Abstract
Bronchopulmonary dysplasia (BPD) is the chronic lung disease associated with premature birth, characterized by impaired vascular and alveolar growth. In neonatal rats bleomycin decreases lung growth and causes pulmonary hypertension (PH), which is poorly responsive to nitric oxide. In the developing lung, through Rho kinase (ROCK) activation, ET-1 impairs endothelial cell function; however, whether ET-1–ROCK interactions contribute to impaired vascular and alveolar growth in experimental BPD is unknown. Neonatal rats were treated daily with intraperitoneal bleomycin with and without selective ETA (BQ123/BQ610) and ETB (BQ788) receptor blockers, nonselective ET receptor blocker (ETRB) (bosentan), or fasudil (ROCK inhibitor). At day 14, lungs were harvested for morphometrics, and measurements of Fulton's index (RV/LV+S), medial wall thickness (MWT), and vessel density. Lung ET-1 protein and ROCK activity (phospho-MYPT-1:total MYPT-1 ratio) were also measured by Western blot analysis. Bleomycin increased lung ET-1 protein expression by 65%, RV/LV+S by 60%, mean linear intercept (MLI) by 212%, and MWT by 140% and decreased radial alveolar count (RAC) and vessel density by 40 and 44%, respectively (P < 0.01 for each comparison). After bleomycin treatment, fasudil and bosentan partially restored RAC and vessel density and decreased MLI, RV/LV+S, and MWT to normal values. Bleomycin increased ROCK activity by 120%, which was restored to normal values by bosentan but not selective ETRB. We conclude that ET-1–ROCK interactions contribute to decreased alveolar and vascular growth and PH in experimental BPD. We speculate that nonselective ETRB and ROCK inhibitors may be effective in the treatment of infants with BPD and PH.
Keywords: endothelin-1 (ET-1), Rho kinase (ROCK) endothelin receptor antagonist (ERA), ETA-selective receptor antagonist, ETB-selective receptor antagonist, bronchopulmonary dysplasia (BPD), pulmonary hypertension
bronchopulmonary dysplasia (BPD) is the chronic lung disease that follows neonatal respiratory distress syndrome (RDS) after premature birth (48). In the postsurfactant era, the “new BPD” is characterized by disrupted pulmonary vascular and alveolar growth resulting in a decreased surface area for gas exchange in the newborn lung (1, 16, 37). In addition, disrupted growth of the pulmonary microvasculature limits pulmonary blood flow and predisposes preterm infants to the development of pulmonary arterial hypertension (PAH) (45). For infants with severe BPD, late PAH is associated with poor prognosis and significant mortality (29). In addition to PAH, early lung injury decreases vascular growth, which results in impaired gas exchange and leads to significant postnatal sequelae including respiratory insufficiency, hypoxemia, exercise intolerance, wheezing, severe developmental delay, and, in some cases, death (1, 6, 7, 45). Thus therapeutic interventions that promote endothelial cell survival, growth, and angiogenesis may decrease the subsequent risk for BPD and late respiratory disease. Inhaled nitric oxide (iNO) improves lung structure and prevents the development of pulmonary hypertension (PH) in experimental BPD (8, 42, 57); however, findings from clinical studies of iNO therapy for the prevention of BPD have been inconsistent (5, 39, 44). Mechanisms responsible for inconsistent responses to iNO in human studies remain unclear.
Bleomycin (Bleo) administration has been used extensively to explore mechanisms of pulmonary fibrosis in adult rodents (11, 12). In adult rats, intratracheal Bleo treatment induces an early inflammatory response that is followed by progressive fibroproliferative changes in lung structure over time (11, 12). Marked elevations in lung ET-1 content occurs early after exposure to Bleo and are associated with initial inflammatory and subsequent fibrotic responses (13, 55). Treatment with nonselective ET receptor antagonists (ERA) prevented the development of lung fibrosis and pulmonary hypertension (PH) in this adult model (55). In neonatal rat pups, we and others have shown that systemic (intraperitoneal) Bleo treatment impairs vascular and alveolar growth and causes PH, providing a useful model for studies of mechanisms relevant to human BPD (41, 60). As observed in clinical trials for the prevention of BPD, iNO therapy only partially improved alveolarization after neonatal Bleo, making it a useful model for studying alternate therapies that may be more effective in the setting of poor responsiveness to inhaled NO.
Endothelins are 21-amino acid vasoconstrictor peptides produced primarily by the vascular endothelium and have a key role in vascular homeostasis. Their actions are mediated through ETA receptor on smooth muscle cells and ETB receptors on the endothelium (62). Clinically, increased ET-1 levels are associated with neonatal RDS, BPD, and persistent pulmonary hypertension of the newborn (PPHN) (20, 22, 23, 38, 47), suggesting the importance of ET-1 in the pathogenesis of BPD and neonatal pulmonary hypertension. However, little is known about the potential efficacy of ERAs for the prevention or treatment of preterm newborns at risk for BPD and PH. Rho kinase (ROCK) is a complex signaling pathway that is responsible for maintaining high pulmonary vascular resistance (PVR) and regulating the myogenic response in the normal fetal lung (50, 59). In experimental PPHN, ROCK activity is increased and contributes to endothelial cell dysfunction, including impaired angiogenesis (34). In addition, Bleo administration in neonatal rat pups increases ROCK activity and ROCK inhibition prevents PH and improves lung structure (41). Recently, we reported that ET-1 causes pulmonary artery endothelial cell dysfunction in PPHN through activation of ROCK (36). Whether Bleo causes PH and reduces alveolarization in neonatal rat pups by increasing ET-1 production is unknown, and whether the adverse effect of Bleo on lung structure is mediated through ET-1 activation of ROCK has not been studied.
Therefore, we hypothesized that Bleo exposure impairs lung vascular and alveolar growth in neonatal rats by increasing lung ET-1 content and activation of ROCK. We further speculate that the effects of ET-1 are mediated by activation of both ETA and ETB receptors, suggesting that nonselective ERA therapy may be more effective than selective ERA treatments for the prevention of BPD and PH.
METHODS
Animals
All procedures and protocols were approved by the Animal Care and Use Committee at the University of Colorado, Denver. Pregnant Sprague-Dawley rats were purchased (Harlan Laboratories, Indianapolis, IN) and maintained at Denver's altitude (1,600 m; barometric pressure 630 mmHg; inspired oxygen pressure 122 mmHg) for at least 1 wk before giving birth. Pups were delivered naturally (on day 21), and the litter size was maintained at 10 pups. Animals were fed ad libitum and exposed to 12:12-h light-dark cycles throughout the study period.
Study Design
We specifically sought to determine whether early treatments with ETA, ETB, or combined ETA and ETB inhibitors could prevent the adverse effects of Bleo on lung structure, pulmonary vascular growth, and PH. We designed this study to assess the separate effects of selective ETA (BQ123, BQ610; 0.5 and 1 mg/kg), selective ETB (BQ788; 0.5 and 1 mg/kg), and nonselective ERA (bosentan; 10 and 100 mg/kg) treatments on distal lung growth and PH in a model of neonatal lung injury induced by daily intraperitoneal Bleo injections. These agents were administered daily intraperitoneal from day 2 to day 14 in combination with Bleo. To determine the relative role of ROCK activation, similar studies were performed with fasudil (15 mg/kg) treatment during Bleo administration. Doses for these studies were determined from prior published reports (9, 19, 28, 41, 54, 60). When no effect was seen with BQ-123, experiments were performed with BQ-610, which has ×100 greater potency than BQ-123 for the ET receptor.
Starting on day 2 of life, pups received Bleo (bleomycin sulfate) at a dose of 0.5 mg/kg in 0.9% saline (2 μl/g body wt by 30-gauge needle) or 0.9% saline (vehicle control) by intraperitoneal injection for 10 days. Five animals were studied within each of the study groups and the effect of bosentan and fasudil were assessed in the absence of Bleo (see Fig. 1). At 14 days of age, animals were killed for studies of PH [by measuring right ventricular hypertrophy (RVH)], pulmonary vascular growth (by measuring pulmonary artery density), medial wall thickness (MWT) and alveolarization [by measuring radial alveolar count (RAC)], and mean linear intercept (MLI).
Fig. 1.
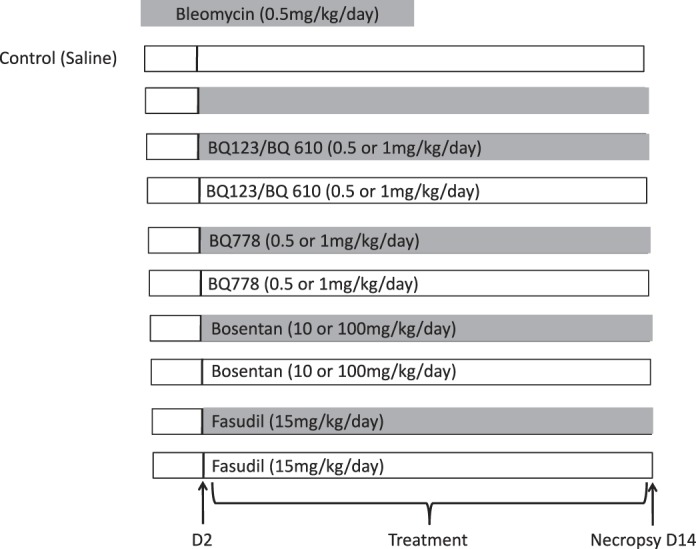
Study designs and treatment protocols. Two-day-old Sprague-Dawley rats received daily bleomycin (0.5 mg/kg) or 0.9% saline by intraperitoneal injection for 14 days. In addition, 2-day-old rat pups also received BQ123 and BQ610 (0.5 and 1 mg/kg) (selective ETA receptor blocker), BQ788 (0.5 and 1 mg/kg) (selective ETB receptor blocker), bosentan (10 and 100 mg/kg) (nonselective endothelin receptor blocker), and fasudil (15 mg/kg) (Rho kinase inhibitor) alone and in combination with bleomycin. Animals were killed at 14 days and lungs and hearts were harvested for histology, morphometric analysis, vessel density, medial wall thickness, and assessment of RVH.
Fixation of Lung Tissue
Animals were killed with intraperitoneal injections of pentobarbital sodium (100 mg/kg). Rat lungs were prepared and fixed in situ at the end of the study (day 14). PBS was infused into the main pulmonary artery through a right ventricular cannula to flush the pulmonary circulation free of blood. A catheter was placed in the trachea, and the lungs were inflated at 25 cmH2O pressure with 4% paraformaldehyde in PBS and maintained under constant pressure for 1 h. This pressure was selected for fixation based on previous studies that demonstrated consistent and uniform lung inflation, and, because the lung was fixed in situ, the chest wall contributes to lower compliance than found in lungs that are completely removed from the thorax. The trachea was ligated to maintain pressure, and the lungs were immersed in paraformaldehyde solution overnight. The lower segment of the left lung was embedded in paraffin, and sections were cut with a microtome set at 5 μm and stained with hematoxylin and eosin. The investigator was blinded to the study group of the sections at the time of morphometric analysis.
Right ventricular hypertrophy.
The heart was removed at autopsy, the right ventricle (RV) and left ventricle plus septum (LV+S) were dissected and weighed, and the ratio of RV to LV+S weights was determined as an assessment of RVH.
Lung morphometric analysis.
Alveolarization was assessed by performing RAC by the method of Emery and Mithal (17). Respiratory bronchioles were identified as bronchioles lined by epithelium in one part of the wall. From the center of the respiratory bronchiole, a perpendicular line was drawn to the edge of the acinus (as defined by a connective tissue septum or the pleura), and the number of septa intersected by this line was counted. Ten counts were performed for each animal from different lung segments. The intra-alveolar distance was measured as the MLI by standard methods, as previously described (4, 17).
Western blot analysis.
We performed Western blot analysis of distal lung homogenates from control, Bleo, and Bleo + selective ETA (BQ123/610), ETB (BQ788), and nonselective endothelin receptor antagonist (ETRA) blockade-treated rat pups after 14 days of treatment. Frozen lung samples were homogenized in ice-cold buffer containing 50 mM Tris·HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA, and 1% Halt protease inhibitor single-use cocktail (Pierce Biotechnology, Rockford, IL). The samples were centrifuged at 1, 500 g for 20 min at 4°C to remove cellular debris. Protein content in the supernatant was determined by the bicinchoninic acid assay [Pierce Biotechnology (catalog no. 23225) Rockford, IL], with bovine serum albumin as the standard. Twenty micrograms of protein sample per lane were resolved by SDS-PAGE, and proteins from the gel were transferred to PVDF membranes.
Phospho-MYPT-1/MYPT-1.
Blots were blocked for 60 min with 5% nonfat dry milk dissolved in TBS with 0.5% Tween 20 (10 mM Tris·HCl, 150 mM NaCl, 0.5% Tween 20, pH 8.0). Blots were then incubated overnight with p-MYPT1 (Thr853) antibody (no. 4563 Cell Signaling, Danvers, MA) (1:1,000) and MYPT-1 (Cell Signaling) (1:1,000). After washing, blots were incubated for 1 h at room temperature with goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary (Santa Cruz Biotechnology, Santa Cruz, CA, SC2054). ROCK activity is expressed as the ratio of p-MYPT-1/β-actin to MYPT-1/β-actin
ET-1.
Blots were blocked for 1 h in 5% nonfat dry milk in TBS with 0.1% Tween 20. These blots were incubated overnight at 4°C with ET-1 (Pierce Antibodies, Thermo Fisher Scientific, Rockford, IL) Antibodies were diluted in 5% nonfat dry milk in TBS with 0.1% Tween 20. After being washed, blots were incubated for 1 h at room temperature with goat anti-mouse IgG HRP antibody (Santa Cruz Biotechnology SC-2064).
p-Akt, Akt.
Blots were blocked for 1 h in 5% nonfat dry milk in TBS with 0.1% Tween 20. These blots were incubated overnight at 4°C with either p-Akt (Cell Signaling, Danvers, MA) or Akt (Santa Cruz Biotechnology) antibodies. Antibodies were diluted in 5% nonfat dry milk in TBS with 0.1% Tween 20. After being washed, blots were incubated for 1 h at room temperature with goat anti-rabbit IgG HRP antibody (Bio-Rad Laboratories, Hercules, CA).
PLC-β.
Blots were blocked for 1 h in 5% nonfat dry milk in TBS with 0.1% Tween 20. These blots were incubated overnight at 4°C with PLC β (Santa Cruz Biotechnology, Santa Cruz, CA) antibody. Antibodies were diluted in 5% nonfat dry milk in TBS with 0.1% Tween 20. After being washed, blots were washed for 1 h at room temperature with goat anti-mouse (Bio-Rad Laboratories).
All blots were stripped and reprobed with an antibody to β-actin (Sigma, A5316) as a loading control.
Bands were visualized by enhanced chemiluminescence (ECL Advance kit; Amersham Pharmacia Biotech, Buckinghamshire, UK). Densitometry was performed using NIH Image (version 1.61) and changes in protein expression were analyzed after normalization for β-actin expression. For all figures representative blots are shown.
IHC.
Immunohistochemistry (IHC) for factor VIII was performed to assess vascular density, and ETAR and ETBR staining was performed to visualize localization with the lung. Paraffin-embedded slides from formalin-fixed tissue were deparaffinized in xylene. The sections were rehydrated by serial immersions in 100% ethanol, 95% ethanol, 70% ethanol, and water. Sections were digested with proteinase K at a concentration of 50 μg/ml for 10 min at room temperature or in Antigen Unmasking Solution (Vector Laboratories, H-3301) boiling for 20 min in a microwave and then washed with phosphate-buffered saline with Tween (PBST) with 2.7 mM KCl, 1.2 mM KH2PO4, 138 mM NaCl, 8.1 mM Na2HPO4, and 0.01% Tween 20. Endogenous peroxidase activity was reduced by immersion in 3% hydrogen peroxide in methanol. An avidin/biotin block (Dako X0590) and Protein Block (Dako X0909) were performed per kit protocols at room temperature. After rinsing, sections were covered in 10% horse serum (factor VII) or 10% goat serum (ETAR and ETBR) for 1 h and incubated with rabbit anti-factor VIII antibody (1:200) or sheep anti-ETAR, ETBR (1:150) diluted in PBST with 10% of the appropriate serum and 1% BSA overnight (no. A0082 Dako USA, Carpenteria, CA). After incubation, the sections were rinsed with PBST and incubated with HRP-labeled secondary antibody diluted 1:2,000 (factor VIII) or 1:500 (ETAR, ETBR) in PBST with 10% appropriate serum and 1% BSA for 1 h. After incubation with the secondary antibody, the sections were rinsed with PBST, incubated in ABC complex (Vector Laboratories, Burlingame, CA) for 30 min at room temperature, rinsed in PBST, and developed with diaminobenzidine and hydrogen peroxide (DAB kit, Vector SK-4100). Slides were lightly counterstained with hematoxylin. The slides were then dehydrated by sequential immersion in 70% ethanol, 95% ethanol, 100% ethanol, and xylene before applying coverslips. For each slide stained for factor VIII, 10 pictures were captured by digital camera for analysis. For assessment of vessel density, images of factor VIII-stained slides were captured with the ×20 objective. The number of factor VIII-positive vessels (<80 μm in size) was counted per each high-power field (HPF) (17).
IF.
Immunofluorescence (IF) for endothelin 1 (ET-1) was performed to localize ET-1 within the lung, and IF for α-smooth muscle actin (α-SMA) was performed to assess vessel thickness. Paraffin-embedded slides from formalin-fixed tissue were deparaffinized in xylene. The sections were rehydrated by serial immersions in 100% ethanol, 95% ethanol, 70% ethanol, and PBS. Antigen retrieval was performed by boiling sections in Antigen Unmasking Solution (Vector Laboratories H-3301) for 20 min in a microwave and then washed with PBST (2.7 mM KCl, 1.2 mM KH2PO4, 138 mM NaCl, 8.1 mM Na2HPO4, and 0.01% Tween 20). Endogenous peroxidase activity was reduced by immersion in 3% hydrogen peroxide in methanol. The sections were the blocked with Protein Block Serum-Free (Dako X0909) per the kit instructions. After rinsing, sections were covered in 10% goat serum for 1 h and then incubated with mouse anti-ET-1 antibody (1:150) or α-SMA (1:200) diluted in PBST with 10% goat serum and 1% BSA overnight (no. A0082 Dako USA). After incubation, the sections were rinsed with PBST and incubated with Alexa Fluor secondary antibody (only done for ET-1 staining) diluted 1:500 in PBST with 10% goat serum and 1% BSA for 1 h. After incubation with the secondary antibody, the sections were rinsed with PBST, incubated with DAPI diluted 1:10,000 in PBST for 5 min, and then rinsed in PBST. Prolong Gold Antifade reagent (Life Technologies, Eugene, OR) was applied to the slides and the coverslip was carefully applied to each slide. For each slide, eight pictures were captured by digital camera for analysis. For assessment of vessel thickness, the images of α-SMA were captured with the ×40 objective. The outside diameter and inside diameter were measured and the vessel thickness percentage was calculated by the following formula: [(external diameter−internal diameter)/external diameter] × 100.
Statistical Analysis
Data are presented as means ± SE. Statistical analysis was performed with the Prism 4 software package (GraphPad Software, San Diego, CA). Statistical comparisons were made using analysis of variance for RAC, MLI, MWT, RV/LV+S, and Western blot analysis using Bonferroni posttest analysis. P < 0.05 was considered significant.
RESULTS
Localization of ET-1 and Endothelin Receptor A and B by IF and IHC Staining in Control Rat Pups and After Bleo Treatment
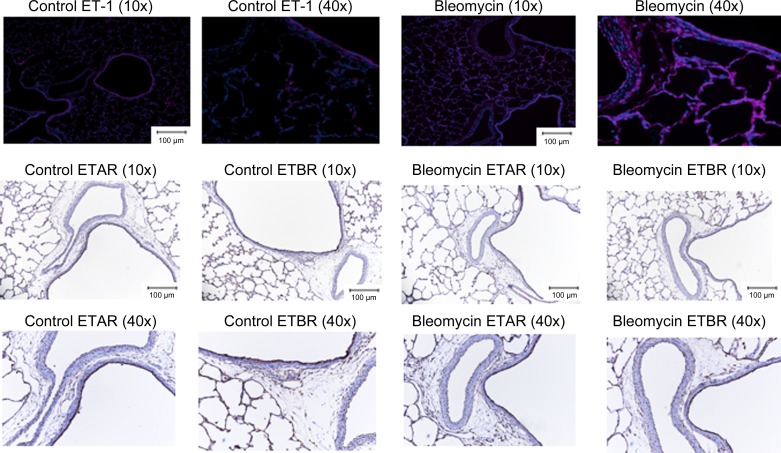
IF and IHC staining was performed on paraffin-embedded slides from formalin-fixed tissue to localize ET-1 (IF staining) (Fig. 2) and ETRA and B (IHC staining) (Fig. 2). In control rats, ET-1 was localized to airway epithelium, endothelium of blood vessels with sporadic cells staining positive in the intestitium. In contrast, after Bleo exposure, ET-1 staining was similar in the airway epithelium, stronger in the endothelium of blood vessels, and much more prevalent throughout the intestitium. In control animals ETAR expression was high in airway lining, light in blood vessel lining, and scattered sporadically through the intestitium. In contrast, in Bleo-exposed animals ETAR expression was increased in the vascular media and airway as well as intestitium. In control animals, ETBR expression was high throughout the intestitium and airway epithelium with low expression in the endothelium and media of blood vessels. In Bleo-exposed animals expression was decreased throughout the interstitium, airway epithelium, and blood vessel endothelium.
Fig. 2.
Localization of lung ET-1, ETA, and ETB receptors by IF and IHC staining in control neonatal rat pups and after 14 days of intraperitoneal bleomycin injection. In control rats, ET-1 was localized to airway epithelium, endothelium of blood vessels with sporadic cells staining positive in the intestitium. In contrast, after bleomycin exposure, ET-1 staining was similar in the airway epithelium, stronger in the endothelium of blood vessels, and much more prevalent throughout the intestitium. In control animals ETAR expression was high in airway lining, light in blood vessel lining, and scattered sporadically through the intestitium. In contrast, in bleomycin-exposed animals ETAR expression was increased in the vascular media and airway as well as intestitium. In control animals, ETBR expression was high throughout the intestitium and airway epithelium with low expression in the endothelium and media of blood vessels. In bleomycin-exposed animals expression was decreased throughout the intestitium, airway epithelium, and blood vessel endothelium.
Intraperitoneal Bleo Increases ET-1 Expression in Neonatal Rat Pups
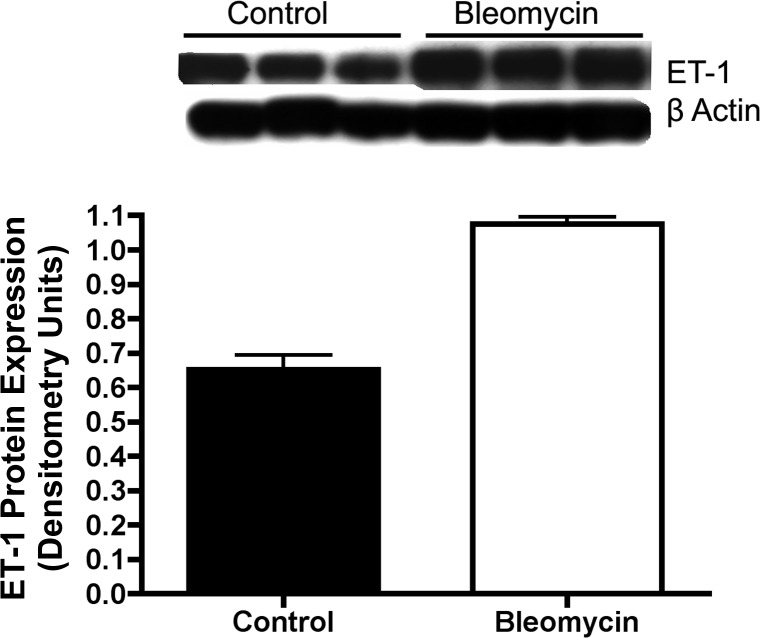
ET-1 protein was measured by Western blot analysis on whole lung homogenates after intraperitoneal Bleo injection in neonatal rat pups. Compared with saline treated controls, Bleo treatment increased whole lung ET-1 expression by 65% (P < 0.01) (Fig. 3).
Fig. 3.
Increased ET-1 protein expression in whole lung homogenates after intraperitoneal bleomycin injection in neonatal rat pups. ET-1 protein was measured by Western blot analysis on whole lung homogenates after 14 days of intraperitoneal bleomycin injections in neonatal rat pups. Compared with controls, bleomycin increased whole lung ET-1 expression by 65% (P < 0.01) (n = 5 animals for each group, representative blot shown).
Effects of Intraperitoneal Bleo, Selective ETA (BQ123/610), Selective ETB (BQ788), and Nonselective (combined) ETA and ETB Blockade on Lung Structure, Vessel Density, and RVH in Neonatal Rat Pups
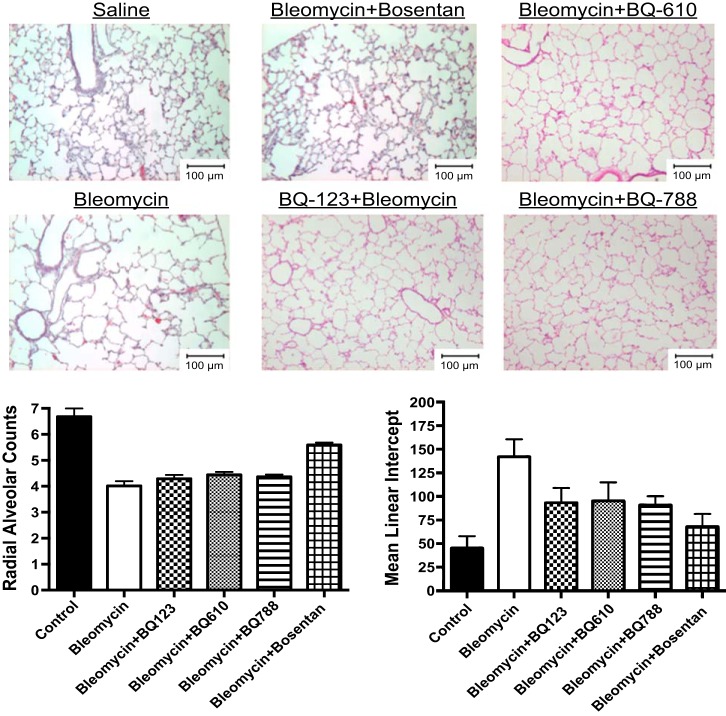
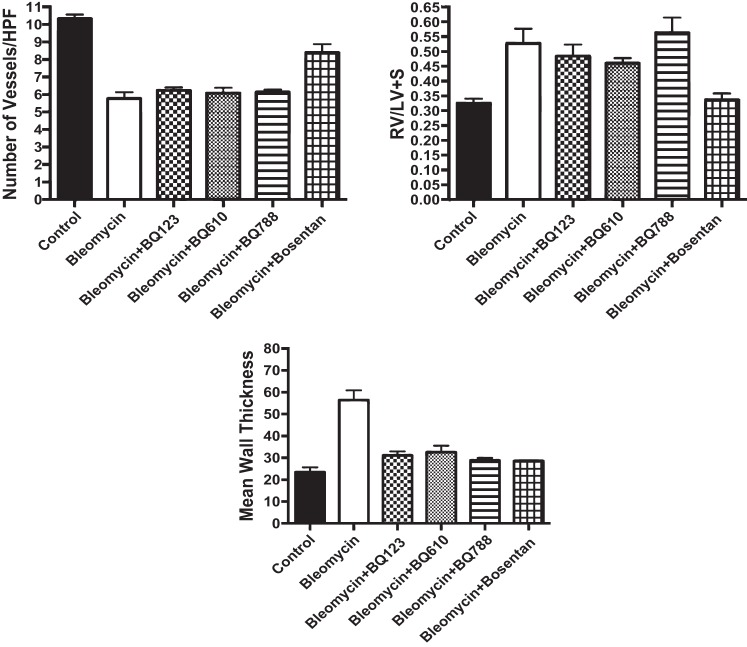
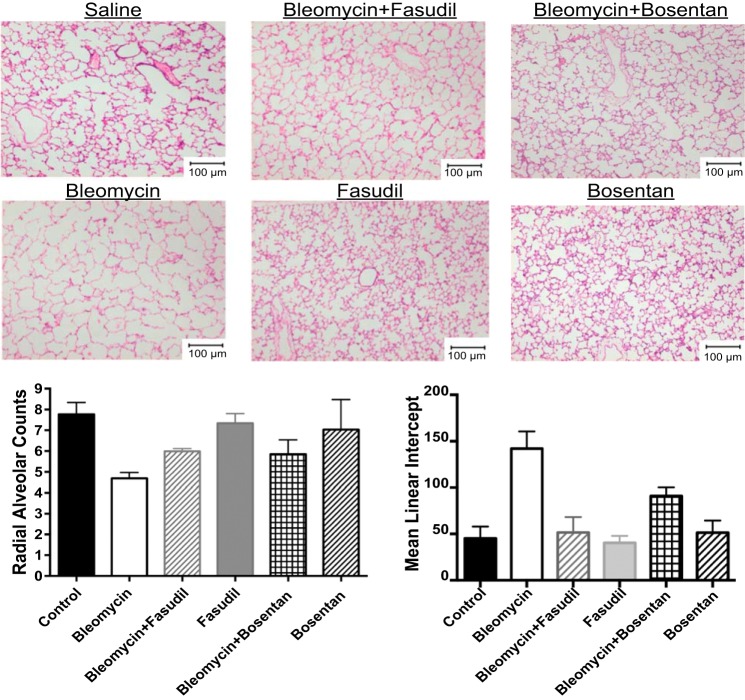
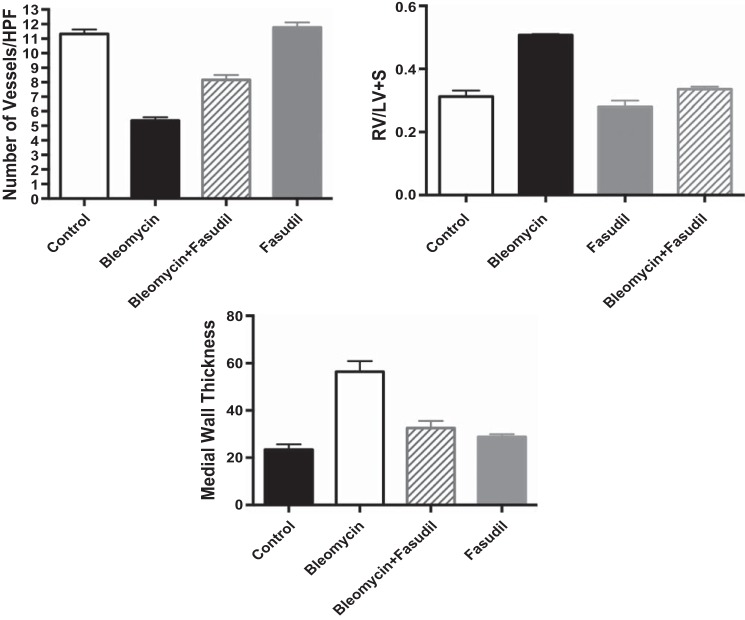
The effect on lung structure was measured by RAC and MLI in neonatal rat pups after exposure to Bleo alone and in combination with BQ123, BQ610, BQ788, and bosentan. Bleo treatment decreased RAC by 40% and increased MLI by 212% (P < 0.01) (Fig. 4). BQ123, BQ610, and BQ788 had no effect on RAC but decreased MLI by 35, 33, and 36%, respectively (P < 0.05 for all comparisons) after Bleo treatment. Bosentan increased RAC by 29% (P < 0.05) and decreased MLI by 52% after Bleo treatment (Fig. 4). Compared with controls, RAC remained decreased by 25% (P < 0.05) and MLI increased by 50% with bosentan treatment. Vessel density was assessed by counting the number of vessels per HPF. Compared with controls, Bleo treatment decreased vessel density by 44% (P < 0.01) (Fig. 5). The addition of bosentan increased vessel density by 40% (P < 0.01); however, compared with controls vessel density remained decreased by 26% (P < 0.05) (Fig. 5). Treatment with BQ123, BQ610, and BQ788 had no effect on vessel density. MWT was calculated by performing staining for smooth muscle actin and calculating (external diameter−internal diameter)/external diameter × 100. Bleo increased MWT by 128% (P < 0.01). Treatment with BQ123, BQ610, BQ788, and bosentan decreased MWT by 45, 42, 49, and 49%, respectively (P < 0.01 for each comparison) restoring MWT to control values (Fig. 5). Bleo treatment increased RV/LV+S by 60% (P < 0.01) (Fig. 5). Bosentan in combination with Bleo decreased RV/LV+S by 36% (P < 0.01), restoring RV/LV+S to control values (Fig. 5). Treatment with BQ123, BQ610, and BQ788 had no effect on RV/LV+S.
Fig. 4.
Bosentan partially restores lung structure after bleomycin exposure in neonatal rat pups. The effect on lung structure was measured by radial alveolar counts (RACs) and mean linear intercepts (MLI) in neonatal rat pups after exposure to bleomycin alone and in combination with BQ123, BQ610, BQ788, and bosentan. Bleomycin treatment decreased RAC by 40% and increased MLI by 212% (P < 0.01) (n = 5 animals for each group, representative histology shown). BQ123, BQ610, and BQ788 had no effect on RAC but decreased MLI by 35, 33, and 36%, respectively (P < 0.05 for all comparisons) after bleomycin treatment (n = 5 animals for each group, representative histology shown). Bosentan increased RAC by 29% (P < 0.05) and decreased MLI by 52% after bleomycin treatment (n = 5 animals for each group, representative histology shown). Compared with controls, RAC remained decreased by 25% (P < 0.05) and MLI increased by 50% (P < 0.05) with bosentan treatment.
Fig. 5.
Effect of intraperitoneal bleomycin, selective ETA (BQ123/610), selective ETB (BQ788), and nonselective ET blockade on vessel density, Fulton's index (RV/LV+S) and medial wall thickness (MWT) in neonatal rat pups. Vessel density was assessed by counting the number of vessels per high-power field (HPF). When compared with controls (n = 5), bleomycin treatment (n = 10) decreased vessel density by 44% (P < 0.01). The addition of bosentan increased vessel density by 40% (P < 0.01) (n = 5); however, compared with controls vessel density remained decreased by 26% (P < 0.05). Treatment with BQ123, BQ610, and BQ788 had no effect on vessel density (n = 5 animals for each group). Bleomycin treatment increased RV/LV+S by 60% (P < 0.01). Bosentan in combination with bleomycin decreased RV/LV+S by 36% (P < 0.01), restoring RV/LV+S to control values. Treatment with BQ123, BQ610, and BQ788 had no effect on RV/LV+S. MWT was calculated by performing staining for α-smooth muscle actin and calculating (external diameter−internal diameter)/external diameter × 100. Bleo increased MWT by 128% (P < 0.01). Treatment with BQ123, BQ610, BQ788, and bosentan decreased MWT by 45, 42, 49, and 49%, respectively (P < 0.01 for each comparison) restoring MWT to control values.
Effect of Intraperitoneal Bleo, Alone or in Combination with Rho Kinase Inhibition (Fasudil) and Nonselective ET Blockade (Bosentan), on Lung Structure Vessel Density, MWT, and RVH in Neonatal Rats
The effect on lung structure was measured by RAC and MLI in neonatal rat pups after exposure to Bleo alone and in combination with fasudil and bosentan (Fig. 6). Bleo treatment decreased RAC by 39% and increased MLI by 212% (P < 0.01 for each comparison). The addition of fasudil increased RAC by 28% (P < 0.05) and decreased MLI by 64% (P < 0.01) after Bleo treatment, restoring MLI to normal values (Fig. 6). Compared with controls, RAC remained decreased by 23% (P < 0.05) with fasudil treatment. This effect on RAC was similar to what was seen with bosentan, which increased RAC by 25% (Fig. 6). Fasudil and bosentan alone had no effect on lung structure in neonatal rat pups. RAC and MLI were unchanged with fasudil and bosentan treatment alone. Vessel density was assessed by counting the number of vessels per HPF. When compared with controls, Bleo treatment decreased vessel density by 53% (P < 0.01) (Fig. 7). The addition of fasudil increased vessel density by 52% (P < 0.01); however, compared with controls vessel density remained decreased by 28% (P < 0.05) (Fig. 7). This effect was similar to what was seen with bosentan, which increased vessel density by 56%. Treatment with fasudil and bosentan alone had no effect on vessel density in neonatal rat pups and values remained similar to controls. Bleo increased MWT by 128% (P < 0.01). Treatment with fasudil decreased MWT 49% (P < 0.01) restoring MWT to control values (Fig. 4). This effect was similar to what was seen with bosentan. Bleo treatment increased RV/LV+S by 63% (P < 0.01) (Fig. 7). Fasudil in combination with Bleo decreased RV/LV+S by 34% (P < 0.01), restoring RV/LV+S to control values (Fig. 7). This effect was similar to what was seen with bosentan, which decreased RV/LV+S by 36%. Treatment with fasudil and bosentan alone had no effect on MWT and RV/LV+S in neonatal rat pups, and values remained similar to controls.
Fig. 6.
Effect of intraperitoneal bleomycin, alone or in combination with Rho kinase inhibition (fasudil) and nonselective ET blockade (bosentan), on lung structure in neonatal rat pups. The effect on lung structure was measured by radial alveolar counts (RACs) and mean linear intercepts (MLI) in neonatal rat pups after exposure to bleomycin alone and in combination with fasudil and bosentan (n = 5 animals for each group, representative histology shown). Bleomycin treatment decreased RAC by 39% and increased MLI by 212% (P < 0.01 for each comparison). The addition of fasudil increased RAC by 28% (P < 0.05) and decreased MLI by 64% (P < 0.01) after bleomycin treatment, restoring MLI to normal values. Compared with controls, RAC remained decreased by 23% (P < 0.05) with fasudil treatment. This effect on RAC was similar to what was seen with bosentan, which increased RAC by 25%. Fasudil and bosentan alone had no effect on lung structure in neonatal rat pups. RAC and MLI were unchanged with fasudil and bleomycin treatment alone.
Fig. 7.
Effect of intraperitoneal bleomycin, alone or in combination with Rho kinase inhibition (fasudil) and nonselective ET blockade (bosentan), on vessel density, Fulton's index (RV/LV+S), and medial wall thickness (MWT) in neonatal rat pups. Compared with controls (n = 5), bleomycin treatment (n = 5) decreased vessel density by 53% (P < 0.01). Fasudil increased vessel density by 52% (P < 0.01) (n = 5 animals); however, compared with controls vessel density remained decreased by 28% (P < 0.05). This effect was similar to what was seen with bosentan, which increased vessel density by 56%. Treatment with fasudil and bosentan alone (n = 5 animals) had no effect on vessel density in neonatal rat pups and values remained similar to controls. Bleo increased MWT by 128% (P < 0.01). Treatment with fasudil decreased MWT 49% (P < 0.01), restoring MWT to control values. This effect was similar to what was seen with bosentan. Bleomycin treatment increased RV/LV+S by 63% (P < 0.01). Fasudil in combination with bleomycin decreased RV/LV+S by 34% (P < 0.01), restoring RV/LV+S to control values. This effect was similar to what was seen with bosentan, which decreased RV/LV+S by 36%. Treatment with fasudil and bosentan alone had no effect on MWT and RV/LV+S in neonatal rat pups, and values remained similar to controls.
Effect of Intraperitoneal Bleo, Alone or in Combination with Nonselective ET Blockade (Bosentan), on Rho Kinase Activity in Neonatal Rat Pups
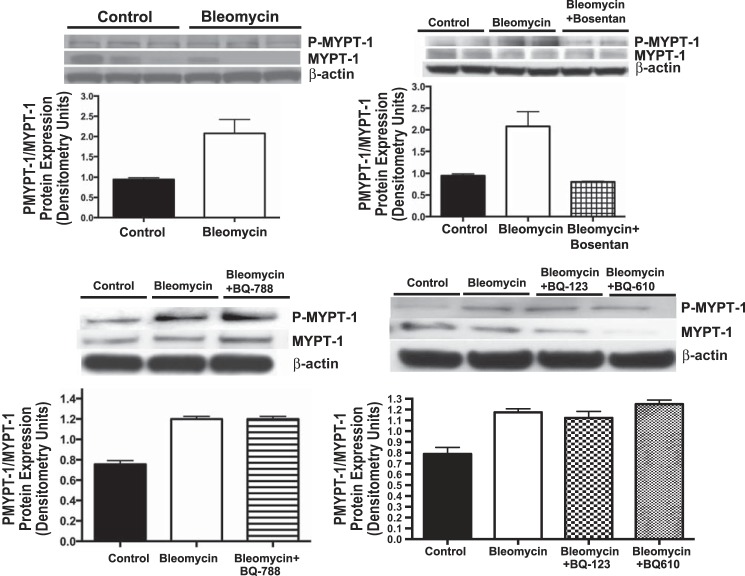
ROCK activity was assessed by measuring the ratio of phosphorylated to total MYPT-1 protein expression (p-MYPT-1:MYPT-1) by Western blot analysis in whole lung homogenates, from neonatal rat pups after Bleo exposure and Bleo in combination with bosentan, BQ123, BQ610, and BQ788. Compared with controls, Bleo increased p-MYPT-1:MYPT-1 protein expression by 119% (P < 0.01) (Fig. 8). Bosentan in combination with Bleo decreased p-MYPT-1:MYPT-1 protein expression by 61% (P < 0.01) restoring ROCK activity to normal (Fig. 8). Compared with Bleo-treated rat pups, BQ123, BQ610, and BQ788 had no effect on p-MYPT-1:MYPT-1 protein expression and ROCK activity remained increased (Fig. 8).
Fig. 8.
Effect of intraperitoneal bleomycin, alone or in combination with selective ETA (BQ123/610), ETB (BQ788), and nonselective ETRB (bosentan), on Rho kinase activity in neonatal rat pups (n = 5 animals for each group, representative blots are shown). Rho kinase activity was assessed by measuring the ratio of phosphorylated to total MYPT-1 protein expression (p-MYPT-1:MYPT-1) by Western blot analysis in whole lung homogenates, from neonatal rat pups. Compared with controls, bleomycin increased p-MYPT-1:MYPT-1 protein expression by 119% (P < 0.01). Bosentan in combination with bleomycin decreased p-MYPT-1:MYPT-1 protein expression by 61% (P < 0.01), restoring Rho kinase activity to normal. Compared with Bleo-treated rat pups, BQ123, BQ610, and BQ788 had no effect on p-MYPT-1:MYPT-1 protein expression, and Rho kinase activity remained increased.
DISCUSSION
In this study we demonstrate a role for ET-1 in the pathogenesis of experimental BPD. ET-1 through activation of ROCK decreases vascular and alveolar growth and contributes to the development of PH. Nonselective ET receptor blockade as well as ROCK inhibition, partially prevent the development of BPD and improve vascular and alveolar growth. Selective ETA and ETB receptor blockade had no effect on lung vascular growth and inconsistent effects on alveolar growth as well as the development of PH. This study provides new insights into the pathogenesis of experimental BPD and provides the rationale for further studies evaluating the efficacy of nonselective ET receptor blockade as well as ROCK inhibition in the setting of BPD.
ET-1 has previously been implicated in the pathogenesis of BPD. In newborn infants with RDS, the degree of elevation in plasma ET-1 concentrations predicted higher risk for the subsequent development of BPD (22, 23). In addition, past work further suggests that lung fibrosis in BPD may also be ET-1 dependent (47). The biological effects of ET-1 are mediated through ET-1 binding to two guanine nucleotide-binding (G-coupled) receptors, ETA and ETB. ETA receptors are present on smooth muscle cells and fibroblasts and mediate proliferation and vasoconstriction (61, 63). ETB receptors are present on the endothelium and to a lesser extent smooth muscle cells, fibroblasts, and macrophages (63). Experimental studies in adult models of PH suggest that ETA receptors mediate many of the detrimental effects of ET-1, such as vasoconstriction and cell proliferation (9, 61, 63). In addition, a previous report has suggested that ETA receptor protein may be expressed in pulmonary artery endothelial cells (PAECs) from adults with idiopathic pulmonary hypertension (30). Several clinical studies have suggested the clinical efficacy of selective ETA receptor blockade in adult and pediatric patients with pulmonary arterial hypertension secondary to a diverse number of disorders (3, 26). Whether these agents have a role in neonatal pulmonary hypertension that occurs in the setting of decreased lung vascular and alveolar growth is unknown. In our study we used two potent selective ETRA antagonists (BQ-123/610) and, compared with nonselective ET receptor blocker (ETRB), demonstrate inconsistent effects on lung alveolar and vascular growth as well as the development of pulmonary hypertension. A possible explanation for this is “receptor cross talk” between ETA and ETB receptors (14). According to this theory, the unopposed ET-1 receptor would compensate for the occupied receptor in the presence of selective antagonism. Evidence supporting this cross talk between ETA and ETB receptors has been obtained in vitro and in vivo (14, 24, 25), and in the case of single ETA blockade a cross talk between ETA and ETB receptors could result in ETB receptor-mediated vasoconstriction and vice versa.
The role of the ETB receptor is more difficult to elucidate. In healthy subjects, ETB receptor activation on the endothelium increases NO and prostacyclin production, prevents apoptosis, inhibits endothelin-converting enzyme, and increases clearance of ET-1 (10, 64). However, the role of these receptors differs considerably in the setting of disease. In adult patients with idiopathic pulmonary hypertension, ETB receptors located on smooth muscle cells and fibroblasts are upregulated in the distal pulmonary vasculature (49) and mediate vasoconstriction, proliferation, and fibrosis (19, 43, 49). Other published studies have shown that ET-1-mediated ETB receptor activation on the endothelium increases reactive oxygen species (21) and thromboxane A2 (TXA2) release (18), proposing detrimental ETB receptor-mediated effects. In addition, we have previously published in isolated PAECs that activation of ROCK and inhibition of PPARγ expression in PPHN PAECs, is secondary to ET-1 activation of the ETB receptor (28, 62). These studies suggest that in the setting of neonatal pulmonary hypertension with impaired vascular growth and alveolar, combined ETA/ETB receptor blockade may be more beneficial than selective ETA receptor blockade alone.
Combined ETRB is frequently used for treating the late manifestations of pulmonary hypertension in BPD (39, 40), with bosentan most commonly used for treating pulmonary hypertension secondary to chronic lung disease (40, 51). These studies are limited to case reports and suggest the efficacy of ETRB in the setting of chronic lung disease and PH. However, these reports are nonrandomized and limited to only a few patients. In addition, ETRBs have been utilized late as rescue therapy rather than early for the prevention of BPD and the long-term sequelae. Our study suggests a role for nonselective ETRB early for the prevention of BPD. When administered in combination with Bleo, nonselective ETRB improved vascular and alveolar growth, prevented the development of pulmonary hypertension, and partially reversed the abnormalities in lung structure seen in the setting of BPD. This study confirms that ET-1 contributes to impaired vascular and alveolar growth in BPD and the importance of both ETA and ETB receptors in the development of BPD. In addition, it is the first report to demonstrate a role for nonselective ETRB for the prevention of BPD.
In addition to elucidating the role of ET-1 and ET receptors in the pathogenesis of BPD, our study suggests that the adverse effects of ET-1 on lung vascular and alveolar growth are mediated through activation of ROCK. Prior studies in adult rats have demonstrated that Bleo administration, through generation of reactive oxygen species, activates ROCK and contributes to the development of pulmonary fibrosis (31). In this study we show that Bleo treatment of neonatal rat pups increased ROCK activity, which was prevented with nonselective ETRB, but not selective ETRA and ETRB receptor inhibition. This finding confirms the importance of combined receptor blockade in experimental BPD and supports the concept of ETA and ETB receptor cross talk. ET-1 is an upstream regulator of ROCK activity (32), and high ET-1 and ROCK activities contribute to high PVR in the normal fetus (33, 50). We have previously demonstrated a link between these two cell signaling pathways in fetal PAECs (28), with ET-1 activation of ROCK decreasing PAEC function and tube formation by PAECs in vitro and ROCK inhibition reversing the adverse effects of ET-1 on tube formation. Our present study extends on this in vitro observation and demonstrates in vivo that ET-ROCK interactions contribute to decreased lung and vascular growth and the development of pulmonary hypertension. By demonstrating in vivo that ROCK inhibition with fasudil increased vascular and alveolar growth, prevented the development of pulmonary hypertension, and partially restored lung structure, we confirm the importance of ET-1–ROCK interactions in the pathogenesis of BPD.
Limitations to the study include the use of Fulton's index as a surrogate for the development of pulmonary hypertension in neonatal rat pups. While invasive measurements of right ventricular systolic pressure would complement the finding of pulmonary hypertension by RV/LV+S, these measurements were not performed. Measurements of MWT were obtained and demonstrate increased MWT with Bleo treatment and restoration to normal with selective and nonselective ETRB and fasudil. PH occurs secondary to increased tone, vascular remodeling, and impaired angiogenesis. Lack of an effect on vessel density with selective ETRB suggests that improved PH after nonselective ETRB and ROCK inhibition is secondary to the effect on vascular growth. Since changes in vascular tone may also contribute to RV remodeling, another potential limitation of the study is that measurements of vascular tone were not performed. Another limitation of the study is that a dose-response curve was not performed with selective ETA and ETB ETRB and established doses of BQ123, BQ610, and BQ-788 were utilized from prior reported studies in the literature. Western blots for PLC β (ETRA) and Akt (ETRB) (data not shown) were performed to confirm blockade of these receptors at these doses. Bleo increased PLC β and Akt expression, which was blocked with these agents, and a more potent effect was seen with BQ610. While it is possible that at higher doses an effect from selective ETRA and ETRB blockade would be seen, based on these data it is unlikely that further receptor blockade would be beneficial. Finally, for morphometric analysis, lungs were inflated at 25 cmH2O pressure with 4% paraformaldehyde in PBS under constant pressure for 1 h. While this is a standard methodology for lung inflation, since flexiVent measurements were not performed to confirm decreased lung compliance in this model, the possibility exists that artifactual “emphysema” may have contributed to changes in RAC and MLI with Bleo treatment.
In conclusion, we demonstrate that ET-1 activation of ROCK decreases lung alveolar and vascular growth and contributes to the development of experimental BPD. Combined but not selective ETRA and ETRB and ROCK inhibition improves vascular and alveolar growth and prevents the development of pulmonary hypertension. This study provides mechanistic insights into the pathogenesis of BPD and confirms the importance of nonselective ETRB in the setting of neonatal pulmonary hypertension and impaired alveolar and vascular growth. We speculate that early treatment with nonselective ETRB and ROCK inhibitors may be effective in preventing the development of BPD.
GRANTS
This study was supported by an Entelligence Young Investigator Award and by National Heart, Lung, and Blood Institute Grants 5K08HL102261 and R01 HL068702-05A2 Basic Science.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.G., N.T., G.J.S., K.K., and S.H.A. conception and design of research; J.G., N.T., G.J.S., K.K., and S.H.A. performed experiments; J.G., N.T., G.J.S., K.K., and S.H.A. analyzed data; J.G., N.T., G.J.S., K.K., and S.H.A. interpreted results of experiments; J.G., K.K., and S.H.A. prepared figures; J.G. drafted manuscript; J.G., N.T., G.J.S., K.K., and S.H.A. edited and revised manuscript; J.G., N.T., G.J.S., K.K., and S.H.A. approved final version of manuscript.
REFERENCES
- 1.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis.” Am J Respir Crit Care Med 164: 1755–1756, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Ambalavanan N, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothelin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res 57: 631–636, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagnall A, Webb D. Are selective endothelin A receptor antagonists better than mixed antagonists? J Cardiovasc Pharmacol 38: S43–S46, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 291: L119–L127, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, Null DR, Hudak ML, Puri AR, Golombek SG, Courtney SE, Stewart DL, Welty SE, Phibbs RH, Hibbs AM, Luan X, Wadlinger SR, Asselin JM, Coburn CE NO. CLD Study Group. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med 357: 1444–1445, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 357: 1946–1955, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 129: e682–e689, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bland RD, Albertine KH, Carlton DP, MacRitchie AJ. Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Respir Crit Care Med 172: 899–906, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonvallet ST, Oka M, Yano M, Zamora MR, Mcmurtry IF, Stelzner TJ. BQ-123, an ETA receptor antagonist, attenuates endothelin-1 induced vasoconstriction in rat pulmonary circulataion. J Cardiovasc Pharmacol 225: 347–350, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Cardillo C, Kilcoyne CM, Cannon RO 3rd, Panza JA. Interactions between nitric oxide and endothelin in the regulation of vascular tone of human resistance vessels in vivo. Hypertension 35: 1237–1241, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med 173: 769–776, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Chen F, Gong L, Zhang L, Wang H, Qi X, Wu X, Xiao Y, Cai Y, Liu L, Li X, Ren J. Short courses of low-dose dexamethasone delay bleomycin-induced lung fibrosis in rats. Eur J Pharmacol 536: 287–295, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol 33: 9–13, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Clozel M, Gray GA. Are there different ETB receptors mediating constriction and relaxation? J Cardiovasc Pharmacol 26, Suppl 3: S262–S264, 2005. [PubMed] [Google Scholar]
- 15.Clozel M, Ramuz H, Clozel JP, Breu V, Hess P, Löffler BM, Coassolo P, Roux S. Pharmacology of tezosentan, new endothelin receptor antagonist designed for parenteral use. J Pharmacol Exp Ther 290: 840–846, 1999. [PubMed] [Google Scholar]
- 16.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 160: 1333–1346, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1-postnatal lung growth. Thorax 37: 572–579, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Orleans-Juste P, Claing A, Telemaque S, Maurice MC, Yano M, Gratton JP. Block of endothelin-1-induced release of thromboxane A2 from the guinea pig lung and nitric oxide from the rabbit kidney by a selective ETB receptor antagonist, BQ-788. Br J Pharmacol 113: 1257–1262, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davie N, Haleen SJ, Upton PD, Polak JM, Yacoub MH, Morrell NW, Wharton J. ETA and ETB receptors modulate the proliferation of human pulmonary artery smooth muscle cells. Am J Respir Crit Care Med 165: 398–405, 2002. [DOI] [PubMed] [Google Scholar]
- 20.de Vroomen M, Lopes Cardozo RH, Steendijk P, Frölich M, Baan J, van Bel F. Endothelin-1 plasma concentration increases in the early phase of pulmonary hypertension development during respiratory distress syndrome: a study in newborn lambs. Early Hum Dev 63: 9–21, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Dong F, Zhang X, Wold LE, Ren Q, Zhang Z, Ren J. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. Br J Pharmacol 145: 323–333, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Sayed M, Sherif L, Said RN, El-Wakkad AS, El-Refay A, Aly H. Endothelin-1 and l-arginine in preterm infants with respiratory distress. Am J Perinatol 2: 129–136, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Figueras-Aloy J, Gómez-Lopez L, Rodríguéz-Miguélez JM, Jordán-García Y, Salvia-Roiges MD, Jiménez W, Carbonell-Estrany X. Plasma endothelin-1 and clinical manifestations of neonatal sepsis. J Perinat Med 32: 522–526, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Fukuroda T, Ozaki S, Ihara M, Ishikawa K, Yano M, Miyauchi T, Ishikawa S, Onizuka M, Goto K, Nishikibe M. Necessity of dual blockade of endothelin ETA and ETB receptor subtypes for antagonism of endothelin-1-induced contraction in human bronchi. Br J Pharmacol 117: 995–999, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuroda T, Ozaki S, Ihara M, Ishikawa K, Yano M, Nishikibe M. Synergistic inhibition by BQ-123 and BQ-788 of endothelin-1-induced contractions of the rabbit pulmonary artery. Br J Pharmacol 113: 336–338, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 117: 3010–3019, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Gien J, Seedorf GJ, Balasubramaniam V, Tseng N, Markham N, Abman SH. Chronic intrauterine pulmonary hypertension increases endothelial cell Rho kinase activity and impairs angiogenesis in vitro. Am J Physiol Lung Cell Mol Physiol 295: L680–L687, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gien J, Tseng N, Seedorf G, Roe G, Abman SH. Endothelin-1 impairs angiogenesis in vitro through Rho kinase activation after chronic intrauterine pulmonary hypertension in fetal sheep. Pediatr Res 73: 252–262, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman G, Perkin RM, Anas NG, Sperling DR, Hicks DA, Rowen M. Pulmonary hypertension in infants with bronchopulmonary dysplasia. J Pediatr 112: 67–72, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Hall SM, Davie N, Klein N, Haworth SG. Endothelin receptor expression in idiopathic pulmonary arterial hypertension: effect of bosentan and epoprostenol treatment. Eur Respir J 38: 851–860, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol 294: L24–L33, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Homma N, Nagaoka T, Morio Y, Ota H, Gebb SA, Karoor V, McMurtry IF, Oka M. Endothelin-1 and serotonin are involved in activation of RhoA/Rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol 50: 697–702, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Ivy DD, Dubus JW, Fox MF, Kinsella JP, Abman SH. Chronic intrauterine pulmonary hypertension alters endothelin receptor activity in the ovine fetal lung. Pediatr Res 39: 435–442, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Ivy DD, le Cras TD, Parker TA, Zenge JP, Jakkula M, Markham NE, Kinsella JP, Abman SH. Developmental changes in endothelin expression and activity in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 278: L785–L793, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Ivy DD, Parker TA, Abman SH. Prolonged endothelin B receptor blockade causes pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 279: L758–L765, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Ivy DD, Parker TA, Ziegler JW, Galan HL, Kinsella JP, Tuder RM, Abman SH. Prolonged endothelin A receptor blockade attenuates chronic pulmonary hypertension in the ovine fetus. J Clin Invest 99: 1179–1186, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jobe AH. The new BPD: an arrest of lung development. Pediatr Res 46: 641–643, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Kambas K, Chrysanthopoulou A, Kourtzelis I, Skordala M, Mitroulis I, Rafail S, Vradelis S, Sigalas I, Wu YQ, Speletas M, Kolios G, Ritis K. Endothelin-1 signaling promotes fibrosis in vitro in a bronchopulmonary dysplasia model by activating the extrinsic coagulation cascade. J Immunol 186: 6568–6575, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, George TN, Southgate WM, Carriedo H, Couser RJ, Mammel MC, Hall DC, Pappagallo M, Sardesai S, Strain JD, Baier M, Abman SH. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med 355: 354–364, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Krishnan U, Krishnan S, Gewitz M. Treatment of pulmonary hypertension in children with chronic lung disease with newer oral therapies. Pediatr Cardiol 29: 1082–1086, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Lee AH, Dhaliwal R, Kantores C, Ivanovska J, Gosal K, McNamara PJ, Letarte M, Jankov RP. ROCK inhibitor prevents bleomycin-induced injury in neonatal rats independent of effects on lung inflammation. Am J Respir Cell Mol Biol 50: 61–73, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 58: 22–29, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Masaki T. Possible role of endothelin in endothelial regulation of vascular tone. Annu Rev Pharmacol Toxicol 35: 235–255, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Mercier JC, Hummler H, Durrmeyer X, Sanchez-Luna M, Carnielli V, Field Greenough A, Van Overmeire B, Jonsson B, Hallman M, Baldassarre J EUNO. Study Group. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet 376: 346–354, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Mourani PM, Mullen M, Abman SH. Pulmonary hypertension in bronchopulmonary dysplasia. Progr Pediatr Cardiol 27: 43–48, 2009. [Google Scholar]
- 46.Mutsaers SE, Foster ML, Chambers RC, Laurent GJ, McAnulty RJ. Increased endothelin-1 and its localization during the development of bleomycin-induced pulmonary fibrosis in rats. Am J Respir Cell Mol Biol 18: 611–619, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Niu JO, Munshi UK, Siddiq MM, Parton LA. Early increase in endothelin-1 in tracheal aspirates of preterm infants: correlation with bronchopulmonary dysplasia. J Pediatr 132: 965–970, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Northway WH, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 276: 357–368, 1967. [DOI] [PubMed] [Google Scholar]
- 49.Ohlstein EH, Arleth A, Bryan H, Elliot JD, Sung CP. The selective ETA receptor antagonist BQ-123 antagonizes endothelin-1 mediated mitogenesis. Eur J Pharmacol 225: 347–350, 1992. [DOI] [PubMed] [Google Scholar]
- 50.Parker TA, Roe G, Grover TR, Abman SH. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 291: L976–L982, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Radicioni M, Bruni A, Camerini P. Combination therapy for life-threatening pulmonary hypertension in a premature infant: first report on bosentan use. Eur J Pediatr 170: 1075–1078, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg AA, Kennaugh J, Koppenhafer SL, Loomis M, Chatfield BA, Abman SH. Elevated immunoreactive endothelin-1 levels in newborn infants with persistent pulmonary hypertension. J Pediatr 123: 109–114, 1993. [DOI] [PubMed] [Google Scholar]
- 53.Rugolotto S, Errico G, Beghini R, Ilic S, Richelli C, Padovani EM. Weaning of epoprostenol in a small infant receiving concomitant bosentan for severe pulmonary arterial hypertension secondary to bronchopulmonary dysplasia. Minerva Pediatr 58: 491–494, 2006. [PubMed] [Google Scholar]
- 54.Sato K, Oka M, Hasunuma K, Ohnishi M, Sato K, Kira S. Effects of separate and combined ETA and ETB blockade on ET-1-induced constriction. Am J Physiol Lung Cell Mol Physiol 269: L668–L672, 1995. [DOI] [PubMed] [Google Scholar]
- 55.Schroll S, Arzt M, Sebah D, Nüchterlein M, Blumberg F, Pfeifer M. Improvement of bleomycin-induced pulmonary hypertension and pulmonary fibrosis by the endothelin receptor antagonist Bosentan. Respir Physiol Neurobiol 170: 32–36, 2010. [DOI] [PubMed] [Google Scholar]
- 56.Takatsuki S, Rosenzweig EB, Zuckerman W, Brady D, Calderbank M, Ivy DD. Clinical safety, pharmacokinetics, and efficacy of ambrisentan therapy in children with pulmonary arterial hypertension. Pediatr Pulmonol 48: 27–34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang JR, Markham NE, Lin YJ, McMurtry IF, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol 287: L344–L351, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Thurlbeck WM. The internal surface area of nonemphysematous lungs. Am Rev Respir Dis 95: 765–773, 1967. [DOI] [PubMed] [Google Scholar]
- 59.Tourneux P, Chester M, Grover T, Abman SH. Fasudil inhibits the myogenic response in the fetal pulmonary circulation.. Am J Physiol Heart Circ Physiol 295: H1505–H1513, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tourneux P, Markham N, Seedorf G, Balasubramaniam V, Abman SH. Inhaled nitric oxide improves lung structure and pulmonary hypertension in a model of bleomycin-induced bronchopulmonary dysplasia in neonatal rats. Am J Physiol Lung Cell Mol Physiol 297: L1103–L1111, 2009. [DOI] [PubMed] [Google Scholar]
- 61.Wedgwood S, Dettman RW, Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 281: L1058–L1067, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Wolf D, Tseng N, Seedorf G, Roe G, Abman SH, Gien J. Endothelin-1 decreases endothelial PPARγ signaling and impairs angiogenesis after chronic intrauterine pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 306: L361–L371, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong J, Fineman JR, Heymann MA. The role of endothelin and endothelin receptor subtypes in regulation of fetal pulmonary vascular tone. Pediatr Res 35: 664–670, 1994. [DOI] [PubMed] [Google Scholar]
- 64.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988. [DOI] [PubMed] [Google Scholar]