Abstract
Influenza severity increases with age, with hospitalization and mortality rates during seasonal influenza epidemics being higher in older men than age-matched women. As it is known that with age, circulating testosterone levels decline in males, we hypothesized that reduced testosterone contributes to age-associated increases in influenza severity. A murine model was used to test this hypothesis. As in men, testosterone concentrations were lower in aged (18 mo) than young (2 mo) male C57BL/6 mice. Following inoculation with influenza A virus (IAV), aged males experienced greater morbidity, clinical disease, and pulmonary inflammation than young males, and had lower neutralizing and total anti-influenza IgG antibody responses. Peak titers of virus in the lungs did not differ between aged and young males, but virus clearance was delayed in aged males. In young males, removal of the gonads increased—whereas treatment of gonadectomized males with testosterone reduced—morbidity, clinical illness, and pulmonary pathology, but viral replication was not altered by hormone manipulation in young males. Treatment of aged males with testosterone improved survival following infection but did not alter either virus replication or pulmonary pathology. These results indicate that low concentrations of testosterone, whether induced surgically in young males or naturally occurring in aged males, negatively impact the outcome of influenza.
Keywords: 2009 H1N1, androgen, elderly, pulmonary inflammation, sex steroids
every year in the united states, the disease burden and cost of influenza is disproportionately highest among individuals over age 65. Ninety percent of deaths from seasonal influenza are in people over 65 years of age (40). Although it is well established that immune protection declines in aged individuals, sex-based differences in immunosenescence are not often considered (13). It has been reported that among unvaccinated individuals 65 years or older, males are more likely than females to be hospitalized and succumb to seasonal influenza (10, 46). During the H7N9 outbreak in China, older males were 2.4 times more likely to die following exposure to H7N9 than their female counterparts (8). In aged individuals who receive either the standard or high-dose trivalent inactivated influenza vaccine, antibody titers are two to three-times lower in males than females (11, 13). The mechanisms that mediate reduced protection against influenza in aged males have not been reported.
As in humans, murine studies suggest that dysregulated immune function in aged individuals underlies severe outcome from influenza A virus (IAV) infection. In response to IAVs, aged mice have elevated pulmonary inflammatory responses (41), reduced numbers, and activity of virus-specific CD8+ T cells during virus clearance (47), as well as lower antibody responses to live virus and vaccines (3) than their young adult counterparts. An informal analysis of the literature revealed that, as in humans, a majority of murine studies on age-associated changes in IAV pathogenesis and vaccination either do not report the sex of the mice (∼50%) or use female mice only (∼35%). A minority of studies use either mixed sexes or male mice, which follows general published trends in the fields of immunology and infectious diseases (6).
In men, there is a gradual decline in testosterone secretion that begins after age 30, with the signs and symptoms of testosterone deficiency typically presenting after age 65 (4). Age-related reductions in testosterone production are associated with symptoms, including decreased libido, erectile dysfunction, fatigue, depression, reduced strength, bone loss, and increased abdominal fat (51). Although concerns about the effects of testosterone replacement on cardiovascular disease have been raised (5), most studies suggest that testosterone replacement in aged males with hypogonadism results in health and quality-of-life benefits (5, 38, 51). To date, there are no studies that have considered the immunological consequences of testosterone replacement in aged males. We hypothesize herein that reduced testosterone concentrations are detrimental for susceptibility to and the outcome of influenza and that susceptibility would be reduced and the outcome improved by testosterone replacement. With the knowledge that testosterone can signal through androgen receptors in immune cells to regulate responses to immunological stimuli, including viruses and vaccines (15, 45), and that exogenous treatment of young adult male mice with testosterone generally reduces the synthesis of proinflammatory cytokines (e.g., IFN-γ and TNF-α), increases anti-inflammatory cytokines (e.g., IL-10), and reduces helper T-cell type 1 (Th1) activity (43), we further hypothesize that elevating serum testosterone levels in both hypogonadal young and aged males would improve the outcome of influenza by mitigating inflammation.
MATERIALS AND METHODS
Animals.
Young (10–12 wk of age) and aged (17–18 mo of age) adult male C57BL/6 mice were obtained from Charles River or the National Institute of Aging, respectively. They were housed 3 to 5 per microisolator cage under standard BSL-2 housing conditions, with food and water available ad libitum. All animal procedures were approved by the Johns Hopkins University Animal Care and Use Committee. Experiments were conducted as a series of replicates, and animal numbers are provided in the legends.
Gonadectomy, testosterone administration, and quantification.
Young adult male mice were anesthetized with ketamine/xylazine cocktail and bilaterally gonadectomized, as previously described (32, 33). All animals were given 2 wk to recover from surgery prior to testosterone treatment. Testosterone was administered by subcutaneously implanting a silicone capsule (0.040 in inner diameter, 0.085 in outer diameter, 7.5 mm length) containing 100% crystalline testosterone propionate (Sigma, St. Louis, MO) between the scapulae. The capsules were equilibrated in sterile physiological saline for 12 h at 37°C prior to implantation. Animals in the placebo group received implants of empty capsules. Testosterone concentrations in serum were measured by radioimmunoassay using antibodies from Fitzgerald (Acton, MA) and tracer testosterone ([1,2,6,7-3H(N)]-testosterone) from PerkinElmer (Waltham, MA) or EIA (Immuno-Biological Laboratories, Minneapolis, MN) following a standard steroid extraction. All males were treated with testosterone for 1 wk prior to infection.
Virus infection and quantification.
The mouse-adapted H1N1 IAVs, A/Puerto Rico/8/34 (PR8: H1N1; courtesy of Dr. Maryna C. Eichelberger) or A/California/4/09/H1N1 [ma2009; H1N1; generated by Dr. Andrew Pekosz using a published sequence (48)] were used in all experiments. Mice were anesthetized and then intranasally inoculated with 30 μl of DMEM for the mock infection or maH1N1 diluted in DMEM (PR8 = 0.05 MLD50; ma2009 = 0.1 MLD50). For virus quantification, log10 dilutions of lung homogenates were plated onto a monolayer of Madin-Darby canine kidney (MDCK) cells in replicates of 6 for 5 days at 32°C. Cells were stained with naphthol blue black (Sigma Aldrich) and scored for cytopathic effects (CPE). The 50% tissue culture infectious dose (TCID50) was calculated according to the Reed-Muench method and was used to back titer all inoculums.
Sample collection.
Body mass and rectal temperature were recorded daily for 21 days, and clinical disease scores were recorded at selected time points during the morbidity studies. Clinical disease scores for IAV-infected mice were based on five points, with one point given for each of the following: dyspnea, piloerection, hunched posture, absent escape response, and a fifth point given if deceased (17). For terminal studies, males were euthanized at designated days postinoculation (dpi), at which time serum and whole lungs were collected.
Lung inflation and histopathology.
Lungs were inflated at constant pressure and then fixed for 48 h with buffered zinc formalin fixative (Z-Fix, Anatech, San Diego, CA). Lungs were embedded in paraffin, cut into 5-μm sections, and mounted on glass slides. Consecutive tissue sections were stained with hematoxylin and eosin for histopathological scoring. Tissue sections were evaluated for vascular, bronchiolar, or alveolar inflammation and edema, and were assigned a score on a 0–3 scale (0 = no inflammation, 1 = mild inflammation, 2 = moderate inflammation, and 3 = severe inflammation). The cumulative inflammation score represented the sum of each individual inflammation parameter. The percentage of affected lung area within each section was calculated from binary images created using ImageJ software (National Institutes of Health, Bethesda, MD), and values represent the average of three random ×10 fields within each tissue section (20). Scoring was performed by a single blinded observer in consultation with a boarded veterinary pathologist. Representative images were taken using a Nikon Eclipse E400 at ×20 magnification.
Antibody neutralization assay.
Serum samples were added to serum-free infection media and serially diluted (1:2 dilutions). One hundred TCID50 units of virus were added to the diluted samples and incubated at room temperature for 1 h. The diluted samples and virus were added to MDCK cells at 100% confluence in 96-well plates and incubated overnight at 37°C. After 16–18 h of incubation, the inoculums were removed, the cells were washed with PBS plus calcium and magnesium, fresh infection media were added, and the cells were incubated for 5 days at 32°C. Each sample dilution series was run in quadruplicate, and the titer was calculated as the highest serum dilution that eliminated virus CPE in two out of four wells per dilution.
Anti-influenza total IgG ELISA.
ELISA plates (Microlon 96-well high-binding plates; Greiner Bio-One) were coated overnight at 4°C with 100 ng of purified PR8 or ma2009 virus, after which plates were washed and blocked for 1 h with blocking solution (10% dry skim milk powder in PBS). Plates were washed, duplicate diluted serum samples were added in a twofold series starting at 1:1,000, and plates were incubated at 37°C for 1 h. Anti-mouse IgG secondary antibody (1:5,000; Peroxidase AffiniPure goat anti-mouse IgG; Jackson Immunoresearch Laboratories, Bar Harbor, ME) was added, and plates were incubated for 1 h at 37°C. Reactions were developed with 3,3′,5,5′ tetramethylbenzidine (TMB; BD Biosciences, San Jose, CA) and stopped using 1N HCL. Plates were read at 450-nm absorbance on a plate reader. To determine the antibody titer, a cutoff value was determined by multiplying the average ELISA values of serum from naïve animals at each dilution by 3. The sample ELISA titer was the highest serum dilution of that sample series with a value above the cutoff.
Statistical analyses.
Morbidity and clinical data were analyzed with a MANOVA with one within-subjects variable (days) and one between-subjects variable (treatment), and significant interactions were further analyzed using planned comparisons. Antibody responses, virus titers, and histopathological data were analyzed using two-way ANOVAs or t-tests, and significant interactions were further analyzed using the Tukey method for pairwise multiple comparisons. Mean differences were considered statistically significant if P < 0.05.
RESULTS
Influenza virus infection is more severe in aged compared with young males.
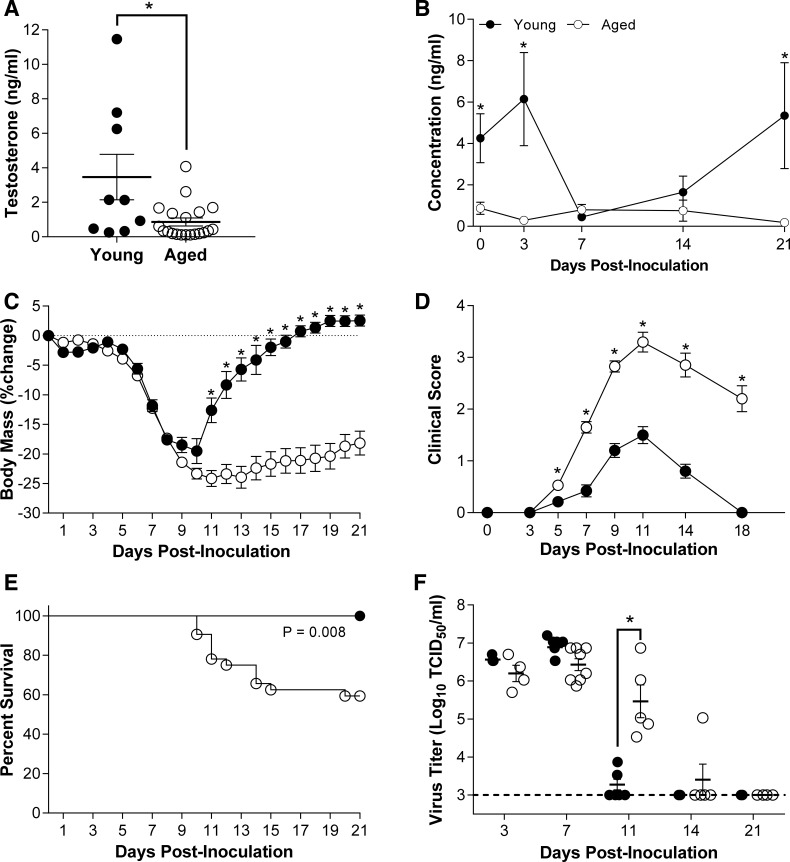
Circulating testosterone concentrations were three-fold higher in uninfected young compared with aged adult male C57BL/6 mice (Fig. 1A; P < 0.05), which are within the ranges reported for inbred laboratory strains of mice (9, 14, 25, 27). Testosterone concentrations were also measured at 3, 7, 14, and 21 dpi in young and aged male mice infected with a low dose of ma2009 (i.e., a dose previously determined to be sublethal in young male mice) and revealed that in young males, testosterone levels dropped during the acute phase of infection (7 dpi), but then rebounded during the recovery phase of infection resulting in testosterone concentrations that were higher than aged males at 0, 3 and 21 dpi only (Fig. 1B; P < 0.05). In contrast, testosterone levels remained low throughout the course of IAV infection in aged male mice.
Fig. 1.
Effects of age on the outcome of ma2009 virus infection. Circulating concentrations of testosterone were measured in aged (17–18 mo; n = 9) and young (10–12 wk; n = 7) male mice prior to infection (A), as well as over the course of infection (B). Following intranasal inoculation with a low dose of mouse-adapted influenza A virus (ma2009), body mass (C), clinical disease (D), and survival (E) were monitored for 21 days in aged and young adult male mice (n = 15–34/age). Pulmonary viral titers were measured at 3, 7, 11, 14, or 21 days postinoculation (dpi) (F; n = 4–8/treatment/time point). *Significant difference between aged and young adult male mice (P < 0.05).
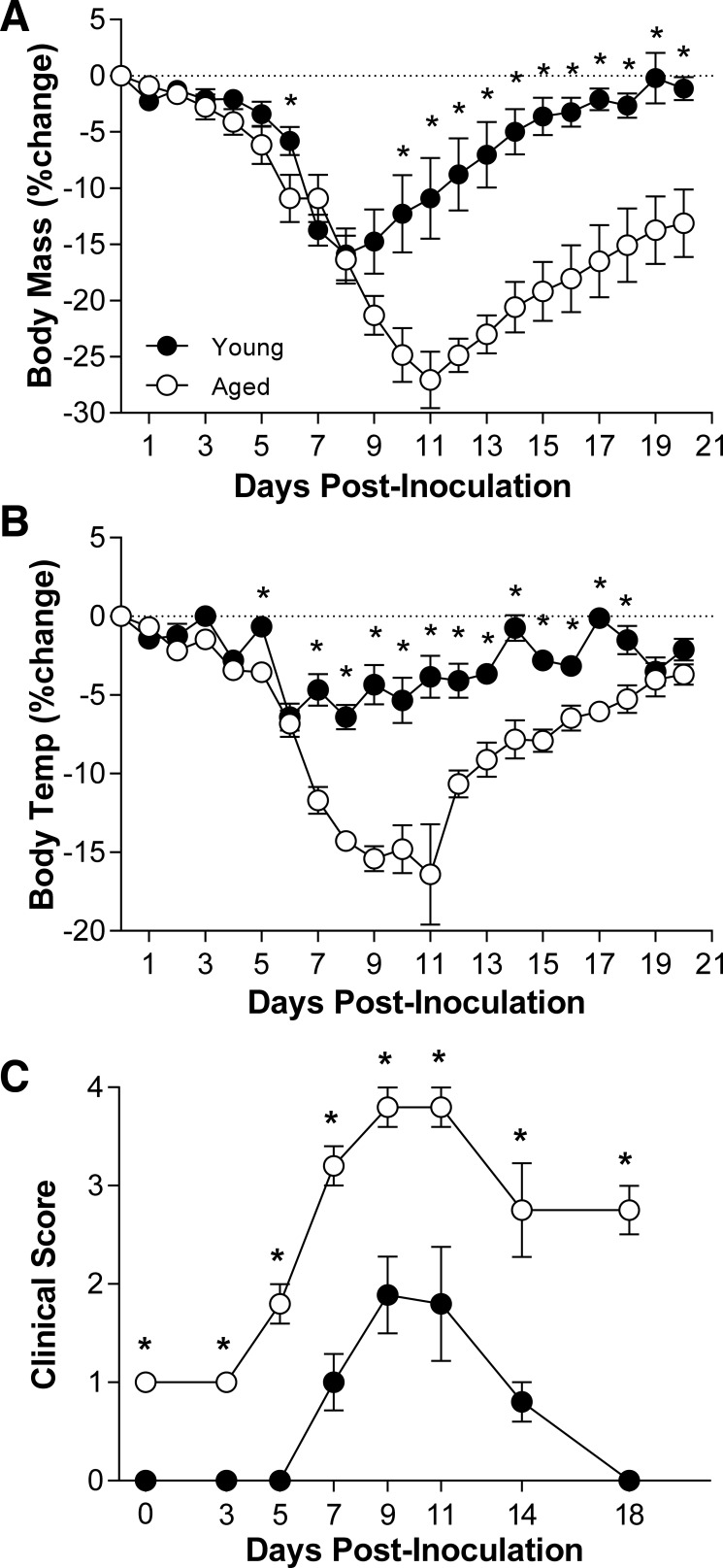
To test the hypothesis that persistently low testosterone levels were associated with a worse outcome of influenza in older than younger males, mice were inoculated with a low dose of ma2009 virus, and body mass, rectal temperature, and clinical disease were monitored for 21 dpi. Aged male mice experienced greater body mass loss, hypothermia, clinical illness, and mortality than young males (Fig. 1, C–E and data not shown; P < 0.05). Titers of ma2009 were measured at select dpi, and although peak viral titers in the lungs did not differ between young and aged male mice, the clearance of infectious virus was significantly delayed in aged males (Fig. 1F; P < 0.05). In response to infection with a low dose of another IAV, PR8, aged males also experienced greater morbidity and clinical illness than young males (Fig. 2, A–C; P < 0.05), illustrating that the effect of aging on severe outcome from influenza was conserved across historic (PR8; isolated in 1934) and contemporary (ma2009) strains of IAV.
Fig. 2.
Effects of age on the outcome of PR8 virus infection. Following intranasal inoculation with a low dose of a historic strain of mouse-adapted influenza A virus (PR8), changes in body mass (A), rectal temperature (B), and clinical disease (C) were monitored for 21 days in aged and young adult male mice (n = 10/group). *Significant difference between aged and young adult male mice are represented (P < 0.05).
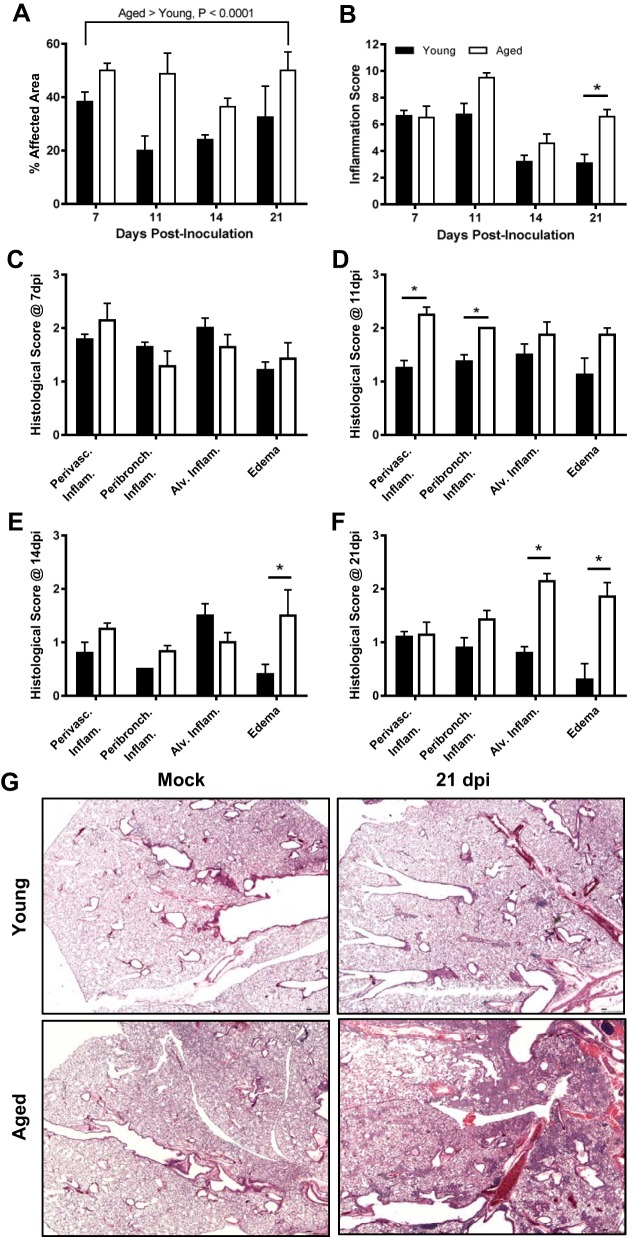
Histological examination of lung tissue was collected at several time points following IAV infection revealed significantly more pulmonary inflammation in the lungs of aged than young males throughout the infection period (Fig. 3A; P < 0.05). In affected areas, similar levels of inflammation were seen between young and aged males at 7 dpi (Fig. 3B); however, by 11, 14, and to the greatest extent at 21 dpi, aged males had significantly more pulmonary inflammation than young males (Fig. 3B; P < 0.05). When scored by structural regions of the lung, no differences in the distribution of inflammation were observed at 7 dpi (Fig. 3C), whereas perivascular and peribronchiolar inflammation were higher in aged than young male mice at 11 dpi (Fig. 3D; P < 0.05). There was a shift in the location of inflammation at 14 and 21 dpi, with increased pulmonary inflammation observed in the terminal bronchioles and alveolar spaces of aged males, predominantly characterized by increased edema at 14 dpi (Fig. 3E; P < 0.05) and increased alveolar inflammation and edema at 21 dpi (Fig. 3, F and G; P < 0.05). We hypothesized that testosterone is associated with protection against IAV in young male mice, based on 1) differences in testosterone concentrations between young and old males and 2) the rebound in testosterone concentrations seen in young, but not aged males (Fig. 1B), which corresponded with improved recovery from IAV-induced pulmonary inflammation (Fig. 3B) during the recovery phase of infection.
Fig. 3.
Effects of age on pulmonary inflammation following infection with ma2009. Lungs were collected from young and aged males that were mock infected or infected with a low dose of influenza A virus (ma2009) and euthanized at 7, 11, 14, or 21 days postinoculation (dpi) (n = 4–7/age/time point). Lung tissues were sectioned, stained with hematoxylin and eosin (H&E), and scored for markers of inflammation, including perivascular inflammation, peribronchiolar inflammation, alveolar inflammation, and edema. Percent affected area (A) and cumulative inflammation scores (B) are presented. Histology scores for individual inflammatory parameters were compared between young and aged males at each time postinoculation (C–F). Representative photomicrographs (G) are shown for lungs collected from young and aged influenza A virus (IAV)- and mock-infected males at 21 dpi. *Significant difference between aged and young adult male mice (P < 0.05).
Protection against influenza in young males is mediated by testosterone.
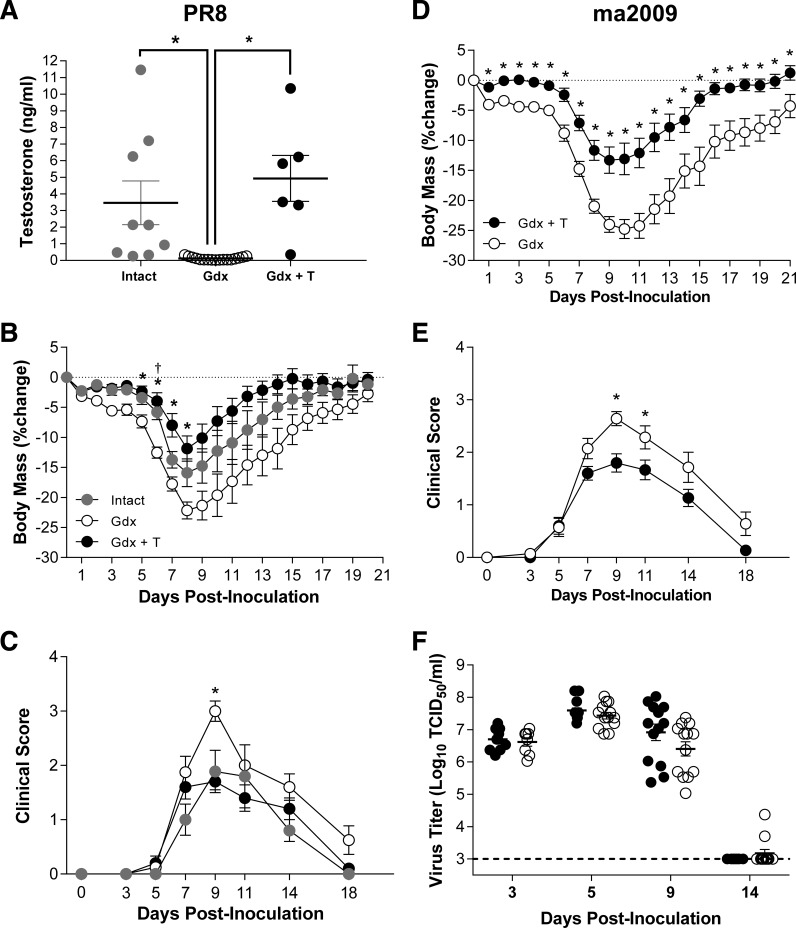
Previous data illustrate that castration of young male mice prior to lethal IAV infection reduces survival compared with testes-intact males (32). To determine whether testosterone directly mediated protection of young males from IAV, young adult male mice were either left intact or castrated to deplete testosterone production, and implanted with either placebo or testosterone capsules prior to infection with a low dose of PR8. Castration reduced, and exogenous testosterone treatment of young adult males restored, circulating testosterone levels to within the physiological range of testes-intact young males (Fig. 4A; P < 0.05). Following infection with either PR8 (Fig. 4, A–C) or ma2009 (Fig 4, D–F), castration of young adult males increased the severity of IAV, and treatment of castrated males with testosterone at physiological doses reduced the severity of IAV to levels comparable with testes-intact male mice (Fig 4, B–E; P < 0.05). Neither peak virus titers nor clearance of infectious virus from the lungs were affected by testosterone replacement in young male mice (Fig. 4F).
Fig. 4.
Effects of exogenous testosterone-treatment on the outcome of IAV infection in young adult male mice. Young adult male mice were left intact or gonadectomized and either treated with placebo (Gdx) or time-released testosterone (Gdx + T) capsules for 1 wk prior to inoculation with influenza A virus (IAV; PR8). A: concentrations of testosterone were measured in serum samples collected 21 days dpi (n = 6–17/treatment). Following intranasal inoculation with PR8, changes in body mass (B) and clinical disease (C) were monitored for 21 dpi (n = 8–10/group). A separate cohort of young adult male mice was gonadectomized and either treated with placebo (Gdx) or time-release testosterone (Gdx + T) capsules for 1 wk prior to inoculation with a contemporary strain of IAV (ma2009). Following intranasal inoculation with ma2009, males (n = 13–15/treatment) were monitored for changes in body mass (D), clinical disease (E), and pulmonary virus titers (F). TCID50, 50% tissue culture infectious dose. *Significant difference between testosterone-treated and placebo-treated Gdx male mice (P < 0.05). †Significant differences between intact and placebo-treated Gdx males are represented by a dagger (P < 0.05).
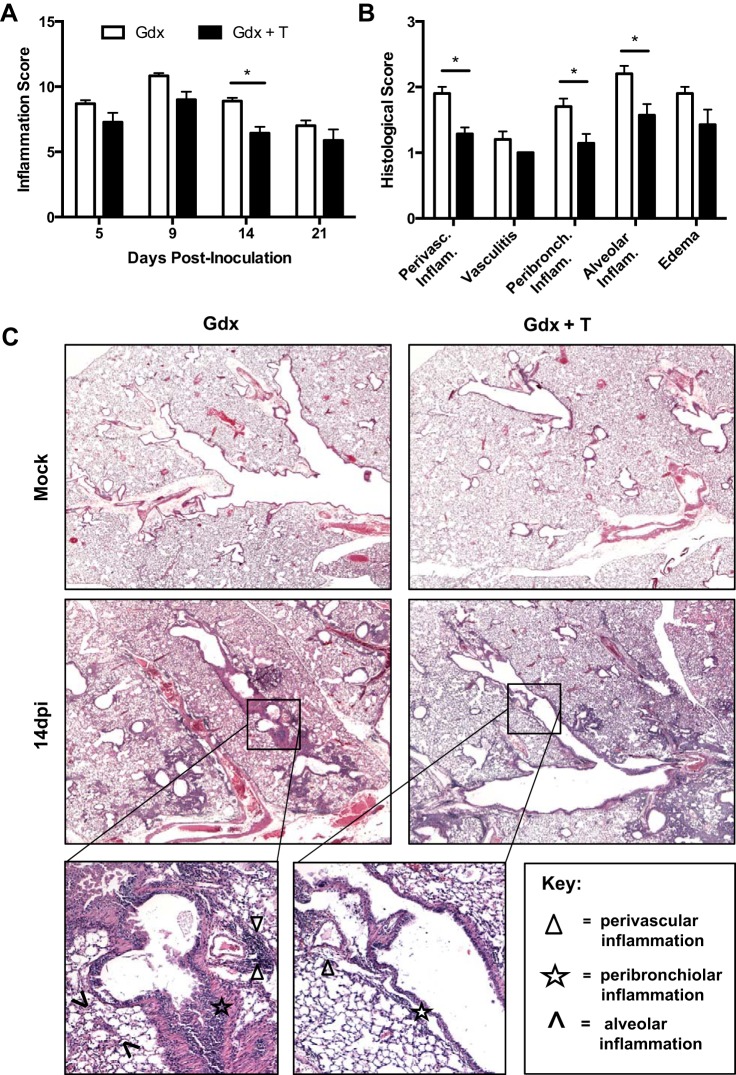
The lungs of castrated males treated with testosterone showed reduced total inflammation and faster resolution of inflammation than the lungs of placebo-treated castrated males (Fig. 5A). Young castrated males treated with testosterone had less pulmonary pathology at 14 dpi compared with placebo-treated males, which presented as less perivascular, peribronchiolar, and alveolar inflammation in the lungs (Fig. 5, B–C; P < 0.05). These data illustrate that testosterone is one host factor contributing to protection against influenza in young adult male mice.
Fig. 5.
Effects of testosterone treatment on pulmonary inflammation following ma2009 virus infection in young adult males. Lungs were collected from young adult males that were gonadectomized and treated with either placebo (Gdx) or testosterone (Gdx + T) and either mock infected or infected with influenza A virus (ma2009) and euthanized at 5, 9, 14, or 21 days dpi (n = 6–10/treatment/time point). Lung tissues were sectioned, stained with H&E, and scored for makers of inflammation, including perivascular inflammation, peribronchiolar inflammation alveolar inflammation, and edema. A: cumulative inflammation scores at 5, 9, 14, or 21 dpi are presented. Histology scores (B) and representative photomicrographs (C) are shown for 14 dpi. *Significant difference between gonadectomized young adult male mice treated with placebo or testosterone (P < 0.05).
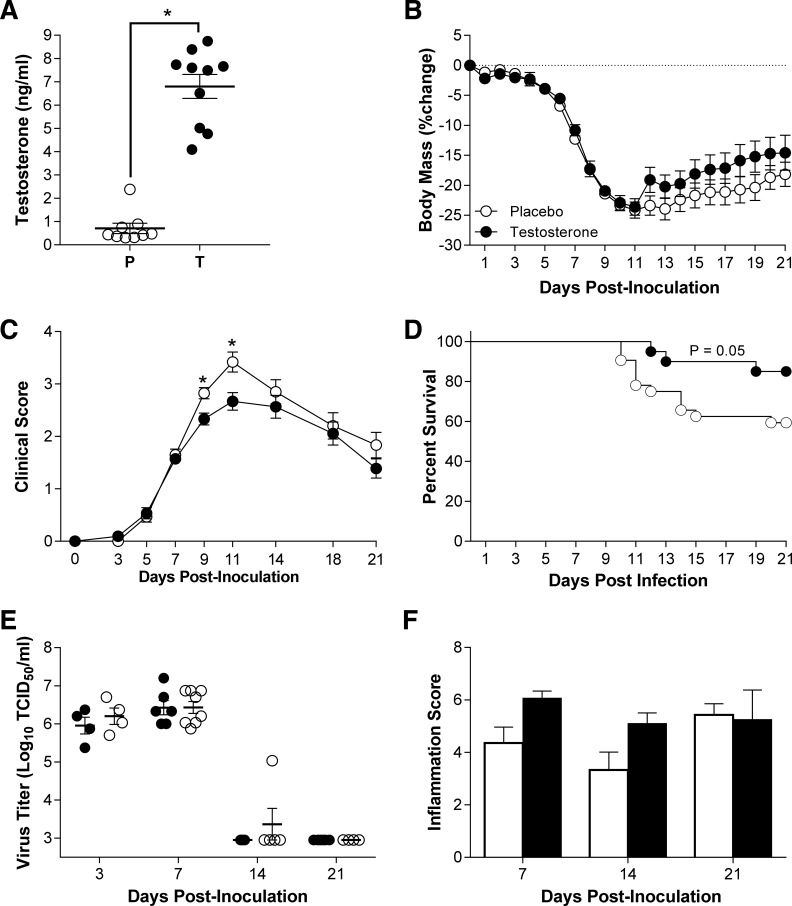
Testosterone replacement improves survival from influenza in aged males.
To determine whether testosterone replacement in aged males could improve the outcome of IAV, aged males were implanted with either placebo or testosterone capsules, and circulating testosterone concentrations were measured 3 wk after implantation. Exogenous testosterone treatment in aged males resulted in circulating testosterone concentrations that were within the physiological range seen in young adult male mice and were significantly higher in the testosterone-treated than in the placebo-treated aged males (Fig. 6A, P < 0.05). Following infection with a low dose of ma2009, aged males treated with testosterone experienced similar body mass loss and hypothermia compared with males treated with placebo (Fig. 6B and data not shown). Despite having similar patterns of morbidity, aged males that were treated with exogenous testosterone experienced less clinical disease (Fig. 6C, P < 0.05) and were significantly more likely to survive infection than aged males treated with placebo (Fig. 6D, P < 0.05). Testosterone treatment did not affect peak virus titers or clearance of infectious virus from the lungs of aged males (Fig. 6E). Replacement of testosterone in aged males also did not significantly alter pulmonary pathology during the course of infection (Fig. 6F). Taken together, these data suggest that testosterone treatment in aged males is associated with reduced clinical illness and mortality from IAV, but not through reduced virus replication or pulmonary inflammation.
Fig. 6.
Effects of testosterone-replacement on the outcome of ma2009 virus infection in aged male mice. Aged male mice were either treated with placebo (P) or time-release testosterone capsules (T) for 1 wk prior to inoculation with IAV (ma2009). A: concentrations of testosterone were measured in serum samples collected 21 days dpi (n = 9–10/treatment). Following intranasal inoculation with IAV, body mass (B), clinical disease (C), and survival (D) were monitored for 21 dpi (n = 24–34). Titers of infectious virus were measured 3, 7, 14, or 21 dpi (E; n = 4–8/treatment/time point). Lung tissue was collected from mock-infected and IAV-infected aged mice that were treated with placebo or testosterone and euthanized 7, 14, or 21 days dpi (n = 5–10/treatment/time point). Lung tissue sections were scored for markers of inflammation, and cumulative inflammation scores are presented (F). *Significant difference between testosterone-treated and placebo-treated aged male mice (P < 0.05).
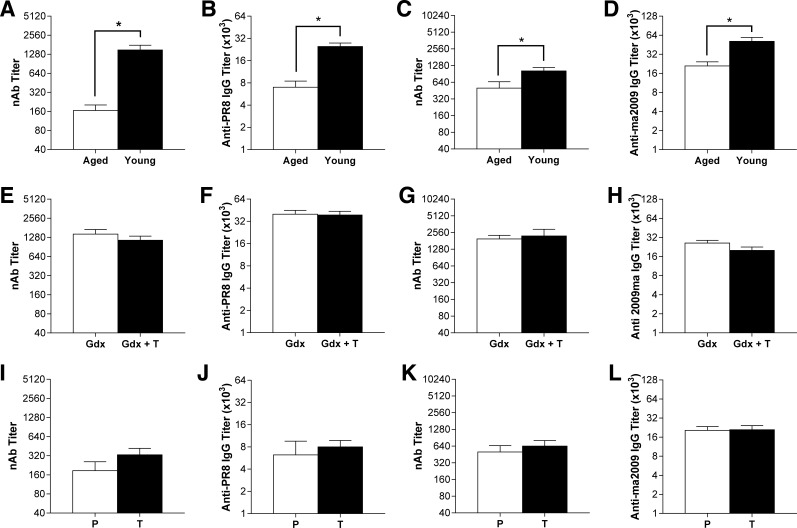
Age rather than testosterone predicts antibody responses to IAVs in males.
Antibody production and the ability of antibodies to neutralize IAVs are relative correlates of protection against subsequent IAV infection and for efficacy of influenza vaccines (18), which are reduced in aged individuals. In the present study, aged males produced significantly lower neutralizing and total anti-IAV serum IgG antibodies than young males against both PR8 and ma2009 (Fig. 7, A–D; P < 0.05 in each case). Treatment of young adult male mice with testosterone had no effect on neutralizing antibody responses or total anti-IAV IgG responses against IAVs (Fig. 7, E–H). Testosterone treatment also did not affect either neutralizing or total anti-IAV IgG antibody responses against IAVs in aged male mice at 21 dpi (Fig. 7, I–L). These data illustrate that despite improving the outcome of influenza in both young and aged males, there was no effect of testosterone on antibody production in either young or aged male mice.
Fig. 7.
Effects of age and testosterone on neutralizing and total IgG antibody responses in young and aged males infected with either PR8 or ma2009 virus. Neutralizing and total anti-influenza IgG antibody titers were measured in serum samples collected 21 days dpi with either PR8 or ma2009 in young and aged adult males (n = 4–12/virus/age; A–D), gonadectomized young adult males treated with either placebo (Gdx) or testosterone (Gdx+T; n = 7–12/virus/treatment; E–H), or in aged male treated with either placebo (P) or testosterone (T; n = 4–10/virus/treatment; I–L). *Significant difference (P < 0.05).
DISCUSSION
Similar to data in female mice (3, 41, 47), aging was associated with worse IAV infection outcomes in male mice. Aged male mice had lower concentrations of testosterone and experienced greater morbidity and mortality following IAV infection compared with young male mice. Changes in body mass were similar between both young and aged mice during the acute phase of infection, with both groups losing ∼20% body mass by 8 dpi. However, during the recovery phase, while body mass in young males returned to baseline by 16 dpi, body mass in aged males did not return to baseline within the 21-day study period. This was associated with delayed viral clearance, persistence of clinical disease symptoms, and impaired resolution of pulmonary inflammation in aged compared with young males. These data suggest a correlation between low testosterone and severity of influenza in aged males. This was further supported by observations that in young males, there was a transient decline in circulating testosterone concentrations during the acute phase of IAV infection when pulmonary inflammation was high, which was followed by a rebound in testosterone levels after (i.e., 21 dpi) recovery from IAV. The association between severe disease and declining androgen levels has been previously reported for IAV (33), HIV (34), and tapeworm infections (19). Presumably, inflammation, and more specifically cytokine secretion, can interfere with steroidogenesis, including testosterone production by the testes (7), which during an acute infection (e.g., IAV) is transient, but during a chronic infection (e.g., HIV) can be long-term and lead to infertility.
To determine the role of testosterone in mitigating IAV-associated disease in the absence of other age-related physiological change, we conducted mechanistic studies in which testosterone concentrations were depleted by castration and replaced with exogenous continuous-release capsules of testosterone that increased testosterone to within the normal physiological range for young adult male mice. In young males, castration followed by treatment with testosterone resulted in a clinical phenotype similar to testes-intact young males after infection with IAV, with testosterone reducing morbidity, clinical disease, and pulmonary inflammation. However, in contrast to aged males, by 21 dpi, gonadectomized young males depleted of testosterone returned to near-baseline body mass, demonstrated minimal evidence of clinical disease, and resolved pulmonary inflammation to levels comparable to testosterone-treated males. The differences between young gonadectomized males and aged males, both of which had low circulating testosterone, suggest that other age-related physiological changes in addition to reduced testosterone may contribute to age-related susceptibility to influenza.
In aged males, testosterone replacement reduced mortality and clinical severity but had minimal effects on morbidity and pulmonary inflammation. It is possible that increased mortality of placebo-treated males created a bias toward animals that survived, and this may have masked differences between treatment groups in recovery from pulmonary inflammation at later time points during infection. Alternatively, other age-related physiological changes may render the aged population more refractory to testosterone's protective effects.
Overall, testosterone improved the outcome of IAV infection in male mice and physiological doses of testosterone were associated with improved outcomes following infection with two different strains of IAV. Although previous studies using a lethal dose of PR8 in young male mice showed a trend for testosterone and the nonaromatizable androgen, dihydrotesosterone, to reduce mortality from influenza virus infection (33), using low doses of both PR8 and ma2009 resulted in more pronounced effects of testosterone on the outcome of infection. The outcome of IAV infection in testosterone-treated young adult castrated males resembled the outcome of infection in testes-intact male mice, suggesting that testosterone is a significant protective factor against influenza.
The biological activity of sex steroids depends on many factors, including the availability of the unbound ligand, receptor expression and distribution, and nuclear translocation and signaling. We hypothesize that testosterone may have more profound effects in young relative to aged male mice due to age-related changes in androgen receptor expression, nuclear translocation, and signaling (21, 36, 39, 42). Sex steroids can only have their biological effects when decoupled from sex hormone binding globulin (SHBG). Studies in humans demonstrate that SHBG levels increase with age and result in decreased bioavailability of circulating testosterone (22, 49). Whether similar regulatory processes may be limiting the efficacy of testosterone treatment in aged male mice warrants future study. With age, if the availability of free testosterone or the activity of androgen receptors and associated signaling pathways are altered, then increasing the concentrations of testosterone may not be sufficient to fully reverse the effects of aging on influenza pathogenesis. Further, whether aging in males is associated with changes in the aromatization of androgens into estrogens requires consideration because at least in female mice, estrogens are anti-inflammatory and improve the outcome of IAV (31, 33). Along these lines, whether the protective effects of testosterone treatment during IAV infection in males are due to signaling through the androgen or estrogen receptor will be investigated in future studies.
In the present study, testosterone treatment of young males conferred protection from IAV-associated disease by reducing pulmonary inflammation and tissue damage during the later stages of infection. The precise cellular mechanisms that mediate immune modulation following testosterone replacement remain to be determined. In response to other inflammatory diseases, such as experimental autoimmune encephalomyelitis (EAE) in mice, testosterone is associated with expansion of regulatory T cell and Th17 populations and the reduction of Th1 activity (12, 50). Generally, castrated male mice have higher numbers of CD4+ and CD8+ T cells than intact males (35). Castrated male mice also have higher numbers of macrophages and antigen-specific CD8+ T cells following viral infection than testes-intact males (23). Although testosterone-induced changes in immune function are beneficial to the outcome of EAE and IAV infection, these same immunological changes can be detrimental to other diseases, including sepsis, trauma-hemorrhage, and wound repair, in which treatment with androgens is associated with depression of cell-mediated immune responses necessary for recovery (2, 16, 30). Characterization of the relative contributions that various immune cell populations play in mediating the protective effects of testosterone against IAV warrants additional study.
Human studies reveal that higher levels of testosterone are correlated with lower antibody responses to the trivalent inactivated seasonal influenza vaccine (15). In the current studies, manipulation of testosterone did not affect either neutralizing or total anti-IAV IgG titers in either young or aged male mice. Whether this reflects differences in androgen signaling in humans and mice, systemic immunization vs. local pulmonary infection, or use of inactive vs. live IAVs requires additional consideration. Instead, age was more likely to predict the antibody response to IAV, with young males having significantly higher antibody titers than aged males.
Study of the hormonal effects on immune responses to IAV has focused exclusively on effects in young adults, and predominantly on hormonal effects in young adult females, which demonstrate profound effects on viral pathogenesis and immune responses to vaccination (17, 28, 29, 33). No studies have evaluated the relative contribution of age-related reductions in sex steroid levels in explaining why influenza pathogenesis and vaccine efficacy are worse in aged males. Whether testosterone also affects susceptibility to pneumonia following secondary bacterial infection, which is often a principle cause of death from influenza (24, 26, 37) warrants further consideration. We now provide systematic evidence of a role for testosterone in mediating IAV pathogenesis in both young and aged males.
Prescription of testosterone replacement therapy has increased as the population of adults aged 65 and older continues to grow (4). To date, clinical studies have focused exclusively on the impact of testosterone on sexual function, mental health, bone health, muscle mass, and metabolic and cardiovascular diseases (1, 4, 44). These data highlight additional impacts of testosterone on the immune response, and suggest that testosterone replacement therapy may have additional benefits in the context of infectious disease.
GRANTS
This work was supported by National Institutes of Health Grants AI112838 (to S. L. Klein) and NIH/NIAID training grant fellowship AI007417 to L. G. vom Steeg.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.G.v.S., M.S.V., O.J.H., O.A., R.M., and H.C. performed experiments; L.G.v.S., M.S.V., O.A., H.C., and S.L.K. analyzed data; L.G.v.S., M.S.V., B.Z., and S.L.K. interpreted results of experiments; L.G.v.S. and M.S.V. prepared figures; L.G.v.S. and S.L.K. drafted manuscript; L.G.v.S., M.S.V., O.J.H., O.A., R.M., B.Z., and S.L.K. edited and revised manuscript; L.G.v.S., M.S.V., O.J.H., O.A., R.M., H.C., B.Z., and S.L.K. approved final version of manuscript; S.L.K. conceived and designed research.
ACKNOWLEDGMENTS
We thank Dr. Cory Brayton for her help with the design of the histological scoring system and Dr. Andrew Pekosz for his critical review of the manuscript. We also thank June Liu of the Zirkin laboratory for her assistance with the RIAs and the members of the Pekosz and Klein laboratories for their detailed discussions of these data.
REFERENCES
- 1.Abadilla KA, Dobs AS. Topical testosterone supplementation for the treatment of male hypogonadism. Drugs 72: 1591–1603, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Angele MK, Wichmann MW, Ayala A, Cioffi WG, Chaudry IH. Testosterone receptor blockade after hemorrhage in males. Restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg 132: 1207–1214, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Asanuma H, Hirokawa K, Uchiyama M, Suzuki Y, Aizawa C, Kurata T, Sata T, Tamura S. Immune responses and protection in different strains of aged mice immunized intranasally with an adjuvant-combined influenza vaccine. Vaccine 19: 3981–3989, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Baer JT. Testosterone replacement therapy to improve health in older males. Nurse Pract 37: 39–44, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Baillargeon J, Urban RJ, Kuo YF, Ottenbacher KJ, Raji MA, Du F, Lin YL, Goodwin JS. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother 48: 1138–1144, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35: 565–572, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bornstein SR, Rutkowski H, Vrezas I. Cytokines and steroidogenesis. Mol Cell Endocrinol 215: 135–141, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Dudley JP, Mackay IM. Age-specific and sex-specific morbidity and mortality from avian influenza A (H7N9). J Clin Virol 58: 568–570, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elefheriou BE, Lucas LA. Age-related changes in testes, seminal vesicles and plasma testosterone levels in male mice. Gerontologia 20: 231–238, 1974. [DOI] [PubMed] [Google Scholar]
- 10.Eshima N, Tokumaru O, Hara S, Bacal K, Korematsu S, Tabata M, Karukaya S, Yasui Y, Okabe N, Matsuishi T. Sex- and age-related differences in morbidity rates of 2009 pandemic influenza A H1N1 virus of swine origin in Japan. PLos One 6: e19409, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 200: 172–180, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Fijak M, Schneider E, Klug J, Bhushan S, Hackstein H, Schuler G, Wygrecka M, Gromoll J, Meinhardt A. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol 186: 5162–5172, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Fink AL, Klein SL. Sex and gender impact immune responses to vaccines among the elderly. Physiology (Bethesda) 30: 408–416, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flood JF, Farr SA, Kaiser FE, La Regina M, Morley JE. Age-related decrease of plasma testosterone in SAMP8 mice: replacement improves age-related impairment of learning and memory. Physiol Behav 57: 669–673, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci USA 111: 869–874, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilliver SC, Wu F, Ashcroft GS. Regulatory roles of androgens in cutaneous wound healing. Thromb Haemost 90: 978–985, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Hall OJ, Limjunyawong N, Vermillion MS, Robinson DP, Wohlgemuth N, Pekosz A, Mitzner W, Klein SL. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog 12: e1005840, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein SL, Pekosz A. Sex-based biology and the rational design of influenza vaccination strategies. J Infect Dis 209 Suppl 3: S114–S119, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larralde C, Morales J, Terrazas I, Govezensky T, Romano MC. Sex hormone changes induced by the parasite lead to feminization of the male host in murine Taenia crassiceps cysticercosis. J Steroid Biochem Mol Biol 52: 575–580, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Laucho-Contreras ME, Taylor KL, Mahadeva R, Boukedes SS, Owen CA. Automated measurement of pulmonary emphysema and small airway remodeling in cigarette smoke-exposed mice. J Vis Exp 52236, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecce L, Lam YT, Lindsay LA, Yuen SC, Simpson PJ, Handelsman DJ, Ng MK. Aging impairs VEGF-mediated, androgen-dependent regulation of angiogenesis. Mol Endocrinol 28: 1487–1501, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leifke E, Gorenoi V, Wichers C, Von Zur Muhlen A, Von Buren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 53: 689–695, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Lin AA, Wojciechowski SE, Hildeman DA. Androgens suppress antigen-specific T cell responses and IFN-γ production during intracranial LCMV infection. J Neuroimmunol 226: 8–19, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louie J, Jean C, Chen TH, Park S, Ueki R, Harper T, Chmara E, Myers J, Catanese C, Farley N, Leis E, DiAngelo C, Fry AM, Finelli L, Carvalho MG, Beall B, Moore M, Whitney C, Blau DM. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic Influenza A (H1N1)—United States, May–August 2009 USA. Morbid Mortal Weekly Rep 58: 1–4, 2009. [PubMed] [Google Scholar]
- 25.Machida T, Yonezawa Y, Noumura T. Age-associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Horm Behav 15: 238–245, 1981. [DOI] [PubMed] [Google Scholar]
- 26.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 198: 962–970, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson JF, Latham KR, Finch CE. Plasma testosterone levels in C57BL/6J male mice: effects of age and disease. Acta Endocrinol 80: 744–752, 1975. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen DC, Sambhara S, Scinicariello F, Attanasio R. Estrogen up-modulates antibody responses to an influenza vaccine in a murine model. In: Immunobiology and Pathogenesis of Influenza Infection. Atlanta, Georgia, 2008. [Google Scholar]
- 29.Pazos MA, Kraus TA, Munoz-Fontela C, Moran TM. Estrogen mediates innate and adaptive immune alterations to influenza infection in pregnant mice. PLos One 7: e40502, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remmers DE, Cioffi WG, Bland KI, Wang P, Angele MK, Chaudry IH. Testosterone: the crucial hormone responsible for depressing myocardial function in males after trauma-hemorrhage. Ann Surg 227: 790–799, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17β-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T-cell responses in the lungs. J Virol 88: 4711–4720, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, Arnold AP, Klein SL. Sex chromosome complement contributes to sex differences in Coxsackievirus B3 but not Influenza A virus pathogenesis. Biol Sex Differ 2: 8, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 7: e1002149, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rochira V, Guaraldi G. Hypogonadism in the HIV-infected man. Endocrinol Metab Clin North Am 43: 709–730, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, Hurwitz AA, McKean DJ, Celis E, Leibovich BC, Allison JP, Kwon ED. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol 173: 6098–6108, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Roehrborn CG, Lange JL, George FW, Wilson JD. Changes in amount and intracellular distribution of androgen receptor in human foreskin as a function of age. J Clin Invest 79: 44–47, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shieh WJ, Blau DM, Denison AM, Deleon-Carnes M, Adem P, Bhatnagar J, Sumner J, Liu L, Patel M, Batten B, Greer P, Jones T, Smith C, Bartlett J, Montague J, White E, Rollin D, Gao R, Seales C, Jost H, Metcalfe M, Goldsmith CS, Humphrey C, Schmitz A, Drew C, Paddock C, Uyeki TM, Zaki SR. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol 177: 166–175, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Cifelli D, Dougar D, Fluharty L, Resnick SM, Storer TW, Anton S, Basaria S, Diem SJ, Hou X, Mohler ER 3rd Parsons JK, Wenger NK, Zeldow B, Landis JR, Ellenberg SS, Testosterone Trials Investigators. Effects of testosterone treatment in older men. N Engl J Med 374: 611–624, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song CS, Rao TR, Demyan WF, Mancini MA, Chatterjee B, Roy AK. Androgen receptor messenger ribonucleic acid (mRNA) in the rat liver: changes in mRNA levels during maturation, aging, and calorie restriction. Endocrinology 128: 349–356, 1991. [DOI] [PubMed] [Google Scholar]
- 40.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289: 179–186, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res 10: 112, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tohgi H, Utsugisawa K, Yamagata M, Yoshimura M. Effects of age on messenger RNA expression of glucocorticoid, thyroid hormone, androgen, and estrogen receptors in postmortem human hippocampus. Brain Res 700: 245–253, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Trigunaite A, Dimo J, Jorgensen TN. Suppressive effects of androgens on the immune system. Cell Immunol 294: 87–94, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Tsujimura A. The relationship between testosterone deficiency and men's health. World J Men's Health 31: 126–135, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.vom Steeg LG, Klein SL. SeXX matters in infectious disease pathogenesis. PLoS Pathog 12: e1005374, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang CS, Wang ST, Chou P. Efficacy and cost-effectiveness of influenza vaccination of the elderly in a densely populated and unvaccinated community. Vaccine 20: 2494–2499, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med 205: 711–723, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye J, Sorrell EM, Cai Y, Shao H, Xu K, Pena L, Hickman D, Song H, Angel M, Medina RA, Manicassamy B, Garcia-Sastre A, Perez DR. Variations in the hemagglutinin of the 2009 H1N1 pandemic virus: potential for strains with altered virulence phenotype? PLoS Pathog 6: e1001145, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeap BB, Almeida OP, Hyde Z, Norman PE, Chubb SA, Jamrozik K, Flicker L. In men older than 70 years, total testosterone remains stable, while free testosterone declines with age. The Health in Men Study. Eur J Endocrinol 156: 585–594, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, Akkermann R, Stanczyk FZ, Prat A, Steinman L, Dunn SE. Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci USA 109: 9505–9510, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zirkin BR, Tenover JL. Aging and declining testosterone: past, present, and hopes for the future. J Androl 33: 1111–1118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]