congenital hereditary endothelial dystrophy (CHED; OMIM no. 217700) is a rare disorder of the corneal endothelium that results in early onset corneal edema and visual impairment. SLC4A11 is the most disparate member of the family of SLC4-gene encoded proteins that includes anion exchangers (AEs) and Na+-HCO3− cotransporters (NBCs). Mutations in SLC4A11 are associated with CHED (9), and genetic ablation of Slc4a11 in mice can recapitulate the CHED disease phenotype (2, 3). Although misfolding mutants of SLC4A11 have been reported, CHED can also result from mutants that are not misfolded, suggesting that impaired transport activity is the most likely basis of disease pathology.
Although, unlike most of its brethren, SLC4A11 does not transport HCO3−, it is well conserved among metazoans. For example, two of the four Caenorhabditis elegans Slc4-like transporters (ABTS-2 and -3) are more closely related to SLC4A11 than to either AE or NBC transporters. Early physiological approaches concluded that SLC4A11 codes for a Na+-coupled borate transporter (7), reminiscent of the boron-transporting action of Slc4-like proteins from plants and yeast. However, more recent approaches have concluded that boron transport is unlikely. While the precise substrate(s) of SLC4A11 remain unsettled, limited consensus is that SLC4A11 is a permeation pathway for H+(OH−) that is influenced by membrane voltage (Vm). However, other modes of operation have been suggested over the last several years that range from 2H+-NH3 cotransport to electrogenic Na+/H+ exchange, and recently SLC4A11 has also been shown to enhance membrane water permeability (8).
Within the past few months, three separate papers were published that studied transport characteristics of SLC4A11/Slc4a11 (4-6). Not surprisingly given the history of this field, each of these has reached somewhat different conclusions. Work from Loganathan et al. (5) in the American Journal of Physiology-Cell Physiology provided evidence of water and electroneutral NH3 transport by human SLC4A11, but failed to detect Vm-activated H+(OH−) flux. Work by Kao et al. (4), also published in the American Journal of Physiology-Cell Physiology, concluded that human SLC4A11 can operate in three modes, mediating Na+-independent and Na+-coupled H+ (OH−) flux as well as electrogenic H+-NH3 cotransport. In this issue of the American Journal of Physiology-Cell Physiology, Myers et al. (6) utilize two-electrode voltage-clamp of Xenopus oocytes with H+-selective microelectrodes to provide evidence that mouse Slc4a11 forms an ideally selective H+(OH−) conduction pathway whose activity is linked to Vm, stimulated by rises in intracellular and extracellular pH (pHi and pHe, respectively), and uncoupled from the transport of any other substrate. Significantly, the Myers work presents a detailed attempt to reconcile the results of these seemingly contradictory studies. The low hanging fruit is of course the difference in species from which the transporter was derived. However, human and mouse proteins are 85% identical at the amino acid level, with the main disparity at the extreme N-terminus of the protein, and three of the four Slc4a11-null mouse strains exhibit signs of CHED. Hence, while possible, it is unlikely that the observed differences can be attributed to species.
The major point of controversy among prior studies was whether H+(OH−) transport occurs in the absence of NH3/NH4+, with additional questions of whether NH3/NH4+ stimulate H+(OH−) transport or are substrates for transport themselves and whether there is a role for Na+. A key observation of the Myers study is that Slc4a11 is stimulated, upon depolarization of Vm, by rises in pHi brought about by its own action. Because NH3/NH4+ application can depolarize Vm and independently raise pHi, Myers suggests that the stimulatory effect of NH3/NH4+ upon SLC4A11 is indirect. Indeed, Myers finds that the H+(OH−) conductance of Slc4a11 can be similarly robust in the absence or the presence of NH3/NH4+. A second critical observation is that the pH-responsiveness of Vm in Slc4a11-expressing cells is ideally Nernstian with respect to H+(OH−), ruling out coupling of H+(OH−) movement with other major physiological ions such as Na+ and Cl−. The authors state that, “we see no electrophysiological evidence in the work of others that cannot be explained by the action of a pHi and pHe-sensitive H+/OH− conductance.” Whether this holds up to the test of time remains to be seen, but Myers et al.'s robust data and detailed analysis supporting this claim are certainly worth considering.
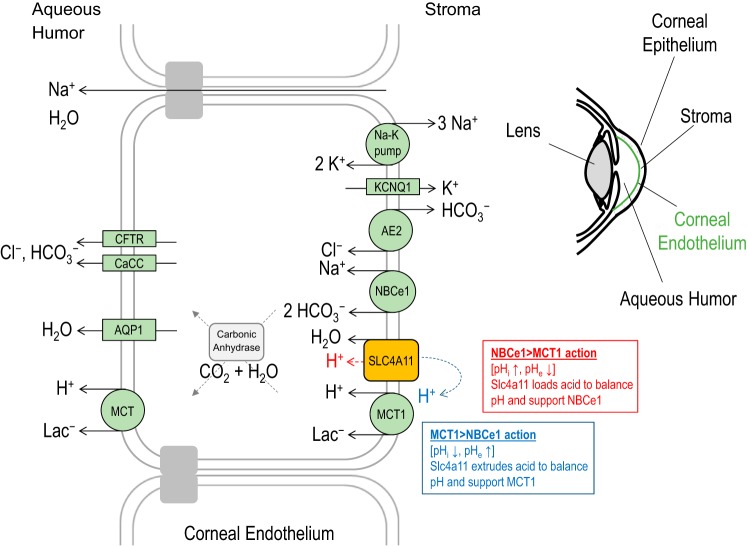
A key question that arises from the conclusion of the Myers study is how might a water-permeable, pH-sensitive H+(OH−) conductance support corneal health? SLC4A11 is expressed in the basolateral membrane of corneal endothelial cells. The endothelial layer counters osmotically driven water movement from the aqueous humor into the collagen matrix of the stroma. This is accomplished through a “pump-leak” mechanism (reviewed in ref. 1), encompassing both water and ion movement back into the aqueous humor. While it is intuitive how water transport by SLC4A11 could support “leak,” the authors provide insightful speculation as to how H+(OH−) transport might support “pump” function. At the basolateral membrane of endothelial cells, the Na+-HCO3− cotransporter NBCe1 and the H+-coupled lactate transporter MCT1 are key components of the reabsorption process. In a system where the movement of acid-base equivalents supports physiologically coupled transporters, pH balancing is not always precise and local substrate gradients can build up. The sensitivity of SLC4A11 to pH and Vm, its ability to operate either as an ideally selective acid-loader or acid-extruder depending on prevailing conditions, and its uncoupling from the transport of any other ion might optimize the efficiency of endothelial fluid transport through two notable mechanisms. First, SLC4A11-mediated acid extrusion could facilitate H+ availability for MCT1 action, particularly during sleep, when eye closure promotes anaerobic metabolism and increased lactate availability. Second, when stromal lactate is low, SLC4A11 could act as an acid loader, helping to pH balance the system without futile cycles of HCO3− exchange that would otherwise result from functional coupling of the acid-loading Cl−/HCO3−-exchanger AE2 to NBCe1. These ideas are summarized in Fig. 1.
Fig. 1.
Role of SLC4A11 in the cornea. The corneal endothelium reabsorbs fluid from the collagen matrix that constitutes the stroma, preventing corneal edema. SLC4A11 in the basolateral membrane functions in local pH balancing and provides conditional support for MCT1 and NBCe1 activity.
A further question is whether SLC4A11 is of physiological importance in other tissues, and if so, whether the underlying mechanism supports its presumptive action as an ideally selective, uncoupled H+(OH−)-conductive pathway. Within this context is the observation that SLC4A11 is expressed in the inner ear, and SLC4A11 mutations are often accompanied by sensorineural hearing loss (Harboyan syndrome; OMIM no. 217400). Similarly, a loss of normal expression in the proximal tubules, the thin descending limb of the loop of Henle, and the collecting ducts may underlie a mild urine-concentrating defect in Slc4a11 mutant mice (intriguingly, this defect has yet to be described in human patients). These observations may motivate further studies of SLC4A11 function and will help to challenge or confirm the role of SLC4A11 in local acid-base signaling and movement.
Finally, there remains the previously published finding that SLC4A11 can move water though the membrane. Even though this aspect of SLC4A11 function was not investigated in the Myers study, it leads to the questions of whether water transport might exhibit pH dependence and how this would contribute to the physiological role of the protein. In addition, the mechanism whereby water transport might be associated with a H+(OH−) conductance pathway is of great interest. The details of this mechanism likely rely on whether SLC4A11 acts as a H+(OH−) channel or a transporter, which remains to be determined. Overall, this work, in conjunction with that of others, highlights a high degree of interest in SLC4A11 and suggests that it utilizes a novel conductance pathway to support corneal physiology and health.
GRANTS
Research in the author's laboratory is supported by the National Science Foundation and the National Institutes of Health (IOS1352836, R01HL127891, and R01GM087483).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
K.N. drafted manuscript.
REFERENCES
- 1.Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res 95: 2–7, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groger N, Frohlich H, Maier H, Olbrich A, Kostin S, Braun T, Boettger T. SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness, and polyuria. J Biol Chem 285: 14467–14474, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han SB, Ang HP, Poh R, Chaurasia SS, Peh G, Liu J, Tan DT, Vithana EN, Mehta JS. Mice with a targeted disruption of Slc4a11 model the progressive corneal changes of congenital hereditary endothelial dystrophy. Invest Ophthalmol Vis Sci 54: 6179–6189, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Kao L, Azimov R, Shao XM, Frausto RF, Abuladze N, Newman D, Aldave AJ, Kurtz I. Multifunctional ion transport properties of human SLC4A11: comparison of the SLC4A11-B and SLC4A11-C variants. Am J Physiol Cell Physiol 311: C820–C830, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loganathan SK, Schneider HP, Morgan PE, Deitmer JW, Casey JR. Functional assessment of SLC4A11, an integral membrane protein mutated in corneal dystrophies. Am J Physiol Cell Physiol 311: C735–C748, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers EJ, Marshall A, Jennings ML, Parker MD. Mouse Slc4a11 expressed in Xenopus oocytes is an ideally selective H+/OH− conductance pathway that is stimulated by rises in intracellular and extracellular pH. Am J Physiol Cell Physiol (September 28, 2016). doi: 10.1152/ajpcell.00259.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park M, Li Q, Shcheynikov N, Zeng W, Muallem S. NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol Cell 16: 331–341, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Vilas GL, Loganathan SK, Liu J, Riau AK, Young JD, Mehta JS, Vithana EN, Casey JR. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum Mol Genet 22: 4579–4590, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DT, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VH, Salto-Tellez M, Venkatraman A, Guo K, Hemadevi B, Srinivasan M, Prajna V, Khine M, Casey JR, Inglehearn CF, Aung T. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet 38: 755–757, 2006. [DOI] [PubMed] [Google Scholar]