Mechanisms of adverse cardiopulmonary interaction in chronic obstructive pulmonary disease (COPD) remain unclear. Using a novel healthy human model of COPD, we demonstrate that lung hyperinflation impairs left ventricular (LV) function to a greater extent than increased negative intrathoracic pressure and provide quantitative evidence for the role of direct ventricular interaction in reducing LV stroke volume.
Keywords: chronic obstructive pulmonary disease, dynamic hyperinflation, direct ventricular interaction, echocardiography, intrathoracic pressure
Abstract
Chronic obstructive pulmonary disease (COPD) is associated with dynamic lung hyperinflation (DH), increased pulmonary vascular resistance (PVR), and large increases in negative intrathoracic pressure (nITP). The individual and interactive effect of these stressors on left ventricular (LV) filling, emptying, and geometry and the role of direct ventricular interaction (DVI) in mediating these interactions have not been fully elucidated. Twenty healthy subjects were exposed to the following stressors alone and in combination: 1) inspiratory resistive loading of −20 cmH2O (nITP), 2) expiratory resistive loading to cause dynamic hyperinflation (DH), and 3) normobaric-hypoxia to increase PVR (hPVR). LV volumes and geometry were assessed using triplane echocardiography. LV stroke volume (LVSV) was reduced during nITP by 7 ± 7% (mean ± SD; P < 0.001) through a 4 ± 5% reduction in LV end-diastolic volume (LVEDV) (P = 0.002), while DH reduced LVSV by 12 ± 13% (P = 0.001) due to a 9 ± 10% reduction in LVEDV (P < 0.001). The combination of nITP and DH (nITP+DH) caused larger reductions in LVSV (16 ± 16%, P < 0.001) and LVEDV (12 ± 10%, P < 0.001) than nITP alone (P < 0.05). The addition of hPVR to nITP+DH did not further reduce LV volumes. Significant septal flattening (indicating DVI) occurred in all conditions, with a significantly greater leftward septal shift occurring with nITP+DH than either condition alone (P < 0.05). In summary, the interaction of nITP and DH reduces LV filling through DVI. However, DH may be more detrimental to LV hemodynamics than nITP, likely due to mediastinal constraint of the heart amplifying DVI.
NEW & NOTEWORTHY
Mechanisms of adverse cardiopulmonary interaction in chronic obstructive pulmonary disease (COPD) remain unclear. Using a novel healthy human model of COPD, we demonstrate that lung hyperinflation impairs left ventricular (LV) function to a greater extent than increased negative intrathoracic pressure and provide quantitative evidence for the role of direct ventricular interaction in reducing LV stroke volume.
chronic obstructive pulmonary disease (COPD) is a progressive, irreversible disease resulting in destruction of the lung parenchyma and vasculature and airway obstruction (42). Expiratory flow limitation worsens with the progression of COPD, resulting in lung hyperinflation and increased negative intrathoracic pressure generation (nITP) (42). Moreover, through parenchymal destruction, alveolar hypoxia, and chronically elevated lung volumes, pulmonary vascular resistance (PVR) is often elevated in this population (35, 58). These pathological changes to the pulmonary system not only increase respiratory symptoms but also may adversely affect left ventricular (LV) function (45, 56, 58) even in the absence of concomitant cardiovascular disease (1, 59).
It is generally established that nITP decreases LV stroke volume (LVSV) (26, 52, 53, 56); however, the etiology of this decrease remains unclear. Increased ventricular afterload (10, 54), collapse of the inferior vena cava (IVC) (41, 64), and direct ventricular interaction (DVI) [an increase in right-ventricular (RV) end-diastolic volume causing a leftward shift of the interventricular septum which reduces LV compliance and constrains LV filling (9)] have all been proposed. Similarly, PVR increases RV afterload, which through series interaction reduces LVSV (7, 19). The hemodynamic effects of lung hyperinflation are less clear, though increased lung volume increases PVR through alveolar capillary compression (18, 66) and may mechanically constrain LV filling (10, 13).
While the isolated hemodynamic effects of nITP and PVR have been investigated, their interactive effect is not known. Similarly, the effects of increased lung volume on LV function are not well established, and previous studies (54, 60) have not comprehensively investigated the potential mechanisms of adverse cardiopulmonary interaction in COPD. Accordingly, the aim of this study was to investigate the hemodynamic effects of nITP, dynamic hyperinflation (DH), and increased PVR alone and in combination on LV function in healthy, spontaneously breathing humans to develop a more comprehensive understanding of hemodynamic compromise in COPD. We hypothesized that 1) DH would reduce LVSV due to an attenuated LV end-diastolic volume (LVEDV), 2) DH in conjunction with nITP would exacerbate DVI and cause larger reductions in LVSV, and 3) the interactive effects of DH, nITP, and normobaric-hypoxia to increase PVR (hPVR) would result in the largest reductions in LVSV due to greater RV volume overload and thus increased DVI.
MATERIALS AND METHODS
Subjects.
Twenty healthy subjects (10:10 male-female, 23 ± 2 yr; mean ± SD) volunteered for the study. Exclusion criteria included the following: current smokers, a history of cardiopulmonary disease, or a body mass index (BMI) >30 kg/m2. This study received ethical approval from the University of British Columbia Clinical Research Ethics Board (H14-01163), and all subjects provided written informed consent prior to participation.
Study design.
Subjects attended the laboratory on two occasions. Visit 1 involved familiarization with the test protocol and equipment, baseline pulmonary function testing, screening to ensure acquisition of quality echocardiographic images, and a hypoxia familiarization trial to determine the fraction of inspired oxygen (FiO2) required to achieve 80% oxygen saturation measured by pulse oximetry (SpO2) for each subject. During visit 2, subjects completed three experimental phases in a standardized order including nITP, DH, and hPVR alone and in combination. Echocardiographic images and ventilatory parameters were measured during each phase. Subjects were given ≥5 min between phases to allow cardiopulmonary parameters [heart rate (HR), minute ventilation (V̇e), and blood pressure] to return to baseline. Additionally, baseline echocardiographic images were acquired at the beginning of each phase and later analyzed to confirm that no changes in LV volumes and geometry occurred between phases across the study duration (repeated baseline measure data not reported).
Inspiratory resistance.
Inspiratory resistance was generated by a custom-built in-line resistance device that reduced diameter and thus increased resistance to airflow, fitted to the inspiratory side of a two-way nonrebreather mouthpiece (model 2700; Hans Rudolph, Shawnee, KS) to achieve a target nITP of −20 cmH2O during normal inspiration. Subjects maintained −20 cmH2O nITP during spontaneous breathing while echocardiographic images were acquired.
Lung hyperinflation.
Lung hyperinflation (DH) was achieved through application of an expiratory resistance (in-line resistor affixed to the expiratory side of the two-way nonrebreather mouthpiece). Subjects had their ventilation paced via metronome (Pro Metronome Application for Apple devices; EUM Lab, Berlin, Germany) such that respiratory rate (RR) matched their resting RR; however, duty cycle was manipulated such that expiratory time (TE) was limited to 50% of total respiratory cycle time (TI/TTOT = 0.5, where TI is inspiratory time). During nITP+DH, duty cycle was paced at TI/TTOT = 0.67 to allow for sustained −20 cmH2O nITP while limiting expiratory flow such that DH also occurred. Echocardiographic measurements were acquired once subjects had reached a constant maximal level of DH that they were able to maintain across the period of echocardiographic image acquisition. Consistent end-expiratory lung volumes across the period of data collection were confirmed by real-time analysis of the subject's expiratory flow and volume and quantified by performing inspiratory capacity (IC) maneuvers at the start and end of echocardiographic image acquisition (68).
Hypoxia.
Subjects were connected to a 150-liter nondiffusion bag and breathed a hypoxic gas for 45 min, as previous research has shown that significant pulmonary vasoconstriction and elevation in pulmonary artery pressures (PAP) occurs within 45 min of acute hypoxic exposure (15, 20). FiO2 was titrated to maintain subjects at an SpO2 of 80% to elicit a moderate increase in PVR similar to what is seen in moderate to severe COPD patients (>GOLD stage II) (43). PAP was estimated from tricuspid regurgitation velocity measured via echocardiography (48), and oxygen saturation was measured continuously via noninvasive pulse oximetry. FiO2 was individualized to achieve SpO2 = 80% at the end of the exposure (mean FiO2 13.6 ± 0.2%). After 45-min wash-in, baseline hypoxic measures were acquired, and then measures were repeated with nITP (nITP+hPVR). Finally, to examine the combined effects of nITP, DH, and hPVR (referred to as our model of COPD), both inspiratory and expiratory resistances were inserted, and nITP+DH was repeated with hPVR (nITP+DH+hPVR).
Ventilatory parameters.
Ventilatory parameters were measured continuously using a two-way low-dead space, low-resistance valve (model 2700; Hans Rudolph) attached to inspiratory and expiratory pneumotachometers via large-bore tubing. Signals from the pneumotachometer were converted to a digital signal using a data acquisition system (Powerlab; ADInstruments, Colorado Springs, CO). The flow signal was integrated over time to obtain volume. All data were sampled at 1 Hz and stored on a computer for later analysis. Routine spirometry was performed according to American Thoracic Society guidelines (2, 39).
Intrathoracic pressure.
ITP was measured via esophageal balloon catheter (Ackrad Laboratories, Cranford, NJ), inserted and positioned as previously described (15). The balloon catheter was connected to a differential pressure transducer (MP45; Validyne, Northridge, CA), which was calibrated before each test using a manometer. Signals from the pressure transducer were converted to a digital signal using a data acquisition system (Powerlab; ADInstruments).
Echocardiography measurements.
Echocardiographic images were acquired by a trained sonographer and recorded on a commercially available ultrasound system (Vivid-E9; GE Medical, Horton, Norway) using a M5S-D 1.5–4.6-MHz probe for two-dimensional (2-D) imaging and a 4V-D 1.5–4.0-MHz probe for 4-D (triplane) imaging. Images were acquired in the left lateral decubitus position in accordance with current guidelines (31) and saved for off-line analysis (EchoPAC version 7; GE Medical). LV parasternal short-axis images at the level of the mitral valve were acquired for assessment of LV geometry. Triplane imaging was used to acquire images in the apical four-, three-, and two-chamber views simultaneously, which were analyzed for LV end-systolic volumes (LVESV), end-diastolic volumes, and ejection fraction (EF) to American Society of Echocardiography standards using the Simpson's triplane method (17, 23, 24). A single trained observer, who was blinded to subject identity and study phase, performed all analysis. The sonographer's technical error of measurement was 2.5% for EF, 1.8 ml for LVEDV, and 4.5 ml for LVESV. Additionally, the intraobserver coefficient of variation for image analysis was 1.6% for LVEDV, 4.1% for LVESV, 2.3% for stroke volume (SV), and 2.7% for EF using the Simpson's triplane method.
Right ventricular systolic pressure (RVSP) was estimated at baseline and after hypoxic exposure using measures of IVC collapse (cIVC) to estimate right atrial pressure (RAP) (27) and the tricuspid regurgitant jet velocity (TRV), using the simplified Bernoulli equation (6, 33). No pulmonic valve or right ventricular outflow tract abnormalities were detected in subjects; thus RVSP was assumed to be equal to systolic pulmonary artery pressure (PAP) (33, 48). RAP was estimated as a range score based on the calculated IVC diameter and cIVC, as has been previously reported (4, 33).
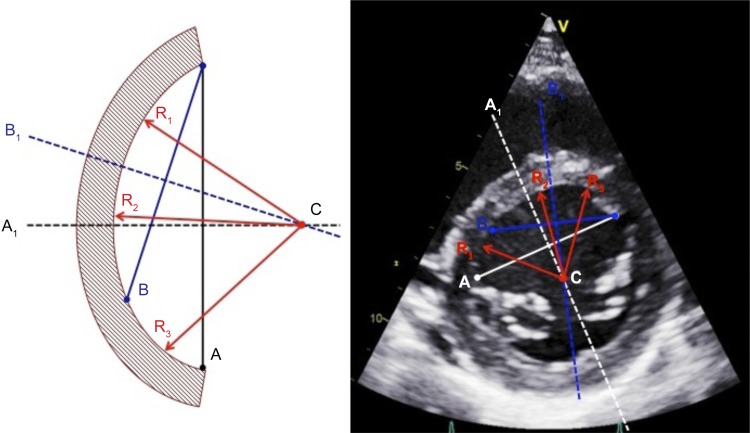
The radius of septal curvature (RSC) was calculated to quantify the degree of septal flattening, and thus presence of DVI, during each intervention. The radius of the septal segment and free-wall segment was determined using a modified technique developed by Brinker et al. (9) and calculated from the parasternal short-axis view at end-diastole and end-systole (Fig. 1).
Fig. 1.
Method for determining radius of septal curvature (RSC). Two chords (A and B) span the septal arc segment and are bisected by perpendicular lines (A1 and B1). The intersection point (C) defines the center of the circle described by the arc segment, from which three radii (R1, R2, and R3) are constructed, the average length of which is calculated to provide the radius.
Statistical analysis.
Descriptive statistics utilized means ± SD. Before applying statistical methods that assume normalcy, a Shapiro-Wilks test was performed. Dependent t-tests were then performed on normally distributed data to determine whether each intervention had a significant effect on cardiopulmonary parameters relative to each specifically measured baseline. If needed, nonparametric data were analyzed using a Wilcoxon signed-rank test. Percent change scores were analyzed using a one-sample t-test to assess whether the mean percent change was different from zero. To determine differences in cardiopulmonary parameters across all phases, repeated-measures one-way ANOVA was used utilizing a Holm-Sidak post hoc comparison to allow for comparison across phases with different sample sizes, relative to baseline parameters. Nonparametric data were analyzed using repeated-measures ANOVA on ranks with a Dunn's post hoc comparison test.
To investigate and quantify the relative contribution of specific interventions in combination, dependent t-tests were used between nITP alone and nITP in conjunction with each independent stressor (e.g., nITP+DH, nITP+hPVR). To quantify the summative effects of nITP+DH+hPVR, one-way repeated-measures ANOVA with three dependent variables was performed between nITP alone, nITP+DH, and nITP+DH+hPVR. When a significant effect was found for the ANOVA, a Tukey post hoc test was used. For nonnormally distributed data, repeated-measures ANOVA on ranks with a Dunn's post hoc comparison was used. LV geometric parameters of septal and LV free-wall radius were analyzed using one-way repeated-measures ANOVA and a Holm-Sidak post hoc comparison relative to baseline for normally distributed data, or a repeated-measures ANOVA on rank with Dunn's post hoc comparison for nonnormally distributed data. The alpha level for all analysis was set a priori at P < 0.05.
RESULTS
Subject characteristics and baseline cardiorespiratory parameters are reported in Tables 1 and 2, respectively. No significant differences were observed between baseline parameters for any phase in normoxia.
Table 1.
Subject characteristics
| Parameter | Value |
|---|---|
| Age | 23 ± 2 |
| Height, m | 1.71 ± 0.10 |
| Weight, kg | 69.0 ± 12.8 |
| BMI, kg/m2 | 23.5 ± 2.4 |
| FVC, liters | 4.53 ± 1.09 |
| FVC, % pred | 100 ± 11 |
| FEV1, liters | 3.52 ± 0.81 |
| FEV1, % pred | 90 ± 12 |
| FEV1/FVC, % | 78 ± 7 |
| IC, liters | 2.65 ± 0.71 |
| MIP, cmH2O | −89 ± 25 |
| MIP, % pred | 87 ± 21 |
| MEP, cmH2O | 115 ± 41 |
| MEP, % pred | 86 ± 25 |
Values are means ± SD, n = 20.
FVC, forced vital capacity; FEV1, forced expired volume in 1 s; % pred, percent of predicted; IC, inspiratory capacity; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure.
Table 2.
Change from baseline cardiopulmonary function during nITP, DH, and nITP+DH
| nITP |
DH |
nITP+DH |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Baseline | Condition | Δ | Baseline | Condition | Δ | Baseline | Condition | Δ |
| RR, beats/min | 14 ± 3 | 9 ± 4 | −5 ± 4† | 13 ± 3 | 14 ± 1 | 1 ± 2 | 13 ± 3 | 14 ± 1 | 1 ± 2 |
| VT, liters | 0.56 ± 0.11 | 1.00 ± 0.47 | 0.44 ± 0.43† | 0.56 ± 0.13 | 0.66 ± 0.15 | 0.09 ± 0.14* | 0.56 ± 0.13 | 0.55 ± 0.14 | −0.01 ± 0.16 |
| VE, l/min | 7 ± 1 | 7 ± 1 | 0 ± 2 | 7 ± 1 | 9 ± 2 | 2 ± 2† | 7. ± 1 | 7 ± 2 | 0 ± 2 |
| TI, s | 1.7 ± 0.3 | 5.2 ± 2.3 | 3.4 ± 2.3† | 1.8 ± 0.5 | 1.3 ± 0.3 | −0.5 ± 0.4† | 1.8 ± 0.5 | 2.3 ± 0.2 | 0.5 ± 0.5† |
| TE, s | 2.8 ± 0.7 | 2.7 ± 1.6 | −0.1 ± 1.6 | 2.6 ± 0.4 | 2.9 ± 0.3 | 0.3 ± 0.5 | 2.6 ± 0.4 | 1.9 ± 0.2 | −0.7 ± 0.4† |
| TI/TTOT | 0.39 ± 0.05 | 0.65 ± 0.06 | 0.26 ± 0.08† | 0.40 ± 0.05 | 0.31 ± 0.06 | −0.09 ± 0.05† | 0.40 ± 0.04 | 0.55 ± 0.04 | 0.15 ± 0.05† |
| Pes.ins, cmH2O | −3.7 ± 1.9 | −22.4 ± 2.8 | −18.7 ± 2.7† | −3.2 ± 1.5 | −13.3 ± 5.4 | −10.1 ± 5.1† | −3.2 ± 1.5 | −23.7 ± 3.3 | −20.5 ± 3.9† |
| Pes.exp, cmH2O | 1.6 ± 1.5 | 2.7 ± 2.0 | 1.1 ± 1.9 | 2.1 ± 1.5 | 3.8 ± 3.4 | 1.8 ± 3.7* | 2.1 ± 1.5 | 7.5 ± 5.6 | 5.4 ± 5.4† |
| IC, liters | – | – | – | 2.90 ± 0.88 | 1.20 ± 0.54 | −1.70 ± 0.81† | 2.90 ± 0.88 | 0.71 ± 0.19 | −2.19 ± 0.83† |
| LVEDV, ml | 112 ± 22 | 108 ± 24 | −5 ± 5† | 113 ± 22 | 102 ± 23 | −10 ± 11† | 111 ± 21 | 97 ± 22 | −14 ± 11† |
| LVESV, ml | 47 ± 10 | 47 ± 10 | 0 ± 4 | 47 ± 9 | 45 ± 12 | −2 ± 5 | 46 ± 8 | 43 ± 9 | −3 ± 4* |
| EF, % | 58 ± 3 | 56 ± 4 | −4 ± 3* | 58 ± 2 | 56 ± 4 | −2 ± 5 | 58 ± 3 | 55 ± 5 | −3 ± 5* |
| LVSV, ml | 65 ± 13 | 61 ± 15 | −4 ± 4† | 66 ± 14 | 57 ± 12 | −9 ± 9† | 65 ± 14 | 54 ± 15 | −10 ± 10† |
| Q, l/min | 3.8 ± 0.7 | 3.9 ± 0.7 | 0.1 ± 0.6 | 3.8 ± 0.6 | 3.5 ± 0.5 | −0.2 ± 0.6 | 3.8 ± 0.6 | 3.6 ± 0.9 | −0.2 ± 0.9 |
| HR, beats/min | 59 ± 12 | 67 ± 14 | 7 ± 9* | 59 ± 11 | 63 ± 12 | 5 ± 5* | 60 ± 11 | 68 ± 13 | 8 ± 9* |
| MAP, mmHg | 76 ± 8 | 77 ± 7 | 1 ± 7 | 76 ± 8 | 80 ± 7 | 3 ± 8 | 76 ± 78 | 85 ± 11 | 8 ± 11* |
| SVR, Δ % | – | – | 4 ± 16 | – | – | 16 ± 25* | – | – | 34 ± 41* |
| SpO2, % | 99 ± 1 | 98 ± 2 | 0 ± 2 | 99 ± 1 | 99 ± 1 | 0 ± 1 | 99 ± 1 | 97 ± 3 | −2 ± 3* |
Values are means ± SD; n = 20, except for echocardiographic volume measurements, n = 19, n = 18, and n = 17, and for LV geometry, n = 19, n = 15, and n = 13, in nITP, DH, and nITP+DH, respectively. Δ, change from baseline; RR, respiratory rate; VT, tidal volume; TI, inspiratory time; TE, expiratory time; TI/TTOT, ratio of inspiratory time to total respiratory cycle time; Pes.ins and Pes.exp, inspiratory and expiratory esophageal pressure; IC, inspiratory capacity; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; EF, ejection fraction; SV, stroke volume; Q, cardiac output; HR, heart rate; MAP, mean arterial pressure; SVR, systemic vascular resistance; SpO2, oxygen saturation measured by pulse oximetry. Significant change from baseline:
P < 0.05,
P < 0.001.
Effects of negative intrathoracic pressure on cardiopulmonary function.
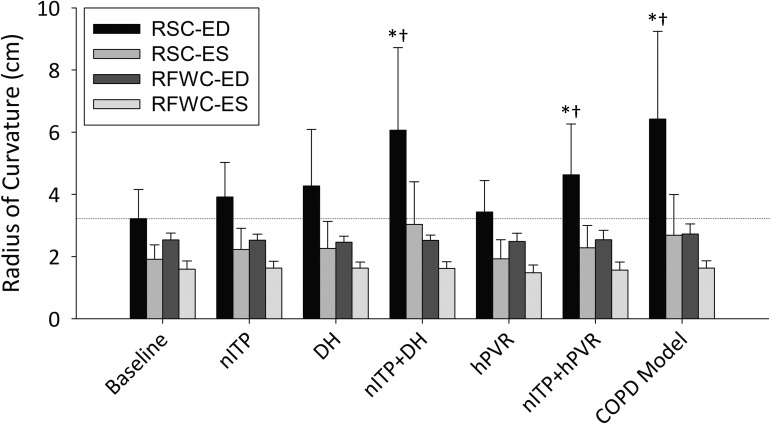
Negative intrathoracic pressure significantly reduced RR and increased tidal volume (VT), but VE was unchanged. LVEDV was reduced by 4 ± 5% (P = 0.002), which, in conjunction with an unchanged LV end-systolic volume (LVESV) (P = 0.992), resulted in a reduction in ejection fraction (EF, P = 0.011) and LVSV (P < 0.001, Table 2). A 13 ± 17% increase in HR (P = 0.004) preserved cardiac output (Q), while mean arterial pressure (MAP) and systemic vascular resistance (SVR; calculated as MAP/Q and expressed as a percent change from baseline) were not changed. The radius of septal curvature (RSC) at end-diastole (RSC-ED) increased by 26 ± 31% (P = 0.002).
Effects of dynamic hyperinflation on cardiopulmonary function.
DH did not change RR; however, VT (P = 0.006) and VE were increased (P < 0.001, Table 2). End-expiratory lung volume (EELV) increased by 1.70 ± 0.81 liters such that inspiratory reserve volume (IRV) was reduced to 0.55 ± 0.43 liters. DH decreased LVEDV by 9 ± 10% (P < 0.001), which was a larger reduction than seen with nITP (P = 0.045), while LVESV did not change significantly. Thus EF was reduced by 2 ± 5% (P = 0.05), while LVSV was decreased by 12 ± 13% (P = 0.001). During DH, Q was maintained by an increase in HR (P = 0.003). SVR was increased (P = 0.02), and there was a trend toward an increased MAP (P = 0.052). DH caused significant septal flattening, as RSC-ED increased by 44 ± 58% (P = 0.017).
Effects of combined nITP and DH on cardiopulmonary function.
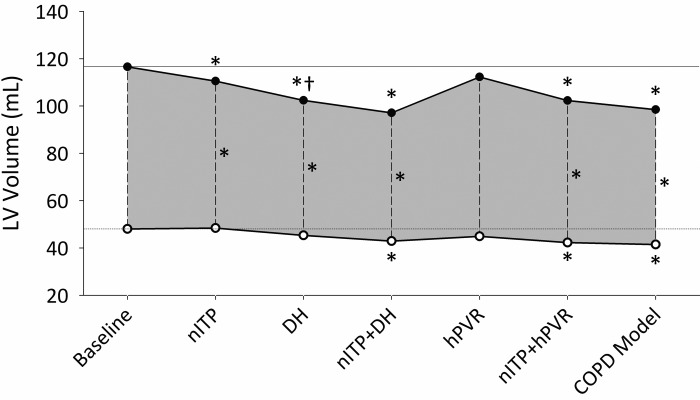
VT and VE were not significantly altered from baseline during nITP+DH (Table 2). IRV was reduced to 0.17 ± 0.15 liters, which was a greater reduction than with DH alone (P < 0.05). LVEDV and LVESV were reduced during nITP+DH by 12 ± 10% (P < 0.001) and 7 ± 9% (P = 0.004), respectively, while EF and SV were reduced by 3 ± 5% (P = 0.038) and 10 ± 10% (P < 0.001), respectively. This was not significantly different from DH alone (LVEDV approached significance, P = 0.066); however, LVEDV, LVESV, and SV were significantly reduced from nITP alone (P < 0.05, Fig. 2). HR increased by 14 ± 17% (P = 0.004), and thus Q was not reduced (P = 0.557). MAP (P = 0.006) and SVR (P = 0.004) were increased during nITP+DH, while SpO2 decreased (P = 0.001). RSC-ED was significantly increased (P < 0.001) and was greater during nITP+DH than during either nITP (59% increase, P = 0.031) or DH (41% increase, P = 0.030) (Fig. 3).
Fig. 2.
Mean LVEDV (●) and LVESV (○) defining LVSV (grey area) across all interventions. *Significant change from baseline (P < 0.05). †Significant change from the previous phase (P < 0.05).
Fig. 3.
Changes in LV geometry across all interventions. RSC-ED, radius of septal curvature at end-diastole; RSC-ES, radius of septal curvature at end-systole; RFWC-ED, radius of LV free-wall curvature at end-diastole; RFWC-ES, radius of free-wall curvature at end-systole. Data are shown as means ± SD. *Significant change from baseline (P < 0.05). †Significant change from the previous phase (P < 0.05).
Effects of hypoxia-induced increased PVR on cardiopulmonary function.
RVSP (assumed synonymous with systolic PAP) increased by 43 ± 37% (P < 0.001), while SpO2 was reduced to 81 ± 2% (P < 0.001, Table 3). Hypoxia resulted in larger nITP than observed in normoxia (−3.5 ± 1.7 cmH2O vs. −6.4 ± 4.0 cmH2O, P = 0.009). There were no significant changes in RR, VT, or VE. Q was increased (P < 0.001) with hPVR due to an increased HR (P < 0.001), while LV volumes did not change significantly from normoxic conditions. No significant effect of hPVR on MAP was found; however, SVR decreased (P = 0.006). LV geometry was not significantly altered by hPVR.
Table 3.
Change from baseline cardiopulmonary function during hPVR alone, nITP+hPVR, and nITP+DH+hPVR (COPD model)
| hPVR |
nITP+hPVR |
nITP+DH+hPVR |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Baseline | Condition | Δ | Baseline | Condition | Δ | Baseline | Condition | Δ |
| RR, beats/min | 14 ± 2 | 14 ± 2 | 0 ± 2 | 14 ± 2 | 9 ± 3 | −5 ± 3† | 14 ± 2 | 14 ± 1 | 0 ± 2 |
| VT, liters | 0.59 ± 0.25 | 0.59 ± 0.11 | −0.01 ± 0.19 | 0.59 ± 0.11 | 0.99 ± 0.39 | 0.4 ± 0.36† | 0.59 ± 0.11 | 0.56 ± 0.09 | 0.03 ± 0.14 |
| VE, l/min | 8 ± 4 | 8 ± 1 | 0 ± 3 | 8 ± 1 | 8 ± 2.6 | 0 ± 3 | 8 ± 1 | 8 ± 1 | 0 ± 1 |
| TI, s | 1.6 ± 0.4 | 1.6 ± 0.4 | −0.1 ± 0.42 | 1.6 ± 0.4 | 4.4 ± 1.7 | 2.8 ± 1.7† | 1.6 ± 0.4 | 2.6 ± 1.2 | 1.0 ± 1.3† |
| TE, s | 2.4 ± 0.4 | 2.5 ± 0.4 | 0.2 ± 0.6 | 2.5 ± 0.4 | 2.7 ± 1.0 | 0.2 ± 1.1 | 2.5 ± 0.4 | 1.8 ± 0.2 | 0.7 ± 0.4† |
| TI/TTOT | 0.41 ± 0.07 | 0.39 ± 0.06 | −0.02 ± 0.09* | 0.39 ± 0.06 | 0.61 ± 0.09 | 0.23 ± 0.13† | 0.39 ± 0.06 | 0.57 ± 0.07 | 0.18 ± 0.1† |
| Pes.ins, cmH2O | −3.5 ± 1.7 | −6.4 ± 4.0 | −2.9 ± 5.2† | −6.4 ± 4.0 | −23.1 ± 3.0 | −16.7 ± 4.7† | −6.4 ± 4.0 | −24.2 ± 4.0 | −17.7 ± 5.8† |
| Pes.exp, cmH2O | 1.9 ± 1.6 | −0.1 ± 2.3 | −2.0 ± 2.5* | −0.1 ± 2.3 | 1.1 ± 2.3 | 1.4 ± 3.0 | −0.1 ± 2.3 | 6.4 ± 5.4 | 6.5 ± 5.9† |
| IC, liters | 2.89 ± 0.88 | 2.54 ± 0.93 | −0.26 ± 0.25† | – | – | – | 2.54 ± 0.93 | 0.62 ± 0.20 | 1.91 ± 0.79† |
| LVEDV, ml | 115 ± 21 | 114 ± 23 | −1 ± 5 | 112 ± 23 | 102 ± 21 | −10 ± 9† | 113 ± 21 | 99 ± 16 | −15 ± 11† |
| LVESV, ml | 47 ± 10 | 45 ± 9 | −2 ± 4 | 45 ± 9 | 42 ± 8 | −3 ± 4* | 45 ± 9 | 42 ± 9 | −3 ± 5* |
| EF, % | 59 ± 2 | 60 ± 3 | 1 ± 3 | 60 ± 3 | 58 ± 4 | −2 ± 3* | 60 ± 3 | 58 ± 5 | −2 ± 4* |
| LVSV, ml | 67 ± 11 | 68 ± 15 | 1 ± 4 | 67 ± 15 | 60 ± 14 | −7 ± 7† | 68 ± 14 | 58 ± 10 | −10 ± 10* |
| Q, l/min | 3.9 ± 0.5 | 4.5 ± 0.6 | 0.6 ± 0.4† | 4.5 ± 0.6 | 4.2 ± 0.8 | −0.2 ± 0.7 | 4.4 ± 0.5 | 3.8 ± 0.7 | −0.7 ± 0.5† |
| HR, beats/min | 59 ± 11 | 68 ± 13 | 8 ± 5† | 68 ± 12 | 73 ± 13 | 4 ± 7* | 67 ± 11 | 66 ± 10 | 0 ± 10 |
| MAP, mmHg | 76 ± 8 | 76 ± 8 | 0 ± 9 | 76 ± 8 | 77 ± 8 | 1 ± 10 | 76 ± 8 | 85 ± 12 | 8 ± 12* |
| SVR, Δ % | – | – | −12 ± 17* | – | – | 7 ± 17 | – | – | 37 ± 19† |
| RVSP, mmHg | 21 ± 5 | 29 ± 4 | 8 ± 5† | – | – | – | – | – | – |
| SpO2, % | 99 ± 1 | 81 ± 2 | −18 ± 2† | 81 ± 2 | 85 ± 6 | 3 ± 5* | 81 ± 2 | 79 ± 5 | −2 ± 6 |
Values are means ± SD; n = 20, except for echocardiographic volume measurements, n = 19 and n = 15, and for LV geometry, n = 19 and n = 13, in hPVR and nITP+DH+hPVR, respectively. Δ, absolute change from baseline (hPVR considered baseline for nITP+hPVR and nITP+DH+hPVR). See Table 2 for abbreviations. Significant change from baseline:
P < 0.05,
P < 0.001.
Effects of hPVR and nITP on cardiopulmonary function.
Relative to hPVR alone, LVEDV (9 ± 7%, P < 0.01), LVESV (5 ± 8%, P < 0.01), EF (2 ± 3%, P = 0.042), and LVSV (10 ± 9%, P < 0.001) were reduced during nITP+hPVR. HR increased (P = 0.021), which maintained Q. LVEDV (P = 0.016) and LVESV (P < 0.001) were reduced to a greater degree during nITP+hPVR than during nITP alone (Fig. 2). MAP and SVR did not change significantly from hypoxic baseline; however, SpO2 increased by 3 ± 5% (P = 0.011). RSC-ED increased by 45 ± 42% (P < 0.001), and RSC increased 20 ± 29% (P = 0.007) at end-systole (RSC-ES); nITP+hPVR caused a greater increase in RSC-ED than nITP (P = 0.032).
Cardiopulmonary function in a model of COPD (nITP+DH+hPVR).
Respiratory parameters during nITP+DH+hPVR were not significantly different from nITP+DH. Relative to hPVR alone, LVEDV was decreased by 12 ± 9% (P < 0.001), LVESV by 7 ± 12% (P = 0.016), EF by 2 ± 4% (P = 0.042), and LVSV by 14 ± 13% (P = 0.002). A greater reduction in LVESV (P = 0.009) and an increased EF (P = 0.014) resulted from nITP+DH+hPVR compared with nITP+DH. With the addition of hPVR, HR did not increase, thus Q was significantly reduced by 15 ± 12% (P < 0.001). MAP and SVR also increased by 11 ± 17% (P = 0.014) and 37 ± 19% (P < 0.001), respectively. SpO2 did not change relative to hypoxic baseline. Greater septal flattening resulted from nITP+DH+hPVR than from any condition in isolation (P < 0.05). RSC-ED increased by 107 ± 85% (P = 0.001), and RSC-ES increased by 44% (P = 0.024). The radius of LV free-wall curvature (RFWC) also changed significantly, with the RFWC-ED increasing by 7 ± 9% (P = 0.026).
DISCUSSION
To our knowledge, this is the first study to provide quantitative evidence for the role of DVI during nITP, elevated lung volumes, and increased PVR in a spontaneously breathing human and to assess the relative contribution of these stressors to reducing LV volumes. The novel findings of this study are as follows: 1) nITP during spontaneous respiration decreases LVSV through a reduction in LVEDV, 2) DH can have profound hemodynamic consequences through exacerbation of DVI and subsequent LV underfilling [a finding which had previously been attributed to changes in pleural pressure rather than lung volume itself (54, 60)], and 3) increased PVR plays a role in hemodynamic compromise as it interacts with nITP and DH to further restrict LV filling.
Effects of increased negative ITP on cardiovascular function.
It is generally accepted that a marked increase in nITP reduces LVSV (8, 11, 54, 56). However, the mechanism(s) responsible for the attenuated LVSV in a spontaneously breathing human remains unclear. Utilizing nITP of −20 cmH2O, we observed marked reductions in LVSV through a reduction in LVEDV, while LVESV did not change. Increased nITP increases afterload through increasing ventricular and arterial transmural wall pressure (45). However, LVESV did not increase, which suggests that afterload may not be primarily responsible for the reduction in LVSV seen in the present study. In contrast, we observed a 26% increase in RSC-ED, which, in conjunction with an unchanged LVESV, supports DVI as a contributing mechanism to the reduction in LVSV. In the absence of data on RV volumes, attenuated venous return as a result of IVC collapse (7, 41) may have also contributed to the observed reduction in LVEDV. While IVC collapse is highly dependent on volume status and abdominal pressure (64), a nITP of −20 cmH2O would greatly increase IVC transmural pressures at the thoracic inlet to a magnitude where IVC collapse is likely to occur in subjects independent of the abdominothoracic pressure gradient (64). Despite this limitation, our data corroborate previous studies in canine models (51, 53, 55, 62, 63) that identify a role for DVI in impairing LV function during increased nITP and provide novel evidence for this mechanism in spontaneously breathing humans.
Effects of increased lung volumes on cardiovascular function.
Few studies have evaluated the effects of DH alone (25, 49) and with nITP on LV function (25, 49, 54). Previous studies utilizing elevated static lung volumes (54) or voluntary hyperinflation (60) have proposed that LVSV reduction is better correlated with increased nITP than lung volume. In contrast, for similar or larger increases in lung volumes in this study, we demonstrated a greater reduction in LVSV during DH than during nITP, which suggests lung hyperinflation during spontaneous breathing is a key mediator of LV function especially when breathing close to TLC.
DVI has been shown to occur as a result of mediastinal constraint from lung hyperinflation, which increases cardiac fossa pressure and constrains ventricular filling (30). More specifically, lung hyperinflation increases pulmonary capillary wedge pressure but simultaneously increases atrial pressures (5). In this scenario, wedge pressure does not accurately reflect LV filling as LV end-diastolic transmural pressure and thus LVEDV have been shown to decrease despite the raised wedge pressure, indicating an external or mediastinal constraint (5, 26, 38). Given that the extent of DH used in this study is greater than that previously reported to increase cardiac fossa pressure in patients with COPD (12), it is likely that considerable mediastinal constraint occurred, resulting in reduced LV compliance, a greater leftward septal shift, and attenuated LVEDV. We observed greater DVI during DH than during nITP, as evidenced by an 18% greater increase in RSC-ED with DH, despite smaller pleural pressure swings (relative to pleural pressure swings seen during nITP alone). As such, our data support that lung volume may have a greater effect on LV hemodynamics than the increased pleural pressure swings seen to occur with DH (42), through mechanical constraint and subsequent exacerbation of DVI. This finding has significant implication for patients with COPD.
Effects of elevated lung volumes and increased negative ITP on cardiovascular function.
In support of our second hypothesis, LVSV was reduced to a greater extent during nITP+DH than during nITP alone, due to a greater reduction in LVEDV. Furthermore, compared with DH alone, nITP+DH saw a trend toward further reduction in LVEDV. Without RV volumetric data, the relative contribution of series interaction and DVI to this response cannot be determined. However, the significantly greater increase in RSC-ED during nITP+DH compared with nITP supports amplification of DVI (Fig. 3). Mediastinal constraint in the presence of RV pressure/volume overload has been shown to amplify DVI in canine models (5), and our data support a similar mechanism in humans. Greater RV filling is likely promoted by nITP+DH than by DH [until the point of IVC collapse (41, 46)] while also increasing RV afterload due to increased LVSV (66). This increase in RV volumes, in the presence of DH causing mediastinal constraint, would amplify DVI as demonstrated by the markedly increased RSC-ED and reductions in LVEDV.
Effects of increased PVR on cardiovascular function.
In COPD, PVR is increased as a result of chronic alveolar hypoxia and destruction of lung parenchyma (36), as well as increased operational lung volumes (57). Yet this critical feature has been neglected in previous studies investigating hemodynamics in healthy human models of COPD (60). Such alterations to the vascular bed would further increase RV afterload, which when combined with the aforementioned effects of nITP+DH, could further compromise LV filling.
Given that LV function is generally preserved in response to acute hypoxia in healthy individuals at rest (14, 40, 61), it was not surprising that hPVR alone did not significantly alter LV function. In contrast, during nITP+hPVR, LVEDV was further decreased from nITP. However, LVSV did not change appreciably, as LVESV was also reduced and EF was significantly increased. These data indicate a greater reduction in LV filling during nITP+hPVR but that a compensatory increase in contractility occurred (61, 65) to maintain LVSV. Further evidence for increased contractility is provided by a significant increase in HR, known to increase inotropy via the Bowditch effect (50), as well as an afterload-mediated increase in contractility (Anrep effect) (3, 28), both of which likely occurred under the given conditions. Thus, while the interaction of nITP+hPVR caused greater impairment to LV filling than under normoxic conditions (through DVI as indicated by increased RSC), LVSV was not significantly affected. Nevertheless, this interaction may translate to considerable LV impairment if contractile reserve is depleted (29) or if ventricular compliance is reduced through mediastinal constraint (12).
Cardiovascular function in a model of COPD.
Investigating the interaction of nITP+DH+hPVR as a novel model of COPD, we observed a significant reduction in LVSV as a result of decreased LVEDV. However, owing to an improved EF, concomitant hPVR did not result in greater impairment to LVSV than was seen with nITP+DH, despite a larger reduction in LVEDV and increase in RSC-ED. An important consideration for interpreting these findings is the ability of the healthy heart to tolerate these hemodynamic stressors, which may differ in individuals with cardiovascular disease. The healthy heart has been shown to be able to compensate for increased afterload by increasing contractility (47); thus the preload-dependent aspect of these mechanisms likely dominated. In contrast, the diseased heart is profoundly afterload sensitive (35, 47), and therefore these mechanisms may be considerably more detrimental to cardiac function in a COPD population with comorbid cardiovascular disease. Thus, while significant, our model may underestimate the interactive effect of these stressors in this population.
Study limitations.
A number of limitations should be acknowledged when extrapolating our findings to COPD. First, utilizing a fixed extrathoracic inspiratory resistance to generate nITP and expiratory resistance to cause DH poorly approximates the dynamic changes in airway resistance and flow that occur during tidal breathing in COPD (44, 67). However, while the respiratory mechanics may be different, the hemodynamic effects of generating −20 cmH2O would likely be similar independent of how that pressure is generated. Second, lung hyperinflation and gas trapping were likely fairly homogeneous throughout the healthy lungs of our subjects. In COPD characterized by heterogeneous emphysema, regional gas trapping occurs due to the severity of disease in specific lung regions (8, 22). Thus the hemodynamic effects of DH in COPD may differ somewhat, depending on the extent and location of gas trapping.
There are some well-documented limitations to noninvasive estimation of right-sided heart and pulmonary pressures using echocardiography. The use of IVC diameter and collapsibility as an indirect measure of RAP is influenced by subject positioning, volume status, and training status. This variability, coupled with a calculated range score for RAP rather than a distinct numerical value, reduces the accuracy of RAP measurement (4). While estimation of RVSP using the modified Bernoulli equation is fairly robust from the perspective of measuring tricuspid valve regurgitation velocity, it suffers the same limitations as does RAP measurement, given that RAP is an inherent component to this equation (33). However, relative to TRV, RAP has relatively little influence on RVSP, and thus overestimation or underestimation of RAP has only minimal impact on calculating RVSP. Additionally, our subjects acted as their own controls, and in this healthy normovolemic population we believe this was a valid method of noninvasively assessing PAP. Measuring PVR also requires the measurement of left atrial pressure (LAP), which was not possible in this study. Thus we cannot accurately quantify the increase in PVR observed with hypoxia. However, assuming LAP did not change in response to acute hypoxia (34, 37) and accounting for the observed increase in Q, the increase in RVSP would translate to a 23 ± 30% increase in PVR (P = 0.004).
Despite these limitations, the data from this study provide novel preliminary insight into the cardiopulmonary interactions that occur as a result of altered pulmonary function similar to that seen with exertion or during exacerbation in patients with COPD, allowing for a better understanding of how these stressors interact to influence hemodynamics.
Conclus ions.
The findings from this study highlight the importance of lung hyperinflation as a primary mediator of LV function and suggest that DH in COPD may have considerable clinical relevance beyond the well-documented impact on dyspnea (16) and exercise tolerance (21, 32) that warrants further investigation.
GRANTS
Grants held by N. D. Eves from the Natural Sciences and Engineering Research Council of Canada and the Canadian Foundation of Innovation provided operating and infrastructure funding for the study. N. D. Eves is also supported by a Clinical Scholar Award from the Michael Smith Foundation for Health. The funding source had no influence on study design, writing of the manuscript or the decision to submit for publication.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
W.S.C. and N.D.E. conceived and designed research; W.S.C., A.M.W., M.H., and N.D.E. performed experiments; W.S.C. analyzed data; W.S.C. and N.D.E. interpreted results of experiments; W.S.C. prepared figures; W.S.C. drafted manuscript; W.S.C., A.M.W., M.H., and N.D.E. edited and revised manuscript; W.S.C., A.M.W., M.H., and N.D.E. approved final version of manuscript.
REFERENCES
- 1.Alter P, van de Sand K, Nell C, Figiel JH, Greulich T, Vogelmeier CF, Koczulla AR. Airflow limitation in COPD is associated with increased left ventricular wall stress in coincident heart failure. Respir Med 109: 1131–1137, 2015. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society/European Respiratory Society. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med 166: 518–624, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Anrep von G. On the part played by the suprarenals in the normal vascular reactions of the body. J Physiol 45: 307–317, 1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel R, Cercek B, Luo H, Siegel RJ. Noninvasive evaluation of right atrial pressure. J Am Soc Echocardiogr 26: 1033–1042, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Belenkie I, Kieser TM, Sas R, Smith ER, Tyberg JV. Evidence for left ventricular constraint during open heart surgery. Can J Cardiol 18: 951–959, 2002. [PubMed] [Google Scholar]
- 6.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol 6: 359–365, 1985. [DOI] [PubMed] [Google Scholar]
- 7.Boussuges A, Molenat F, Burnet H, Cauchy E, Gardette B, Sainty JM, Jammes Y, Richalet JP. Operation Everest III (Comex '97): modifications of cardiac function secondary to altitude-induced hypoxia. An echocardiographic and Doppler study. Am J Respir Crit Care Med 161: 264–270, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Boutou AK, Zoumot Z, Nair A, Davey C, Hansell DM, Jamurtas A, Polkey MI, Hopkinson NS. The impact of homogeneous versus heterogeneous emphysema on dynamic hyperinflation in patients with severe COPD assessed for lung volume reduction. COPD 12: 598–605, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinker JA, Weiss JL, Lappé DL, Rabson JL, Summer WR, Permutt S, Weisfeldt ML. Leftward septal displacement during right ventricular loading in man. Circulation 61: 626–633, 1980. [DOI] [PubMed] [Google Scholar]
- 10.Brookhart JM, Boyd TE. Local differences in intrathoracic pressure and their relation to cardiac filling pressure in the dog. Am J Physiol 148: 434–444, 1947. [DOI] [PubMed] [Google Scholar]
- 11.Buda AJ, Pinsky MR, Ingels NB, Daughters GT, Stinson EB, Alderman EL. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med 301: 453–459, 1979. [DOI] [PubMed] [Google Scholar]
- 12.Butler J, Schrijen F, Henriquez A, Polu JM, Albert RK. Cause of the raised wedge pressure on exercise in chronic obstructive pulmonary disease. Am Rev Respir Dis 138: 350–354, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy SS, Ramanathan M. Dimensional analysis of the left ventricle during PEEP: relative septal and lateral wall displacements. Am J Physiol Heart Circ Physiol 246: H792–H805, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Dedobbeleer C, Hadefi A, Naeije R, Unger P. Left ventricular adaptation to acute hypoxia: a speckle-tracking echocardiography study. J Am Soc Echocardiogr 26: 736–745, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Eves ND, Petersen SR, Haykowsky MJ, Wong EY, Jones RL. Helium-hyperoxia, exercise, and respiratory mechanics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174: 763–771, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Faisal A, Alghamdi BJ, Ciavaglia CE, Elbehairy AF, Webb KA, Ora J, Neder JA, O'Donnell DE. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am J Respir Crit Care Med 193: 299–309, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Folland ED, Parisi AF, Moynihan PF, Jones DR, Feldman CL, Tow DE. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography: a comparison of cineangiographic and radionuclide techniques. Circulation 60: 760–766, 1979. [DOI] [PubMed] [Google Scholar]
- 18.Frank NR, Radford EP, Whittenberger JL. Static volume-pressure interrelations of the lungs and pulmonary blood vessels in excised cats' lungs. J Appl Physiol 14: 167–173, 1959. [DOI] [PubMed] [Google Scholar]
- 19.Ghignone M, Girling L, Prewitt RM. Effect of increased pulmonary vascular resistance on right ventricular systolic performance in dogs. Am J Physiol Heart Circ Physiol 246: H339–H343, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Grünig E, Mereles D, Hildebrandt W, Swenson ER, Kübler W, Kuecherer H, Bärtsch P. Stress Doppler echocardiography for identification of susceptibility to high altitude pulmonary edema. J Am Coll Cardiol 35: 980–987, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Guenette JA, Jensen D, Webb KA, Ofir D, Raghavan N, O'Donnell DE. Sex differences in exertional dyspnea in patients with mild COPD: physiological mechanisms. Respir Physiol Neurobiol 177: 218–227, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Herth FJF, Valipour A, Shah PL, Eberhardt R, Grah C, Egan J, Ficker JH, Wagner M, Witt C, Liebers U, Hopkins P, Gesierich W, Phillips M, Stanzel F, McNulty WH, Petermann C, Snell G, Gompelmann D. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med 4: 185–193, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs LD, Salgo IS, Goonewardena S, Weinert L, Coon P, Bardo D, Gerard O, Allain P, Zamorano JL, de Isla LP, Mor-Avi V, Lang RM. Rapid online quantification of left ventricular volume from real-time three-dimensional echocardiographic data. Eur Heart J 27: 460–468, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins C, Bricknell K, Hanekom L, Marwick TH. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol 44: 878–886, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Jörgensen K, Müller MF, Nel J, Upton RN, Houltz E, Ricksten SE. Reduced intrathoracic blood volume and left and right ventricular dimensions in patients with severe emphysema: an MRI study. Chest 131: 1050–1057, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Kingma I, Smiseth OA, Frais MA, Smith ER, Tyberg JV. Left ventricular external constraint: relationship between pericardial, pleural and esophageal pressures during positive end-expiratory pressure and volume loading in dogs. Ann Biomed Eng 15: 331–346, 1987. [DOI] [PubMed] [Google Scholar]
- 27.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol 66: 493–496, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Knowlton FP, Starling EH. The influence of variations in temperature and blood-pressure on the performance of the isolated mammalian heart. J Physiol 44: 206–219, 1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosmala W, Rojek A, Przewlocka-Kosmala M, Mysiak A, Karolko B, Marwick TH. Contributions of nondiastolic factors to exercise intolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol 67: 659–670, 2016. [DOI] [PubMed] [Google Scholar]
- 30.Kroeker CAG, Shrive NG, Belenkie I, Tyberg JV. Pericardium modulates left and right ventricular stroke volumes to compensate for sudden changes in atrial volume. Am J Physiol Heart Circ Physiol 284: H2247–H2254, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, Pérez de Isla L, Kamp O, Kasprzak JD, Lancellotti P, Marwick TH, McCulloch ML, Monaghan MJ, Nihoyannopoulos P, Pandian NG, Pellikka PA, Pepi M, Roberson DA, Shernan SK, Shirali GS, Sugeng L, Cate Ten FJ, Vannan MA, Zamorano JL, Zoghbi WA. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 25: 3–46, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Langer D, Ciavaglia CE, Neder JA, Webb KA, O'Donnell DE. Lung hyperinflation in chronic obstructive pulmonary disease: mechanisms, clinical implications and treatment. Expert Rev Respir Med 8: 731–749, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr 23: 685–713, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Lejeune P, De Smet JM, de Francquen P, Leeman M, Brimioulle S, Hallemans R, Melot C, Naeije R. Inhibition of hypoxic pulmonary vasoconstriction by increased left atrial pressure in dogs. Am J Physiol Heart Circ Physiol 259: H93–H100, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Light RW, Mintz HM, Linden GS, Brown SE. Hemodynamics of patients with severe chronic obstructive pulmonary disease during progressive upright exercise. Am Rev Respir Dis 130: 391–395, 1984. [DOI] [PubMed] [Google Scholar]
- 36.Magee F, Wright JL, Wiggs BR, Paré PD, Hogg JC. Pulmonary vascular structure and function in chronic obstructive pulmonary disease. Thorax 43: 183–189, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malik AB, Kidd BS. Pulmonary arterial wedge and left atrial pressures and the site of hypoxic pulmonary vasoconstriction. Respiration 33: 123–132, 1976. [DOI] [PubMed] [Google Scholar]
- 38.Marini JJ, Culver BH, Butler J. Mechanical effect of lung distention with positive pressure on cardiac function. Am Rev Respir Dis 124: 382–386, 1981. [DOI] [PubMed] [Google Scholar]
- 39.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, ATS Task Force/ERS. Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Naeije R, Dedobbeleer C. Pulmonary hypertension and the right ventricle in hypoxia. Exp Physiol 98: 1247–1256, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Natori H, Tamaki S, Kira S. Ultrasonographic evaluation of ventilatory effect on inferior vena caval configuration. Am Rev Respir Dis 120: 421–427, 1979. [DOI] [PubMed] [Google Scholar]
- 42.O'Donnell DE. Ventilatory limitations in chronic obstructive pulmonary disease. Med Sci Sports Exerc 33, Suppl: S647–S655, 2001. [DOI] [PubMed] [Google Scholar]
- 43.O'Donnell DE, Laveneziana P, Webb K, Neder JA. Chronic obstructive pulmonary disease. Clin Chest Med 35: 51–69, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Paredi P, Goldman M, Alamen A, Ausin P, Usmani OS, Pride NB, Barnes PJ. Comparison of inspiratory and expiratory resistance and reactance in patients with asthma and chronic obstructive pulmonary disease. Thorax 65: 263–267, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Permutt S, Wise RA. Mechanical Interaction of Respiration and Circulation. Hoboken, NJ: John Wiley, 1986. [Google Scholar]
- 46.Pinsky MR. Cardiovascular issues in respiratory care. Chest 128, Suppl 2: 592S–597S, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Pouleur H, Covell JW, Ross JJ. Effects of nitroprusside on venous return and central blood volume in the absence and presence of acute heart failure. Circulation 61: 328–337, 1980. [DOI] [PubMed] [Google Scholar]
- 48.Pyxaras SA, Pinamonti B, Barbati G, Santangelo S, Valentincic M, Cettolo F, Secoli G, Magnani S, Merlo M, Giudice Lo F, Perkan A, Sinagra G. Echocardiographic evaluation of systolic and mean pulmonary artery pressure in the follow-up of patients with pulmonary hypertension. Eur J Echocardiogr 12: 696–701, 2011. [DOI] [PubMed] [Google Scholar]
- 49.Ranieri VM, Dambrosio M, Brienza N. Intrinsic PEEP and cardiopulmonary interaction in patients with COPD and acute ventilatory failure. Eur Respir J 9: 1283–1292, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Richmond DR, Angus JA, Goodman AH, Cobbin LB. The effect of heart rate on indices of myocardial contractility in the dog. Clin Exp Pharmacol Physiol 2: 469–479, 1975. [DOI] [PubMed] [Google Scholar]
- 51.Robotham JL, Badke FR, Kindred MK, Beaton MK. Regional left ventricular performance during normal and obstructed spontaneous respiration. J Appl Physiol Respir Environ Exerc Physiol 55: 569–577, 1983. [DOI] [PubMed] [Google Scholar]
- 52.Scharf S. The effect of decreased intrathoracic pressure on ventricular function. J Sleep Res 4: 53–58, 1995. [DOI] [PubMed] [Google Scholar]
- 53.Scharf SM, Brown R, Saunders N, Green LH. Effects of normal and loaded spontaneous inspiration on cardiovascular function. J Appl Physiol Respir Environ Exerc Physiol 47: 582–590, 1979. [DOI] [PubMed] [Google Scholar]
- 54.Scharf SM, Brown R, Tow DE, Parisi AF. Cardiac effects of increased lung volume and decreased pleural pressure in man. J Appl Physiol Respir Environ Exerc Physiol 47: 257–262, 1979. [DOI] [PubMed] [Google Scholar]
- 55.Scharf SM, Brown R, Warner KG, Khuri S. Intrathoracic pressures and left ventricular configuration with respiratory maneuvers. J Appl Physiol 66: 481–491, 1989. [DOI] [PubMed] [Google Scholar]
- 56.Scharf SM. Cardiovascular effects of airways obstruction. Lung 169: 1–23, 1991. [DOI] [PubMed] [Google Scholar]
- 57.Schrijen FV, Henriquez A, Carton D, Delorme N, Butler J. Pulmonary vascular resistance rises with lung volume on exercise in obstructed airflow disease. Clin Physiol 9: 143–150, 1989. [DOI] [PubMed] [Google Scholar]
- 58.Sietsema K. Cardiovascular limitations in chronic pulmonary disease. Med Sci Sports Exerc 33, Suppl: S656–S661, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Smith BM, Prince MR, Hoffman EA, Bluemke DA, Liu CY, Rabinowitz D, Hueper K, Parikh MA, Gomes AS, Michos ED, Lima JAC, Barr RG. Impaired left ventricular filling in COPD and emphysema: is it the heart or the lungs? The Multi-Ethnic Study Of Atherosclerosis COPD Study. Chest 144: 1143–1151, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol 96: 1920–1927, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Stembridge M, Ainslie PN, Hughes MG, Stöhr EJ, Cotter JD, Tymko MM, Day TA, Bakker A, Shave R. Impaired myocardial function does not explain reduced left ventricular filling and stroke volume at rest or during exercise at high altitude. J Appl Physiol 119: 1219–1227, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strohl KP, Scharf SM, Brown R, Ingram RH. Cardiovascular performance during bronchospasm in dogs. Respiration 51: 39–48, 1987. [DOI] [PubMed] [Google Scholar]
- 63.Takata M, Mitzner W, Robotham JL. Influence of the pericardium on ventricular loading during respiration. J Appl Physiol 68: 1640–1650, 1990. [DOI] [PubMed] [Google Scholar]
- 64.Takata M, Wise RA, Robotham JL. Effects of abdominal pressure on venous return: abdominal vascular zone conditions. J Appl Physiol 69: 1961–1972, 1990. [DOI] [PubMed] [Google Scholar]
- 65.Wauthy P, Pagnamenta A, Vassalli F, Naeije R, Brimioulle S. Right ventricular adaptation to pulmonary hypertension: an interspecies comparison. Am J Physiol Heart Circ Physiol 286: H1441–H1447, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Whittenberger JL, McGregor M, Berglund E, Borst HG. Influence of state of inflation of the lung on pulmonary vascular resistance. J Appl Physiol 15: 878–882, 1960. [DOI] [PubMed] [Google Scholar]
- 67.Yamauchi Y, Kohyama T, Jo T, Nagase T. Dynamic change in respiratory resistance during inspiratory and expiratory phases of tidal breathing in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 7: 259–269, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan S, Kaminski D, Sliwinski P. Reliability of inspiratory capacity for estimating end-expiratory lung volume changes during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 156: 55–59, 1997. [DOI] [PubMed] [Google Scholar]