Abstract
The SLC4A11 gene encodes the bicarbonate-transporter-related protein BTR1, which is mutated in syndromes characterized by vision and hearing loss. Signs of these diseases [congenital hereditary endothelial dystrophy (CHED) and Harboyan syndrome] are evident in mouse models of Slc4a11 disruption. However, the intrinsic activity of Slc4a11 remains controversial, complicating assignment of its (patho)physiological role. Most studies concur that Slc4a11 transports H+ (or the thermodynamically equivalent species OH−) rather than HCO3−, but disparities have arisen as to whether the transport is coupled to another species such as Na+ or NH3/NH4+. Here for the first time, we examine the action of mouse Slc4a11 in Xenopus oocytes. We simultaneously monitor changes in intracellular pH, membrane potential, and conductance as we alter extracellular pH, revealing the electrical and chemical driving forces that underlie the observed ion fluxes. We find that mSlc4a11 is an ideally selective H+/OH− conductive pathway, the action of which is uncoupled from the cotransport of any other ion. We also find that the activity of mSlc4a11 is independently enhanced by both extracellular and intracellular alkalinization, suggesting OH− as the most likely substrate and providing a novel explanation for the apparent NH3-dependence of Slc4a11-mediated currents reported by others. We suggest that the unique properties of Slc4a11 action underlie its value as a pH regulator in corneal endothelial cells.
Keywords: acid-base, cornea, fluid transport, NaBC1, proton
one of the most tightly regulated parameters in the body is pH. Extracellular pH (pHe) is predominantly maintained by the CO2/HCO3− buffering system and is set at a value close to 7.4 by the balance between Pco2 (controlled by the lungs) and [HCO3−] (controlled by the kidneys) in blood plasma (5, 18). Intracellular pH (pHi) is maintained at values slightly lower than pHe, a value that is determined by the balanced actions of acid loaders [such as Cl−/HCO3− exchangers of the solute carrier 4 (Slc4) family] that counter rises in pHi and acid extruders [such as Na+/H+ exchangers (Slc9 family) and Na+-HCO3− cotransporters (also Slc4 family)] that counter falls in pHi (14, 48). Beyond regulation of pHi, tightly coupled acid-extruding and acid-loading activities produce a net influx of Na+ and Cl− that can contribute to cell volume homeostasis (16, 23, 29).
The extent of the Slc4 family of solute carriers was finalized in 2001 with the cloning of human SLC4A11 (46). Of the transporters encoded by the other nine genes in the family (Slc4a1–Slc4a10; there is no Slc4a6), three are Cl−/HCO3− exchangers (AE1, AE2, and AE3) and five are Na+-coupled HCO3− transporters (NBCe1, NBCe2, NBCn1, NBCn2/NCBE, and NDCBE). The molecular actions of the Slc4a9 and Slc4a11 gene products, however, remain controversial (44, 47). Slc4a11—aka the bicarbonate-transporter-related protein BTR1—is the most divergent of the 10 Slc4 members, sharing less than 20% sequence identity with other members at the amino-acid level (48). Moreover, unlike most other Slc4 proteins, Slc4a11 does not transport HCO3− (28, 34, 42). Nonetheless, Slc4a11 is a clinically important protein: individuals carrying SLC4A11 mutations exhibit congenital hereditary endothelial dystrophy (CHED) and Harboyan syndrome: both rare forms of corneal dystrophy, the latter also being associated with progressive deafness (12, 53). Signs of these diseases (corneal edema from birth followed by endothelial cell dysmorphia and loss with age as well as decreased endocochlear potential and auditory brainstem response) are recapitulated in strains of Slc4a11-null mice (19, 20, 35, 51), along with a mild urine-concentrating defect (19, 20) that has yet to be observed in human probands (33). Consistent with its pathological roles, Slc4a11 is expressed in corneal endothelial cells (19, 51), fibroblasts of the inner ear (35), and in renal epithelia (8, 19).1 However, assignment of a precise physiological or pathophysiological role for Slc4a11 at any of these locations has been hindered by a lack of consensus regarding its molecular action and its roster of substrates.
Slc4-like genes that are predicted to encode proteins which are identifiably Slc4a11-like, as opposed to AE-like or NBC-like, can be found in most metazoan genomes, indicating a long-held specialization of role (44). Because mammalian Slc4a11 does not appear capable of supporting HCO3− transport, and because Slc4-like proteins from Arabidopsis (a plant) and Saccharomyces (a yeast) are boric-acid rather than bicarbonate transporters, the original assignment of human Slc4a11 as a Na+-coupled borate transporter seemed fitting (17). However, doubts were subsequently voiced in the literature about the physiological requirement for a borate transporter in mammalian cells and none of the four groups, including ourselves, that have subsequently investigated Slc4a11 action have been able to produce any evidence for borate transport by human Slc4a11 in cell lines or oocytes (6, 28, 30, 34, 42, 51).2 Even the original study that favored borate transport stopped short of demonstrating the translocation of borate across the plasma membrane, reporting only that borate enhances Slc4a11-dependent pHi changes (43). One element of the original paper that has persisted is the concept that, regardless of the presence of borate, Slc4a11 confers a H+/OH− (the two are thermodynamically indistinguishable) permeability to its host cell that is under the influence of membrane potential (30, 43, 59). However, each of these subsequent studies has ultimately arrived at different conclusions regarding the molecular action of Slc4a11 and no consensus has yet emerged. Some find evidence that its H+/OH− permeation is uncoupled from any other substrate (30). Some find evidence that its action is coupled to (or at least additionally permeable to) Na+ (42, 43) and yet others report that Slc4a11 is an electrogenic NH3-2H+ cotransporter (59). The authors of a parallel line of investigation find Slc4a11 to be H2O and NH3 permeable (34, 51).
With studies of Slc4a11-null mice in mind, we focused our attention on discerning the molecular action of mouse Slc4a11 (mSlc4a11) in Xenopus oocytes. The observation of enhanced H+/OH− permeability in mSlc4a11-expressing cells also forms the starting point of the present study. Here we test the current hypotheses regarding mammalian Slc4a11 action using a combination of two-electrode voltage-clamp and H+-selective microelectrodes. We examine the H+/OH− fluxes associated with Slc4a11 action while considering the chemical and electrical driving forces acting upon the pathway. This approach allows us to demonstrate for the first time that mouse Slc4a11 is a unique entity in the realm of pH regulation: an uncoupled and ideally selective H+/OH− conductive pathway, whose action is acutely stimulated by rises in extracellular and intracellular pH. We use our new insights to reconcile previous observations of Slc4a11-expressing cells and propose a single, unifying model of Slc4a11 action.3
MATERIALS AND METHODS
cDNA preparation.
Full-length mouse Slc4a11 cDNA (IMAGE clone no. 40060299) was purchased from Thermo Fisher Scientific (Grand Island, NY) and sent for automated DNA sequencing (Eurofins MWG Operon, Louisville, KY). The results revealed three silent and two nonsilent base mismatches compared to the reference mouse sequence (GenBank DNA accession no. BC111884). The two missense substitutions, predicted to result in the missense substitutions Ala151Thr and Ala850Ser, were corrected by QuikChange mutagenesis, performed according to the manufacturer's instructions using the complementary primer pairs no. 1/2 (T151A conversion) and no. 3/4 (S850A conversion) in Table 1. The corrected open-reading frame was amplified by PCR and subcloned into the pGH19 expression vector (38) between XmaI and HindIII restriction sites. The forward subcloning primer (no. 5 in Table 1) included a consensus Kozak sequence before the ATG and the reverse primer (no. 6 in Table 1) included the natural termination codon. The resulting clone was named mSlc4a11.pGH19. The equivalent of the human CHED-linked mutation R125H (R99H in the mouse sequence) was introduced by QuikChange mutagenesis using primers no. 7 and no. 8 in Table 1. The resulting clone was named R99H/mSlc4a11.pGH19.
Table 1.
Primers used for the subcloning of mSlc4a11 and R99H/mSlc4a11
| Primer | Sequence (5′–3′) |
|---|---|
| 1 | GACCTGGACCTGCTCATGGCTAAGCTCTTTACAGATG |
| 2 | CATCTGTAAAGAGCTTAGCCATGAGCAGGTCCAGGTC |
| 3 | CTACCCCGAATCATTGAGGCCAAGTACTTGGATGTC |
| 4 | GACATCCAAGTACTTGGCCTCAATGATTCGGGGTAG |
| 5 | CGAAGCCCGGGCCACCATGTCACAGAATGAACAC |
| 6 | GCAACAAGCTTTCAGGGCCTATGCTCAGC |
| 7 | CTTTGAGGAAGAGGTCCATGCACACCGGGACCTGGATG |
| 8 | ACATCCAGGTCCCGGTGTGCATGGACCTCTTCCTCAAAG |
cRNA synthesis.
pGH19 clones were linearized using NotI and the linearized cDNA was purified using a MinElute PCR Purification Kit (QIAGEN, Valencia, CA). Capped cRNA was generated using the T7 mMessage mMachine Transcription Kit (Thermo Fisher Scientific). The quantity and quality (A260/A280 ratio) of the cRNA was assessed using a Nanodrop 2000 Spectrophotometer (Thermo Fisher Scientific).
Xenopus oocytes.
The extraction of ovaries from Xenopus laevis frogs was performed in accordance with the rules and recommendations of the Institutional Animal Care and Use Committee (IACUC) at the University at Buffalo, which reviewed and approved the protocol. Oocytes were extracted from female Xenopus laevis frogs (Xenopus Express, Brooksville, FL) as described elsewhere (38). In brief, frogs were anesthetized by immersion in 0.2% tricaine solution for 15 min (or until unresponsive to toe pinch) and small sections of ovary were surgically extracted for washing in Ca2+-free “NRS” buffer (in mM: 82 NaCl, 2 KCl, 20 MgCl2, 5 HEPES, adjusted to pH 7.45 with NaOH). Frogs were subsequently euthanized by exsanguination. Following the Ca2+-free washes, oocytes were liberated from the tissue sections by digestion in NRS buffer plus 2 mg/ml type-IA collagenase (C2674: Sigma-Aldrich, St. Louis, MO) for 15–35 min. Finally, liberated cells were washed in NRS buffer, followed by ND96 solution (aka pHe = 7.5 solution: Table 2), followed by OR3 medium. OR3 medium contains 14 g/l of Leibovitz's L-15 medium (10-045-CV: Thermo Fisher Scientific), 100 U/ml penicillin, 100 μg/ml streptomycin, 5 mM HEPES, adjusted to pH 7.5 using NaOH. The osmolality of OR3, measured using a Vapro vapor pressure osmometer (Wescor, Logan, UT), was adjusted to 195 ± 5 mOsmol/kg osmolality with H2O.
Table 2.
Composition (in mM) of solutions used in electrophysiological assays
| Solution |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na+ | NMDG+ | K+ | Ca2+ | Mg2+ | Cl− | Gluconate− | Buffer | pH | |
| pHe = 6.5 | 96 | 2 | 1.8 | 1 | 103.1 | 5 (Bis Tris) | 6.5 | ||
| pHe = 7.5 | 96 | 2 | 1.8 | 1 | 103.1 | 5 (HEPES) | 7.5 | ||
| pHe = 8.5 | 96 | 2 | 1.8 | 1 | 103.1 | 5 (Bicine) | 8.5 | ||
| Na-free | 96 | 2 | 1.8 | 1 | 103.1 | 5 (Bicine) | 8.5 | ||
| Cl-free | 96 | 2 | 1.8 | 1 | 103.1 | 5 (Bicine) | 8.5 | ||
Biotinylation.
Biotinylation of oocytes was performed with the Pierce Cell Surface Protein Isolation Kit (Thermo Fisher Scientific, Rockford, IL) using a version of the manufacturer's protocol adjusted for oocytes. Briefly, groups of fifteen oocytes were rinsed in ice-cold PBS (diluted to 200 mOsmol/kg to be isosmotic with oocyte cytosol) and each group was transferred into a separate well of six-well plate that contained the same PBS solution plus 0.24 mg/ml of Sulfo-NHS-SS-Biotin biotinylation reagent. After a 1 h incubation in the dark at 4°C with gentle agitation, the reactions were quenched by adding 10% vol/vol of the supplied quenching solution and the cells were washed in ice-cold TBS (pH 7.5). The cells were lysed in lysis buffer (Roche Diagnostics cOmplete, EDTA-free Protease Inhibitor cocktail tablet + 1% Triton X-100 in TBS pH 7.5) and insoluble debris was pelleted by centrifugation. The supernatant containing soluble material was incubated with neutravidin beads for 1 h using the supplied columns. Unbound protein was washed from the columns with lysis buffer, and the bound (i.e., biotinylated) protein was eluted from the beads using 1× SDS buffer containing 50 mM DTT. The eluted material was the “biotinylated fraction” sample. Samples were resolved by polyacrylamide gel electrophoresis using 3–8% Tris-acetate protein gels (Thermo Fisher Scientific) and transferred to PVDF membranes using an iBlot dry blotting system (Invitrogen). Membranes were probed for Slc4a11 protein using a custom anti-BTR1 antibody (Thermo Fisher Scientific) that was generated in rabbits against the extreme C-terminal epitope “LPRIIEAKYLDVMDAEHRP” in the cytosolic Ct of the protein. Immunoreactivity was visualized using an HRP-conjugated goat anti-rabbit secondary antibody (no. 1706515 Bio-Rad, Hercules, CA) and Pierce ECL 2 Western-blotting substrate (Thermo Fisher Scientific) in conjunction with a Pierce MyECL imager (Thermo Fisher Scientific).
Electrophysiological assays.
Xenopus oocytes were placed in a recording chamber (no. RC-3Z, Warner Instruments, Hamden, CT) and impaled with microelectrodes pulled from Clark borosilicate capillary glass (no. GC200TF-10, Warner Instruments) that were mounted in half-cell holders. Glass was pulled using a P-1000 micropipette puller (Sutter Instrument, Novato, CA) to achieve a tip resistance, when filled with saturated KCl of 0.5 MΩ. Voltage measuring and current-passing electrodes were used filled with saturated KCl (no. SP138-500, Thermo Fisher Scientific). pH-measuring electrodes were filled at the tip with a short (∼0.5 mm) column of H+-selective ionophore cocktail B (Sigma Aldrich) and backfilled with a solution that contained (in mM) 40 KH2PO4, 15 NaCl (pH adjusted to 7.0 with NaOH). Voltage-measuring and current-passing electrodes were connected to an OC-725C oocyte clamp (Warner Instruments) to measure membrane potential (Vm) and currents (Im). The H+-selective microelectrode was connected to a Duo 773 electrometer, with pH being reported as the difference between that signal and the signal from the voltage-sensing electrode. Voltage-clamp protocols were set and run from the pClamp 10.4 software suite (Molecular Devices, Sunnyvale, CA): oocytes were clamped at the desired value of V (typically the spontaneous membrane potential) prior to initiation of a protocol in which the potential was stepped to +20 mV for 100 ms before returning to its initial value for 100–400 ms before being stepped to 0 mV and so on in −20 mV intervals, with the final step being to − 160 mV. Superfusing solutions were changed using a six-channel perfusion pinch-valve control system (no. VC-6, Warner Instruments). Detailed descriptions of solutions used in this study (e.g., osmolality and ion composition) are shown in Table 2.
Data analysis.
Current-voltage (I–V) relationships (generated using Clampfit 10) and pHi/Vm traces (acquired using custom software written by Dale Huffman from the laboratory of Walter Boron, Case Western Reserve University, Cleveland, OH) were transferred to Excel for calculation of Gm or d[H+]i/dt. Statistical analysis was performed by MiniTab17 using a general linear model with Tukey's post hoc comparison in which injected material (H2O vs. cRNA) and extracellular solution were treated as two interacting, fixed factors. The cell number (e.g., 1 through 6 of n = 6) was considered as a random factor to allow for pairing of responses to serial solution changes by individual cells. The analysis output assigns a letter to each data group to denote its statistical difference from other data groups; we show these letters above each data point in our figures. Data points that share a letter are considered to be statistically indistinguishable (confidence interval 95%).
RESULTS
Raising extracellular pH (pHe) increases the H+/OH− permeability of mSlc4a11-expressing oocytes.
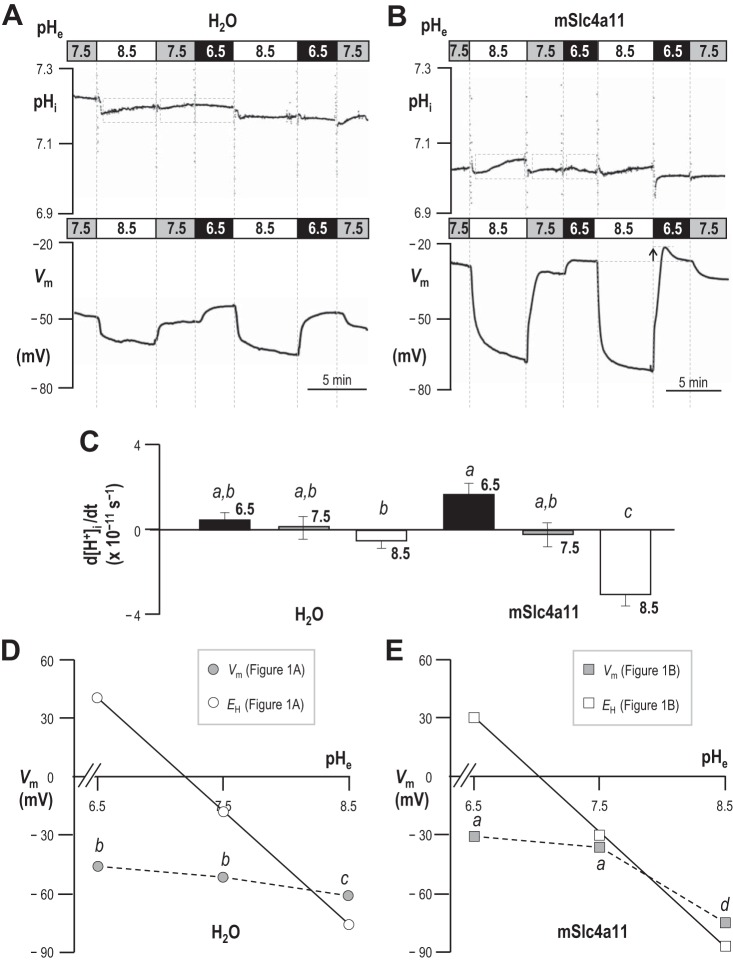
Figure 1A shows a representative experiment in which we simultaneously monitored intracellular pH (pHi) and spontaneous membrane potential (Vm) of a H2O-injected oocyte during exposure to pHe = 6.5, pHe = 7.5, and pHe = 8.5 solutions. The average resting value of pHi in pHe = 7.5 solution was 7.15 ± 0.03 (n = 6) and the average Vm was −46 ± 3 mV (n = 6). During exposure to pHe = 6.5 solution, pHi of these cells tended to acidify and Vm tended to depolarize (Fig. 1A). During exposure to pHe = 8.5 solution, the pHi of H2O-injected cells tended to alkalinize4 and Vm tended to hyperpolarize. The small changes in [H+]i that we detected while changing pHe did not achieve statistical significance in our sample (Fig. 1C, n = 6; groups that share the same letter were not significantly different from each other; 95% confidence; result of a general linear model analysis with Tukey's pairwise comparison). On the other hand, the more hyperpolarized Vm in pHe = 8.5 solution was significantly different from the Vm in either pHe = 6.5 or pHe = 7.5 solution (gray data points in Fig. 1D, n = 6). The white data points in Fig. 1D represent the averaged calculated values of EH (the equilibrium potential for H+/OH−) given the average measured pHi of each cell (from data such as those in Fig. 1A) at each value of pHe. The slope of the line drawn through these points has the expected Nernstian ideal value of −58 mV/decade. The slope of the dotted line that describes the actual relationship between Vm and pHe in H2O-injected cells, as pHe increases from 6.5 to 7.5, is −5 mV/decade. The slope of the dotted line that describes the relationship between Vm and pHe as pHe increases from 7.5 to 8.5 is −8 mV/decade. Thus we observe no evidence of substantial H+/OH− permeability in H2O-injected oocytes.
Fig. 1.
Extracellular pH (pHe)-dependence of membrane potential (Vm) and intracellular pH (pHi) in H2O-injected and mSlc4a11-expressing oocytes. A and B: pHi and Vm of an oocyte that had been injected with H2O (A) or mSlc4a11 cRNA (B) was monitored while the cell was exposed to extracellular solutions of pH 6.5, 7.5, or 8.5. The gray dashed boxes exemplify the periods over which the rates of [H+] change were calculated for C (i.e., omitting rapid changes close to the time of solution change that could be associated with artifacts or other non-Slc4a11-dependent phenomena). The arrow in B shows an enhanced depolarization of cells in pHe = 6.5 solution following exposure to pHe = 8.5 solution. C: the rate of change of intracellular [H+] during exposure to pHe = 6.5, 7.5, or 8.5 solutions (n = 6). Rates were calculated from the change in [H+] that occurred during the periods such as enclosed by gray boxes in A and B. The letters above each bar denote statistically indistinguishable groups reported by general linear model with Tukey's post hoc analysis (confidence interval 95%). D and E: average Vm of H2O-injected (D) or mSlc4a11-expressing (E) cells during exposure to pHe = 6.5, 7.5, or 8.5 solutions (gray data points), with letters to denote statistically indistinguishable groups. Vm values were taken at the end of the exposure period to each solution. For each value of pHe, each “n” represents an averaged value from the 2–3 exposure periods to which each cell was subjected. The white data points indicate the equilibrium potential for H+ determined at the average pHi from A or B at each value of pHe. Note that standard error bars are included but are eclipsed by the data point markers.
Figure 1B shows an equivalent experiment to that in Fig. 1A, performed on an mSlc4a11-expressing oocyte. The average pHi of these cells was 6.95 ± 0.01 (n = 6) and the average resting Vm was −34 ± 3 mV (n = 6). Thus these cells are more acidic and more depolarized at rest than H2O-injected oocytes (P < 0.05; n = 6; unpaired t-test for each parameter). During exposure to pHe = 6.5 solution, the rate of acidification of mSlc4a11-expressing cells tended to be greater than, but was not significantly different from, that of H2O-injected cells (Fig. 1C) even when assessed with the less conservative t-test (P = 0.10). We find that the relationship between Vm and pHe in mSlc4a11-expressing cells vs. H2O-injected cells is described by a similar slope (i.e., −5 mV/decade), as pHe increases from 6.5 to 7.5 (gray data points in Fig. 1E). On the other hand, mSlc4a11-expressing cells behave very differently from H2O-injected cells during exposure to pHe = 8.5 solution. Firstly, mSlc4a11-expressing cells exhibit a significant alkalinization (Fig. 1, B and C). Secondly, the slope of the dotted line that describes the relationship between Vm and pHe (as pHe increases from 7.5 to 8.5) is −38 mV/decade (Fig. 1D), indicating substantial H+/OH− permeability of the mSlc4a11-expressing plasma membrane. A final notable feature of the behavior of mSlc4a11-expressing cells is that the extent of depolarization upon exposure to pHe = 6.5 solution is transiently exaggerated (in the direction of EH) by ∼3 mV if the cell is bathed in pHe = 8.5 (see arrow in lower panel of Fig. 1B) rather than pHe = 7.5 solution immediately prior to the solution change. This indicates that the loss of H+/OH− permeability upon extracellular acidification is not instantaneous.
Raising pHe increases the membrane conductance of mSlc4a11-expressing oocytes.
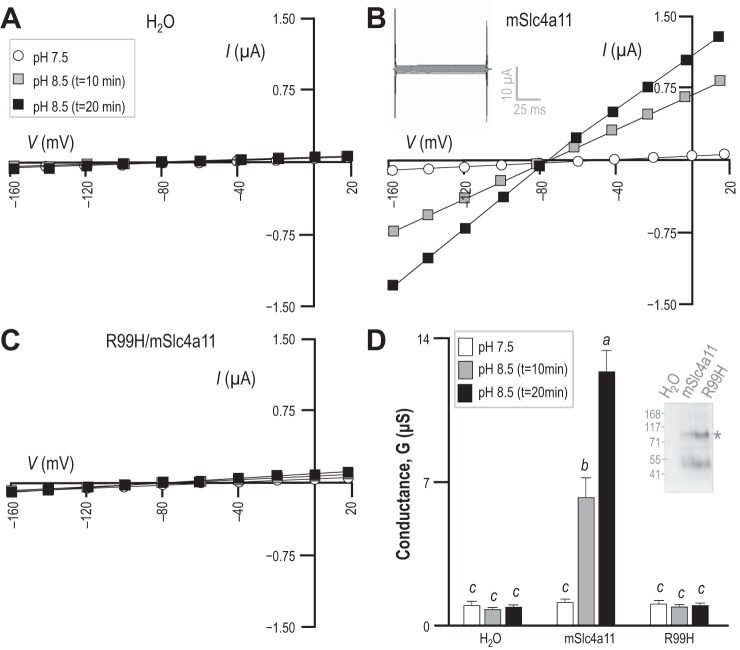
To examine the membrane currents (Im) responsible for the pH-dependent shifts in Vm observed in Fig. 1, we subjected oocytes to our voltage-clamping protocol. Figure 2A shows a set of current-voltage (I–V) relationships gathered from a H2O-injected oocyte during exposure to pHe = 7.5 and pHe = 8.5 solutions. The conductance of the membrane (Gm) was unaltered by the rise in pHe, as summarized for a larger number of cells in Fig. 2D (n = 6; groups that share the same letter are not significantly different from each other; 95% confidence; result of a general linear model analysis with Tukey's pairwise comparison). Figure 2B shows an equivalent set of I–V plots gathered from an mSlc4a11-expressing oocyte. In pHe = 7.5 solution, the Gm of mSlc4a11-expressing cells is not significantly different from that of H2O-injected cells (white bars in Fig. 2D). However, exposure of mSlc4a11-expressing oocytes to pHe = 8.5 solution resulted in the expected hyperpolarization of Vm (evident in the negative shift of the zero-current value of V in Fig. 2B) as well as a gradual increase in the magnitude of Gm throughout the 20 min exposure period (gray vs. black data in Fig. 2, B and D). We will return to this phenomenon of rising Gm vs. time in the section entitled “Raising intracellular pH increases the H+/OH− conductance of mSlc4a11-expressing oocytes.” An example of the current steps elicited by our voltage-clamp protocol after 20 min of exposure to pHe = 8.5 solution is shown in the gray inset in Fig. 2B, and reveals no acute time- or voltage-dependence of Im. A mutant mSlc4a11 [R99H/mSlc4a11: equivalent to the CHED-causing mutation R125H in human Slc4a11 (24, 52)] accumulated to a similar extent as wild-type mSlc4a11 in the plasma membrane of oocytes (P = 0.23, paired two-tailed t-test, n = 4: example of biotinylation results shown in Fig. 2D/gray inset). However, oocytes expressing the mutant did not exhibit any rise in Gm (Fig. 2, C and D) nor did their Vm hyperpolarize to any greater degree than of H2O-injected cells in response to the application of pHe = 8.5 solution (P = 0.27 vs. the ΔVm of H2O-injected cells, unpaired two-tailed t-test, data not shown).
Fig. 2.
pHe-dependence of membrane conductance (Gm) in H2O-injected and mSlc4a11-expressing oocytes. A–C: representative sets of current-voltage (I–V) relationships for an oocyte that had been injected with H2O (A), mSlc4a11 cRNA (B), or cRNA encoding an “R99H” mutant of mSlc4a11 (C). A representative current ladder obtained for the Slc4a11-expressing oocyte bathed for 20 min in pHe = 8.5 solution is shown in the inset in B. D: average membrane conductance calculated for six of each cell type from data such as that in A, B, and C. Letters above each bar denote statistically indistinguishable groups. The inset in D shows one of three Western blots of biotinylated oocyte protein, probed for mSlc4a11 immunoreactivity.
mSlc4a11 is ideally permeable to H+/OH−.
The equation below (Eq. 1) restates the Goldman-Hodgkin-Katz equation. Equation 1 predicts that, if mSlc4a11 is perfectly selective for H+ or OH− (i.e., PH = 1; PK, PNa, and PCl = 0), the slope of the relationship between the transmembrane H+ gradient and Vm will approach the Nernstian ideal of 58 mV/pH unit:
| (1) |
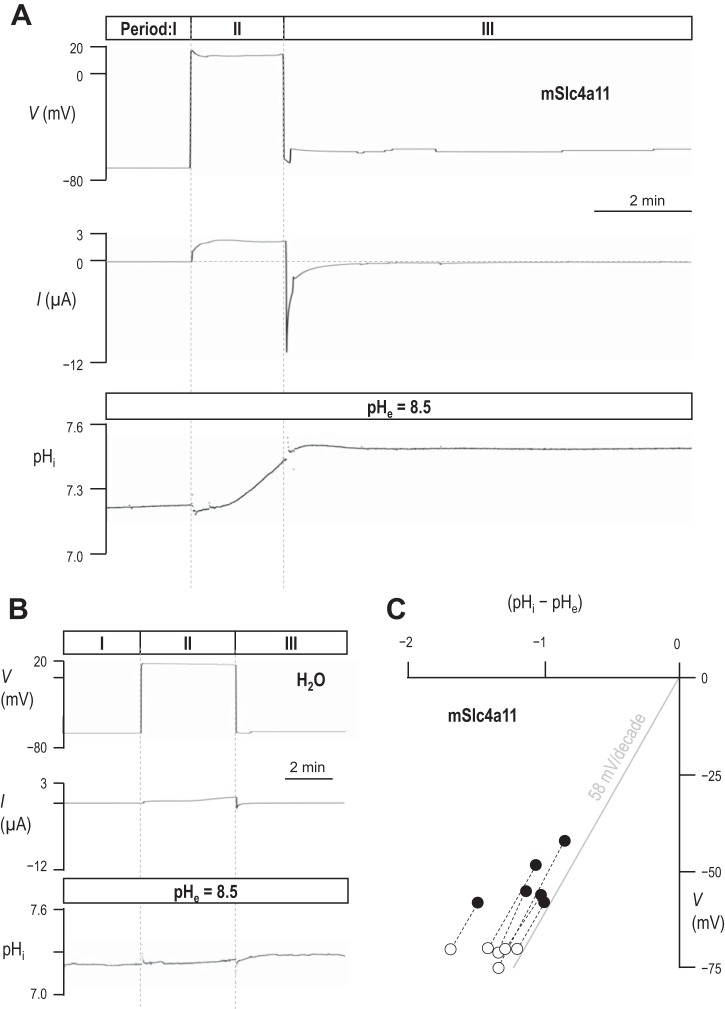
Acidic shifts in pHe prevent mSlc4a11 action from dominating Vm (Fig. 1E); therefore, instead of monitoring the response of pHi and Vm to changes in pHe, we opted to monitor the response of Vm at constant pHe as we altered pHi. Figure 3A shows a representative experiment in which we bathed an mSlc4a11-expressing oocyte in pHe = 8.5 solution, allowed Vm to hyperpolarize (per Fig. 1B) and, when we observed no further change in Vm, applied a voltage-clamp to maintain Vm at its spontaneous (“zero-current” or “steady-state”) value. This steady state is shown in Period I of Fig. 3A and provides us with the first of two data points that we gathered from each cell that describes the relationship between the transmembrane pH gradient (pHi - pHe) and Vm, that are plotted for each cell in Fig. 3C (open circles). To obtain the second data point (filled circles in Fig. 3C), we caused pHi to rise by taking advantage of the H+/OH− permeability of mSlc4a11-expressing cells; clamping V to +20 mV for a short period of time (Period II in Fig. 3A) provides the necessary acid-extruding driving force. Once pHi had risen by a substantial amount (on average 0.29 ± 0.04 units, n = 6, which approximates to a halving of [H+]i), we interrupted the pHi trajectory by clamping V to a new value that represented the zero-current value at the new value of pHi (Period III in Fig. 3A). As seen in the traces in Fig. 3A, we made several fine adjustments to the value of V until we located its zero-current value. We held the cell in clamp at this value of V until we observed no further change in pHi, and then noted our second “pHi − pHe” vs. Vm data point for the cell (filled circles in Fig. 3C). Figure 3B shows one of five equivalent experiments that we performed on H2O-injected oocytes. Note that the pHi in this cell does not change substantially in response to the voltage-clamp maneuvers. Figure 3C shows the accumulated data points gathered from experiments such as those in Fig. 3A, as well as the ideal Nernstian relationship between Vm and the transmembrane pH gradient (gray line). The average slope of the experimentally determined “pHi − pHe” vs. Vm relationships is 64 ± 4 mV/pH unit (n = 6).
Fig. 3.
The relationship between pHi and Vm in H2O-injected and mSlc4a11-expressing oocytes. A and B: representative voltage, current, and pHi traces recorded from an mSlc4a11-expressing (A) and a H2O-injected oocyte (B) exposed to pHe = 8.5 solution. During Period I, Vm was clamped at its spontaneous (zero-current) value. During Period II, Vm was clamped to +20 mV to drive electrogenic acid extrusion. During Period III, Vm was clamped at its new zero-current value, reflecting the increased pHi. C: a plot of zero-current Vm vs. transmembrane pH gradient from six cells such as that represented in A. White data points were collected from each cell during Period I in A. Black data points were collected towards the end of Period III in A.
Raising intracellular pH increases the H+/OH− conductance of mSlc4a11-expressing oocytes.
Because pHi rises in mSlc4a11-expressing oocytes during exposure to pHe = 8.5 solution, we hypothesized that the apparent time-dependent increase of Gm, represented in Fig. 2, could represent a dependence on pHi. Figure 4A shows one of six experiments in which we monitored the pHi of an mSlc4a11-expressing oocyte bathed in pHe = 8.5 solution. At regular intervals throughout the recording (typically each minute, see deflections in V trace in Fig. 4A), we initiated a 5-s step-wise voltage-clamp protocol to monitor changes in Gm (bottom trace in Fig. 4A). During Period I of the experiment we allowed each cell to spontaneously hyperpolarize and alkalinize for 5 min in response to the application of pHe = 8.5 solution. During Period II, we applied an electrical driving force to promote faster mSlc4a11-mediated acid-extrusion by voltage-clamping the membrane potential at −60 mV (EH = −84 mV in the example shown). Gm increases nearly 100-fold over the 0.45 pH unit rise. The evolution of the current steps evoked by our voltage-clamp protocol over the course of the experiment is represented in the inset in Fig. 4A. The average relationship between pHi and Gm for all six cells tested is plotted in Fig. 4B.
Fig. 4.
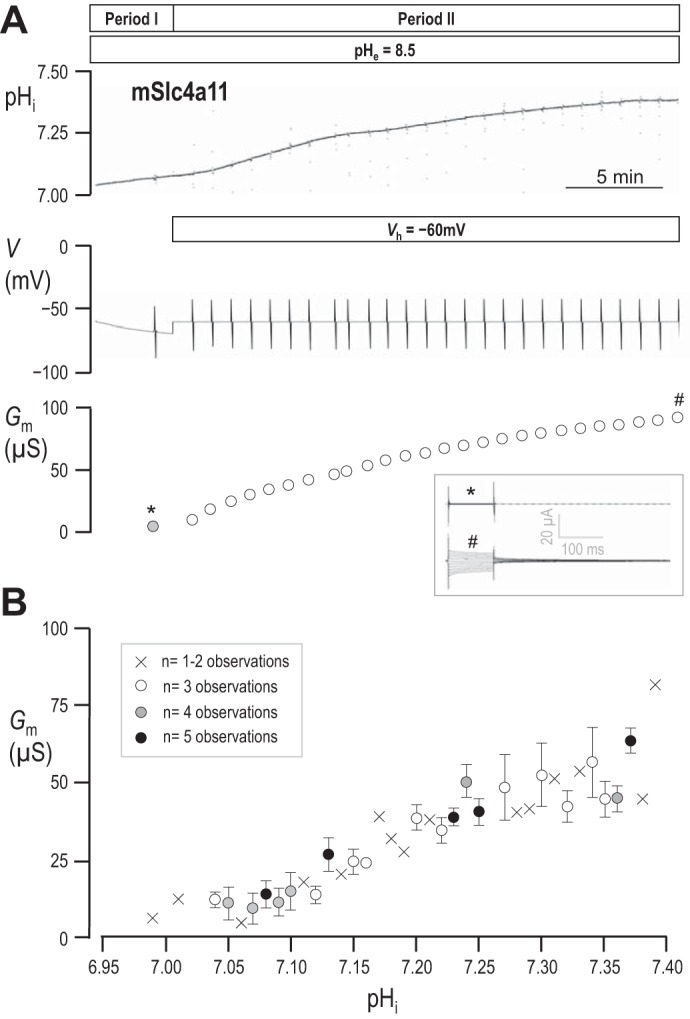
The relationship between pHi and Gm in mSlc4a11-expressing oocytes. A: representative pHi and voltage traces recorded from an mSlc4a11-expressing cell during exposure to pHe = 8.5 solution prior to, and during, voltage-clamp at −60 mV to drive electrogenic acid-extrusion. The deflections in the voltage trace denote the points at which current-voltage relationships were obtained. The values of G calculated for the cell at each point are plotted in bottom panel; the gray data point was obtained prior to the application of the voltage-clamp while the cell was spontaneously hyperpolarizing towards initial EH. The inset shows the current ladders at the measurement points labeled * and #. B: the relationship between pHi (rounded to 2 decimal places) vs. Gm averaged from data gathered from six mSlc4a11-expressing cells. Low confidence observations are marked with crosses, higher confidence observations are marked with open or filled circles accompanied by standard error bars.
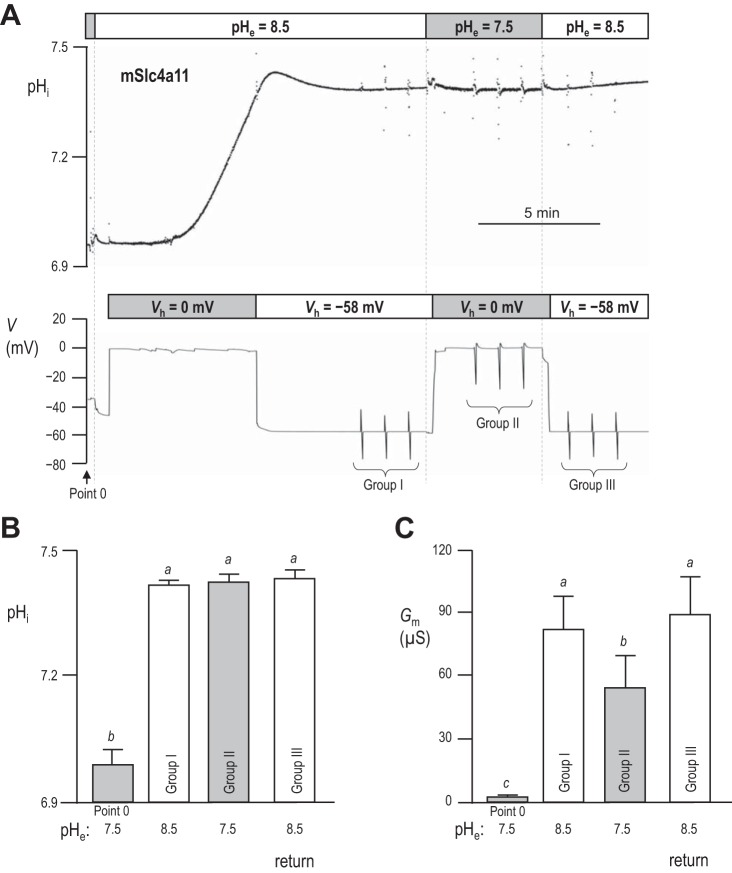
Given the apparent pHi sensitivity of mSlc4a11, we hypothesized that the lack of mSlc4a11 conductivity at physiological pH (pHe = 7.5 solution in Fig. 2) could be a function of pHi rather than pHe per se. In a set of experiments represented by the example shown in Fig. 5A, we compared Gm in pHe = 8.5 vs. pHe = 7.5 solutions while pHi was held at a constant elevated value close to 7.5. We first obtained a value for Gm in an mSlc4a11-expressing oocyte at rest in pHe = 7.5 solution (“Point 0” in Fig. 5, A and C). Next, we exposed the cell to pHe = 8.5 solution and applied a voltage-clamp (Vh = 0 mV) to rapidly alkalinize pHi. Once pHi was close to 7.5, we voltage-clamped the cell to −58 mV (the predicted value of EH for the condition pHe = 8.5/pHi = 7.5) to hold pHi constant. When pHi was stable, we made three consecutive measurements of Gm, 1 min apart (“Group I” in Fig. 5A). Next, we exposed the oocyte to our pHe = 7.5 solution, immediately switching the voltage-clamp to 0 mV (the predicted value of EH for the new condition pHe = 7.5/pHi = 7.5) to prevent changes in pHi and again measured Gm (“Group II” in Fig. 5A). Finally, we returned to our pHe = 8.5 solution, reclamped the cell to −58 mV, and measured Gm again (“Group III” in Fig. 5A). Figure 5B reports the average pHi at which each group of Gm values was obtained from six cells and shows that the pHi was not different in pHe = 7.5 solution (Group II) vs. either bracketing measurements in pHe = 8.5 solution (Groups I and III). We note that pHi was slightly more acidic than predicted if H+ was at electrochemical equilibrium under these conditions (see discussion). Figure 5C reports the average values of Gm determined at each value of pHe at this constant, elevated value of pHi. These data show two things:
Fig. 5.
pHi-dependence vs. pHe-dependence of membrane conductance (Gm) in mSlc4a11-expressing oocytes. A: representative pHi and voltage traces recorded from an mSlc4a11-expressing oocyte during exposure to pHe = 7.5 and pHe = 8.5 solution under voltage-clamp. When the extracellular solution was pH 8.5, Vm was initially held at 0 mV to promote a mSlc4a11-mediated rise in pHi to ∼7.5, at which point Vm was clamped to the predicted value of EH (0 mV) to stabilize pHi at ∼7.5. When the extracellular solution was pH 7.5, Vm was held at −58 mV to maintain pHi at ∼7.5. Point 0 and Groups I–III denote regions of the trace where I–V relationships were measured to allow calculation of Gm measurements. There was no significant change in pHi or Gm between the first and last measurement in Groups I, II, or III and thus we took the average of the three readings for each group to be a single replicate to be reported in B and C. B: average values (n = 7 for all) of pHi measured at each of the points (or group, in which case the three values in each group were averaged) noted in A, with letters to indicate groups that are statistically indistinguishable (general linear model, confidence interval 95%). C: average values (n = 7 for all) of Gm measured at each of the points noted in A, with letters to indicate groups that are statistically indistinguishable.
1) Gm is significantly greater when pHi ∼7.5 vs. ∼7.0 (Group II vs. Point 0); i.e., the conductivity of mSlc4a11 at pHe = 7.5 increases with pHi.
2) Gm is substantially greater when pHe = 8.5 vs. 7.5 even at elevated values of pHi (Groups I and III vs. Group II); i.e., the enhancement of Gm in Fig. 2 is not solely a reflection of increased pHi; mSlc4a11 is truly and additionally pHe sensitive.
Removing extracellular Na+ or Cl− does not greatly influence the H+/OH− conductance of mSlc4a11-expressing oocytes.
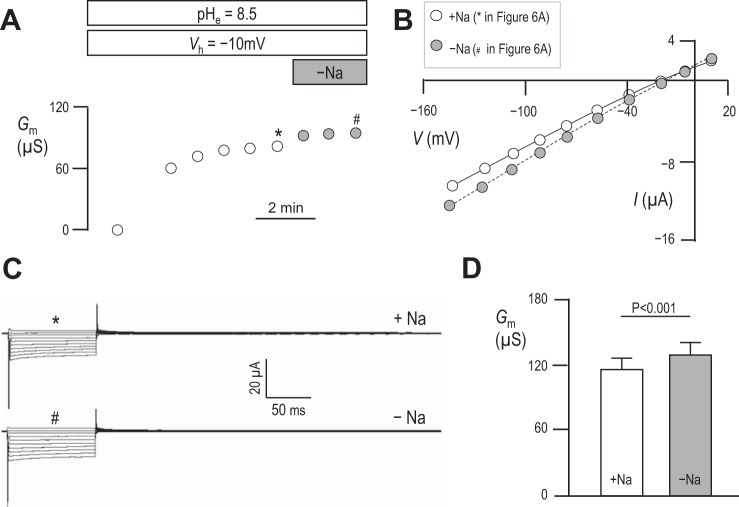
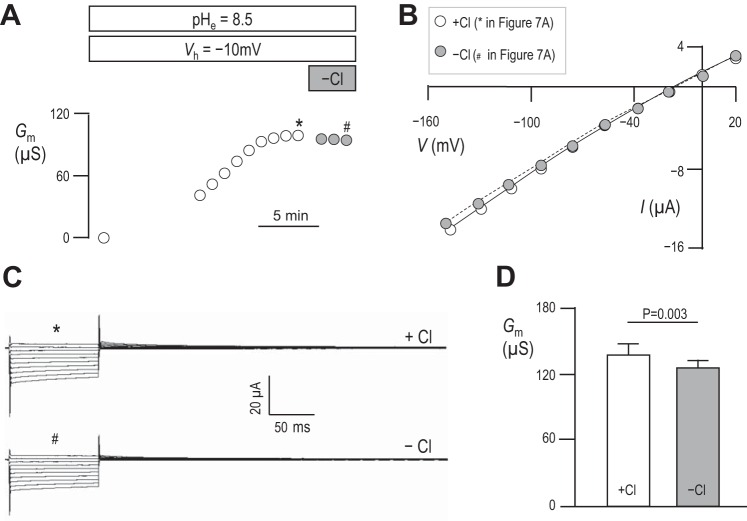
Results in Fig. 3 show that mSlc4a11 is ideally permeable to H+/OH− and therefore not substantially permeable to Na+. However, these results do not rule out the possibility that mSlc4a11 H+/OH− permeability is Na+ dependent. Figure 6A shows a representative experiment in which we exposed an mSlc4a11-expressing oocyte to pHe = 8.5 solution, clamped Vm at −10 mV to promote acid-extrusion, and monitored Gm. Once Gm had reached its maximum value, as judged from 3 consecutive readings 1 min apart that showed no substantial change, we applied a bath solution identical to our pHe = 8.5 solution except for the replacement of all Na+ with NMDG+. We monitored Gm for a further 3 min and, noting no obvious alteration in Gm, terminated the experiment. The Gm data points with (*) or without (#) Na+ in Fig. 6A were generated from the I–V plots shown in Fig. 6B, which in turn were generated from the current ladders shown in Fig. 6C. The averaged values of Gm with or without Na+ are shown for a larger number of cells in Fig. 6D. Although Gm was significantly greater in mSlc4a11-expressing cells in the absence of Na+ (result of a paired two-tailed t-test, n = 6), the change is insubstantial and the currents are qualitatively similar in the presence vs. the absence of Na+. Figure 7, A–D, shows a similar suite of experiments designed to probe the influence of extracellular Cl− on mSlc4a11 action. Here we find that the complete replacement of Cl− with gluconate− causes a significant (result of a paired two-tailed t-test, n = 6) yet insubstantial fall in Gm with, again, no obvious qualitative change in the currents.
Fig. 6.
The influence of extracellular Na+ on Gm in mSlc4a11-expressing oocytes. A: representative experiment showing progressive changes in Gm in an mSlc4a11-expressing oocyte clamped at −10 mV in pHe = 8.5 solution. Gray data points indicate measurements made in Na+-free solution. B and C: data points in A marked with * and # are generated from data similarly marked in B accompanying representative I–V plots and C accompanying current ladders. D: averaged data from six cells showing the influence of extracellular Na+ on Gm. Statistic is the result of a two-tailed, paired t-test. Data equivalent to that shown in A–D, but showing the influence of extracellular Na+.
Fig. 7.
The influence of extracellular Cl− on Gm in mSlc4a11-expressing oocytes. A–D: data equivalent to that shown in Fig. 6, but showing the influence of extracellular Cl−.
NH3/NH4+ indirectly increases the H+/OH− conductance of mSlc4a11-expressing oocytes by depolarizing Vm.
Others have presented data in support of the hypothesis that Slc4a11 is an NH3-2H+ cotransporter (59). To reproduce these data, we obtained I–V relationships from H2O-injected cells (example in Fig. 8A) and cells expressing mSlc4a11 (example in Fig. 8B) during exposure to our pHe = 7.5 and pHe = 8.5 solutions in the presence of 5 mM NH4Cl. The initial response to NH3/NH4+ application at pHe = 7.5 of both H2O-injected and mSlc4a11-expressing cells was a rapid depolarization of Vm. The Vm of H2O-injected oocytes depolarized from −51 ± 2 mV to −16 ± 2 mv (n = 6) and mSlc4a11-expressing oocytes depolarized from −38 ± 1 mV to −3 ± 1 mV (n = 6). Only in mSlc4a11-expressing cells was this depolarization accompanied by a rapid increase in Gm that became statistically significant when cells were subsequently exposed to NH3/NH4+ at pHe = 8.5 (Fig. 8C). Note that the magnitude of the NH3/NH4+-induced Gm in pHe = 8.5 solution is similar to that which can be achieved by depolarization induced alkalinization in the absence of NH3/NH4+ (e.g., Fig. 6D).
Fig. 8.
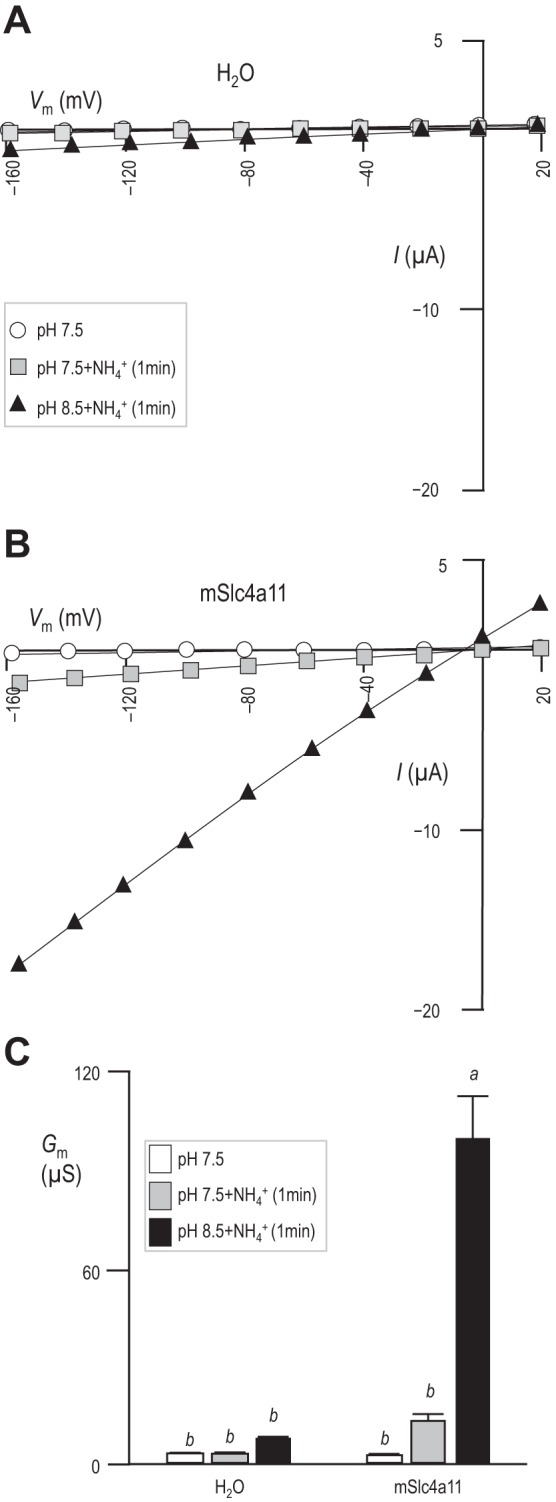
NH3/NH4+-dependence of Gm in mSlc4a11-expressing oocytes. A and B: representative sets of I–V relationships for an oocyte that had been injected with H2O (A) or mSlc4a11 cRNA (B) and sequentially exposed to pHe = 7.5 solution, pHe = 7.5 solution containing 5 mM NH4Cl, and pHe = 8.5 solution containing 5 mM NH4Cl. C: average membrane conductance calculated for six of each cell type from data such as that in A and B, with letters above each bar to denote statistically indistinguishable groups (general linear model, confidence interval 95%).
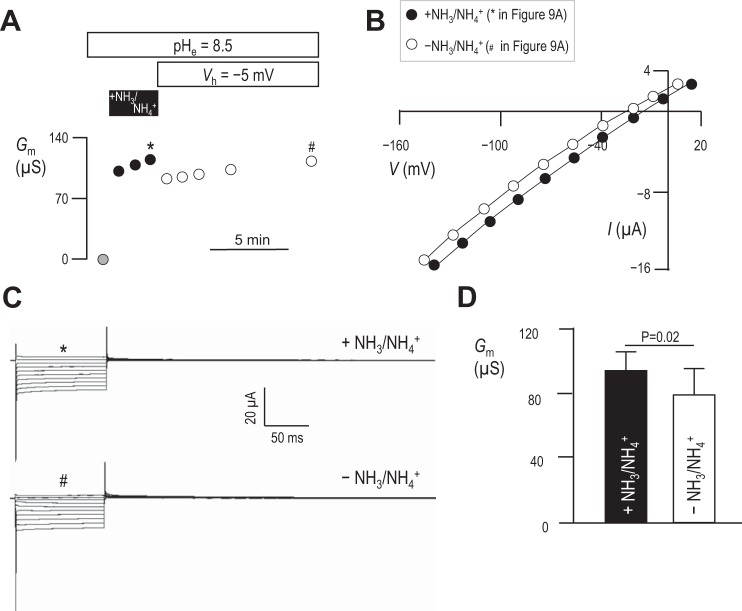
We hypothesized that, if this conductance does represent the action of an electrogenic, NH3/NH4+-coupled transport process (rather than an indirect stimulation of uncoupled H+/OH− conductance that follows depolarization) the NH3/NH4+-induced rise in Gm ought to be reversed by removal of NH3/NH4+. Figure 9A shows a representative experiment in which we monitored the Gm of an mSlc4a11-expressing oocyte in pHe = 8.5 solution during and after exposure to NH3/NH4+. The first I–V relationship was obtained at spontaneous Vm, 1 min into an exposure to pHe = 8.5 solution; the calculated Gm is the gray data point in Fig. 9A. The cell was then exposed to pHe = 8.5 solution containing 5 mM NH4Cl, which caused Vm to substantially depolarize. Gm was determined in these conditions from further I–V relationships obtained 1 min apart from each other (filled symbols). Finally, the original pHe = 8.5 solution (lacking NH3/NH4+) was restored to the bath while Vm was voltage-clamped at its new depolarized value (−5 mV in the example shown in Fig. 9A, to mimic the depolarizing effect of NH3/NH4+ presence) and five further I–V relationships were obtained at 1, 2, 3, 5, and 10 min following NH3/NH4+ removal (open symbols). The Gm data points with (*) or without (#) NH3/NH4+ in Fig. 9A were generated from the I–V plots shown in Fig. 9B, which in turn were generated from the current ladders shown in Fig. 9C. Figure 9D shows the average Gm values measured at each point and demonstrates that, while the removal of NH3/NH4+ causes a significant decrease in Gm, the majority of the conductance persists even 10 min after extracellular NH3/NH4+ removal.
Fig. 9.
NH3/NH4+-independence of Gm in mSlc4a11-expressing oocytes. A: representative experiment showing progressive changes in Gm in an mSlc4a11-expressing oocyte exposed to pHe = 8.5 solution containing 5 mM NH4Cl at spontaneous Vm (black data points), or subsequently to NH3/NH4+-free pHe = 8.5 solution (white data points). Data points marked with * or # were generated from data similarly marked in B accompanying representative I–V plots and C accompanying current ladders. D: averaged data from six cells showing the influence of extracellular NH3/NH4+ removal on NH3/NH4+-induced Gm. Statistic is the result of a two-tailed, paired t-test.
DISCUSSION
Our conclusions regarding the molecular action of mSlc4a11.
Our data show that Slc4a11 is a pHi and pHe-stimulated H+/OH− conductance. A major strength of our approach that allows us to extend this observation beyond previous studies with mammalian cells is that we simultaneously monitor and control Vm as we observe pHi changes. Thus for the first time we report that mSlc4a11-expressing cells exhibit an exaggerated Vm response to pHe changes. By matching our observations to the predicted Vm response of an ideally H+/OH−-permeable membrane, we make the novel observation that the apparent H+/OH− permeability of mSlc4a11-expressing cells increases as pHe rises (Fig. 1D). Furthermore, at pHe = 8.5—the value in our assay at which H+/OH− permeability appears to be greatest—the relationship between the transmembrane pH gradient and Vm is close to Nernstian (Fig. 3). This is a particularly powerful result when considering the relative scarcity of H+/OH− compared to other ions in our solutions such as Na+. Equation 1 predicts that even if mSlc4a11 had a PNa/PH/OH permeability ratio of 10−6, Vm would tend towards ENa [∼ +60 mV (57)] rather than EH and would be essentially unresponsive to changes in the transmembrane pH gradient. The same holds true for H+/OH− vs. all other components of our solutions that are present at mM concentration. Our data also rule out any obligate Na+, or Cl−, dependence of the mSlc4a11-mediated H+/OH− current (Figs. 6 and 7).
Another unique finding of our study is that the mSlc4a11 conductance is acutely and intrinsically sensitive to changes in pHe and pHi, with rises in either parameter, independently resulting in an increase in Gm (Figs. 4 and 5). This observation suggests that OH− is the charge carrier, but there is clearly an allosteric effect of pHi and pHe because under no circumstances did we observe a rectification in the I–V relationships that would be expected if the pHe- or pHi-dependent rises in Gm were simply caused by a rise in OH− (or even HCO3−)5 on one side of the membrane. The diminution of Gm caused by lowering pHi explains one confounding observation in Fig. 2: the reason that mSlc4a11 does not appear to be substantially conductive at physiological pHe. At rest, mSlc4a11-expressing oocytes are unusually acidified (pHi = 6.95), far below the values of pHi in our H2O-injected oocytes (pHi = 7.22) or the value expected in, for example, a corneal endothelial cell [pHi ∼ 7.3 (4, 26)]. The lower steady-state pHi in oocytes in culture at pHe = 7.5 is consistent with the unopposed action of a H+/OH− channel: the driving force acting on mSlc4a11 expressed in a cell with initial conditions pHi = 7.22, and Vm = −50 mV (per our H2O-injected cells) will promote acid loading. mSlc4a11-mediated acid-loading should continue until electrochemical equilibrium is reached, or until pHi falls to a value at which mSlc4a11 is not substantially conductive. As Vm (−34 mV) is close to EH (−32 mV) at rest in these cells, it is likely a combination of these factors apply. Thus the more acidified and depolarized state of mSlc4a11 expressing cells vs. H2O-injected cells is a predictable consequence of the expression of a H+/OH− conductance.
The predominant electrogenic permeability of the membrane of mSlc4a11-expressing cells is to H+/OH−. Accordingly, pHi of these cells is almost exclusively under electrical control. However, under zero-current conditions we observe that pHi is always slightly more acidic than predicted if H+ were at electrochemical equilibrium (e.g., non-zero x-axis intercepts extrapolated from data in Fig. 3C and the lower-than-7.5 value of pHi in Fig. 5B). This phenomenon is consistent with one or a combination of the following factors:
1) The action of an electroneutral acid-loading activity such as an endogenous Cl−/HCO3− exchanger (under these particular assay conditions, pHi is typically more alkaline than that of a H2O-injected oocyte, which would tend to promote such endogenous acid loading mechanisms),
2) The pHi sensed by mSlc4a11 at the plasma membrane following a period of robust acid-extrusion being more alkaline than the bulk pHi sensed by our pHi electrode, the tip of which is likely positioned tens of μm away from the inner surface of the membrane, or
3) The pH at the cell surface (pHs) sensed by mSlc4a11 being more acidic than bulk pHe; indeed even in H2O-injected oocytes pHs is slightly more acidic than bulk pHe (15).
Importantly, none of our observations are consistent with mSlc4a11 mediating acid-extruding activities such as Na+/H+ exchange or Na+-OH− cotransport. Thus we conclude that mSlc4a11 is an ideally selective H+/OH− conductance. Whether this conductance represents the action of a facilitative carrier (or H+/OH− exchanger) rather than a bona fide ion channel could be experimentally determined in future work by study of single-channel events in membrane patches expressing mSlc4a11.
To our knowledge, although many proteins are capable of conducting H+/OH−, the major passive H+-selective conductance in animals is the voltage-gated H+-channel Hv1, the time and voltage-dependence of which are not features of the currents associated with mSlc4a11 (9, 10, 50). In summary, we find mSlc4a11 to be a unique entity: an ideally H+/OH−-selective, voltage and time-independent conductance that is independently stimulated by rises in pHe and pHi.
Comparisons with the data of others regarding Slc4a11 action.
We must preface any discussion of previous studies with a disclaimer that ours uses mouse rather than human Slc4a11. The mouse and human orthologs of Slc4a11 are 85% identical at the amino -acid level, with a major disparity at the extreme N-terminus of the protein, which is 29–72 aa shorter in the mouse ortholog depending on which human variant is considered [SLC4A11-A, B, or C (30)]. The role, if any, of these N-terminal appendages is unknown, but comparison with other Slc4 proteins suggests that these are more likely to play a regulatory role than to determine intrinsic transport action (e.g., refs. 37, 45, 54). We must also point out that most previous studies draw on experiments that have been conducted using mammalian cells, whereas we are using oocytes. The use of oocytes provides the advantage of studying mSlc4a11 in the absence of complications from substantial endogenous pH-regulating mechanisms. The use of oocytes also requires us to note that we are studying mSlc4a11 action in the absence of any natural binding partners/accessory subunits; thus our study considers the intrinsic properties of the mSlc4a11 polypeptide.
Our data are in best agreement with those of Kao and coworkers (30), who studied human SLC4A11-C in HEK cells. As we find with oocytes, the pHi of SLC4A11-expressing HEK cells is also more acidic at rest than that of mock-transfected cells, although not to the same extent as the oocytes in our study. This difference is explained by the acid-extruding action of an EIPA-sensitive NHE that is endogenous to HEK cells; in the presence of EIPA the pHi of these cells falls to a value that is presumably close to EH (30). The findings of Kao et al. are exemplified by the observation that lowering extracellular K+ (or Na+ in gramicidin-permeabilized cells), a maneuver that ought to hyperpolarize Vm, results in an acidification of Slc4a11-expressing cells. This response is consistent with expression of electrogenic H+/OH− permeability. A potential disparity between the behaviors of human SLC4A11-C in HEK cells vs. mSlc4a11 in oocytes is that, unlike the conductance mediated by mSlc4a11, the H+/OH− fluxes mediated by the human clone show no obvious sign of pH-dependence. However, methodological and technical differences between the two studies complicate a direct data comparison.
Other studies report that mSlc4a11-mediated H+/OH− transport is coupled to the transport of another molecular species. Several groups have reported that mSlc4a11 interacts with Na+ (28, 42, 43). One group found that removing extracellular Na+ caused HEK cells to acidify. However, Na+ removal also caused a rapid depletion of Na+ in both mock- and mSlc4a11-transfected cells in that study (43); both were thus likely to be hyperpolarized by this maneuver. Therefore, with hindsight, the acidification could be explained as the predictable effect of hyperpolarization on Slc4a11-mediated H+/OH− conductance rather than a direct Na+-dependence of Slc4a11 action. Consistent with this hypothesis, Kao et al. (30) only observe acidification upon Na+ removal in cells that have been permeabilized with gramicidin. Beyond the observation of apparent Na+-dependence, that same group also reported that intracellular [Na+] increased in mSlc4a11-expressing cells when they were exposed to a pHe = 8.2 solution and decreased when exposed to a pHe = 6.2 solution: an observation seemingly consistent with Na+/H+ exchange or Na+-OH− cotransport. The caveat to this result is that these maneuvers elicit dramatic pHi changes in these cells and the observed changes in the fluorescence of the SBFI Na+ indicator could be explained by the pH sensitivity of SBFI (13). The robustness of a second report of Slc4a11 Na+ permeability in mammalian cells (42) is unclear as that activity was unusually sensitive to EIPA and may represent the action of an endogenous protein (59). Finally, two groups do not find any evidence for the influence Na+ on Slc4a11 action (30, 34). In summary, taken together with our data, there is no robust evidence for the Na+ permeability of Slc4a11 or the obligate Na+-dependence of Slc4a11-mediated H+-OH− transport.
Two recent studies report an interaction of Slc4a11 with NH3 (34, 59) Both groups of researcher find, as we do (Fig. 8), that the application of NH3/NH4+ induces the appearance of currents and that the magnitude of these currents increase as pHe rises. The authors of the first study (performed in PS120-NHE-deficient Chinese hamster ovary-cells) conclude that these currents represent the coupled cotransport of NH3 and H+, (59) while the authors of the second study (performed in oocytes) find against either NH3-coupled or uncoupled H+/OH− conductance and suggest instead that these currents could represent an endogenous pH-sensitive NH4+ channel (34) Because we only observe these currents in Slc4a11-expressing cells (Fig. 2 and Fig. 3), because we observe these currents in the absence of NH3/NH4+ (Figs. 2– 7), and because we find that the NH3/NH4+-induced currents persist long after the removal of NH3/NH4+ (Fig. 9), we conclude that the currents observed in the presence of NH3/NH4+ do not involve mSlc4a11-mediated NH3/NH4+ transport or the action of an endogenous NH4+ channel. There are three mechanisms by which NH3/NH4+ application could indirectly enhance Slc4a11-mediated H+/OH− conductance:
1) Independently of the presence of heterologously expressed Slc4a11, the application of NH3/NH4+ rapidly and robustly depolarizes both mammalian cells and oocytes (1, 7). This depolarization in itself is sufficient to increase mSlc4a11-mediated H+/OH− currents by increasing the driving force for mSlc4a11-mediated acid-extrusion, as we observe in mSlc4a11-expressing oocytes that are voltage-clamped at values more positive than spontaneous Vm (e.g., Fig. 4A).
2) The rise in pHi brought about by Slc4a11-mediated acid-extrusion further increases Gm.
3) The influx of NH3 across the plasma membrane would exert an additional stimulatory effect by further alkalinizing pHi (22, 27, 55)6.
Interpretation aside, the major disparity between our study and the findings of the two NH3-related reports lies in their lack of evidence for H+/OH− conductance in the absence of NH3/NH4+. Actually, the authors of the study in PS120 cells did detect evidence for uncoupled H+/OH− conductance, but concluded that it was relatively small and therefore of less physiological significance than NH3/H+ cotransport (59). However, the assay used is not conducive to the appearance of these currents: cells were voltage-clamped at − 60 mV while the cells were exposed to a pHe = 8.5 solution. We would not expect to detect substantial currents during a brief exposure to these solutions, especially when voltage-clamped at a value that is presumably close to EH. In fact we find that the H+/OH− conductance can be similarly robust in the absence vs. the presence of NH3/NH4+ (Fig. 9). The authors of the oocyte study find that the magnitude of the change in zero-current potential in NH3/NH4+ solution as pHe rises from 7.4 to 8.4 (expected to be −58 mV, if pHi remains constant) is inconsistent with coupled NH3-H+ cotransport or uncoupled H+/OH− conduction. However, two factors should be taken into account when considering these data:
1) Vm is probably not dominated by Slc4a11 action in these cells as the elicited currents are 10 times smaller than those that we observe in the presence of NH3/NH4+ and are undetectable in the absence of NH3/NH4+ (i.e., Vm may not be the same as the reversal potential of Slc4a11).
2) In the presence of NH3/NH4+ when the currents are greatest in those experiments, Vm includes a contribution from the depolarizing influence of NH3/NH4+ (i.e., Vm may not be the same as the reversal potential of Slc4a11 even in the presence of NH3/NH4+). Furthermore, pHi is not the same when pHe = 7.4 vs. 8.4. In fact when maximally conductive, Slc4a11 is likely capable of rapidly dissipating transmembrane pH gradients in which case no change in Erev would be expected as pHe is changed.
In summary, we see no electrophysiological evidence in the work of others that cannot be explained by the action of a pHi and pHe-sensitive H+/OH− conductance.
The pathophysiological role of mSlc4a11.
An interesting and parallel line of investigation finds Slc4a11 to be permeable to both H2O (at pHe = 7.4 in the absence of NH3/NH4+) and NH3 (34, 51), like members of the aquaporin protein family (40). As many ion transporters exhibit H2O-permeability (31, 58) and as it is theoretically possible for a membrane protein to conduct both water and H+ (32), these two hypotheses are not mutually exclusive. Indeed, many H2O-carrying transport proteins such as Na+-glucose and H+-lactate cotransporters have been described to carry H2O during transport cycles at rates not dissimilar to that reported for Slc4a11 (58). In the case of H2O-carrying transporters, as opposed to H2O channels, molecular dynamic simulations indicate that the large-scale conformational changes associated with alternating access transport mechanisms permit the transient formation of water-permeable pathways (31). As we do not yet know the mechanism by which H+/OH− is carried by Slc4a11 (channel vs. transporter), it is premature to speculate how the H+/OH− and H2O conducting pathways are related. It is even possible that the two pathways are entirely discrete, e.g., one passes through each monomer and another passes through the interface between two monomers in a dimer. However, as both actions are blocked by mutations equivalent to the CHED-causing mutation R125H, it is entirely likely that both activities are related to the physiological function of Slc4a11.
A key question is how might a H2O-permeable, pH-sensitive H+/OH− conductance support corneal health? The role of the corneal endothelial cells in which Slc4a11 is expressed—and which are dysfunctional or lost in CHED—is to promote fluid re-secretion from the collagenous corneal stroma (49). The stroma has the potential to draw water from the aqueous humor due to the oncotic pressure exerted by its proteinaceous matrix: if unopposed, as in CHED, the fluid accumulation distorts the matrix causing loss of vision. The endothelial cells transport Na+, HCO3−, and lactate− (and thence water) from the stromal fluid and back into the aqueous humor to maintain a normal state of stromal hydration (Fig. 10A). Leading this action in the basolateral (stromal facing) membrane of endothelial cells are the Na+-2HCO3− cotransporter NBCe1 and the H+-coupled lactate transporter MCT1 (3). The basolateral membrane is also the location of Slc4a11. The role of H2O permeability in this membrane is obvious and has been discussed previously by its original proponents (51), so we will confine our discussion to consideration of the value of a pH-sensitive H+/OH− conductance.
Fig. 10.
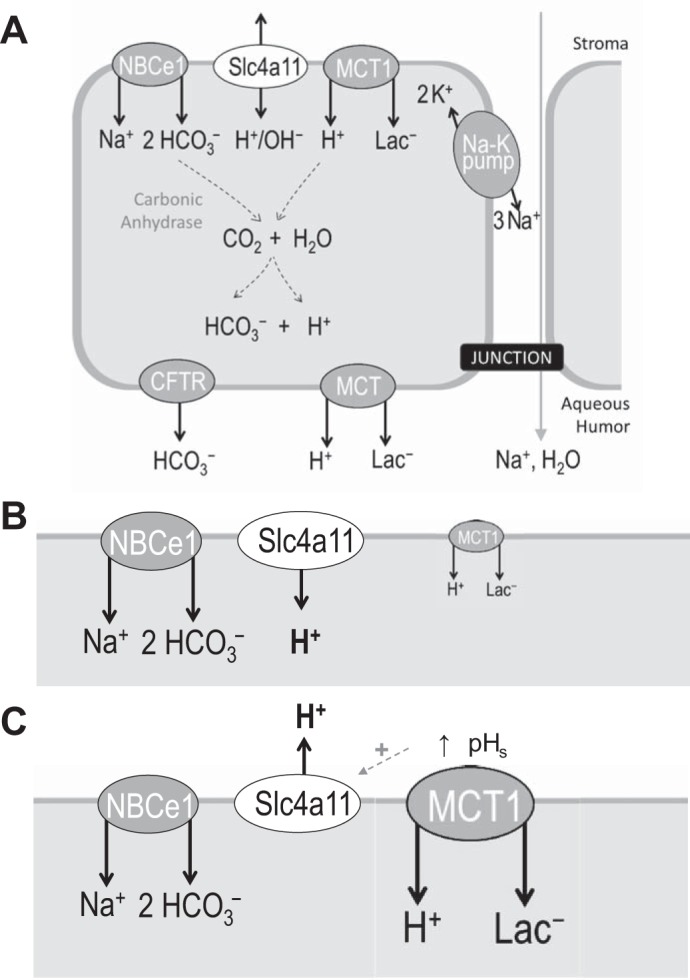
Simplified model of ion transport across the corneal endothelium. A: Slc4a11 is expressed in the same membrane as the Na+-2HCO3− cotransporter (NBCe1) and H+-Lac− cotransporter (MCT1). Although the actions of these two transporters are coupled, stromal [Lac−] can vary throughout the day. Imbalances in NBCe1/MCT1 action could disturb pHi and transendothelial fluid movement. B and C: Slc4a11 action could respond to and compensate for such imbalances to maintain pHi and endothelial function, preventing a rise in pHi when stromal lactate is low and MCT1 action is suboptimal (B) and supporting MCT1 action when stromal lactate is elevated (C).
The acid-base status of the fluid compartments on either side of the basolateral membrane is under constant challenge from the transmembrane flux of H+ and HCO3−: the pH in the immediate vicinity of a robust acid-base transport can be as much as 1 pH unit different from bulk pH (11, 36). Although the actions of NBCe1 and MCT1 are functionally coupled (2), they are presumably not always perfectly balanced as stromal [lactate] varies substantially throughout the day (21). Basolateral NHE1 and AE2 are situated to respond to and counter changes in pHi in these cells so the uniqueness of the role of Slc4a11 presumably lies with its unique molecular action, i.e., the ability to respond to changes in pHe/pHs and Vm and, being uncoupled from other ion gradients, its ability to operate either as an acid loader or an acid extruder depending on prevailing conditions. Stromal pH is 7.5 and endothelial pHi is 7.3, meaning that EH ∼ −12 mV. Unfortunately, there is a great deal of uncertainty concerning the Vm of endothelial cells; values have been reported that are more negative than − 35 mV (56), in which case Slc4a11 would act as an acid loader unless pHe was to rise above 7.9, and as depolarized as 0 mV (25), in which case Slc4a11 would act as an acid extruder. There could be numerous roles for such a protein; we suggest two scenarios in which a pH-sensitive H+/OH− conductance could be useful:
1) As an electrogenic acid-loader, Slc4a11 would provide a constant and preemptive influx of H+ that, along with MCT1 action, would minimize pH changes caused by the constant electrogenic influx of HCO3−, especially when stromal [lactate] is low (Fig. 10B). This would presumably be preferable to balancing the pH gradient across the basolateral membrane via the action of the Cl−/HCO3− exchanger AE2. pH balance via the functional coupling of AE2 to NBCe1 would result in futile cycles of HCO3−/HCO3− exchange, thereby reducing the efficiency of transendothelial bicarbonate transport. Furthermore, the resulting net uptake of NaCl could disturb endothelial cell volume and transendothelial fluid transport.
2) MCT1 action requires support from an extracellular carbonic anhydrase to maintain an adequate supply of H+ to drive H+/Lac− cotransport (41). During periods of elevated MCT1 activity, such as during sleep when eye closure promotes anaerobic metabolism and lactate production, Slc4a11 could provide additional support in response to the depletion of surface H+ (elevation in pHs) to extrude and recycle H+ (Fig. 10C). This action would promote MCT1 activity, while preemptively countering intracellular acidification.
Such actions could contribute to pHi and cell volume homeostasis, while optimizing the efficiency of endothelial fluid transport. Thus loss of Slc4a11 would be expected to have an immediate impact on endothelial function with longer-term detrimental consequences for endothelial health. This hypothesis could explain why both loss of endothelial function and subsequent loss of endothelial cells occurs in CHED.
GRANTS
This work was supported by startup funding from the Dean of the School of Medicine and Biomedical Sciences and the Department of Physiology and Biophysics at UB:SUNY (to M. D. Parker). Salary support was provided to E. J. Myers via a Carl W. Gottschalk Research Scholar Grant from the ASN Foundation for Kidney Research (to M. D. Parker). The subcloning of mSlc4a11.pGH19 and the generation of the anti-Slc4a11 antibody were supported by an R21 award (EY021646) from the National Institutes of Health (to M. L. Jennings and M. D. Parker).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.J.M., M.L.J., and M.D.P. conceived and designed the research; E.J.M., A.M., and M.D.P. performed experiments; E.J.M. and M.D.P. analyzed data; E.J.M. and M.D.P. interpreted results of experiments; E.J.M. and M.D.P. prepared figures; E.J.M. and M.D.P. drafted manuscript; E.J.M., M.L.J., and M.D.P. edited and revised manuscript; E.J.M., A.M., M.L.J., and M.D.P. approved final version of manuscript.
ACKNOWLEDGMENTS
M. D. Parker thanks Walter Boron at Case Western Reserve University for the use of laboratory facilities during the subcloning of pGH19.mSlc4a11.
Footnotes
Exactly which renal epithelia express Slc4a11 has not been fully resolved. One report places Slc4a11 in the apical membrane of human proximal tubule cells and the basolateral membrane of human collecting duct epithelia (8), while the other finds evidence for Slc4a11 expression in the thin descending limb of the loop of Henle in mice (19). Both studies used the same custom anti-Slc4a11 antibody raised by Damkier and coworkers against the Ct epitope “LPRIIEAKYLDVMDAEHRP” that is common to both mouse and human Slc4a11.
Our 2014 abstract also included evidence that yeast transformed with mSlc4a11 cDNA in the pYES vector accumulated more boron than nontransformed yeast, albeit at supraphysiological concentrations of boron. We have since come to appreciate that this is an unexpected feature of this strain of yeast transformed with even the empty pYES plasmid.
This article is the topic of an Editorial Focus by Keith Nehrke (40a).
Prior to the gradual alkalinization, the switch from pHe = 7.5 (or pHe = 6.5) solution to pHe = 8.5 solution caused a paradoxical sharp fall in pHi in both H2O-injected and mSlc4a11-expressing cells. The reasons behind this are not clear, but as this phenomenon is not specific to mSlc4a11-expressing cells we have omitted it from our analysis of Fig. 1 data.
Because these solutions are equilibrated with the atmosphere (Pco2 = 0.03 mmHg), raising pHe from 7.5 to 8.5 would also cause a 10-fold increase in [HCO3−] from ∼0.25 to 2.5 mM, although there is no evidence to suggest that Slc4a11 is permeable to HCO3−.
The influence of NH3/NH4+ application on oocyte pHi is complex, sometimes resulting in a paradoxical acidification of both pHs and pHi (39). However, the authors of the second (oocyte) study show that NH3/NH4+ application alkalinizes pHi of Slc4a11-expressing oocytes (34). As a further note, those authors interpret the unexpected alkalinization as evidence that Slc4a11 is NH3 permeable. This observation must be interpreted cautiously because NH3/NH4+ application depolarizes oocytes, and thus the alkalinization could result from increasing the driving force for acid-extrusion via mSlc4a11-mediated H+/OH− conductance.
REFERENCES
- 1.Allert N, Köller H, Siebler M. Ammonia-induced depolarization of cultured rat cortical astrocytes. Brain Res 782: 261–270, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Becker HM, Bröer S, Deitmer JW. Facilitated lactate transport by MCT1 when coexpressed with the sodium bicarbonate cotransporter (NBC) in Xenopus oocytes. Biophys J 86: 235–247, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res 95: 2–7, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. I. Na+/H+ exchange in the absence and presence of HCO3−. Invest Ophthalmol Vis Sci 33: 3058–3067, 1992. [PubMed] [Google Scholar]
- 5.Boron WF. Acid base physiology. In: Medical Physiology. A Cellular and Molecular Approach, edited by Boron WF, Boulpaep EL. Philadelphia, PA: Elsevier Saunders, 2012, p. 652–671. [Google Scholar]
- 6.Brotski C, Marshall A, Jennings ML, Patel SP, Parker MD. The mammalian bicarbonate transporter related protein BTR1 (Slc4a11) promotes boron accumulation in yeast (Abstract). FASEB J 29: 845–23., 2015. [Google Scholar]
- 7.Burckhardt BC, Frömter E. Pathways of NH3/NH4+ permeation across Xenopus laevis oocyte cell membrane. Pflügers Arch 420: 83–86, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol 293: R2136–R2146, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev 83: 475–579, 2003. [DOI] [PubMed] [Google Scholar]
- 10.DeCoursey TE. The voltage-gated proton channel: a riddle, wrapped in a mystery, inside an enigma. Biochemistry (Mosc) 54: 3250–3268, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De-la-Rosa V, Suárez-Delgado E, Rangel-Yescas GE, Islas LD. Currents through Hv1 channels deplete protons in their vicinity. J Gen Physiol 147: 127–136, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desir J, Moya G, Reish O, Van Regemorter N, Deconinck H, David KL, Meire FM, Abramowicz MJ. Borate transporter SLC4A11 mutations cause both Harboyan syndrome and non-syndromic corneal endothelial dystrophy. J Med Genet 44: 322–326, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diarra A, Sheldon C, Church J. In situ calibration and [H+] sensitivity of the fluorescent Na+ indicator SBFI. Am J Physiol Cell Physiol 280: C1623–C1633, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol Aspects Med 34: 236–251, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endeward V, Musa-Aziz R, Cooper GJ, Chen LM, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF, Gros G. Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J 20: 1974–1981, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Friedman PA, Andreoli TE. CO2-stimulated NaCl absorption in the mouse renal cortical thick ascending limb of Henle. Evidence for synchronous Na+/H+ and Cl−/HCO3− exchange in apical plasma membranes. J Gen Physiol 80: 683–711, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frommer WB, von Wirén N. Plant biology: ping-pong with boron. Nature 420: 282–283, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Giebisch G, Windhager E. Transport of acids and bases. In: Medical Physiology. A Cellular and Molecular Approach, edited by Boron WF, Boulpaep EL. Philadelphia, PA: Elsevier Saunders, 2012, p. 851–865. [Google Scholar]
- 19.Gröger N, Fröhlich H, Maier H, Olbrich A, Kostin S, Braun T, Boettger T. SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness, and polyuria. J Biol Chem 285: 14467–14474, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han SB, Ang HP, Poh R, Chaurasia SS, Peh G, Liu J, Tan DTH, Vithana EN, Mehta JS. Mice with a targeted disruption of Slc4a11 model the progressive corneal changes of congenital hereditary endothelial dystrophy. Invest Ophthalmol Vis Sci 54: 6179–6189, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Harper CL, Boulton ME, Bennett D, Marcyniuk B, Jarvis-Evans JH, Tullo AB, Ridgway AE. Diurnal variations in human corneal thickness. Br J Ophthalmol 80: 1068–1072, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey EN. Studies on the permeability of cells. J Exp Zool 10: 507–556, 1911. [Google Scholar]
- 23.Hebert SC. Hypertonic cell volume regulation in mouse thick limbs. II. Na+-H+ and Cl−-HCO3− exchange in basolateral membranes. Am J Physiol Cell Physiol 250: C920–C931, 1986. [DOI] [PubMed] [Google Scholar]
- 24.Hemadevi B, Veitia RA, Srinivasan M, Arunkumar J, Prajna NV, Lesaffre C, Sundaresan P. Identification of mutations in the SLC4A11 gene in patients with recessive congenital hereditary endothelial dystrophy. Arch Ophthalmol 126: 700–708, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Hodson S, Wigham C. A near-zero membrane potential in transporting corneal endothelial cells of rabbit. J Physiol 412: 365–374, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hull DS, Green K, Bowman K, Csukas S, Riley MV. Intracellular pH and glutathione levels in rabbit corneal endothelium following storage in moist chamber and MK medium. Invest Ophthalmol Vis Sci 24: 214–217, 1983. [PubMed] [Google Scholar]
- 27.Jacobs MH. The influence of ammonium salts on cell reaction. J Gen Physiol 5: 181–188, 1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalimarada SS, Ogando DG, Vithana EN, Bonanno JA. Ion transport function of SLC4A11 in corneal endothelium. Invest Ophthalmol Vis Sci 54: 4330–4340, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang L, Chernova MN, Alper SL. Secondary regulatory volume increase conferred on Xenopus oocytes by expression of AE2 anion exchanger. Am J Physiol Cell Physiol 272: C191–C202, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Kao L, Azimov R, Abuladze N, Newman D, Kurtz I. Human SLC4A11-C functions as a DIDS-stimulatable H+(OH−) permeation pathway: partial correction of R109H mutant transport. Am J Physiol Cell Physiol 308: C176–C188, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Shaikh SA, Enkavi G, Wen PC, Huang Z, Tajkhorshid E. Transient formation of water-conducting states in membrane transporters. Proc Natl Acad Sci USA 110: 7696–7701, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Licsandru E, Kocsis I, Shen YX, Murail S, Legrand YM, van der Lee A, Tsai D, Baaden M, Kumar M, Barboiu M. Salt-excluding artificial water channels exhibiting enhanced dipolar water and proton translocation. J Am Chem Soc 138: 5403–5409, 2016. [DOI] [PubMed] [Google Scholar]
- 33.Liskova P, Dudakova L, Tesar V, Bednarova V, Kidorova J, Jirsova K, Davidson AE, Hardcastle AJ. Detailed assessment of renal function in a proband with Harboyan syndrome caused by a novel homozygous SLC4A11 nonsense mutation. Ophthalmic Res 53: 30–35, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Loganathan SK, Schneider HP, Morgan PE, Deitmer JW, Casey JR. Functional assessment of SLC4A11, an integral membrane protein mutated in corneal dystrophies. Am J Physiol Cell Physiol 311: C735–C748, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez IA, Rosenblatt MI, Kim C, Galbraith GC, Jones SM, Kao L, Newman D, Liu W, Yeh S, Pushkin A, Abuladze N, Kurtz I. Slc4a11 gene disruption in mice: cellular targets of sensorineuronal abnormalities. J Biol Chem 284: 26882–26896, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makani S, Chesler M. Endogenous alkaline transients boost postsynaptic NMDA receptor responses in hippocampal CA1 pyramidal neurons. J Neurosci 27: 7438–7446, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol 127: 639–658, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musa-Aziz R, Boron WF, Parker MD. Using fluorometry and ion-sensitive microelectrodes to study the functional expression of heterologously-expressed ion channels and transporters in Xenopus oocytes. Methods 51: 134–145, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musa-Aziz R, Jiang L, Chen LM, Behar KL, Boron WF. Concentration-dependent effects on intracellular and surface pH of exposing Xenopus oocytes to solutions containing NH3/NH4+. J Membr Biol 228: 15–31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Transport of NH3/NH in oocytes expressing aquaporin-1. Am J Physiol Renal Physiol 281: F255–F263, 2001. [DOI] [PubMed] [Google Scholar]
- 40a.Nehrke K. H(OH), H(OH), H(OH): a holiday perspective. Focus on “Mouse Slc4a11 expressed in Xenopus oocytes is an ideally selective H+/OH− conductance pathway that is stimulated by rises in intracellular and extracellular pH.” Am J Physiol Cell Physiol (September 28, 2016). doi: 10.1152/ajpcell.00259.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen TT, Bonanno JA. Bicarbonate, NBCe1, NHE, and carbonic anhydrase activity enhance lactate-H+ transport in bovine corneal endothelium. Invest Ophthalmol Vis Sci 52: 8086–8093, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogando DG, Jalimarada SS, Zhang W, Vithana EN, Bonanno JA. SLC4A11 is an EIPA-sensitive Na+ permeable pHi regulator. Am J Physiol Cell Physiol 305: C716–C727, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park M, Li Q, Shcheynikov N, Zeng W, Muallem S. NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol Cell 16: 331–341, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93: 803–959, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker MD, Bouyer P, Daly CM, Boron WF. Cloning and characterization of novel human SLC4A8 gene products encoding Na+-driven Cl−/HCO3− exchanger variants NDCBE-A, -C, and -D. Physiol Genomics 34: 265–276, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker MD, Ourmozdi EP, Tanner MJ. Human BTR1, a new bicarbonate transporter superfamily member and human AE4 from kidney. Biochem Biophys Res Commun 282: 1103–1109, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Peña-Münzenmayer G, George AT, Shull GE, Melvin JE, Catalán MA. Ae4 (Slc4a9) is an electroneutral monovalent cation-dependent Cl−/HCO3− exchanger. J Gen Physiol 147: 423–436, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO3−) transporters. Mol Aspects Med 34: 159–182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swan JS, Hodson SA. Rabbit corneal hydration and the bicarbonate pump. J Membr Biol 201: 33–40, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Thomas RC, Meech RW. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature 299: 826–828, 1982. [DOI] [PubMed] [Google Scholar]
- 51.Vilas GL, Loganathan SK, Liu J, Riau AK, Young JD, Mehta JS, Vithana EN, Casey JR. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum Mol Genet 22: 4579–4590, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilas GL, Morgan PE, Loganathan SK, Quon A, Casey JR. A biochemical framework for SLC4A11, the plasma membrane protein defective in corneal dystrophies. Biochemistry (Mosc) 50: 2157–2169, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DTH, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VHK, Salto-Tellez M, Venkatraman A, Guo K, Hemadevi B, Srinivasan M, Prajna V, Khine M, Casey JR, Inglehearn CF, Aung T. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nat Genet 38: 755–757, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Wang DK, Liu Y, Myers EJ, Guo YM, Xie ZD, Jiang DZ, Li JM, Yang J, Liu M, Parker MD, Chen LM. Effects of Nt-truncation and coexpression of isolated Nt domains on the membrane trafficking of electroneutral Na+/HCO3− cotransporters. Sci Rep 5: 12241, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warburg O. Über die Oxydationen in lebenden Zellen nach Versuchen am Seeigelei. Hoppe Seylers Z Physiol Chem 66: 305–340, 2009. [Google Scholar]
- 56.Watsky MA, Rae JL. Resting voltage measurements of the rabbit corneal endothelium using patch-current clamp techniques. Invest Ophthalmol Vis Sci 32: 106–111, 1991. [PubMed] [Google Scholar]
- 57.Weber W. Ion currents of Xenopus laevis oocytes: state of the art. Biochim Biophys Acta 1421: 213–233, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Zeuthen T. Water-transporting proteins. J Membr Biol 234: 57–73, 2010. [DOI] [PubMed] [Google Scholar]
- 59.Zhang W, Ogando DG, Bonanno JA, Obukhov AG. Human SLC4A11 is a novel NH3/H+ co-transporter. J Biol Chem 290: 16894–16905, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]