Abstract
Chromatin-modifying enzymes mediate DNA methylation and histone modifications on recruitment to specific target gene loci in response to various stimuli. The key enzymes that regulate chromatin accessibility for maintenance of modifications in DNA and histones, and for modulation of gene expression patterns in response to cigarette smoke (CS), are not known. We hypothesize that CS exposure alters the gene expression patterns of chromatin-modifying enzymes, which then affects multiple downstream pathways involved in the response to CS. We have, therefore, analyzed chromatin-modifying enzyme profiles and validated by quantitative real-time PCR (qPCR). We also performed immunoblot analysis of targeted histone marks in C57BL/6J mice exposed to acute and subchronic CS, and of lungs from nonsmokers, smokers, and patients with chronic obstructive pulmonary disease (COPD). We found a significant increase in expression of several chromatin modification enzymes, including DNA methyltransferases, histone acetyltransferases, histone methyltransferases, and SET domain proteins, histone kinases, and ubiquitinases. Our qPCR validation data revealed a significant downregulation of Dnmt1, Dnmt3a, Dnmt3b, Hdac2, Hdac4, Hat1, Prmt1, and Aurkb. We identified targeted chromatin histone marks (H3K56ac and H4K12ac), which are induced by CS. Thus CS-induced genotoxic stress differentially affects the expression of epigenetic modulators that regulate transcription of target genes via DNA methylation and site-specific histone modifications. This may have implications in devising epigenetic-based therapies for COPD and lung cancer.
Keywords: chromatin modifications, epigenetics, lungs, cigarette smoke, epithelial cells, COPD
chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the developed world. Cigarette smoking is the main cause of this debilitating disease; other major risk factors include indoor air pollution (biomass fuel), outdoor air pollution, occupational dusts, and noxious chemicals. The molecular pathways involved in development of COPD are unclear; in particular, the consequences of the gene-tobacco interaction on long-term gene deregulation via the epigenome have not been extensively studied. Epigenomic effects include covalent posttranslational histone modifications (PTMs), such as acetylation, phosphorylation, methylation, ubiquitination, SUMOylation, and ADP-ribosylation, and DNA methylation, which play complex regulatory roles in transcription (17, 24). These PTMs may act alone or in concert in a context-dependent manner facilitating the activation or repression of chromatin-mediated gene expression (4).
Specific patterns of histone modification have been proposed to constitute a “histone code,” which can be recognized via “decoding machinery” composed of chromatin-associated proteins that recognize modified histones. Core histones are modified by chromatin-modifying enzymes, which exist in cells as parts of multicomponent protein complexes, and these complexes are frequently recruited to chromatin by DNA-bound transcription factors (4). Modified core histones can then be recognized by specific regulatory factors, which in turn can alter the structural properties of chromatin. Chromatin modification enzymes are classified into groups, including histone acetyltransferases (HATs), histone deacetylases (HDACs), histone methyltransferases (HMTs: targeting both lysine and arginine residues), histone demethylases (HDMs), and protein kinases, while DNA methylation is mediated by DNA methyltransferases (DNMTs). The possible link for epigenetic modifications in different disease phenotypes is thought to be due to environmentally induced alterations in gene expression patterns (16), mediated at least in part via epigenomic modification of chromatin.
Chromatin-modifying enzymes direct DNA methylation and histone modifications to specific regions of the genome in response to environmental stimuli, such as cigarette smoke (CS). Chromatin modifications are thus associated with inflammatory, immune, oxidative stress and antioxidant-responsive genes (28). Previous studies have described global gene expression/transcriptome profiling, DNA methylation, and/or histone modifications by CS in lung cells (12, 18, 27, 35, 37), but the key enzymes that regulate chromatin accessibility, gene expression profiles, and histone modifications in response to CS are not known. We, therefore, hypothesize that CS exposure alters the gene expression profiles of chromatin-modifying enzymes in human bronchial epithelial cells (H292) and mouse lungs, which subsequently play crucial roles in the regulation of the epigenome and transcription. CS-induced alteration in the transcription of chromatin-modifying enzymes (DNMTs, HATs/HDACs, and HMTs/HDMs) is likely an important mechanism contributing to modulation of expression of genes involved in the pathogenesis of smoking-induced chronic lung diseases, including COPD and lung cancer. Hence, the aim of this study is to identify the gene expression pattern of epigenetic chromatin modification enzymes in CS extract (CSE)-treated H292 cells and CS-exposed mouse lung (acute and chronic), specifically in reference to their gene expression profiles and downstream effects on target biological functions and distinct signaling pathways. Quantitative real-time PCR (qPCR) approach combined with EpiFactors database searches were used to provide additional insights into how CS-induced differential gene expression of chromatin-modifying enzymes can affect target DNA regions and associated histones, thereby affecting their biological functions.
MATERIALS AND METHODS
Ethics statement and scientific rigor/reproducibility.
All experiments for animal studies were performed in accordance with the standards established by the United States Animal Welfare Act, as set forth by the National Institutes of Health guidelines. The research protocol for mouse studies was approved by the University Committee on Animal Research Committee of the University of Rochester.
We used a rigorous/robust and unbiased approach throughout the experimental plans (e.g., in vitro cells and in vivo mouse model) and during analyzing the results so as to ensure that our data are reproducible, along with full and detailed reporting of both methods and raw/analyzed data. All of the key biological and/or chemical resources that are used in this study were validated and authenticated (methods and resources) and are of scientific standard from commercial sources. Our results adhere to National Institutes of Health standards of reproducibility and rigor.
Materials.
Unless otherwise stated, all biochemical reagents used in this study were purchased from Sigma Chemical (St. Louis, MO). Penicillin-streptomycin, l-glutamine, and RPMI-1640 were obtained from Gibco BRL (Grand Island, NY). Fetal bovine serum (FBS) was obtained from HyClone Laboratories (Logan, UT). Anti-acetyl histone H3 (Lys9) (catalog no. ABE18) and anti-acetyl histone H3 (Lys56) (catalog no. 07–677 and catalog no. 04–1135) were obtained from Millipore. Di-methyl-histone H3 (Lys36) (catalog no. 2901), di-methyl-histone H3 (Lys79) (catalog no. 9757), acetyl histone H4 (Lys12) (catalog no. 2591), histone H3 (catalog no. 9715), and histone H4 (catalog no. 2592) antibodies were obtained from Cell Signaling (Danvers, MA). Anti-acetyl histone H4 (Lys16) (ab61240) and anti-histone H4 (tri-methyl Lys20) (ab9053) antibodies were obtained from Abcam (Cambridge, MA). The antibodies listed above were previously validated for specificity using human and mouse samples (7, 23, 26, 39).
Cell culture.
Human bronchial epithelial (H292) cells derived from human lung mucoepidermoid carcinoma were obtained from the American Type Culture Collection (Manassas, VA). H292 cells were grown in RPMI-1640 medium supplemented with FBS (10%), l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). The cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
Preparation of CSE for treatment.
Research-grade cigarettes (3R4F) were obtained from the Kentucky Tobacco Research and Development Center (Lexington, KY). Ten percent aqueous CSE was prepared by bubbling smoke from one cigarette into 10 ml of serum-free RPMI-1640 at a rate of one cigarette per 2 min, as described previously with modifications (6, 26). Human bronchial epithelial cells were grown in 100-mm culture dishes to 80–90% confluency in respective culture media with 0.5% FBS. Then cells were treated with CSE (1% or 2%) for different duration (time points: 1, 4, and 24 h) at 37°C with 5% CO2. At the end of the treatment, cells were washed with cold sterile Ca2+/Mg2+-free 1× PBS, and harvested cell pellets were store in −80°C for RNA isolation, cDNA synthesis, and qPCR analysis.
Mouse CS exposure.
C57BL6/J mice (Jackson Laboratory, Bar Harbor, ME) was bred and maintained with a 12:12-h light-dark cycle in the vivarium facility of the University of Rochester. Eight- to ten-week-old C57BL/6J mice were exposed to CS using research grade cigarettes (3R4F), according to the Federal Trade Commission protocol (1 puff/min of 2-s duration and 35-ml volume). A Baumgartner-Jaeger CSM2072i automatic CS generating machine (CH Technologies, Westwood, NJ) was used for acute 3-day CS exposure, as described previously (23, 42). The mainstream smoke concentration was set at a value of ∼300 mg/m3 total particulate matter (TPM) by adjusting the flow rate of the diluted medical air, and the level of carbon monoxide in the chamber was ∼350 ppm (42). Mice received two 1-h exposures (1 h apart) daily for 3 consecutive days and were killed at 24 h after the last exposure. For chronic 6 mo of CS exposure, research 3R4F cigarettes were used to generate a mixture of sidestream smoke (89%) and mainstream smoke (11%) using a Teague smoking machine (model TE-10, Teague Enterprises, Woodland, CA) at a concentration of ∼100 mg/m3 TPM (41). Each smoldering/burning cigarette was puffed for 2 s, once every minute, for a total of eight puffs, at a flow rate of 1.05 l/min, to provide a standard puff of 35 cm3. Mice received 5-h exposures per day, 5 days/wk, for 6 mo and were killed 24 h after the last CS exposure. Control mice were exposed to filtered air in an identical chamber, according to the same protocol described for CS exposure.
RNA extraction and real-time PCR.
Total RNA was prepared using RNeasy miniprep kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. RNA samples were quantified by Nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE). Human and mouse epigenetic chromatin modification enzymes RT2 Profiler PCR array was obtained from SABiosciences (Frederick, MD). One microgram of total RNA was used for reverse transcription using RT2 first-strand kit, and the entire cDNA reaction was diluted and distributed amongst the 96 wells of the PCR array plates. All of the real-time PCR reactions were performed with RT2 SYBR Green/ROX PCR master mix either using the ABI 7900HT 384-well block (Applied Biosystems, Carlsbad, CA), or using CFX96 real-time system (Bio-Rad). The results were analyzed using software provided by the manufacturer (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php). Differentially regulated genes between CSE and control at different time points (1, 4, and 24 h) were identified by statistical significance and fold change > 1.5 for in vitro experiments and > 1.2 for in vivo experiments. Fold-change [2^(−delta delta Ct)] is the normalized gene expression [2^(−delta Ct)] in the test sample (CSE/CS) divided the normalized gene expression [2^(−delta Ct)] in the control sample (control/air). The P values are calculated based on a Student's t-test of the replicate 2^(−delta Ct) values for each gene in the control group and treatment groups, and P < 0.01 is considered statistically significant. The discrepancies in RT2 Profiler PCR array data and kinetics of expression (temporal statistics) is due to normalization of housekeeping genes provided in the array. The array data housekeeping genes, such as β2-microglobulin, hypoxanthine phosphoribosyltransferase 1, ribosomal protein L13a (RPL13A), glyceraldehyde-3-phosphate dehydrogenase, and β-actin, were used, and for temporal statistical analysis we used RPL13A as housekeeping control for all of the kinetics of expression data. The selected mouse epigenetic chromatin modification enzyme genes were validated by qPCR primers obtained from a quantitative real-time PCR primer database (qPrimerDepot: https://mouseprimerdepot.nci.nih.gov/). The primer sequences used in this study are given below which were synthesized by IDT (www.idtdna.com): Dnmt1 forward (F) 5′-TTTTCTGTTAAGCCATCTCTTTCC-3′, reverse (R) 5′-AACAGCTCCAGCCCGAGT-3′; Dnmt3a (F) 5′-GCTTTCTTCTCAGCCTCCCT-3′, (R) 5′-CCATGCCAAGACTCACCTTC-3′; Dnmt3b (F) 5′-CTGGCACCCTCTTCTTCATT-3′, (R) 5′-ATCCATAGTGCCTTGGGACC-3′; Hdac2 (F) 5′-TCATCCGGATTCTATGAGGC-3′, (R) 5′-CGGCAAGAAGAAAGTGTGCT-3′; Hdac4 (F) 5′-AGGATTCAGCAGCTCCACAG-3′, (R) 5′-TGAACTTAAGGCACTGACGC-3′; Hat1 (F) 5′-TTTCATCATCCCCAAAGAGC-3′, (R) 5′-AACACAGCAATCGAGCTGAA-3′; Ncoa3 (F) 5′-TTCGCCTAGTCCACTCATCC-3′, (R) 5′-GTGGACTCCGAGATCGGTAA-3′; Prmt1 (F) 5′-GAAACTTCTTCAAGAGGCGG-3′, (R) 5′-GACTCGGGTGAAGATGGC-3′; Prmt5 (F) 5′-TGATTAGACGGGAGGTCAGC-3′, (R) 5′-ACATGGATGTGGTGGCATAA-3′; Prmt8 (F) 5′-GCCAGTAGAGAACACGACCC-3′, (R) 5′-CCCTGAGAACCACAAAGACG-3′; Nsd1 (F) 5′-CCTGGGTGGAGTTTCTTTTTC-3′, (R) 5′-TTTGAGACTCCAGATTGCGA-3′; Aurkb (F) 5′-TTCCGTAGGACTCTCTGGGA-3′, (R) 5′-CCTGGAGAATGGCTCAGAAG-3′; and Usp22 (F) 5′-CAGACATGGCAGACACAGGA-3′, (R) 5′-GGACAACTGGAAGCAAAACC-3′.
Immunoblot analysis.
The levels of proteins were measured using the bicinchoninic acid kit. Twenty-five micrograms of protein from whole lung homogenate were prepared as described (23), subjected to electrophoresis on 14% SDS-PAGE gels, and transferred onto nitrocellulose membranes (Amersham, Arlington Heights, IL). The nitrocellulose membrane was blocked with 5% BSA or milk and subsequently incubated overnight at 4°C with specific primary antibodies (1:1,000 dilution). After three washing steps (10 min each), the levels of protein were detected by probing with specific secondary anti-rabbit, -mouse, or -goat antibody (1:20,000 dilution) linked to horseradish peroxidase (Dako, Santa Barbara, CA) for 1 h, and bound complexes were detected using the enhanced chemiluminescence method (Perkin-Elmer, Waltham, MA).
Human samples.
The lung tissue specimens from normal, life-long nonsmokers, smokers, and patients with COPD were collected by the Department of Medicine and Pathology, Helsinki University Central Hospital (41). The clinical characteristics of the patients used were described previously in detail (41).
EpiFactors database search.
The differentially expressed chromatin modification enzymes in this study from both in vitro and in vivo were searched using a database of human epigenetic factors and complexes available online (http://epifactors.autosome.ru) (21). In brief, the EpiFactors is a web-accessible database that provides broad information about human proteins and complexes involved in epigenetic regulation. This includes epigenetic regulators, protein complexes, targets, and products. EpiFactors contains information on 815 proteins, including 95 histones and protamines (21). We performed a database search using the EpiFactors database for the differentially expressed epigenetic chromatin modification enzymes altered under different experiment conditions used in this study. We determined their possible role in modulating site-specific histone modifications, along with other known protein complexes and targets reported earlier in the literature summaries in the database.
Statistical analysis.
Statistical analysis of significance was calculated using unpaired Student's t-test. Probability of significance compared with control was based on two-tail t-tests. The Benjamini-Hochberg procedure was used to adjust the original P values from qPCR array data to control the false discovery rate at 5%. The multiplicity adjustments were conducted for both in vitro and in vivo array data at each time point to help reduce the overall type I error rate in qPCR array data. Temporal statistical differences (P < 0.05) using in vitro and in vivo qPCR data were analyzed by two-way ANOVA (Tukey's multiple-comparison test) using GraphPad Prism 6 (La Jolla, CA). The results are shown as means ± SE, unless otherwise indicated. P < 0.05 is considered as statistically significant.
RESULTS
CS-induced altered gene expression profiles of human epigenetic chromatin modification enzymes in human bronchial epithelial cells in vitro.
We and others have shown that CSE/CS causes distinct and differential PTMs (e.g., histone acetylation) in vitro and in vivo using immunoblot analysis and mass spectrometry approaches (14, 27). We hypothesized that CSE alters gene expression pattern of epigenetic chromatin modification enzymes, thereby affecting the process of either addition (writer) or removal (eraser) of specific histone modifications in a time-dependent manner in human bronchial epithelial cells (H292). PTMs, such as histone acetylation process, have been well documented to correlate with regulation of proinflammatory gene expression, in both in vitro and in vivo models (8, 11, 38, 39). Human bronchial epithelial cells were treated with CSE at different time points: 1 h (2% CSE), and 4 h and 24 h (1% CSE). Cells were harvested, and RNA isolated to synthesize cDNA, followed by qPCR analysis for human epigenetic chromatin modification enzymes. Interestingly, qPCR array data analysis from H292 cells revealed time-dependent differential modulation of epigenetic chromatin modification enzymes in response to CSE treatment in H292 cells. At early time points (1 or 4 h), expression levels of a smaller number of chromatin modification genes were significantly altered that belong to HDAC (Hdac2) and HAT (Myst4) (data not shown).
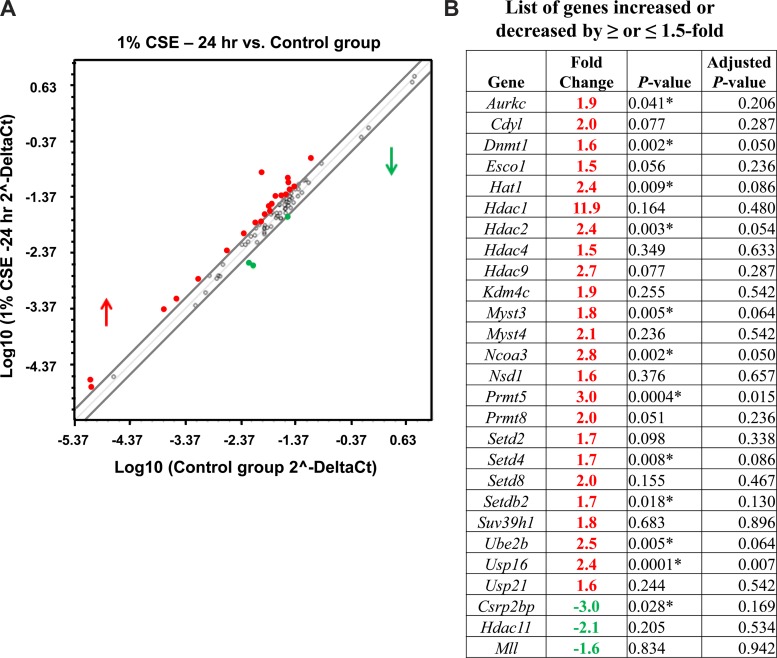
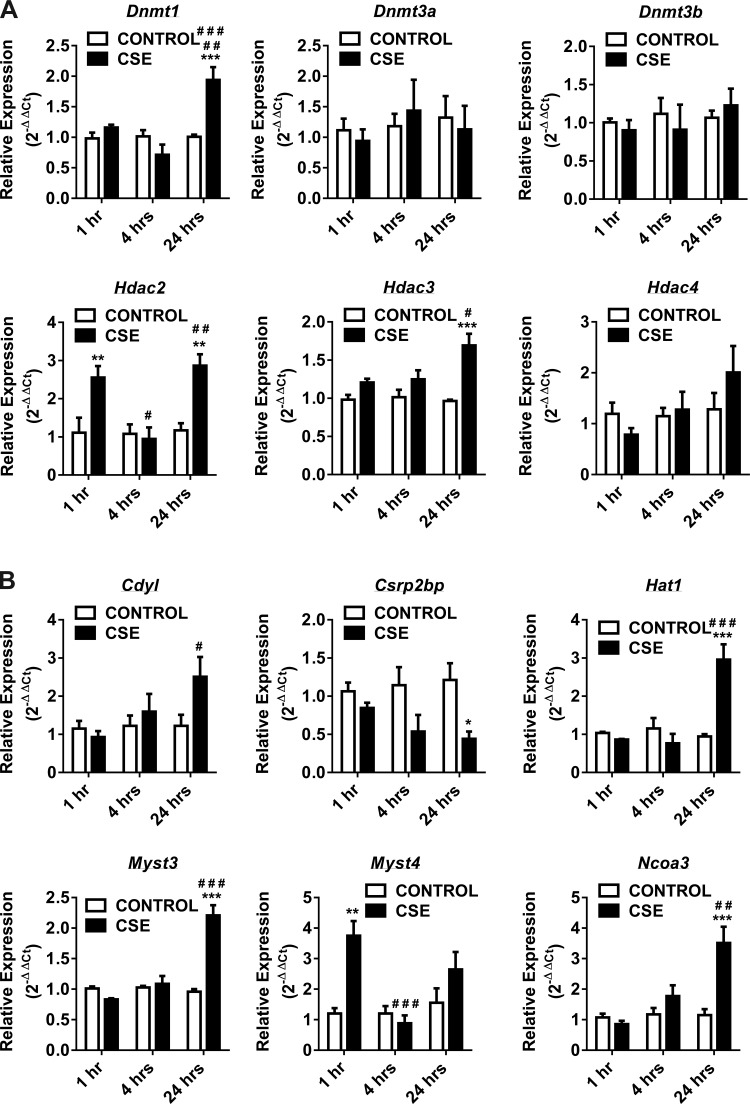
Similarly, when we analyzed qPCR data from the 24-h CSE-treated H292 cells normalized to control, a significant number of human epigenetic chromatin modification enzyme genes were upregulated compared with the genes that were downregulated (Fig. 1). The genes that were significantly upregulated belong to DNMT (Dnmt1), HDACs (Hdac2 and Hdac3) (Fig. 2A), HATs (Cdyl, Hat1, Myst3, and Ncoa3) (Fig. 2B), HMTs (Prmt5 and Nsd1), SET domain proteins (Setdb2 and SetD4) (Fig. 2C), histone phosphorylation (Aurkc), histone ubiquitination (Ube2b), and histone deubiquitination (Usp16) (Fig. 2D). Among them, only Csrp2bp gene expression, which is associated with HAT, was significantly downregulated (Fig. 2B). The key human epigenetic chromatin modification enzyme genes that were significantly up- or downregulated in response to CSE treatment (4 or 24 h) ≥ or ≤1.5-fold changes normalized to control are summarized, along with the scatterplot data (Fig. 1). When we closely compared gene expression profiles from short-time CSE treatment (1 or 4 h) vs. long-time CSE treatment (24 h), most of the upregulated human epigenetic chromatin modification genes belong to HATs and a few HDACs. These data suggest that HATs and HDACs play a crucial role in maintaining the threshold between histone acetylation and deacetylation that is required for transcriptional gene regulation during CSE-induced stress response in vitro in H292 cells.
Fig. 1.
Gene expression profiles of chromatin modification enzymes that were increased above or below 1.5-fold cutoff in 24-h control vs. CSE-treated H292 cells. Human bronchial epithelial cells were treated for 24 h with or without CSE. RNA extracted from control and CSE-treated (1%) cells was analyzed using RT2 Profiler PCR array for human epigenetic chromatin modification enzymes. A: scatterplot analysis of human chromatin modification enzymes show marked upregulation or downregulation of genes by ≥ or ≤1.5-fold in 24-h control vs. CSE-treated (1%) H292 cells. Red denotes high expression (upregulated), and green denotes low expression (downregulated). Values are means ± SE (n = 4/group). B: table shows gene symbol, fold change, P value (Student's t-test; P < 0.01), and Benjamini-Hochberg adjusted P value (multiple-target analysis) for genes altered by CSE compared with control.
Fig. 2.
Gene expression of DNA methyltransferases, histone deacetylases, histone acetyltransferases, histone methyltransferases and SET domain proteins, histone phosphorylation, and histone ubiquitination enzymes at 1, 4, and 24 h in CSE-treated H292 cells. Human bronchial epithelial cells were treated with and without CSE for 1 h (2% CSE) and 4 and 24 h (1% CSE). RNA extracted from control and CSE-treated cells was analyzed using RT2 Profiler PCR array for human epigenetic chromatin modification enzymes. A: the transcription levels of genes encoding specific DNMTs (Dnmt1, Dnmt3a, and Dnmt3b) and HDACs (Hdac2, Hdac3, and Hdac4) were examined by qPCR using the 2−ΔΔCt method. B: the transcription levels of genes encoding specific HATs (Cdyl, Csrp2bp, Hat1, Myst3, Myst4, and Ncoa3) were examined by qPCR using the 2−ΔΔCt method. C: the transcription levels of genes encoding specific HMTs (Prmt1, Prmt5, and Nsd1) and SET domain proteins (Setdb2, Setd4, and Setd5) were examined by qPCR using the 2−ΔΔCt method. D: the transcription levels of genes encoding specific histone phosphorylation (Aurkc and Nek6) and histone ubiquitination (Ube2b, Usp16, and Usp22) were examined by qPCR using the 2−ΔΔCt method. Bar graphs represent the mean normalized expression of samples in control vs. CSE-treated H292 cells. Data were normalized using the endogenous housekeeping gene ribosomal protein L13a (Rpl13a) as reference and controls as calibrators. Statistical significance (P < 0.05) was analyzed by two-way ANOVA (Tukey's multiple-comparison test) using GraphPad Prism 6. Values are means ± SE (n = 4/group). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control. #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. CSE (1 or 4 h).
CS-induced altered gene expression profiles of mouse epigenetic chromatin modification enzymes in mouse lung in vivo.
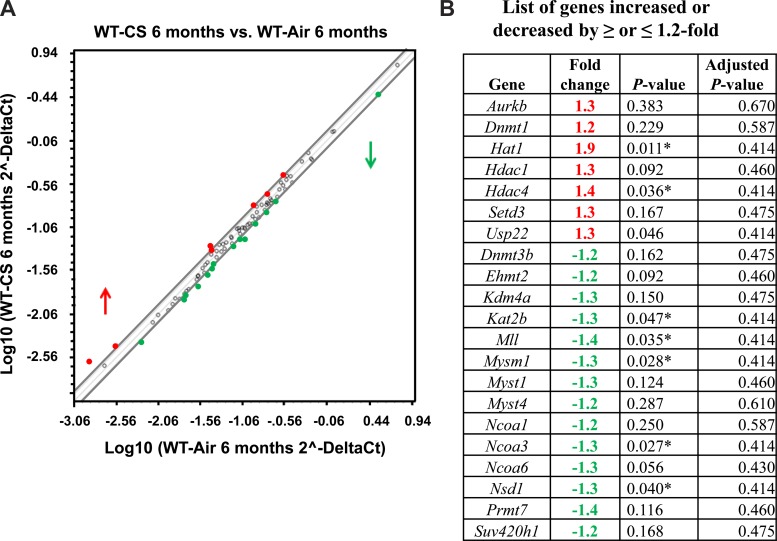
CS exposure leads to hyperacetylation and decreased HDAC activity in the lungs of smokers and patients with COPD, as well as in vivo in rodent lungs exposed to CS and human lung epithelial cells (1, 15, 29). Another report showed elevated hyperacetylation of histone H3.3 in lungs of patients with COPD that were extracellular and degradation resistant (3). We hypothesized that CS exposure alters the gene expression pattern of chromatin modification enzymes, thereby affecting the dynamics of DNA methylation and histone modifications that regulate transcriptional control in vivo in the mouse lung. C57BL/6J mice were exposed to acute (∼300 mg/m3 TPM for 3 days) and chronic (∼100 mg/m3 TPM for 6 mo) CS using different exposure setups, as described in materials and methods. Lung tissues were harvested, and RNA isolated to synthesize cDNA, followed by qPCR analysis for mouse epigenetic chromatin modification enzymes. qPCR array data analysis from mouse chromatin modification enzymes for acute air- and CS-exposed mouse lung showed downregulation of most genes (data not shown), but the chronic model of CS exposure had modest alterations (Fig. 3).
Fig. 3.
Gene expression profiles of chromatin modification enzymes that were increased above or below 1.2-fold cutoff in chronic (6 mo) air- vs. CS-exposed mouse lungs. C57BL6/J mice were exposed to chronic air or CS exposure. RNA extracted from air- and CS-exposed mice were analyzed using RT2 Profiler PCR array for mouse epigenetic chromatin modification enzymes. A: scatterplot analysis of mouse chromatin modification enzymes showing marked upregulation or downregulation of genes by ≥ or ≤1.2-fold in air- vs. CS-exposed mouse lungs. Red denotes high expression (upregulated), and green denotes low expression (downregulated). Values are means ± SE (n = 4/group). B: table showing gene symbol, fold change, P value (Student's t-test; P < 0.01), and Benjamini-Hochberg adjusted P value (multiple-target analysis) for genes altered by CS compared with air.
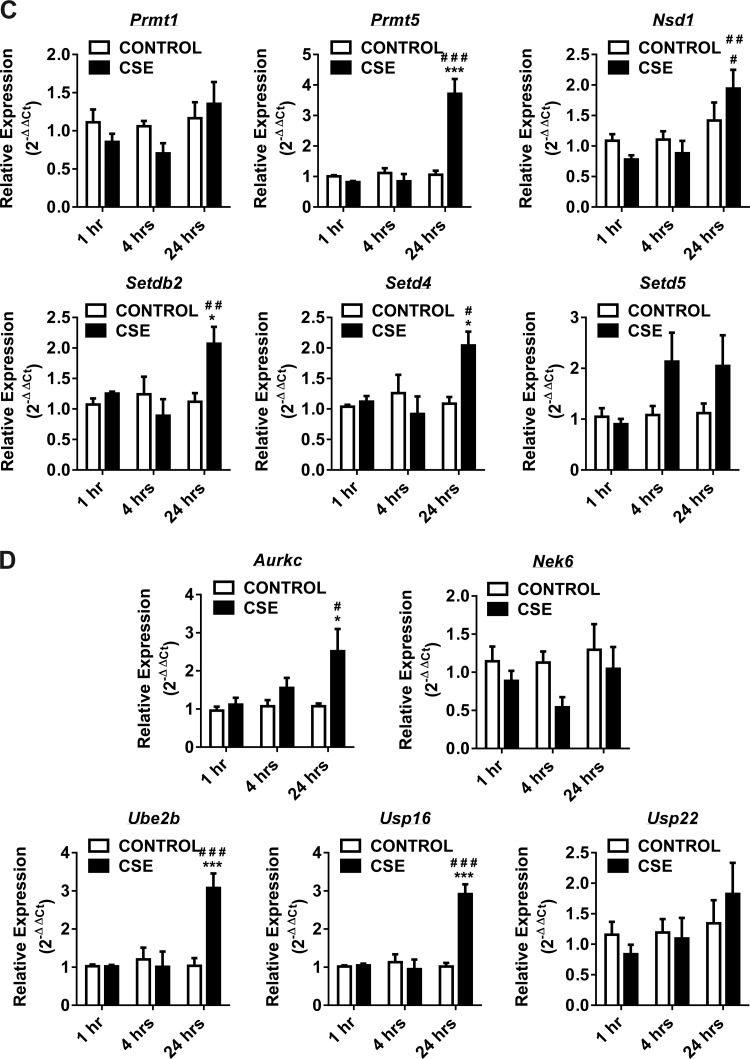
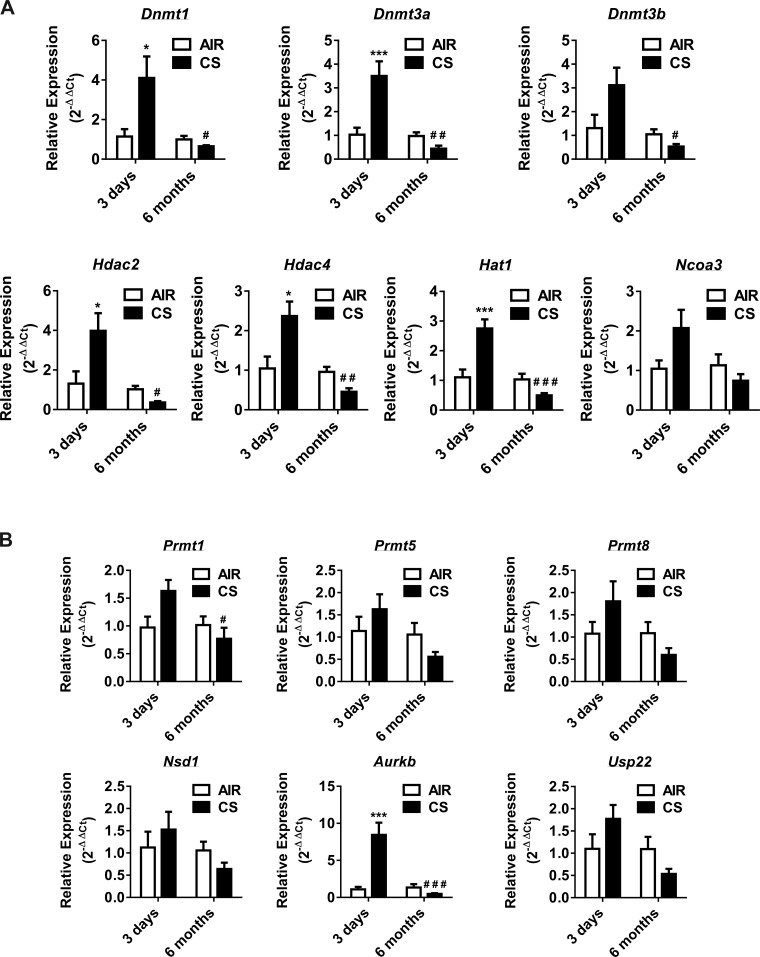
However, no significant differences were evident between acute vs. chronic model in their relative mRNA expression levels analyzed by qPCR array. Based on the initial in vitro and in vivo gene expression data analyzed from qPCR array, we chose the selected epigenetic regulators that showed differential expression pattern under each category (i.e., DNMTs, HDACs, HATs, HMTs, histone phosphorylation, and ubiquitination) for validation by qPCR. In the acute (3 days) CS exposure model, the expression levels for most of the chromatin modification enzyme genes validated, which were significantly upregulated, belonged to DNMTs (Dnmt1 and Dnmt3a), HDACs (Hdac2 and Hdac4), HAT (Hat1), and histone phosphorylation (Aurkb) (Fig. 4). The key mouse epigenetic chromatin modification enzyme genes that were significantly upregulated in response to acute CS exposure (3 days) ≥1.5-fold change normalized to control are shown in Fig. 4.
Fig. 4.
Gene expression of DNA methyltransferases, histone deacetylases, histone acetyltransferases, histone methyltransferases, histone phosphorylation, and histone ubiquitination in acute and chronic air- vs. CS-exposed mouse lungs. C57BL6/J mice were exposed to acute (3 days) and chronic (6 mo) air or CS. RNA extracted from air- and CS-exposed mice were analyzed using qPCR primers for specific mouse epigenetic chromatin modification enzymes. A: the transcription levels of genes encoding specific DNMTs (Dnmt1, Dnmt3a, and Dnmt3b), HDACs (Hdac2 and Hdac4), and HATs (Hat1 and Ncoa3) were examined by qPCR using the 2−ΔΔCt method. B: the transcription levels of genes encoding specific HMTs (Prmt1, Prmt5, Prmt8, and Nsd1), histone phosphorylation (Aurkc), and ubiquitination (Usp22) were examined by qPCR using the 2−ΔΔCt method. Bar graphs represent the mean normalized expression of samples in air group vs. CS-exposed mouse lung. Data were normalized using the endogenous housekeeping gene 18S rRNA as reference and air group control as calibrators. Statistical significance (P < 0.05) was analyzed by two-way ANOVA (Tukey's multiple-comparison test) using GraphPad Prism 6. Values are means ± SE (n = 4–6/group). *P < 0.05 and ***P < 0.001 vs. air. #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. CS (3 days).
Furthermore, when we analyzed the qPCR validation data from the chronic CS-exposed mouse lung normalized to the air group, all of the analyzed genes showed modest changes, but no significant differences were seen in the relative expression of selected mouse epigenetic chromatin modification enzyme genes (Fig. 4). When we compared the relative transcription of these genes with acute CS-exposed mouse lungs, most of the genes were downregulated in chronic CS-exposed mouse lung. The genes that were significantly downregulated belong to DNMTs (Dnmt1, Dnmt3a, and Dnmt3b), HDACs (Hdac2 and Hdac4), HAT (Hat1), HMT (Prmt1), and histone phosphorylation (Aurkb) (Fig. 4). The key mouse epigenetic chromatin modification enzyme genes that were significantly up- or downregulated in response to acute and chronic CS exposure ≥ or ≤1.5-fold change normalized to the air group are shown in Fig. 4. When we closely compared gene expression profiles from acute vs. chronic CS-exposed mouse lungs, most of the downregulated mouse epigenetic chromatin modification genes belong to DNMT, HDACs, HATs, and HMTs, including histone phosphorylation. We noted that there were some discrepancies between mouse lung epigenetic chromatin modification enzyme gene expression array data vs. validation of selective epigenetic regulators. The reason could not be ascertained, but this may be due to different experimental batches of lung tissues used for qPCR array vs. validation of gene expression for acute and chronic exposures. Another reason may be as a result of the temporal and dynamic effect of CS on epigenetic modifications, as seen from our data.
The gene expression profiles were distinct in acute and chronic CS-exposed mouse lungs. This altered transcription of epigenetic regulators may directly or indirectly contribute to the epigenetic state of the lung tissue affecting transcriptional gene regulation of both the upstream and downstream targets during CS-induced stress response in vivo in mouse lung.
CSE-induced changes in histone modifications in mouse lung and patients with COPD.
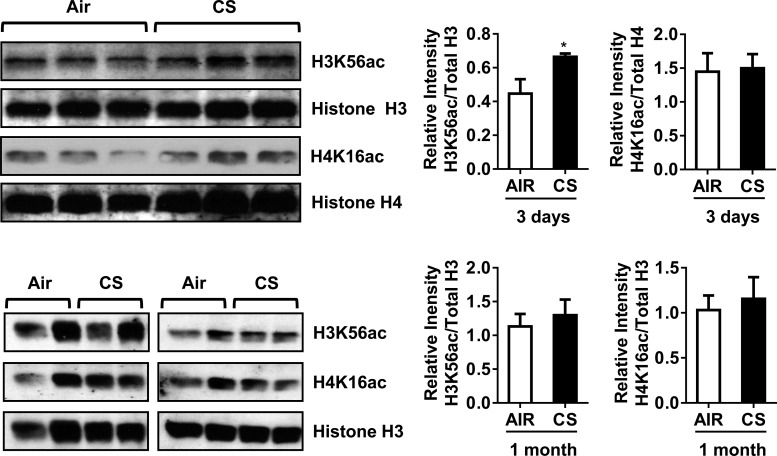
Previously, we have shown that CS induces phospho-acetylation of histone H3S10/H3K9 and acetylation of histone H4K12 in H292 cells (26). Similarly, in rodent lung exposed to acute or subchronic CS, we found distinct histone modifications in H3S10 and H3K9 (phospho-acetylation) and H4K12 acetylation (23). From the qPCR data for human and mouse epigenetic chromatin modification enzymes, we found several HATs and HDACs were significantly affected during acute vs. chronic CS exposure. CS exposure resulted in gene expression of the HATs, such as Cdyl, Hat1, Myst3, and Ncoa3 in vitro or in vivo that could possibly affect the acetylation status of histone H3 and H4. Therefore, we decided to measure the abundance of some the key histone marks, such as H3K56ac and H4K16ac in acute and subchronic (1 mo) CS-exposed mouse lungs in vivo. Acute 3-day CS exposure significantly increased the levels of H3K56ac in mouse lungs compared with air-exposed controls, but we did not observe any significant increase in H4K16ac abundance (Fig. 5).
Fig. 5.
Acute CS exposure increased histone H3K56 acetylation in mouse lungs. Whole lung homogenates were used for immunoblot analysis against anti-histone H3K56 acetylation. The level of H3K56ac, but not H4K16ac, was increased in response to acute (3 days) CS exposure in mouse lungs. Both H3K56ac and H4K16ac remained unaltered in subchronic (1 mo) air- and CS-exposed mouse lungs. The band intensity was measured by densitometry, and data are shown as relative intensity to total histones H3/H4. Values are means ± SE (n = 4/group). *P < 0.05 vs. air.
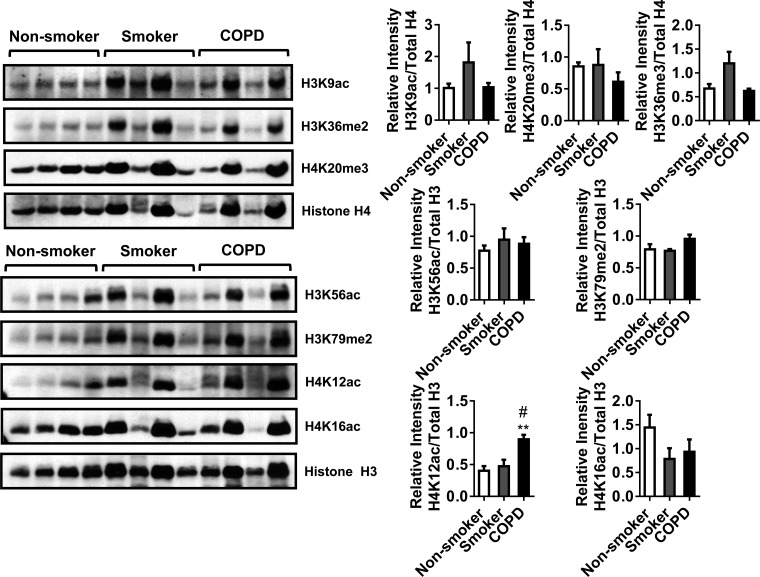
Furthermore, we measured the abundance of H3K56ac and H4K16ac in subchronic 1-mo CS-exposed mouse lung. We did not see any significant changes in the levels of both the histone marks. CS exposure affected the gene expression of specific HATs and HMTs, including SET domain proteins in vitro or in vivo, which possibly could have repercussions on both acetylation and methylation status of histone H3 and H4. Additionally, we measured other histone modifications (H3K9ac, H3K56ac, H3K36me2, H3K79me2, H4K12ac, H4K16ac, and H4K20me3) in human lung homogenates from nonsmokers, smokers, and patients with COPD to determine whether these modifications are significantly altered during the disease state. We found H4K12ac was significantly increased in patients with COPD compared with nonsmokers and smokers. All of the other histone modifications were not significantly altered (Fig. 6). The identified PTMs (histone acetylation) that were found to be altered by CS in mouse lung and in lungs of patients with COPD may be caused by specific HATs that mediate histone acetylation. These data suggest that the gene expression pattern of epigenetic modulators in acute vs. subchronic CS influences the state of PTMs in vivo in the lung.
Fig. 6.
Histone modifications in nonsmokers, smokers, and patients with COPD and with histone H4K12 acetylation. Whole lung homogenates from nonsmokers, smokers, and patients with COPD were used for immunoblot analysis against anti-histone H3K9ac, H3K56ac, H3K36me2, H3K79me2, H4K12ac, H4K16ac, H4K20me3, and total histone H3 and H4. Of all of the histone marks analyzed, the level of H4K12ac was significantly increased in lungs of patients with COPD. The band intensity was measured by densitometry, and data are shown as relative intensity to total histones H3/H4. Values are means ± SE (n = 4/group). **P < 0.01 vs. nonsmoker. #P < 0.05 vs. smoker.
EpiFactors database search.
Based on the available resources from the EpiFactors database reported recently (http://epifactors.autosome.ru/) (21), we performed searches only for the genes that were significantly up- or downregulated in epigenetic chromatin modification enzyme profiling from human bronchial epithelial cells (H292) treated with CSE (4 h and 24 h) or mouse exposed to acute and chronic CS to correlate with their role as “Writer” and “Eraser” of specific PTMs in vitro and in vivo. The EpiFactors database search revealed the specific role of epigenetic chromatin modification enzymes as writers that can cause the marks or erasers that can remove the marks (5, 17, 20); the type of modification they mediate in DNA or histones (such as DNA methylation, acetylation or deacetylation, methylation or demethylation, phosphorylation, ubiquitination or deubiquitination) and epigenetic regulators; and protein complexes that will complement during the process of PTMs in target DNA or histone that are modified at specific sites/residues based on the recent literature.
Interestingly, the database analysis from the significantly up- or downregulated chromatin modification enzyme genes in vitro and in vivo shows differential patterns and possible target-specific modifications that could play a crucial role during CS stress-induced epigenetic gene regulation. In H292 cells treated with CSE (2%) for 1 h, only two genes were significantly upregulated. One was HATs (Myst4), and the other one was HDAC (Hdac2) (Fig. 2, A and B). When we analyzed H292 cells treated with CSE (1%) at 4 h, seven genes were altered. This includes five genes that were upregulated and two genes that were downregulated by CS. Among the upregulated genes, Cdyl and Ncoa3 belong to HATs, which specifically modify histones, including histone H4. The only HAT gene that was significantly downregulated is Csrp2bp. Along with protein complex ATAC, it regulates histone H4 acetylation (data not shown). One of the well-known histone phosphorylation genes, Aurkc, which is known to modify H3S10 and H3S28, was upregulated. The other histone phosphorylation gene, Nek6, that phosphorylates histones H1 and H3, was downregulated by CS. Among the SET domain proteins, Setd5 plays an important role, causing methylation of histones along with other HMTs that were upregulated. Finally, the only HDAC that was upregulated at this time point was Hdac3, which deacetylates H2A, H2B, H3, and H4 (data not shown).
In H292 cells treated with CSE (1%) for 24 h, we found 11 genes that were significantly upregulated. This include writers such as DNMTs (Dnmt1: DNA), HATs (Hat1: H4, H2A acetylation; Myst3: H3 and H4 acetylation along with MOZ/MORF protein complex; Ncoa3: acetylation of histones; Csrp2bp: histone H4 acetylation along with ATAC protein complex), HMTs (Prmt5: H3R8 and H4R3 arginine methyltransferase along with methylosome protein complex; Setdb2: H3K9 methylation), histone phosphorylation (Aurkc: H3S10 and H3S28), histone ubiquitination (Ube2b: H2A, H2BK121 ubiquitination), and the remaining two erasers, including histone deubiquitination (Usp16: H2Aub) and HDAC (Hdac2: H2A, H2B, H3, and H4), remove acetylation of histone marks in H2A, H2B, H3, and H4, along with the coordinated action/interaction of protein complexes such as SWI/SNF_Brg1(I), SWI/SNF-Brg1(II), SWI/SNF_Brm, NuRD, BHS, MeCP1, mSin3A, core HDAC, mSin3A-like complex, RING2-L3MBTL2, and LSD-CoREST (Table 1).
Table 1.
EpiFactors database search for differentially expressed human chromatin modification enzyme qPCR array data, and their functional role as writers or erasers, mediating site-specific histone modifications in 24-h CSE-treated H292 cells
| Gene Symbol | HGNC-approved Name | Writer(s) or Eraser(s) | Type of Modification | Protein Complex | Target (Histones/DNA) |
|---|---|---|---|---|---|
| H292 cells by CSE (1%) at 24 h and control experimental condition | |||||
| ↑Aurkc | Aurora kinase C | Writer | Histone phosphorylation | H3S10, H3S28 | |
| ↑Dnmt1 | DNA (cytosine-5-)-methyltransferase 1 | Writer | DNA methylation | DNA | |
| ↑Hat1 | Histone acetyltransferase 1 | Writer | Histone acetylation | H4, H2A | |
| ↑Myst3 | K(lysine) acetyltransferase 6A | Writer | Histone acetylation | MOZ/MORF | H3, H4 |
| ↑Ncoa3 | Nuclear receptor coactivator 3 | Writer | Histone acetylation | Histone | |
| ↑Prmt5 | Protein arginine methyltransferase 5 | Writer | Histone methylation | Methylosome | H3R8, H4R3 |
| ↑Setdb2 | SET domain, bifurcated 2 | Writer | Histone methylation | H3K9 | |
| ↑Ube2b | Ubiquitin-conjugating enzyme E2B | Writer | Histone ubiquitination | H2A, H2BK121 | |
| ↑Usp16 | Ubiquitin specific peptidase 16 | Eraser | Histone deubiquitination | H2Aub | |
| ↓Csrp2bp | CSRP2 binding protein | Writer | Histone acetylation | ATAC | H4 |
| ↑Hdac2 | Histone deacetylase 2 | Eraser | Histone deacetylation | SWI/SNF_Brg1(I), SWI/S NF_Brg1(II), SWI/SNF_Br m, NuRD, BHC, MeCP1, mSin3A, core HDAC, mSin3A-like complex, RING2-L3MBTL2, LSD-CoREST | H2AKac, H2BKac, H3Kac, H4Kac |
The genes that are significantly up- or downregulated by ≥ or ≤1.5-fold in H292 cells treated with CSE for 24 h are denoted by an up arrow (upregulated) or a down arrow (downregulated) next to the gene symbol.
Additionally, when we analyzed acute (3 days) CS-exposed mouse lung epigenetic chromatin modification enzymes gene expression profiles, only one writer that mediates histone phosphorylation (Aurkb: H3S10 and H3S28) was upregulated. All of the remaining 13 genes that belong to other classes of chromatin modification enzymes, such as DNMT (Dnmt3a: DNA), HAT (Kat5: H2AK5, H3K14, H4K5, K4K8, K4K12, H4K16 acetylation in coordination with SWR, NUA4, Piccolo_NuA4 protein complex), HMTs, including SET domain protein (Mll5: H3K4; PRMT1: H4R3; Setd1b: H3K4 methylation along with COMPASS protein complex), HDM (erasers, Kdm5c: H3K4me3; Kdm4a: H3K4me3 and H3K36me3), histone phosphorylation (Rps6ka5: H2AS1, H3S10, H3S28), histone deubiquitination (eraser, Usp21: H2Aub), and HDACs (erasers, Hdac8: H2a, H2B, H3 and H4; Hdac9 H3 and H4 deacetylation), were significantly downregulated in acute CS-exposed mouse lung (data not shown). When we analyzed the chronic (6 mo) CS-exposed mouse lung epigenetic chromatin modification enzymes gene expression profiles, only two genes were upregulated. One belonged to writer HAT (Hat1: H2A and H4 acetylation), and the other was an eraser HDAC (Hdac4: H2A, H2B, H3 and H4 deacetylation). The remaining five other chromatin modification genes (writers, Kat2b and Ncoa3: histone acetylation; Mll: H3K4 methylation mediated by interaction with MLL-HCF, CHD8, COMPASS-like MLL1,2 protein complexes; Nsd1: H3K36 and H4K20 methylation; eraser, Mysm1: histone deubiquitination H2Aub) were significantly downregulated (Table 2).
Table 2.
EpiFactors database search for differentially expressed chromatin modification enzyme qPCR array data, and their functional role as writers or erasers, mediating site-specific histone modifications in 6-mo chronic CS-exposed mouse lungs
| Gene Symbol | HGNC-approved Name | Writer(s) or Eraser(s) | Type of Modification | Protein Complex | Target (Histones) |
|---|---|---|---|---|---|
| Chronic CS exposure (6 mo) vs. air exposure experimental condition | |||||
| ↑Hat1 | Histone acetyltransferase 1 | Writer | Histone acetylation | H4, H2A | |
| ↓Kat2b | K(lysine) acetyltransferase 2B | Writer | Histone acetylation | Histone | |
| ↓Mll (Kmt2a) | Lysine (K)-specific methyltransferase 2A | Writer | Histone methylation | MLL-HCF, CHD8, COMPASS-like MLL1,2 | H3K4 |
| ↓Mysm1 | Myb-like, SWIRM and MPN domains 1 | Eraser | Histone deubiquitination | H2Aub | |
| ↓Ncoa3 | Nuclear receptor coactivator 3 | Writer | Histone acetylation | Histone | |
| ↓Nsd1 | Nuclear receptor binding SET domain protein 1 | Writer | Histone methylation | H3K36, H4K20 | |
| ↑Hdac4 | Histone deacetylase 4 | Eraser | Histone deacetylation | H2AKac, H2BKac, H3Kac, H4Kac | |
The genes that are significantly up- or downregulated by ≥ or ≤1.2-fold in chronic CS-exposed mouse lung are denoted by an up arrow (upregulated) or a down arrow (downregulated) next to the gene symbol.
Additionally, when we validated gene expression of selective mouse lungs epigenetic chromatin modification enzymes from acute CS-exposed mouse lungs, three writers that mediate epigenetic alterations include DNMTs (Dnmt1 and Dnmt3a), HAT (Hat1), and histone phosphorylation (Aurkb: H3S10 and H3S28) genes that were significantly upregulated. The remaining two genes belong to erasers, including HDACs (Hdac2 and Hdac4: H2a, H2B, H3, and H4 deacetylation) that were significantly upregulated in acute CS-exposed mouse lungs. Finally, we analyzed the qPCR validation data from chronic CS-exposed mouse lungs. Almost all were modestly altered, but were not statistically significant compared with air group controls. We then compared them with acute 3-day CS-exposed mouse lung, and we found all the four writers and two erasers were significantly downregulated. The writers that were downregulated include DNMTs (Dnmt1, Dnmt3a, and Dnmt3b), HATs (Hat1: H2A and H4 acetylation), HMTs (Prmt1: H4R3), and histone phosphorylation (Aurkb: H3S10, H3S28). The other two erasers belong to HDACs (Hdac2 and Hdac4: H2A, H2B, H3, and H4 deacetylation) that were significantly downregulated compared with acute CS-exposed mouse lungs (Table 3).
Table 3.
EpiFactors database search for differentially expressed mouse chromatin modification enzymes validated by qPCR, and their functional role as writers or erasers, mediating site-specific histone modifications in acute vs. chronic CS-exposed mouse lungs
| Gene Symbol | HGNC-approved Name | Writer(s) or Eraser(s) | Type of Modification | Protein Complex | Target (Histones) |
|---|---|---|---|---|---|
| Acute CS exposure (3 days) vs. chronic CS exposure (6 mo) | |||||
| ↓DNMT1, DNMT3A, and DNMT3B | DNA (cytosine-5-)-methyltransferase 1 | Writer | DNA methylation | DNA | |
| ↓Hdac2 | Histone deacetylase 2 | Eraser | Histone deacetylation | SWI/SNF_Brg1(I), S WI/SNF_Brg1(II), SW I/SNF_Brm, NuRD, BHC, MeCP1, mSin3A, core HDAC, mSin3A-like complex, RING2-L3MBTL2, LSD-CoREST | H2AKac, H2BKac, H3Kac, H4Kac |
| ↓Hdac4 | Histone deacetylase 4 | Eraser | Histone deacetylation | H2AKac, H2BKac, H3Kac, H4Kac | |
| ↓Hat1 | Histone acetyltransferase 1 | Writer | Histone acetylation | H4, H2A | |
| ↓Prmt1 | Protein arginine methyltransferase 1 | Writer | Histone methylation | H4R3 | |
| ↓Aurkb | Aurora kinase B | Writer | Histone phosphorylation | H3S10, H3S28 | |
The genes that are significantly downregulated in chronic vs. acute CS-exposed mouse lung are denoted by a down arrow (downregulated) next to the gene symbol.
DISCUSSION
Several studies have been reported previously investigating gene expression/transcription using either individual components of CS or CSE/CS condensate in cultured lung epithelial cells, in monocytes, or in vivo mouse models (14, 18, 33, 37). None of these studies, however, focused on the gene expression profiles of chromatin-modifying enzymes affected by CS. In this study, we provide a comprehensive analysis of gene expression profiles from human and mouse chromatin modifiers using two different approaches. The first approach involved H292 cells to determine at the cellular level the specific effects of CSE on human lung epithelial cells. In the other approach, we evaluated the effects of CS on whole lung in acute vs. chronic models of CS exposure. We measured altered gene expression profiles by quantitative real-time RT-PCR analysis of expression of chromatin-modifying enzymes. Furthermore, targeted histone modifications mediated by chromatin modification enzymes were evaluated by immunoblot analysis of lungs of mice exposed to CS, along with analysis of lung tissue from nonsmokers, smokers, and patients with COPD.
We find higher expression of HDAC (Hdac2) and HAT (Myst4) in acute (1 h) CSE-treated H292 cells, whereas other genes examined are not significantly altered at this time point. We see early onset of HAT/HDAC alteration due to CSE treatment in H292 cells. Prior reports demonstrated an abnormal inflammatory response and steroid resistance, linking aberrant expression of Hdac2 (2, 28) and Myst4 with tumorigenesis (40). Additional evidence showed that MYST4 was required for RUNX2-dependent transcriptional activation and interaction with p53 in cancer (34, 40). We also observed a time-dependent increase in upregulation of several genes in H292 cells treated with CSE for 1, 4, and 24 h. At 24 h, 11 different chromatin modifier genes were upregulated, including a DNMT (Dnmt1), HATs (Hat1, Myst3, and Ncoa3), HMTs (Prmt5, Setd4, and Setdb2), a histone kinase (Aurkc), histone ubiquitinase (Ube2b) and deubiquitinase (Usp16), and a HDAC (Hdac2). These data revealed that 24-h CSE treatments in human lung epithelial cells could differentially affect the gene expression levels of DNMTs, HATs/HDACs, HMTs/HDMs, histone kinases, and histone ubiquitination/deubiquitination enzymes, which could directly affect transcriptional regulation of downstream targets.
We found a significant difference in chromatin modifier expression between acute CS- vs. chronic CS-exposed mouse lung. Almost all of the genes validated (Dnmt1, Dnmt3a, Dnmt3b, Hdac2, Hdac4, Hat1, Prmt1, and Aurkb) are downregulated in chronic CS-exposed mouse lung compared with acute CS-exposed mouse lung. The genes validated in acute air- vs. CS-exposed mouse lung that were significantly upregulated include Dnmt1, Dnmt3a, Hdac2, Hdac4, Hat1, and Aurkb. We also compared gene expression data from H292 cells treated with CSE for 24 h with 3-day CS-exposed mouse lungs. We found that most of the genes upregulated in both groups were HATs, HDACs, and histone kinases. The differences in gene expression patterns observed in in vitro data vs. in vivo data could be due to cell-type specific effects (primary vs. transformed cells), or might arise from heterogeneity of cell type in lung tissue samples. Furthermore, other factors, such as exposure to cells via air-liquid interface in in vitro cell culture mod els or varying doses of CS used in in vivo exposures, could affect epigenetic modifications. Our chronic CS-exposed mice showed a modest change, but were not significantly different in the relative transcription levels of chromatin-modifying enzymes. This is consistent with the two prior reports that showed a subtle difference (statistically not significant) in the transcription of epigenetic modifiers between secondhand smoke-exposed and controls in mice determined by microarray and RT-PCR analyses (31, 32). Consequently, our data on chromatin-modifying genes after acute and chronic CS exposures explain the differences in their expression patterns.
We have previously shown that CS-mediated activation of mitogen- and stress-activated kinase 1 causes phospho-acetylation of histone H3S10/H3K9 and acetylation of histone H4K12 in bronchial epithelial cells (26). Similarly, in acute or subchronic CS-exposed rodent lung, we observed histone modification in H3S10 and H3K9 (phospho-acetylation) and H4K12 acetylation (23). In the present study, we found a strong correlation between increased gene expression of Aurkc in 24 h. CSE-treated H292 cells and increased gene expression of Aurkb in acute CS-exposed mouse lung; both gene products mediate phosphorylation of H3S10. In another report, we showed histone modifications at H3S10/K9 on NF-κB-inducing kinase promoter in response to CSE in H292 cells (7). We believe that the prior results of increased acetylation and methylation of distinct histone marks upon in vitro and in vivo exposure (CSE/CS) are likely related to upregulation of specific HATs (Hat1, Cdyl, Myst3, Myst4, and Ncoa3) and HMTs (Prmt5, Nsd1, Setdb2, Setd4, and Setdb2) that can induce histone H3 and H4 acetylation and methylation, observed in our data from CSE-treated H292 cell in vitro and mouse lung in vivo (27).
Previously, we used a bottom-up mass spectrometry approach to identify novel site-specific histone modifications in histone H3 and H4 in CSE/CS-exposed vs. control/air-exposed H292 cells and mouse lungs (27). We found that CSE-treated H292 cells exhibited distinct histone PTMs (H3K27me2, H3K36me, H3K56me2, H3K79ac, H4K12ac, H4K20me2, H3K31me2, H4R35me2, and H4R36me2). Our delineation here of altered expression of specific chromatin-modifying enzymes in vitro in H292 cells is likely linked to this prior observation. The altered expression of chromatin modification enzymes directly affects histone PTMs, potentially influencing downstream signaling targets, such as CS-induced DNA damage response, cell cycle, proinflammatory gene regulation, telomeric DNA damage (genomic instability), and cellular senescence (9, 13, 30, 43). The known histone marks that are reported to be involved in CS-induced DNA damage response and replicative stress in human lung epithelial cells include H3K9ac, H3K14ac, H3K56ac, H3K79me2, H4K8ac, H4K12ac, H4K16ac, and H4K20me2 (26, 27) (Tables 1–3).
Our data on acute air- and CS-exposed mouse lung showed a significant increase in histone H3K56ac, but not H4K16ac, even though both are linked to the DNA damage response. We also measured the levels of other PTMs in H3 and H4 (H3K9ac, H3K56ac, H3K36me2, H3K79me2, H4K12ac, H4K16ac, and H4K20me3) in lungs of nonsmokers, smokers, and patients with COPD. We found a significant increase in H4K12ac in the lungs of patients with COPD compared with nonsmokers and smokers, along with an increase in H3K56ac in mouse lung after acute CS exposure. This dynamic nature of histone PTMs in mouse and human lung samples could possibly be a result of alterations in gene expression of specific epigenetic regulators (HATs, HMTs, and SET domain proteins) and coactivator/corepressor protein complexes that coordinately perform their roles. The changes in the site-specific histone modifications can ultimately affect replication, chromatin compaction, transcriptional regulation, and DNA damage response that normally occurs to maintain homeostasis during CS-induced oxidative stress.
There is limited information available regarding the expression of chromatin-modifying enzymes in in vitro and in vivo models of CS exposure. It was previously reported that dose- and time-dependent treatments of long-term CS condensate affected histone modifications (H4K16ac, H4K20me3) and coincided with decreased Dnmt1 and increased Dnmt3b expression. In the same study, the authors have shown that CS induces cancer-associated epigenomic alterations (DNA methylation: hyper- and hypomethylation) in cultured lung epithelial cells (18). CS condensate treatment in human lung cells caused promoter methylation of several genes, including hsa-let-7a-3, Chd1, Cxcl12, Pax5, Rassf2, and Tcf21 (19). Thus our data derived from in vitro and in vivo models of acute vs. chronic CS exposure might be related to epigenomic alterations in target inflammatory and prosenescent genes and their promoters [histone modifications and DNA methylation (hypo- or hypermethylation]). This would then influence the epigenetic mechanisms regulating gene expression during the pathogenesis of smoking-related chronic lung diseases (10, 22, 25, 35). Recently, it has been shown that histone modifications can be modulated by other epigenetic mechanisms; for example, miRNA-218 mediates H3K27 trimethylation through EZH2 (enhancer of zeste 2 polycomb repressive complex 2 subunit) during CSE-induced malignant transformation of human bronchial epithelial cells in vitro (36). Thus the possibility that CS could affect other target-specific epigenetic modulators and protein complexes cannot be ruled out. These molecules may play an important role in coordinating the expression of epigenetic modification enzymes and subsequently affecting target DNA and histones. Further studies are required to identify the potential epigenetic histone marks in a larger cohort of human samples from nonsmokers, smokers, and patients with COPD that can be used as a novel biomarker for severity of the disease.
Limitations of the study.
Our study has several limitations: e.g., the use of human bronchial epithelial (H292) cells rather than primary human cells, although our data are corroborated in mouse and human lungs. Most of the genes that were statistically significant by t-test were not significant after performing the multiple target analysis by the Benjamini-Hochberg procedure to adjust the original P values from the qPCR data to control the false discovery rate at 5% in both in vitro and in vivo. However, we selected specific targets under each category for validation by qPCR. Another limitation of the study is the temporal and dynamic changes in chromatin-modifying gene expression profiles in PCR array vs. validation data based on experimental batches and tissues used. In addition, further studies are required to confirm the involvement of specific chromatin modifier(s) in CS-induced lung pathological responses via mechanistic studies using cell culture and/or animal models.
In conclusion, we show that CSE/CS causes differential expression of chromatin modifiers in vitro in H292 cells and in vivo in mouse lung. A significant increase in gene expression was observed among several chromatin modification enzymes, including DNMTs, HATs, HMTs and SET domain proteins, histone kinases, and ubiquitinases. CS induced histone modifications specifically on H3K56ac and H4K12ac. Thus CS-induced genotoxic stress differentially affects the expression of epigenetic modulators that regulate transcription of target genes via site-specific histone modifications. The findings described have implications in devising potential therapies via epigenetic-based drugs for treating smoking-related chronic lung diseases such as COPD, including lung cancer.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grants 2R01-HL-085613 (to I. Rahman) and 3R01-HL-085613-07S1, American Heart Association Grant RG-305393 (to I. K. Sundar), and National Institute of Environmental Health Sciences Grant P30-ES01247.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
I.K.S. and I.R. conception and design of research; I.K.S. performed experiments; I.K.S. analyzed data; I.K.S. and I.R. interpreted results of experiments; I.K.S. prepared figures; I.K.S. and I.R. drafted manuscript; I.K.S. and I.R. edited and revised manuscript; I.K.S. and I.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Michael Bulger and Janice Gerloff for editing the manuscript, and Dr. Dongmei Li at the Clinical and Translational Science Institute, University of Rochester, for biostatistical analyses.
REFERENCES
- 1.Adenuga D, Rahman I. Protein kinase CK2-mediated phosphorylation of HDAC2 regulates co-repressor formation, deacetylase activity and acetylation of HDAC2 by cigarette smoke and aldehydes. Arch Biochem Biophys 498: 62–73, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adenuga D, Yao H, March TH, Seagrave J, Rahman I. Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am J Respir Cell Mol Biol 40: 464–473, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrero CA, Perez-Leal O, Aksoy M, Moncada C, Ji R, Lopez Y, Mallilankaraman K, Madesh M, Criner GJ, Kelsen SG, Merali S. Histone 3.3 participates in a self-sustaining cascade of apoptosis that contributes to the progression of COPD. Am J Respir Crit Care Med 188: 673–683, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger SL. Histone modifications in transcriptional regulation. Curr Opin Genet Dev 12: 142–148, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Butler JS, Koutelou E, Schibler AC, Dent SY. Histone-modifying enzymes: regulators of developmental decisions and drivers of human disease. Epigenomics 4: 163–177, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carp H, Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis 118: 617–621, 1978. [DOI] [PubMed] [Google Scholar]
- 7.Chung S, Sundar IK, Hwang JW, Yull FE, Blackwell TS, Kinnula VL, Bulger M, Yao H, Rahman I. NF-kappaB inducing kinase, NIK mediates cigarette smoke/TNFalpha-induced histone acetylation and inflammation through differential activation of IKKs. PLoS One 6: e23488, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton AL, Hazzalin CA, Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell 23: 289–296, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Dimauro T, David G. Chromatin modifications: the driving force of senescence and aging? Aging (Albany NY) 1: 182–190, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasanelli F, Baglietto L, Ponzi E, Guida F, Campanella G, Johansson M, Grankvist K, Johansson M, Assumma MB, Naccarati A, Chadeau-Hyam M, Ala U, Faltus C, Kaaks R, Risch A, De Stavola B, Hodge A, Giles GG, Southey MC, Relton CL, Haycock PC, Lund E, Polidoro S, Sandanger TM, Severi G, Vineis P. Hypomethylation of smoking-related genes is associated with future lung cancer in four prospective cohorts. Nat Commun 6: 10192, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447: 972–978, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Hackett NR, Shaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, Witover B, Salit J, Crystal RG. The human airway epithelial basal cell transcriptome. PLoS One 6: e18378, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humpal SE, Robinson DA, Krebs JE. Marks to stop the clock: histone modifications and checkpoint regulation in the DNA damage response. Biochem Cell Biol 87: 243–253, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Ibuki Y, Toyooka T, Zhao X, Yoshida I. Cigarette sidestream smoke induces histone H3 phosphorylation via JNK and PI3K/Akt pathways, leading to the expression of proto-oncogenes. Carcinogenesis 35: 1228–1237, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, Barnes PJ. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 352: 1967–1976, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet 8: 253–262, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouzarides T. SnapShot: histone-modifying enzymes. Cell 131: 822, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Killian JK, Yang M, Walker RL, Hong JA, Zhang M, Davis S, Zhang Y, Hussain M, Xi S, Rao M, Meltzer PA, Schrump DS. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene 29: 3650–3664, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyn-Cook L, Word B, George N, Lyn-Cook B, Hammons G. Effect of cigarette smoke condensate on gene promoter methylation in human lung cells. Tob Induc Dis 12: 15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin-Subero JI, Esteller M. Profiling epigenetic alterations in disease. Adv Exp Med Biol 711: 162–177, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Medvedeva YA, Lennartsson A, Ehsani R, Kulakovskiy IV, Vorontsov IE, Panahandeh P, Khimulya G, Kasukawa T; FANTOM Consortium, Drablos F. EpiFactors: a comprehensive database of human epigenetic factors and complexes. Database (Oxford) 2015: bav067, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu W, Baccarelli A, Carey VJ, Boutaoui N, Bacherman H, Klanderman B, Rennard S, Agusti A, Anderson W, Lomas DA, DeMeo DL. Variable DNA methylation is associated with chronic obstructive pulmonary disease and lung function. Am J Respir Crit Care Med 185: 373–381, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajendrasozhan S, Chung S, Sundar IK, Yao H, Rahman I. Targeted disruption of NF-κB1 (p50) augments cigarette smoke-induced lung inflammation and emphysema in mice: a critical role of p50 in chromatin remodeling. Am J Physiol Lung Cell Mol Physiol 298: L197–L209, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rando OJ, Chang HY. Genome-wide views of chromatin structure. Annu Rev Biochem 78: 245–271, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato T, Arai E, Kohno T, Takahashi Y, Miyata S, Tsuta K, Watanabe S, Soejima K, Betsuyaku T, Kanai Y. Epigenetic clustering of lung adenocarcinomas based on DNA methylation profiles in adjacent lung tissue: its correlation with smoking history and chronic obstructive pulmonary disease. Int J Cancer 135: 319–334, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundar IK, Chung S, Hwang JW, Lapek JD Jr, Bulger M, Friedman AE, Yao H, Davie JR, Rahman I. Mitogen- and stress-activated kinase 1 (MSK1) regulates cigarette smoke-induced histone modifications on NF-kappaB-dependent genes. PLoS One 7: e31378, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundar IK, Nevid MZ, Friedman AE, Rahman I. Cigarette smoke induces distinct histone modifications in lung cells: implications for the pathogenesis of COPD and lung cancer. J Proteome Res 13: 982–996, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundar IK, Yao H, Rahman I. Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases. Antioxid Redox Signal 18: 1956–1971, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szulakowski P, Crowther AJ, Jimenez LA, Donaldson K, Mayer R, Leonard TB, MacNee W, Drost EM. The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174: 41–50, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Tjeertes JV, Miller KM, Jackson SP. Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J 28: 1878–1889, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tommasi S, Zheng A, Besaratinia A. Exposure of mice to secondhand smoke elicits both transient and long-lasting transcriptional changes in cancer-related functional networks. Int J Cancer 136: 2253–2263, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Tommasi S, Zheng A, Besaratinia A. Expression of epigenetic modifiers is not significantly altered by exposure to secondhand smoke. Lung Cancer 90: 598–603, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tommasi S, Zheng A, Yoon JI, Besaratinia A. Epigenetic targeting of the Nanog pathway and signaling networks during chemical carcinogenesis. Carcinogenesis 35: 1726–1736, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Troke PJ, Kindle KB, Collins HM, Heery DM. MOZ fusion proteins in acute myeloid leukaemia. Biochem Soc Symp 73: 23–39, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Vucic EA, Chari R, Thu KL, Wilson IM, Cotton AM, Kennett JY, Zhang M, Lonergan KM, Steiling K, Brown CJ, McWilliams A, Ohtani K, Lenburg ME, Sin DD, Spira A, Macaulay CE, Lam S, Lam WL. DNA methylation is globally disrupted and associated with expression changes in chronic obstructive pulmonary disease small airways. Am J Respir Cell Mol Biol 50: 912–922, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B, Liu Y, Luo F, Xu Y, Qin Y, Lu X, Xu W, Shi L, Liu Q, Xiang Q. Epigenetic silencing of microRNA-218 via EZH2-mediated H3K27 trimethylation is involved in malignant transformation of HBE cells induced by cigarette smoke extract. Arch Toxicol 90: 449–461, 2016. [DOI] [PubMed] [Google Scholar]
- 37.Wright WR, Parzych K, Crawford D, Mein C, Mitchell JA, Paul-Clark MJ. Inflammatory transcriptome profiling of human monocytes exposed acutely to cigarette smoke. PLoS One 7: e30120, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature 423: 655–659, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Yang SR, Valvo S, Yao H, Kode A, Rajendrasozhan S, Edirisinghe I, Caito S, Adenuga D, Henry R, Fromm G, Maggirwar S, Li JD, Bulger M, Rahman I. IKK alpha causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol 38: 689–698, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang XJ, Ullah M. MOZ and MORF, two large MYSTic HATs in normal and cancer stem cells. Oncogene 26: 5408–5419, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Ronty M, Kinnula VL, Rahman I. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest 122: 2032–2045, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174–L1186, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Yuan J, Pu M, Zhang Z, Lou Z. Histone H3-K56 acetylation is important for genomic stability in mammals. Cell Cycle 8: 1747–1753, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]