Abstract
Clinical studies indicate that smoking combustible cigarettes promotes progression of renal and cardiac injury, leading to functional decline in the setting of chronic kidney disease (CKD). However, basic studies using in vivo small animal models that mimic clinical pathology of CKD are lacking. To address this issue, we evaluated renal and cardiac injury progression and functional changes induced by 4 wk of daily combustible cigarette smoke exposure in the 5/6th partial nephrectomy (PNx) CKD model. Molecular evaluations revealed that cigarette smoke significantly (P < 0.05) decreased renal and cardiac expression of the antifibrotic microRNA miR-29b-3 and increased expression of molecular fibrosis markers. In terms of cardiac and renal organ structure and function, exposure to cigarette smoke led to significantly increased systolic blood pressure, cardiac hypertrophy, cardiac and renal fibrosis, and decreased renal function. These data indicate that decreased expression of miR-29b-3p is a novel mechanism wherein cigarette smoke promotes accelerated cardiac and renal tissue injury in CKD. (155 words)
Keywords: combustible cigarettes, physiology, tobacco-related disease, toxicology, fibrosis, microRNA
cigarette smoking is the most common cause of preventable disease and premature death in the United States. Despite several tobacco control programs intended to reduce consumption, cigarette smoking still causes 480,000 deaths each year in the United States (19). Smoking rates have fallen from over 50% of US adults smoking in the 1960s to ∼15–16% smoking in 2015 (19). But while rates of conventional smoking have fallen, the rates of nicotine inhalation via electronic (e)-cigarette use (vaping) have meteorically risen to 10% of the adult population (19). The marketing of e-cigarettes as a healthy alternative to combustible cigarette smoking is one cause of increased use of e-cigarettes among adults and children (8, 10, 14, 18, 20, 27). Whereas toxic and lethal effects of traditional combustible cigarettes have been demonstrated in respiratory, cancer, and cardiovascular settings, limited data are available that evaluate the potential toxicity of e-cigarettes in these settings (6, 29). Given the dramatic rise in popularity of e-cigarettes and the perception of these nicotine delivery devices as safe alternatives to combustible cigarettes (3, 8, 10, 14, 18, 20, 27, 37), additional studies are warranted to determine whether these popular devices are dangerous to human health.
Clear evidence supports the existence of a “cardiorenal” axis in which toxicity in the heart and kidneys interact to induce injury in both organ systems (9). In point of fact, renal insufficiency worsens congestive heart failure (CHF) and is directly associated with morbidity and mortality in CHF patients (39). Patients with chronic kidney disease (CKD) clinically present with left ventricular dysfunction, diastolic dysfunction, cardiac hypertrophy, and cardiac fibrosis, a condition referred to as uremic cardiomyopathy (2). These interactions have significant impacts on human health and should be included in the evaluation of toxicity of tobacco and novel nicotine delivery devices.
The assumptions of nephrotoxic effects of combustible cigarettes are of a controversial nature (5, 40). Clinically correlations between combustible cigarette smoke and accelerated progression of cardiac and renal functional decline and pathology in the setting of CKD are known (15, 31, 40). However, mechanistic understanding of this phenomenon and whether it also occurs with use of e-cigarette have been significantly limited by the lack of inhalation toxicology studies in appropriate CKD models that mimic clinical pathology (11–13). Certainly, previous studies on cigarette smoking in healthy models revealed renal toxicity due to exposure to combustible cigarette smoke (32–35). However, these studies did little to analyze the mechanisms involved in accelerated progression of cardiorenal injury in the setting of CKD that is observed with tobacco use (35). On the basis of these findings it is important to conduct well-planned studies using clinically relevant small animal models with pre-existing CKD to understand the pathological mechanisms that lead to worsened cardiorenal injury and toxicity and as an entry point to extend these findings to e-cigarettes to determine novel toxicity of these increasingly popular nicotine delivery devices.
Our previous studies have shown that formation of cardiac and renal fibrosis and pathological changes in the 5/6th partial nephrectomy (PNx) surgical rodent model of CKD, which is known to be clinically relevant (2), involves increases in endogenous circulating cardiotonic steroids and activation of Na/K-ATPase signaling (17, 23). Additionally, we have recently found a significant role of Na/K-ATPase signaling in mediating decreased expression of the antifibrotic microRNA (miRNA or miR) miR-29b-3p in the development of uremic cardiomyopathy induced by this mechanism (12). This decreased expression, both in vivo and in vitro, regulated cardiac fibrosis in the setting of CKD (12).
By combining our well-characterized model of CKD used in the studies indicated above with exposure to combustible cigarette smoke, the current study evaluated the novel hypothesis that inhalation of combustible cigarette smoke worsens both cardiac and renal tissue injury and functional changes in the setting of CKD via mediation of the antifibrotic miRNA miR-29b-3p. Overall this novel miR-29b-3p-mediated mechanism, which results in dysregulation of fibrosis-related mRNA expression (targets of miR-29b-3p), represents a novel, common, molecular pathway underlying accelerated cardiac and renal tissue fibrosis following exposure to combustible cigarettes.
METHODS
Animals.
Animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals under protocols approved by the Institutional Animal Care and Use Committee at the University of Toledo. A total of 32 eight-week-old male Sprague-Dawley rats obtained from Charles River (Spencerville, OH) weighing 300–350 g were used for experiments. The rats were divided at random into four groups: 1) Sham (control) operation plus room air exposure (abbreviated ShamA); 2) Sham plus exposure to combustible cigarette smoke (abbreviated ShamS); 3) 5/6th partial nephrectomy (PNx) surgery exposed to room air (treatment abbreviated PNxA); and 4) PNx plus combustible cigarette smoke exposure (abbreviated PNxS). All animals were reared under a 12 h dark/light cycle, fed standard chow (Teklad standard maintenance diet), and provided water ad libitum.
We used CKD by performing the segmental arterial infarction model of PNx in rats as previously described (11, 13, 16). For Sham surgeries incisions were made as in both steps of the PNx and the kidneys isolated; however, neither ligation nor removal of the opposing kidney was performed.
Smoking protocol.
To perform the exposure studies for combustible cigarettes we employed a side stream, whole body smoking apparatus and an exposure protocol designed based on relevant literature (5, 30, 41, 42). Animals were exposed to the smoke of five cigarettes each day (1 cigarette every 30 min for 2.5 h). This protocol was carried out 5 days per week for 4 wk. Control animals were placed in similar chambers but exposed to room air only.
Echocardiographic imaging and blood pressure measurement.
Echocardiography was performed on combustible cigarette exposed rats or air-exposed controls just prior to death as previously indicated (13) using an Acuson Sequoia C512 machine (Siemens). End diastolic and end-systolic dimensions, interventricular septal wall and posterior wall thickness, ejection fraction (EF), myocardial performance index (i.e., Tei), and velocity of circumferential shortening were measured by standard techniques and formulae (36).
Additionally, just prior to sacrificing the rats, we performed blood pressure measurement via tail-cuff, using a CODA High Through-put 8 Channel Non-invasive Blood Pressure System (Kent Scientific, Torrington, CT) as described previously (13, 22, 23).
Final disposition of animals and organ collection.
Following the 4th week of the exposure protocol rats were anesthetized with a mixture of ketamine and xylazine (100 mg/kg and 10 mg/kg body wt, respectively) injected intraperitoneally. Animals were then euthanized by exsanguination following removal of the heart while under anesthesia. Organs were collected and either frozen or fixed for use in biochemical, molecular, or histological analyses as previously described (17, 22, 23).
RNA isolation and real time-quantitative polymerase chain reaction.
We homogenized 30 mg of frozen left ventricular or renal tissues in QIAzol, and total RNA was isolated using the miRNeasy mini kit (catalog no. 217004; Qiagen, Germantown, MD) according to the instructions provided by the manufacturer. Immediately following RNA isolation two sets of cDNA were synthesized; first for mRNA (to evaluate changes in extracellular matrix component expression), cDNA was synthesized using the RT2 First Strand cDNA synthesis kit from Qiagen according to the manufacturer's protocol using 1 μg of total RNA as the input for the reaction (catalog no. 330404, Qiagen). Following reverse transcription, cDNA was diluted according to the manufacturer's protocol for storage at −20°C and later use in quantitative real-time polymerase chain reaction (qPCR). For synthesis of cDNA from miRNA, 250 ng of total RNA isolated above was used in the miScript II RT kit (catalog no. 218160, Qiagen) according to the manufacturer's protocol.
To determine changes in extracellular matrix gene expression in rat cardiac and renal tissues species specific primers for collagen 1A1 (Col1a1; catalog no. PPR42922A), collagen 3a1 (Col3a1; catalog no. PPR43017A), collagen 4a1 (Col4a1; catalog no. PPR50699A), matrix metalloprotease 2 (Mmp2; catalog no. PPR43605D), integrin beta 1 (Itgb1; catalog no. PPR48046F), fibrillin 1 (Fbn; catalog no. PPR45703A) and elastin (Eln; catalog no. PPR50786A), in addition to glyceraldehyde 3-phosphate dehydrogenase (GAPDH; catalog no. PPR06557B) as a control were purchased from Qiagen and were used in the RT2 SYBR Green qPCR reaction mix (catalog no. 330529, Qiagen) according to the manufacturer's protocol.
Determinations of changes in miR-29b-3p expression in rat left ventricular or renal tissue made use of species specific Qiagen miScript primer assays for miR-29b-3p (catalog no. MS00005544) and small RNA, RNU6 (catalog no. MS00033740) as a control. These primers were used in the miScript Sybr Green PCR kit (catalog no. 218161, Qiagen) according to the manufacturer's protocol. Reactions were carried out on an ABI 7500 Fast platform (Life Technologies, Boston, MA). Determinations of mRNA and miRNA expression conducted by comparing the relative change in cycle threshold value of the target (ΔCt) from the internal control, GAPDH (for mRNA expression), or RNU6 (internal control for miR-29b-3p expression) was computed. Fold change in expression vs. control was then calculated for each mRNA and miRNA in each sample using the equation, expression = 2−ΔΔCt methodology (21).
Measurement of urinary cotinine.
At the conclusion of the study, 24 h urine samples were collected for rats exposed to combustible cigarette smoke or associated controls. At the end of urine collection, animals were killed as detailed above, and blood samples were obtained from the abdominal aorta of each rat. Urine samples were then assessed for levels of cotinine (a metabolic product of nicotine that accumulates after smoking) as a measure of combustible cigarette smoke exposure (5) using a commercially available direct competitive enzyme linked immunosorbent assay (item no. CO096D-100; Cal Biotech, Spring Valley, CA) assay.
Urinary protein excretion.
Assessments of 24 h urinary protein excretion (UPE) were performed as previously described (25).
Measurement of serum cystatin C.
Cystatin C was measured in plasma from blood samples collected at the time of euthanasia using a commercially available kit from Abcam (Cambridge, MA, catalog no. ab201281) performed according to the manufacturer's protocol.
Creatinine and creatinine clearance.
Plasma and urine creatinine were measured using a commercial kit from Teco Diagnostics (Anaheim, CA, catalog no. C515-480). Creatinine clearance was calculated using the following formula: urine creatinine × urine volume (ml)/plasma Cr × 24 h × 60 min.
Histology.
Rat left ventricle and renal tissues were immediately fixed in 4% formaldehyde Tris-buffered solution (pH 7.2) and paraffin-embedded after 48 h of fixation. Masson's trichrome staining for cardiac and renal fibrosis was conducted and computer-aided morphometry used to quantify the percent of fibrosis in the tissue as previously described (13, 17, 22, 23).
Statistical analyses.
Data are presented as means ± SE. Data obtained were analyzed by t-test or by two-way ANOVA with an unbalanced design, where appropriate. Analyses were conducted using Graph Pad Prism 7 software.
RESULTS
Combustible cigarette smoke exposure and PNx significantly increase cardiac and renal fibrosis.
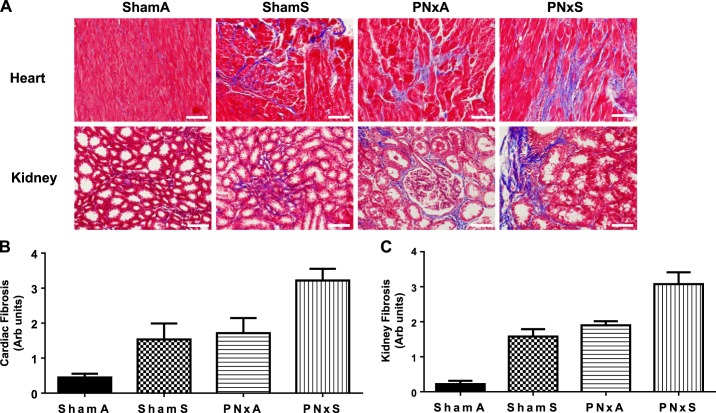
Cardiac and renal fibrosis are major causes of renal and cardiac functional changes in the setting of CKD (2). To assess whether exposure to combustible cigarette smoke led to increased cardiac and renal fibrosis in the setting of CKD, we used Masson's trichrome stains on tissue sections. As shown in Fig. 1, both cardiac and renal fibrosis are significantly increased with cigarette smoking [ShamS; effect size 19.9% (P = 0.0010) and 22.6% (P < 0.0001), respectively; Table 1] and CKD [PNxA; effect size 25.8% (P = 0.0003) and 35.3% (P < 0.0001), respectively; Table 1] groups. Combining cigarette smoking and CKD (PNxS) resulted in increased cardiac and renal fibrosis versus that observed for PNxA or ShamS animals; however, significant interactions between the surgery and exposure were not observed [Fig. 1, A–C; effect size: 0.5% (P = 0.5584) and 0.2 (P = 0.6745); Table 1]. Compared with ShamA animals, PNxS animals had a sevenfold increase in cardiac fibrosis and 14-fold increase in renal fibrosis (Fig. 1, A–C). These data show that both smoking and CKD effect cardiac and renal fibrosis.
Fig. 1.
PNx and smoking induce significant increases in cardiac and renal fibrosis. Representative Masson's trichrome ×20 photomicrographs of cardiac and renal tissue fibrosis (white scale bar equals 25 μm) (A) and quantification of fibrosis by calculating the mean percent fibrotic area using computer-aided morphometry for heart (B) and kidney (C). Means ± SE are shown from measurements in n = 9 ShamA, 8 ShamS, 5 PNxA, and 10 PNxS animals. Data were analyzed with 2-way ANOVA; full results can be found in Table 1. Abbreviations: ShamA, sham-operated room air exposure only (no combustible cigarette exposure); ShamS, sham-operated exposed to 5 cigarettes per day 5 days per week for 4 wk; PNxA, 5/6th partial nephrectomy room air exposure only (no combustible cigarette exposure); PNxS, 5/6th partial nephrectomy exposed to 5 cigarettes per day 5 days per week for 4 wk.
Table 1.
Two-way ANOVA analysis effect sizes for physiological parameters for Fig. 1
| Variable | Interaction % of Total Variation (P value) | Surgery % of Total Variation (P value) | Exposure % of Total Variation |
|---|---|---|---|
| Cardiac fibrosis (Fig. 1A) | 0.5143 (P = 0.5584) | 25.76 (P = 0.0003) | 19.86 (P = 0.0010) |
| Kidney fibrosis (Fig. 1B) | 0.1509 (P = 0.6745) | 35.29 (P < 0.0001) | 22.59 (P < 0.0001) |
Items in boldface are significant.
Smoke exposure and PNx elicit significant decreases in expression of antifibrotic miR-29b-3p and increases in fibrosis related mRNA expression.
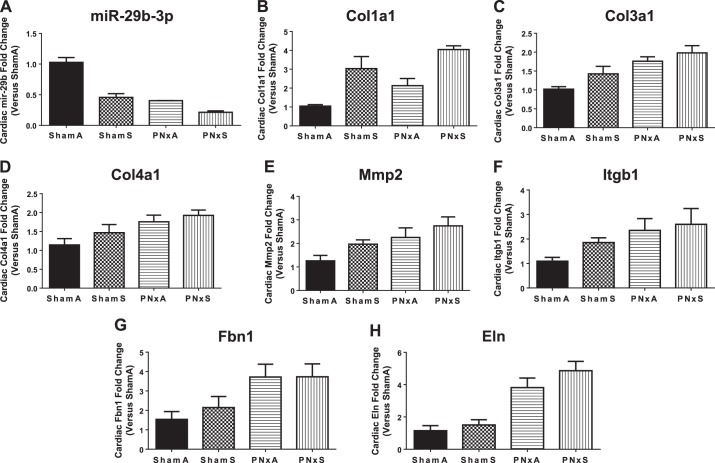
Our previous studies identified a mechanistic link between CKD induced cardiac fibrosis and significant decreases in cardiac tissue expression of the antifibrotic miRNA miR-29b-3p (12). We linked decreased expression of miR-29b-3p to increased expression of the fibrosis marker (and miR-29b-3p target) collagen 1A1 (12). To demonstrate a mechanistic link between CKD, cardiac and renal fibrosis, and combustible cigarette smoke, we evaluated expression of miR-29b-3p in cardiac and renal tissues from rats exposed to combustible cigarette smoke. miR-29b-3p expression was significantly decreased by more than twofold in cardiac tissues of CKD [PNxA; effect size 25.8% (P = 0.0275); Table 2] and cigarette smoke exposed (ShamS) rats [Fig. 2A; effect size 30.3% (P < 0.0001); Table 2]. The combination of cigarette smoke and CKD (PNxS) led to an even more significant approximately fivefold decrease in miR-29b-3p expression vs. ShamA, this level of expression was significantly lower than any other group considered [Fig. 2A; interaction effect size 7.7% (P = 0.0052); Table 2]. To further link combustible cigarette smoke induced miR-29b-3p decreases with increases in cardiac fibrosis and extracellular matrix remodeling, we measured the expression of several targets of miR-29b-3p that have increased expression following induction of fibrosis (12, 24). The expressions of Col1a1, Col3a1, Col4a1, Mmp2, Itgb1, Fbn, and Eln mRNA were all significantly elevated in the PNxA and PNxS groups (Fig. 2, B–H, P < 0.05; see Table 2 for effect sizes), consistent with CKD causing lower levels of miR-29b-3p, and thus higher levels of fibrosis. Analysis of the smoke exposed rats found that expression of Col1a1, Mmp2, and Itgb1 was significantly elevated in the ShamS group, and Col1a1 expression was particularly elevated in the PNxS group over any other group evaluated [Fig. 2B; effect size 49.8% (P < 0.0001); Table 2]. These data confirm that the significant decreases observed in cardiac tissue expression of miR-29b-3p have effects on fibrosis formation.
Table 2.
Two-way ANOVA analysis effect sizes for physiological parameters for Fig. 2
| Variable | Interaction % of Total Variation (P value) | Surgery % of Total Variation (P value) | Exposure % of Total Variation |
|---|---|---|---|
| Cardiac miR-29b (Fig. 2A) | 7.676 (P = 0.0052) | 39.41 (P < 0.0001) | 30.25 (P = 0.0275) |
| Cardiac Col1a1 mRNA (Fig. 2B) | 0.01983 (P = 0.9180) | 14.34 (P = 0.0125) | 49.77 (P < 0.0001) |
| Cardiac Col3a1 mRNA (Fig. 2C) | 0.9161 (P = 0.5488) | 44.13 (P = 0.0006) | 10.33 (P = 0.0564) |
| Cardiac Col4a1 mRNA (Fig. 2D) | 0.645 (P = 0.6772) | 31.45 (P = 0.0092) | 6.722 (P = 0.1899) |
| Cardiac Mmp2 mRNA (Fig. 2E) | 0.421 (P = 0.7265) | 28.34 (P = 0.01) | 13.17 (P = 0.0638) |
| Cardiac Itgb1 mRNA (Fig. 2F) | 1.618 (P = 0.5326) | 24.98 (P = 0.0234) | 6.282 (P = 0.2269) |
| Cardiac Fbn1 mRNA (Fig. 2G) | 0.9713 (P = 0.6092) | 38.98 (P = 0.0045) | 1.055 (P = 0.5944) |
| Cardiac Eln mRNA (Fig. 2H) | 0.875 (P = 0.46) | 67.32 (P < 0.0001) | 3.611 (P = 0.1436) |
Items in boldface are significant.
Fig. 2.
Smoking decreases microRNA (miR)-29b-3p and regulates its targets mRNAs in rat cardiac tissue. RT-quantitative (q)PCR based assessment of miR-29b-3p expression (A); collagen 1a1 mRNA (Col1a1, B); collagen 3a1 (Col3a1, C); collagen 4a1 (Col4a1, D); matrix metalloprotease 2 (Mmp2, E); integrin beta 1 (Itgb1, F); fibrillin (Fbn1, G); and elastin (Eln, H). Means ± SE of n = 5 independent samples assessed in triplicate per group. Data were analyzed with 2-way ANOVA; full results can be found in Table 2. Abbreviations: ShamA, sham-operated room air exposure only (no combustible cigarette exposure); ShamS, sham-operated exposed to 5 cigarettes per day 5 days per week for 4 wk; PNxA, 5/6th partial nephrectomy room air exposure only (no combustible cigarette exposure); PNxS, 5/6th partial nephrectomy exposed to 5 cigarettes per day 5 days per week for 4 wk.
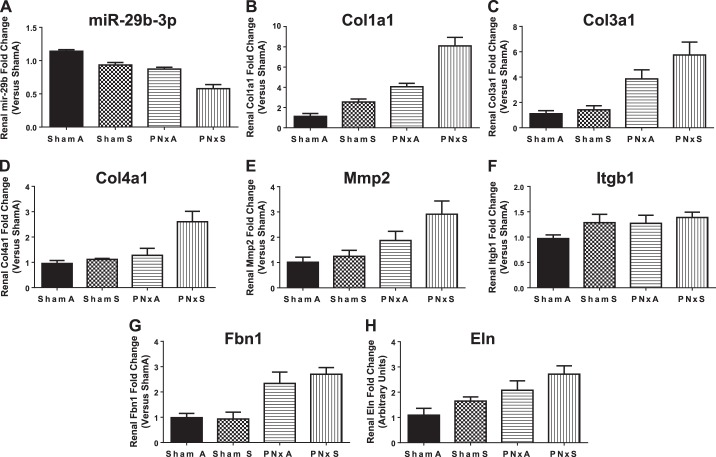
In rat renal tissues there was a significant decrease in miR-29b-3p expression induced by cigarette smoke [ShamS; Fig. 3A; effect size 34.6% (P = 0.0275); Table 3]. CKD (PNxA) also significantly decreased miR-29b-3p expression by more than 20% [Fig. 3A; effect size 53.6% (P < 0.0001); Table 3]. Combining cigarette smoke with CKD led to a 300% decrease in miR-29b-3p expression, which was lower than all other groups; however, two-way analysis of variance indicated that there is no significant interaction between smoke exposure and surgery [Fig. 3A, interaction effect size 1.1% (P = 0.2735), Table 3]. Analyzing kidney tissues for expression of miR-29b-3p targets that are involved in fibrosis formation and extracellular matrix remodeling revealed that CKD (PNxA) led to elevated expression of Col1a1, Col3a1, Col4a1, Mmp2, Fbn1, and Eln vs. ShamA controls (P < 0.05, Fig. 3, B–E and G and H; see Table 3 for effect sizes). Cigarette smoke exposure alone led to increased renal expression of Col1a1 and Col4a1 [ShamS; Fig. 3, B and D; effect sizes 22.7% (P < 0.0001) and 20% (P = 0.0103), respectively; Table 3]. Cigarette smoke exposure on a CKD (PNxS) background led to elevations in expression of Col1a1 and Col4a1 greater than that seen in any other group analyzed [Fig. 3, B and D; interaction effect sizes 5.2% (P = 0.0229) and 12.3% (P = 0.0364), respectively; Table 3]. These data link reduction of miR-29b-3p induced by combustible cigarette smoke to significant changes in the expression of fibrosis-related mRNAs.
Fig. 3.
Smoking decreases miR-29b-3p and regulates its targets mRNAs in rat renal tissue. RT-qPCR based assessment of miR-29b-3p expression (A); collagen 1a1 mRNA (Col1a1, B); collagen 3a1 (Col3a1, C); collagen 4a1 (Col4a1, D); matrix metalloprotease 2 (Mmp2, E); integrin beta 1 (Itgb1, F); fibrillin (Fbn1, G); and elastin (Eln, H). Means ± SE of n = 5 independent samples assessed in triplicate per group. Data were analyzed with 2-way ANOVA; full results can be found in Table 3. Abbreviations: ShamA, sham-operated room air exposure only (no combustible cigarette exposure); ShamS, sham-operated exposed to 5 cigarettes per day 5 days per week for 4 wk; PNxA, 5/6th partial nephrectomy room air exposure only (no combustible cigarette exposure); PNxS, 5/6th partial nephrectomy exposed to 5 cigarettes per day 5 days per week for 4 wk.
Table 3.
Two-way ANOVA analysis effect sizes for physiological parameters for Fig. 3
| Variable | Interaction % of Total Variation (P value) | Surgery % of Total Variation (P value) | Exposure % of Total Variation |
|---|---|---|---|
| Renal miR-29b (Fig. 3A) | 1.083 (P = 0.2735) | 53.58 (P < 0.0001) | 34.6 (P < 0.0001) |
| Renal Col1a1 mRNA (Fig. 3B) | 5.207 (P = 0.0229) | 54.77 (P < 0.0001) | 22.7 (P < 0.0001) |
| Renal Col3a1 mRNA (Fig. 3C) | 2.919 (P = 0.2487) | 58.84 (P < 0.0001) | 5.649 (P = 0.1153) |
| Renal Col4a1 mRNA (Fig. 3D) | 12.31 (P = 0.0364) | 29.94 (P = 0.0026) | 19.97 (P = 0.0103) |
| Renal Mmp2 mRNA (Fig. 3E) | 3.883 (P = 0.2733) | 38.18 (P = 0.0026) | 9.659 (P = 0.0925) |
| Renal Itgb1 mRNA (Fig. 3F) | 2.716 (P = 0.4547) | 10.83 (P = 0.1455) | 12.43 (P = 0.1207) |
| Renal Fbn1 mRNA (Fig. 3G) | 1.123 (P = 0.4806) | 64.05 (P < 0.0001) | 0.6244 (P = 0.5976) |
| Renal Eln mRNA (Fig. 3H) | 0.05805 (P = 0.8986) | 38.59 (P = 0.0048) | 12.95 (P = 0.073) |
Items indicated in boldface are significant.
Exposure to combustible cigarette smoke or CKD significantly elevated systolic blood pressure and urine cotinine excretion.
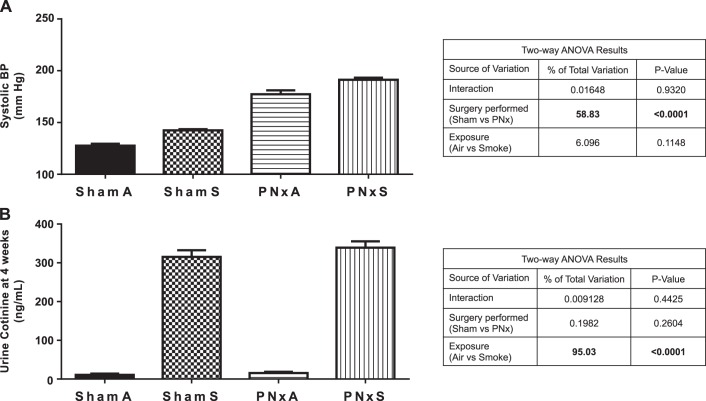
To determine the potential effects of depressed renal function (PNx model of CKD) and combustible cigarette smoke on cardiovascular and renal parameters in rats, systolic blood pressure and urinary cotinine excretion were analyzed 4 wk following the start of the smoke exposure protocol. As shown in Fig. 4A, systolic blood pressure was elevated in ShamS [142.4 ± 3.5 mmHg; effect size 6.1% (P = 0.1148)] and PNxA [177.3 ± 16.3 mmHg; effect size 58.83% (P < 0.0001)] groups, vs. ShamA controls (127.7 ± 5.6 mmHg). No significant interaction between smoke exposure and surgery was found in PNxS animals; however, systolic blood pressure was elevated [191.3 ± 10.3 mmHg, interaction effect size 0.02% (P = 0.9320)] vs. all other groups.
Fig. 4.
Smoking and PNx increased systolic blood pressure and urinary cotinine excretion. A: systolic blood pressure (BP) (mmHg) as determined by tail cuff 4 wk after surgery and implementation of smoking exposure. B: mean cotinine concentration measured in urine collected over a 24 h period just prior to organ collection. Means ± SE are shown from measurements in n = 9 ShamA, 8 ShamS, 5 PNxA, and 10 PNxS animals. Data were analyzed with 2-way ANOVA; full results can be found to the right of each graph. Abbreviations: mm HG, millimeters of mercury; ShamA, sham-operated room air exposure only (no combustible cigarette exposure); ShamS, sham-operated exposed to 5 cigarettes per day 5 days per week for 4 wk; PNxA, 5/6th partial nephrectomy room air exposure only (no combustible cigarette exposure); PNxS, 5/6th partial nephrectomy exposed to 5 cigarettes per day 5 days per week for 4 wk.
Nicotine is the major addictive component of combustible cigarette smoke and is known to induce pathogenic stimuli, like oxidative stress; the major metabolite of nicotine, cotinine, can be measured in the urine as a biomarker of tobacco exposure (28). As shown in Fig. 4B, smoke exposure was a significant factor [effect size 95.0% (P < 0.0001)] leading to elevated 24 h urine cotinine excretion, regardless of surgery, confirming the accumulation of nicotine following cigarette smoke inhalation in this model of CKD.
Combustible cigarette smoke leads to increased left ventricular hypertrophy.
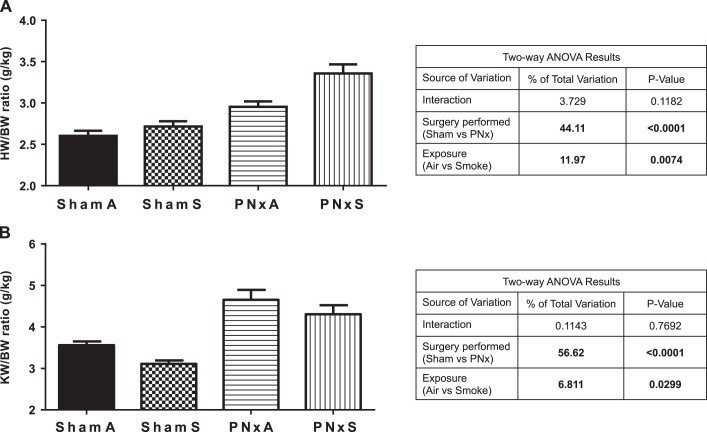
To further assess the physiological effects of combustible cigarette smoke exposure in a model of CKD, we evaluated the effects of combustible cigarette smoke inhalation on total body weight (BW), as a marker of overall health in these animals and heart weight (HW) and kidney weight (KW) as markers of hypertrophy. Smoking resulted in significant weight loss in rats with normal kidney function, with ShamS rats weighing 331.0 ± 8.7 g vs. ShamA rats weighing 366.9 ± 9.1 g [effect size 16.6% (P = 0.0155), Tables 4 and 5]. Two-way ANOVA analysis revealed that surgical manipulation alone did not have significant effects in BW [effect size 9.3% (P = 0.0635); Table 5], resulting in a final BW of 337.2 ± 10.0 g vs. 325.3 ± 7.9 g in PNxS rats (Table 4). Rats with reduced renal function (PNxA) had significantly increased cardiac hypertrophy as evidenced by an increased HW-to-BW ratio [2.95 ± 0.06 g/kg PNxA vs. 2.60 ± 0.06 g/kg ShamA; effect size 44.1% (P < 0.0001); Fig. 5A], consistent with reduction in renal function leading to pathologic changes in the myocardium. Smoking was revealed to have independent significant effects on this ratio [effect size 12.0% (P = 0.0074), Fig. 5B]. Left ventricular (LV) hypertrophy, based on LV weight (LVW) and LVW/BW ratios, followed the same trend as that for overall cardiac hypertrophy (Tables 4 and 5). The final heart and LV weights in the PNxS group were 22 and 26% higher, respectively, than that for the ShamS animals (Tables 4 and 5). These data demonstrate that smoking induces cardiac hypertrophy in the setting of pre-existing CKD.
Table 4.
Physiological and cardiac Doppler imaging results
| Variable | ShamA (n = 9) |
ShamS (n = 8) |
PNxA (n = 5) |
PNxS (n = 10) |
|---|---|---|---|---|
| Heart rate, beats/min | 335 ± 9 | 339 ± 8 | 365 ± 10 | 347 ± 8 |
| LVW/BW, g/kg | 2.10 ± 0.05 | 2.20 ± 0.04 | 2.47 ± 0.04 | 2.82 ± 0.11 |
| Final BW, g | 367 ± 9 | 331 ± 9 | 337 ± 10 | 325 ± 8 |
| Final HW, mg | 954 ± 28 | 897 ± 23 | 998 ± 51 | 1091 ± 44 |
| LVW, mg | 769 ± 23 | 727 ± 15 | 835 ± 38 | 917 ± 44 |
| KW, g | 1.30 ± 0.27 | 1.03 ± 0.03 | 1.58 ± 0.12 | 1.39 ± 0.05 |
| EDV, μl | 208 ± 10 | 181 ± 12 | 204 ± 12 | 165 ± 10 |
| ESV, μl | 56 ± 2 | 37 ± 5 | 41 ± 5 | 18 ± 5 |
| WTI | 0.39 ± 0.01 | 0.42 ± 0.03 | 0.42 ± 0.03 | 0.52 ± 0.02 |
| EF, % | 73 ± 1 | 80 ± 2 | 80 ± 2 | 89 ± 2 |
| VCF, s−1 | 0.76 ± 0.03 | 0.77 ± 0.03 | 0.88 ± 0.07 | 0.92 ± 0.07 |
| Tei index | 0.69 ± 0.02 | 0.69 ± 0.02 | 0.75 ± 0.07 | 0.78 ± 0.04 |
Data presented in this table are: heart rate, left ventricle hypertrophy [LVW (g) to BW (kg) ratio], final body weight in g, final heart weight in mg, LVW in mg, KW in g, end-diastolic volume (ml), end-systolic volume (ml), wall thickness index, ejection fraction, velocity of circumferential shortening, and Tei index, as determined prior to sacrifice by echocardiography. Means ± SE are shown from measurements in n = 9 ShamA, 8 ShamS, 5 PNxA, and 10 PNxS animals. Data analyze with 2-way ANOVA, results of analyses found in Table 5. Abbreviations: LVW, left ventricle weight; BW, body weight; HW, heart weight; KW, kidney weight; EDV, end-diastolic volume; ESV, end-systolic volume; WTI, wall thickness index; EF, ejection fraction; VCF, velocity of circumferential shortening; Tei index, myocardial performance index; ShamA, sham operated room air exposure only (no combustible cigarette exposure); ShamS, sham-operated exposed to 5 cigarettes per day 5 days per week for 4 wk; PNxA, 5/6th partial nephrectomy room air exposure only (no combustible cigarette exposure); PNxS, 5/6th partial nephrectomy exposed to 5 cigarettes per day 5 days per week for 4 wk.
Table 5.
Two-way ANOVA analysis effect sizes for parameters in Table 4
| Variable | Interaction % of Total Variation (P value) | Surgery % of Total Variation (P value) | Exposure % of Total Variation |
|---|---|---|---|
| Heart rate, beats/min | 4.271 (P = 0.2352) | 12.74 (P = 0.0453) | 1.73 (P = 0.4466) |
| LVW/BW, g/kg | 3.14 (P = 0.1367) | 49.24 (P < 0.0001) | 10.17 (P = 0.0101) |
| Final BW, g | 4.145 (P = 0.2082) | 9.327 (P = 0.0635) | 16.58 (P = 0.0155) |
| Final HW, mg | 9.077 (P = 0.0626) | 22.85 (P = 0.0046) | 0.5228 (P = 0.6452) |
| LVW, mg | 7.034 (P = 0.0861) | 29.98 (P = 0.0010) | 0.732 (P = 0.5707) |
| KW, g | 0.171 (P = 0.8122) | 10.94 (P = 0.0653) | 5.652 (P = 0.1788) |
| EDV, μl | 0.7403 (P = 0.6028) | 2.056 (P = 0.3878) | 22.39 (P = 0.0073) |
| ESV, μl | 0.301 (P = 0.6677) | 21.75 (P = 0.0010) | 33.19 (P < 0.0001) |
| WTI | 4.973 (P = 0.1412) | 17.15 (P = 0.0089) | 17.15 (P = 0.0089) |
| EF, % | 0.4393 (P = 0.5984) | 28.11 (P = 0.0002) | 28.11 (P = 0.0002) |
| VCF, s−1 | 0.7647 (P = 0.4418) | 61.94 (P < 0.0001) | 2.124 (P = 0.2040) |
| Tei index | 0.4959 (P = 0.6919) | 12.4 (P = 0.0551) | 0.4959 (P = 0.6919) |
Fig. 5.
Smoking and PNx have effects on overall cardiac and renal organ weight. Data shown are heart weight (g)-to-body weight (kg) ratio (A), and the left kidney weight (g)-to-body weight (kg) ratio (B) as determined at time of organ collection. Means ± SE are shown from measurements in n = 9 ShamA, 8 ShamS, 5 PNxA, and 10 PNxS animals. Data were analyzed with 2-way ANOVA; full results can be found to the right of each graph. Abbreviations: BW, body weight measured in kg; HW, heart weight measured in g; KW, kidney weight measured in g; ShamA, sham-operated room air exposure only (no combustible cigarette exposure); ShamS, sham-operated exposed to 5 cigarettes per day 5 days per week for 4 wk; PNxA, 5/6th partial nephrectomy room air exposure only (no combustible cigarette exposure); PNxS, 5/6th partial nephrectomy exposed to 5 cigarettes per day 5 days per week for 4 wk.
Increased cardiac hypertrophy following smoke exposure (ShamS and PNxS) was apparent in measurements of end-diastolic volume and end-systolic volume [Tables 4 and 5, effect size 22.4% (P = 0.0073)]. The wall thickness index (a measure of overall ventricular wall thickness evaluated at diastole) was significantly increased in independently by both PNx and smoke exposure animals over that observed in any other group [Tables 4 and 5; effect sizes 17.2% (P = 0.0089) for both variables]. In the setting of CKD or smoking, the EF significantly increased compared with control [72.6 ± 0.8% ShamA, 80.4 ± 1.7% PNxA, 80.2 ± 1.9% ShamS; Tables 4 and 5; effect sizes 28.1% (P = 0.0002) for both variables]. To determine the presence of diastolic dysfunction, the velocity of circumferential shortening and Tei index (myocardial performance index) were assessed and found to be elevated in PNxS rats vs. ShamA controls (Tables 4 and 5), confirming diastolic dysfunction in the setting of CKD and smoking.
Exposure to combustible cigarette smoke as well as PNx decrease renal function.
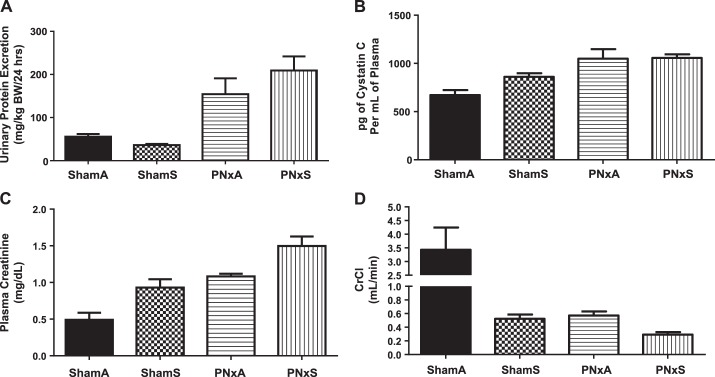
To determine whether exposure to combustible cigarette smoke worsens renal function in rats with pre-existing CKD, we assessed several measures of kidney function at the end of the 4th week of the cigarette smoke exposure protocol. Total UPE over a 24 h period was significantly increased in PNxA and PNxS animals (154.4 ± 36.7 and 209.2 ± 32.7 mg/kg BW/24 h, respectively) vs. both ShamA and ShamS groups [55.6 ± 6.2 and 36.0 ± 2.9 mg/kg BW/24 h, respectively; Fig. 6; effect size of surgery 49.8% (P < 0.0001); Table 6].
Fig. 6.
Proteinuria, plasma cystatin C, and plasma creatinine and clearance are significantly worsened with PNx and smoking. All data were determined 4 wk following the start of PNx and smoking regimen. A: urinary protein excretion; B: plasma cystatin C levels; C: level of plasma creatinine; D: creatinine clearance calculated with the following formula: urine creatinine × urine vol (ml)/plasma Cr × 24 h × 60 min. Means ± SE are shown from measurements in n = 9 ShamA, 8 ShamS, 5 PNxA, and 10 PNxS animals. Data were analyzed with 2-way ANOVA, full results can be found in Table 6. Abbreviations: BW, body weight; CrCl, Creatinine clearance; ShamA, sham-operated room air exposure only (no combustible cigarette exposure); ShamS, sham-operated exposed to 5 cigarettes per day 5 days per week for 4 wk; PNxA, 5/6th partial nephrectomy room air exposure only (no combustible cigarette exposure); PNxS, 5/6th partial nephrectomy exposed to 5 cigarettes per day 5 days per week for 4 wk.
Table 6.
Two-way ANOVA analysis effect sizes for parameters in Fig. 6
| Variable | Interaction % of Total Variation (P value) | Surgery % of Total Variation (P value) | Exposure % of Total Variation |
|---|---|---|---|
| Urinary protein excretion, mg/kg BW/24 h | 3.723 (P = 0.1419) | 49.8 (P < 0.0001) | 0.8359 (P = 0.4798) |
| Pg of Cystatin C per ml of plasma | 4.523 (P = 0.1085) | 45.82 (P < 0.0001) | 5.347 (P = 0.0822) |
| Plasma creatinine, mg/dl | 0.01691 (P = 0.9174) | 36.77 (P < 0.0001) | 19.98 (P = 0.0012) |
| CrCL, ml/min | 20.35 (P = 0.0027) | 28.14 (P = 0.0008) | 29.91 (P = 0.0006) |
Items indicated in boldface are significant.
Plasma cystatin C levels assessed at the time of death showed that smoking induced a 28% increase [ShamS vs. ShamA, Fig. 6B, effect size 5.3% (P = 0.0822), Table 6]. However, while PNx resulted in an ∼58% increase in serum cystatin C levels vs. ShamA, no further increase was induced by the addition of smoke exposure in the setting of PNx [Fig. 6B, effect size 45.8% (P < 0.0001), Table 6].
Plasma creatinine levels were significantly increased by either CKD or cigarette smoke exposure [ShamS and PNxA; Fig. 6C; effect sizes 36.8% (P < 0.0001) and 20% (P = 0.0012), respectively, Table 6]. Combining the insults (PNxS group) led to serum creatinine levels of 1.50 ± 0.13 mg/dl, which was higher than any other group analyzed [Fig. 6C, interaction effect size 0.02% (P = 0.9174), Table 6]. Assessments of creatinine clearance (Fig. 6D), revealed that CKD [effect size 28.1% (P = 0.0008), Table 6] or cigarette smoke exposure [effect size 29.9% (P = 0.0006), Table 6] significantly decreased creatinine clearance, additionally a significant interaction between these two variables was observed [effect size 20.4% (P = 0.0027), Table 6]. These data indicate that, even in healthy models, cigarette smoking can lead to diminished renal function and that imposing cigarette smoking onto a background of pre-existing CKD may lead to more severe decrements in renal function.
DISCUSSION
The current study distinguished several factors related to CKD that were significantly affected by combustible cigarette smoke. More importantly these studies revealed that cardiac miR-29b-3p expression, renal Col1a1 and Col4a1 mRNA expression, and creatinine clearance all displayed significant interactions, indicating that smoking worsened these important factors in CKD-induced pathology. Both combustible cigarettes and e-cigarettes induced significant decreases in the important epigenetic antifibrotic mediator miR-29b-3p. This is a significant and novel finding illustrating the effects of tobacco and nicotine on epigenetic regulation of fibrosis formation in cardiac and renal tissues.
Recently published work from our lab revealed that decreased tissue expression of miR-29b-3p is a mechanism of cardiorenal toxicity and organ fibrosis in CKD (12). miRNAs have been shown to play roles in the progression of many diseases, including fibrotic, hypertrophic, and apoptotic disorders, and can serve as biomarkers of disease (26). Recently, several studies have focused on miR-29b's ability to regulate fibrosis, a known effect of organ injury due to toxicity (38, 43). The data from the current study are the first to indicate that smoking leads to significant decreases in miR-29b-3p in cardiac and renal tissues, and the first to indicate that these effects synergized with CKD induced decreases in miR-29b-3p to lead to worsening tissue fibrosis as indicated by increased fibrosis related mRNA targets of miR-29b-3p in both cardiac and renal tissue. This novel finding indicates that decreased expression of miR-29b-3p may be a novel mechanism of nicotine delivery that can mediate renal injury, which should be evaluated in e-cigarette-exposed animals. It is important to note that previous publications on cigarette smoking showed that endothelial cell dysfunction, activation of growth factors (angiotensin II, endothelin-1, and TGFB1), tubulotoxic effects, oxidative stress, increased clotting of platelets, impaired lipoprotein and glycosaminoglycan metabolism, modulation of immune mechanisms, vasopressin-mediated antidiuresis, and insulin resistance all are affected by smoking exposure (32–35). These factors could also play a role in mediating worsened cardiorenal injury and functional decline following cigarette smoke exposure and need to be evaluated following e-cigarette exposure. Future detailed studies are planned to evaluate these mechanisms.
A major limitation of the current study should be noted in that multiple testing correction has not been applied to the study, in particular when investigating the effects of surgery and smoke exposure on the expression of seven gene targets of miR-29b-3p. While these data are strongly indicative that smoking worsens several features of cardiorenal pathology and function, a clearer picture would be gained with more animals included in the study.
In terms of exposure to combustible cigarette smoke, miR-29b-3p levels are altered in lung tissue and blood of patients with chronic obstructive pulmonary disease (1). Given that increased nicotine levels are common to both combustible cigarette and e-cigarette exposure and that nicotine alone is known to mediate global miRNA expression in circulation and in various cardiac, gastric, stem, and pulmonary cell lines and tissues (28), our data suggest that inhalation of combustible cigarette smoke leads to downregulation of the fibrosis suppressor miR-29b-3p, which has the potential to cause dysregulation of profibrosis mediators, possibly culminating in cardiac and renal tissue fibrosis. Lastly, the current data also showed that using the rat PNx animal model of CKD is a promising new tool that can be used to perform future in-depth mechanistic studies to determine other epigenetic and biomolecular factors that lead to accelerated cardiorenal injury and functional decline in the setting of pre-existing CKD, following nicotine, combustible cigarette and/or e-cigarette exposure.
Future studies designed to understand the roles that combustible cigarette smoke and e-cigarette vapor exposure play in mediating cardiorenal injury, in healthy individuals as well as those with pre-existing CKD, will need to distinguish the contributions of individual factors within smoke and vapor, such as nicotine, tar, arsenic, hydrogen cyanide, and other harmful compounds that are commonly found at high levels in combustible cigarette smoke (28), in contrast to the nicotine, formaldehyde, acrolein, and nitrosamines that have been found in varying quantities in e-cigarette vapor (4). Studies that utilize in vitro and in vivo models to evaluate the contributions of one or more of these factors are necessary to rapidly determine the potential toxicities of novel nicotine delivery devices and popular tobacco products, such as e-cigarettes, e-hookahs, water pipes, cigarillos, etc., which are rapidly gaining in popularity (7), and are desperately needed, as it will be decades before epidemiologic data are available to guide user choice and governmental regulations.
GRANTS
This work was supported by National Institutes of Health Grants (1F32DK-104615-01 to C. A. Drummond, HL-105649 to J. Tian, and HL-109015 to J. I. Shapiro). Additional support was from the National and Ohio Valley Affiliate of the American Heart Association (AHA; 13POST16860035 to S. T. Haller), Veterans Affairs BLR&D Career Development Award (1IK2BX001313 to L. E. Crotty Alexander), AHA Beginning Grant-in-aid (16BGIA27790079 to L. E. Crotty Alexander), and O'Brien Center Daniel O'Connor Memorial Pilot Award to L. E. Crotty Alexander. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.A.D., L.E.C.A., S.T.H., X.F., J.X.X., D.J.K., J.L., Y.Y., D.-A.H., and D.P.M. performed experiments; C.A.D. analyzed data; C.A.D., C.J.C., J.I.S., and J.T. interpreted results of experiments; C.A.D. prepared figures; C.A.D., L.E.C.A., and J.T. drafted manuscript; C.A.D., L.E.C.A., S.T.H., J.X.X., D.J.K., C.J.C., J.I.S., and J.T. edited and revised manuscript; C.A.D., L.E.C.A., S.T.H., X.F., J.X.X., D.J.K., J.L., Y.Y., D.-A.H., D.P.M., C.J.C., J.I.S., and J.T. approved final version of manuscript.
REFERENCES
- 1.Badrnya S, Baumgartner R, Assinger A. Smoking alters circulating plasma microvesicle pattern and microRNA signatures. Thromb Haemost 112: 128–136, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev 61: 9–38, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr ER. E-cigarettes: facts, perceptions, and marketing messages. Clin J Oncol Nurs 18: 112–116, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Cheng T. Chemical evaluation of electronic cigarettes. Tob Control 23, Suppl 2: ii11–ii17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coggins CRE. A further review of inhalation studies with cigarette smoke and lung cancer in experimental animals, including transgenic mice. Inhal Toxicol 22: 974–983, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Crotty Alexander LE, Malhotra A. The civil liberty of smoking cigarettes. Chest 148: 6–8, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Crotty Alexander LE, Vyas A, Schraufnagel DE, Malhotra A. Electronic cigarettes: the new face of nicotine delivery and addiction. J Thorac Dis 7: E248–E251, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Marco C, Invernizzi G, Bosi S, Pozzi P, Di Paco A, Mazza R, Ruprecht AA, Munarini E, Boffi R. The electronic cigarette: potential health benefit or mere business? Tumori 99: 299e–301e, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Di Lullo L, Gorini A, Russo D, Santoboni A, Ronco C. Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med 5: 254–266, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle C, Patterson S, Scott J. Electronic cigarettes and smoking cessation: a quandary? Lancet 383: 408, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Drummond CA, Budny GV, Haller ST, Liu J, Yan Y, Xie JX, Malhotra D, Shapiro JI, Tian J. Gender differences in the development of uremic cardiomyopathy following partial nephrectomy: role of progesterone. J Hypertens (Los Angel) 2: 109–117, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond CA, Hill MC, Shi H, Fan X, Xie JX, Haller ST, Kennedy DJ, Liu J, Garrett MR, Xie Z, Cooper CJ, Shapiro JI, Tian J. Na/K-ATPase signaling regulates collagen synthesis through microRNA-29b-3p in cardiac fibroblasts. Physiol Genomics 48: 220–229, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond CA, Sayed M, Evans KL, Shi H, Wang X, Haller ST, Liu J, Cooper CJ, Xie Z, Shapiro JI, Tian J. Reduction of Na/K-ATPase affects cardiac remodeling and increases c-kit cell abundance in partial nephrectomized mice. Am J Physiol Heart Circ Physiol 306: H1631–H1643, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiore MC, Schroeder SA, Baker TB. Smoke, the chief killer–strategies for targeting combustible tobacco use. N Engl J Med 370: 297–299, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall ME, Wang W, Okhomina V, Agarwal M, Hall JE, Dreisbach AW, Juncos LA, Winniford MD, Payne TJ, Robertson RM, Bhatnagar A, Young BA. Cigarette smoking and chronic kidney disease in African Americans in the Jackson Heart Study. Journal of the American Heart Association 5: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller ST, Drummond CA, Yan Y, Liu J, Tian J, Malhotra D, Shapiro JI. Passive immunization against marinobufagenin attenuates renal fibrosis and improves renal function in experimental renal disease. Am J Hypertens 27: 603–609, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haller ST, Kennedy DJ, Shidyak A, Budny GV, Malhotra D, Fedorova OV, Shapiro JI, Bagrov AY. Monoclonal antibody against marinobufagenin reverses cardiac fibrosis in rats with chronic renal failure. Am J Hypertens 25: 690–696, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampton T. Experts call for research plus regulation of e-cigarettes. JAMA 311: 123–124, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Jamal A, Homa DM, O'Connor E, Babb SD, Caraballo RS, Singh T, Hu SS, King BA. Current cigarette smoking among adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep 64: 1233–1240, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Jo CL, Ayers JW, Althouse BM, Emery S, Huang J, Ribisl KM. US consumer interest in non-cigarette tobacco products spikes around the 2009 federal tobacco tax increase. Tob Control 24: 395–399, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones JA, Stroud RE, O'Quinn EC, Black LE, Barth JL, Elefteriades JA, Bavaria JE, Gorman JH 3rd, Gorman RC, Spinale FG, Ikonomidis JS. Selective microRNA suppression in human thoracic aneurysms: relationship of miR-29a to aortic size and proteolytic induction. Circ Cardiovasc Genet 4: 605–613, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy DJ, Elkareh J, Shidyak A, Shapiro AP, Smaili S, Mutgi K, Gupta S, Tian J, Morgan E, Khouri S, Cooper CJ, Periyasamy SM, Xie Z, Malhotra D, Fedorova OV, Bagrov AY, Shapiro JI. Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am J Physiol Renal Physiol 294: F450–F454, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY, Shapiro JI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension 47: 488–495, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics 44: 237–244, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumarasamy S, Gopalakrishnan K, Toland EJ, Yerga-Woolwine S, Farms P, Morgan EE, Joe B. Refined mapping of blood pressure quantitative trait loci using congenic strains developed from two genetically hypertensive rat models. Hypertens Res 34: 1263–1270, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latronico MV, Condorelli G. Therapeutic use of microRNAs in myocardial diseases. Curr Heart Fail Rep 8: 193–197, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Lee YO, Hebert CJ, Nonnemaker JM, Kim AE. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med 62: 14–19, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Mattes W, Yang X, Orr MS, Richter P, Mendrick DL. Biomarkers of tobacco smoke exposure. Adv Clin Chem 67: 1–45, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation 129: 28–41, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minicucci MF, Azevedo PS, Polegato BF, Paiva SAR, Zornoff LAM. Cardiac remodeling induced by smoking: concepts, relevance, and potential mechanisms. Inflamm Allergy Drug Targets 11: 442–447, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Noborisaka Y. Smoking and chronic kidney disease in healthy populations. Nephrourol Mon 5: 655–667, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odoni G, Ogata H, Viedt C, Amann K, Ritz E, Orth SR. Cigarette smoke condensate aggravates renal injury in the renal ablation model. Kidney Int 61: 2090–2098, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Orth SR. Cigarette smoking: an important renal risk factor - far beyond carcinogenesis. Tob Induc Dis 1: 137–155, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orth SR. Smoking and the kidney. J Am Soc Nephrol 13: 1663–1672, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients-absence of evidence or evidence of absence? Clin J Am Soc Nephrol 3: 226–236, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Pachon RE, Scharf BA, Vatner DE, Vatner SF. Best anesthetics for assessing left ventricular systolic function by echocardiography in mice. Am J Physiol Heart Circ Physiol 308: H1525–H1529, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polosa R, Caponnetto P. Regulation of e-cigarettes: the users' perspective. Lancet Respir Med 1: e26, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Ramdas V, McBride M, Denby L, Baker AH. Canonical transforming growth factor-beta signaling regulates disintegrin metalloprotease expression in experimental renal fibrosis via miR-29. Am J Pathol 183: 1885–1896, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saltzman HE, Sharma K, Mather PJ, Rubin S, Adams S, Whellan DJ. Renal dysfunction in heart failure patients: what is the evidence? Heart Fail Rev 12: 37–47, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Staplin N, Haynes R, Herrington WG, Reith C, Cass A, Fellström B, Jiang L, Kasiske BL, Krane V, Levin A, Walker R, Wanner C, Wheeler DC, Landray MJ, Baigent C, Emberson J, SHARP Collaborative Group. Smoking and adverse outcomes in patients with CKD: the study of heart and renal protection (SHARP). Am J Kidney Dis 68: 371–380, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuji H, Fujimoto H, Matsuura D, Nishino T, Lee KM, Yoshimura H. Comparison of biological responses in rats under various cigarette smoke exposure conditions. J Toxicol Pathol 26: 159–174, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuji H, Lee KM, Yoshino K, Nakamura H, Lulham G, Renne R, Yoshimura H. Comparison of the physiological and morphological effects of cigarette smoke exposure at comparable weekly doses on Sprague-Dawley rats. Inhal Toxicol 23: 17–32, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Zhu JN, Chen R, Fu YH, Lin QX, Huang S, Guo LL, Zhang MZ, Deng CY, Zou X, Zhong SL, Yang M, Zhuang J, Yu XY, Shan ZX. Smad3 inactivation and miR-29b upregulation mediate the effect of carvedilol on attenuating the acute myocardium infarction-induced myocardial fibrosis in rat. PLoS One 8: e75557, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]