Abstract
Vascular smooth muscle cell (SMC) migration is an essential step involved in neointimal formation in restenosis and atherosclerosis. Lysophosphatidic acid (LPA) is a bioactive component of oxidized low-density lipoprotein and is produced by activated platelets, implying that LPA influences vascular remodeling. Our previous study revealed that matricellular protein CCN1, a prominent extracellular matrix (ECM) protein, mediates LPA-induced SMC migration in vitro. Here we examined the role of CCN1 in LPA-induced neointimal formation. By using LPA infusion of carotid artery in a mouse model, we demonstrated that LPA highly induced CCN1 expression (approximately six- to sevenfold) in neointimal lesions. Downregulation of CCN1 expression with the specific CCN1 siRNA in carotid arteries blocked LPA-induced neointimal formation, indicating that CCN1 is essential in LPA-induced neointimal formation. We then used LPA receptor knockout (LPA1−/−, LPA2−/−, and LPA3−/−) mice to examine LPA receptor function in CCN1 expression in vivo and in LPA-induced neointimal formation. Our data reveal that LPA1 deficiency, but not LPA2 or LPA3 deficiency, prevents LPA-induced CCN1 expression in vivo in mouse carotid arteries. We also observed that LPA1 deficiency blunted LPA infusion-induced neointimal formation, indicating that LPA1 is the major mediator for LPA-induced vascular remodeling. Our in vivo model of LPA-induced neointimal formation established a key role of the ECM protein CCN1 in mediating LPA-induced neointimal formation. Our data support the notion that the LPA1-CCN1 axis may be the central control for SMC migration and vascular remodeling. CCN1 may serve as an important vascular disease marker and potential target for vascular therapeutic intervention.
Keywords: lysophosphatidic acid, CCN1, smooth muscle cells, vascular neointimal formation
smooth muscle cell (SMC) migration is an essential process involved in neointimal formation in atherosclerosis and in restenosis after angioplasty or vascular stent implantation (15, 19, 23, 38). Oxidized LDL (oxLDL) (3, 26), various growth factors, and extracellular matrix (ECM) proteins are potent inducers contributing to SMC migration (23). Lysophosphatidic acid (LPA) is an important bioactive lipid present abundantly in serum (42, 49) and has been found in mild oxLDL (43). LPA highly accumulates in human and mouse atherosclerotic lesions (7, 43). LPA is also produced in platelet activation (20, 22, 31, 41). Therefore, LPA is implicated in the pathological process of vascular disease and is suggested to have a profound function on vascular SMCs and on artery walls.
Indeed, LPA induces SMC migration and proliferation (1, 6, 18, 47). Our recent study revealed that LPA, via the activation of a specific cell membrane receptor, LPA receptor 1 (LPA1), upregulates matricellular protein Cyr61 (CCN1) expression, which, in turn, activates integrin signal cascades, leading to SMC migration (54). These results reveal that CCN1 is a key component of LPA signaling, suggesting that CCN1 is an important regulator of in vivo, LPA-mediated functions. It has been shown that LPA induces vascular neointimal formation in vivo (55–57); however, the molecular mechanisms underlying LPA induction of neointimal formation are not well understood. We hypothesized that the de novo synthesized CCN1 is the key mediator of LPA-induced vascular neointimal formation.
The function of LPA in cells is mediated by its cognate G protein-coupled receptors (GPCRs), including the Edg family of LPA receptors LPA1–3, LPA4 (GPR23), LPA5 (GPR92), and LPA6 (P2Y5), the latter three belonging to the purinoceptor cluster (13, 14). In addition, some of the effects of LPA have been reported to be activated by the nuclear receptor peroxisome proliferator-activated receptor (PPAR-γ) (32, 56). Using primary SMCs isolated from mouse aorta of wild-type mice and LPA receptor knockout mice, we previously demonstrated that LPA1 is the key regulator mediating LPA-induced CCN1 expression and SMC migration (54). In the present study, we evaluated the contribution of LPA receptors in CCN1 expression in mouse carotid arteries and in neointimal formation using the unique LPA receptor knockout mouse model.
In this study, we examined an array of lipid components reportedly present in oxLDL to determine their effects on SMC migration and proliferation. LPA, with a variable-length carbon chain that is either saturated or unsaturated, has the most prominent effect on SMC proliferation and migration compared with other established phospholipids/oxidized lipids of oxLDL (21, 35, 51–53). The effects of LPA infusion on vascular neointimal formation in mouse carotid arteries and on CCN1 expression in neointimal lesions were examined. Furthermore, the roles of CCN1 and LPA receptors in LPA-induced neointimal formation were determined.
MATERIALS AND METHODS
Materials.
16:0 LPA (1-palmitoyl-2-hydroxy-sn-glycero-3-phosphate), c18:1 LPA [1-O-9-(Z)-octadecenyl-2-hydroxy-sn-glycero-3-phosphate], 18:1 LPA (1-oleoyl-2-hydroxy-sn-glycero-3-phosphate), 20:4 LPA (1-arachidonoyl-2-hydroxy-sn-glycero-3-phosphate), 4-hydroxynonenal (HNE), 16:0 lysophosphatidylcholine (LPC) (1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine), 16:0 lysophosphatidylethanolamine (LPE) (1-palmitoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine), 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC), 1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine (PGPC), and 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC) were purchased from Avanti Polar Lipids (Alabaster, AL). oxPAPC was made from oxidation of PAPC (52). Nonsilencing siRNA and CCN1 siRNA were from Qiagen (Gaithersburg, MD). siRNA transfection reagent RNAiMAX was from Life Technologies (Grand Island, NY). Pluronic gel (F127), anti-α-smooth muscle actin (cat. no. MA1-37207), and von Willebrand factor (cat. no. HPA001815) were from Sigma (St. Louis, MO). Anti-CCN1 antibody was from R&D Systems (cat. no. AF4055; Minneapolis, MN). TRIzol reagent and the ThermoScript RT-PCR system were from Invitrogen (Carlsbad, CA). Biotinylated secondary antibodies and peroxidase diaminobenzidine (DAB) kits were from Vector Laboratories (Burlingame, CA).
Cell culture.
Mouse aortic SMCs were prepared from explants of excised aortas of mice as described previously (8). Briefly, 6-wk-old mice (C57BL/6J, both male and female, body wt 20–25 g) were used. Cells were maintained in DMEM containing 10% fetal bovine serum. For migration and proliferation assays, SMCs of passages 7–8 were used. Cells were made quiescent by incubation in serum-free DMEM for 24 h.
Cell migration assay.
Cell migration was performed as described previously (54). Briefly, SMCs were cultured in serum-free DMEM medium for 24 h and then were trypsinized and plated onto the Transwell migration plates (Corning, New York, NY) for migration assays. Cells (2 × 105 cells) were added to the upper chamber. Cells were allowed to migrate through filters (8-μm pore size) precoated on both sides with gelatin in the presence of either medium (600 μl) alone or medium with inducers at designated concentrations in the lower chamber. Cell migration was carried out at 37°C in 5% CO2 for 6 h. Cells remaining on the upper surface of the filter were carefully removed by mechanical scraping. Cells that migrated to the lower side were fixed with methanol and then stained with Harris hematoxylin and eosin (H and E). The cells that had migrated to the lower surface of the filter were counted in four random objective fields (×200 magnification) using a Nikon Eclipse E600 microscope.
Cell proliferation assay.
SMC proliferation was determined in a [3H]thymidine incorporation assay as described previously (9). Cells, cultured for 24 h in serum-free DMEM, were stimulated with or without inducers for 24 h. During the last 6-h incubation period, cells were labeled with 1 μCi/ml methyl-[3H]thymidine. After being labeled, cells were washed three times with ice-cold PBS and two times with 10% trichloroacetic acid. Trichloroacetic acid-insoluble material was hydrolyzed by 0.25 N NaOH, and radioactivity was assayed in a liquid scintillation counter.
Noninjury common carotid artery surgery for LPA infusion.
The mouse surgery was performed following the procedures reported previously (55). Both sexes of C57BL/6J mice were used in this study. Animal experiments, including euthanasia, were conducted following the ethical guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Tennessee. LPA1+/−, LPA2+/−, and LPA3+/− mice were generously provided by Dr. Jerold Chun, The Scripps Research Institute, and have been characterized previously (16, 17). The mice were backcrossed for 12–15 generations to LPA1−/−, LPA2−/−, and LPA3−/− mice with the C57BL/6J background. Wild-type mice (C57BL/6J) were from Jackson Laboratory (Bar Harbor, ME). Wild-type (C57BL/6J, 8 wk old), LPA1−/−, LPA2−/−, and LPA3−/− mice (8 wk old, body wt 25–30 g) were anesthetized with ketamine (80 mg/kg)/xylazine (5 mg/kg). Carotid artery surgery was performed under a dissecting microscope. The left carotid artery and its branches were exposed, and the left internal carotid artery (ICA) and the common carotid artery (CCA), distal to the bifurcation, were temporarily clamped. A transverse arteriotomy was made in the left external carotid artery (ECA), and a 33-G needle tip connected with a vascular catheter (Strategic Applications) was carefully inserted into the left ECA without any injury to the intima of the CCA. The CCA was washed three times with 100 μl of the base medium (saline with 0.2% BSA) and then filled with 100 μl of the base medium or 10 μM LPA dissolved in base medium through the catheter. After 30 min, the left ECA was tied off, the clamps removed, and blood circulation restored. Fourteen days later, control mice (saline treated) and LPA-treated mice were perfused intracardially with 4% paraformaldehyde in PBS. The carotid artery was excised and embedded in paraffin.
Hematoxylin and eosin staining, neointimal measurement, and immunohistochemical staining.
For visualization of neointimal area, CCA sections were stained with H and E. Briefly, paraffin-embedded sections (10 μm) were deparaffinized with xylene and processed in gradient ethanol, followed by H and E staining. Neointima quantitation was performed as described previously (34, 50, 58); the mean of five sections was taken. The areas of the lumen and internal elastic lamina (IEL) were determined by planimetry using Image Pro Plus software; neointima area was calculated by subtracting the lumen area from the IEL area. For detection of CCN1 expression and specific tissue markers [α-smooth muscle actin and von Willebrand factor (vWF)], 5-μm-thick sections were used for immunohistochemical analysis, as described previously (29, 58). Briefly, deparaffinized tissue sections were incubated with the primary antibodies and biotinylated secondary antibodies. The reactions were visualized with peroxidase DAB kits. For quantitative immunohistochemical analysis (36), the areas of staining signals in images were measured using Image Pro Plus.
siRNA delivery to CCA in vivo.
CCN1 siRNA (100 nM) (catalog no. SI00193452, target sequence 5′- CCGACTGTACAGCCTATTCAA-3′; sense strand 5′-GACUGUACAGCCUAUUCAATT-3′; antisense strand 5′-UUGAAUAGGCUGUACA GUCGG-3′) and nonsilencing siRNA (100 nM) (catalog no. 1027310) (Qiagen, Valencia, CA) were delivered to the left CCA following the methods reported previously (24, 37, 44), with infusion of siRNA solution first and then application of pluronic gel mixed with siRNA. Briefly, siRNAs were mixed with transfection reagent Lipofectamine RNAiMax (Life Technologies). Pluronic gel (F127) solutions were prepared at 1 mg/ml with siRNA. The mixture was stored at 4°C (liquid at 4°C). siRNA solution (200 μl) (without pluronic gel) was first infused into the left CCA for 30 min, and then the siRNA solution was removed followed by 30-min LPA infusion. Upon restoring blood flow, siRNA solution and pluronic gel mixture were pasted peripherally around the exposed segment of the CCA. The muscle and skin incision was then closed immediately after the application of the gel. The mice were recovered, and CCAs were harvested in 2 wk.
RT-PCR assay.
Total RNA was isolated from SMCs, aorta, and CCA using TRIzol reagent. The first strand of cDNA was reverse transcribed using a reverse transcription system from Invitrogen. The cDNA products were amplified using GoTaq Flexl DNA polymerase. The LPA receptor primers used for conventional PCR were as follows: LPA1, 5′-AGC TGC CTC TAC TTC CAG C-3′ (forward) and 5′-TTG CTG TGA ACT CCA GCC AG-3′ (reverse); LPA2, 5′-ATG GGC CAG TGC TAC TAC AAC G-3′ (forward) and 5′-AGG GTG GAG TCC ATC AGT G-3′ (reverse); LPA3, 5′-GAC AAG CGC ATG GAC TTT-3′ (forward) and 5′-CAT GTC CTC GTC CTT GTA CG-3′ (reverse); LPA4, 5′-GTT GTA TTC ATC CTG GGT CT-3′ (forward) and 5′-AGC GAC TCC ATC CTT ATA TG-3′ (reverse); and LPA5, 5′-TGC TCT GAC CTT GTT GTT CC-3′ (forward) and 5′-AGC AAC CCA TAT ACA GCC AGC G-3′ (reverse). Amplification conditions were as follows: 5 min at 95°C and 27–33 cycles of 30 s at 95°C, 30 s at 55°C, and 1 min at 72°C. The reaction was followed by a final extension for 10 min at 72°C. The PCR products were analyzed by electrophoresis on a 1.0% agarose gel.
Statistical analysis.
Results are means ± SD. Comparisons between multiple groups were performed using one-way ANOVA with post hoc Newman-Keuls tests. Single comparisons were made using two-tailed, unpaired Student's t-tests. P < 0.05 was considered statistically significant.
RESULTS
LPA, with a variable-length carbon chain that is either saturated or unsaturated, has a prominent effect on SMC proliferation and migration compared with other established phospholipids/oxidized lipids of oxLDL.
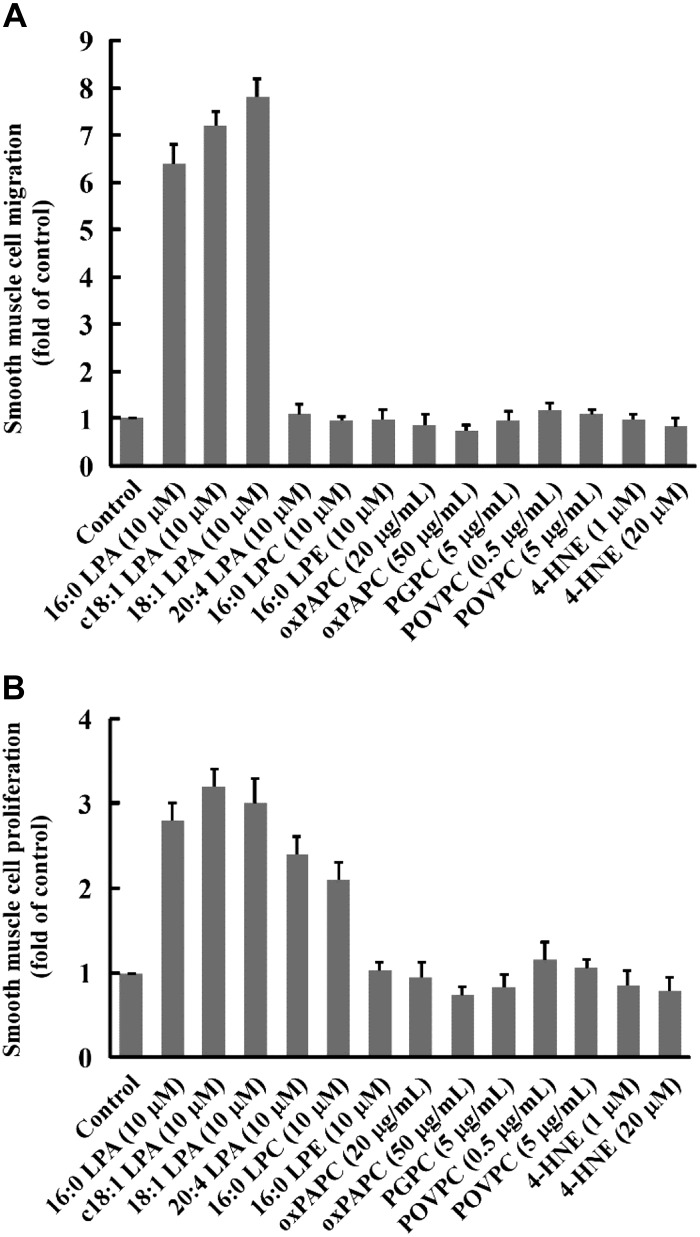
HNE, LPC, POVPC, PGPC, and oxPAPC are established phospholipids/oxidized lipids in oxLDL (21, 35, 51–53). It has been reported that these bioactive lipids induce SMC proliferation and migration (2, 9, 10, 12, 25, 40). LPA accumulates in human atherosclerotic lesions (39, 43). The majority (80%) of total LPA found in human atherosclerotic plaques is acyl-LPA, whereas 18:0 and 18:1 are the major forms of acyl LPA (39). Acyl-LPA 16:0 and 18:1 have been detected in animal atherosclerotic lesions (7). Acyl-LPA 16:0, 18:1, and 20:4 are present in human plasma and serum (4, 5, 41). We compared effects of acyl-LPA 16:0, 18:1, and 20:4 and the alkyl c18:1 ether-linked LPA analog on SMC migration and proliferation with other lipid components of oxLDL, LPC, oxPAPC, PGPC, POVPC, and 4-HNE. As illustrated in Fig. 1, we observed that 1) all forms of LPAs have prominent effects on SMC proliferation and migration compared with other established phospholipids/oxidized lipids of oxLDL and 2) acyl-LPA 16:0 and 18:1 and alkyl c18:1 LPA analog highly induced SMC migration and proliferation; however, acyl-LPA 20:4 increased only SMC proliferation and had a neglect effect on SMC migration. Therefore, we chose acyl 18:1 LPA in the following in vivo studies to examine whether LPA induces CCN1 expression and whether CCN1 mediates LPA-induced neointimal formation.
Fig. 1.
Effects of various lipids on SMC migration and proliferation in vitro. LPA's effect on SMC migration (A) and SMC proliferation (B) was compared with other lipid components of oxLDL. 16:0 LPA, c18:1 LPA, 18:1 LPA, 20:4 LPA, HNE, LPC, POVPC, PGPC, and oxPAPC were used to stimulate mouse SMCs. Acyl LPAs used were 16:0 LPA, 18:1 LPA, and 20:4 LPA; alkyl LPA was c18:1 LPA. LPE was used as a structure-related lipid control. Values are means ± SD, n = 5.
LPA significantly induces neointimal formation in mouse carotid arteries in a noninjury model.
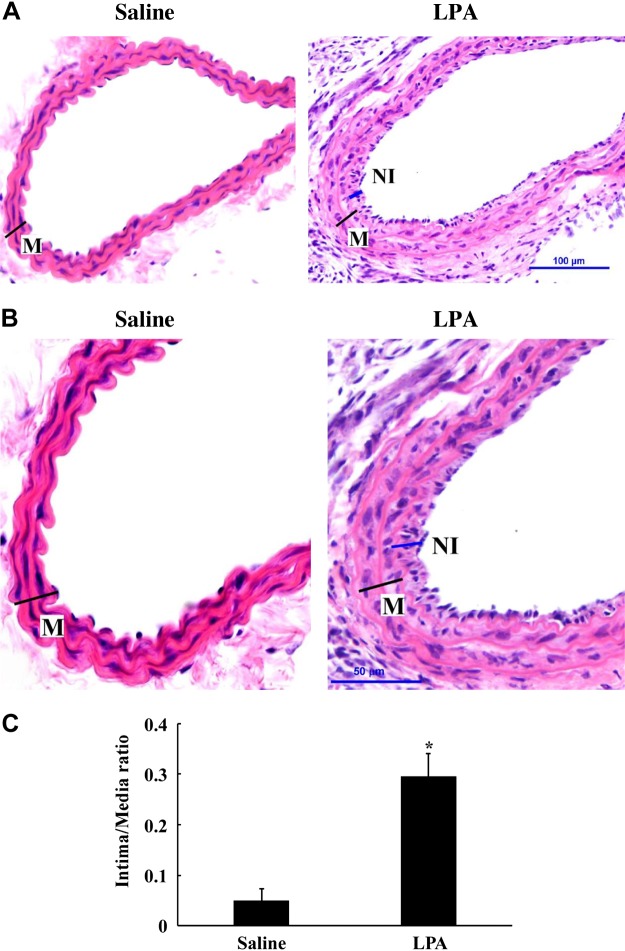
The left mouse CCA was infused with either saline or 10 μM LPA in base medium (saline with 0.2% BSA) for 30 min and then blood circulation restored. Fourteen days later, the CCAs were collected and embedded in paraffin. The sections were stained with H and E. As shown in Fig. 2, A–C, LPA significantly induced neointimal formation compared with the saline-infused control group.
Fig. 2.
LPA induces neointimal formation in mouse CCAs. Mouse CCA was infused with saline or LPA (10 μM) for 30 min, and blood flow was restored. Foureen days later, control mice (saline-treated) and LPA-treated mice were perfused intracardially with 4% paraformaldehyde in PBS. The carotid artery was excised and embedded in paraffin, and the sections were stained with hematoxylin and eosin (H and E) for detection of neointimal formation. M, media; NI, neointima. A: neointimal formation in saline- and LPA-infused mouse CCAs. B: magnified images of saline- and LPA-infused mouse CCAs. Scale bars are illustrated in the right panels. C: digitized graph illustration of intima/media area comparison between saline group and LPA group. Comparisons between groups were performed using Student's t-test. Values are means ± SD; five sections were analyzed in each mouse CCA; n = 5 mice. *P < 0.05 vs. saline group.
LPA-induced neointima in mouse carotid arteries is mainly composed of SMCs.
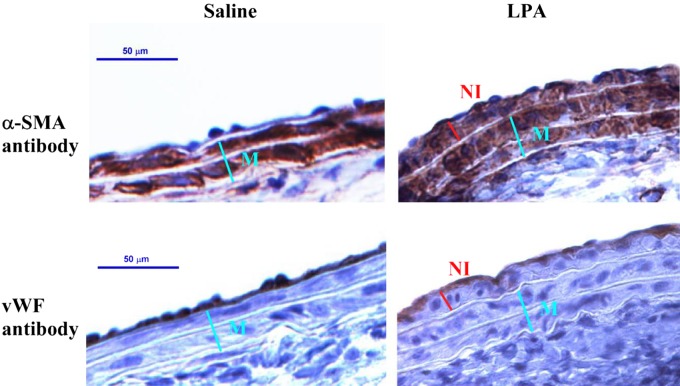
As shown in Fig. 3, top, cells in the neointima and the tunica media, which is composed of SMCs, were strongly stained with α-smooth muscle actin antibody specific for SMCs, indicating that the LPA-induced neointima is chiefly composed of SMCs. In contrast, vWF antibody specific for endothelial cells stained only the endothelium of the carotid arteries of both control and LPA-treated cells (Fig. 3, bottom). The latter results demonstrate that the vascular endothelium remained intact in the noninjury LPA infusion model, which is different from a wire-injury model.
Fig. 3.
Immunohistochemical analysis of LPA-induced neointima. Sections were immunostained either for α-smooth muscle-actin (α-SMA, top) or for von Willebrand factor (vWF) specific for endothelial cells (bottom). Nuclei were counterstained with hematoxylin. Right: CCA infused with LPA. Left: CCA infused with saline (controls).
LPA induces CCN1 expression in vascular neointimal lesions.
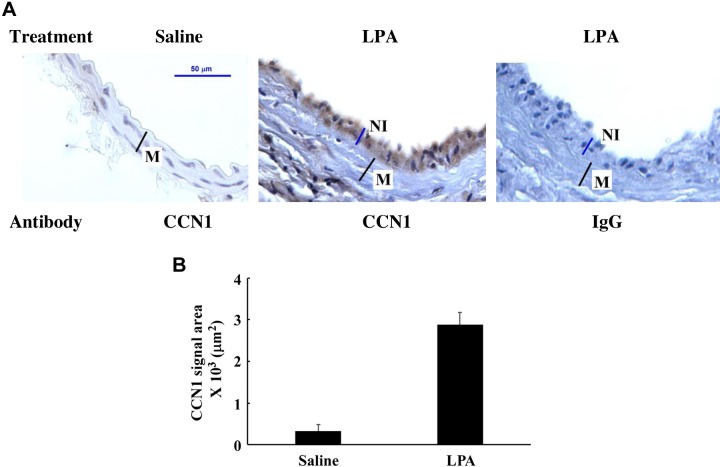
In our previous study, we observed that LPA highly induces CCN1 expression in the ECM of SMCs and that CCN1 is the key mediator for LPA-induced SMC migration (54). We hypothesized that LPA induces CCN1 expression in vascular neointimal lesions. In this nonmechanical injury model, we observed that transient infusion of LPA in mouse carotid arteries highly induced CCN1 expression in neointimal lesions in mouse carotid arteries (Fig. 4). The immunohistochemistry data clearly indicate that CCN1 was mainly expressed in neointimal lesions but not in media SMCs (Fig. 4, A, middle, and B).
Fig. 4.
LPA induction of CCN1 expression in mouse CCA. CCA sections from LPA-infused (middle and right) or saline-infused (left) mouse carotid arteries were immunostained either with Cyr61 antibody (left and middle) or negative control, rabbit IgG (right). Nuclei were counterstained with hematoxylin. A: representative image of CCN1 expression detected by immunohistochemistry. B: graph illustration of detected CCN1 signal area. Values are means ± SD, n = 5 mice.
CCN1 is the key regulator mediating LPA-induced neointimal formation in mouse carotid arteries.
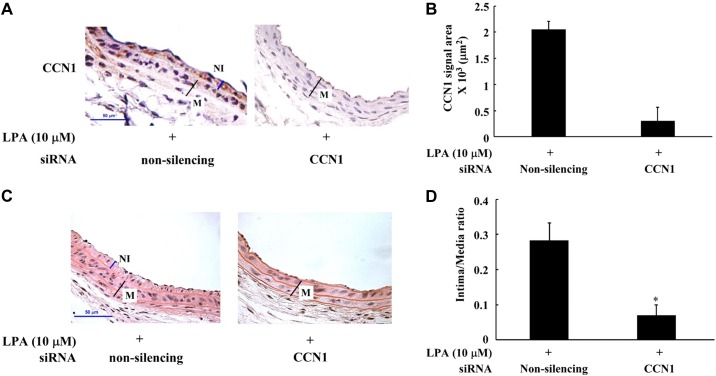
LPA induces CCN1 expression in neointimal lesions, suggesting a possible role of CCN1 in LPA-induced vascular neointimal formation. To examine whether LPA-triggered CCN1 expression in vascular neointima area contributes to neointimal formation, we evaluated the effect of CCN1 depletion in vivo on LPA-induced neointimal formation in mouse carotid arteries. The in vivo knockdown of CCN1 was achieved by transient infusion of CCN1 siRNA into the CCA and then the application of Pluronic gel onto the outside of the CCA (details described in materials and methods). The assessment of CCN1 protein expression indicates that the specific CCN1 siRNA could efficiently knock down the expression of CCN1 in vivo (Fig. 5, A and B). We observed that knockdown of CCN1 expression significantly blocked LPA-induced neointimal formation (about 80%, Fig. 5, C and D). These data indicate that CCN1 is a key mediator for LPA-induced neointimal formation.
Fig. 5.
Knockdown of CCN1 with siRNA reduced LPA-induced neointimal formation. Nonsilencing siRNA or CCN1 siRNA was transfected into mouse CCA followed by LPA infusion into CCA; after LPA infusion, siRNA solution and pluronic gel mixture were pasted peripherally around the exposed segment of the left CCA. In 2 wk, the harvested CCA was detected by H and E staining and immunohistochemistry. A: expression of CCN1 in nonsilencing or CCN1-specific, siRNA-treated mouse CCAs detected by immunohistochemistry. B: graph illustration of CCN1 in vivo expression. Immunohistochemical staining area was measured and compared between nonsilencing and CCN1 siRNA groups. Comparisons between groups were performed using Student's t-test. Values are means ± SD, n = 5 mice. C: effect of nonsilencing siRNA or CCN1 siRNA on LPA-induced neointimal formation examined by H and E staining. D: digitized graph illustration of intima/media area comparison between nonsilencing and CCN1 siRNA groups. Comparisons between groups were performed using Student's t-test. Values are means ± SD; five sections were analyzed in each mouse CCA; n = 7 mice. *P < 0.05 vs. the nonsilencing siRNA group.
LPA1 is the most responsible receptor mediating LPA-induced CCN1 expression in neointimal lesions in mouse carotid arteries and LPA-induced neointimal formation.
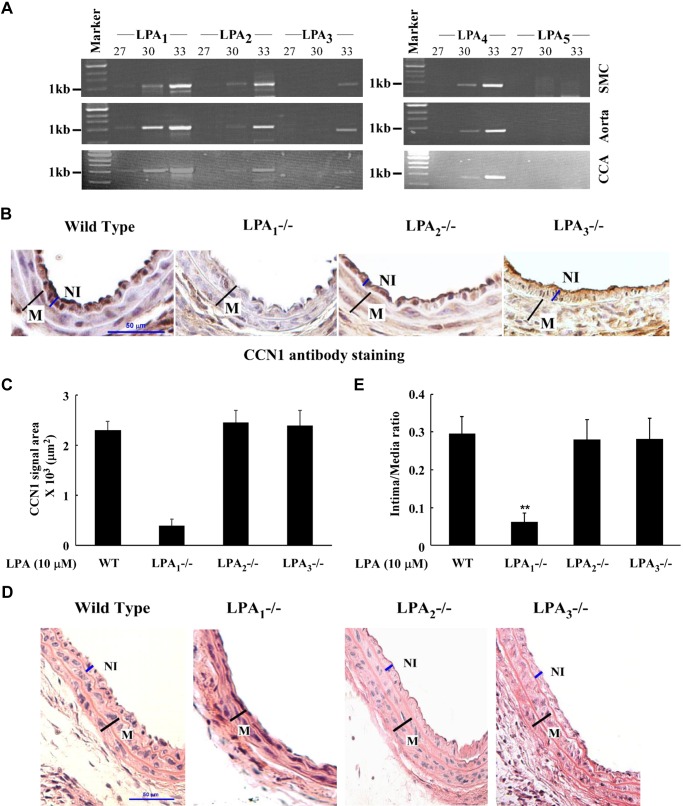
LPA exerts various biological functions on cells via its cognate receptors in plasma membranes (13, 14). We identified LPA1 as responsible for LPA-induced CCN1 expression and CCN1-mediated SMC migration in an in vitro cell culture system (54). In the present study, our data demonstrate that CCN1 was a key mediator for LPA-induced neointimal formation in vivo in mouse carotid arteries (Fig. 5). We hoped to identify which LPA receptor mediates CCN1 expression in vivo in neointimal formation. We first determined LPA receptor expression levels in mouse carotid arteries compared with those from aortas and isolated primary SMCs. As shown in Fig. 6A, first, the patterns of the expression levels of these LPA receptors were similar among carotid arteries, aortas, and SMCs. Second, LPA1, LPA2, and LPA4 were highly expressed in carotid arteries, aortas, and SMCs; LPA3 had very low expression levels, and LPA5 was not expressed in carotid arteries, aortas, and SMCs. We then compared CCN1 expression levels in carotid arteries in response to LPA infusion in LPA1−/−, LPA2−/−, LPA3−/−, and wild-type mice. As shown in Fig. 6, B and C, CCN1 was highly expressed in neointimal lesions in carotid arteries in wild-type, LPA2−/−, and LPA3−/− mice, but not in LPA1−/− mice, indicating that LPA1 is responsible for LPA-induced CCN1 expression. We further assessed the effect of LPA receptor deficiency on neointimal lesion formation. As demonstrated in Fig. 6, D and E, LPA1 deficiency, but not LPA2 or LPA3 deficiency, failed to respond to LPA stimulation; i.e., significant neointimal formation was not detected in LPA1−/− mice. Taken together, these data indicate that LPA1 is responsible for CCN1 expression in carotid arteries in vivo and responsible for neointimal formation.
Fig. 6.
LPA receptor expression in mouse carotid arteries and effects of LPA receptor deficiency on CCN1 expression and neointimal formation. A: expression profiles of LPA receptors in mouse SMC, aorta, and CCA were detected by RT-PCR in various amplification cycles labeled at the top of each lane. B: expression of CCN1 in CCA of WT, LPA1−/−, LPA2−/−, and LPA3−/− mice infused with LPA. WT, LPA1−/−, LPA2−/−, and LPA3−/− mice were used for LPA infusion, and CCAs were harvested in 2 wk. CCN1 expression was examined with immunohistochemical staining. C: graph illustration of CCN1 in vivo expression. Immunohistochemical staining area was measured and compared between WT, LPA1−/−, LPA2−/−, and LPA3−/− groups. Values are means ± SD, n = 5 mice. D: neointimal formation in CCAs of WT, LPA1−/−, LPA2−/−, and LPA3−/− mice infused with LPA was detected by H and E staining. E: intima/media area was measured, and a graph illustration is shown. Data of all groups were analyzed using ANOVA, and comparisons between groups were performed using Dunnett's t-test. Values are means ± SD; five sections were analyzed in each mouse CCA; n = 7 mice. **P < 0.01 vs. WT group.
DISCUSSION
With this study, we provided the first evidence that LPA induces CCN1 expression in neointimal lesions in carotid arteries in vivo. Our data demonstrated that CCN1 is the key mediator controlling LPA-induced vascular remodeling and that LPA1 is responsible for both CCN1 expression in vivo and neointimal formation in carotid arteries. These results support a conclusion that the LPA receptor-CCN1 axis mediates vascular remodeling.
Since the discovery that LPA is formed during mild oxidation of LDL, is the active compound in mildly oxidized LDL, and accumulates in the core of atherosclerotic lesions (43), the effect of LPA in vascular neointimal formation has been assessed in rat and mouse models. It has been shown that LPA and its ether analog alkyl-glycerophosphate induce vascular neointima formation in rat and mouse carotid arteries (11, 45, 55, 56). The major cellular components of neointimal lesions induced by LPA transient infusion are SMCs, indicating that SMC migration and proliferation principally contribute to neointimal formation. The molecular mechanism by which LPA induces SMC migration in vivo in the artery wall has been largely unknown. Our recent work revealed that LPA-induced SMC migration in vitro is mediated by CCN1, a matricellular protein that binds to integrins and has diverse roles in many biological processes, including angiogenesis, proliferation, apoptosis, differentiation, migration, and survival (27, 28). We tested whether LPA infusion in carotid artery induces CCN1 expression and, if so, whether CCN1 contributes to neointimal formation. We have now demonstrated that LPA infusion highly induces CCN1 expression in vivo in mouse carotid arteries. CCN1 siRNA knockdown prevented LPA-induced neointimal formation, indicating the functional role of CCN1 in LPA-induced vascular remodeling. These results suggest that matricellular protein CCN1 is a therapeutic target for oxLDL/oxidized lipid-provoked vascular remodeling. Consistent with our finding, a previous report showed that CCN1 mediates mechanical injury-induced vascular remodeling (30). Therefore, de novo synthesized ECM-related proteins are likely playing an important role in mediating artery remodeling in restenosis and atherosclerosis.
LPA exerts its biological functions via its plasma membrane receptors. Acyl-LPA 18:0 and 18:1 are the major forms of LPA found in human atherosclerotic plaques (39). Acyl-LPA 18:1 and 16:0 are major forms of LPA detected in animal atherosclerotic lesions (7). Acyl-LPA 18:1, 16:0, and 20:4 are present in human plasma and serum (4, 5, 41). The majority of LPA characterized in biological fluids and tissues is the acyl form; however, the alkyl ether glycerophosphate analog, alkyl-GP, has also been detected (46). Alkyl-GP has biological properties distinct from acyl-LPA (39, 48). A previous study reported that the ether analog of LPA, alkyl-GP, mediates neointimal formation via the PPAR-γ pathway (11). Whether LPA cognate receptors play a role in acyl-LPA-triggered neointimal formation has been an uncertainty. A recent study suggests that LPA1 and LPA3 may play a role in mediating acyl-LPA-induced neointimal formation using a chemical inhibitor of LPA receptors and a gene knockdown approach (45). Assessments of which LPA receptors are responsible for LPA-induced neointimal formation rely on the availability of LPA receptor knockout animal models. We have now used this LPA receptor knockout model to evaluate the responsibility of these LPA receptors. The results from our experiments reveal that LPA1 is the essential receptor mediating the function of acyl-LPA on neointimal formation. In a previous study, LPA1 and LPA2 have been shown to mediate vascular ligation injury-induced neointimal formation (33). Taken together, these results pointed out that LPA receptors are therapeutic targets for treatment of vascular remodeling.
Although the possibility of functional redundancy/compensation of LPA receptors exists, the results from our previous study, using multiple approaches, including RT-PCR and Northern and Western analyses, indicated that, in mouse primary SMCs, neither LPA1 deficiency nor LPA2 deficiency has an effect on LPA3 expression (54). Also deficiency of any one LPA receptor (LPA1-3) does not affect expression of other LPA receptors in SMCs (54). In the present study, we compared LPA receptor expression levels among carotid arteries, aortas, and SMCs. The results show that LPA receptor expression patterns are similar in carotid arteries, aortas, and SMCs. LPA1, LPA2, and LPA4 are the predominantly expressed forms; however, LPA1 mediates LPA signaling, leading to CCN1 expression and neointimal formation.
LPA signaling is complex. It has been shown that various LPA forms with saturated or nonsaturated or long- or short-side carbon chains are in oxLDL, atherosclerotic lesions, and plasma/serum (4, 5, 7, 41). Various LPA forms may have distinct affinity toward plasma membrane LPA receptors and may exert different functions on cells. As revealed in the present study, 20:4 LPA has a profound effect on SMC proliferation; however, it has little effect on primary SMC migration. A recent report showed that 20:4 LPA-induced neointimal formation depends on smooth muscle progenitor cell migration via a CXCL12-mediated mechanism (45). 18:1 LPA, present richly in atherosclerotic lesions, human plasma, and serum (4, 5, 7, 39, 41), has a prominent effect on SMC migration and proliferation, contributing to neointimal formation via a CCN1-mediated SMC migration mechanism. Our results demonstrated that LPA1 and CCN1 are important mediators for neointimal formation. CCN1 may be an emerging neointimal marker and promising therapeutic target for intervention toward curing vascular remodeling in restenosis, atherosclerosis, and even cancers.
GRANTS
This work was supported by National Institutes of Health Grants HL107466 (to M.-Z. Cui) and AG026640 (to X. Xu). This work was also supported by the University of Tennessee Center of Excellence in Livestock Diseases and Human Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.H., F.Z., D.D.W., D.A., and J.S., performed experiments; F.H., F.Z., D.D.W., D.A., J.S., G.L., X.X., and M.-Z.C. analyzed data; F.H., F.Z., G.L., X.X., and M.-Z.C. interpreted results of experiments; F.H. and M.-Z.C. prepared figures; F.H. and M.-Z.C. drafted manuscript; F.H., F.Z., D.A., J.S., X.X., and M.-Z.C. edited and revised manuscript; F.H., F.Z., D.D.W., D.A., J.S., G.L., X.X., and M.-Z.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank pathologist Dr. Michael McEntee (University of Tennessee) for help with vascular morphological analysis of mouse carotid arteries. We also thank Misty Bailey (University of Tennessee) for critical reading of the manuscript.
REFERENCES
- 1.Ai S, Kuzuya M, Koike T, Asai T, Kanda S, Maeda K, Shibata T, Iguchi A. Rho-Rho kinase is involved in smooth muscle cell migration through myosin light chain phosphorylation-dependent and independent pathways. Atherosclerosis 155: 321–327, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shawaf E, Naylor J, Taylor H, Riches K, Milligan CJ, O'Regan D, Porter KE, Li J, Beech DJ. Short-term stimulation of calcium-permeable transient receptor potential canonical 5-containing channels by oxidized phospholipids. Arterioscler Thromb Vasc Biol 30: 1453–1459, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autio I, Jaakkola O, Solakivi T, Nikkari T. Oxidized low-density lipoprotein is chemotactic for arterial smooth muscle cells in culture. FEBS Lett 277: 247–249, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Baker DL, Desiderio DM, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal Biochem 292: 287–295, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Baker DL, Umstot ES, Desiderio DM, Tigyi GJ. Quantitative analysis of lysophosphatidic acid in human blood fractions. Ann NY Acad Sci 905: 267–269, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Boguslawski G, Grogg JR, Welch Z, Ciechanowicz S, Sliva D, Kovala AT, McGlynn P, Brindley DN, Rhoades RA, English D. Migration of vascular smooth muscle cells induced by sphingosine 1-phosphate and related lipids: Potential role in the angiogenic response. Exp Cell Res 274: 264–274, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bot M, Bot I, Lopez-Vales R, van de Lest CH, Saulnier-Blache JS, Helms JB, David S, van Berkel TJ, Biessen EA. Atherosclerotic lesion progression changes lysophosphatidic acid homeostasis to favor its accumulation. Am J Pathol 176: 3073–3084, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock TA, Alexander RW, Ekstein LS, Atkinson WJ, Gimbrone MA Jr. Angiotensin increases cytosolic free calcium in cultured vascular smooth muscle cells. Hypertension 7: I105–I109, 1985. [DOI] [PubMed] [Google Scholar]
- 9.Chai YC, Howe PH, DiCorleto PE, Chisolm GM. Oxidized low density lipoprotein and lysophosphatidylcholine stimulate cell cycle entry in vascular smooth muscle cells. Evidence for release of fibroblast growth factor-2. J Biol Chem 271: 17791–17797, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S, Berliner JA, Subbanagounder GG, Bhunia AK, Koh S. Identification of a biologically active component in minimally oxidized low density lipoprotein (MM-LDL) responsible for aortic smooth muscle cell proliferation. Glycoconj J 20: 331–338, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y, Makarova N, Tsukahara R, Guo H, Shuyu E, Farrar P, Balazs L, Zhang C, Tigyi G. Lysophosphatidic acid-induced arterial wall remodeling: Requirement of PPARgamma but not LPA1 or LPA2 GPCR. Cell Signal 21: 1874–1884, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherepanova OA, Pidkovka NA, Sarmento OF, Yoshida T, Gan Q, Adiguzel E, Bendeck MP, Berliner J, Leitinger N, Owens GK. Oxidized phospholipids induce type VIII collagen expression and vascular smooth muscle cell migration. Circ Res 104: 609–618, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: Subtypes and biological actions. Annu Rev Pharmacol Toxicol 50: 157–186, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev 62: 579–587, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clowes AW, Schwartz SM. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res 56: 139–145, 1985. [DOI] [PubMed] [Google Scholar]
- 16.Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the LPA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci USA 97: 13384–13389, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of LPA(2) (Edg4) and LPA(1)/LPA(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: Signaling deficits without obvious phenotypic abnormality attributable to LPA(2). Mol Cell Biol 22: 6921–6929, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damirin A, Tomura H, Komachi M, Liu JP, Mogi C, Tobo M, Wang JQ, Kimura T, Kuwabara A, Yamazaki Y, Ohta H, Im DS, Sato K, Okajima F. Role of lipoprotein-associated lysophospholipids in migratory activity of coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol 292: H2513–H2522, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol 28: 812–819, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J 291: 677–680, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esterbauer H, Jurgens G, Quehenberger O, Koller E. Autoxidation of human low density lipoprotein: Loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res 28: 495–509, 1987. [PubMed] [Google Scholar]
- 22.Gerrard JM, Robinson P. Identification of the molecular species of lysophosphatidic acid produced when platelets are stimulated by thrombin. Biochim Biophys Acta 1001: 282–285, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res 100: 607–621, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Hlawaty H, San Juan A, Jacob MP, Vranckx R, Letourneur D, Feldman LJ. Local matrix metalloproteinase 2 gene knockdown in balloon-injured rabbit carotid arteries using nonviral-small interfering RNA transfection. J Gene Med 11: 92–99, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone SR, Ross J, Rizzo MJ, Straub AC, Lampe PD, Leitinger N, Isakson BE. Oxidized phospholipid species promote in vivo differential Cx43 phosphorylation and vascular smooth muscle cell proliferation. Am J Pathol 175: 916–924, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohno M, Yokokawa K, Yasunari K, Kano H, Minami M, Ueda M, Yoshikawa J. Effect of natriuretic peptide family on the oxidized LDL-induced migration of human coronary artery smooth muscle cells. Circ Res 81: 585–590, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Lau LF. CCN1/CYR61: The very model of a modern matricellular protein. Cell Mol Life Sci 68: 3149–3163, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau LF. Cell surface receptors for CCN proteins. J Cell Commun Signal 10: 121–127, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Wang Q, Chai W, Chen MH, Liu Z, Shi W. Hyperglycemia in apolipoprotein E-deficient mouse strains with different atherosclerosis susceptibility. Cardiovasc Diabetol 10: 117, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumae H, Yoshida Y, Ono K, Togi K, Inoue K, Furukawa Y, Nakashima Y, Kojima Y, Nobuyoshi M, Kita T, Tanaka M. CCN1 knockdown suppresses neointimal hyperplasia in a rat artery balloon injury model. Arterioscler Thromb Vasc Biol 28: 1077–1083, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Mauco G, Chap H, Simon MF, Douste-Blazy L. Phosphatidic and lysophosphatidic acid production in phospholipase C-and thrombin-treated platelets. Possible involvement of a platelet lipase. Biochimie 60: 653–661, 1978. [DOI] [PubMed] [Google Scholar]
- 32.McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci USA 100: 131–136, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panchatcharam M, Miriyala S, Yang F, Rojas M, End C, Vallant C, Dong A, Lynch K, Chun J, Morris AJ, Smyth SS. Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ Res 103: 662–670, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips JW, Barringhaus KG, Sanders JM, Yang Z, Chen M, Hesselbacher S, Czarnik AC, Ley K, Nadler J, Sarembock IJ. Rosiglitazone reduces the accelerated neointima formation after arterial injury in a mouse injury model of type 2 diabetes. Circulation 108: 1994–1999, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: A chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci USA 85: 2805–2809, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall'Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab 11: 379–389, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redmond EM, Hamm K, Cullen JP, Hatch E, Cahill PA, Morrow D. Inhibition of patched-1 prevents injury-induced neointimal hyperplasia. Arterioscler Thromb Vasc Biol 33: 1960–1964, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 362: 801–809, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G, Siess W. Subtype-selective antagonists of lysophosphatidic Acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation 108: 741–747, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Ruef J, Rao GN, Li F, Bode C, Patterson C, Bhatnagar A, Runge MS. Induction of rat aortic smooth muscle cell growth by the lipid peroxidation product 4-hydroxy-2-nonenal. Circulation 97: 1071–1078, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem 277: 21197–21206, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Schumacher KA, Classen HG, Spath M. Platelet aggregation evoked in vitro and in vivo by phosphatidic acids and lysoderivatives: Identity with substances in aged serum (DAS). Thromb Haemost 42: 631–640, 1979. [PubMed] [Google Scholar]
- 43.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci USA 96: 6931–6936, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smolock EM, Korshunov VA, Glazko G, Qiu X, Gerloff J, Berk BC. Ribosomal protein L17, RpL17, is an inhibitor of vascular smooth muscle growth and carotid intima formation. Circulation 126: 2418–2427, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramanian P, Karshovska E, Reinhard P, Megens RT, Zhou Z, Akhtar S, Schumann U, Li X, van Zandvoort M, Ludin C, Weber C, Schober A. Lysophosphatidic acid receptors LPA1 and LPA3 promote CXCL12-mediated smooth muscle progenitor cell recruitment in neointima formation. Circ Res 107: 96–105, 2010. [DOI] [PubMed] [Google Scholar]
- 46.Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokumura A, Hanahan DJ. Occurrence of lysophosphatidic acid and its alkyl ether-linked analog in rat brain and comparison of their biological activities toward cultured neural cells. Biochim Biophys Acta 1440: 194–204, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Tokumura A, Iimori M, Nishioka Y, Kitahara M, Sakashita M, Tanaka S. Lysophosphatidic acids induce proliferation of cultured vascular smooth muscle cells from rat aorta. Am J Physiol Cell Physiol 267: C204–C210, 1994. [DOI] [PubMed] [Google Scholar]
- 48.Tokumura A, Sinomiya J, Kishimoto S, Tanaka T, Kogure K, Sugiura T, Satouchi K, Waku K, Fukuzawa K. Human platelets respond differentially to lysophosphatidic acids having a highly unsaturated fatty acyl group and alkyl ether-linked lysophosphatidic acids. Biochem J 365: 617–628, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogt W. Pharamacologically active acidic phospholipids and glycolipids. Biochem Pharmacol 12: 415–420, 1963. [DOI] [PubMed] [Google Scholar]
- 50.Wang D, Oparil S, Chen YF, McCrory MA, Skibinski GA, Feng W, Szalai AJ. Estrogen treatment abrogates neointima formation in human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol 25: 2094–2099, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, Navab M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 96: 2882–2891, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 272: 13597–13607, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Watson AD, Subbanagounder G, Welsbie DS, Faull KF, Navab M, Jung ME, Fogelman AM, Berliner JA. Structural identification of a novel pro-inflammatory epoxyisoprostane phospholipid in mildly oxidized low density lipoprotein. J Biol Chem 274: 24787–24798, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Wu DD, Zhang F, Hao F, Chun J, Xu X, Cui MZ. Matricellular protein Cyr61 bridges lysophosphatidic acid and integrin pathways leading to cell migration. J Biol Chem 289: 5774–5783, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshida K, Nishida W, Hayashi K, Ohkawa Y, Ogawa A, Aoki J, Arai H, Sobue K. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation 108: 1746–1752, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD, Byun HS, Bittman R, Tigyi G. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med 199: 763–774, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Z, Subramanian P, Sevilmis G, Globke B, Soehnlein O, Karshovska E, Megens R, Heyll K, Chun J, Saulnier-Blache JS, Reinholz M, van Zandvoort M, Weber C, Schober A. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab 13: 592–600, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Zhu B, Kuhel DG, Witte DP, Hui DY. Apolipoprotein E inhibits neointimal hyperplasia after arterial injury in mice. Am J Pathol 157: 1839–1848, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]