish can be challenged by a rapid change in water pH. Using the zebrafish model, the present findings revealed that the compensatory mechanism for Na+ uptake by exposure to a low pH environment is different between chronic acid acclimation and acute acid exposure; NHE3b is important for Na+ compensation following acid acclimation (20), whereas NCC is involved after acute exposure. Based on our findings, we propose that acute acidosis disrupts Na+ and Cl− homeostasis, and that activation of NCC would allow compensation for both ions. However, the mechanism for the rapid regulation of NCC by acid exposure is not clear and remains to be fully elucidated in future studies. Overall, the present study suggests that the zebrafish model may prove useful in evaluating the homeostatic regulation and pathophysiology of ions associated with acid stress.
GRANTS
This work was supported by the Natural Sciences and Engineering Research Council Discovery and Natural Sciences and Engineering Research Council Research Tools and Instrumentation Grants to S. F. Perry.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.W.K. and S.F.P. conceived and designed research; R.W.K. performed experiments; R.W.K. analyzed data; R.W.K. and S.F.P. interpreted results of experiments; R.W.K. prepared figures; R.W.K. drafted manuscript; R.W.K. and S.F.P. edited and revised manuscript; R.W.K. and S.F.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Vishal Saxena, Christine Archer, and Bill Fletcher for help with animal husbandry, and Andrew Ochalski for help in microscopy. We also thank Dr. M. Uchiyama (University of Toyama) for the H+-ATPase antibody. We acknowledge Dr. Yusuke Kumai (1985–2015) for inspiration in the zebrafish acidosis research.
REFERENCES
- 1.Audet C, Wood CM. Do rainbow trout (Salmo gairdneri) acclimate to low pH? Can J Fish Aquat Sci 45: 1399–1405, 1988. [Google Scholar]
- 2.Bayaa M, Vulesevic B, Esbaugh A, Braun M, Ekker ME, Grosell M, Perry SF. The involvement of SLC26 anion transporters in chloride uptake in zebrafish (Danio rerio) larvae. J Exp Biol 212: 3283–3295, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Chang WJ, Wang YF, Hu HJ, Wang JH, Lee TH, Hwang PP. Compensatory regulation of Na+ absorption by Na+/H+ exchanger and Na+-Cl− cotransporter in zebrafish (Danio rerio). Front Zool 10: 1–12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang WJ, Horng JL, Yan JJ, Hsiao CD, Hwang PP. The transcription factor, glial cell missing 2, is involved in differentiation and functional regulation of H+-ATPase-rich cells in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 296: R1192–R1201, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Duarte RM, Ferreira MS, Wood CM, Val AL. Effect of low pH exposure on Na+ regulation in two cichlid fish species of the Amazon. Comp Biochem Physiol A Mol Integr Physiol 166: 441–448, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Dymowska AK, Boyle D, Schultz AG, Goss GG. The role of acid-sensing ion channels (ASICs) in epithelial Na+ uptake in adult zebrafish (Danio rerio). J Exp Biol 2015. [DOI] [PubMed] [Google Scholar]
- 7.Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 4: 21–40, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Esaki M, Hoshijima K, Kobayashi S, Fukuda H, Kawakami K, Hirose S. Visualization in zebrafish larvae of Na+ uptake in mitochondria-rich cells whose differentiation is dependent on foxi3a. Am J Physiol Regul Integr Comp Physiol 292: R470–R480, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Esaki M, Hoshijima K, Nakamura N, Munakata K, Tanaka M, Ookata K, Asakawa K, Kawakami K, Wang W, Weinberg ES, Hirose S. Mechanism of development of ionocytes rich in vacuolar-type H+-ATPase in the skin of zebrafish larvae. Dev Biol 329: 116–129, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez RJ, Wilson RW, Wood CM. Ionoregulation in tropical fishes from ion poor, acidic blackwaters. In: Fish Physioliology. The Physiology of Tropical Fishes, edited by Val A, Fonseca de Almeida e Val VM, and Rall D. New York: Academic, 2005, vol. 21, p. 397–442. [Google Scholar]
- 11.Hanaoka R, Ohmori Y, Uyemura K, Hosoya T, Hotta Y, Shirao T, Okamoto H. Zebrafish gcmb is required for pharyngeal cartilage formation. Mech Dev 121: 1235–1247, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Hirata T, Kaneko T, Ono T, Nakazato T, Furukawa N, Hasegawa S, Wakabayashi S, Shigekawa M, Chang MH, Romero MF, Hirose S. Mechanism of acid adaptation of a fish living in a pH 3.5 lake. Am J Physiol Regul Integr Comp Physiol 284: R1199–R1212, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Horng JL, Lin LY, Hwang PP. Functional regulation of H+-ATPase-rich cells in zebrafish embryos acclimated to an acidic environment. Am J Physiol Cell Physiol 296: C682–C692, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Hwang PP. Ion uptake and acid secretion in zebrafish (Danio rerio). J Exp Biol 212: 1745–1752, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Hwang PP, Lee TH, Lin LY. Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol 301: R28–R47, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Hwang PP, Chou MY. Zebrafish as an animal model to study ion homeostasis. Pflügers Arch 465: 1233–1247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito Y, Kato A, Hirata T, Hirose S, Romero MF. Na+/H+ and Na+/NH4+ exchange activities of zebrafish NHE3b expressed in Xenopus oocytes. Am J Physiol Regul Integr Comp Physiol 306: R315–R327, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumai Y, Bahubeshi A, Steele S, Perry SF. Strategies for maintaining Na+ balance in zebrafish (Danio rerio) during prolonged exposure to acidic water. Comp Biochem Physiol A 160: 52–62, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Kumai Y, Bernier NJ, Perry SF. Angiotensin-II promotes Na+ uptake in larval zebrafish, Danio rerio, in acidic and ion-poor water. J Endocrinol 220: 195–205, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Kumai Y, Perry SF. Ammonia excretion via Rhcg1 facilitates Na+ uptake in larval zebrafish, Danio rerio, in acidic water. Am J Physiol Regul Integr Comp Physiol 301: R1517–R1528, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Kwong RWM, Kumai Y, Perry SF. The physiology of fish at low pH: the zebrafish as a model system. J Exp Biol 217: 651–662, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Kwong RWM, Perry SF. Cortisol regulates epithelial permeability and sodium losses in zebrafish exposed to acidic water. J Endocrinol 217: 253–264, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Kwong RWM, Perry SF. An essential role for parathyroid hormone in gill formation and differentiation of ion-transporting cells in developing zebrafish. Endocrinology 156: 2384–2394, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Kwong RWM, Perry SF. The tight junction protein claudin-b regulates epithelial permeability and sodium handling in larval zebrafish, Danio rerio. Am J Physiol Regul Integr Comp Physiol 304: R504–R513, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao BK, Chen RD, Hwang PP. Expression regulation of Na+-K+-ATPase α1-subunit subtypes in zebrafish gill ionocytes. Am J Physiol Regul Integr Comp Physiol 296: R1897–R1906, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Lin LY, Horng JL, Kunkel JG, Hwang PP. Proton pump-rich cell secretes acid in skin of zebrafish larvae. Am J Physiol Cell Physiol 290: C371–C378, 2006. [DOI] [PubMed] [Google Scholar]
- 27.McDonald DG. The effects of H+ upon the gills of freshwater fish. Can J Zool 61: 691–703, 1983. [Google Scholar]
- 28.McDonald DG, Walker RL, Wilkes PRH. The interaction of environmental calcium and low pH on the physiology of the rainbow trout, Salmo Gairdneri. II. Branchial ionoregulatory mechanisms. J Exp Biol 102: 141–155, 1983. [Google Scholar]
- 29.Meneton P. Comparative roles of the renal apical sodium transport systems in blood pressure control. J Am Soc Nephrol 11: S135–S139, 2000. [PubMed] [Google Scholar]
- 30.Shih TH, Horng JL, Liu ST, Hwang PP, Lin LY. Rhcg1 and NHE3b are involved in ammonium-dependent sodium uptake by zebrafish larvae acclimated to low-sodium water. Am J Physiol Regul Integr Comp Physiol 302: R84–R93, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Shono T, Kurokawa D, Miyake T, Okabe M. Acquisition of glial cells missing 2 enhancers contributes to a diversity of ionocytes in zebrafish. PLoS One 6: e23746, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YF, Tseng YC, Yan JJ, Hiroi J, Hwang PP. Role of SLC12A10.2, a Na-Cl cotransporter-like protein, in a Cl uptake mechanism in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 296: R1650–R1660, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Wang YF, Yan JJ, Tseng YC, Chen RD, Hwang PP. Molecular physiology of an extra-renal Cl(-) uptake mechanism for body fluid Cl(-) homeostasis. Int J Biol Sci 11: 1190–1203, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood CM. The physiological problems of fish in acid waters. In: Acid Toxicity and Aquatic Animals, edited by Morris R, Brown DJA, Taylor EW, and Brown JA. Cambridge, UK: Cambridge University Press, 1989, p. 125–152. [Google Scholar]
- 35.Yan JJ, Chou MY, Kaneko T, Hwang PP. Gene expression of Na+/H+ exchanger in zebrafish H+-ATPase-rich cells during acclimation to low-Na+ and acidic environments. Am J Physiol Cell Physiol 293: C1814–C1823, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Zall DM, Fisher D, Garner MQ. Photometric determination of chlorides in water. Anal Chem 28: 1665–1668, 1956. [Google Scholar]