Abstract
Introduction
The aim of the study was to study the long-term effect on cardiovascular disease risk factors of stress from direct experience of an earthquake as a young person.
Material and methods
We selected workers born between July 1, 1958 and July 1, 1976 who were examined at Kailuan General Hospital between May and October of 2013. Data on cardiovascular events were taken during the workers’ annual health examination conducted between 2006 and 2007. All subjects were divided into three groups according to their experience of the Tangshan earthquake of July 28, 1976, as follows: control group; exposed group 1 and exposed group 2. We compared cardiovascular disease risk factors between the three groups as well as by gender and age.
Results
One thousand one hundred and ninety-six workers were included in the final statistical analysis. Among all subjects, resting heart rate (p = 0.003), total cholesterol (p < 0.001), and fasting blood glucose (p < 0.001) were significantly higher among those who experienced the earthquake compared with unexposed controls, but were unrelated to loss of relatives. No significant difference in triglyceride levels was observed between the three groups (p = 0.900). Further refinement showed that the effects were restricted to males 40 years of age or older at the time of analysis, but were due primarily to age at the time of earthquake exposure (p = 0.002, p < 0.001 and p = 0.002).
Conclusions
Earthquake experience in the early years of life has long-term effects on adult resting heart rate, total cholesterol, and fasting plasma glucose, especially among men.
Keywords: earthquake stress, cardiovascular disease, risk factors, long-term effect
Introduction
Earthquakes are catastrophic natural events that can cause strong physiological and psychological responses, especially from the autonomic nervous system [1, 2]. The emergency responses of the cardiovascular system include instantaneous effects and delayed responses. The instantaneous cardiovascular reaction is more likely to result in death from cardiovascular disease, while the delayed response leads to exacerbation of cardiovascular disease risk factors, such as increases in resting heart rate, blood triglycerides and total cholesterol levels [2–4]. Previous studies show significant increases during months when natural disasters occur in the incidence of cardiovascular disease, including angina pectoris, stroke, myocardial infarction, heart failure, and aortic dissection [5–7], and these diseases increase 1.5- to 3-fold after earthquakes [8]. However, there are few data on the relation between natural disaster-induced stress and cardiovascular disease risk factors, and even fewer concerning the relation between earthquake experience in the early years of life and cardiovascular disease risk factors. We therefore set out to study cardiovascular disease risk factors in individuals who experienced an earthquake 30 years previously, when they were 18 years old or younger.
Material and methods
Subjects
This study was approved by the medical ethics committee of the research center. The subjects of the study were Kailuan workers who were examined in the health examination center of Kailuan General Hospital between May 2013 and October 2013. Criteria for inclusion in the exposed groups were: 1) signature of informed consent form; 2) residency in Tangshan during and since the earthquake; and 3) birth between July 1, 1958 and July 1, 1976. Criteria for inclusion in the control group were: 1) signature of informed consent form; 2) having moved to Tangshan after the earthquake, and resided there since; and 3) birth between July 1, 1958 and July 1, 1976. Exclusion criteria were as follows: 1) injury during the earthquake of 2013; 2) history of any of the following: use of antihypertensive or lipid-lowering drugs, drugs for hypoglycemia, or of insulin to control blood glucose; 3) sinus arrhythmia or definitive cardiovascular disease; 4) incomplete information related to the earthquake; or 5) incomplete data for physiological and biochemical indicators such as waist circumference, body mass index, blood pressure, or resting heart rate. Grouping: subjects were placed into one of three groups depending on their experience of the Tangshan earthquake of July 28, 1976, as follows: control group: no experience; exposed group 1: experienced the earthquake without loss of relatives; exposed group 2: experienced the earthquake with loss of relatives.
Materials
The questionnaire used was generated by the authors. Information on the subjects’ general condition and earthquake-related parameters was collected one-on-one by trained investigators. General information included name, gender, date of birth, smoking history, history of alcohol consumption, physical training, educational background, job, and family income. Smoking was defined as consumption of at least 1 cigarette per day for at least 1 year. Drinking was defined as consumption on average of 100 ml/day white spirits (alcohol content above 50%) for at least one year. Smokers or drinkers were still defined as such if they ceased either habit for less than 1 year. Earthquake-related parameters included: whether subjects experienced the Tangshan earthquake of July 28th, 1976, whether they were injured in it, and whether they lost any relatives. We compared the subjects’ general and earthquake information with the Kailuan workers’ physical examination data obtained in 2006–2007, and then performed statistical analysis on the combined data.
Methods
Health assessment
Height and weight were measured using the corrected RGZ-120 scale. Subjects were weighed in light clothing without shoes or hats; body mass index (BMI) = weight/height2 (kg/m2). Waist circumference was measured using a horizontal tape at the slenderest part of the waist, with an accuracy of 0.1 cm. Blood pressure was measured using a corrected mercury sphygmomanometer applied to the brachial artery of the right arm. Smoking, tea and coffee were prohibited, and subjects were seated quietly for 15 min before the measurement was taken. Systolic pressure (SBP) was read at the first Korotkoff sound and diastolic (DBP) at the fifth. Three consecutive measurements were taken at 1–2 min intervals, then averaged. Resting heart rate (RHR) was measured after 5 min of inactivity and is expressed as beats per minute (bpm). An electrocardiograph (Japanese Light-Electric Company) was used to record a 12-lead ECG with subjects in a horizontal position; II lead was chosen to trace five QRS waves continually; RR deviation was measured, and the RHR calculated according to average RR deviation.
Determination of biochemical indices
Blood was drawn from the cubital veins of fasting subjects into EDTA vacuum tubes at 7:00–9:00 a.m. on the same day as the physical examination. Blood was centrifuged at 3000 × g for 10 min at room temperature, and supernatant collected for assaying fasting blood glucose (FBG), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C). Total cholesterol and TG were measured by the oxidase method, using oxidase provided by Shanghai Classical Biological Engineering Co., LTD. The hexokinase method was used to measure FBG, with reagent provided by Born in Beikong Biotechnology Co., LTD. A Hitachi 7600 Automatic Biochemical Analyzer was used for analysis, with quality control performed on each batch. All instruments were operated by a professional laboratory technician.
Statistical analysis
All the questionnaire information and health examination data were entered at a Kailuan General Hospital terminal, and an Oracle 10.2 g database was created by uploading them to a server in Kailuan General Hospital. A file of DBF format was generated by exporting data by standard procedures. We used SAS9.2 software for statistical analysis. One-way analysis of variance, Kruskal-Wallis test and Student’s t-test were used for comparison of continuous variables between workers in the exposed and control group if it was appropriate. A χ2 test was used for comparison of categorical variables between workers in the exposed and control group. Logistic regression analyses were performed to examine the association between earthquake and cardiovascular disease-related factors (RHR, TG and FBG). A two-sided p-value less than 0.05 was considered statistically significant.
Results
Long-term cardiovascular health is affected by earthquake experience
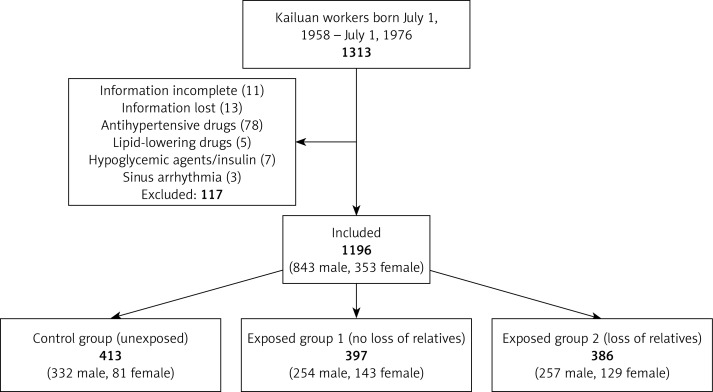
We enrolled 1313 workers in this study, of whom 117 were excluded, as shown in Figure 1, leaving 1196 workers on whom statistical analysis was ultimately conducted (843 male and 353 female). General information on the subjects is shown in Table I.
Figure 1.
Enrollment, exclusion, and inclusion of Kailuan workers as study subjects
Table I.
General information on workers
| Characteristic | Control group (N = 413) | Exposed group 1 (N = 397) | Exposed group 2 (N = 386) | P-value** |
|---|---|---|---|---|
| Age, mean ± SD [years] | 40.00 ±4.72 | 40.34 ±4.86 | 40.34 ±4.32 | 0.327 |
| Male, n (%) | 332 (80.39) | 254 (63.98) | 257 (66.58) | < 0.001 |
| Physical training, n (%) | 365 (88.38) | 340 (85.64) | 342 (88.6) | 0.373 |
| Junior high school education or above, n (%) | 148 (35.84) | 164 (41.31) | 123 (31.87) | 0.022 |
| Monthly family income ≥ 600 Yuan, n (%) | 256 (61.99) | 208 (52.39) | 194 (50.26) | 0.002 |
| Smoking, n (%)* | 212 (51.33) | 200 (50.38) | 194 (50.26) | 0.945 |
| Drinking, n (%)* | 276 (66.83) | 234 (58.94) | 240 (62.18) | 0.065 |
| History of family hypertension, n (%) | 115 (27.85) | 148 (37.28) | 105 (27.2) | 0.003 |
As defined in Materials.
P-values for comparison among control group, exposed group 1 and exposed group 2.
We also analyzed physiological parameters to discover which of them were affected by earthquake experience. As shown in Table II, we determined that three, namely RHR, TC, and FBG, were significantly different in workers exposed to the Tangshan earthquake compared to unexposed controls 30 years after the event, with workers in both exposed groups having higher heart rates, cholesterol levels, and glucose levels than unexposed controls. Although earthquake exposure produced elevated values, comparison of workers in exposed groups 1 and 2 demonstrated no significant differences in cardiovascular parameters at the time of analysis (p > 0.05). Since loss of relatives did not appear to contribute to the changes we did observe (see footnote to Table II), the two exposed groups were combined in all subsequent analyses. No significant differences between the control and exposed groups were observed in blood pressure, specific cholesterol fractions, triglycerides, waistline, or body mass index.
Table II.
Comparison of cardiovascular disease-related factors between workers in exposed groups and controls
| Cardiovascular disease-related factors | Control group (N = 413) | Exposed group 1 (N = 397) | Exposed group 2 (N = 386) | P-value* |
|---|---|---|---|---|
| Waistline [cm] | 83.48 ±10.71 | 83.31 ±10.66 | 84.14 ±10.58 | 0.661 |
| BMI [kg/m2] | 25.07 ±3.35 | 24.62 ±3.59 | 24.78 ±3.64 | 0.116 |
| RHR [bpm] | 72.06 ±9.45 | 73.64 ±10.13 | 74.54 ±10.79** | 0.003 |
| SBP [mm Hg] | 118.13 ±15.76 | 117.59 ±15.19 | 118.26 ±18.20 | 0.788 |
| DBP [mm Hg] | 78.10 ±9.47 | 77.57 ±9.07 | 77.62 ±10.47 | 0.727 |
| TC [mmol/l] | 4.83 ±0.98 | 5.01 ±0.97 | 5.05 ±0.86** | < 0.001 |
| LogTG | 1.40 ±1.06 | 1.51 ±1.25 | 1.43 ±1.07 | 0.900 |
| LDL-C [mmol/l] | 2.18 ±0.65 | 2.16 ±0.64 | 2.22 ±0.66 | 0.497 |
| HDL-C [mmol/l] | 1.51 ±0.53 | 1.52 ±0.52 | 1.54 ±0.52 | 0.762 |
| FBG [mmol/l] | 5.12 ±1.24 | 5.43 ±1.39 | 5.36 ±1.64** | < 0.001 |
Data are means ± SD. Abbreviations explained in Methods.
P-values for comparison among control group, exposed group 1 and exposed group 2.
Compared with group 1 p > 0.05.
Earthquake effects are specific to older males
We further stratified the affected cardiovascular disease-related factors in the control and earthquake-experienced groups with respect to both gender and age. We reasoned that an earthquake might affect long-term cardiovascular health differently depending on age at the time of exposure, because developmental stage at the time of the event could influence physiological responses to stress. The age of 40 (at the time of biochemical analysis) was chosen to demarcate subjects, because individuals younger than 40 would have been younger than 10 years old during the earthquake, and thus preadolescent. Those 40 years of age or older would include all postpubertal subjects. This analysis, shown in Table III, revealed that earthquake exposure specifically affected males 40 years of age or older at the time of biochemical analysis for all three factors, with higher values measured among exposed workers in the combined groups 1 and 2 than in controls. No significant effects were observed either in female workers or in males younger than 40.
Table III.
Comparison of RHR, TC and FBG between workers in exposed and control groups, stratified by gender and age
| Cardiovascular disease-related factors | Female (N = 353) | P-value | Male (N = 843) | P-value | ||
|---|---|---|---|---|---|---|
| Control group (n = 81) | Exposed group 1 + 2 (n = 272) | Control group (n = 332) | Exposed group 1 + 2 (n = 501) | |||
| RHR, mean ± SD [bpm]: | ||||||
| Age < 40 | 74.06 ±10.75 | 73.53 ±9.79 | 0.795 | 72.35 ±9.25 | 73.95 ±9.432 | 0.129 |
| Age ≥ 40 | 72.60 ±9.12 | 74.65 ±12.21 | 0.230 | 71.35 ±9.38 | 74.07 ±10.38 | 0.002 |
| TC, mean ± SD [mmol/l]: | ||||||
| Age < 40 | 4.46 ±0.81 | 4.69 ±0.77 | 0.112 | 4.72 ±1.11 | 4.81 ±0.91 | 0.447 |
| Age ≥ 40 | 4.79 ±1.00 | 4.95 ±0.92 | 0.33 | 4.97 ±0.89 | 5.32 ±0.89 | < 0.001 |
| FBG, mean ± SD [mmol/l]: | ||||||
| Age < 40 | 5.00 ±0.61 | 5.02 ±0.53 | 0.857 | 5.04 ±0.71 | 5.10 ±0.87 | 0.437 |
| Age ≥ 40 | 5.17 ±0.54 | 5.39 ±1.44 | 0.116 | 5.19 ±1.65 | 5.70 ±1.96 | 0.002 |
Given that the effects were gender-specific, we hypothesized that males might be more likely than females to experience extreme changes in cardiovascular risk factors after earthquake exposure. Odds ratios for high values of RHR, TC, and FBG were stratified by gender. We chose 80 beats/min as a value for RHR above which cardiovascular disease risk increases significantly [6–8]. The values used as cutoffs for TC and FBG are widely considered to delineate clinical abnormality. The results, shown in Table IV, confirm that male workers, but not females, were significantly more likely to experience high-value increases in all three parameters if exposed to the earthquake than were unexposed workers.
Table IV.
Odds ratios (OR) of high-value RHR, TC and FBG for workers in exposed and control groups
| Cardiovascular disease-related factors | Group | Total | Female | Male | |||
|---|---|---|---|---|---|---|---|
| OR* (95% CI) | P-value | OR* (95% CI) | P-value | OR* (95% CI) | P-value | ||
| RHR > 80 bpm | Control | 1 | 1 | 1 | |||
| Exposed | 1.450 (1.051–2.001) | 0.029 | 1.111 (0.551–2.239) | 0.769 | 1.605 (1.105–2.330) | 0.013 | |
| TC > 5.98 mmol/l | Control | 1 | 1 | 1 | |||
| Exposed | 1.493 (1.035–2.153) | 0.032 | 1.100 (0.426–2.845) | 0.843 | 1.581 (1.057–2.365) | 0.026 | |
| FBG > 6.1 mmol/l | Control | 1 | 1 | 1 | |||
| Exposed | 1.594 (0.915–2.776) | 0.099 | 1.346 (0.279–6.494) | 0.711 | 1.842 (1.001–3.386) | 0.049 | |
OR were derived from a logistic analysis model adjusting for age, income, education, physical training, smoking and drinking.
Age at the time of earthquake exposure influences long-term cardiovascular effects
We recognize that, in addition to any effect of age or development at the time of earthquake exposure, differences in cardiovascular health between control and exposed subjects might be more easily detected after the age of 40, because cardiovascular pathology manifests more frequently in middle age than younger.
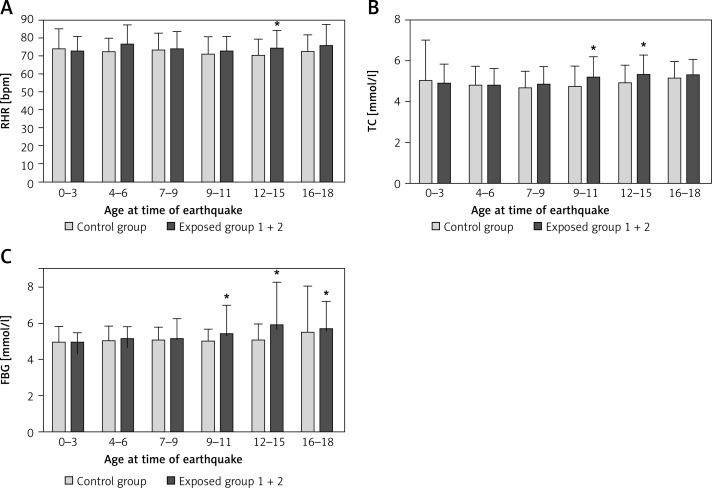
In an attempt to distinguish these factors, we stratified our comparison of cardiovascular parameters in the control and exposed groups by more specific ages at the time of the earthquake (shown in Figure 2; note that all subjects ≥ 10 years old in Figure 2 are in the group ≥ 40 years old in Table III). While this stratification confirms that earthquake-associated effects are mostly confined to those ≥ 40 years old when analyzed, it also demonstrates that within this group, some long-term effects are observed only if subjects were exposed to the earthquake at a particular time in adolescence. Thus RHR is affected long term only in those exposed at 12–15 years of age, and TC is affected only by exposure between ages 9 and 15. This analysis suggests that susceptibility to increases in long-term cardiovascular risk due to earthquake trauma is affected, at least in part, by specific age when exposed to the earthquake, not merely by age at the time of biochemical analysis.
Figure 2.
Effect of earthquake 30 years later on RHR, TC, and FBG of male workers age 18 or younger at time of earthquake
*P < 0.05 compared with control group.
Discussion
On July 28, 1976, a 7.0-magnitude earthquake occurred in Tangshan, China, leveling the whole of the city in an instant. The loss of traffic, telecommunications, power, and water supply resulted in the deaths of 242,000 and serious injury of 164,000. This study demonstrates that those who experienced the Tangshan earthquake in their early years suffered an increased risk of elevated RHR, fasting blood sugar and total cholesterol as adults. After an earthquake in the southern Italian city of Naples, a series of longitudinal studies targeting male workers in the Olivetti factory showed an increase in RHR, fasting blood sugar and total cholesterol 2–8 weeks after the earthquake [9]. Examination of the subjects 4 years after that earthquake showed that property damage and/or interruption of social networks are risk factors for an increase in RHR [10]. A follow-up study of the Olivetti workers 7 years after the Naples earthquake indicated that the resulting stress had no significant effect on resting heart rate, fasting blood sugar or total cholesterol [11]. Our study differs from the Italian one, however, in that our subjects experienced the earthquake as adolescents and children at less than 18 years of age, while the Olivetti factory workers were traumatized as adults. Our data therefore suggest that earthquake experience in the early years has a longer-term effect on RHR than does a similar experience during adulthood.
Resting heart rate is defined as heartbeats per minute in a state of sobriety and inactivity. The frequency range of the heartbeat in adults is 60–100 beats/min. As a single risk factor for hypertension, an elevated RHR can increase blood pressure in various ways [12–14]. In addition, the occurrence and development of coronary artery disease are closely related to resting heart rate. A higher rate increases myocardial oxygen consumption, thereby increasing harmful free radicals; vascular endothelial cells are damaged, and atherosclerosis is accelerated. Atherosclerosis increases peripheral vascular resistance, and with it the risk of plaque rupture and thrombosis [15]. Thus an increase in RHR not only can affect the incidence of cardiovascular events and hypertension, but also plays an important role in the development of heart failure [16–18].
We also found that earthquake experience at a young age has a long-term effect on fasting blood sugar in adult males. There is a significant increase in blood glucose level in patients with traumatic brain injury, with the size of the increase being related to severity of the injury, according to a study from Harun Haron et al. [19]. It is well established that stress can promote excitement of the sympathetic nervous system: increases in catecholamine promote gluconeogenesis, thus increasing blood glucose level. In addition, lipid metabolism becomes disordered, resulting in insulin resistance. A meta-analysis by Einarson et al. [20] showed that among 191,249 non-diabetic patients who had an increase in blood glucose, 6.6% died of cardiovascular diseases, 7.6% died of cardiovascular events, 3.6% received cardiac stimulant treatment, and 1.7% experienced sudden death. The mortality of patients with cardiovascular disease increases even with a random rise in blood glucose within a normal range [21]. Such a rise can damage vascular endothelial cells, leading to cholesterol deposition in the injured area and promoting atherosclerosis. In addition, this study shows that with increasing stress, problematic behaviors such as smoking and drinking increase, exacerbating the risk of cardiovascular disease. In our study cohort, the percentage of workers who were smokers or drinkers was no higher in those exposed to the earthquake than in controls (Table I), so the effects we observed are not attributable to those behaviors.
We found no long-term effect of earthquake experience during early years on cardiovascular disease among adult females. This may be due to a protective effect of female physiology against kidney and cardiovascular diseases [22]. Sympathetic activation is related to the menstrual cycle, and estrogen inhibits such activation according to a recent report [23]. By signaling to reduce sympathetic activity the estrogen receptor, especially the β form, diminishes the hypertensive effects of aldosterone and high salt, and confers protection from stress [24]. In addition, estrogen can dilate the smooth muscle of the coronary artery directly, and protect the cardiovascular system by decreasing LDL and increasing HDL, thus reducing the occurrence of cardiovascular diseases.
Limitations and future research directions: Many non-pharmacological factors affect resting heart rate, fasting blood glucose and total cholesterol, such as hormones and diseases (including infectious and immune system diseases), with the magnitude of comorbidity depending in part on disease severity. The effect of such diseases on these cardiovascular risk factors is hard to exclude. In addition, the sample size of our female cohort was relatively small, and needs to be enlarged in future analyses of the effects of early earthquake experience on adult cardiovascular risk factors and the mechanisms that underlie them.
Acknowledgments
Na Li and Yumei Wang contributed equally to this paper.
This study was supported by the National Natural Science Foundation of China (code: 81271489) and Hebei Province Natural Science Foundation of China (H2014206280).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Bradley BA, Cubrinovski M. Near-source strong ground motions observed in the 22 February 2011 Christchurch earthquake. Seismological Research Letters. 2011;82:853–65. [Google Scholar]
- 2.Cooney MT, Vartiainen E, Laakitainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–9. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Wang Z, Liu X, et al. Effects of high resting heart rate on the stability of carotid artery plaque in a middle and advanced aged population. Zhonghua Yi Xue Za Zhi. 2014;94:2308–11. [PubMed] [Google Scholar]
- 4.Palatini P, Casiglia E, Julius S, Pessina AC. High heart rate: a risk factor for cardiovascular death in elderly men. Arch Intern Med. 1999;159:585–92. doi: 10.1001/archinte.159.6.585. [DOI] [PubMed] [Google Scholar]
- 5.Kario K, McEwen BS, Pickering TG. Disasters and the heart: a review of the effects of earthquake-induced stress on cardiovascular disease. Hypertens Res. 2003;26:355–67. doi: 10.1291/hypres.26.355. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz BG, French WJ, Mayeda GS, et al. Emotional stressors trigger cardiovascular events. Int J Clin Pract. 2012;66:631–9. doi: 10.1111/j.1742-1241.2012.02920.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown DL. Disparate effects of the 1989 Loma Prieta and 1994 Northridge earthquakes on hospital admissions for acute myocardial infarction: importance of superimposition of triggers. Am Heart J. 1999;137:830–6. doi: 10.1016/s0002-8703(99)70406-0. [DOI] [PubMed] [Google Scholar]
- 8.Nishizawa M, Hoshide S, Shimpo M, Kario K. Disaster hypertension: experience from the great East Japan earthquake of 2011. Curr Hypertens Rep. 2012;14:375–81. doi: 10.1007/s11906-012-0298-z. [DOI] [PubMed] [Google Scholar]
- 9.Trevisan M, Celentano E, Meucci C, et al. Short-term effect of natural disasters on coronary heart disease risk factors. Arteriosclerosis. 1986;6:491–4. doi: 10.1161/01.atv.6.5.491. [DOI] [PubMed] [Google Scholar]
- 10.Bland SH, Farinaro E, Krogh V, Jossa F, Scottoni A, Trevisan M. Long term relations between earthquake experiences and coronary heart disease risk factors. Am J Epidemiol. 2000;151:1086–90. doi: 10.1093/oxfordjournals.aje.a010152. [DOI] [PubMed] [Google Scholar]
- 11.Trevisan M, Jossa F, Farinaro E, et al. Earthquake and coronary heart disease risk factors: a longitudinal study. Am J Epidemiol. 1992;135:632–7. doi: 10.1093/oxfordjournals.aje.a116342. [DOI] [PubMed] [Google Scholar]
- 12.Grassi G. Sympathetic neural activity in hypertension and related diseases. Am J Hypertens. 2010;23:1052–60. doi: 10.1038/ajh.2010.154. [DOI] [PubMed] [Google Scholar]
- 13.Koskela JK, Tahvanainen A, Haring A, et al. Association of resting heart rate with cardiovascular function: a cross-sectional study in 522 Finnish subjects. BMC Cardiovasc Disord. 2013;13:102. doi: 10.1186/1471-2261-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dibona GF. Neural control of the kidney past, present, and future. Hypertension. 2003;41:621–4. doi: 10.1161/01.HYP.0000047205.52509.8A. [DOI] [PubMed] [Google Scholar]
- 15.Wu X. The significance of heart rate in cardiovascular disease. Chin J Int Med. 2006;45:601–2. [Google Scholar]
- 16.Kario K, Matsuo T, Shimada K, Pickering TG. Factors associated with the occurrence and magnitude of earthquake-induced increases in blood pressure. Am J Med. 2001;111:379–84. doi: 10.1016/s0002-9343(01)00832-4. [DOI] [PubMed] [Google Scholar]
- 17.Gerin W, Chaplin W, Schwartz JE, et al. Sustained blood pressure increase after an acute stressor: the effects of the 11 September 2001 attack on the New York City World Trade Center. J Hypertens. 2005;23:279–84. doi: 10.1097/00004872-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Logue JN, Hansen H. A case-control study of hypertensive women in a post-disaster community: Wyoming Valley, Pennsylvania. J Human Stress. 1980;6:28–34. doi: 10.1080/0097840X.1980.9934533. [DOI] [PubMed] [Google Scholar]
- 19.Harun Haron R, Imran MK, Haspani MS. An observational study of blood glucose levels during admission and 24 hours post-operation in a sample of patients with traumatic injury in a hospital in Kuala Lumpur. Malays J Med Sci. 2011;18:69–77. [PMC free article] [PubMed] [Google Scholar]
- 20.Einarson TR, Machado M, Henk Hemels ME. Blood glucose and subsequent cardiovascular disease: update of a meta-analysis. Curr Med Res Opin. 2011;27:2155–63. doi: 10.1185/03007995.2011.626760. [DOI] [PubMed] [Google Scholar]
- 21.Kadowaki S, Okamura T, Hozawa A, et al. NIPPON DATA Research Group Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. Diabetologia. 2008;51:575–82. doi: 10.1007/s00125-007-0915-6. [DOI] [PubMed] [Google Scholar]
- 22.Denton KM, Hilliard LM, Tare M. Sex-related differences in hypertension: seek and ye shall find. Hypertension. 2013;62:674–7. doi: 10.1161/HYPERTENSIONAHA.113.00922. [DOI] [PubMed] [Google Scholar]
- 23.Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension. 2013;61:395–9. doi: 10.1161/HYPERTENSIONAHA.112.202598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue B, Zhang Z, Beltz TG, et al. Estrogen receptor-beta in the paraventricular nucleus and rostroventrolateral medulla plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension. 2013;61:1255–62. doi: 10.1161/HYPERTENSIONAHA.111.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]