Abstract
Purpose
It is hypothesized that robotic gastrectomy may surpass laparoscopic gastrectomy after the operators acquire long-term experience and skills in the manipulation of robotic arms. This study aimed to evaluate the long-term learning curve of robotic distal gastrectomy (RDG) for gastric cancer compared with laparoscopic distal gastrectomy (LDG).
Materials and Methods
From October 2008 to December 2015, patients who underwent LDG (n=809) were matched to patients who underwent RDG (n=232) at a 1:1 ratio, by using a propensity score matching method after stratification for the operative year. The surgical outcomes, such as trends of operative time, blood loss, and complication rate, were compared between the two groups.
Results
The RDG group showed a longer operative time (171.3 minutes vs. 147.6 minutes, P<0.001) but less estimated blood loss (77.6 ml vs. 116.6 ml, P<0.001). The complication rate and postoperative recovery did not differ between the two groups. The RDG group showed a longer operative time and similar estimated blood loss compared with the LDG group after 5 years of experience (operative time: 159.2 minutes vs. 136.0 minutes in 2015, P=0.003; estimated blood loss: 72.9 ml vs. 78.1 ml in 2015, P=0.793).
Conclusions
In terms of short-term surgical outcomes, RDG may not surpass LDG after a long-term experience with the technique.
Keywords: Stomach neoplasms, Gastrectomy, Laparoscopy, Robotics, Learning curve
Introduction
During the last decade, developments in laparoscopic instruments and technologies have led to major surgical breakthroughs, and many reports have demonstrated the clinical advantages of laparoscopic gastrectomy for gastric cancer compared with open surgery.1,2,3 Although the long-term oncological safety of this technique is yet to be established, many retrospective studies demonstrated that laparoscopic distal gastrectomy (LDG) was comparable to open distal gastrectomy in terms of short-term and long-term outcomes.4,5,6,7 Thereby, LDG is now considered a safe and technically feasible treatment option for early-stage gastric cancer.
Further refinements in the surgical environment have been attempted with the robotic surgical platform to overcome the shortcomings of conventional laparoscopic surgery. These improvements include three-dimensional imaging, enhanced dexterity with articulated robotic arms, comfortable operator position, and integrated emerging technologies such as indocyanine green fluorescence. However, a recent multicenter phase II trial failed to show the superiority of robotic gastrectomy over laparoscopic gastrectomy.8 Thus, the clinical advantage of robotic gastrectomy for gastric cancer remains unclear, and several clinical trials are under way to determine the adjunct benefits of robotic gastrectomy in specific clinical settings.
One of the ignored advantages of robotic surgery in previous studies is that the surgeon can manipulate the four robotic arms including the camera view at will. This advantage allows the surgeon to have a relatively independent ability from that of the first assistant or the scopist, compared with conventional laparoscopic surgery. Thus, it is hypothesized that robotic gastrectomy may surpass laparoscopic gastrectomy after the operators acquire long-term experience and skills in the manipulation of robotic arms; however, no studies on the long-term learning curve of robotic gastrectomy have been undertaken yet.
Therefore, the aim of this study was to evaluate the long-term learning curve of robotic distal gastrectomy (RDG) for gastric cancer compared with LDG by means of a case-matched study with propensity score analysis.
Materials and Methods
This study was approved by the Institutional Review Board of Ajou University Hospital, and adhered to the principles of the Declaration of Helsinki and its revisions. Written informed consent was obtained from all patients undergoing the surgery.
1. Patients
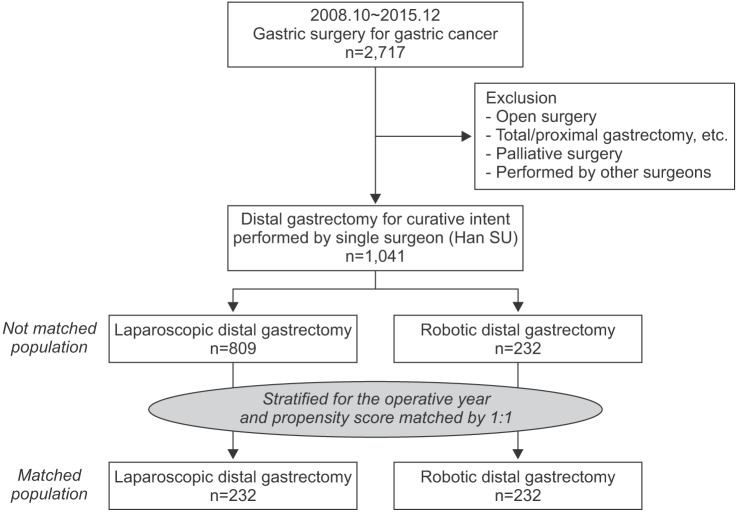
The study design is summarized in Fig. 1. From October 2008 to December 2015, a total of 2,717 patients underwent gastric surgery for gastric cancer at Ajou University Hospital. Of them, 1,676 patients were excluded because of undergoing an open approach, having treatment with a palliative intent, being treated by another operator who did not perform robotic gastrectomy, and undergoing other gastrectomies except distal gastrectomy. The remaining 1,041 patients who underwent LDG or RDG were eligible for inclusion to this study (809 laparoscopic distal gastrectomies and 232 robotic distal gastrectomies).
Fig. 1. Population flowchart.
Before the matching, we stratified the patients for operative years to reflect the time variations in the surgical environment, such as surgical devices, techniques, and operator experience. Then, patients who underwent LDG were matched to patients who underwent RDG at a 1:1 ratio, by using a propensity score matching (PSM) method. The matched variables included age, sex, body mass index, previous abdominal surgery, comorbidity, American Society of Anesthesiologists score, and preoperative T and N stages. Intraoperative variables such as combined resection and extent of lymph node dissection were also included because these variables could considerably influence the surgical outcomes, which are the primary end points of this study.
Finally, 464 patients (232 patients for each group) were analyzed for the present study. The surgical outcomes, including operative time, blood loss, and postoperative complications, were compared between the LDG and RDG groups.
2. Statistical analysis
All statistical analyses were performed by using IBM SPSS Statistics ver. 18.0 (IBM Co., Armonk, NY, USA) and the R program (R Foundation for Statistical Computing, Vienna, Austria). The χ2 test and Student's t-test were used for comparisons between the two groups. PSM was performed by using the MatchIt package in the R program (R Foundation for Statistical Computing). The propensity scores were estimated by running a logit model, and the nearest neighboring matching algorithm was applied with a caliper of 0.01. A P-value of <0.05 was considered statistically significant.
Results
1. Patient characteristics
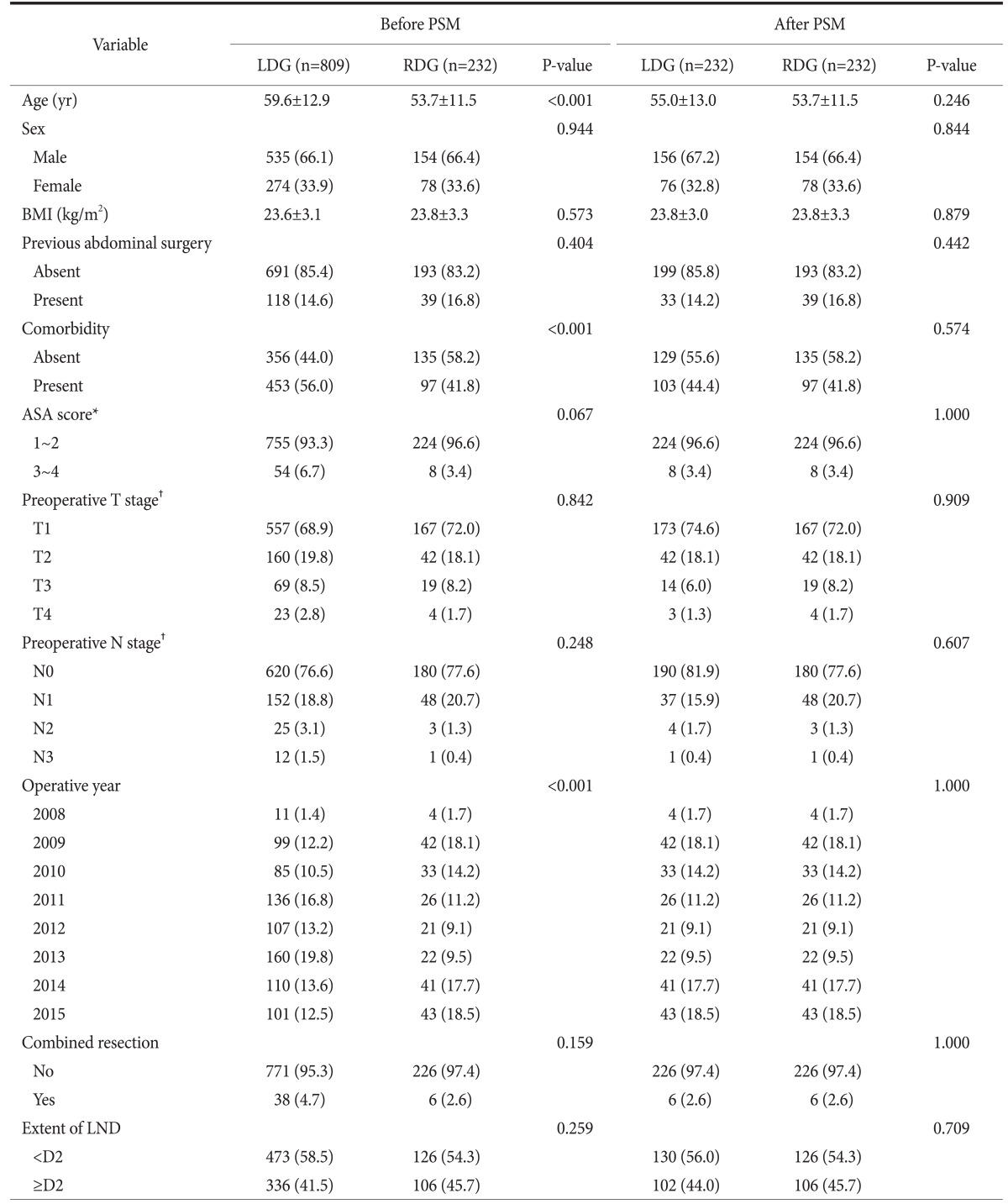
The patient characteristics before and after the PSM are listed in Table 1 (detailed PSM data at each operative year are shown in Supplement 1). Before the matching, the LDG group showed older age and a higher rate of comorbidity than the RDG group. Moreover, there was a significant difference in the distribution of operative year between the two groups. However, these disparities were resolved after the matching, and no difference in patient characteristics was found in the matched groups.
Table 1. Patient characteristics before and after PSM.
Values are presented as mean±standard deviation or number (%). PSM = propensity score matching; LDG = laparoscopic distal gastrectomy; RDG = robotic distal gastrectomy; BMI = body mass index; LND = lymph node dissection. *Classification according to the American Society of Anesthesiologist. †Classification according to the Union for International Cancer Control/American Joint Committee on Cancer 7th edition. The stage was judged by radiologists based on preoperative computed tomography images.
2. Comparison of operative outcomes
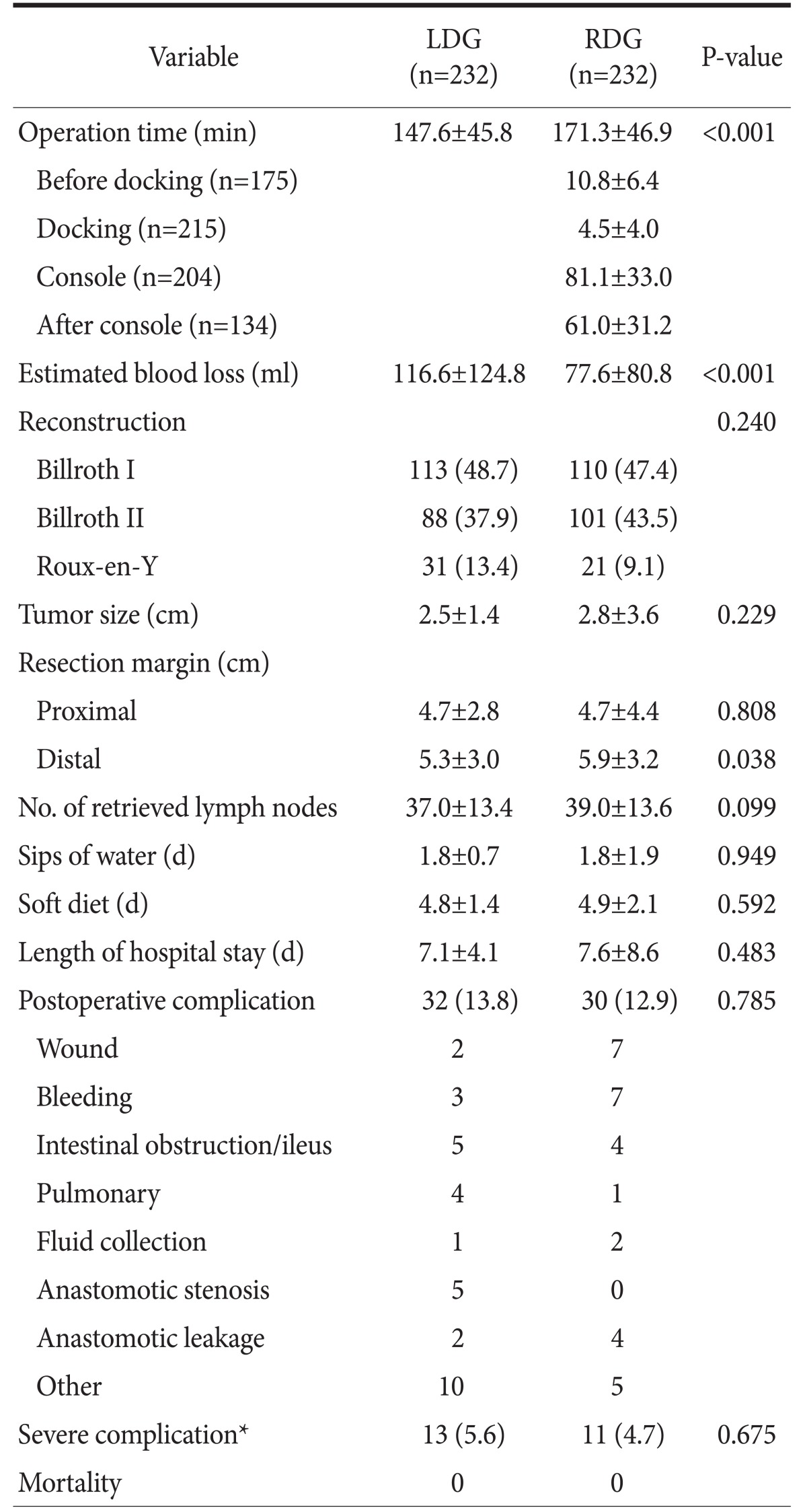
Table 2 summarizes the comparisons of surgical outcomes between the LDG group and the RDG group. The RDG group showed a longer operative time (171.3 minutes vs. 147.6 minutes, P<0.001) but less estimated blood loss (77.6 ml vs. 116.6 ml, P<0.001). The complication rate and postoperative recovery did not differ between the two groups, and there was no operative mortality in both groups.
Table 2. Comparison of surgical outcomes between laparoscopic distal gastrectomy and robotic distal gastrectomy after propensity score matching.
Values are presented as mean±standard deviation or number (%). LDG = laparoscopic distal gastrectomy; RDG = robotic distal gastrectomy. *Clavien-Dindo classification grade ≥IIIa.
3. Trends of operative time, blood loss, and procedural time
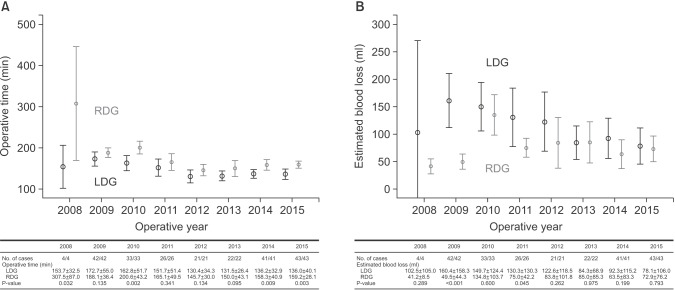
The RDG group was matched to the LDG group at each operative year. Then, the operative time and blood loss were compared between the two groups (Fig. 2). Concerning operative time, the RDG group showed a trend of longer operative times than those of the LDG group in all operative years. The differences in operative times between the two groups were distinct in the early operative years (2008~2010) and decreased gradually over time. However, the differences were significant even in the late operative years. The mean operative times of RDG and LDG were 158.3 minutes and 136.2 minutes in 2014 (P=0.009) and 159.2 minutes and 136.0 minutes in 2015 (P=0.003), respectively. Concerning estimated blood loss, the RDG group showed a trend of less blood loss than that of the LDG group in the early operative years (2008~2012); however, there were no differences in the late operative years. The mean estimated blood loss of RDG and LDG were 85.0 ml and 84.3 ml in 2013 (P=0.975), 63.5 ml and 92.3 ml in 2014 (P=0.199), and 72.9 ml and 78.1 ml in 2015 (P=0.793), respectively.
Fig. 2. Trends of operative time and blood loss between laparoscopic distal gastrectomy (LDG) and robotic distal gastrectomy (RDG). (A) Trends of operative time. (B) Trends of blood loss. Values are presented as number only or mean±standard deviation.
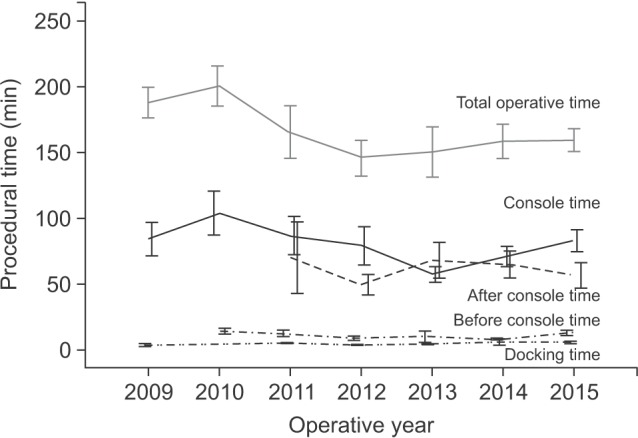
The detailed procedural times of the RDG group were analyzed (Table 2). The mean “before docking” time and “docking” time were 10.8 minutes and 4.5 minutes, respectively. These procedural times did not fluctuate according to the operative years, whereas the trend of total operative times was similar to that of the “console” times (Fig. 3).
Fig. 3. Trends of each procedural time in the robotic distal gastrectomy group.
Discussion
Despite improvements of the surgical environment in robotic surgery, the clinical advantage of robotic gastrectomy for gastric cancer remains unclear. Recently, the results of a multicenter prospective study comparing robotic gastrectomy with laparoscopic gastrectomy was published.8 A total of 434 patients (223 robotic gastrectomies and 211 laparoscopic gastrectomies) were analyzed on intention-to-treat and per-protocol bases. The operative time was longer (221 minutes vs. 178 minutes, P<0.001) and the total cost was higher in the robotic group (13,432 United States dollar [USD] vs. 8,090 USD, P<0.001), whereas the complication rate and estimated blood loss were not different between the two groups (11.9% vs. 10.3% [P=0.619] and 50 ml vs. 55 ml [P=0.318] in per-protocol analysis, respectively). Consequently, this study failed to prove the theoretical superiority of robotic surgery in gastric cancer surgery.
Many efforts have been made to determine the clinical benefits of robotic gastrectomy over laparoscopic gastrectomy during the past decade. Kim et al.9 reported the result of a retrospective study comparing 87 cases of RDG to 288 cases of LDG, with a focus on the performance of lymphadenectomy in the N2 area. The mean harvested number of N2 was significantly higher in the RDG group (16.3 vs. 13.2, P=0.001). Similarly, Son et al.10 reported a higher number of retrieved lymph nodes of the suprapancreatic area in robotic total gastrectomy (14.5 vs. 11.3, P=0.023). More recently, Suda et al.11 reported that robotic gastrectomy showed lower rates of local complications, such as pancreatic fistula, than did laparoscopic surgery (1.1% vs. 9.8%, P=0.007).
In the same context, we conducted this retrospective study to determine the clinical benefits of robotic gastrectomy for gastric cancer. In contrast to laparoscopic gastrectomy, the surgeon can manipulate all robotic arms. The steady camera view and consistent movement of the third robotic arm offers good operative fields, which seem better than those provided by human assistants. Moreover, its maneuverability could be improved through the accumulation of experience and reproduced through the operator's independent ability from that of the assistant. Thus, we hypothesized that robotic gastrectomy may surpass laparoscopic gastrectomy after the operators acquire long-term experience and skills in the manipulation of the robotic surgical platform. However, previous studies on the learning curve of robotic gastrectomy has focused on the cut-off points for surgical stability.12,13,14
To our best knowledge, this study is the first to evaluate the long-term learning curve of RDG for gastric cancer. In the present study, we failed to show that RDG can surpass LDG in terms of surgical outcomes after acquiring long-term experience. However, we first performed stratified analysis in robotic gastrectomy for the operative years, and showed how the operative time and estimated blood loss changed during the long-term experience. Notably, the estimated blood loss of the RDG group was less than that of the LDG group in the early operative years; however, this advantage of RDG remarkably decreased and there was no difference between the two groups in the late operative years. In the present study, the assumed gap of operative times between RDG and LDG was about 20 to 25 minutes. This was larger than the sum of the “before docking” and “docking” times, which implied that the other procedural times in robotic gastrectomy were also longer than those of laparoscopic gastrectomy. We postulated that this was due to several reasons. First, the surgical wounds were closed with knot-burying sutures in robotic gastrectomy but not in laparoscopic gastrectomy. Furthermore, the hemostasis and irrigation after reconstruction were more difficult in some cases of robotic gastrectomy owing to the trocar placement.
The present study has some limitations. This study was confined to a single center and the experience of a single surgeon. Furthermore, some data such as the before docking time and after console time were missed in certain patients owing to the nature of a retrospective study design. In addition, the long-term learning curve in robotic total gastrectomy was not evaluated in the present study, nevertheless the potential advantage of robotic total gastrectomy was assumed to achieve sufficient lymphadenectomy especially in the splenic hilum. Therefore, further studies are needed to confirm our preliminary results on the long-term learning curve of robotic gastrectomy for gastric cancer.
In conclusion, RDG showed a trend of longer operative times and similar estimated blood loss compared with LDG even after 5 years of experience. Moreover, the complication rates in the studied period were not different between the two groups. These results imply that RDG may not surpass LDG simply through the accumulation of long-term experience. Efforts to determine the adjunct benefits of the robotic surgical platform or surgical standardization in robotic gastrectomy would be needed in the future.
Acknowledgments
This study was supported by a grant from the National R & D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1320270). The funding source hand no role in the design of this article and will not have any role during its execution or publication.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
Electronic Supplementary Material
The online version of this article (doi: https://doi.org/10.5230/jgc.2016.16.4.240) contains supplementary materials.
Details of propensity score matching for each operative year
References
- 1.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 2.Kim YW, Yoon HM, Yun YH, Nam BH, Eom BW, Baik YH, et al. Long-term outcomes of laparoscopy-assisted distal gastrectomy for early gastric cancer: result of a randomized controlled trial (COACT 0301) Surg Endosc. 2013;27:4267–4276. doi: 10.1007/s00464-013-3037-x. [DOI] [PubMed] [Google Scholar]
- 3.Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01) Ann Surg. 2016;263:28–35. doi: 10.1097/SLA.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 4.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 5.Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014;32:627–633. doi: 10.1200/JCO.2013.48.8551. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34:1350–1357. doi: 10.1200/JCO.2015.63.7215. [DOI] [PubMed] [Google Scholar]
- 7.Kataoka K, Katai H, Mizusawa J, Katayama H, Nakamura K, Morita S, et al. Non-randomized confirmatory trial of laparoscopy-assisted total gastrectomy and proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group Study JCOG1401. J Gastric Cancer. 2016;16:93–97. doi: 10.5230/jgc.2016.16.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, et al. Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann Surg. 2016;263:103–109. doi: 10.1097/SLA.0000000000001249. [DOI] [PubMed] [Google Scholar]
- 9.Kim YW, Reim D, Park JY, Eom BW, Kook MC, Ryu KW, et al. Role of robot-assisted distal gastrectomy compared to laparoscopy-assisted distal gastrectomy in suprapancreatic nodal dissection for gastric cancer. Surg Endosc. 2016;30:1547–1552. doi: 10.1007/s00464-015-4372-x. [DOI] [PubMed] [Google Scholar]
- 10.Son T, Lee JH, Kim YM, Kim HI, Noh SH, Hyung WJ. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc. 2014;28:2606–2615. doi: 10.1007/s00464-014-3511-0. [DOI] [PubMed] [Google Scholar]
- 11.Suda K, Man-I M, Ishida Y, Kawamura Y, Satoh S, Uyama I. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc. 2015;29:673–685. doi: 10.1007/s00464-014-3718-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Shi Y, Qian F, Tang B, Hao Y, Zhao Y, et al. Cumulative summation analysis of learning curve for robot-assisted gastrectomy in gastric cancer. J Surg Oncol. 2015;111:760–767. doi: 10.1002/jso.23876. [DOI] [PubMed] [Google Scholar]
- 13.Kim HI, Park MS, Song KJ, Woo Y, Hyung WJ. Rapid and safe learning of robotic gastrectomy for gastric cancer: multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur J Surg Oncol. 2014;40:1346–1354. doi: 10.1016/j.ejso.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Park SS, Kim MC, Park MS, Hyung WJ. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc. 2012;26:60–67. doi: 10.1007/s00464-011-1828-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of propensity score matching for each operative year