Different methods to estimate lung strain from computed tomography measurements produce distinct strain magnitudes and topographical distributions in healthy mechanically ventilated pigs. Middependent lung regions are those subjected to larger strain during mechanical ventilation, and thus at risk for ventilator-induced injury. Strain estimates are also differently sensitive to applied tidal volume and positive end-expiratory pressure. Values consistent with lung injury/inflammation can occur regionally with settings encountered clinically even when global strain is below critical levels.
Keywords: anesthesia, computed tomography, mechanical ventilation, regional ventilation, strain measurements
Abstract
Parenchymal strain is a key determinant of lung injury produced by mechanical ventilation. However, imaging estimates of volumetric tidal strain (ε = regional tidal volume/reference volume) present substantial conceptual differences in reference volume computation and consideration of tidally recruited lung. We compared current and new methods to estimate tidal volumetric strains with computed tomography, and quantified the effect of tidal volume (VT) and positive end-expiratory pressure (PEEP) on strain estimates. Eight supine pigs were ventilated with VT = 6 and 12 ml/kg and PEEP = 0, 6, and 12 cmH2O. End-expiratory and end-inspiratory scans were analyzed in eight regions of interest along the ventral-dorsal axis. Regional reference volumes were computed at end-expiration (with/without correction of regional VT for intratidal recruitment) and at resting lung volume (PEEP = 0) corrected for intratidal and PEEP-derived recruitment. All strain estimates demonstrated vertical heterogeneity with the largest tidal strains in middependent regions (P < 0.01). Maximal strains for distinct estimates occurred at different lung regions and were differently affected by VT-PEEP conditions. Values consistent with lung injury and inflammation were reached regionally, even when global measurements were below critical levels. Strains increased with VT and were larger in middependent than in nondependent lung regions. PEEP reduced tidal-strain estimates referenced to end-expiratory lung volumes, although it did not affect strains referenced to resting lung volume. These estimates of tidal strains in normal lungs point to middependent lung regions as those at risk for ventilator-induced lung injury. The different conditions and topography at which maximal strain estimates occur allow for testing the importance of each estimate for lung injury.
NEW & NOTEWORTHY
Different methods to estimate lung strain from computed tomography measurements produce distinct strain magnitudes and topographical distributions in healthy mechanically ventilated pigs. Middependent lung regions are those subjected to larger strain during mechanical ventilation, and thus at risk for ventilator-induced injury. Strain estimates are also differently sensitive to applied tidal volume and positive end-expiratory pressure. Values consistent with lung injury/inflammation can occur regionally with settings encountered clinically even when global strain is below critical levels.
mechanical ventilation is used by a large number of patients with initially normal lungs who receive general anesthesia and intensive care. It is well established that mechanical ventilation with large tidal volumes can result in lung injury (24). However, the mechanism linking regional tidal expansion of lung parenchyma and lung injury is not fully established, and its investigation requires accurate assessment of regional tidal expansion.
Excessive stretch of lung parenchyma is a basic mechanism of lung injury (11, 26). In vitro, large cyclic biaxial stretch of alveolar epithelial cells results in the reduction of cell viability (7, 8, 26). In vivo, global tidal lung expansion [defined as the ratio between tidal volume (VT) and functional residual capacity (FRC)] with values above 2.2 applied for 54 h to initially normal lungs leads to increased lung weight, cardiopulmonary deterioration, and multiorgan dysfunction (22). Using positron emission tomography (PET) imaging, we showed during early lung injury that higher regional tidal strain (defined as regional VT divided by regional end-expiratory volume) resulted in higher regional inflammation, that the regional inflammatory effects of strain were greatly amplified by endotoxemia, and that the effect of positive end-expiratory pressure (PEEP) in reducing lung inflammation could be due to a reduction in regional tidal strain (31). Thus, regional tidal strains appear to play a key role in early ventilator-induced lung injury. A recent study implicating large driving pressures in the increase in postoperative pulmonary complications reinforces the importance of tidal strain in causing or contributing to lung injury in surgical patients with initially normal lungs (17).
In vivo assessment of tidal lung strain involves major challenges due to topographical and temporal inhomogeneity of lung inflation. The presence of a gravitational gradient in lung aeration and regional differences in tidal expansion even in normal lungs imply a spatial distribution of strain and the requirement for regional measurements for accurate quantification. In addition, alveolar tidal recruitment results in a portion of the regional VT being delivered to alveolar units collapsed at end-expiration. Consequently, accounting for it should be included in the computation of tidal strain of units continuously aerated during breathing. Regional volume changes have been assessed with computed tomography (CT) (10) and PET (29), and the alveolar strain (4) has been proposed to assess regional lung tissue deformation during mechanical ventilation. Although these measurements are based on the ratio between a tidal change in volume over a reference volume as the estimate of tidal strain, they present substantial conceptual differences in meaning and in addressing the inhomogeneous expansion of lung parenchyma. This is because they differ on the reference volume used to estimate the regional strain: either the end-expiratory lung volume present at the applied PEEP level (10, 31) or the FRC (presumed as the end-expiratory lung volume at PEEP = 0) (4). Additionally, they do not always account for tidal alveolar recruitment (4, 10, 31). Accordingly, those estimates of tidal strain could result in distinct assessments of the magnitude and distribution of regional lung deformation. Yet, although those measurements have been related to relevant injury to the lung in the form of edema or inflammation (4, 10, 31) and are potentially translatable to humans, they have never been directly compared. In-depth understanding of such measurements is necessary to establish the strain correlates of lung injury during mechanical ventilation (e.g., through partitioning of the contributions of deformations associated with different reference volumes and recruitment to ultimate injury).
Changes in ventilator settings affect both the regional inflation of the lungs and the regional distribution of ventilation, thus influencing regional tidal strain. Increased VT may increase global tidal strain. However, regional changes in strain with VT may not equal global changes in the presence of heterogeneous regional lung mechanical impedances. Increased PEEP could result in either a reduction in tidal strain when local VT is referred to end-expiratory volume, or to increased strain if PEEP would result in increased local VT referred to a constant local FRC. Thus, modifications in VT and PEEP could compound to determine spatially heterogeneous distributions of strain. Knowledge of these distributions are relevant because injurious regional levels could be reached even when global tidal strain is limited (18, 31).
To understand the differences between the various estimates of tidal strains in normal lungs and the regional effects of VT and PEEP on those strain measurements, we studied mechanically ventilated pigs using CT imaging. We used large animals to investigate relevant regional lung inflation heterogeneity analogous to that of humans. Our goals were to 1) compare CT-based measures of tidal strain during mechanical ventilation of pigs with normal lungs, 2) introduce an estimate of regional tidal strain that accounts for the local effect of tidal lung recruitment, and 3) determine the effect of PEEP and VT on those regional measures of tidal lung strain along a stepwise incremental PEEP and VT maneuver.
METHODS
The experimental protocol follows the requirements of the German law for animal studies and was approved by the Institutional Animal Care Committee of University Hospital Carl Gustav Carus and the Government of the State of Saxony, Germany.
Anesthesia and Mechanical Ventilation
Eight female pigs (weight, 38.3 ± 3.6 kg) were premedicated and induced with an intramuscular injection of 10 mg/kg ketamine and 1 mg/kg midazolam (Ratiopharm, Ulm, Germany). After endotracheal intubation (8.0 mm internal diameter; Malinckrodt, Athlone, Ireland), anesthesia was maintained via an intravenous (auricular vein) continuous infusion of midazolam (1 to 2 mg·kg−1·h−1) and ketamine (15-20 mg·kg−1·h−1). Muscle paralysis was achieved by continuous administration of atracurium (0.5–2.5 mg·kg−1·h−1; Ratiopharm). Animals were ventilated using an EVITA XL 4 Lab (Dräger Medical, Lübeck, Germany) with volume-controlled ventilation: VT = 10 ml/kg, FiO2 = 1.0, inspiratory:expiratory ratio = 1:1, inspiratory pause of 15–20%, and PEEP = 5 cmH2O for approximately 30–40 min. Respiratory rate was adjusted to achieve a PaCO2 in the range of 35–45 mmHg. The minute ventilation determined at this stage was maintained for the remainder of the study. Crystalloid solution (E153; Serumwerk Bernburg) was administered at 5–10 ml·kg−1·h−1 to keep pulmonary capillary wedge pressure (measured at end-expiration) within the range of 8–12 mmHg.
Computed Tomography
Helical CT scans of the entire lung were obtained with a Somatom Sensation 16 (Siemens, Erlangen, Germany). The CT scanner was set as follows: collimation, 16 × 0.75 mm; pitch, 1.35; bed speed, 38.6 mm/s; voltage, 120 kV; and tube current-time product, 120 mAs. Images were reconstructed with contiguous slices of 5-mm thickness yielding images with 512 × 512 voxels with dimensions of 0.443 × 0.443 mm × 5 mm.
Mechanical Ventilation Protocol for Imaging
Our protocol aimed to examine strains at different VT-PEEP combinations. The sequence of applied ventilatory settings started with a progressive selection of two levels of VT (6 and 12 ml/kg). For each VT, three stepwise incremental levels of PEEP (0, 6, and 12 cmH2O) were applied. An FiO2 = 1.0 was used throughout, and each VT-PEEP combination was maintained for 10 min. Following this 10-min stabilization period, end-expiratory and end-inspiratory images were acquired during breath hold produced by clamping of the endotracheal tube. Other ventilatory settings included an inspiratory:expiratory ratio of 1:1, and a respiratory rate set to achieve the minute ventilation established with the preimaging settings. No lung recruitment was performed between different VT-PEEP combinations.
Image Segmentation and Region of Interest Definition
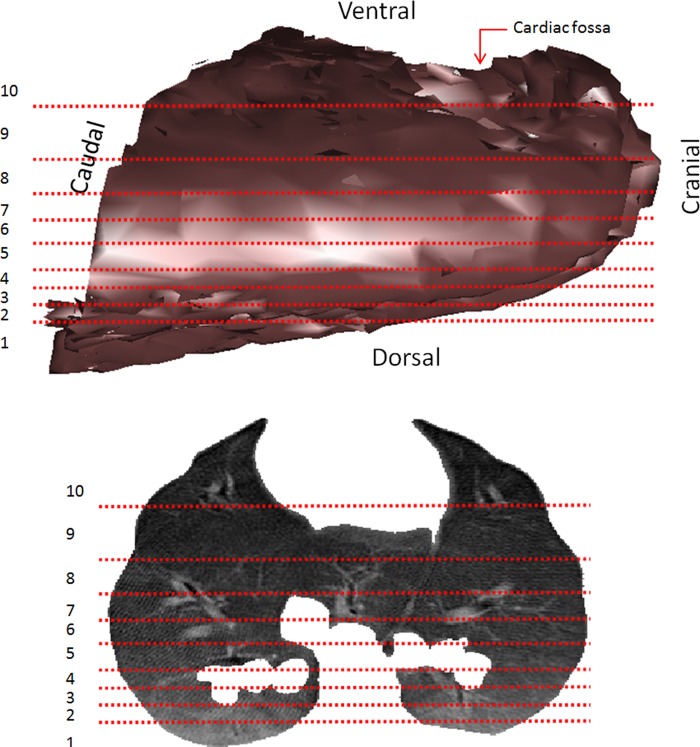
Segmentation of the lung was performed semiautomatically, with dedicated software (CHRISTIAN II, Technical University Dresden). Following this, the three-dimensional lung field was divided into 10 regions of interest (ROIs) with equal tissue volume along the ventral-dorsal axis. Regional measurements in the present manuscript were calculated at the ROI level. Each ROI spans the full cross-sectional area of the lungs in the coronal plane. The most dorsal and ventral ROIs were discarded due to larger boundary effects, resulting in 8 isogravitational ROIs with equal tissue volume along the gravitational axis (Fig. 1).
Fig. 1.
Regions of interest (ROIs) used in the analysis. Computed tomography (CT) three-dimensional reconstructed images of whole lungs (top) and one slice of the lungs (bottom). Horizontal lines represent boundaries of 10 ROIs with equal tissue volume. The most dorsal (1) and the most ventral (10) ROIs were excluded from the analyses due to larger boundary effects. Gray scale (top) represents a three-dimensional light shadow effect. Gray scale (bottom) is based on Hounsfield units, where black = air and white = dense bone.
Regional Aeration Analysis
To characterize aeration distribution of the studied lungs, each ROI was divided into 4 aeration compartments: hyperinflated (−1,000 to −901 HU), normally aerated (−900 to −501 HU), poorly aerated (−500 to −101), and nonaerated (−100 to +100) (13). ROI quantification of each of these compartments was expressed as a fraction of the regional volume of lung tissue (VTisr) VTisr was computed as the sum of the product of the voxel volume (Vvox) and the individual voxel tissue fraction (FTis,vox = 1 − FGas,vox, FGas,vox = voxel gas fraction) for all voxels within the ROI, assuming a tissue density (dimensionless) equal to 1:
| (1) |
where Nvox is the number of voxels in the ROI.
The volume of lung tissue of each aeration compartment (VTis,cptr) within a given ROI was estimated from the voxels composing the compartment as:
| (2) |
where Nvox,cpt is the number of ROI voxels in a given aeration compartment.
From these, the fractional regional volume of lung tissue for each compartment was computed and used for presentation of the results (expressed in %, = 100·VTis,cptr/VTisr). Compartments were quantified at end-inspiration and end-expiration for all ROIs.
Computation of Regional Strain Measures
Regional tidal alveolar strain.
The regional tidal alveolar strain (εALV) measure conceptualizes tidal lung strain as the change in regional volume referred to the regional resting lung volume (i.e., at the end of a complete passive expiration when alveolar pressure reached atmospheric pressure) corrected for recruited gas volume [i.e., both PEEP-induced and tidal recruitment (estimated alveolar strain = VT/gas volume at FRC + recruited gas volume)] (4). εALV was derived from CT images at end-expiration and end-inspiration for all ROIs using the tidal component of the formulation proposed by Caironi et al. (4):
| (3) |
where regional tidal volume (VTr) was computed as the difference between gas volume at the end-inspiratory and end-expiratory CT images within the ROI. Regional gas volume at FRC (VFRCr) was determined experimentally as the regional gas volumes derived from the CT images at end-expiration for PEEP = 0 [in contrast to the original work, which used an extrapolation to PEEP = 0 from images at PEEP = 5 cmH2O (4)]. Regional gas volume recruited tidally and corrected for end-expiration gas fractions (VRec,EE,VTr) and regional gas volume recruited with the application of PEEP (VRec,PEEPr) were determined from measurements of the decrease in volume of nonaerated lung tissue caused by the application of tidal volume (ΔVTis,NA,VTr) and (ΔVTis,NA,PEEPr) in each ROI. ΔVTis,NA,VTr was calculated as the difference between end-expiratory and end-inspiratory regional volumes of nonaerated tissue (i.e., VTis,cptr for the nonaerated compartment at end-expiration and end-inspiration). ΔVTis,NA,PEEPr was calculated as the difference between regional volume of nonaerated tissue at PEEP = 0 (VTis,NA,PEEP0r) end-expiration and the regional volume of nonaerated tissue at end-expiration on the studied PEEP (VTis,NA,PEEPir, PEEPi = 6 and 12 cmH2O and ΔVTis,NA,PEEPr = VTis,NA,PEEP0r − VTis,NA,PEEPir). We assumed that recruited alveoli equilibrate to a level of expansion equivalent to that of other open alveoli within the same ROI for the same PEEP and VT conditions. Accordingly, we computed VRec,EE,VTr as the gas volume that recruited tissue would have contained at the start of the breathing cycle (end-expiration) with a mean regional end-expiratory gas fraction of aerated regions (FGas,EE,Aerr):
| (4) |
And VRec,PEEPr for the studied PEEP levels was calculated as:
| (5) |
where FGas,EE,Aerr in Eqs. 4 and 5 refers to the value corresponding to the specific VT-PEEP combination considered.
Regional-specific volume change.
Regional-specific volume change, represented as εsVol, conceptualizes strain as the tidal change in regional volume referred to the regional gas volume at end-expiration (VGasEEr), i.e, εsVol = VTr/VGasEEr·εsVol] has been formulated in terms of regional gas fractions at end-inspiration (FGasEIr) and end-expiration (FGasEEr) aiming at a measure less sensitive to small registration errors (10). We used this formulation to calculate εsVol in each ROI according to the equation found in Fuld et al. (10):
| (6) |
Regional-specific volume change corrected for tidal recruitment.
This measure, εsVol,Rec, is adjusted εsVol for the presence of tidal recruitment to separate the change in lung volume associated with recruited areas from the change in volume that will produce expansion of areas already open at the end of expiration. εsVol as originally formulated (see above) would result in an overestimation of strain if tidal recruitment occurred because a portion of the gas volume at end-inspiration would be distributed into recruited units causing no additional stretch of units open at end-expiration (the reference volume). To account for this effect, we subtracted the gas volume going into tidally recruited units (VRec,EI,VTr) at end-inspiration from the tidal change in gas volume (VTr). This yields the volumetric strain imposed on continuously aerated regions throughout the breathing cycle (εsVo,lRec), to be differentiated from regions undergoing cyclic recruitment. εsVol,Rec is computed as:
| (7) |
| (8) |
Thus, εsVol,Rec was computed from regional volumes (in contrast to εsVol, computed from gas fractions), with VGas,EEr estimated as the sum of the product between voxel gas fraction and voxel volume within the ROI in the end-expiratory CT images (14). This approach allows for the partition of the regional and total intratidal change in lung volume into that associated with recruited areas (computed as VRec,EI,VTr) from that due to expansion of areas already open at the end of expiration (undergoing strain εsVol,Rec).
Regional tissue-normalized tidal volume.
This measure (VTr/VTisr) expresses the distribution of the regional tidal volume per unit volume of lung parenchymal tissue. If the regional tissue volume (VTisr) was a measure of the regional number of alveolar units, then VTr/VTisr would be proportional to the regional tidal volume per alveolar unit. εsVol, VTr/VTisr can also be expressed in terms of gas fractions:
| (9) |
Estimation of Tidal Recruitment and Tidal Hyperinflation
Tidal lung recruitment and hyperinflation were computed from end-expiratory and end-inspiratory CT images using previously described methods (20). In short, the volume of tissue undergoing tidal recruitment was estimated as the difference in tissue volume of nonaerated regions between end-expiratory and end-inspiratory images. The volume of tissue undergoing tidal hyperinflation was estimated as the difference in tissue volume of hyperinflated regions between end-inspiratory and end-expiratory images. We expressed tidal recruitment and hyperinflation as the fraction of the total regional lung tissue volume, computed as described in Eq. 1.
Statistical Analysis
All data are expressed as the mean ± SD. PEEP levels, VT levels, and position along the vertical axis (ROIs) for all variables were compared by means of a generalized linear model. Global hemodynamic and respiratory data, as well as whole-lung tidal recruitment and hyperinflation, strain measures, and regional aeration differences were tested by pairwise comparisons with paired t-tests, and multiple comparisons were corrected with the Holm-Bonferroni method. For all comparisons a critical P < 0.05 was considered significant [MATLAB and Statistics Toolbox Release 2010a and R Core Team (2013)].
RESULTS
Global measurements of respiratory and hemodynamics were consistent with those of normal lungs (Table 1). As expected, peak, mean, and plateau airway pressures increased with PEEP and VT. For all combinations of VT and PEEP, PaO2 was greater than 400 mmHg, with no differences between individual VT-PEEP combinations in PaO2 or mean arterial pressure. PaCO2 at PEEP = 6 and 12 cmH2O and respiratory system elastances at all PEEP levels were lower for VT = 12 ml/kg than VT = 6 ml/kg.
Table 1.
Gas exchange, hemodynamic, and mechanical respiratory variables
| VT 6 ml/kg |
VT 12 ml/kg |
|||||
|---|---|---|---|---|---|---|
| PEEP 0 | PEEP 6 | PEEP 12 | PEEP 0 | PEEP 6 | PEEP 12 | |
| PaO2, mmHg | 458 ± 41 | 441 ± 28 | 448 ± 14 | 429 ± 73 | 414 ± 44 | 473 ± 49 |
| PaCO2, mmHg | 43.8 ± 4.2 | 51.4*‡ ± 5.5 | 52.3*‡ ± 4.7 | 43.9 ± 3 | 41.2 ± 2.8 | 38† ± 2.5 |
| MAP, mmHg | 93 ± 19 | 97 ± 16 | 97 ± 18 | 104 ± 13 | 102 ± 16 | 95 ± 18 |
| Ers, cmH2O/l | 52 ± 11‡ | 53 ± 13 | 55‡ ± 9 | 43 ± 8 | 43 ± 6 | 39 ± 6 |
| Ppeak, cmH2O | 15.9‡ ± 2.3 | 21.7*‡ ± 2.5 | 28.1†‡ ± 1.8 | 24.3 ± 3 | 29.8* ± 1.9 | 34† ± 2.2 |
| Pmean, cmH2O | 6.9‡ ± 1.2 | 12.7*‡ ± 1.2 | 19.1†‡ ± 0.9 | 10.3 ± 1.4 | 16* ± 0.8 | 21.2† ± 0.9 |
| Pplat, cmH2O | 8.9‡ ± 2 | 15.7*‡ ± 2.2 | 20.7†‡ ± 2.9 | 17.5 ± 3.1 | 21.9* ± 3.3 | 25.5† ± 2.9 |
Data are shown as mean ± SD.
Significantly different from PEEP = 0 cmH2O; †significantly different from PEEP = 0 and 6 cmH2O; ‡significantly different from VT = 12 ml/kg.
VT, tidal volume; PEEP, positive-end expiratory pressure; PaO2, partial pressure of arterial oxygen; PaCO2, partial pressure of carbon dioxide; MAP, mean arterial pressure; Ers, respiratory system elastance; Ppeak, peak inspiratory airway pressure; Pmean, mean inspiratory airway pressure; Pplat, plateau inspiratory airway pressure;
Regional Aeration, Tidal Recruitment, and Hyperinflation
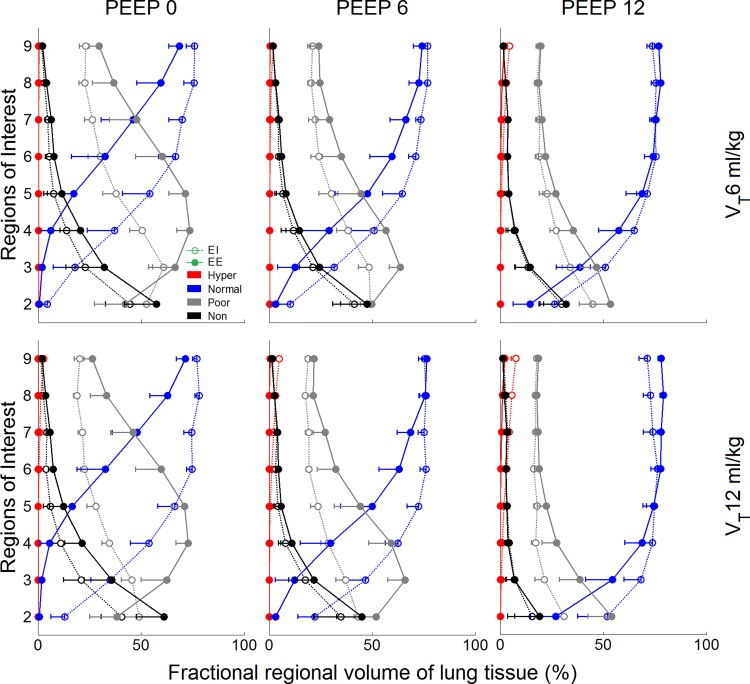
For all VT-PEEP conditions, nondependent ROIs were predominantly composed of normally aerated tissue, followed by poorly aerated, and a small fraction of nonaerated and hyperinflated tissue, both at end-inspiration and end-expiration (Fig. 2). The most dependent ROIs (ROIs 2 and 3) instead presented mostly poorly aerated tissue, followed by nonaerated and a small percentage of normally aerated tissue, which increased with PEEP. Hyperinflated and normally aerated lung tissue corresponded with a larger fraction of lung tissue volume with increased PEEP and VT, whereas poorly aerated and nonaerated tissue decreased in volume with PEEP and VT (Fig. 2). No significant difference between lung tissue volumes at end-inspiration (486 ± 54 ml) and at end-expiration (487 ± 51 ml) was found.
Fig. 2.
Regional distribution of aeration compartments for the studied combinations of positive end-expiratory pressure (PEEP) and tidal volume (VT) levels. The x-axes show the fractional regional volume of lung tissue corresponding to each aeration compartment, and the y-axes shows eight ROIs from the most dependent (ROI 2) to the most nondependent (ROI 9). Open circles represent end-inspiration (EI) data; closed circles represent end-expiration (EE) data. Normally aerated regions (blue) predominated in these normal lungs, mainly in nondependent ROIs. In general, normal aeration increased from exhalation to inhalation. An exception was observed at PEEP = 12 cmH2O, in which normally aerated regions at EE represented a larger fraction of the regional lung tissue volume than at EI for the most nondependent ROIs due to hyperinflation. Poorly aerated regions (gray) predominated on dependent ROIs. Poor aeration decreased with inhalation with the exception of the most dependent ROI (ROI 2), in which poor aeration increased in some cases from exhalation to inhalation due to increase in nonaerated regions. Nonaerated regions (black) usually represented a small fraction of the total lung tissue volume, but it reached ∼60% at PEEP = 0 in the most dependent ROI. It always decreased from exhalation to inhalation. Hyperinflated regions (red) were negligible in most cases with a maximum at nondependent ROIs of 7.5% (PEEP = 12 cmH2O and VT = 12 ml/kg). Data are shown as the mean of all animals.
Poorly aerated tissue had a maximum regional value for PEEP = 0 at midlung levels along the vertical axis, representing 73.5 ± 6.7% of the regional (isogravitational) tissue volume and 28.4 ± 0.4% of whole lung tissue volume (Fig. 2). Normally aerated tissue in nondependent regions ranged between 59.5% and 79.1% of the regional tissue volume (Fig. 2) depending on ventilatory settings (60.4 ± 1.8% whole lung tissue volume). Nonaerated tissue was predominantly localized in dependent regions constituting up to 61.2 ± 14.5% of the regional tissue volume (Fig. 2) at PEEP = 0 cmH2O and VT = 12 ml/kg end-expiration (18.8 ± 5.8% whole lung tissue volume). Hyperinflation was present only in nondependent regions, with a maximum of 7.5 ± 2.3% of the ROI's tissue volume (Fig. 2) at PEEP = 12 cmH2O and VT = 12 ml/kg end-inspiration (1.2 ± 0.4% whole lung tissue volume).
Changes in the percentage of regional lung tissue volume attributed to each aeration compartment from end-expiration to end-inspiration were highest in normally and poorly aerated lung, and increased with VT (Fig. 2). This can be visualized by the distance in the x-axis direction between end-inspiratory and end-expiratory curves for each aeration compartment (Fig. 2). PEEP decreased the tidal changes in regional aeration for all ROIs except for hyperinflated regions. Interestingly, for PEEP = 12 cmH2O at the most nondependent ROIs, normally aerated regions represented slightly higher fractions of regional lung tissue volume at end-expiration than at end-inspiration (Fig. 2). This was due to the increase in hyperinflation during inhalation.
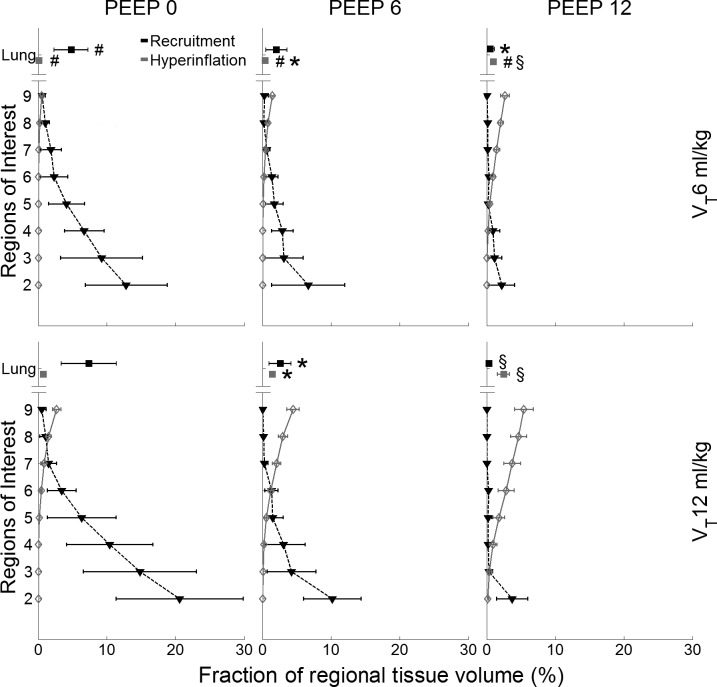
The isolated effect of tidal recruitment is presented in Figure 3 showing a gravitational dependence, with largest values (20.6 ± 9.9% of the regional tissue volume) in the most dependent regions for PEEP = 0 and VT = 12 ml/kg. Substantial dependent tidal recruitment (12.8 ± 6.4% of regional tissue volume) was also observed for PEEP = 0 cmH2O and VT = 6 ml/kg. Tidal hyperinflation increased toward nondependent regions (Fig. 3), with higher values (5.2 ± 1.5% of the regional tissue volume) for PEEP = 12 cmH2O and VT = 12 ml/kg. The magnitude of tidal hyperinflation increased significantly with VT for the entire lung (P < 0.05 for all PEEP levels, Fig. 3). PEEP significantly reduced tidal recruitment, whereas it increased tidal hyperinflation (Fig. 3).
Fig. 3.
Tidal recruitment and tidal hyperinflation for the studied combinations of PEEP and VT levels. The x-axes show the fraction of regional tissue volume corresponding to both measurements, and the y-axes shows eight ROIs from the most dependent (ROI 2) to the most nondependent (ROI 9) and whole lung data (Lung). Tidal recruitment (black) showed the largest values in the most dependent regions for PEEP = 0; whereas tidal hyperinflation (gray) increased toward nondependent regions with highest values for PEEP = 12 cmH2O. Both measurements increased with VT. Data are shown as the mean ± SD of all animals. Global effects: *P < 0.05 vs. PEEP = 0 cmH2O; §P < 0.05 vs. PEEP = 0 and 6 cmH2O; #P < 0.05 VT = 6 vs. 12 ml/kg.
Regional Lung Strain
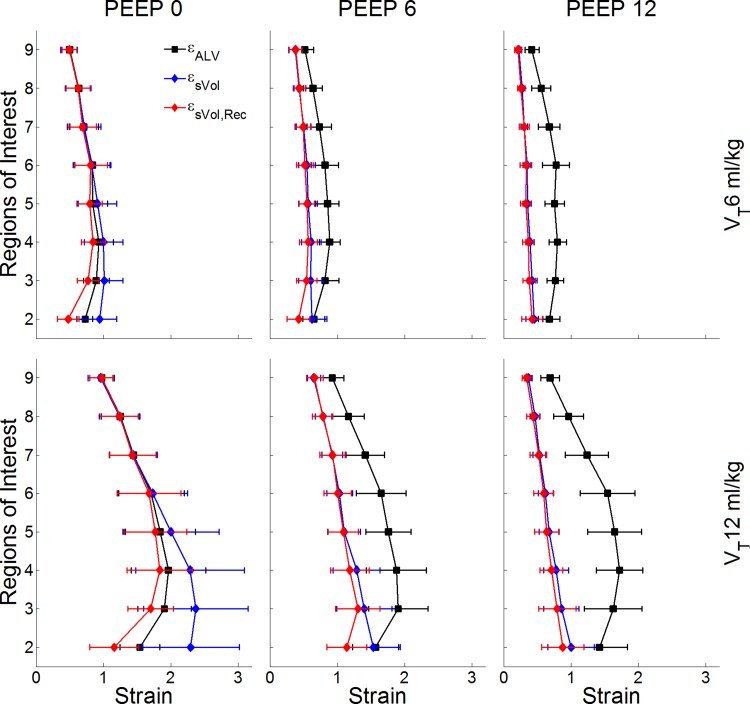
The different strain measurements showed substantially distinct patterns of change with PEEP, VT, and vertical position (Fig. 4). εsVol demonstrated gravitational dependence with increased strain from nondependent ROIs to dependent ROIs (Fig. 4). Instead, while also showing gravitational dependence, εsVol,Rec and εALV presented maxima at mid- to dependent-lung levels along the vertical axis. Maxima at mid to dependent levels was present for εsVol,Rec at conditions of PEEP = 0 and 6 cmH2O, and for εALV in all conditions. This is consistent with the correction for recruited tissue in dependent regions (Fig. 3 and Fig. 5). εsVol,Rec provided a new metric yielding smaller strain values than εsVol, a likely better descriptive of tissue expansion in dependent regions, combined with the relatively similar regional tidal volumes in mid and dependent regions (Fig. 5). Accordingly, εsVol and εsVol,Rec were significantly different in dependent regions at PEEP = 0 (P < 0.001). εALV was significantly different from εsVol and εsVol,Rec at PEEP = 6 and 12 cmH2O for both VTs (P < 0.05). Instead, εALV, εsVol, and εsVol,Rec were equal in nondependent regions at PEEP = 0 for both VTs (Fig. 4), consistent with the absence of tidal recruitment in those regions (Fig. 2), and with the equivalence between end-expiratory volumes and lung volumes at FRC with PEEP = 0.
Fig. 4.
Regional strain estimates for the studied combinations of PEEP and VT levels. The x-axes shows strain values (dimensionless), and the y-axes shows eight ROIs from the most dependent (ROI 2) to the most nondependent (ROI 9). Regional specific volume change (εsVol) in blue demonstrated gravitational dependence with increased strain from nondependent to dependent ROIs, and decrease with increased PEEP. Regional-specific volume change corrected for tidal recruitment (εsVol,Rec, red) and tidal alveolar strain (εALV, black), while also showing gravitational dependence, presented maxima at middependent lung levels. εsVol,Rec decreased with PEEP, whereas εALV increased. For all VT-PEEP conditions, a significant difference was found between methods (P < 0.001), and between ROIs (P < 0.01). Data are shown as the mean ± SD.
Fig. 5.
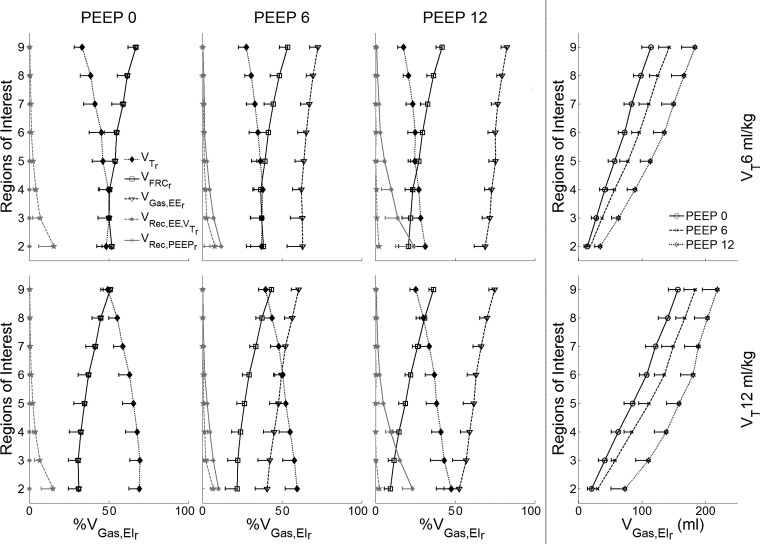
Gas volumes used in the strain calculations for the studied combinations of PEEP and VT levels. Plots in the first three columns (left) depict combinations of VT and PEEP. In these plots, the x-axes represent the gas volume of the depicted gas volume component as a percentage of the regional end-inspiratory gas volume (Vgas,EIr). The y-axes show eight ROIs from the most dependent (ROI 2) to the most nondependent (ROI 9). Represented are the gas volumes corresponding to the regional tidal volume (VTr), regional gas volume at functional residual capacity (VFRCr), regional end-expiratory gas volume (Vgas,EEr), regional gas volume recruited with PEEP (Vrec,PEEPr), and regional gas volume recruited with VT at end-expiration (Vrec,EE,VTr). The rightmost column displays the absolute regional gas volume at end-inspiration in each ROI for the three studied PEEP and VT conditions. Although Vgas,EEr and VFRC are superimposed for PEEP = 0, Vgas,EEr > VFRC as PEEP increases. Data are shown as the mean ± SD of all animals.
Of note, maximum values for εsVol and εsVol,Rec occurred at the same ventilatory settings at PEEP = 0 and VT = 12 ml/kg but on distinct ROIs that were significantly different from other settings (P < 0.001). εsVol maximum was at the most dependent ROI (2.3 ± 0.8). In contrast, εsVol,Rec had its maximum at mid to dependent regions (1.8 ± 0.5). Although εALV also had a numerical maximum at PEEP = 0 and VT = 12 ml/kg (2.0 ± 0.6, Fig. 4) in a middependent region, that value was not significantly different from εALV obtained at larger PEEP values for the same VT. Largest numerical strains values for the different estimates and VT-PEEP condition occurred between regions 2 (most dependent) to 5 (mid lung).
The regional gas volume components of the strain measurements were examined as a fraction of the regional gas volume at end-inspiration, VGas,EIr (Fig. 5). Regional tidal volume, VTr, corresponded to an increasing fraction of VGas,EIr toward dependent regions. Similarly, both recruited gas volumes by tidal volume (VRec,EE,VTr) and by PEEP (VRec,PEEPr) increased from nondependent to depended ROIs (Fig. 5). As expected, VRec,PEEPr increased with applied PEEP, and VRec,EE,VTr with absolute VT. This can be derived from the fact that relative change in VTr with end-inspiratory volume was similar for VT = 6 and 12 ml/kg (three leftmost columns in Fig. 5), combined with the larger end-inspiratory absolute volumes with VT = 12 ml/kg (rightmost column in Fig. 5). Note the presence of substantial recruited gas volume with VT in dependent regions, particularly in conditions with PEEP = 0 and 6 cmH2O, which relate to the regional reduction in the fraction of VT distributed to units continuously open during breathing. Maximum recruited volume by VT (VRec,EE,VTr) occurred in the most dependent regions for conditions of VT = 6 and 12 ml/kg and PEEP = 0 cmH2O (∼15.1% of the end-inspiratory regional volume, Fig. 5), while maximum recruited volume by PEEP (VRec,PEEPr) occurred in the same regions for conditions of VT = 6 and 12 ml/kg and PEEP = 12 cmH2O (∼23.5% of the end-inspiratory regional volume, Fig. 5). Because nondependent regions have larger absolute volumes than dependent regions (rightmost panel in Fig. 5), the absolute distribution of VT results in larger nondependent than dependent intratidal volume changes.
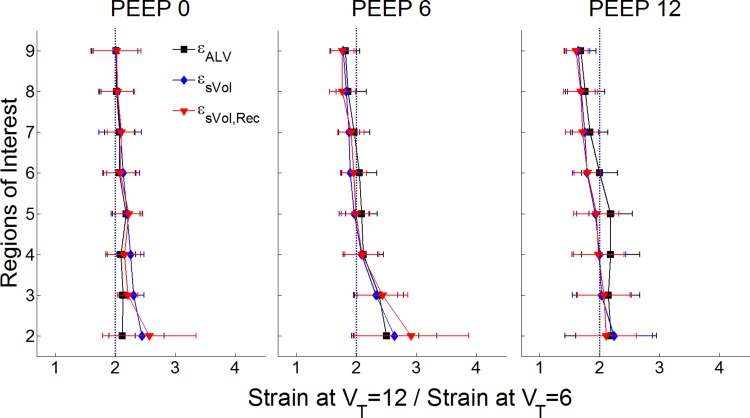
As expected, an increase in VT from 6 to 12 ml/kg produced a significant increase in all regional strain measures (Fig. 4 and 6), approximately doubling them for all ROIs. The degree of gravitational dependence of all strain measures was influenced by VT, with larger vertical gradients for larger VT (P < 0.01). Interestingly, the increase in regional strains produced by an increased VT also showed a gravitational dependence, higher in dependent than in nondependent ROIs for all measures (Fig. 6).
Fig. 6.
VT effect on strain estimates for the studied combinations of PEEP levels. The x-axes show the ratio of the regional strain estimates obtained at VT = 12 and VT = 6 ml/kg. The y-axes show eight ROIs from the most dependent (ROI 2) to the most nondependent (ROI 9). Regional-specific volume change (εsVol, blue) and regional-specific volume change corrected for tidal recruitment (εsVol,Rec, red) showed ratios above 2 in dependent regions particularly at PEEP = 0 and 6 cmH2O. Instead, εALV increased by a factor above 2 in midlung regions for a PEEP = 12 cmH2O. Data are shown as the mean ± SD of all animals.
Changes in PEEP affected predominantly εsVol and εsVol,Rec (Fig. 4). εsVol fell with increased PEEP in all ROIs (P < 0.001) (Fig. 4), in line with its inverse relationship with regional end-expiratory volume (Fig. 5). The same was observed for εsVol,Rec (P < 0.001). Instead, εALV did not change with PEEP. This is consistent with its formulation that relates tidal volume changes to the sum of the resting lung volume, which is consists of the FRC (at PEEP = 0) and the regions recruited by PEEP and the regional VT (Fig. 6), but not by expansion of units already open. PEEP decreased the ratio between regional strains at VT = 12 ml/kg and VT = 6 ml/kg for all strain measures in nondependent ROIs (P < 0.001) and numerically increased that ratio from PEEP = 0 to 6 cmH2O in dependent regions (Fig. 6).
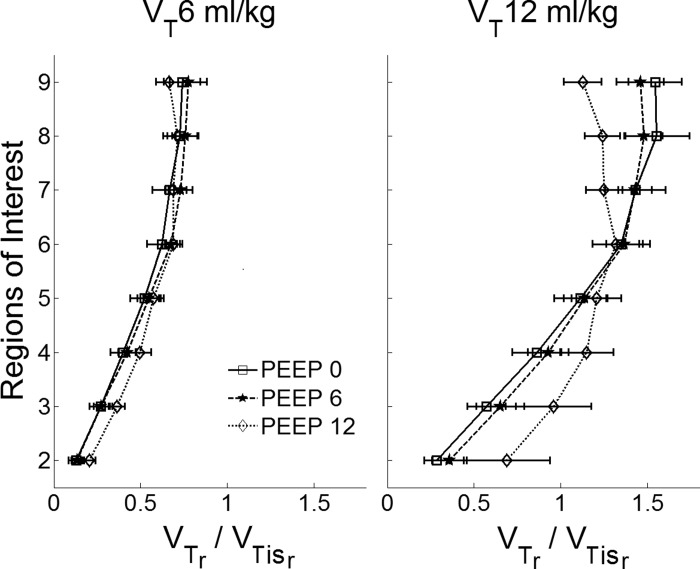
The tissue-normalized regional tidal volume (VTr/VTisr) decreased from non-dependent to dependent regions (Fig. 7) in all conditions. VTr/VTisr was mostly not affected by changes in PEEP, with the exception of PEEP = 12 cmH2O at VT = 12 ml/kg in which VTr/VTisr was approximately constant in nondependent regions and decreased less toward dependent regions compared with those cases for PEEP = 0 and 6 cmH2O (Fig. 7). This is consistent with the more homogeneous distribution of regional tidal volume relative to tissue volume at higher PEEPs (Fig. 7). Overall, changes in VTr/VTisr with PEEP did not reach statistical significance in these normal lungs (P = 0.18). VTr/VTisr significantly increased with VT (Fig. 7) with highest values for low PEEPs on nondependent ROIs.
Fig. 7.
Regional tissue-normalized tidal volume (VTr/VTisr) for VT = 6 and 12 ml/kg. The x-axes show tissue normalized tidal volume (dimensionless), the y-axes show eight ROIs from the most dependent (ROI 2) to the most nondependent (ROI 9). VTr/VTisr decreased from nondependent to dependent regions in all conditions, and was mostly not affected by changes in PEEP. The exception was at PEEP = 12 cmH2O and VT = 12 ml/kg, which homogenized the distribution of VTr/VTisr. Data are shown as the mean ± SD of all animals.
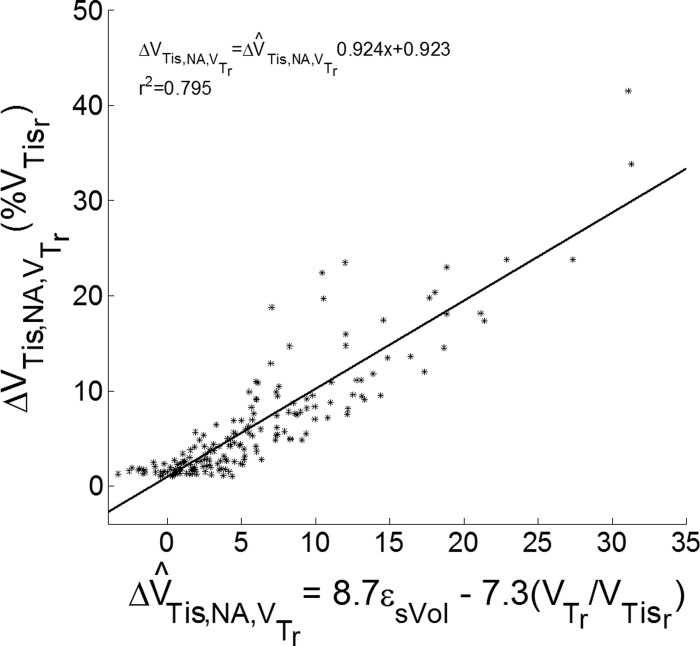
Regional tidal recruitment was highly correlated (r2 = 0.80) with a linear combination of εsVol and VTr/VTisr (Fig. 8). This was computed for data from all animals at all ROIs, PEEP, and VT in regions with tidal recruitment >1% of regional tissue volume.
Fig. 8.
Relationship between experimental and estimated tidal recruitment. Experimental tidal recruitment was computed as the decrease in volume of nonaerated lung tissue caused by the application of tidal volume (ΔVTis,NA,VTr). Values are expressed as a percentage of regional tissue volume (%VTisr). Estimated tidal recruitment (ΔV^T^is,NA,VTr) was calculated using linear regression with specific volume change (εsVol) and regional tissue-normalized tidal volume (VTr/VTisr) as independent variables, and ΔVTis,NA,VTr as dependent variables. Points represent measurements from all animals at all regions of interest, PEEP, and VT with tidal recruitment >1%. r2 = Pearson coefficient of determination.
DISCUSSION
Our main findings in mechanically ventilated pigs with heterogeneous normal lungs with inflation comparable to that of humans are as follows. First, regional tidal strains are distributed heterogeneously along the vertical axis, with maxima at mid to dependent lung regions for all studied estimates suggesting these as regions of highest risk for mechanical injury. Second, the methods to compute regional lung strain result in estimates that are markedly different in magnitude and dependence on VT, PEEP, and location of the ROI. The differences are significant enough to produce, for the same ventilatory conditions, maxima of strain measures at distinct lung regions for the different methods. Third, increases in tidal volumes from 6 to 12 ml/kg approximately doubles regional tidal strains with regional heterogeneity, larger in dependent regions for estimates referenced to end-expiratory volume. Fourth, PEEP homogenizes the spatial distribution of regional strain estimates for methods using the end-expiratory volume of the region of interest as the reference volume while it increases strain heterogeneity when estimates are referenced to FRC. Fifth, regional lung recruitment can be estimated from regional gas fractions at end-inspiration and end-expiration.
Lung Strain
There is increasing evidence that lung strain is an essential variable determining the degree of lung injury resulting from mechanical ventilation (23, 31). The large number of patients with initially normal lungs undergoing mechanical ventilation in operating rooms and the increasing evidence for a relationship between ventilatory settings and postoperative pulmonary complications in surgical patients (17) emphasize the need to understand the determinants of strain in normal lungs.
Whereas the ideal measurement of strain would be performed at the cellular level, this is not feasible in vivo. Accordingly, imaging techniques that can be used with humans have been used to estimate lung volumetric strain. Because the quantitative relationship between strain and lung injury is unknown, it is uncertain what component of strain (e.g., static, tidal) or magnitude range with or without a threshold would best indicate risk for tissue injury. Recent results pointing to the relevance of driving pressure to postoperative pulmonary complications in surgical patients mostly with initially normal lungs (17), as well as to mortality in patients with acute respiratory distress syndrome (2) imply a key role for tidal deformation in producing clinically important lung injury. Indeed, the basic physiological justification for driving pressure as a relevant variable is its meaning as a normalized global strain (i.e., driving pressure = VT/respiratory system compliance) (2, 17). Such results are consistent with previous studies of epithelial cell monolayers indicating a relationship between cyclic stretch and alveolar epithelial permeability (6) and cell death (26). Yet, the effect of chest wall contribution and regional lung mechanical heterogeneity would result in essential differences between regional lung strains and global driving pressure. The importance of such regional measurements of tissue deformation used in our study is exemplified by the fact that two of them have been recently related to severe pulmonary edema (4) and inflammation (31). Our results clearly indicate that those strain metrics are not equivalent. Therefore, it is essential to understand the concept, magnitude, and distribution of those different metrics to identify conditions and lung regions related to risk of injury by tidal expansion.
Although the imaging estimates we studied attempted to assess strain from its physical definition as the deformation of a structure relative to its starting state, they clearly differed in their vertical distribution and change with VT and PEEP. The differences ultimately result from the imaging measurements of volume change and reference volume used in the computations. Overall, a topographically heterogeneous distribution of lung deformation was observed, which implies that during usual ventilatory settings different lung regions are at distinct risk of strain injury. In fact, values reached within the studied settings have been associated with increased lung inflammation (31), particularly with additional exposure to a systemic inflammatory stimulus. The largest values were observed at middependent regions for all strain measures, suggesting these to be regions of largest susceptibility to tidal mechanical injury, consistent with previous observations in initially normal lungs subjected to tidal expansion and systemic inflammation (31). Yet, strain maxima occurred at different lung regions implying a testable effect of the different strain estimates.
Strain Measures
Specific tidal volume change (εsVol).
In previous studies, we showed that εsVol was related to the magnitude of regional metabolic activity, a marker of neutrophilic inflammation, during mechanical ventilation and endotoxemia (31). This implied the relevance of this variable in conditions commonly related to lung injury. In the current study, we show that in normal lungs εsVol is heterogeneously affected by an increase in VT at different PEEP levels with dependent regions presenting higher relative changes in εsVol than nondependent regions. Because the reference lung volume to compute εsVol is the end-expiratory volume, increases in PEEP resulted in reductions in εsVol by increasing end-expiratory volume even if regional elastance was changed. This is consistent with the previous reduction of inflammation with PEEP (9, 32). PEEP also caused the reduction of spatial heterogeneity of lung strain and tidal recruitment consistent with a lung with more uniformly distributed mechanical impedances. The consequent homogeneous expansion occurs not only in large regions but also at the microscale level (5, 19, 30) likely contributing to the beneficial effects of PEEP. Use of end-expiratory volume as a reference volume is partially founded on the presence of lung stress relaxation (10), which allows the lung to achieve a new mechanical state at end-expiration at different PEEP levels.
A limitation of εsVol as originally described (10) is that it does not account for the effect of tidal recruitment on regional lung strain. This results in an overestimation of strains because, in the presence of tidal recruitment, part of the gas volume at end-inspiration is due to recruited regions and not to expansion of regions open at the end-expiratory volume. Importantly, this also means that εsVol is a parameter computed under the assumption that all units are open along the breathing cycle, and implies that the measurement conceptually refers to units aerated at end-expiration, presumed the same as those aerated at end-inspiration.
Recruitment corrected εsVol (εsVol,Rec).
To correct for that tidally recruited gas volume, we introduced the variable εsVol,Rec as the recruitment corrected-specific tidal volume change. This required estimation of εsVol from the CT regional gas volumes instead of from regional gas fractions as occurred in the original CT and PET studies (10, 31). εsVol estimates made on the basis of gas volume can produce errors due to nonlinearity of CT density changes with increasing gas volume (10) and these errors are exacerbated by movement of tissue out of the ROI with lung expansion (16). However, we found no difference between estimates computed with gas volumes and gas fractions in the current study (i.e., εsVol and εsVol,Rec were superimposed in Fig. 4 for nondependent regions). This is due to the use of ROIs with equal tissue volume along the ventral-dorsal axis, which minimize the effect of tissue movement. Such an approach leads to very similar tissue volumes within each ROI at end-inspiration and end-expiration, and consequently, approximately the same tissue being used for measurement in the absence of substantial tissue distortions off the isogravitational plane. Of note, our correction for lung recruitment differed from that proposed by Caironi et al. (4), who presumed that recruited nonaerated lung expands to a gas fraction of 70% at end-inspiration. Instead, we used experimental measurements to estimate the average aeration of recruited regions as the average regional gas fraction of all aerated units (e.g., poorly aerated, normally aerated, and hyperinflated) at end-inspiration.
As expected, introduction of the correction for recruited volume in the studied conditions showed the largest effects in dependent regions during PEEP = 0 cmH2O and VT = 6, and particularly VT = 12 ml/kg conditions. The latter was the condition in which also the largest values for εsVol and εsVol,Rec were observed. The largest regional effect was observed in dependent regions, in which correction for recruited volume resulted in a 44.4 ± 28.0% decrease in the strain estimated for PEEP = 0 cmH2O. At the whole lung level, this corresponded to ∼19% of nonaerated lung tissue volume, which is consistent with dependent lung derecruitment observed in normal humans (15, 25) and animals (21) during general anesthesia and muscle paralysis. In these normal lungs, that correction predominantly reduced the gradient of strain along the ventral-dorsal axis and evidenced maxima still in dependent ROIs, but above the most dependent one. As for εsVol, εsVol,Rec is a measure specifically related to regions open at end-expiration. Yet, differently from εsVol and εALV, εsVol,Rec refers exclusively to continuously aerated regions during tidal breathing. Those undergoing tidal recruitment are topographically characterized and used for the computations, but the εsVol,Rec measure does not apply to them. Accordingly, this approach allows for the partition of these two distinct biomechanical processes—expansion of aerated regions vs. tidal recruitment. In this way, each can be separately used for further investigation of the contribution of those processes to mechanical injury (e.g., for correlation with regional edema or inflammation).
Considering that trends in εsVol and εsVol,Rec along the vertical axis were mostly maintained, our results support the contribution of regional tidal strain to inflammation even when corrected for recruitment. Although tidal recruitment estimated with PET did not appear to contribute additionally to inflammation (31), use of higher-resolution techniques such as PET/CT and synchrotron radiation (3) to quantify tidal recruitment will be needed to more accurately partition the effects of strain and recruitment. Data on the regional association between lung injury and strain measures referenced to the regional resting lung are not available, and its contribution will need to be studied.
Alveolar strain.
A previous study used whole-lung measurements to estimate a so called “alveolar strain” (εALV), which showed a clear relationship with intensity of lung injury, cardiopulmonary instability, and organ failure in initially healthy animals (4). εALV uses FRC at 0 cmH2O airway pressure as its reference gas volume, presumed to represent the resting volume of the lung (4). To this volume is added the gas volume recruited by PEEP and VT to account for tidal recruitment. Consequently, this measure uses as its reference the volume at end-expiration that corresponds to all aerated units at end-inhalation. This contrasts with the exclusive use of aerated end-expiratory units for εsVol and εsVol,Rec. Thus, the formulation of εALV implies that the tidal strain generated during breathing results in equal magnitudes of strain in regions continuously aerated as well as those intratidally recruited. In the present study, we applied the concept to assess tidal strains at the regional level. By using CT to measure regional gas volumes at end-expiration and PEEP = 0 we increased the accuracy of the estimated reference volume compared with the original work, which did not carry out measurements at PEEP = 0 and had to apply an extrapolation to PEEP = 0 from images at PEEP = 5 cmH2O (4). In these normal lungs, we estimated the difference between the experimental and approximated methods to be small (4.5 ± 6.6% for VT = 6 ml/kg, and 0.1 ± 5.8% for VT = 12 ml/kg).
The largest εALV (1.9 ± 0.6) was observed for VT = 12 ml/kg and PEEP = 0 and 6 cmH2O, and at the level of middependent lung regions. This is due to the reference volume of εALV, taken as the regional gas volume present at FRC, combined with the increased regional tidal ventilation in middependent regions for similar global VT produced by PEEP. Note that differently from end-expiratory volume used to compute εsVol, regional gas volume at FRC does not change regionally with PEEP, producing a relationship between εALV and regional VT different from that between εsVol or εsVol,Rec and regional VT. Whereas the association between local εALV and regional lung injury is not known, observation of a reduction in dependent markers of inflammation (18F-fluorodeoxyglucose uptake) with increased PEEP for equal tidal volumes during mechanical ventilation (9, 32) suggests that this measure of strain does not appear to be as sensitive to regional lung injury as εsVol, at least within short time frames and relatively lower tidal volume conditions. The report that whole-lung total alveolar strain in patients with acute respiratory distress syndrome was higher only in patients with a very high percentage of potentially recruitable lung, with no association to mortality (4), suggests that this strain measure may reveal only a portion of relevant strain-related phenomena, and that other strain measures could provide additional insights into the relationship between strain and lung injury.
A critical aspect in the computation of all strain estimates is the determination of a reference volume corresponding to the resting state of lung parenchyma. This determination is hampered by the fact that the lung is always prestressed. Because transpulmonary pressures are not only not zero but they also variable regionally, the resting volume of the lung even at FRC (i.e., at end-expiration with PEEP = 0) does not correspond to the resting state of the parenchyma both at the global and regional levels. An alternative manner to apply that concept, to εALV for instance, would be to consider an average global value as the resting lung volume. In this case, εALV would be increased in nondependent regions and reduced in dependent regions according to values shown in Fig. 5.
Although they are statistically different, the distinct strain measures presented the common features of low-magnitude and homogeneous distribution along the vertical axis for lower VT. This finding may explain why in a recent large, single-center study of a surgical population, predominantly with initially normal lungs, VT had limited relevance for major postoperative respiratory complications (17). Our results also provide insight into the regional strain effects of an increase in driving pressure in the normal lung. The physiological justification for the relevance of the driving pressure to outcomes relies on the concept that it provides an indirect measure of global strain (VT/static compliance of the respiratory system, compliance understood as a measure of lung volume). Figure 6 shows that a VT increase (related to a driving pressure increase) produces a magnification of tidal strains in dependent lung regions for lower PEEP values = 0 and 6 cmH2O beyond the mostly homogeneous and proportional increase of strains with VT. This demonstrates that regional strain changes are not necessarily proportional to global driving changes even in the normal lung.
Regional Tidal Volume Normalized by Tissue Volume
The use of tissue volume instead of lung gas volume as a normalization factor would be advantageous if it could be assumed that tissue volume represented predominantly alveolar units. In this case, VTr/VTisr would represent the deformation of tissue per alveolar unit. We found a clearly distinct pattern in VTr/VTisr compared with the other strain measurements, predominantly with larger values in nondependent regions, increased with VT, and mostly independent from PEEP. Thus, it is a measure also with enough distinct topographical characteristics to allow for testing its relationship to regional injury. Findings of increased glucose phosphorylation rate associated with neutrophilic activation in nondependent regions of sheep with normal lungs subjected to 16 h of mechanical ventilation with physiological tidal volumes suggest the potential relevance of this measure to lung injury (27).
Of note, we showed that tidal-recruited volume can be estimated from εsVol and VTr/VTisr (Fig. 8). This is relevant because estimates of recruited volume as described in the current paper are limited to CT techniques (3, 33). However, other imaging techniques such as PET do not provide a direct estimate of recruited volume, whereas they allow for estimates of εsVol and VTr/VTisr in addition to providing important molecular information. Figure 8 indicates that even in the absence of CT images, availability of measurements of gas and tissue fractions at end-inspiration and end-expiration such as obtained with gated PET (31) could allow for the estimates of recruited volume.
Limitations
The use of static CT images taken during inspiratory and expiratory pauses allows redistribution of gases and recruitment/derecruitment of lung and may incompletely characterize events occurring during dynamic conditions. Also, CT does not allow differentiation between the various sources of density such as collapsed and edematous tissue, and subvoxel recruitment would be measured as a change in aeration. Additionally, although ROIs with equal amounts of tissue divided by coronal planes along the dorsal-ventral axis are easily defined without the need for tracking specific anatomical features and are well established in CT image analysis (4, 27, 29), their use presumes that for different lung volumes, corresponding ROIs contain the same lung tissue. Changes in lung tissue caused by tissue distortion across coronal planes, regional blood volume distribution, and variability of image segmentation could introduce error measurements. Because the vertical axis is a main direction of lung expansion (30) and we used lung tissue volume as the criterion for delimitation of the ROI, most of the ROI volume likely corresponds to the same sample volume of lung parenchyma at the measured time points, supporting an expected small measurement error caused by tissue deformation. Of note, even when different portions of lung tissue cross the boundaries of the ROI, estimates are still accurate if the tissue moving in has similar characteristics to the tissue moving out of the ROI.
We used an incremental VT-PEEP protocol without recruitment maneuvers and with FiO2 = 1.0 to magnify dependent lung collapse and lung expansion heterogeneity, consistent with our goal of generating a model with a wide range of strains and tidal recruitment to be used for comparisons between strain estimates. This model likely resulted in larger inflation heterogeneity at each VT-PEEP condition than what would be obtained if volume history would have been standardized with recruitment maneuvers before each condition and lower FiO2. Of note, stepwise increases in PEEP have been used in recent clinical trials (1, 12, 28), and our experimental conditions would provide an estimate of regional strain distributions in those cases. Finally, changes in lung tissue volume due to edema could have affected comparisons of VT-PEEP conditions. Only the last experimental point showed a small increase in lung tissue volume of 20 ± 22 ml (5% tissue volume) compared with the first time point. The effect of such an increase in tissue volume would have been a small underestimation of PEEP and VT effect on poorly aerated and nonaerated regions. Changes in lung tissue volume directly affecting strain measures would be relevant particularly if observed between end-inspiratory and end-expiratory images. Lung tissue volumes at those time points were essentially equally supporting the quality of the measurements. Considering that there were no systematic changes in lung tissue volume or PaO2/FiO2 throughout the study, the results were normalized to regional absolute tissue volume, and the magnitude of that last tissue volume change was small, we expect a small effect of edema on our results.
Conclusion
In summary, lung strain is spatially heterogeneously distributed in mechanically ventilated normal lungs. This implies that the different lung regions are at different risks for mechanical injury. The maxima for different strain estimates occur at distinct locations along the ventral-dorsal axis and with different dependence on VT-PEEP conditions, offering a quantitatively testable approach to assess the relevance of those specific strain estimates to regional injury. Based on the results, strains referenced to end-expiratory lung volume appear to be relevant for lung injury. Increased VT results in a spatially heterogeneous increase in lung strain, larger in mid- to dependent-lung regions. In contrast, PEEP reduces tidal strain estimates referenced to end-expiratory lung volumes, although it does not affect tidal strain referenced to resting lung volume.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1 HL-121228 (to M. F. Vidal Melo), and by Research Support Foundation of the State of Rio de Janeiro, Brazilian National Council for Scientific and Technological Development, and German Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.F.S.d.C.P., M.G.d.A., A.R.C., and M.F.V.M. conception and design of research; P.M.S., A.G., M.G.d.A., and A.R.C. performed experiments; L.F.S.d.C.P., T.J.W., P.M.S., A.G., M.F.V.M., and A.R.C. analyzed data; L.F.S.d.C.P., T.J.W., T.W., J.G.V., A.R.C., and M.F.V.M. interpreted results of experiments; L.F.S.d.C.P. prepared figures; L.F.S.d.C.P. and M.F.V.M. drafted manuscript; L.F.S.d.C.P., T.J.W., T.W., J.G.V., A.R.C., and M.F.V.M. edited and revised manuscript; L.F.S.d.C.P., T.W., and M.F.V.M. approved final version of manuscript.
REFERENCES
- 1.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338: 347–354, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372: 747–755, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Bayat S, Porra L, Albu G, Suhonen H, Strengell S, Suortti P, Sovijarvi A, Petak F, Habre W. Effect of positive end-expiratory pressure on regional ventilation distribution during mechanical ventilation after surfactant depletion. Anesthesiology 119: 89–100, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Caironi P, Cressoni M, Chiumello D, Ranieri M, Quintel M, Russo SG, Cornejo R, Bugedo G, Carlesso E, Russo R, Caspani L, Gattinoni L. Lung opening and closing during ventilation of acute respiratory distress syndrome. Am J Respir Crit Care Med 181: 578–586, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Carney DE, Bredenberg CE, Schiller HJ, Picone AL, McCann UG, Gatto LA, Bailey G, Fillinger M, Nieman GF. The mechanism of lung volume change during mechanical ventilation. Am J Respir Crit Care Med 160: 1697–1702, 1999. [PubMed] [Google Scholar]
- 6.Cavanaugh KJ, Cohen TS, Margulies SS. Stretch increases alveolar epithelial permeability to uncharged micromolecules. Am J Physiol Cell Physiol 290: C1179–C1188, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen TS, Cavanaugh KJ, Margulies SS. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur Respir J 32: 854–861, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Cohen TS, Gray Lawrence G, Khasgiwala A, Margulies SS. MAPK activation modulates permeability of isolated rat alveolar epithelial cell monolayers following cyclic stretch. PLoS One 5: e10385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Prost N, Costa EL, Wellman T, Musch G, Tucci MR, Winkler T, Harris RS, Venegas J, Kavanagh B, Vidal Melo MF. Effects of ventilation strategy on distribution of lung inflammatory cell activity. Crit Care 17: R175, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuld MK, Easley RB, Saba OI, Chon D, Reinhardt JM, Hoffman EA, Simon BA. CT-measured regional specific volume change reflects regional ventilation in supine sheep. J Appl Physiol 104: 1177–1184, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Fung YC. Stress, deformation, and atelectasis of the lung. Circ Res 37: 481–496, 1975. [DOI] [PubMed] [Google Scholar]
- 12.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant JY, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin JE, Pereira B, Jaber S; IMPROVE Study Group . A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 369: 428–437, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354: 1775–1786, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 164: 1701–1711, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Hedenstierna G, Rothen HU. Respiratory function during anesthesia: effects on gas exchange. Compr Physiol 2: 69–96, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Kaczka DW, Simon BA, Thompson BT. Regional lung strain and inflammation. Am J Respir Crit Care Med 185: 228–230, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Ladha K, Vidal Melo MF, McLean DJ, Wanderer JP, Grabitz SD, Kurth T, Eikermann M. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 351: h3646, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 28: 596–608, 1970. [DOI] [PubMed] [Google Scholar]
- 19.Mertens M, Tabuchi A, Meissner S, Krueger A, Schirrmann K, Kertzscher U, Pries AR, Slutsky AS, Koch E, Kuebler WM. Alveolar dynamics in acute lung injury: heterogeneous distension rather than cyclic opening and collapse. Crit Care Med 37: 2604–2611, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 164: 122–130, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Protti A, Andreis DT, Milesi M, Iapichino GE, Monti M, Comini B, Pugni P, Melis V, Santini A, Dondossola D, Gatti S, Lombardi L, Votta E, Carlesso E, Gattinoni L. Lung anatomy, energy load, and ventilator-induced lung injury. Intensive Care Med Exp 3: 34, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Protti A, Andreis DT, Monti M, Santini A, Sparacino CC, Langer T, Votta E, Gatti S, Lombardi L, Leopardi O, Masson S, Cressoni M, Gattinoni L. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med 41: 1046–1055, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Protti A, Cressoni M, Santini A, Langer T, Mietto C, Febres D, Chierichetti M, Coppola S, Conte G, Gatti S, Leopardi O, Masson S, Lombardi L, Lazzerini M, Rampoldi E, Cadringher P, Gattinoni L. Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med 183: 1354–1362, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Sutherasan Y, Vargas M, Pelosi P. Protective mechanical ventilation in the non-injured lung: review and meta-analysis. Crit Care 18: 211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tokics L, Hedenstierna G, Strandberg A, Brismar B, Lundquist H. Lung collapse and gas exchange during general anesthesia: effects of spontaneous breathing, muscle paralysis, and positive end-expiratory pressure. Anesthesiology 66: 157–167, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 162, 2 Pt 1: 357–362, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Tucci MR, Costa EL, Wellman TJ, Musch G, Winkler T, Harris RS, Venegas JG, Amato MB, Vidal Melo MF. Regional lung derecruitment and inflammation during 16 hours of mechanical ventilation in supine healthy sheep. Anesthesiology 119: 156–165, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valente Barbas CS. Lung recruitment maneuvers in acute respiratory distress syndrome and facilitating resolution. Crit Care Med 31, 4 Suppl: S265–S271, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Venegas JG, Melo MF. Measurement of regional specific lung volume change using respiratory-gated PET of inhaled 13N-nitrogen. J Nucl Med 51: 646–653, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Venegas JG, Vidal Melo MF. Effect of regional lung inflation on ventilation heterogeneity at different length scales during mechanical ventilation of normal sheep lungs. J Appl Physiol 113: 947–957, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Zheng H, Venegas JG, Vidal Melo MF. Effect of local tidal lung strain on inflammation in normal and lipopolysaccharide-exposed sheep. Crit Care Med 42: e491–e500, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Venegas J, Vidal Melo MF. Ventilation heterogeneity is reduced with application of PEEP in healthy supine sheep. Am J Respir Crit Care Med 183: A3214, 2011. [Google Scholar]
- 33.Wrigge H, Zinserling J, Muders T, Varelmann D, Gunther U, von der Groeben C, Magnusson A, Hedenstierna G, Putensen C. Electrical impedance tomography compared with thoracic computed tomography during a slow inflation maneuver in experimental models of lung injury. Crit Care Med 36: 903–909, 2008. [DOI] [PubMed] [Google Scholar]