Administration of exogenous H2S was unable to increase the pools of combined H2S in the heart of rats, even after H2S intoxication. Any long-lasting effects of H2S on the heart, previously reported in the literature, do not require a measurable accumulation of H2S in cardiac tissue.
Keywords: H2S intoxication, protein S-sulfuration, acid-labile sulfide
Abstract
In this study, we have tried to characterize the limits of the approach typically used to determine H2S concentrations in the heart based on the amount of H2S evaporating from heart homogenates—spontaneously, after reaction with a strong reducing agent, or in a very acidic solution. Heart homogenates were prepared from male rats in control conditions or after H2S infusion induced a transient cardiogenic shock (CS) or cardiac asystole (CA). Using a method of determination of gaseous H2S with a detection limit of 0.2 nmol, we found that the process of homogenization could lead to a total disappearance of free H2S unless performed in alkaline conditions. Yet, after restoration of neutral pH, free H2S concentration from samples processed in alkaline and nonalkaline milieus were similar and averaged ∼0.2–0.4 nmol/g in both control and CS homogenate hearts and up to 100 nmol/g in the CA group. No additional H2S was released from control, CS, or CA hearts by using the reducing agent tris(2-carboxyethyl)phosphine or a strong acidic solution (pH < 2) to “free” H2S from combined pools. Of note, the reducing agent DTT produced a significant sulfide artifact and was not used. These data suggest that 1) free H2S found in heart homogenates is not a reflection of H2S present in a “living” heart and 2) the pool of combined sulfides, released in a strong reducing or acidic milieu, does not increase in the heart in a measurable manner even after toxic exposure to sulfide.
NEW & NOTEWORTHY
Administration of exogenous H2S was unable to increase the pools of combined H2S in the heart of rats, even after H2S intoxication. Any long-lasting effects of H2S on the heart, previously reported in the literature, do not require a measurable accumulation of H2S in cardiac tissue.
exogenous hydrogen sulfide (H2S) or H2S releasing agents have been administered to various animal models and in vitro preparations in an attempt to determine whether this molecule possesses physiological and pharmacological properties beneficial to human health, such as preventing ischemic cardiac insult (27, 33, 51, 52). This novel field of research must, however, resolve a difficult conundrum (11, 39): as there is a clear overlap between the blood and tissue concentrations of H2S that have been reported to be associated with measurable physiological/pharmacological effects and those producing toxic effects (15, 24), the fundamental question remains of whether it is possible for H2S to increase in a measurable manner in tissues without producing some signs of toxicity (11). In addition, it is difficult to reconcile long-term effects that could result from the direct accumulation of H2S in tissues with the very rapid disappearance of dissolved H2S from the blood (2, 24) and tissue (58) after exogenous administration. The latter disappears in minutes because of the high rate of H2S oxidation in the blood (10, 60, 62) and its profound interaction with hemoglobin in the blood (16, 59). As a result and since only the dissolved form of exogenous H2S (at neutral pH, H2S/HS−) is able to diffuse from the blood into the tissues, we can anticipate that only a very small fraction of this dissolved/free exogenous H2S/HS− that is administered can eventually diffuse into tissues (16, 24). Furthermore, the pool of H2S/HS− diffusing into any tissue is rapidly oxidized in the mitochondria (17) or can combine with metallo-compounds (45). It comes therefore as no surprise that many studies have suggested that 1) only a trivial amount of free/dissolved H2S (in the nM range at best), if any, can be found in baseline conditions in various tissues including the heart (6, 18, 28, 57) and 2) no significant change in the pools of combined H2S should be expected to be recovered after administration of exogenous H2S. More recent studies have, however, reported values that were at odds with this view, showing concentrations of free H2S in the high micromolar range in the heart (22, 25, 42) and much higher levels of combined H2S after administration of a relatively small quantity of exogenous H2S (34, 41). The origin of this discrepancy, which is sometimes of several orders of magnitude, is not clear.
Most of the studies designed to determine the concentrations of H2S in the heart after H2S administration rely on a similar principle: the determination of gaseous H2S diffusing from heart homogenates. The premise of this approach is that free H2S in cardiac tissues, i.e., dissolved H2S, is present in the form of gaseous H2S and HS− in a pH-dependent manner. Since the acidic dissociation constant (pKa) of H2S is ∼7 (1, 32), gaseous H2S, which is about a third of the total amount of dissolved H2S at physiological pH, evaporates in keeping with the difference in partial pressure of H2S between the milieu where it is supposed to be present and the gaseous phase. HS− converts to gaseous H2S as the latter evaporates, until all soluble H2S/HS− disappears from the milieu. For the pools of sulfide that are unable to evaporate spontaneously (20), different methods have been used to free combined HS− from proteins and metalloproteins. For instance, sulfane sulfur, bound sulfur (42, 55, 54), or sulfurated/sulfhydrated proteins (31, 40, 56)—terms coined to describe a pool of H2S combined with the cysteine residues of proteins—have been suggested to be produced after sulfide administration. Strong reducing agents, such as dithiothreitol (DTT) or tris(2-carboxyethyl)phosphine (TCEP), by cleaving disulfide bonds have been shown to be able to release this pool of sulfide (8, 30, 44, 61), which then could be measured as gaseous H2S. It should be pointed out that the view that H2S/HS− can per se create in vivo sulfhydrated proteins has been challenged on various grounds (55); rather, it has been suggested that it is the oxidized form of H2S, S0, that can achieve such sulfhydration (55).
Another pool of combined sulfide has been described as “acid labile,” from which gaseous H2S can only be released at low pH, typically below 4 (18). This “acid-labile” pool comprises various metallo-sulfide compounds, which reflect the propensity of free H2S to combine with the multitude of metalloproteins present in and outside cells. H2S released at extremely low pH should not be confused with the effect of decreasing pH on the transformation of HS−, already present in dissolved form, into gaseous H2S. In addition to these pools, polysulfides can be formed after exposure to exogenous H2S and have been suggested to contribute to some of the effects of H2S (21, 32). More importantly, most free H2S is rapidly oxidized and eventually eliminated as sulfate (7) and thiosulfate (7, 17, 29) and cannot be recovered by evaporation. We have estimated that the rate of disappearance of free H2S from the blood is so rapid that soluble H2S concentration in the blood can fall to zero within minutes after sulfide intoxication (16).
The purpose of the present study was to determine the fate of exogenous H2S in the heart in control conditions and after infusion of levels of H2S producing an acute cardiac failure during which H2S must interact with cardiac proteins (19) and metalloproteins (16), using a methodology based on the principle described above, i.e., H2S evaporation. We sought to characterize the limits of this approach. We have focused our interest on the processes involved in preparing tissue homogenates, which require an extreme alteration of dying cardiac tissues, which are cut, sliced, crushed, and then homogenized, conditions during which a significant amount of free sulfide could evaporate. Our general objective was to determine whether the changes in pools of H2S in the heart can be used as a tool to evaluate the benefits of H2S at low levels (26, 52) as well as its toxicity at higher levels of exposure (2, 12, 16). Our specific aims were to describe 1) the effects of the process of homogenization on H2S evaporation; 2) the use of alkaline solution while processing the cardiac tissue to limit this evaporation; 3) the effects of DTT, the reducing agent typically used to free H2S from cysteine residues; and 4) the change in various pools of H2S in the heart in rats exposed to H2S-induced cardiac toxicity. Finally, we sought to integrate and discuss these data in light of models accounting for the interaction of H2S with proteins and metallo-compounds in vitro and in vivo.
METHODS
Determination of H2S
Description of apparatus and equipment.
Samples [10 or 20 ml of sodium hydrosulfide (NaSH) solutions or heart homogenate] were placed in a 50-ml leakproof chamber sealed with an airtight plastic stopper and equipped with two catheters and inlet-outlet ports for gas flow. Samples were introduced into the chamber with these catheters; unless otherwise mentioned, the pH of the solution or the homogenate was maintained between 6 and 7, even when the samples were processed at higher pH (see below). The “fluid phase” was constantly agitated with a magnetic stirrer bar, while the headspace was ventilated by a continuous flow of N2 to limit the oxidation of H2S. This flow was set at 500 ml/min to overcome the rate of sampling of the gas analyzer while maintaining the highest possible H2S partial pressure gradient between the fluid and gas phases. In few instances, a 500-ml Erlenmeyer flask was used for some validation experiments as described below. The actual flow rate of N2 was determined with a pneumotachograph connected to a pressure transducer (Pneumotach amplifier 1, series 1100, Hans Rudolph, Shawnee, KS). The concentration of H2S flowing out of the headspace was directly measured by a H2S gas analyzer [Interscan RM series, Interscan, range 0–1 parts per million (ppm), resolution 0.001 ppm]. The analog output of the H2S analyzer and the flow rate signal were recorded with an A/D data acquisition system (PowerLab 16/35, ADInstruments) and were analyzed on LabChart7 (ADInstruments). The concentration of H2S (ppm) in the gas phase multiplied by the gas flow rate through the chamber headspace (l/s) allowed the computation of the rate of H2S diffusing from the solution. The total amount of H2S evaporating at any given time t could then be computed by integrating the rate of diffusion of H2S during this time t. Results are expressed as concentration of H2S per gram of tissue or per liter of solution as well as total quantity of H2S produced.
Validation of the measurement.
The linearity and limits of detection of our experimental setup were tested with known concentrations of H2S in 10 ml or 20 ml of a buffer solution (final concentration of 0.05, 0.1, 2, 20, 100, and 200 μM H2S) added in the chamber as described in Description of apparatus and equipment. Stock solution of H2S was freshly prepared from NaSH (Sigma) in PBS.
To determine potential artifacts produced on the H2S signal related to the nature and pH of the solution, the same analysis was repeated by injecting 2–5 ml of solution containing HCl (final 0.3 M), glycine-NaOH buffer (25 mM), TCEP (5 mM), or DTT (50 mM) into the chamber containing 10 or 20 ml of buffer solution (for TCEP or DTT) without any H2S present. The total quantity of H2S is presented as “equivalent H2S.” All compounds were tested in triplicate.
Effects of pH of solution on H2S and effects of homogenization.
With a pKa around 7, soluble H2S is present in solution in the forms of HS− and gaseous H2S, with about one-third of gaseous H2S at physiological pH (1, 32). At pH of 9, most H2S is under the form HS−, minimizing the loss of H2S in tissue, but with little or no S0 (1, 32). Since homogenization was done at a pH of 9 in fresh tissue, we have determined the maximal rate of disappearance of H2S from a solution containing 100 nmol of H2S that was constantly agitated at a pH of 6, 7, or 9. In addition, to clarify the effect of homogenization per se on the loss of H2S during tissue processing, solutions of H2S in PBS (5 ml) or 50 mM glycine-NaOH buffer (pH 9.3) were exposed to the same protocol as heart homogenates, with a tapered glass tissue homogenizer (Wheaton) for 15 min, and then were introduced into the chamber to measure H2S remaining after this process. A solution of 100 mM sodium phosphate buffer was used to restore neutral pH. Again, all our measurements were done at neutral pH, even when the processing of fresh tissue was performed in alkaline solution.
In Vivo Animal Experiments and Tissue Sampling
Animal preparation.
Fifteen male Sprague-Dawley rats (Charles River) weighing 499 ± 95 g (∼12–16 wk) were used. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (8th ed., National Research Council Institute for Laboratory Animal Research). The study was approved by the Penn State Hershey Medical Center Institutional Animal Care and Use Committee.
Rats were anesthetized by inhalation of 3–5% isoflurane in O2 for few minutes before intraperitoneal injection of urethane (700 mg/kg) + α-chloralose (60 mg/kg). The rats were tracheostomized and were breathing spontaneously. Expiratory flow was measured with a pneumotachograph (series 1100, Hans Rudolph), and mixed expired O2, CO2, and H2S fractions were determined by specific analyzers as previously described (14, 24). A catheter (PE-50) was inserted into the right femoral artery to measure arterial blood pressure (ABP). A similar catheter was advanced to the left ventricle through the right carotid artery to monitor ventricular pressure via pressure transducers (46). An additional catheter was inserted into the right femoral vein for continuous H2S or saline infusion. Electrocardiogram was recorded by a bioelectric amplifier (Differential AC Amplifier, model 1700, A-M Systems). All signals were digitized with PowerLab and were visualized online. Data were stored for further analysis with LabChart7.
Six animals were used as control, and nine animals were exposed to H2S by continuous infusion of NaSH solution. H2S infusion was started at a rate of 0.4 mg/min (5 μmol/min, 0.8 mg/ml NaSH in saline), and then the rate of infusion was increased by 0.08 mg/min (1 μmol/min) every 2 min. In three rats, the infusion was stopped when mean ABP (MABP) reached 50 mmHg with a decrease in left ventricular contractility (dP/dtmax) (cardiogenic shock, CS). These three rats were euthanized precisely 5 min after the end of sulfide infusion. The animals were transcardially perfused with 60 ml of cold PBS to wash out the blood, and the heart was removed from the rat within a minute and weighed. The heart was immediately immersed in a total volume of 5 ml of 50 mM glycine-NaOH buffer (pH 9.3). In the six remaining animals, the infusion was continued until the animal presented a complete cardiac asystole (CA), typically by pulseless electrical activity (PEA). Animals presenting apnea during the infusion were mechanically ventilated. These six rats were perfused with PBS according to the same protocol as soon the PEA occurred. Three of these six hearts were immediately immersed in glycine-NaOH buffer, while the three other hearts were snap-frozen by liquid nitrogen and stored in a −80°C freezer. In the six control animals, three hearts were immediately used for H2S measurement, while the other three hearts were snap-frozen.
H2S measurement in the heart.
The fresh hearts were homogenized at a pH of 9 with a tapered tissue grinder and transferred to the 50-ml chamber. The pH of the tissue homogenate was restored to ∼7 as soon as it was introduced into the chamber with sodium phosphate buffer. As described in Effects of pH of solution on H2S and effects of homogenization, the total amount of free H2S spontaneously evaporating was determined at neutral pH in all fresh samples. The total time between the death of the animal and the recording was <30 min.
After 1 h of spontaneous H2S evaporation, TCEP was added to the chamber at a final concentration of 5 mM (pH was adjusted to neutral by NaOH) while H2S signal was continuously recorded. An additional injection of 50 mM TCEP was performed 10 min later, and the total TCEP-released signal was measured over 30 min. The pool of acid-labile H2S was then identified by injecting HCl (final concentration of 0.3 M) into the chamber. A small aliquot (∼50 μl) of the sample was withdrawn from the chamber through the catheter each time H2S signal returned to baseline for determination of the pH of the solution. With this approach, the pH of the solution reached values in each instance <1.5. Each test was performed in triplicate.
Frozen cardiac tissues were homogenized with a mortar. Ground tissue was mixed with 10 ml of saline and kept in an airtight syringe. The homogenate was then injected into the 50-ml chamber to measure free H2S.
The “equivalent H2S” level produced by glycine-NaOH buffer injection or the injections of TCEP or HCl was subtracted from the corresponding H2S signal. The quantity of H2S in the heart homogenate was expressed as nanomoles per gram of tissue wet weight. To compare our data to the levels of free or bound H2S in the heart reported in the literature, we assumed that the weight of proteins was ∼10% of the total wet weight of the heart in rats (28, 48).
H2S in Vitro Interaction with Proteins and Metalloproteins.
We also tested the limit of detection for H2S bound to protein (albumin) or metalloprotein [hydroxocobalamin or methemoglobin (MetHb)]. The flask was prefilled with N2 for 20 min, and both air inlet and outlet ports were clamped to allow 10 min of incubation. H2S (final concentration of 200 μM) was injected into the 500-ml chamber containing a solution of 10% albumin (1.52 mM; bovine albumin fraction V, DOT Scientific), hydroxocobalamin (1 mM; Sigma), or 10% MetHb (1.55 mM; human-derived Hb, Sigma). Free H2S was determined for 30 min; then TCEP was injected, followed by an injection of HCl (0.3 M). The same protocol was repeated in albumin with 60 min of incubation instead of 10 min. Each condition was studied in triplicate.
Statistical Analysis
Linear regression and Bland-Altman plots were used to compare the values of H2S in our solutions and expected concentrations obtained by weighing NaSH. The time constant of the change in the concentrations of H2S in solution was determined at different pH values as the time required for the concentrations to decrease by 63%. The concentrations of H2S evaporating from the solutions were compared between PBS and 10% albumin by unpaired t-test. The effects of incubation time (10 min or 1 h) in PBS or albumin solution were also analyzed by unpaired t-test. H2S recoveries in solution containing albumin, MetHb, hydroxocobalamin, or PBS alone were also compared with one-way ANOVA followed by Bonferroni's post hoc comparisons. P < 0.05 was regarded as significant for any of these comparisons.
All statistical analyses were conducted with GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
RESULTS
Validation of H2S Detection Method
Detection limit and linearity in H2S determination.
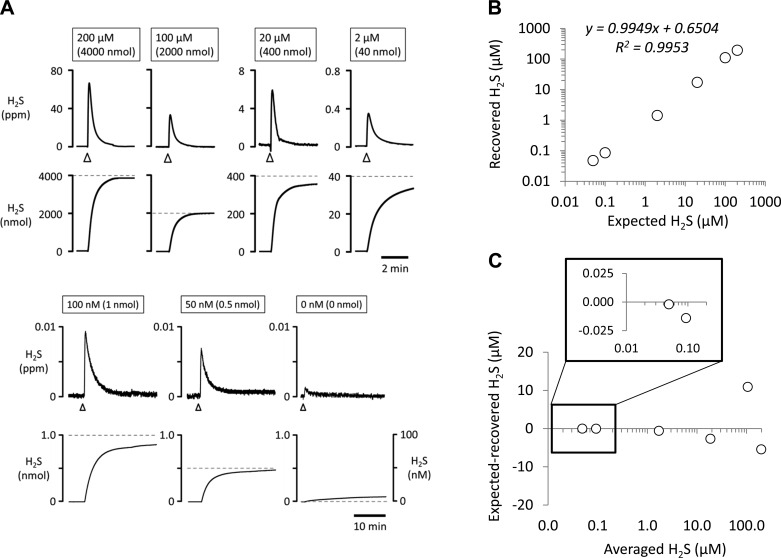
The linearity and detection limits were established by injecting known quantities of H2S into the chamber (Fig. 1). The expected concentrations of H2S were determined by precise weighing of NaSH (see methods) corresponding to a final concentration of 200, 100, 20, and 2 μM (in 20 ml) and 0.1 and 0.05 μM (in 10 ml) of H2S. The amount of H2S injected ranged therefore from 0.5 nmol to 4 μmol. The total quantities of free H2S evaporating/recovered over a 30-min period are given in Fig. 1 for each concentration (in triplicate) as a function of the expected values. Dropping the pH of the solution to 1.2 after 30 min of recording (not shown in Fig. 1), transforming any remaining HS− to gaseous H2S, allowed the recovery of an additional 5% across all H2S levels. Of note, the recovery of total H2S for the lowest levels of H2S that we used, i.e., 0.5 and 1 nmol (corresponding to 50 and 100 nM) were 0.48 and 0.86 nmol, respectively, as shown in Fig. 1.
Fig. 1.
A: H2S signal expressed in parts per million (ppm; top) and the corresponding quantity in nanomoles (bottom) in response to injection in the chamber of solutions containing different concentrations of H2S (200, 100, 20, and 2 μM in 20 ml and 100 and 50 nM in 10 ml of buffer solution). Arrowheads indicate the moment of injection. B: correlation between the concentrations of H2S solution injected (expected values obtained by weighing NaSH) into the chamber and H2S recovered from evaporation (measured values). Each data point is the average of results obtained in triplicate. C: Bland-Altman plot of the data shown in B. The approach used to determine H2S concentrations allows a recovery of most of the H2S present in solution. Inset: magnification of the plot for the lowest concentrations (0.05 and 0.1 μM H2S).
Effects of saline, HCl, glycine-NaOH buffer, and TCEP solutions on H2S signal.
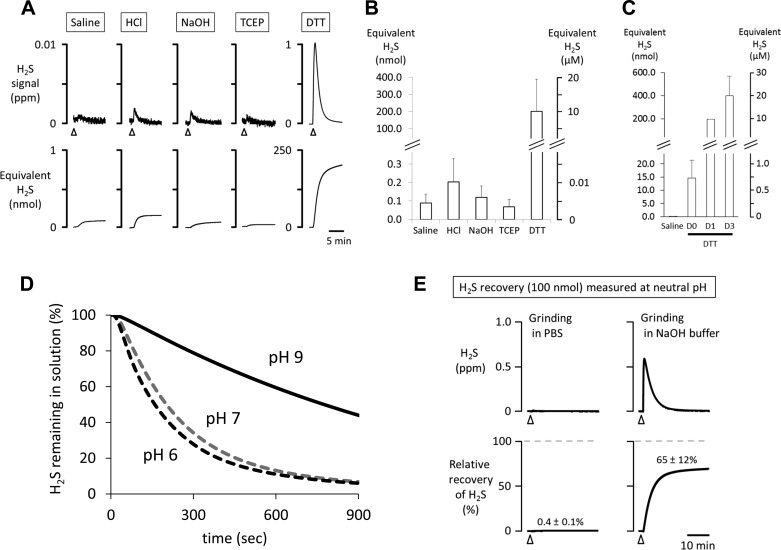
As illustrated in Fig. 2A, injection of 2 ml of saline into 10 or 20 ml of PBS produced a small artifact, corresponding to an equivalent level of H2S of 0.089 ± 0.047 nmol (or 4.5 ± 2.4 nM in the 20-ml chamber). Injection of HCl (final 0.3 M) into the chamber also produced a very small and transient artifact, which averaged 0.204 ± 0.126 nmol (10.2 ± 6.3 nM), while the injection of 25 mM glycine-NaOH buffer (buffer used in our fresh homogenate) produced an artifact of 0.123 ± 0.061 nmol (6.2 ± 3.1 nM). These artifacts from HCl and glycine-NaOH buffer were similar to saline. Finally, in contrast to DTT, 5 mM TCEP injection produced 0.068 ± 0.041 nmol (3.4 ± 2.1 nM in the chamber), which was similar to the error obtained after injection of saline. TCEP at 50 mM produced the same artifact as the solution containing 5 mM. Data presented below are reported after subtraction of this artifact.
Fig. 2.
A: H2S signal after injection in the chamber of 2–5 ml of solutions containing saline, HCl (0.3 M final concentration), glycine-NaOH buffer (25 mM), TCEP (5 mM), and DTT (50 mM). H2S signal is expressed in ppm (top) and the corresponding quantity in nanomoles (bottom). Arrowheads indicate the moment of injection. B: averaged signals (triplicate measurement) expressed in equivalent H2S produced by saline, HCl, glycine-NaOH, TCEP, and DTT. Note that the injection of saline, HCl, glycine-NaOH buffer, and TCEP produced an artifact ranging from 0.07 to 0.20 nmol of equivalent H2S (in 10 or 20 ml, see methods). In contrast, significant levels of H2S appear to be produced by DTT. C: averaged signals produced by saline and DTT which was prepared at the time of the experiment (D0) or 1 day (D1) or 3 days (D3) before the experiment. Freshly prepared DTT solution (D0) released an amount of H2S that was 150 times higher than the artifact produced by saline. H2S recovered from the DTT solution was even much larger when 1- to 3-day-old DTT was used. D: H2S remaining in solution at neutral and alkaline pH while constantly agitated at relevant pH values. At pH of 6 and 7 very similar kinetics of H2S were found (with time constant of 2.7 and 4.1 min, respectively), and H2S remaining in tissues reached the same level at 15 min. Using a solution pH of 9 dramatically slowed down the rate of evaporation, with a longer time constant (21.8 min). E: H2S measured at neutral pH from 100 nmol of H2S-mixed solution after 15 min of “processing” in glass homogenizer. Almost all the H2S virtually disappeared from the solution prepared with PBS after 15 min of grinding. In contrast, the loss of H2S was prevented by using an alkaline solution, allowing the recovery of 65% of H2S present in solution.
Amount of H2S produced by DTT.
As developed in methods, DTT has been used in many studies as the primary reducing agent to release H2S from cysteine residues (18, 42, 61). DTT produced very significant H2S artifact: injecting 50 mM DTT (1 mmol) into the chamber produced an error corresponding to 201.88 ± 187.13 nmol of H2S, or a concentration of 10.1 ± 9.4 μM (Fig. 2, A and B). Fresh DTT immediately prepared before the measurement produced an average of 15 nmol H2S, leading to error in concentration in our setting of 0.75 μM and 10 times more if the solution was >1 day old (Fig. 2C).
Effect of pH on rate of H2S evaporation.
The maximal rate of H2S evaporation obtained during vigorous and constant agitation was similar in solutions mixed with 100 nmol H2S measured at pH of 6 and 7, with a time constant of disappearance from the solution of 2.7 and 4.1 min, respectively (Fig. 2D). At a pH of 9, the maximal rate of H2S evaporation was dramatically slowed down, with a much more longer time constant (21.8 min).
Mechanical effects of tissue grinder on the loss of dissolved H2S.
The mechanical effects of grinding on the recovery of H2S from a known concentration of H2S in PBS and in alkaline solution (100 nmol, 10 μM in 10 ml) are illustrated in Fig. 2E: all dissolved H2S has virtually vanished after 15 min of grinding when performed at neutral pH. In contrast, the loss of H2S was limited by grinding at pH of 9 (with glycine-NaOH buffer), allowing recovery of 65 ± 12% of the expected H2S amount. Of note, all measurements were done at neutral pH.
Summary of characterization.
Our method of determination of H2S was able to accurately determine concentrations of free H2S in solutions across a range of 50 nM to 200 μM. The limit of detection that we considered in this study was 0.2 nmol. The addition of DTT released a large amount of H2S into the measurement chamber, in contrast to TCEP. Finally, the use of alkaline solution at pH 9 during the process of homogenization prevented in part, but not totally, the loss of H2S from the sample solution.
Determination of H2S Concentrations in Heart After Sulfide Intoxication-Induced Cardiac Failure in Rats
Circulatory effects of H2S.
Nine animals received an intravenous infusion of H2S at a rate of ∼0.8 mg/min (10 μmol/min), which we found to produce a rapid depression in cardiac function (19, 46, 47). In six of these nine rats the infusion was continued until the animal presented CA, while in three rats the infusion was stopped as soon as MABP reached 50 mmHg (Fig. 3). As presented in methods, six rats received saline infusion and were used as the control group.
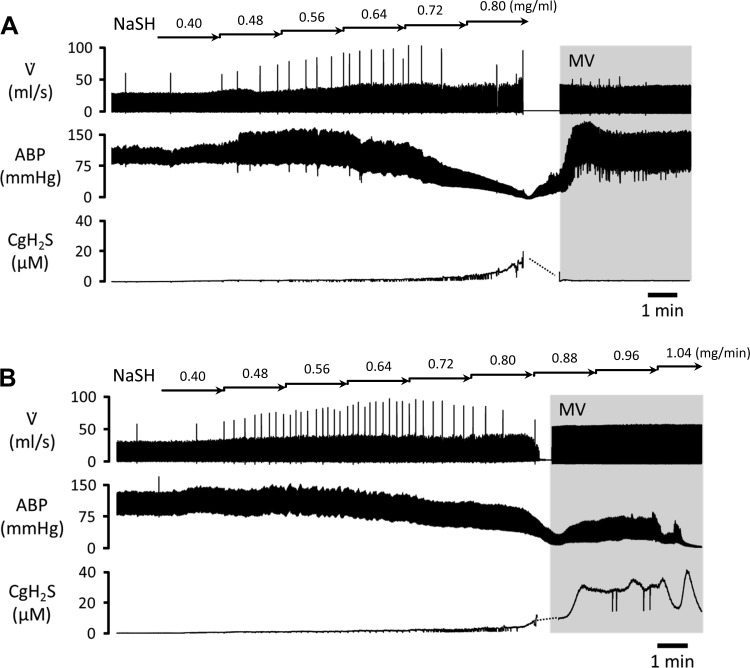
Fig. 3.
Examples of the effects of continuous infusion of H2S, starting at 0.4 mg/min (5 μmol/min) NaSH in saline and then increasing by 0.08 mg/min (1 μmol/min) every 2 min, on respiratory flow (V̇), arterial blood pressure (ABP), and concentration of gaseous H2S in the blood (CgH2S) estimated from expired H2S. H2S infusion induced decrease in ABP and increase in CgH2S. A: in the cardiogenic shock (CS) protocol, infusion was interrupted when mean ABP dropped below 50 mmHg. In this example, the rat was mechanically ventilated (MV, gray area) because it presented apnea. ABP recovered rapidly. Expired H2S disappeared within a few minutes after the cessation of the infusion. Animals were euthanized 5 min after the cessation of sulfide infusion. B: cardiac asystole (CA) conditions. H2S infusion was maintained, increasing the rate of infusion by 0.08 mg/min (1 μmol/min) every 2 min, until CA. CgH2S reached a peak of 40 μM before sharply decreasing, likely related to a decrease in pulmonary blood flow.
The effects of H2S infusion were identical to those we previously reported (46, 47). Baseline MABP was 95 ± 18 mmHg with oxygen consumption (V̇o2) of 11 ± 2 ml/min. MABP decreased within <5 min, reaching 50 mmHg at 12 ± 4 min into the infusion, while estimated arterial concentrations of gaseous H2S (CgH2S) increased to 5.8 ± 1.6 μM. Of note, V̇o2 was still unchanged at this time (12 ± 4 ml/min). Cardiac contractility decreased during this period, as reflected by a drop in dP/dtmax from 4,394 ± 1,011 to 3,134 ± 636 mmHg/s. In the three rats in which infusion was stopped at this level, MABP and dP/dtmax continued to decrease, reaching their nadir (35 ± 13 mmHg and 1,779 ± 1,270 mmHg/s, respectively) within 2–3 min. Two of three animals presented apnea requiring mechanical ventilation. When the animals were euthanized (5 min after the cessation of H2S exposure), all the circulatory parameters had already recovered (Fig. 3), while V̇o2 remained unchanged (12 ± 6 ml/min). MABP and dP/dtmax were back to baseline (94 ± 18 mmHg and 4,593 ± 1,180 mmHg/s, respectively). Expired H2S was undetectable at the time of euthanasia. The total amount of H2S that was infused averaged 94 ± 26 μmol (7 ± 2 mg) in these three rats.
In the six remaining rats, H2S infusion was maintained with all the animals under mechanical ventilation. MABP and dP/dtmax continued to decrease until a PEA or ventricular fibrillation occurred. Cardiac arrest was observed 18 ± 4 min into the infusion. CgH2S reached a peak value of 37 ± 10 μM. The total amount of H2S injected under this condition was 178 ± 58 μmol (13 ± 4 mg).
H2S concentrations in heart.
control animals.
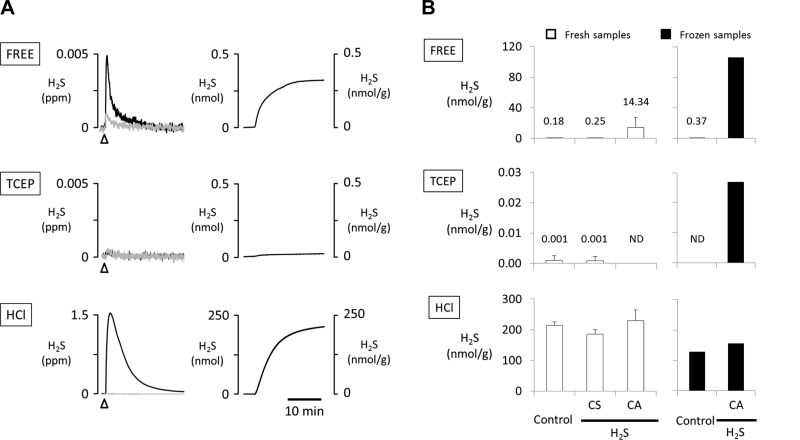
Figure 4A shows original traces of H2S evaporating from control heart homogenates. Free H2S was measured in three control animals from freshly harvested hearts (1.1–1.8 g) after homogenization in glycine-NaOH buffer and from frozen hearts in the other three rats. The amount of H2S that we could recover from fresh control hearts (processed in alkaline solution) averaged 0.50 ± 0.46 nmol (0.15–1.02 nmol), corresponding to a concentration of free H2S of 0.18 ± 0.24 nmol/g tissue (Fig. 4B). The total amount of H2S released after TCEP (5 mM) was within our limits of detection, corresponding to 0.001 ± 0.002 nmol/g. Higher concentrations of TCEP (50 mM) had no additional effects. Gaseous H2S abruptly increased after addition of 0.3 M HCl (286.5 ± 68.5 nmol). The concentration of H2S produced by an acidic environment (pH < 1.5) was 214.5 ± 11.6 nmol/g.
Fig. 4.
A: example of the changes in H2S concentrations in ppm (left) and in the quantity of H2S expressed in nanomoles and nanomoles per gram of tissue (right), released from control heart homogenates. Top: H2S levels were obtained without any intervention (spontaneous release of free H2S). Gray line presents signal produced by the vehicle solution. Middle: TCEP (5 mM) injection did not produce any measurable change in H2S. Bottom: effects of 0.3 M HCl injection on release of H2S. Note that reducing the pH abruptly to very low levels produced the release of H2S corresponding to concentrations of H2S of ∼200 nmol/g. B: average + SD concentrations of H2S released from hearts that were freshly homogenized or snap-frozen and homogenized. The levels of free H2S (spontaneously released) were similar in hearts harvested from the animals with H2S-induced cardiogenic shock (CS) and the control group. Higher levels of H2S were found in the hearts obtained from the animals with cardiac asystole (CA). In contrast, H2S levels measured after the injection of 5 mM TCEP and 0.3 M HCl were similar in all groups. Note that H2S was not detected (ND) in the fresh CA hearts and the frozen control hearts after TCEP in control conditions. The release of H2S and the effect of TCEP and HCl injection were similar in the frozen samples and the fresh samples in control conditions.
Results were of the same order of magnitude with the three pooled frozen hearts (total 2.9 g) while the hearts were processed at neutral pH. Free H2S averaged 0.37 nmol/g, while H2S released after TCEP and HCl injection were 0 and 128.3 nmol/g, respectively.
intoxicated animals.
The level of free H2S in the three hearts (0.9–1.6 g) harvested after H2S-induced acute CS and processed at pH 9 averaged 0.25 ± 0.10 nmol/g and was not different from the levels found in the control hearts. In contrast, in the three hearts (1.1–1.6 g) obtained from the animals that died from H2S-induced complete CA the level of free H2S was ∼10 times higher, reaching 14.34 ± 12.48 nmol/g. Much higher levels were even found in the pooled homogenate from the hearts (2.9 g) obtained from the three remaining animals that died from H2S exposure (106 nmol/g, ∼600 times both the control level and the level found during sublethal intoxication).
However, 5 mM TCEP released no H2S (0.001 ± 0.001 nmol/g, under detection limit) from the hearts that presented acute cardiac failure or CA. HCl (0.3 M) injection induced a large increase in H2S from samples of both acute cardiac failure and CA, averaging 186.1 ± 13.2 and 229.9 ± 34.6 nmol/g, respectively, values that were similar to the levels found in the frozen hearts (Fig. 4B).
Interaction Between H2S with Albumin and Metallo-compounds
Ten percent albumin solution.
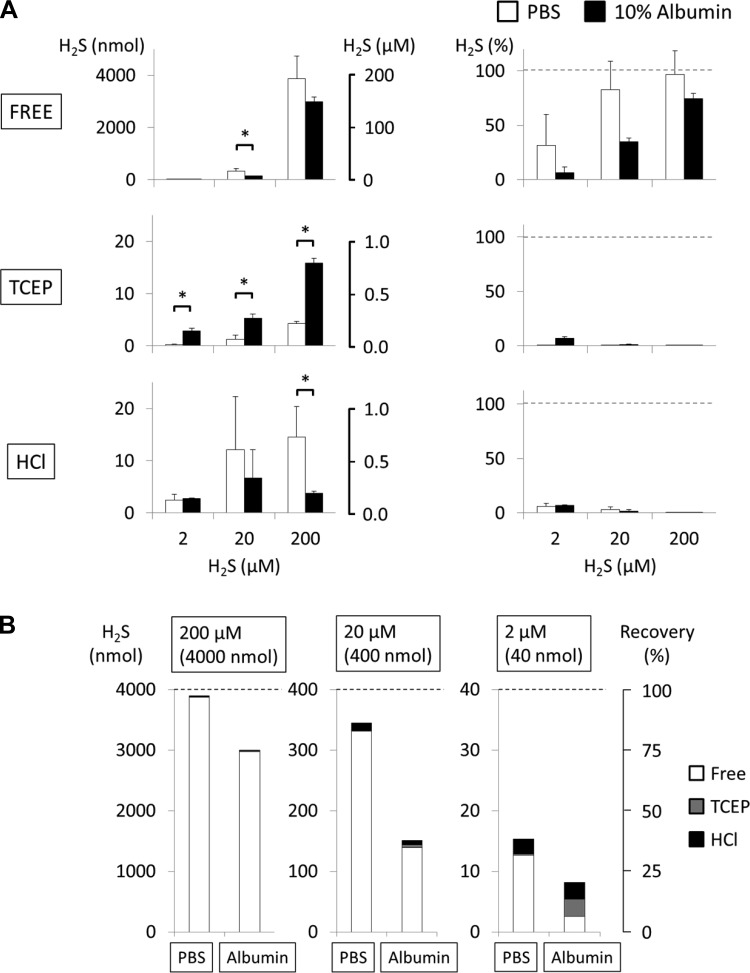
A 10% albumin solution (1.52 mM) was incubated for 10 min with 2, 20, and 200 μM H2S and for 1 h with 200 μM H2S only (Fig. 5 and Fig. 6). After 10 min of incubation the recovery of gaseous H2S was only 6 ± 5%, 35 ± 4%, and 74 ± 5% of the initial amount of H2S for 2, 20, and 200 μM, respectively. Addition of 5 mM TCEP and 0.3 M HCl did not produce any relevant recovery of H2S; as a result, 80 ± 5%, 62 ± 3%, and 25 ± 5% of the initial H2S concentration was not recovered after incubation with 2, 20, and 200 μM H2S. After 1 h of incubation, the recovery of a solution of 200 μM H2S was only 6 ± 2% (Fig. 6). H2S evaporating after TCEP and HCl represented not more than 2% and 1% of the total initial amount of H2S, respectively.
Fig. 5.
H2S recovery from PBS and 10% albumin solution after 10 min of incubation with various concentrations of H2S (2, 20, 200 μM). A, left: H2S recovered is expressed in absolute values. Right: H2S recovered is expressed in % of initial concentrations. The level of H2S recovered in albumin solution was decreased compared with PBS, and although administration of 5 mM TCEP allow recovery of some H2S from the protein solution as a function of the initial H2S concentration, the largest part of H2S was not recovered by TCEP. *P < 0.05, significant difference between PBS and 10% albumin. B: various pools of H2S recovered from PBS or albumin solution at various H2S concentrations. This representation illustrates that the major part of H2S “disappearing” from H2S mixed with albumin was not recovered after TCEP or HCl.
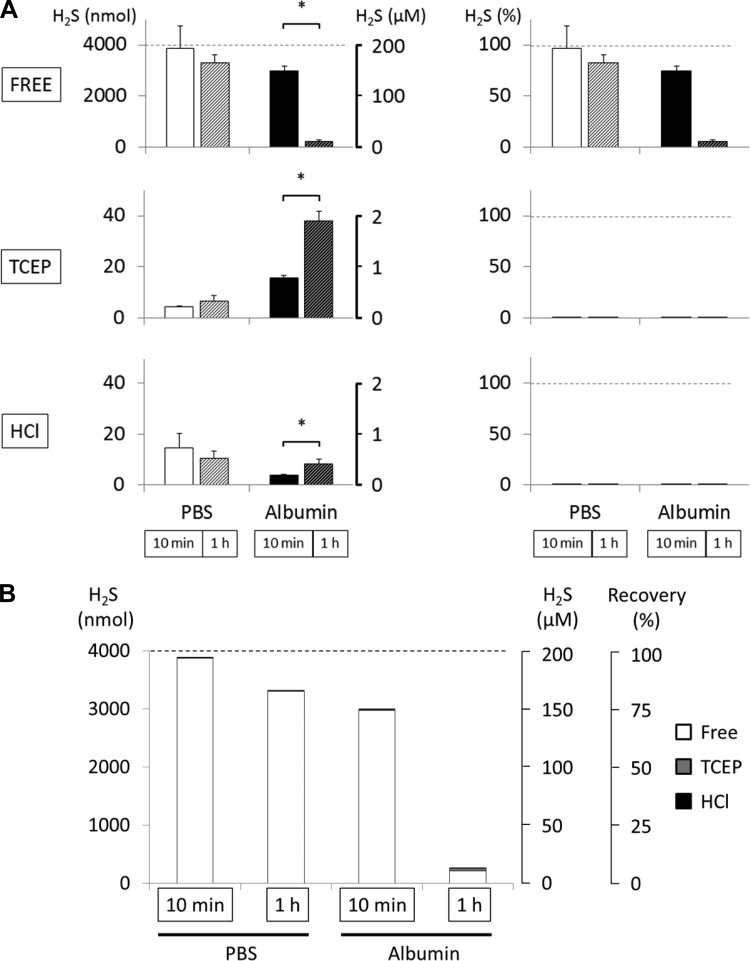
Fig. 6.
H2S recovery in PBS and 10% albumin solution after 1 h of incubation with 200 μM H2S (data obtained at 10 min are given for comparison and are the same as in Fig. 5). Data presented similarly to Fig. 5. A: in PBS, recovery of free H2S was 97% and 83% of original concentration after 10 min and 1 h of incubation, respectively. TCEP (5 mM) and HCl (0.3 M) injection did not produce any significant levels of H2S. In albumin, 94% of initial concentration of free H2S was not recovered after 1 h of incubation. H2S produced by TCEP or HCl corresponded to a tiny proportion of H2S that was not recovered. *P < 0.05, significant difference between 10-min and 1-h incubation. B: various pools of H2S recovered from PBS or albumin solution after 10-min and 1-h incubation. This representation illustrates that the major part of H2S “disappearing” from H2S mixed with albumin was not recovered after TCEP or HCl.
MetHb.
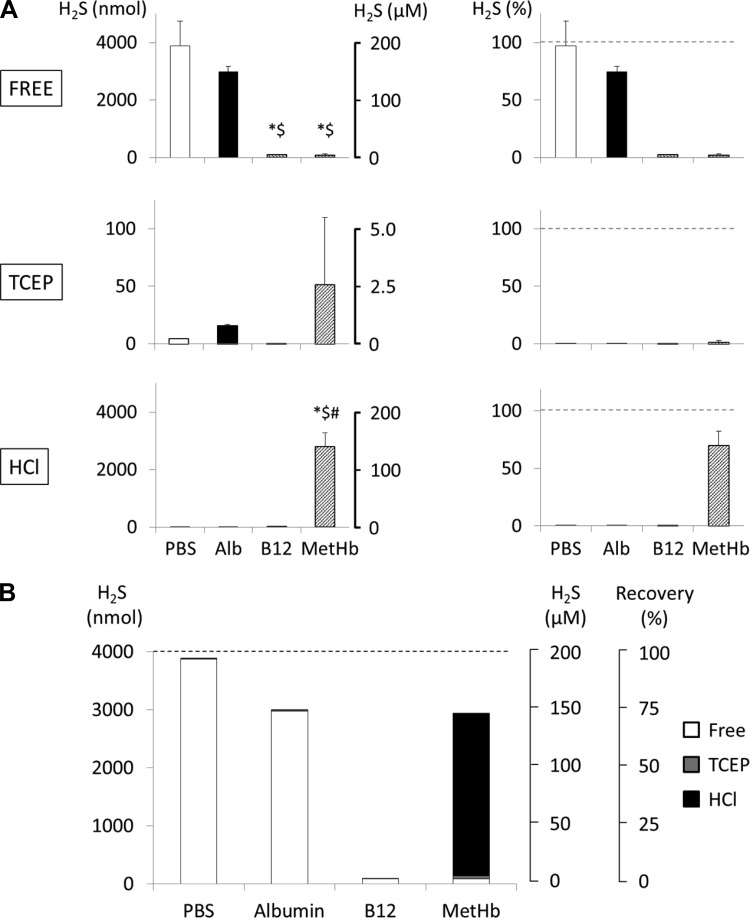
MetHb solution (1.55 mM) was incubated with 200 μM (4,000 nmol) H2S for 10 min. As illustrated in Fig. 7, only 2 ± 1% of the initial quantity of H2S was spontaneously recovered. Injection of 5 mM TCEP had virtually no effect and allowed the recovery of no more than 1 ± 2% of initial H2S. However, in major contrast to the solution of albumin and as shown in Fig. 7, 70 ± 12% of H2S incubated with MetHb solution was immediately recovered after addition of 0.3 M HCl.
Fig. 7.
H2S recovery from a solution of hydroxocobalamin (B12; 1 mM) and methemoglobin (MetHb; 1.55 mM) incubated for 10 min. Data presented similarly to Fig. 6. A: 98% of the initial amount of H2S was not recovered from hydroxocobalamin solution, and neither 5 mM TCEP nor 0.3 M HCl released any additional H2S. Similarly, most of the free H2S (98% of initial concentration) was not recovered in MetHb solution. However, ∼70% of H2S was recovered after addition of HCl. *Significantly different from PBS (P < 0.05); $significantly different from albumin (Alb) (P < 0.05); #significantly different from hydroxocobalamin (P < 0.05). B: various pools of H2S recovered from the solution of hydroxocobalamin and MetHb after 10-min incubation. This panel shows that the major part of H2S “disappearing” from H2S mixed with the cobalt compound was not recovered after TCEP or HCl, in contrast to the reaction with the ferric iron of MetHb.
Hydroxocobalamin.
As shown in Fig. 7, incubating a 1 mM solution of hydroxocobalamin with 200 μM H2S solution for 10 min led to the spontaneous recovery of only 2 ± 1% of initial H2S concentration, akin to the effects of MetHb, and no additional H2S (0.003 ± 0.003%) was recovered after addition of 5 mM TCEP. However, the addition of 0.3 M HCl did not produce any relevant evaporation of H2S (0.12 ± 0.07%), leaving 98% of the initial H2S unrecovered.
DISCUSSION
To determine the concentrations of the various pools of H2S present in the heart after sulfide intoxication, our approach, which has been used previously by different groups (18, 28, 42), relies on the assumption that molecules of dissolved H2S present in an homogenate of tissues, in this instance cardiac tissue, are capable of diffusing outside the tissue and then can be measured as gaseous H2S. Various pools of H2S have been found to be present in tissues based on this method (6, 18, 28, 42, 44, 57): 1) a pool of dissolved H2S (gaseous H2S and HS−) spontaneously diffusing from the tissue homogenate and 2) a pool of combined H2S that must be first transformed into a dissolved form before being measured. Two different interventions are typically applied: A first approach uses a strong reducing agent (typically DTT or TCEP) with the aim of recovering H2S combined on cysteine residues of proteins. This approach was first used in brain tissues by Warenycia et al. (61). A second procedure consists of acutely decreasing pH to extremely low levels (<2) and, by doing so, releasing H2S from a pool of sulfide, which comprises sulfide-metals (18). It should be pointed out that this methodology is being used on “dead tissue” homogenate, sometimes prepared in alkaline conditions to avoid initial evaporation of H2S (6).
What we found is that numerous limitations exist with this approach, affecting our interpretations of the presence of dissolved and combined H2S and the effects of infusion of exogenous sulfide, as discussed in the following paragraphs.
Baseline Levels of H2S in Heart: Is Free Sulfide Present in the Heart in Control Conditions?
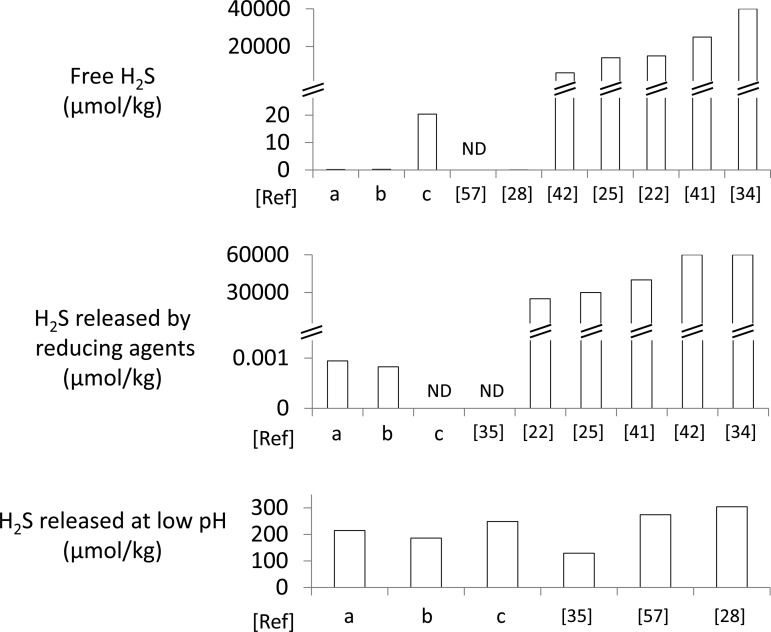
As shown in Fig. 4, the level of H2S spontaneously released from the heart in baseline conditions was within the same order of magnitude as in the studies of Levitt et al. (28) and Ubuka et al. (57), in which levels of baseline H2S were found to be extremely small (0.055 nmol/g tissue) or close to the detection limit. However, as mentioned in the introduction and as illustrated in Fig. 8, some studies have reported baseline levels ranging from 10,000 to 40,000 nmol/g (22, 25, 34, 41, 42). In most of these studies, the concentration of gaseous H2S was typically measured by gas chromatography with a single sampling from the headspace in a closed vial. The debate on the actual amount of “free” sulfide present in tissues is not a new one (9, 38). In 2008, Furne et al. (6) and Whitfield et al. (62) independently published two papers aimed at challenging the view that gaseous/dissolved H2S endogenously produced is present in the blood and in tissues at concentrations previously suggested to range from a few to hundreds of micromolar (39). A similar argument has been made by Olson (37), who also confirmed, by direct measurement of the level of gaseous H2S “diffusing” from tissue homogenates or from the blood, that the concentration of free H2S was, if anything, lower than a few hundred nanomolar. This contention is also supported by the observation that concentrations in the low micromolar range of gaseous/dissolved H2S in solution are enough to impede mitochondrial function (3, 64). Furthermore, from a toxicological standpoint, H2S poisoning provokes life-threatening symptoms at concentrations of gaseous H2S in the blood around 5 μM (16, 24, 47).
Fig. 8.
Concentrations of H2S in rodent hearts from the literature. Concentrations are expressed in micromoles per kilogram. Whenever data were expressed per gram of protein, we assumed a 10% ratio between the weight of the heart and the weight of proteins (28, 48). Free H2S, H2S produced in reducing environment, and H2S released in very acidic condition were identified according to the methods used in each paper. H2S concentrations from references a, b, and c are the concentrations measured in the present study in control, CS, and CA conditions, respectively. ND, undetectable. This figure shows that 2 different ranges of concentrations of free H2S can be found in the literature (see discussion): a first group of studies including ours identified levels of free H2S below 0.2 μmol/kg, while a second group of studies reported levels between 6,000 and 40,000 μmol/kg. Similarly very high concentrations of H2S have been found after addition of a reducing agent, which in most cases was DTT (in contrast to TCEP in our study), corresponding to levels of H2S even higher than the entire amount of H2S that was administered (see also Table 1). The acid-labile H2S concentrations are all within a similar range (high micromolar) in all studies.
In our hands, the process of preparing the homogenate tissue, if performed at neutral pH, got rid of all free H2S present by evaporation, as expected from previous works from Olson's group (4). As shown in Fig. 2, using NaOH during the heart processing clearly slowed down this evaporation process, in keeping with the expected effects of an alkaline pH on H2S-to-HS− ratio (32). However, loss by evaporation is still present even at pH 9 during the grinding process.
Finding free H2S in postmortem conditions may therefore say little about the presence of free H2S in a living heart, as it is the result of the balance between H2S released and unable to be oxidized by dying tissues and H2S evaporating, trapped, and/or oxidized before, during, and after the homogenization procedure. The alkaline condition that was used to limit evaporation could have also contributed to the postmortem H2S/HS− production from tissue homogenate containing cysteine molecules (36, 62). Of note, we found similar levels of H2S in our frozen tissues that were not prepared in glycine-NaOH buffer (Fig. 4), in which all dissolved H2S should have been expected to evaporate.
No additional H2S was recovered after incubation with TCEP in baseline conditions. This strong reducing agent was preferred over DTT, which produced a large H2S signal “artifact” (Fig. 2). A similar effect was observed previously (39) and has already been suggested to take place in other tissues (53), making disputable any inference about the tissular concentrations of H2S found in the micromolar range after DTT.
Decreasing pH below 2 with ∼0.3 M HCl led to the recovery of a relatively large amount of gaseous H2S, corresponding to a tissue concentrations of sulfide around the 200 μM (or rather μmol/kg) range. These levels of H2S after treatment with a strong acid are consistent with the results of Levitt et al. (28), Ubuka et al. (57), and Ogasawara et al. (35) (see Fig. 8). This pool of H2S, by analogy with the effects of HCl on our solution of MetHb, may have originated from H2S already present/combined on myoglobin or may have originated from a large pool of thiols or proteins denatured at very low pH.
Effects of H2S Administration
Only after lethal intoxication (CA conditions) was the level of free H2S elevated (∼100 times higher than in control and CS conditions). Although this still represents a very low level (low μM), these concentrations would be consistent with the levels we found in the blood to be associated with high toxicity for the heart (15, 16, 47). Keeping in mind the limitations of this approach, these data suggest that the ability of the heart and most tissues to oxidize H2S disappears as soon as the mitochondrial function is abolished (as in CA conditions), allowing the accumulation of exogenous H2S. Nevertheless, the total amount of infused H2S leading to an acute CS in a 500-g animal averaged 94,000 nmol delivered within 10 min; 178,000 nmol was required to produce a terminal CA. Free H2S found in the heart from our intoxicated animals was not more than 0.04 nmol and 20.18 nmol in CS and CA conditions, respectively. In other words, at best 0.00004% and 0.011% of the total amount of H2S that was infused was found in the heart in CS and CA conditions, respectively, despite the massive H2S exposure.
Table 1 illustrates the different levels of H2S found in the heart after H2S administration in keeping with the dose administered in various studies. In contrast to studies reporting the quantity of H2S recovered in combined forms that were sometimes higher than the amount administered, our main finding was that the amount of combined H2S in the hearts harvested a few minutes after recovery from a sulfide exposure that led to a profound depression in cardiac function (CS conditions) was not different from that in control animals. The same amount of H2S was recovered after TCEP or after HCl (Fig. 4 and Fig. 8) in all conditions, despite the fact that H2S must have interacted with the heart, at least with L-type calcium channels, during H2S-induced cardiac failure (19, 50, 65), leading to a profound depression in cardiac contractility. Clearly, data on the cobalt compounds (Fig. 7) suggest that the interactions between metallo-compounds and H2S cannot be reduced to a simple trapping effect, as H2S oxidation in air may have taken place during the few minutes the heart was harvested, a phenomenon that could have been catalyzed by the presence of some metallo-compounds. The lack of effects of TCEP also requires some clarification. Following original publication of Warenycia et al. (61), it has been accepted that in a reducing environment H2S combined with the free cysteine residues of proteins can be released. Although the existence of this mechanism in vivo has been challenged, strong reducing agents appear to be able to release free H2S from proteins (18, 42, 44). We found that this pool does not represent a significant sink for H2S as was previously hypothesized (63), at least within a range of concentrations of H2S producing a cardiac arrest. The fate of H2S in a simple solution of albumin, which may possess one free cysteine for creation of a disulfide bond with H2S per molecule of albumin (49), does support the view that most exogenous H2S reacting in tissues can be recovered (Fig. 5 and Fig. 6). This implies that the major part, if not all H2S, could have been oxidized. Indeed, while H2S mixed with PBS for 1 h had a recovery rate that averaged ∼80% (Fig. 6), free H2S released from an albumin solution barely reached a few percent. In our study, >90% of H2S was still unrecovered from the albumin solution even after TCEP, indicating that the presence of albumin may have catalyzed H2S oxidation into sulfide compounds that do not reversibly release H2S under reducing or acidic conditions.
Table 1.
Quantity of H2S in the heart after H2S administration
| Pool of H2S | Reference | Species | Protocol of Administration | Amount of Exogenous H2S Administered, nmol | Change in Amount of H2S in Heart, nmol | H2S Concentration in Heart, % dose administered |
|---|---|---|---|---|---|---|
| Free | b | Rats | IV H2S-induced CS | 94,000 | +0.04 | +0.00004 |
| c | Rats | IV H2S-induced CA | 178,000 | +20.18 | +0.011 | |
| 34 | Mice | Intra-LV and IV 7 days | 224 | +1,000 | +446 | |
| 41 | Diabetic mice | IV 1 day | 64 | +50 | +78 | |
| IV 7 days | 448 | +50 | +11 | |||
| Bound (reducing agent) | b | Rat (TCEP) | IV H2S-induced CS | 94,000 | 0 | 0 |
| c | Rat (TCEP) | IV H2S-induced CA | 178,000 | 0 | 0 | |
| 34 | Mice (DTT) | Intra-LV and IV 7 days | 224 | +8,000 | +3,571 | |
| 41 | Diabetic mice (DTT) | IV 1 day | 64 | +1,000 | +1,563 | |
| IV 7 days | 448 | +3,500 | +781 |
Values are amount of H2S injected (mice or rats) and change in the amount of H2S found in the heart from baseline conditions expressed in nanomoles or in % of the amount administered from the literature. References b and c summarize our data obtained during H2S-induced acute cardiogenic shock (CS) and cardiac asystole (CA), respectively. Note that in many studies the additional amount of H2S recovered from the heart after H2S administration was much higher than the amount of H2S that was administered. IV, intravenous; LV, left ventricle.
Finally, as mentioned in the introduction, it should be kept in mind that the amount of H2S required to interact with the heart to acutely reduce its contractility is actually extremely small.
Perspectives
This study does not support the view that exogenous H2S increases the pools of free or combined H2S in the heart in a significant manner, even at toxic levels. This conclusion does not preclude the formation of such pools, which if affecting key proteins may exert important physiological as well as toxic effects, but we interpret our finding as evidence that this pool represents, if anything, a trivial quantity within the limit of detection of methods using H2S evaporation. Obviously, considering that only a very small fraction of exogenously administered H2S would be “available” to the heart, it would require a considerable amount of H2S (higher than in lethal intoxication) to increase the pool of H2S in the heart anyway. At these levels, the brain, the most sensitive organ to H2S toxicity, would long have been intoxicated. The previously reported prevention of ischemia-reperfusion injury by H2S (41, 43) could well be mediated by mechanisms that are not directly related to the accumulation of H2S per se in the heart but via specific interaction with key proteins or key mechanisms of transduction. It is also possible that the acute effects of H2S on mitochondrial respiration (3), via decrease in ATP (23) along with reactive oxygen species production (5), could be effective by triggering for instance a preconditioning effect in the heart rather than by acting through a persistent accumulation of a measurable pool of sulfide.
In terms of H2S poisoning, H2S provokes a very rapid depression in cardiac contractility. The therapeutic agents trapping H2S, cobalt compounds or MetHb (15), are most effective when H2S is present in a free form. We have shown that the rapidly diffusible nature of H2S creates a small window for antidotes to be administered with some effectiveness (2, 12, 16). Our present results indicate that free H2S can be found in the heart but only after lethal exposure conditions in which the animals presented a PEA (12, 46) but not in the animals surviving the intoxication. These observations, along with the still unresolved issue of whether free H2S found in the heart is actually present in vivo, challenge the use of trapping agents alone in the treatment of sulfide intoxication-induced acute CS (13, 47) if administered minutes after H2S exposure.
Conclusions
Methods using evaporation of free H2S from cardiac homogenates do not allow a meaningful determination of dissolved H2S in the heart. In addition, H2S released after application of the strong reducing agent TCEP was hardly detectable in postmortem conditions. Acid-labile H2S was found in the high micromolar range, but its origin remains unclear and this pool of H2S was not affected after H2S administration even at high or toxic levels. These data can be understood in light of in vitro interactions between H2S and proteins or metallo-compounds and the very low levels of H2S able to diffuse into tissues during and after H2S administration even in toxic ranges. These results suggest that the major part, if not all, of exogenous H2S administered in vivo vanishes in forms that cannot be recovered.
GRANTS
This work was supported by the National Institutes of Health Office of the Director and the National Institute of Neurological Disorders and Stroke (Grants 1R21 NS-090017-02 and U01 NS-097162).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.S. and P.H. performed experiments; T.S. and P.H. analyzed data; T.S. and P.H. interpreted results of experiments; T.S. and P.H. prepared figures; T.S. and P.H. drafted manuscript; T.S. and P.H. edited and revised manuscript; T.S. and P.H. approved final version of manuscript; P.H. conceived and designed research.
ACKNOWLEDGMENTS
The authors thank Dr. Matthew Rannals for his comments and suggestions and Nicole Tubbs for her skillful technical assistance.
REFERENCES
- 1.Carroll JJ, Mather AE. The solubility of hydrogen sulphide in water from 0 to 90°C and pressures to 1 Mpa. Geochim Cosmochim Acta 53: 1163–1170, 1989. [Google Scholar]
- 2.Chenuel B, Sonobe T, Haouzi P. Effects of infusion of human methemoglobin solution following hydrogen sulfide poisoning. Clin Toxicol (Phila) 53: 93–101, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr 40: 533–539, 2008. [DOI] [PubMed] [Google Scholar]
- 4.DeLeon ER, Stoy GF, Olson KR. Passive loss of hydrogen sulfide in biological experiments. Anal Biochem 421: 203–207, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Eghbal MA, Pennefather PS, O'Brien PJ. H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. Toxicology 203: 69–76, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 295: R1479–R1485, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem Pharmacol 62: 255–259, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Getz EB, Xiao M, Chakrabarty T, Cooke R, Selvin PR. A comparison between the sulfhydryl reductants tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry. Anal Biochem 273: 73–80, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Guidotti TL. Occupational exposure to hydrogen sulfide in the sour gas industry: some unresolved issues. Int Arch Occup Environ Health 66: 153–160, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Haggard HW. The fate of sulfides in the blood. J Biol Chem 49: 519–529, 1921. [Google Scholar]
- 11.Haouzi P. Is exogenous hydrogen sulfide a relevant tool to address physiological questions on hydrogen sulfide? Respir Physiol Neurobiol 229: 5–10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haouzi P, Chenuel B, Sonobe T. High-dose hydroxocobalamin administered after H2S exposure counteracts sulfide-poisoning-induced cardiac depression in sheep. Clin Toxicol (Phila) 53: 28–36, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haouzi P, Sonobe T. Cardiogenic shock induced reduction in cellular O2 delivery as a hallmark of acute H2S intoxication. Clin Toxicol (Phila) 53: 416–417, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haouzi P, Sonobe T, Chenuel B. Oxygen-related chemoreceptor drive to breathe during H2S infusion. Respir Physiol Neurobiol 201: 24–30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haouzi P, Sonobe T, Judenherc-Haouzi A. Developing effective countermeasures against acute hydrogen sulfide intoxication: challenges and limitations. Ann NY Acad Sci 1374: 29–40, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haouzi P, Sonobe T, Torsell-Tubbs N, Prokopczyk B, Chenuel B, Klingerman CM. In vivo interactions between cobalt or ferric compounds and the pools of sulphide in the blood during and after H2S poisoning. Toxicol Sci 141: 493–504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275: 3352–3361, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 11: 205–214, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Judenherc-Haouzi A, Zhang XQ, Sonobe T, Song J, Rannals MD, Wang J, Tubbs N, Cheung JY, Haouzi P. Methylene blue counteracts H2S toxicity-induced cardiac depression by restoring L-type Ca channel activity. Am J Physiol Regul Integr Comp Physiol 310: R1030–R1044, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabil O, Motl N, Banerjee R. H2S and its role in redox signaling. Biochim Biophys Acta 1844: 1355–1366, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura Y, Mikami Y, Osumi K, Tsugane M, Oka J, Kimura H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J 27: 2451–2457, 2013. [DOI] [PubMed] [Google Scholar]
- 22.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA 111: 3182–3187, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiss L, Deitch EA, Szabo C. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci 83: 589–594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klingerman CM, Trushin N, Prokopczyk B, Haouzi P. H2S concentrations in the arterial blood during H2S administration in relation to its toxicity and effects on breathing. Am J Physiol Regul Integr Comp Physiol 305: R630–R638, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G Sr, Gojon G Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 127: 1116–1127, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavu M, Bhushan S, Lefer DJ. Hydrogen sulfide-mediated cardioprotection: mechanisms and therapeutic potential. Clin Sci (Lond) 120: 219–229, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Lefer DJ. A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc Natl Acad Sci USA 104: 17907–17908, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levitt MD, Abdel-Rehim MS, Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal 15: 373–378, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Libiad M, Yadav PK, Vitvitsky V, Martinov M, Banerjee R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J Biol Chem 289: 30901–30910, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu P, O'Mara BW, Warrack BM, Wu W, Huang Y, Zhang Y, Zhao R, Lin M, Ackerman MS, Hocknell PK, Chen G, Tao L, Rieble S, Wang J, Wang-Iverson DB, Tymiak AA, Grace MJ, Russell RJ. A tris(2-carboxyethyl) phosphine (TCEP) related cleavage on cysteine-containing proteins. J Am Soc Mass Spectrom 21: 837–844, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy P, Palinkas Z, Nagy A, Budai B, Toth I, Vasas A. Chemical aspects of hydrogen sulfide measurements in physiological samples. Biochim Biophys Acta 1840: 876–891, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson CK, Calvert JW. Hydrogen sulfide and ischemia-reperfusion injury. Pharmacol Res 62: 289–297, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson CK, Lambert JP, Molkentin JD, Sadoshima J, Calvert JW. Thioredoxin 1 is essential for sodium sulfide-mediated cardioprotection in the setting of heart failure. Arterioscler Thromb Vasc Biol 33: 744–751, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogasawara Y, Isoda S, Tanabe S. Tissue and subcellular distribution of bound and acid-labile sulfur, and the enzymic capacity for sulfide production in the rat. Biol Pharm Bull 17: 1535–1542, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta 1787: 856–863, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal 17: 32–44, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson KR. The therapeutic potential of hydrogen sulfide: separating hype from hope. Am J Physiol Regul Integr Comp Physiol 301: R297–R312, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Olson KR, DeLeon ER, Liu F. Controversies and conundrums in hydrogen sulfide biology. Nitric Oxide 41: 11–26, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Peake BF, Nicholson CK, Lambert JP, Hood RL, Amin H, Amin S, Calvert JW. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am J Physiol Heart Circ Physiol 304: H1215–H1224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME, Lefer DJ. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol 302: H2410–H2418, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Predmore BL, Lefer DJ. Hydrogen sulfide-mediated myocardial pre- and post-conditioning. Expert Rev Clin Pharmacol 4: 83–96, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen X, Peter EA, Bir S, Wang R, Kevil CG. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic Biol Med 52: 2276–2283, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith RP. The oxygen and sulfide binding characteristics of hemoglobins generated from methemoglobin by two erythrocytic systems. Mol Pharmacol 3: 378–385, 1967. [PubMed] [Google Scholar]
- 46.Sonobe T, Haouzi P. H2S induced coma and cardiogenic shock in the rat: Effects of phenothiazinium chromophores. Clin Toxicol (Phila) 53: 525–539, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonobe T, Haouzi P. Sulfide intoxication-induced circulatory failure is mediated by a depression in cardiac contractility. Cardiovasc Toxicol 16: 67–78, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starnes JW, Beyer RE, Edington DW. Myocardial adaptations to endurance exercise in aged rats. Am J Physiol Heart Circ Physiol 245: H560–H566, 1983. [DOI] [PubMed] [Google Scholar]
- 49.Streeter AJ, Dahlin DC, Nelson SD, Baillie TA. The covalent binding of acetaminophen to protein. Evidence for cysteine residues as major sites of arylation in vitro. Chem Biol Interact 48: 349–366, 1984. [DOI] [PubMed] [Google Scholar]
- 50.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res 79: 632–641, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Szabo G, Veres G, Radovits T, Gero D, Modis K, Miesel-Groschel C, Horkay F, Karck M, Szabo C. Cardioprotective effects of hydrogen sulfide. Nitric Oxide 25: 201–210, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Togawa T, Ogawa M, Nawata M, Ogasawara Y, Kawanabe K, Tanabe S. High performance liquid chromatographic determination of bound sulfide and sulfite and thiosulfate at their low levels in human serum by pre-column fluorescence derivatization with monobromobimane. Chem Pharm Bull (Tokyo) 40: 3000–3004, 1992. [DOI] [PubMed] [Google Scholar]
- 54.Toohey JI. Sulphane sulphur in biological systems: a possible regulatory role. Biochem J 264: 625–632, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toohey JI. Sulfur signaling: is the agent sulfide or sulfane? Anal Biochem 413: 1–7, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Toohey JI. The conversion of H2S to sulfane sulfur. Nat Rev Mol Cell Biol 13: 803, 2012. [DOI] [PubMed] [Google Scholar]
- 57.Ubuka T, Abe T, Kajikawa R, Morino K. Determination of hydrogen sulfide and acid-labile sulfur in animal tissues by gas chromatography and ion chromatography. J Chromatogr B Biomed Sci Appl 757: 31–37, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Van de Louw A, Haouzi P. Oxygen deficit and H2S in hemorrhagic shock in rats. Crit Care 16: R178, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van de Louw A, Haouzi P. Ferric iron and cobalt(III) compounds to safely decrease hydrogen sulfide in the body? Antioxid Redox Signal 19: 510–516, 2013. [DOI] [PubMed] [Google Scholar]
- 60.Vitvitsky V, Kabil O, Banerjee R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid Redox Signal 17: 22–31, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warenycia MW, Goodwin LR, Francom DM, Dieken FP, Kombian SB, Reiffenstein RJ. Dithiothreitol liberates non-acid labile sulfide from brain tissue of H2S-poisoned animals. Arch Toxicol 64: 650–655, 1990. [DOI] [PubMed] [Google Scholar]
- 62.Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol 294: R1930–R1937, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, VandenEkart E, Toombs CF, Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol 160: 941–957, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yong R, Searcy DG. Sulfide oxidation coupled to ATP synthesis in chicken liver mitochondria. Comp Biochem Physiol B Biochem Mol Biol 129: 129–137, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Zhang R, Sun Y, Tsai H, Tang C, Jin H, Du J. Hydrogen sulfide inhibits L-type calcium currents depending upon the protein sulfhydryl state in rat cardiomyocytes. PLoS One 7: e37073, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]