Abstract
Secreted hormones play pivotal roles in tissue cross talk to maintain physiologic blood glucose and lipid levels. We previously showed that C1q/TNF-related protein 12 (CTRP12) is a novel secreted protein involved in regulating glucose metabolism whose circulating levels are reduced in obese and insulin-resistant mouse models. Its role in lipid metabolism, however, is unknown. Using a novel heterozygous mouse model, we show that the loss of a single copy of the Ctrp12 gene (also known as Fam132a and adipolin) affects whole body lipid metabolism. In Ctrp12 (+/−) male mice fed a control low-fat diet, hepatic fat oxidation was upregulated while hepatic VLDL-triglyceride secretion was reduced relative to wild-type (WT) littermates. When challenged with a high-fat diet, Ctrp12 (+/−) male mice had impaired lipid clearance in response to acute lipid gavage, reduced hepatic triglyceride secretion, and greater steatosis with higher liver triglyceride and cholesterol levels. Unlike male mice, Ctrp12 (+/−) female mice fed a control low-fat diet were indistinguishable from WT littermates. When obesity was induced by high-fat feeding, Ctrp12 (+/−) female mice developed mild insulin resistance with impaired insulin tolerance. In contrast to male mice, hepatic triglyceride secretion was increased in Ctrp12 (+/−) female mice fed a high-fat diet. Thus, in different dietary and metabolic contexts, loss of a single Ctrp12 allele affects glucose and lipid metabolism in a sex-dependent manner, highlighting the importance of genetic and environmental determinants of metabolic phenotypes.
Keywords: adipokine, lipid metabolism, triglyceride secretion, obesity, diabetes
systemic energy metabolism is regulated by both central and peripheral mechanisms (13). In the periphery, secreted hormones play especially critical roles in tissue crosstalk to maintain blood glucose and lipid levels within physiological range (33, 34). Dysregulated glucose and/or lipid metabolism is causally linked to metabolic disorders such as Type 2 diabetes mellitus (T2DM) and dyslipidemia (35, 40). Adipokines, an important class of secreted proteins with metabolic functions, are produced by adipose tissue (33, 36). Circulating adipokines (e.g., leptin, adiponectin, resistin, RBP4, TNF-α) regulate metabolism either directly, by acting on metabolic tissues (e.g., liver and skeletal muscle), or indirectly, by modulating local and systemic inflammatory responses (16, 18, 33, 34, 36). The circulating levels and functions of adipokines are frequently disrupted by obesity, thereby contributing to and exacerbating obesity-linked insulin resistance, T2DM, dyslipidemia, and cardiovascular diseases.
To better understand tissue cross talk and hormonal control of energy balance, we have characterized an evolutionarily conserved family of secreted plasma proteins of the C1q family, referred to as C1q/TNF-related proteins (CTRP1-15) (3, 31, 39, 50–55). Using genetic gain- and loss-of-function mouse models, as well as the infusion of recombinant protein, we have previously demonstrated important metabolic functions for several members of the CTRP family (3, 4, 19, 26–30, 32, 38, 39, 48).
CTRP12 (encoded by the FAM132A gene) was identified on the basis of shared sequence homology with other previously characterized CTRP family members (49, 50). Enomoto et al. (10) also identified CTRP12/adipolin as an adipose-enriched transcript downregulated in obesity. In humans, CTRP12 is predominantly expressed by adipose tissue; in mice, it is more widely expressed (10, 50). Expression of CTRP12 is downregulated in mouse models of obesity and diabetes (10, 50); conversely, its expression is markedly upregulated in mouse models with enhanced insulin sensitivity (i.e., Krüppel-like factor 3-knockout mice) (2). In healthy lean human subjects, induction of the hyperinsulinemic state by insulin infusion increases circulating levels of CTRP12 (45). In vivo, as well as ex vivo, exposure of human adipose tissue explants to the anti-diabetic drugs rosiglitazone and metformin also increases the expression and secretion of CTRP12 (43–45). Intriguingly, humans with a deletion spanning chromosome 1p36.33, which includes the CTRP12/FAM132A locus, develop hyperphagia and obesity (5, 6, 11, 56).
Functional studies in mice suggest that CTRP12 is an adipokine with antidiabetic (50) and anti-inflammatory (10) activities. We showed that CTRP12 acts via insulin-dependent and -independent pathways to control glucose metabolism in liver and adipose tissue (50). A short-term overexpression of CTRP12 improves insulin sensitivity in wild-type mice, as well as in genetic (ob/ob) and dietary models of obesity and diabetes (10, 50). Independently of insulin, CTRP12 stimulates glucose uptake in adipocytes and suppresses glucose output in hepatocytes (50).
In humans, CTRP12 (FAM132A) gene lies within the Chromosome 1p36.33 locus, a region commonly deleted in monosomy 1p36 (15). A subset of these patients present with a Prader-Willi-like phenotypes that includes obesity, hyperphagia, and in some cases, T2DM resulting from hyperinsulinemia and impaired glucose tolerance (5, 41). While a larger patient sample size is needed for high-resolution mapping of the genes responsible for the obesity phenotype, it is beneficial to examine how the loss of one copy of a gene within the deleted locus, such as CTRP12, might contribute to the metabolic phenotypes seen in 1p36 monosomy. While a short-term CTRP12 overexpression improves glucose metabolism in mice (10, 50), the effects of CTRP12 deficiency in whole body lipid metabolism has not been previously studied. Therefore, using a novel heterozygous mouse model, we sought to address how partial deficiency of CTRP12 expression would alter systemic glucose and lipid metabolism. Our results reveal a novel and previously unappreciated role for CTRP12 in modulating hepatic and systemic lipid metabolism.
MATERIALS AND METHODS
Animals.
The CTRP12 heterozygous (+/−) mouse strain was generated on a C57BL/6 background via homologous recombination in ES cells by the Mouse Biology Program at UC Davis. The Ctrp12 (also known as Fam132a) gene, located on mouse chromosome 4, contains eight exons. To create a null allele, a gene trap cassette with a splice acceptor was inserted between exons 1 and 2, disrupting the gene. Genotyping primers for the Ctrp12 wild-type (WT) allele were: forward 5′-CTGTCCATTCCACAGGCTGCTG-3′ (within intron 1) and reverse 5′-CCTTGGGCCTTCGTGAAAATGACC-3′ (within intron 1). The expected size of the WT band was 250 bp. Genotyping primers for the Ctrp12 targeting vector were: forward (LacZ) 5′-GGTAAACTGGCTCGGATTAGGG-3′ and reverse (LacZ) 5′-TTGACTGTAGCGGCTGATGTTG-3′. The expected size of the null allele was 211 bp. To confirm that CTRP12 mRNA expression was reduced in Ctrp12 (+/−) mice, we performed quantitative PCR analysis on cDNA isolated from gonadal and inguinal adipose depots and kidneys using the following primer pair: 5′-GCGTGTGGATTCCCCCAATA-3′ (forward) and 5′-CCGTCGGACAAAGTTCAACC-3′ (reverse). All mice used in this study were generated by crossing Ctrp12 (+/−) to WT C57BL6/J mice. Ctrp12 (+/−) mice and WT (+/+) littermate controls were housed in polycarbonate cages on a 12 h light-dark photocycle with ad libitum access to water and food. Littermates were used throughout the study as WT controls. Mice were fed a high-fat diet (HFD; 60% kcal derived from fat, Research Diets #D12492) or a matched control low-fat diet (LFD; 10% kcal derived from fat, Research Diets #D12450B). Diet was provided for a period of 24 wk, beginning between 5 and 6 wk of age. At termination of the study, animals were fasted for 4 h and euthanized. At this time, tissues were collected, snap-frozen in liquid nitrogen, and kept at −80°C until analysis. All animal protocols were submitted to and approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Body composition analysis.
Body composition analyses for fat and lean mass were performed on mice at 25–28 wk using Echo-MRI-100 (Echo Medical Systems, Waco, TX) at the Johns Hopkins University School of Medicine mouse phenotyping core facility.
Indirect calorimetry.
HFD- and LFD-fed Ctrp12 (+/−) and WT (+/+) mice (n = 7–12/group) at 25–28 wk of age were used for simultaneous assessments of daily body weight change, food intake (corrected for spillage), physical activity, and whole body metabolic profile in the Comprehensive Laboratory Animal Monitoring System (CLAMS) system (Columbus Instruments). Data were collected for 3–4 days to confirm that mice were acclimated to the calorimetry chambers (indicated by stable body weights, food intake, and diurnal metabolic patterns), and data were analyzed from the 4th day onward. Rates of oxygen consumption (V̇o2; ml·kg−1·h−1) and carbon dioxide production (V̇co2; ml·kg−1·h−1) in each chamber were measured every 24 min throughout the studies. Respiratory exchange ratio (RER = V̇co2/V̇o2) was calculated by CLAMS software (version 4.02) to estimate relative oxidation of carbohydrates (RER = 1.0) vs. fats (RER ∼0.7), not accounting for protein oxidation. Energy expenditure (EE) was calculated as EE = V̇o2 × [3.815 + (1.232 × RER)] (kcal·kg−1·h−1) (24) and normalized for lean body mass (kcal·lean kg−1·h−1) as recommended (46). Physical activities were measured by infrared beam breaks in the metabolic chamber. Average metabolic values were calculated per subject and averaged across subjects for statistical analysis by Student's t-test.
Quantitative PCR analysis.
Total RNA was isolated from mouse tissues with TRIzol (Life Technologies, Carlsbad, CA). We reverse-transcribed 2 μg of total RNA using GoScript reverse transcriptase (Promega, Madison, WI). We used 10 ng of cDNA in each quantitative PCR (qPCR) reaction with SYBR Green PCR master mix on a CFX Connect system (Bio-Rad Laboratories, Hercules, CA). Results were analyzed by the 2−ΔΔCt method (37). Real-time PCR data were normalized to 36B4 (adipose tissue) or β-actin (liver, kidney, and skeletal muscle) levels. Primer sequences for genes related to lipid uptake, synthesis, and oxidation are shown in Table 1.
Table 1.
Primers used in quantitative real-time PCR analyses
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| Acc | TGACAGACTGATCGCAGAGAAAG | TGGAGAGCCCCACACACA |
| Fasn | GCTGCGGAAACTTCAGAAAAT | AGAGACGTGTCACTCCTGGACTT |
| Gpat1 | CAACACCATCCCCGACATC | GTGACCTTCGATTATGCGATCA |
| Lipc | ATGGGAAATCCCCTCCAAATCT | GTGCTGAGGTCTGAGACGA |
| Lrp1 | GACCAGGTGTTGGACACAGATG | AGTCGTTGTCTCCGTCACACTTC |
| Ldlr | CGCGGATCTGATGCGTCGCT | CGGCCCTGGCAGTTCTGTGG |
| Sort1 | CCCGGACTTCATCGCCAAG | AGGACGAGAATAACCCCAGTG |
| Pcsk9 | TTGCAGCAGCTGGGAACTT | CCGACTGTGATGACCTCTGGA |
| ApoE | CTGACAGGATGCCTAGCCG | CGCAGGTAATCCCAGAAGC |
| Cd36 | ATGGGCTGTGATCGGAACTG | GCTCCGTACAGAGTGTAGCAAG |
| Fatp5 | GTTCTCCCGTCCAAGACCATT | GCTCCGTACAGAGTGTAGCAAG |
| Hnf-4 | CACGCGGAGGTCAAGCTAC | CCCAGAGATGGGAGAGGTGAT |
| Ppar-γ | CCAGAGTCTGCTGATCTGCG | GCCACCTCTTTGCTCTGCTC |
| Fxr | GCTTGATGTGCTACAAAAGCTG | CGTGGTGATGGTTGAATGTCC |
| Srebp1c | GGAGCCATGGATTGCACATT | GGCCCGGGAAGTCACTGT |
| Acox1 | AGATTGGTAGAAATTGCTGCAAA | ACGCCACTTCCTTGCTCTTC |
| Cpt1a | CACCAACGGGCTCATCTTCTA | CAAAATGACCTAGCCTTCTATCGAA |
| Lcad | TCTTTTCCTCGGAGCATGACA | GACCTCTCTACTCACTTCTCCAG |
| Pgc1 | CAGCCTCTTTGCCCAGATCT | CCGCTAGCAAGTTTGCCTCA |
| Ppar-α | ACAAGGCCTCAGGGTACCA | GCCGAAAGAAGCCCTTACAG |
| aP2 | CCGCAGACGACAGGA | CTCATGCCCTTTCATAAACT |
| Lpl | CCCTGAAGACACAGCTGAGG | GGCTGTACCCTAAGAGGTGG |
| Vldlr | GAGCCCCTGAAGGAATGCC | CCTATAACTAGGTCTTTGCAGATATGG |
| Atgl | TGTGGCCTCATTCCTCCTAC | TCGTGGATGTTGGTGGAGCT |
| Hsl | GCTGGGCTGTCAAGCACTGT | GTAACTGGGTAGGCTGCCAT |
| B-actin | GGCACCACACCTTCTACAATG | GGGGTGTTGAAGGTCTCAAAC |
| 36B4 | AGATTCGGGATATGCTGTTGGC | TCGGGTCCTAGACCAGTGTTC |
Glucose tolerance test.
Mice 18–19 wk of age that had been fed their respective diet for 12–13 wk were fasted overnight (12–16 h) before glucose injection. Glucose (Sigma) was reconstituted in saline (0.9 g NaCl/l) and injected intraperitoneally (ip) into mice at a dose of 1 mg/g body wt. Blood glucose was measured at 0, 15, 30, 60, and 120 min after glucose injection using a glucometer (NovaMax Plus, Billerica, MA). Serum was collected from the tail vein at 0 and 15 min postinjection into Microvette CB 300 tubes (Sarstedt). Serum insulin levels during the glucose tolerance tests were measured using an ELISA kit (Millipore, Billerica, MA).
Insulin and pyruvate tolerance test.
Insulin tolerance tests were performed on mice at 18 wk of age that had been fed their respective diet for 12 wk. Pyruvate tolerance tests were performed on mice that were 20 wk of age that had been fed their respective diet for 14 wk. Food was removed 2 h before insulin injection. Insulin was injected ip at a dose of 1.0 U/kg ip (LFD) or 1.5 U/kg (HFD) body wt. Blood glucose was measured at the indicated time points using a glucometer (NovaMax Plus). To measure glucose output during a pyruvate tolerance test, mice were fasted overnight for 16 h before sodium pyruvate injection. Sodium pyruvate (Sigma) was reconstituted in saline and injected ip into mice at a dose of 1 g/kg body wt. Blood glucose was measured at the indicated time points with a glucometer.
Lipid tolerance test.
Lipid tolerance tests were performed on mice at 22 wk of age, after 16 wk on their respective diets. Mice were fasted overnight for 12–16 h before receiving an oral gavage of Intralipid (20% lipid emulsion soybean oil, Sigma) at a dose of 10 μl/g body wt. Serum was collected at 0, 1, 2, 3, and 4 h postgavage via tail bleed, and serum lipid species were measured as described below.
Blood and tissue chemistry analysis.
Tail vein blood samples were allowed to clot on ice and were then centrifuged for 10 min at 10,000 g. Serum samples were stored at −80°C. Serum triglycerides (TG) and cholesterol were measured according to the manufacturer's instructions using an Infinity kit (Thermo Fisher Scientific, Middletown, VA). Nonesterified free fatty acids (NEFA) were measured using a Wako kit (Wako Chemicals, Richmond, VA). Serum β-hydroxybutyrate (ketone) concentrations were measured with a StanBio Liquicolor kit (StanBio Laboratory, Boerne, TX). To measure tissue TG and cholesterol content, frozen tissue was weighed and homogenized in water and an equal amount of homogenate was used for lipid extraction using the Folch method (12). The chloroform layer was dried in a Speedvac and resuspended in Triton X-100 (5% in saline, vol/vol). TG and cholesterol measurements were normalized to total protein content.
TG/VLDL secretion assay (poloxamer 407 injections).
Poloxamer injections were administered to mice at 26 wk of age, after 20 wk on the diet. Food was removed for 4 h, beginning 2 h into the light cycle. Poloxamer 407 (Sigma) was solubilized in saline at 4°C overnight, then injected ip into mice at a dose of 1,000 mg/kg 6 h into the light cycle. Serum was collected at 0, 1, 2, 4, and 8 h (LFD) or at 0, 1, 2, 6, and 9 h (HFD) via tail vein.
Statistical analyses.
All results are expressed as means ± SE. Statistical analysis was performed with Prism 5 software (GraphPad). Data were analyzed with two-tailed Student's t-tests or by repeated-measures ANOVA. Values were considered to be significant at P < 0.05.
RESULTS
Generation of Ctrp12 (+/−) mice.
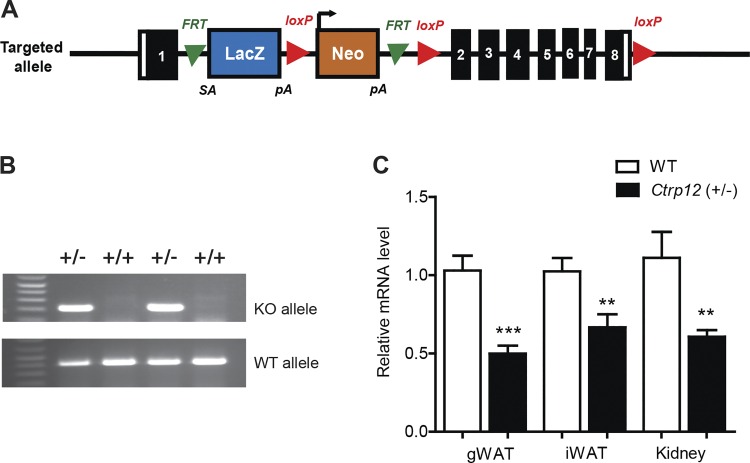
To generate a heterozygous mouse model for CTRP12, which better models the human condition compared with a full knockout, we disrupted a single copy of the Ctrp12 gene (also known as Fam132a and adipolin) by inserting a promoter-less LacZ reporter with a splice acceptor downstream of exon 1 via standard homologous recombination (Fig. 1A). Expression of the LacZ reporter was driven by the endogenous Ctrp12 gene promoter. Full-length mouse CTRP12 comprises 308 amino acids; Ctrp12 exon 1 encodes the first 53 amino acids that make up the signal peptide and a very small portion of the NH2-terminal region. Splicing of exon 1 to a LacZ reporter containing a polyadenylation signal on the 3′-end yields a severely truncated polypeptide if translated. Genotypes of mice carrying one copy of the targeting vector were verified by PCR; the heterozygotes were crossed to WT mice to generate mice heterozygous at the Ctrp12 locus (+/−) and WT (+/+) littermates (Fig. 1B). Ctrp12 (+/−) mice were born at the expected Mendelian frequencies, with no apparent gross phenotype. In the mouse, CTRP12 is most abundantly expressed in adipose tissue and kidney. qPCR analysis on gonadal and subcutaneous adipose depots as well as kidney showed that, in Ctrp12 (+/−) mice, Ctrp12 mRNA levels were reduced ∼50% compared with their WT littermates (Fig. 1C).
Fig. 1.
Generation of CTRP12 heterozygous mice. A: schematic of the gene trap targeting the Ctrp12 allele. B: representative agarose gels displaying the PCR products of the Ctrp12 wild-type (WT) and targeted [knockout (KO)] alleles for mouse genotyping. C: relative mRNA expression levels of Ctrp12 in gonadal (gWAT) and subcutaneous (inguinal; iWAT) adipose tissue and kidney (n = 8–11 per group). Real-time PCR data were normalized to 36B4 (adipose tissue) or β-actin (Kidney). **P < 0.01; ***P < 0.005.
Whole body metabolic parameters of Ctrp12 (+/−) male mice fed an LFD.
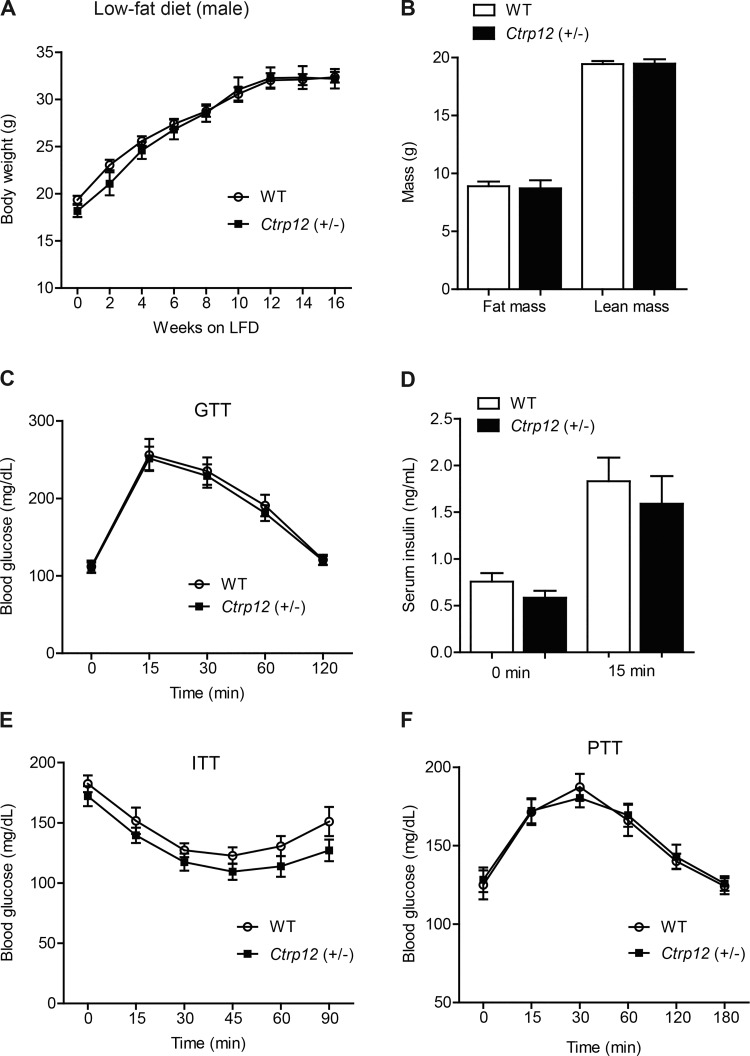
Loss of a single copy of the Ctrp12 gene did not affect body weight or composition in heterozygous male mice fed a control LFD. We monitored body weights over the course of 16 wk and observed no differences in body weight, total fat, or lean mass in Ctrp12 (+/−) male mice compared with WT littermates (Fig. 2, A and B). We also observed no changes in food intake, metabolic rate (V̇o2 and V̇co2), EE, or physical activity levels between WT and Ctrp12 (+/−) male mice (Table 2). Organ weights did not differ between genotypes (see Table 4).
Fig. 2.
Body weight, body composition, and whole body glucose metabolism in low fat diet (LFD)-fed WT and Ctrp12 (+/−) male mice. A: body weight gain over time in WT (+/+) and Ctrp12 (+/−) littermates. B: NMR measurements of total lean and fat mass in WT and Ctrp12 (+/−) mice at 20 wk of age. C: blood glucose levels of WT and Ctrp12 (+/−) at 0, 15, 30, 60, and 120 min following glucose injection in a glucose tolerance test (GTT). D: serum insulin levels of WT and Ctrp12 (+/−) mice at 0 and 15 min postglucose injection. E: blood glucose levels of WT and Ctrp12 (+/−) mice at 0, 15, 30, 45, 60, and 90 min following 1 U/kg body wt insulin injection in an insulin tolerance test (ITT). F: blood glucose levels of WT and Ctrp12 (+/−) mice at 0, 15, 30, 60, 120, and 180 min following injection of 1 g/kg body wt sodium pyruvate in a pyruvate tolerance test (PTT). (n = 9–14 per group.)
Table 2.
Food intake, oxygen consumption, carbon dioxide production, RER, energy expenditure, and physical activity levels in WT and Ctrp12 (+/−) male and female mice fed LFD
| LFD | Male |
Female |
||
|---|---|---|---|---|
| WT (n = 10) | +/− (n = 7) | WT (n = 8) | +/− (n = 7) | |
| Food intake, g | 4.87 ± 0.39 | 4.58 ± 0.19 | 3.46 ± 0.16 | 3.60 ± 0.52 |
| V̇o2, ml·kg−1·h−1 | 2,968.48 ± 120.25 | 3,016.37 ± 139.41 | 3,858.23 ± 47.0 | 3,613.79 ± 199.88 |
| V̇co2, ml·kg−1·h−1 | 2,696 ± 115.56 | 2,728.63 ± 117.1 | 3,711.45 ± 70.97 | 3,427.3 ± 206.09 |
| RER (V̇o2/V̇co2) | 0.90 ± 0.0094 | 0.9 ± 0.11 | 0.96 ± 0.013 | 0.95 ± 0.028 |
| Energy expenditure, kcal·kg−1·h−1 | 14.64 ± 0.60 | 14.87 ± 0.67 | 19.29 ± 0.25 | 18.01 ± 0.99 |
| Physical activity, beam breaks | 40,006 ± 3,307 | 38,679 ± 3,431 | 57,588 ± 8,009 | 55,756 ± 9,800 |
CTRP12, C1q/TNF-related protein 12; LFD, low-fat diet; RER, respiratory exchange ratio; WT, wild type; V̇o2, oxygen consumption; V̇co2, carbon dioxide production.
Table 4.
Organ weights of WT and Ctrp12 (+/−) male mice fed LFD or HFD
| Male Mice | LFD | HFD | ||
|---|---|---|---|---|
| WT (n = 11) | +/− (n = 13) | WT (n = 16) | +/− (n = 17) | |
| Kidney, g | 0.17 ± 0.004 | 0.17 ± 0.004 | 0.2 ± 0.0087 | 0.18 ± 0.0068 |
| Liver, g | 1.79 ± 0.084 | 1.75 ± 0.090 | 2.71 ± 0.17 | 2.53 ± 0.16 |
| Liver/BW | 0.048 ± 0.002 | 0.047 ± 0.002 | 0.055 ± 0.0029 | 0.052 ± 0.0020 |
| eWAT, g | 0.83 ± 0.048 | 0.78 ± 0.056 | 0.98 ± 0.042 | 1.011 ± 0.047 |
| iWAT, g | 0.59 ± 0.040 | 0.57 ± 0.041 | 1.138 ± 0.047 | 1.146 ± 0.036 |
BW, body weight; eWAT, epididymal white adipose tissue; iWAT, inguinal white adipose tissue.
Impact of CTRP12 partial deficiency on glucose homeostasis in male mice fed an LFD.
Short-term administration of recombinant CTRP12 to mice improves glucose tolerance and insulin sensitivity (50). Loss of a single Ctrp12 allele, however, did not affect glucose disposal in peripheral tissues in a glucose tolerance test (Fig. 2C). Neither overnight fasting insulin levels nor glucose-induced insulin secretion were different between WT and Ctrp12 (+/−) male mice (Fig. 2D). Insulin sensitivity, as judged by insulin tolerance tests, was comparable between WT and Ctrp12 (+/−) mice (Fig. 2E). Pyruvate tolerance tests were performed to assess hepatic glucose output in response to overnight fasting; no differences were observed between genotypes (Fig. 2F).
Altered hepatic lipid metabolism in Ctrp12 (+/−) male mice fed an LFD.
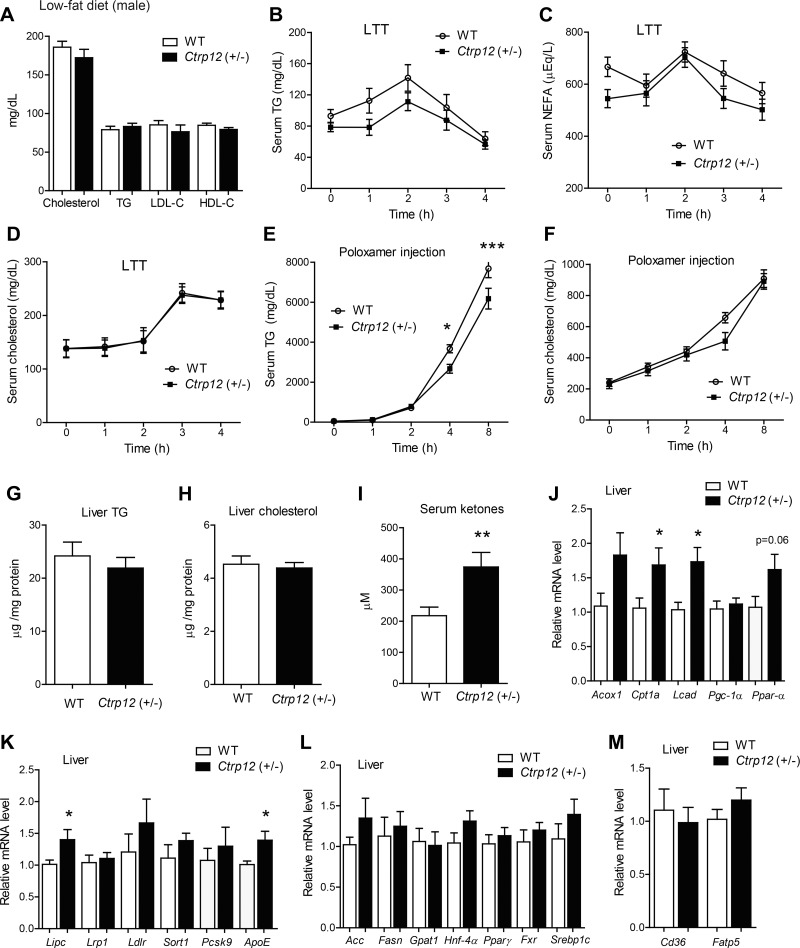
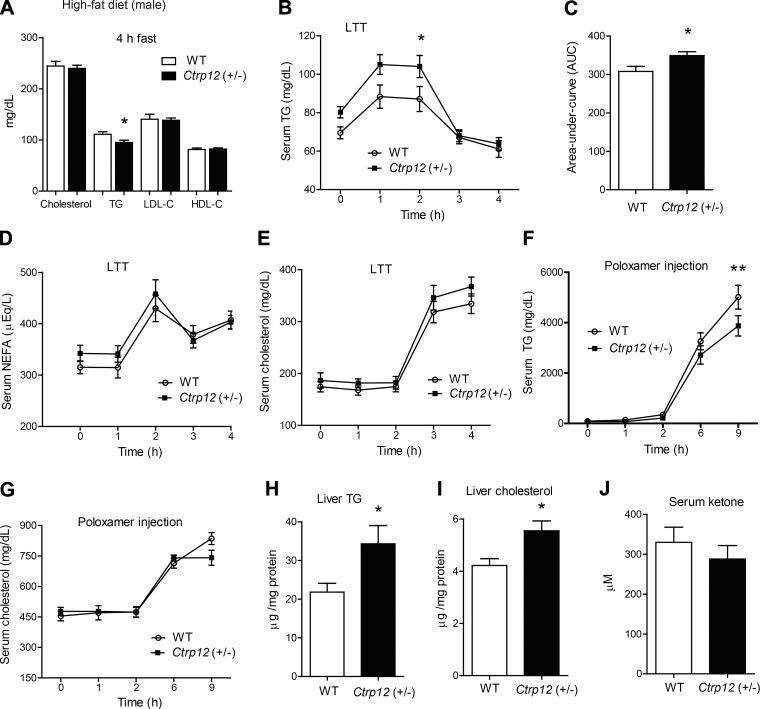
Next, we addressed the impact of CTRP12 partial deficiency on systemic lipid metabolism. Measurements of steady-state serum cholesterol, TG, LDL-cholesterol, and HDL-cholesterol levels in mice fasted for 4 h revealed no differences between WT and Ctrp12 (+/−) animals (Fig. 3A). Following an overnight (16 h) fast, serum NEFA levels were significantly lower in Ctrp12 (+/−) mice relative to WT controls (667 ± 37 vs. 544 ± 35 μeq/l, P < 0.05). To determine if the ability to clear postprandial lipids was altered in Ctrp12 (+/−) mice, we performed a lipid tolerance test where mice were given an oral gavage of Intralipid following an overnight fast, and serum TG and free fatty acid levels were subsequently measured at various time points. No differences in circulating levels of TGs, free fatty acids, or cholesterol were observed between genotypes over the course of 4 h following oral lipid gavage (Fig. 3, B–D). Hepatic triglyceride secretion, in the form of VLDL-TG, can be quantified when lipid uptake in peripheral tissues is inhibited with Poloxamer 407 (an inhibitor of lipoprotein lipase activity) (25). Interestingly, we found that Poloxamer-injected Ctrp12 (+/−) mice had significantly lower TG secretion compared with WT littermates at the 4 and 8 h time points (Fig. 3E), despite comparable levels of serum cholesterol (Fig. 3F). Liver TG and cholesterol content, however, were similar between WT and Ctrp12 (+/−) mice (Fig. 3, G and H). Serum ketone (β-hydroxybutyrate) levels, a marker for hepatic fat oxidation, were significantly increased in fasted (4 h) Ctrp12 (+/−) mice (Fig. 3I). Consistent with enhanced hepatic fat oxidation, expression of fat oxidation genes (Cpt1a, Lcad, and Ppar-α) was also upregulated in the liver of Ctrp12 (+/−) animals (Fig. 3J). Furthermore, we observed a modest, but significant, increase in the expression of genes involved in VLDL clearance, such as hepatic lipase (Lipc and ApoE) (Fig. 3K). A combination of increased fat oxidation and VLDL clearance may result in lower VLDL-TG export from the liver of Ctrp12 (+/−) mice. In contrast to fat oxidation genes, the expression of genes involved in lipid synthesis (Acc, Fasn, Gpat1, Hnf-4α, Ppar-γ, Srebp1c) and uptake (Cd36 and Fatp5) were unchanged in the livers of WT and Ctrp12 (+/−) male mice (Fig. 3, L and M).
Fig. 3.
Lipid metabolism in LFD-fed WT and Ctrp12 (+/−) male mice. A: serum total cholesterol, triglyceride (TG), LDL-cholesterol, and HDL-cholesterol levels in WT and Ctrp12 (+/−) mice following a 4 h food removal. Serum levels of TG (B), nonesterified free fatty acids (NEFA, C), and cholesterol (D) in WT and Ctrp12 (+/−) male mice at 0, 1, 2, 3, and 4 h following an oral gavage of 20% Intralipid in a lipid tolerance test (LTT). Serum levels of TG (E) and cholesterol (F) in WT and Ctrp12 (+/−) male mice at 0, 1, 2, 4, and 8 h following injection of Poloxamer 407 (1 g/kg body wt). Liver TG (G) and cholesterol (H) in 4 h fasted WT and Ctrp12 (+/−) male mice. I: serum ketone (β-hydroxybutyrate) levels in WT and Ctrp12 (+/−) male mice fasted for 4 h. Quantitative PCR analysis of hepatic genes involved in fat oxidation (J), VLDL clearance (K), lipid synthesis (L), and lipid uptake (M) in WT and Ctrp12 (+/−) male mice. All quantitative PCR data were normalized to β-actin levels (n = 10–13 per group). *P < 0.05; **P < 0.01; ***P < 0.005.
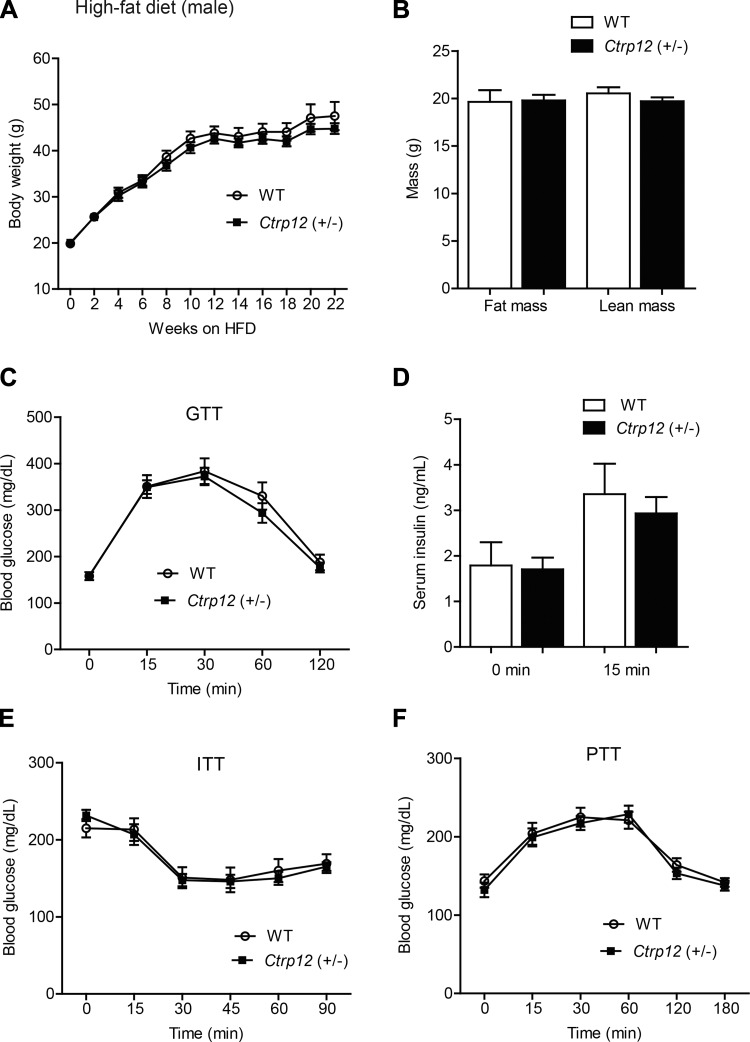
Whole body metabolic parameters of Ctrp12 (+/−) male mice challenged with an HFD.
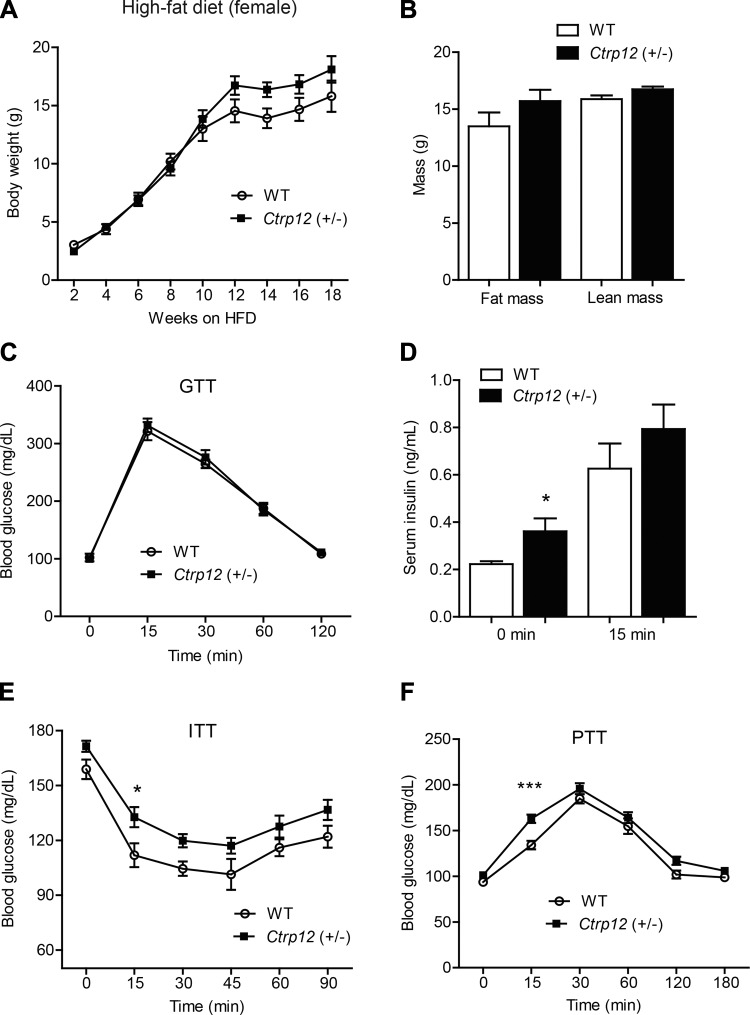
Circulating levels of CTRP12 have been shown to be reduced in diet-induced obese mouse models (10, 50); conversely, administration of the antidiabetic drug metformin to humans increases CTRP12 serum levels (44). To assess the impact of CTRP12 partial deficiency on the pathophysiological state of obesity and insulin resistance, we placed Ctrp12 (+/−) and WT littermates on an HFD for 12 wk. No differences were observed between genotypes in body weight (Fig. 4A), body composition (Fig. 4B), glucose tolerance (Fig. 4C), glucose-induced insulin secretion (Fig. 4D), insulin tolerance (Fig. 4E), or pyruvate tolerance (Fig. 4F). WT and Ctrp12 (+/−) mice were also not different in food intake, metabolic rate (V̇o2 and V̇co2), energy expenditure, physical activity levels, or organ weights (Tables 3 and 4).
Fig. 4.
Body weight, body composition, and whole body glucose metabolism in high fat diet (HFD)-fed WT and Ctrp12 (+/−) male mice. A: body weight gain over time in WT and Ctrp12 (+/−) male mice. B: NMR analysis of total lean and fat mass in WT and Ctrp12 (+/−) male mice at 20 wk of age. C: blood glucose levels of WT and Ctrp12 (+/−) male mice at 0, 15, 30, 60, and 120 min following glucose injection in a glucose tolerance test (GTT). D: serum insulin levels of WT and Ctrp12 (+/−) male mice at 0 and 15 min postglucose injection. E: blood glucose levels of WT and Ctrp12 (+/−) male mice at 0, 15, 30, 45, 60, and 90 min following insulin (1 U/kg body wt) injection in an insulin tolerance test (ITT). F: blood glucose levels of WT and Ctrp12 (+/−) male mice at 0, 15, 30, 60, 120, and 180 min following sodium pyruvate (1 g/kg body wt) injection in a pyruvate tolerance test (PTT) (n = 7–9 per group).
Table 3.
Body weight, food intake, oxygen consumption, carbon dioxide production, RER, energy expenditure, and physical activity levels in WT and Ctrp12 (+/−) male and female mice fed HFD
| HFD | Male |
Female |
||
|---|---|---|---|---|
| WT (n = 11) | +/− (n = 12) | WT (n = 7) | +/− (n = 10) | |
| Food intake, g | 2.54 ± 0.14 | 2.46 ± 0.09 | 2.04 ± 0.17 | 1.93 ± 0.15 |
| V̇o2, ml·kg−1·h−1 | 2,313.75 ± 87.10 | 2,259.0 ± 29.10 | 2,902.38 ± 70.27 | 2,653.22 ± 100.23 |
| V̇co2, ml·kg−1·h−1 | 1,736.82 ± 60.87 | 1,725.76 ± 25.05 | 2,124.55 ± 47.29 | 1,940.20 ± 75.46 |
| RER (V̇o2/V̇co2) | 0.75 ± 0.0039 | 0.76 ± 0.0059 | 0.73 ± 0.0053 | 0.73 ± 0.0059 |
| Energy expenditure, kcal·kg−1·h−1 | 10.97 ± 0.41 | 10.74 ± 0.14 | 13.69 ± 0.32 | 12.51 ± 0.47 |
| Physical activity, beam breaks | 28,000 ± 2,332 | 25,040 ± 1,396 | 46,581 ± 4,193 | 42,495 ± 5,935 |
HFD, high-fat diet.
Altered hepatic lipid metabolism in Ctrp12 (+/−) male mice fed an HFD.
In LFD-fed Ctrp12 (+/−) male mice, hepatic fat oxidation was increased and TG secretion was reduced. We subsequently examined the impact of diet-induced obesity on lipid metabolism in Ctrp12 (+/−) mice. In 4 h fasted mice, serum TG levels were significantly lower in Ctrp12 (+/−) mice compared with WT controls (Fig. 5A). When subjected to a lipid tolerance test, circulating TG levels were significantly higher in Ctrp12 (+/−) mice gavaged with a bolus of emulsified Intralipid, indicative of impaired clearance of postprandial lipids (Fig. 5, B and C). Circulating levels of free fatty acids and cholesterol were not different between genotypes in the lipid tolerance test (Fig. 5, D and E). As with the LFD-fed male mice, hepatic TG secretion was also significantly reduced at 9 h following injection of Poloxamer 407 in Ctrp12 (+/−) mice, while serum cholesterol levels were comparable between genotypes (Fig. 5, F and G). Given that hepatic TG secretion was reduced in Ctrp12 (+/−) mice, we determined if hepatic TG and cholesterol content would be altered in these animals. Indeed, liver TG and cholesterol levels were significantly elevated in Ctrp12 (+/−) mice compared with WT littermates (Fig. 5, H and I). Fasting serum ketone levels, a marker of hepatic fat oxidation, were similar between WT and Ctrp12 (+/−) mice (Fig. 5J). Unlike the LFD-fed male mice, we observed no differences in the expression of genes involved in lipid synthesis (Acc, Fasn, Ppar-γ, Srebp1c, Hnf-4α), oxidation (Acox1, Cpt1a, Lcad, Ppar-α), uptake (Cd36), or clearance (ApoE and Sort1) in the liver of Ctrp12 (+/−) mice (data not shown). Hepatic expression of proinflammatory genes (Il-1β, Il-6, Tnf-α) was also not different between genotypes (data not shown).
Fig. 5.
Whole body lipid metabolism in HFD-fed WT and Ctrp12 (+/−) male mice. A: serum total cholesterol, triglyceride (TG), LDL-cholesterol, and HDL-cholesterol levels in WT and Ctrp12 (+/−) male mice. Food was removed 4 h before serum collection. Serum levels of TG (B) and the corresponding area under curve (C), NEFA (D), and total cholesterol (E) in WT and Ctrp12 (+/−) male mice at 0, 1, 2, 3, and 4 h following an oral gavage of 20% Intralipid in a lipid tolerance test (LTT). Mice were overnight fasted (16 h) before oral lipid gavage. Serum levels of TG (F) and total cholesterol (G) in WT and Ctrp12 (+/−) male mice at 0, 1, 2, 6, and 9 h following injection of Poloxamer 407 (1 g/kg body wt). Food was removed 4 h before Poloxamer injection. Liver TG (H) and cholesterol (I) levels in WT and Ctrp12 (+/−) male mice. J: serum ketone levels in 4 h fasted WT and Ctrp12 (+/−) male mice (n = 11–16 per group). *P < 0.05; **P < 0.01.
Metabolic parameters of Ctrp12 (+/−) female mice fed an LFD.
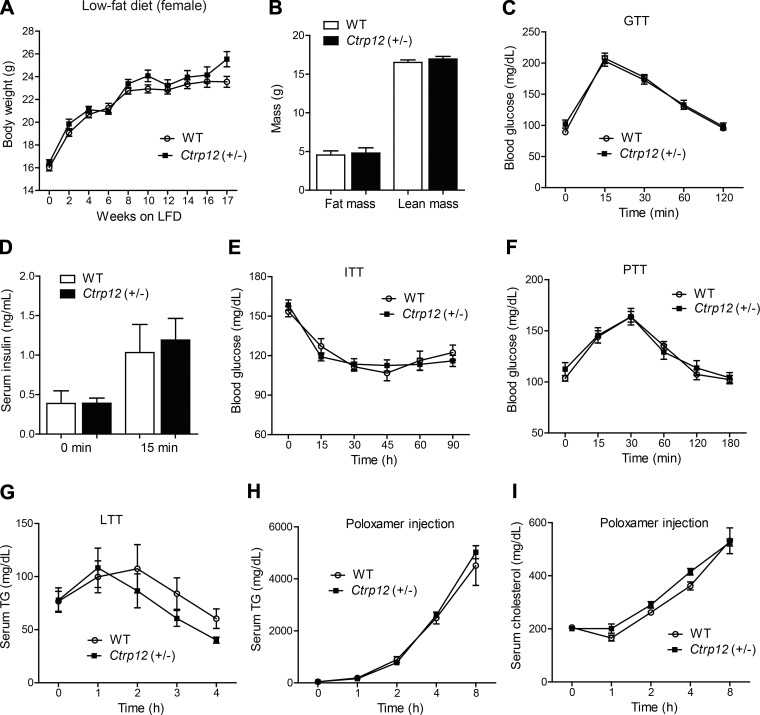
The metabolic responses to changes in energy intake and hormone levels in mice and humans can vary significantly depending on sex (9, 23, 47). To determine whether sex influences the phenotypic outcomes, we also included LFD-fed and HFD-fed female mice in our analyses. As with the male mice, no differences in body weight or body composition were noted between WT and Ctrp12 (+/−) female mice fed a control LFD (Fig. 6, A and B). Other metabolic parameters, food intake, metabolic rate (V̇o2 and V̇co2), EE, and physical activity levels, as well as organ weights, were also comparable between genotypes (Tables 2 and 5). We did not observe any differences between LFD-fed WT and Ctrp12 (+/−) female mice in glucose tolerance (Fig. 6C), glucose-stimulated insulin secretion (Fig. 6D), insulin tolerance (Fig. 6E), pyruvate tolerance (Fig. 6F), lipid tolerance (Fig. 6G), or hepatic TG secretion (Fig. 6, H and I).
Fig. 6.
Body weight, body composition, and whole body glucose and lipid metabolism in LFD-fed WT and Ctrp12 (+/−) female mice. A: body weight gain over time of WT and Ctrp12 (+/−) female mice. B: NMR analysis of total lean and fat mass in WT and Ctrp12 (+/−) female mice at 20 wk of age. C: blood glucose levels of WT and Ctrp12 (+/−) females at 0, 15, 30, 60, and 120 min following glucose injection in a glucose tolerance test (GTT). D: serum insulin levels of WT and Ctrp12 (+/−) female mice at 0 and 15 min postglucose injection. E: blood glucose levels of WT and Ctrp12 (+/−) female mice at 0, 15, 30, 45, 60, and 90 min following insulin (1 U/kg body wt) injection in an insulin tolerance test (ITT). F: blood glucose levels of WT and Ctrp12 (+/−) female mice at 0, 15, 30, 60, 120, and 180 min following sodium pyruvate (1 g/kg body wt) injection in a pyruvate tolerance test (PTT). Serum levels of TG (G) and NEFA (H) in WT and Ctrp12 (+/−) female mice at 0, 1, 2, 3, and 4 h following an oral gavage of 20% Intralipid in a lipid tolerance test (LTT). I: serum levels of TG in WT and Ctrp12 (+/−) female mice at 0, 1, 2, 6, and 9 h following injection of Poloxamer 407 (1 g/kg body wt). Food was removed 4 h before Poloxamer injection (n = 10–13 per group).
Table 5.
Organ weights of WT and Ctrp12 (+/−) female mice fed LFD or HFD
| Female Mice | LFD |
HFD |
||
|---|---|---|---|---|
| WT (n = 12) | +/− (n = 10) | WT (n = 10) | +/− (n = 13) | |
| Kidney, g | 0.12 ± 0.0026 | 0.13 ± 0.004 | 0.133 ± 0.0050 | 0.14 ± 0.0042 |
| Liver, g | 1.19 ± 0.056 | 1.19 ± 0.080 | 1.24 ± 0.042 | 1.31 ± 0.066 |
| Liver/BW | 0.046 ± 0.001 | 0.046 ± 0.002 | 0.030 ± 0.00087 | 0.033 ± 0.0014 |
| eWAT, g | 0.38 ± 0.025 | 0.35 ± 0.031 | 1.59 ± 0.096 | 1.68 ± 0.12 |
| iWAT, g | 0.26 ± 0.019 | 0.26 ± 0.023 | 0.92 ± 0.061 | 0.93 ± 0.0078 |
Reduced insulin sensitivity in Ctrp12 (+/−) female mice fed an HFD.
We next examined the impact of diet-induced obesity in female mice fed an HFD for 12 wk. Body weight, body composition, and glucose tolerance were comparable between WT and Ctrp12 (+/−) female mice (Fig. 7, A–C). Other metabolic parameters (Table 3) and organ weights (Table 5) were also similar between genotypes. However, Ctrp12 (+/−) female mice fed an HFD had reduced insulin sensitivity, as indicated by higher fasting insulin levels (Fig. 7D), as well as reduced glucose clearance in insulin tolerance tests (Fig. 7E). Consistent with mild insulin resistance, hepatic glucose output during a pyruvate tolerance test was also higher at the 15 min time point in Ctrp12 (+/−) mice compared with WT controls (Fig. 7F).
Fig. 7.
Body weight, body composition, and whole body glucose metabolism in HFD-fed WT and Ctrp12 (+/−) female mice. A: body weight gain over time in WT and Ctrp12 (+/−) female mice. B: NMR analysis of total lean and fat mass in WT and Ctrp12 (+/−) female mice at 20 wk of age. C: blood glucose levels of WT and Ctrp12 (+/−) females at 0, 15, 30, 60, and 120 min following glucose injection in a glucose tolerance test (GTT). D: serum insulin levels of WT and Ctrp12 (+/−) female mice measured at 0 and 15 min postglucose injection. E: blood glucose levels of WT and Ctrp12 (+/−) female mice during at 0, 15, 30, 45, 60, and 90 min following insulin (1 U/kg body wt) injection in an insulin tolerance test (ITT). F: area under curve of data shown in E. F: blood glucose levels of WT and Ctrp12 (+/−) female mice at 0, 15, 30, 60, 120, and 180 min following sodium pyruvate (1 g/kg body wt) injection in a pyruvate tolerance test (PTT).(n = 7–13 per group). *P < 0.05; ***P < 0.005.
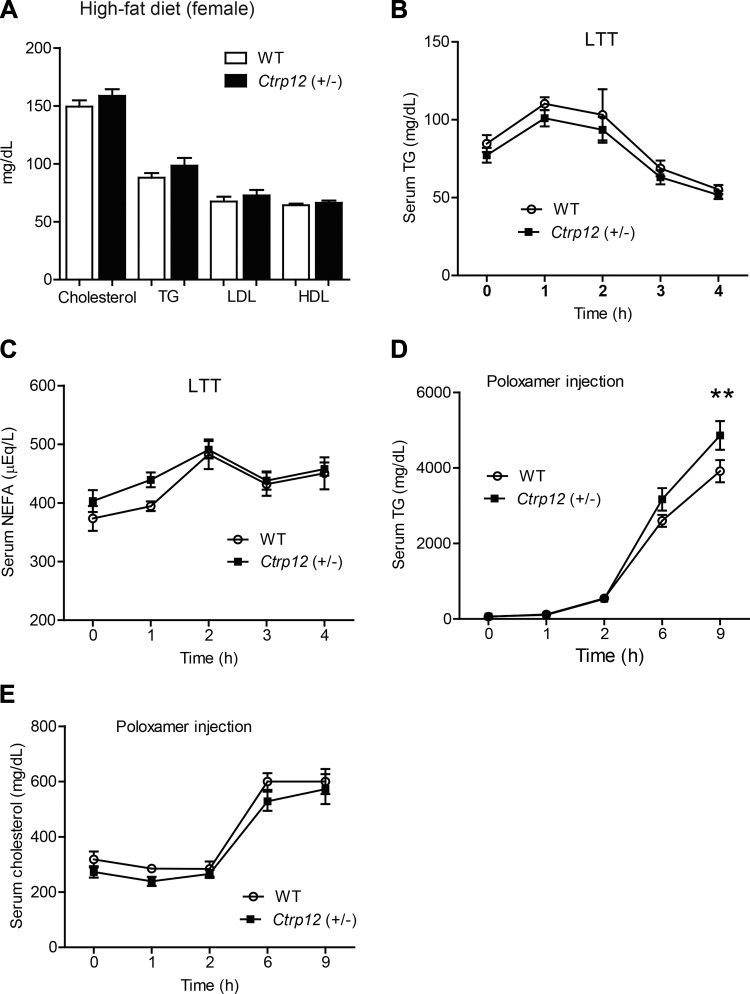
Increased hepatic TG secretion in Ctrp12 (+/−) female mice fed an HFD.
Unlike the HFD-fed male mice, serum total cholesterol, TGs, HDL, and LDL-cholesterol levels were not different between WT and Ctrp12 (+/−) female mice fed an HFD (Fig. 8A). Furthermore, the ability to handle an acute lipid gavage in a lipid tolerance test was unaffected in Ctrp12 (+/−) female mice (Fig. 8, B and C). In striking contrast to male mice, we observed enhanced hepatic TG, but not cholesterol, secretion in Poloxamer-injected Ctrp12 (+/−) female mice (Fig. 8, D and E).
Fig. 8.
Whole body lipid metabolism in HFD-fed WT and Ctrp12 (+/−) female mice. A: serum total cholesterol, TG, LDL-cholesterol, and HDL-cholesterol levels in WT and Ctrp12 (+/−) female mice. Food was removed 4 h before serum collection. Serum levels of TG (B) and NEFA (C) in WT and Ctrp12 (+/−) female mice at 0, 1, 2, 3, and 4 h following an oral gavage of 20% Intralipid in a lipid tolerance test (LTT). Serum levels of TG (D) and total cholesterol (E) in WT and Ctrp12 (+/−) female mice at 0, 1, 2, 6, and 9 h following Poloxamer 407 (1 g/kg body wt) injection. Food was removed 4 h before Poloxamer injection (n = 10–15). **P < 0.01.
DISCUSSION
Previous gain-of-function mouse models employing adenoviral overexpression (10, 50), as well as correlative human studies (43–45), have implicated CTRP12 in regulating glucose metabolism. The role of CTRP12 in modulating lipid metabolism, however, is unknown; furthermore, the impact of its deficiency on whole body metabolism has also not been studied. Complete loss of function (homozygous mutations) of any given gene in the human genome is relatively rare, whereas partial loss of function of one copy of a gene is much more common in humans (14, 20). One notable example is monosomy 1p36, which occurs ∼1 in every 5,000 births (15), wherein varying lengths of DNA are deleted from one copy of the p36.33 locus on Chromosome 1. In addition to the congenital physical dysmorphisms, deletion of this locus, which includes the CTRP12 (Fam132a) gene, also results in obesity and impaired glucose tolerance in a subset of 1p36 monosomy patients (7, 41). To understand the contribution of the loss of a single copy of CTRP12 to syste mic lipid and glucose metabolism, we sought to address the impact of partial CTRP12 deficiency on metabolic homeostasis using a novel mouse model heterozygous for Ctrp12 gene.
While there were no overall differences in gross morphology, body weight, body composition, or organ size, loss of a single Ctrp12 allele resulted in modest, but significant, changes in lipid metabolism in male mice. Notably, hepatic VLDL-TG secretion was reduced in male mice that were fed either a control LFD or challenged with an HFD to induce obesity. Several mechanisms could account for the observed phenotypes in Ctrp12 (+/−) male mice: reduced free fatty acid flux from adipose tissue to liver, decreased lipid synthesis, impaired VLDL assembly, or increased hepatic fatty acid oxidation. In LFD-fed Ctrp12 (+/−) male mice, fasting levels of serum free fatty acids were lower, suggesting that reduced hepatic TG secretion may be partly attributed to decreased influx of fatty acids into liver. No changes in lipolytic gene (Atgl and Hsl) expression, however, were noted in the adipose tissue of Ctrp12 (+/−) male mice (data not shown). Increased fatty acid oxidation, indicated by elevated serum ketone levels (a direct marker of hepatic fat oxidation) and upregulated expression of fat oxidation genes (Cpt1a, Lcad, Ppar-α), in the liver of Ctrp12 (+/−) male mice could, in part, account for the reduced TG secretion. Enhanced hepatic fat oxidation is independent of AMPK activation, as no differences were observed in the levels of phospho-AMPK in the liver of WT and Ctrp12 (+/−) male mice (data not shown). Interestingly, the expression of hepatic lipase (Lipc), ApoE (ApoE), and Cd36, involved in the uptake and clearance of TG-rich lipoproteins, were modestly upregulated in the adipose tissue (not shown) and liver of Ctrp12 (+/−) male mice. Thus, loss of a single Ctrp12 allele affects hepatic lipid metabolism in the normal, nonobese, state. While the effects of partial CTRP12 deficiency are mild, it must be noted that an ∼50% reduction in the vast majority of genes, as found in many heterozygous mouse models, have no metabolic consequences when the animals are consuming a standard chow diet. The mild, but significant, phenotypes we observed are consistent with and support the notion that each variant of a gene that reduces the corresponding mRNA and protein level contributes modestly, at best, to the overall metabolic phenotypes in humans based on recent genome-wide association studies (22).
Changes in metabolic state as a result of dietary intake clearly influence the phenotypic outcomes of Ctrp12 (+/−) male mice. When fed a calorie-dense HFD to induce obesity, the capacity of Ctrp12 (+/−) male mice to handle and dispose of an acute oral lipid load following an overnight fast was attenuated, an effect not observed in the LFD-fed animals. In the postprandial state, intestinal TG-rich lipoproteins (i.e., chylomicrons) are the main contributors to serum lipid levels (8). The fact that lipid clearance is impaired in HFD-fed Ctrp12 (+/−) male mice even though hepatic VLDL-TG secretion is lower suggests that intestinal chylomicron-TG output could be higher in Ctrp12 (+/−) animals in the immediate postprandial state. In support of this, we observed the greatest differences in plasma TG levels within the first 2 h following Intralipid gavage, whereas these differences disappeared by the 3 and 4 h time points. This suggests that the increase in plasma TG immediately following Intralipid ingestion is likely influenced by dietary lipids derived from chylomicrons, and the differences between genotypes disappeared once the chylomicron-derived lipids are taken up by the liver, repackaged into VLDL, and secreted into blood. Future studies in which the clearance of lipoprotein-associated TG in Ctrp12 (+/−) mice is inhibited before lipid gavage will help to determine the intestinal contribution to TG-rich lipoprotein export in the postprandial state. Since total fat mass was not different between genotypes, reduced lipid clearance seen in the Ctrp12 (+/−) animals in response to lipid gavage is unlikely to be due to decreased lipid uptake in adipose tissue.
Unlike the LFD-fed Ctrp12 (+/−) male mice with lower fasting free fatty acids, the NEFA levels were not different between fasted WT and Ctrp12 (+/−) male mice fed an HFD. This was likely due to the high steady-state levels of serum free fatty acids derived from the HFD. In the LFD-fed group, reduced hepatic TG secretion did not alter hepatic TG and cholesterol content in the liver of Ctrp12 (+/−) male mice; this was likely due, in part, to upregulated hepatic fat oxidation. In the HFD-fed Ctrp12 (+/−) male mice, however, reduced hepatic TG secretion was not accompanied by increased hepatic fat oxidation, as neither serum ketone levels nor the expression of fat oxidation genes (Cpt1a, Acox1, Lcad, Ppar-α) differed between the two groups. Consistent with reduced hepatic VLDL-TG secretion without increased lipid utilization, HFD-fed Ctrp12 (+/−) male mice had elevated liver TG and cholesterol content.
Sex differences play important roles in metabolic disease susceptibility and outcomes (9, 47), and there is an increasing need to better understand sex as an independent variable contributing to phenotypic differences in animal models (23). For this reason, we also aimed to address whether partial deficiency of CTRP12 influences whole body metabolism in a sex-dependent manner. Unlike male mice, Ctrp12 (+/−) female mice fed a control LFD were indistinguishable from WT littermates. However, when challenged with an HFD to induce obesity, Ctrp12 (+/−) female mice developed a modest, but significant, insulin resistance, as indicated by elevated fasting insulin levels and a mild impairment in insulin and pyruvate tolerance. These results support previous studies showing that CTRP12 plays a positive role in glucose metabolism (50). In contrast, no changes in whole body glucose metabolism were noted in Ctrp12 (+/−) male mice. Under normal conditions when animals were fed a control LFD, or when obesity was induced with HFD, a ∼50% baseline expression of Ctrp12 appears to be sufficient to maintain normal glucose homeostasis in male mice. Interestingly, in contrast to male mice, HFD-fed Ctrp12 (+/−) female mice had increased hepatic TG secretion compared with WT littermates; the basis for these sex-dependent differences may be complex and is presently unknown but is likely related to sex hormones. In humans, sex differences are known to contribute to differences in insulin sensitivity, distribution of fat in various fat depots, and energy metabolism (21). It is therefore not surprising that we observed phenotypic differences in how male and female mice respond to the loss of a single Ctrp12 allele. Given that sex hormones regulate a variety of physiological processes, future studies will help uncover the basis for sex-dependent requirement for CTRP12 in maintaining lipid homeostasis.
In summary, partial deficiency of CTRP12 affects glucose and lipid metabolism in a manner that is dependent on dietary contexts and the sex of the animals. We have summarized key metabolic differences between genotypes in Table 6. Future studies examining the complete loss of CTRP12 in homozygous (−/−) knockout mice will provide additional insights into the requirement of CTRP12 for metabolic homeostasis. In humans, partial gene deficiencies in multiple loci, rather than complete loss of function of a given gene, underlie complex diseases such as obesity and Type 2 diabetes (22). Other studies examining the effects of heterozygosity on glucose and lipid metabolism have underscored the additional insights gained from mouse models of partial gene deficiency that are relevant to human disease variants (1, 17, 42). Therefore, our results highlight the value of examining the consequences of partial gene deficiency in preclinical animal models of complex diseases, as these data will provide a basis to help interpret and evaluate functional consequences of reduced gene expression due to polymorphisms or epigenetic changes in the gene regulatory regions uncovered by large-scale genome-wide studies in humans.
Table 6.
Summary of metabolic phenotypes in Ctrp12 (+/−) male and female mice fed LFD or HFD
| Male |
Female |
||
|---|---|---|---|
| HFD | LFD | HFD | LFD |
| Decreased serum TG (4 h food removal) | Decreased hepatic VLDL-TG secretion (poloxamer-injected mice) | Higher fasting insulin levels | No observable phenotype |
| Moderate improvements in lipid tolerance tests | Increased serum ketones (4 h food removal) | Moderate decrease in insulin sensitivity | |
| Decreased hepatic VLDL-TG secretion (poloxamer-injected mice) | Upregulation of hepatic fatty acid oxidation genes | Moderate increase in hepatic glucose output (pyruvate tolerance tests) | |
| Increased hepatic TG and cholesterol content | Increased hepatic VLDL-TG secretion (poloxamer-injected mice) | ||
TG, triglyceride.
GRANTS
This work was supported, in part, by grants from the National Institutes of Health (DK-084171) and Novo Nordisk to G. W. Wong. S. Y. Tan was supported by a predoctoral fellowship from the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.Y.T. and G.W.W. conception and design of research; S.Y.T., H.C.L., X.L., S.L., and S.R. performed experiments; S.Y.T. analyzed data; S.Y.T. and G.W.W. interpreted results of experiments; S.Y.T. prepared figures; S.Y.T. and G.W.W. drafted manuscript; S.Y.T., H.C.L., X.L., S.R., and G.W.W. edited and revised manuscript; S.Y.T., H.C.L., X.L., S.L., S.R., and G.W.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Susan Aja for help with indirect calorimetry.
REFERENCES
- 1.Baker DJ, Atkinson AM, Wilkinson GP, Coope GJ, Charles AD, Leighton B. Characterization of the heterozygous glucokinase knockout mouse as a translational disease model for glucose control in type 2 diabetes. Br J Pharmacol 171: 1629–1641, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell-Anderson KS, Funnell AP, Williams H, Mat Jusoh H, Scully T, Lim WF, Burdach JG, Mak KS, Knights AJ, Hoy AJ, Nicholas HR, Sainsbury A, Turner N, Pearson RC, Crossley M. Loss of Kruppel-like factor 3 (KLF3/BKLF) leads to upregulation of the insulin-sensitizing factor adipolin (FAM132A/CTRP12/C1qdc2). Diabetes 62: 2728–2737, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byerly MS, Petersen PS, Ramamurthy S, Seldin MM, Lei X, Provost E, Wei Z, Ronnett GV, Wong GW. C1q/TNF-related protein 4 (CTRP4) is a unique secreted protein with two tandem C1q domains that functions in the hypothalamus to modulate food intake and body weight. J Biol Chem 289: 4055–4069, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byerly MS, Swanson R, Wei Z, Seldin MM, McCulloh PS, Wong GW. A central role for C1q/TNF-related protein 13 (CTRP13) in modulating food intake and body weight. PLoS One 8: e62862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Angelo CS, Da Paz JA, Kim CA, Bertola DR, Castro CI, Varela MC, Koiffmann CP. Prader-Willi-like phenotype: investigation of 1p36 deletion in 41 patients with delayed psychomotor development, hypotonia, obesity and/or hyperphagia, learning disabilities and behavioral problems. Eur J Med Genet 49: 451–460, 2006. [DOI] [PubMed] [Google Scholar]
- 6.D'Angelo CS, Kohl I, Varela MC, de Castro CI, Kim CA, Bertola DR, Lourenco CM, Koiffmann CP. Extending the phenotype of monosomy 1p36 syndrome and mapping of a critical region for obesity and hyperphagia. Am J Med Genet A 152A: 102–110, 2010. [DOI] [PubMed] [Google Scholar]
- 7.D'Angelo CS, Koiffmann CP. Copy number variants in obesity-related syndromes: review and perspectives on novel molecular approaches. J Obes 2012: 845480, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demignot S, Beilstein F, Morel E. Triglyceride-rich lipoproteins and cytosolic lipid droplets in enterocytes: key players in intestinal physiology and metabolic disorders. Biochimie 96: 48–55, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295: 1288–1299, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Enomoto T, Ohashi K, Shibata R, Higuchi A, Maruyama S, Izumiya Y, Walsh K, Murohara T, Ouchi N. Adipolin/C1qdc2/CTRP12 functions as an adipokine that improves glucose metabolism. J Biol Chem 286: 34552–34558, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eugster EA, Berry SA, Hirsch B. Mosaicism for deletion 1p36.33 in a patient with obesity and hyperphagia. Am J Med Genet 70: 409–412, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 13.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998. [DOI] [PubMed] [Google Scholar]
- 14.1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature 526: 68–74, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heilstedt HA, Ballif BC, Howard LA, Kashork CD, Shaffer LG. Population data suggest that deletions of 1p36 are a relatively common chromosome abnormality. Clin Genet 64: 310–316, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Jelinek DA, Maghsoodi B, Borbon IA, Hardwick RN, Cherrington NJ, Erickson RP. Genetic variation in the mouse model of Niemann Pick C1 affects female, as well as male, adiposity, and hepatic bile transporters but has indeterminate effects on caveolae. Gene 491: 128–134, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei X, Rodriguez S, Petersen PS, Seldin MM, Bowman CE, Wolfgang MJ, Wong GW. Loss of CTRP5 improves insulin action and hepatic steatosis. Am J Physiol Endocrinol Metab 310: E1036–E1052, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O'Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG, Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536: 285–291, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ 6: 14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy MI. Genomics, type 2 diabetes, obesity. N Engl J Med 363: 2339–2350, 2010. [DOI] [PubMed] [Google Scholar]
- 23.McCullough LD, de Vries GJ, Miller VM, Becker JB, Sandberg K, McCarthy MM. NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol Sex Differ 5: 15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLean JA, Tobin G. Animal and Human Calorimetry. New York: Cambridge University Press, 1987. [Google Scholar]
- 25.Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res 46: 2023–2028, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Peterson JM, Aja S, Wei Z, Wong GW. C1q/TNF-related protein-1 (CTRP1) enhances fatty acid oxidation via AMPK activation and ACC inhibition. J Biol Chem 287: 1576–1587, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson JM, Seldin MM, Tan SY, Wong GW. CTRP2 overexpression improves insulin and lipid tolerance in diet-induced obese mice. PLoS One 9: e88535, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson JM, Seldin MM, Wei Z, Aja S, Wong GW. CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am J Physiol Gastrointest Liver Physiol 305: G214–G224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol 305: R522–R533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem 285: 39691–39701, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson JM, Wei Z, Wong GW. CTRP8 and CTRP9B are novel proteins that hetero-oligomerize with C1q/TNF family members. Biochem Biophys Res Commun 388: 360–365, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez S, Lei X, Petersen PS, Tan SY, Little HC, Wong GW. Loss of CTRP1 disrupts glucose and lipid homeostasis. Am J Physiol Endocrinol Metab 311: E678–E697, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444: 847–853, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 156: 20–44, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 148: 852–871, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55: 1537–1545, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Seldin MM, Lei X, Tan SY, Stanson KP, Wei Z, Wong GW. Skeletal muscle-derived myonectin activates the mTOR pathway to suppress autophagy in liver. J Biol Chem 289: 36073–36082, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 287: 11968–11980, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell 104: 531–543, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Stagi S, Lapi E, Pantaleo M, Chiarelli F, Seminara S, de Martino M. Type II diabetes and impaired glucose tolerance due to severe hyperinsulinism in patients with 1p36 deletion syndrome and a Prader-Willi-like phenotype. BMC Med Genet 15: 16, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talukder MA, Preda M, Ryzhova L, Prudovsky I, Pinz IM. Heterozygous caveolin-3 mice show increased susceptibility to palmitate-induced insulin resistance. Physiol Rep 4: e12736, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan BK, Chen J, Adya R, Ramanjaneya M, Patel V, Randeva HS. Metformin increases the novel adipokine adipolin/CTRP12: role of the AMPK pathway. J Endocrinol 219: 101–108, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Tan BK, Chen J, Hu J, Amar O, Mattu HS, Ramanjaneya M, Patel V, Lehnert H, Randeva HS. Circulatory changes of the novel adipokine adipolin/CTRP12 in response to metformin treatment and an oral glucose challenge in humans. Clin Endocrinol (Oxf) 81: 841–846, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Tan BK, Lewandowski KC, O'Hare JP, Randeva HS. Insulin regulates the novel adipokine adipolin/CTRP12: in vivo and ex vivo effects. J Endocrinol 221: 111–119, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Muller TD, Munzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods 9: 57–63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varlamov O, Bethea CL, Roberts CT Jr. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne) 5: 241, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei Z, Lei X, Petersen PS, Aja S, Wong GW. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am J Physiol Endocrinol Metab 306: E779–E790, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei Z, Lei X, Seldin MM, Wong GW. Endopeptidase cleavage generates a functionally distinct isoform of C1q/tumor necrosis factor-related protein-12 (CTRP12) with an altered oligomeric state and signaling specificity. J Biol Chem 287: 35804–35814, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem 287: 10301–10315, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei Z, Peterson JM, Wong GW. Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): activation OF AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J Biol Chem 286: 15652–15665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei Z, Seldin MM, Natarajan N, Djemal DC, Peterson JM, Wong GW. C1q/tumor necrosis factor-related protein 11 (CTRP11), a novel adipose stroma-derived regulator of adipogenesis. J Biol Chem 288: 10214–10229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J 23: 241–258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 416: 161–177, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci USA 101: 10302–10307, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zagalo A, Dias P, Pereira C, Sampaio Mde L. Morbid obesity in a child with monosomy 1p36 syndrome. BMJ Case Rep 2012: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]