Abstract
Lung branching morphogenesis relies on a number of factors, including proper epithelial cell proliferation and differentiation, cell polarity, and migration. Rac1, a small Rho GTPase, orchestrates a number of these cellular processes, including cell proliferation and differentiation, cellular alignment, and polarization. Furthermore, Rac1 modulates both noncanonical and canonical Wnt signaling, important pathways in lung branching morphogenesis. Culture of embryonic mouse lung explants in the presence of the Rac1 inhibitor (NSC23766) resulted in a dose-dependent decrease in branching. Increased cell death and BrdU uptake were notably seen in the mesenchyme, while no direct effect on the epithelium was observed. Moreover, vasculogenesis was impaired following Rac1 inhibition as shown by decreased Vegfa expression and impaired LacZ staining in Flk1-Lacz reporter mice. Rac1 inhibition decreased Fgf10 expression in conjunction with many of its associated factors. Moreover, using the reporter lines TOPGAL and Axin2-LacZ, there was an evident decrease in canonical Wnt signaling in the explants treated with the Rac1 inhibitor. Activation of canonical Wnt pathway using WNT3a or WNT7b only partially rescued the branching inhibition. Moreover, these results were validated on human explants, where Rac1 inhibition resulted in impaired branching and decreased AXIN2 and FGFR2b expression. We therefore conclude that Rac1 regulates lung branching morphogenesis, in part through canonical Wnt signaling. However, the exact mechanisms by which Rac1 interacts with canonical Wnt in human and mouse lung requires further investigation.
Keywords: Rac1, canonical WNT, lung, branching morphogenesis
lung branching morphogenesis is coordinated by physical, cellular, and molecular events, essential for the formation of a functional breathing organ. Adequate lung development results from a series of spatial and temporal events occurring in conjunction with signal transduction pathways such as BMP, FGF, and Wnt, as well as cross talk between the epithelium and mesenchyme (18, 39). The Wnt signaling pathway is one of the major pathways governing lung development and repair (12). Canonical Wnt signaling is mediated by the translocation of β-catenin to the nucleus, where it activates transcription of several factors regulating cell proliferation and differentiation, while noncanonical Wnt signaling is β-catenin independent. In a model of intrauterine nitrofen-induced pulmonary hypoplasia, canonical Wnt signaling was significantly downregulated, suggesting that alteration in this pathway results in defective lung development (38). Either diminished or excessive canonical Wnt signaling in the developing lung, achieved through deletion or constitutive activation of β-catenin, respectively, impair lung branching morphogenesis and alveolar formation (8, 11, 13, 25, 30). Furthermore, canonical Wnt signaling through β-catenin plays a major role in the differentiation and fate specification of both epithelial and mesenchymal lung progenitors (25, 30). This has been reviewed in detail by De Langhe and Reynolds (12). β-Catenin regulates the expression of Fgfr2b and Bmp4 in the distal lung epithelium (35), important signaling molecules in the maintenance and expansion of the progenitor cell pool. β-Catenin also interacts with Fgf10 signaling, and it has been shown that mesenchymal deletion of β-catenin decreases Fgf10 expression, alters the differentiation of angioblasts into mature endothelial cells, and reduces the amplification of Fgf10-expressing smooth muscle cells, though the total pool of smooth muscle cells continues to expand (11).
Several molecules modulate canonical Wnt signaling, including the small Rho GTPase, Ras-related C3 botulinum toxin substrate 1, or Rac1. Rac1 regulates several signaling pathways including the noncanonical Wnt pathway, nuclear factor-κB (NF-κB), phosphatidylinositol 3-kinase (PI3K), and mitogen-activated protein kinase (MAPK). However, recent studies showed that Rac1 also modulates canonical Wnt signaling by increasing nuclear localization of β-catenin (41). Moreover, Esufali et al. (16) showed that dominant negative forms of Rac1 in colon cancer cells cause a marked inhibition of canonical Wnt signaling. Rac1 can be activated by Wnt ligands such as Wnt3a (41) and increases gene expression of β-catenin and Wnt target genes such as Axin2, c-Myc, Lef-1, and Cyclin D1, by stimulating the nuclear β-catenin/LEF-1 complex formation (22). Rac1 regulates several cellular events including cell proliferation, adhesion, migration, and differentiation as well as gene expression. Rac1 plays an important role in development, particularly branching morphogenesis, of several organ systems including mammary gland, tooth, and salivary gland (3, 20, 32). In an in vitro model of mammary gland branching, Zhu et al. (45) showed that activated Rac1 is required for branch elongation where it then localizes at the tip of the new branches. A more recent study also demonstrated that Rac1 is required for adequate epithelial tube elongation in Drosophila (36).
Since canonical Wnt signaling is important for proper lung branching morphogenesis, and Rac1 is necessary for adequate canonical Wnt signaling (41), we hypothesize that Rac1 regulates lung branching morphogenesis via canonical Wnt signaling. The role of Rac1 in the developing lung is still unclear and poorly studied. Herein, we investigated the effect of Rac1 inhibition on lung branching morphogenesis and showed that chemical inhibition of Rac1 alters branching in the lung, decreases canonical Wnt signaling as well as Fgf signaling targets, and causes expansion of the smooth muscle cell pool. Moreover, Rac1 inhibition impaired vasculogenesis in both mouse and human lung explants. However, activation of canonical Wnt signaling using WNT3a or WNT7b resulted in a modest rescue of the branching defects. Using human fetal lung explants, we showed that RAC1 inhibition impaired branching in human explants and decreased AXIN2 expression. Further studies are needed to determine the precise mechanism by which Rac1 interacts with canonical Wnt signaling in the developing lung.
METHODS
Ethics statement.
Deidentified human fetal samples were obtained under IRB approval (CHLA-14-2211). The only collected clinical information was the gestational age or whether there are any known fetal anomalies. Specimens obtained from procedures performed on HIV- or viral hepatitis-positive patients were excluded.
Animal experiments were performed under research protocols (31-11 and 253-11) approved by the Animal Research Committee at Children's Hospital Los Angeles and in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The approval identification for Children's Hospital Los Angeles is AAALAC A3276-01.
Mice.
C57BL6 mice were crossed in the evening, and the following morning identification of a copulation plug was considered day 0.5 of gestation. The animals were then collected at embryonic day (E)12.5 for whole-mount culturing, or at E11.5 for epithelial tip/mesenchymal separation. Flk1-LacZ animals were obtained from Jackson Laboratories (B6.129-Kdrtm1Jrt/J, stock number 002938). Wnt reporter mice TOPGAL [Tg(Fos-lacZ)34Efu/J, stock number 004623] and Axin2-LacZ (B6.129P2-Axin2tm1Wbm/J, stock number 009120) were also obtained from Jackson Laboratories (10, 28). TOPGAL, Axin2-LacZ, and Flk1-LacZ males were time-mated with C57BL6 females, as described above, and the embryos were collected at E12.5. Wif1−/−LacZ animals were a generous gift from Dr. Igor B. Dawid (23). The animals were crossed between each other to expand the colony and time-mated to collect E12.5 embryos. Wif1-null animals present no abnormalities and are viable and fertile.
Whole lung explants culture.
The explant cultures were performed as previously described (15). Briefly, embryos were isolated at day E12.5 from timed-pregnant females and the lungs were microdissected in ice-cold HBSS, assuring that the tracheas remained intact. The lungs were then placed atop an 8.0-μm, 13-mm Nuclepore polycarbonate track-etch membrane (Whatman, Florham Park, NJ) and cultured in Dulbecco's modified Eagle's medium: nutrient mix F-12 (DMEM/F-12) HEPES-free and containing l-glutamine (Invitrogen, Carlsbad, CA), supplemented with 50 units/ml of penicillin-streptomycin (Invitrogen) and 0.5% FBS. Explants were treated with different concentrations (6.25–100 μM) of the Rac1 inhibitor: NSC23766 (Calbiochem/EMD Millipore, Billerica, MA) for 48 h to assess dose effect. The media and inhibitor were changed daily. Explants treated with (8 μM or 10 μM) EHop-016 (Selleckhem, Houston, TX) were cultured under similar conditions, with controls being cultured with comparable amounts of DMSO given that the inhibitor is dissolved in DMSO. Wnt reporter lung explant cultures (TOPGAL and Axin2-LacZ) and Flk1-LacZ explants were treated with 25 μM of NSC23766. In subsequent experiments, in which rescue of the inhibited epithelial branches was being tested, the explants were treated with 25 μM of NSC23766 and/or recombinant Wnt ligand WNT3a (200 ng/ml) (R&D, Minneapolis, MN). Controls in all experiments were cultured with supplemented culture media only.
Human fetal lung explant cultures.
Cultures for human fetal lung were performed from samples at gestational ages ranging between 10–12 wk of age. Intact edges were carefully cut into uniform pieces, leaving the outer edge of the explant intact, and were cultured at air-liquid interface atop a Nuclepore polycarbonate membrane, as described above for mouse lung explants, in DMEM/F-12 HEPES-free and containing l-glutamine (Invitrogen), supplemented with 50 units/ml of penicillin-streptomycin (Invitrogen) and 1% FBS. Explants were treated with 25 μM of NSC23766 for 48 h. The media and inhibitor were changed daily.
Detection of β-galactosidase activity.
Staining for β-galactosidase activity was performed as previously described (2, 9). Briefly, lungs from Flk1-LacZ, TOPGAL, or Axin2-LacZ animals were washed in PBS, followed by a quick 3-min fixation in 4% paraformaldehyde. The lungs were again washed in PBS, followed by a brief wash in warm LacZ buffer. To evaluate the vasculature or canonical Wnt signaling, the lungs were incubated overnight at 37°C with LacZ buffer containing 2 mg/ml X-gal (RPI Research Products, Mount Prospect, IL). After sufficient staining, the samples were then washed in PBS, fixed in 4% PFA, and photographed using a color camera attached to a bright-field microscope.
Immunohistochemistry.
Cultured explants were washed in PBS, fixed overnight in 4% PFA at 4°C, dehydrated in graded ethanol solutions, cleared in xylene, and embedded in paraffin. For immunostaining, 5-μm sections were cleared twice with xylene and rehydrated. Antigen retrieval was performed using a sodium citrate buffer (10 mM, pH 6.0), in which the slides were boiled at 95°C for 12 min. Slides were then washed in 0.1% Tris-buffered saline/Tween (0.1% Tween) and blocked using 3% bovine serum album/0.1% Triton. Slides were incubated with primary antibodies anti-CD31 (1:50, Thermo Scientific, Waltham, MA), anti-α-smooth muscle actin (1:200, Dako Cytomation, Carpinteria, CA), and Phosphohistone H3 (1:200, Cell Signaling, Danvers, MA) at 4°C overnight. For immunofluorescence, Cy-3 and FITC-conjugated secondary antibodies (1:200, Jackson Immunoresearch Laboratories, West Grove, PA) were incubated for 1 h at room temperature in a humidifying chamber. Human tissue slides stained with activated Rac1 were processed in a similar manner and were incubated overnight with primary antibody (IgM) anti-active Rac-GTP (1:100, NewEast Biosciences, Malvern, PA), and Alexa488 goat anti-mouse IgM secondary (Abcam, Cambridge, UK). Slides were mounted with Vectashield containing DAPI (Vector, Burlingame, CA). Fluorescent images were acquired using a Leica monochrome camera attached to a Leica DM4000B microscope.
The GST-PAK-PBD staining was performed as previously described (45). Following rehydration, antigen unmasking, and washing, slides were incubated with 10% goat serum for 1 h at room temperature. The slides were then incubated with GST-PAK-PBD (Cytoskeleton, Denver, CO) (2.5 μg in 100 μl of goat serum) overnight at 4°C, followed by a rabbit anti-GST antibody (Sigma, St. Louis, MO) (1:200) for 1 h at room temperature, and finally a Cy3 goat anti-rabbit antibody (Cell Signaling) (1:300). Negative controls were incubated with rabbit anti-GST antibody followed by Cy3 goat anti-rabbit antibody.
TUNEL staining.
Sections of lung explants treated with Rac1 inhibitor or controls were cleared twice in xylene and rehydrated. Sodium citrate antigen retrieval was then performed on them as previously described. The slides were then washed in running deionized H2O for 5 min. Specimens were then covered in a 2 μg/ml proteinase K solution for 3 min at room temperature and placed in a humidifying chamber. Slides were immediately washed in cold PBS and fixed in 4% PFA for 5 min. Slides were again washed in PBS and TUNEL staining was performed using the In Situ Cell Death Detection Kit, Fluorescein (Roche, Switzerland) as recommended by the manufacturer for 1 h at 37°C in a humidifying chamber. The slides were then washed three times in PBS and mounted with Vectashield containing DAPI (Vector).
For the explant cultures, TUNEL was incubated for 2 h at 37°C in a four-well plate. The wells containing the explants were then washed, stained with DAPI, and imaged.
BrdU staining.
Media of control and treated wild-type (WT) E12.5 lung explants was treated with 2 μl Amersham Cell Proliferation Labeling Reagent (GE Healthcare, Buckinghamshire, UK) at 45 h in culture. The explants remained in culture until the completion of the 48-h culture period, allowing incorporation of the labeling reagent within the proliferating cells. Explants were processed similarly as previously described for immunohistochemistry. Briefly, slides were rehydrated and were then covered in the Amersham anti-BrdU antibody (GE Healthcare) according to the manufacturer's instructions, for 1 h at room temperature. The slides were then washed and detected using a Cy3 secondary antibody (1:200, Jackson Immunoresearch Laboratories). Slides were mounted with Vectashield containing DAPI (Vector).
Real-time PCR analyses.
Whole mouse lungs were used for RNA extraction. For human samples, slices containing both proximal and distal areas were used for RNA extraction. RNA was extracted from control and treated lung explants after 48 h in culture. This was done using a hand-held homogenizer as well as the protocol and materials of the iNtRon Biotechnology easy-spin Total RNA Extraction Kit. RNA (250 or 500 ng/μl depending on the concentration of the RNA) was reverse-transcribed into cDNA using Tetro cDNA Synthesis Kit (Bioline, Taunton, MA) according to the manufacturer's instructions; 500 ng cDNA was used for dual color Hydrolysis Probe-Universal probe library-based quantitative real-time PCR, using the LightCycler 480 from Roche Applied Science. Primers were designed using Roche Applied Science Probe-based assay design center. GAPDH assay, commercially available from Roche Applied Science, was used as the reference gene. Mouse primers are as follows: Acta2 (R: ACATAGCTGGAGCAGCGTCT; L: CCCACCCAGAGTGGAGAA), β-catenin (R: AAGAACGGTAGCTGGGATCA; L: TGCAGATCTTGGACTGGACA), Bmp4 (R: GAGGAGTTTCCATCACGAAGA; L: GCTCTGCCGAGGAGATCA), Dkk1 (R: CCAAGGTTTTCAATGATGCTT; L: CCGGGAACTACTGCAAAAAT), Etv4 (R: GGGGAGTCATAGGCACTGG; L: CAGAGTCCCCGCACAGAC), Etv5 (R: GCAGCTCCCGTTTGATCTT, L: GCAGTTTGTCCCAGATTTTCA), Fgf10 (R: AACAACTCCGATTTCCACTGA, L: CGGGACCAAGAATGAAGACT), Fgfr2b (R: CATCCATCTCCGTCACATTG; L: CCCTACCTCAAGGTCCTGAA), Fgfr2c (R: TGCAGGCGATTAAGAAGACC; L: TGCATGGTTGACAGTTCTGC), Rac1 (R: GAGCAGGCAGGTTTTACCAA; L: AGATGCAGGCCATCAAGTGT), Spry2 (R: CTCCATCAGGTCTTGGCAGT; L: GAGAGGGGTTGGTGCAAAG), Spry4 (R: CACCAAGGGACAGGCTTCTA; L: GTGGAGCGATGCTTGTGAC), Vegfa (R: AGAGGTCTGGTTCCCGAAA; L: TTAAACGAACGTACTTGCAGATG), Axin2 (R: TGCCAGTTTCTTTGGCTCTT; L: CTGCTGGTCAGGCAGGAG) and Wif1 (R: GGCAGACACTGCAATAAGAGG; L: TTAAGTGAAGGCGTGTGTCG). Human primers are as followss: AXIN2 (R: ACTGCCCACACGATAAGGAG; L: GCTGACGGATGATTCCATGT), FGF10 (R: ACGGCAACAACTCCGATT; L: CGGGACCAAGAAGGAGAACT), FGFR2b (R: CTGGACTCAGCCGAAACTGT; L: GCACAAGCTGACCAAACGTA), WIF1 (R: TGGGAAACCAACTTGAACAAC; L: AGATCCAACCGTCAATGTCC), VEGFA (R: CCGTCTCTCTCTTCCTCGAC; L: GGATTTTGGAAACCAGCAGA) and RAC1 (R: CAGGAAATGCATTGGTTGTG; L: CTGATGCAGGCCATCAAGT).
Murine epithelial tip and mesenchymal cultures.
The isolated epithelial tips and mesenchymal cultures were performed as previously described (15, 23) with minor modifications. Briefly, embryos were isolated at day E11.5 from timed-pregnant females and the lungs were microdissected in HBSS. The bottoms of four-well plates were coated with a thin layer of growth factor reduced Matrigel (Corning, Corning, NY) and placed in an incubator to polymerize. Meanwhile, lungs were transferred onto a glass petri dish containing DMEM/F-12 (Invitrogen) supplemented with 10% FBS. Using insulin syringe needles, the mesenchyme was manually separated from the epithelium. A pure population of either mesenchyme or epithelium was pipetted up with 15 μl of media that would be expelled simultaneously into a precoated well alongside 15 μl of reduced growth factor Matrigel, creating a dome, assuring pieces of tissue were being submerged in Matrigel. The cultures were immediately placed in the incubator to allow for polymerization of the Matrigel and were then immersed in DMEM/F-12 medium, supplemented with 50 units/ml of penicillin-streptomycin (Invitrogen) and 0.5% FBS. To assure adequate growth, the mesenchymal cultures were supplemented with FGF9 (200 ng) whereas the epithelial cultures were supplemented with FGF10 (200 ng) (for both the control and treated condition wells). Experimental groups were treated with 25 μM NSC23766 for up to 4 days. The media, growth factor supplementation, and treatment were changed daily. The isolated epithelium and mesenchyme cultures were incubated in at 37°C, 5% CO2, and relatively high humidity.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism software. Nonparametric tests were used to compare two groups and one way-ANOVA was used to compare multiple groups. Data are presented as average values ± SE. The results were considered significant if P ≤ 0.05.
RESULTS
Rac1 is expressed in the developing lung in human and mouse.
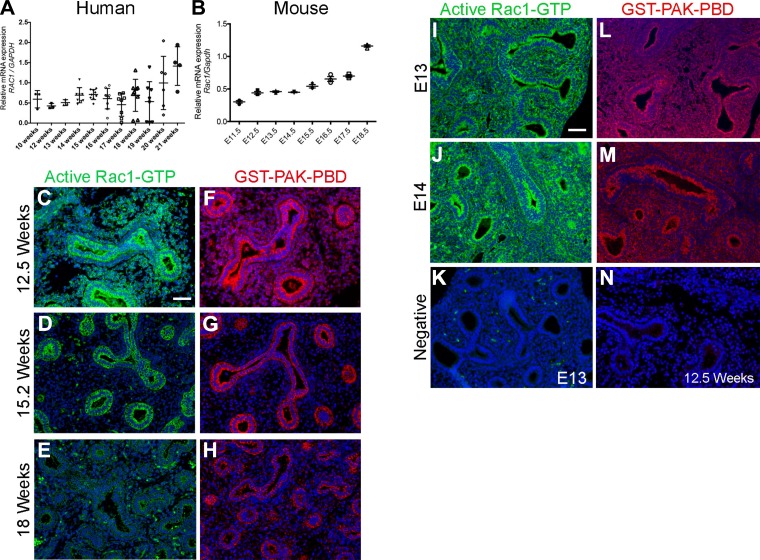
To determine whether Rac1 plays a role in lung branching morphogenesis, we sought to determine its expression in the developing lung in both human and mouse. Human fetal samples at different gestational ages, corresponding to late pseudoglandular/early canalicular stage (10–21 wk), were obtained under IRB approval and used herein. We demonstrated that RAC1 is expressed in human fetal lungs starting from 10 wk through 21 wk of gestation (Fig. 1A). We observed a significant increase in RAC1 expression at 21 wk compared with the other stages studied (P ≤ 0.05). In mouse lung, we investigated the expression of Rac1 from E11.5 through E18.5. Expression was constant between E11.5 and E13.5 (Fig. 1B) and started to increase at E14.5. By E18.5 Rac1 expression levels had increased approximately threefold compared with E11.5 (Fig. 1B). We next analyzed the expression and distribution of the active GTP-bound form of Rac1 in the developing human and mouse lung by immunofluorescence. In the human lung, active Rac1 was strongly expressed in both epithelium and mesenchyme at 12.5 wk gestation (Fig. 1C). At 15.2 wk gestation, active RAC1 expression became more restricted to the epithelium (Fig. 1D), whereas by 18 wk gestation its expression was very low throughout the lung tissue (Fig. 1E). This was confirmed using the GST-PAK-PBD fusion protein allowing the detection of GTP-bound Rac1 (Fig. 1, F–H). No staining was detected when the tissue was incubated with either secondary alone (Fig. 1K, E12 mouse lung section) or anti-GST followed by secondary antibody only (Fig. 1N, 12.5 wk human lung section). However, in the mouse lung, staining with active Rac1 antibody showed active Rac1 expression in both epithelium and mesenchyme similarly at E13 (Fig. 1F) and E14 (Fig. 1G). Similar results were obtained when using the fusion protein GST-PAK-PBD (Fig. 1, L and M).
Fig. 1.
Rac1 is expressed in mouse and human developing lungs. A and B: qRT-PCR showing the expression of RAC1 in human fetal lungs between gestational weeks 10 and 21 (A) n = 3 at least, and in the developing mouse lung between embryonic day E11.5 and E18.5 (B), n = 3 for each time point. C–E: immunofluorescent staining showing active Rac1 (green) and DAPI (blue) in 12.5 (C), 15.2 (D), and 18 wk (E) gestational age human fetal lung. F–H: fluorescent staining using the fusion protein GST-PAK-PBD (red) and DAPI (blue) on 12.5 (F), 15.2 (G), and 18 wk (H) human fetal lung. I–J: immunofluorescent staining showing active Rac1 (green) and DAPI (blue) in E13 (I) and E14 mouse lung (J). K: negative control in absence of active-Rac1 antibody and only secondary antibody on E13 mouse lung. L and M: staining using the fusion protein GST-PAK-PBD (red) and DAPI (blue) on E13 (L) and E14 (M) mouse lung. N: negative control in presence of anti-GST and secondary antibody only on 12.5 wk human fetal lung. Staining was performed on slides from at least 3 independent samples. Scale bar is 50 μm.
Rac1 inhibition decreases lung branching in a dose-dependent manner.
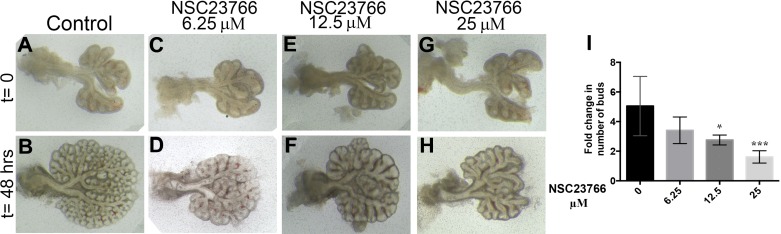
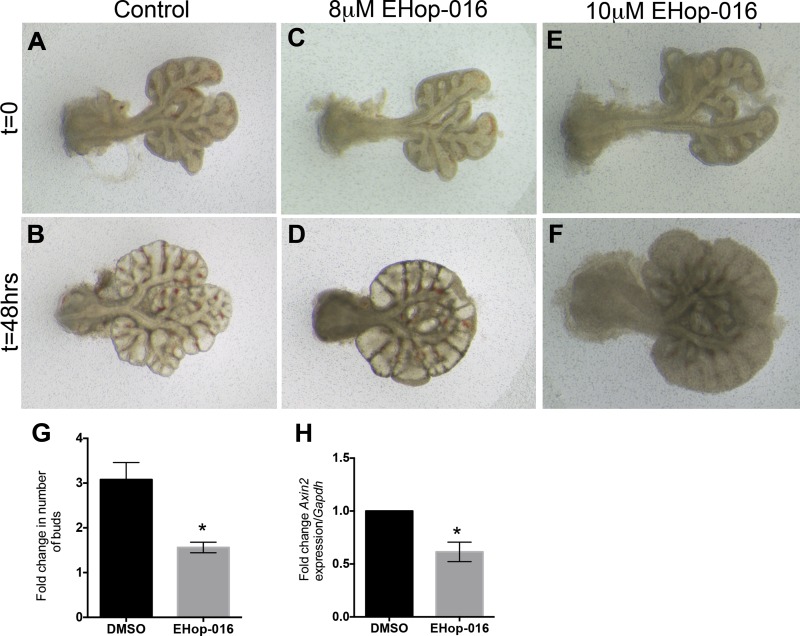
To test the effect of Rac1 inhibition on lung branching morphogenesis, we cultured embryonic day 12.5 lung explants in air-liquid interface as previously described (15) and treated them with different concentrations of the Rac1 inhibitor NSC23766. NSC23766 does not affect the activity of RhoA or Cdc42 and impedes the action of Rac1 by preventing the GDP/GTP exchange required for Rac1 activity by disrupting the interaction between Rac1 and the Rac-specific GEFs. After 48 h of culture in presence of 6.25 μM of NSC23766, the lung explants displayed fewer branches than the control explants (Fig. 2, D vs. B). This inhibition in branching was more pronounced as the concentrations of inhibitor increased (Fig. 2, F and H vs. B). Quantification of the fold increase in buds between t = 0 and t = 48 h showed a dose-dependent decrease in the number of buds. No change in the number of buds at t = 48 h was observed in presence of 6.25 μM of NSC23766 compared with control (3.41 ± 0.52 vs. 5.05 ± 0.82, P = 0.1910, n = 5, Fig. 2I), whereas, a significant decrease in budding was demonstrated in presence of 12.5 and 25 μM of NSC23766, compared with control (2.75 ± 0.16 and 1.61 ± 0.15 vs. 5.05 ± 0.82, n = 8, P = 0.0394 and P < 0.0001) (Fig. 2I). Higher concentrations of NSC23766, 50 μM (the widely used IC50) and 100 μM, resulted in the death of the explants after 48 h in culture (data not shown). For all the subsequent experiments hereafter, we used 25 μM of NSC23766, the concentration that decreased branching the most at 48 h but did not cause complete tissue necrosis. To confirm that this effect is mediated mainly through Rac1 as opposed to off target pathways of NSC23766, we used another common Rac1 inhibitor EHop-016, a potent inhibitor of Rac1 but also Rac3 (7). EHop-016 inhibited branching and induced distal bud dilation at a concentration of 8 μM (Fig. 3, D vs. B); and resulted in tissue necrosis at 10 μM (Fig. 3, F vs. B). When the growth was quantified by counting the number of new branches at t = 48 h compared with t = 0 in the explants treated with 8 μM EHop-016 compared with DMSO controls (Fig. 3G), a significant decrease in branching was observed (1.56 ± 0.117 vs. 3.078 ± 0.4 in DMSO, n = 4, P = 0.0035). Since tissues were severely necrotic at 10 μM, the branches were not clearly defined and thus not possible to count.
Fig. 2.
Rac1 inhibition decreased embryonic mouse lung branching in a dose-dependent manner. Whole-mount view of E12.5 mouse lung explants at 0 h (A, C, E, and G) and following 48 h in culture (B, D, F, and H) in the absence (A and B) or presence of 6.25 (C and D), 12.5 (E and F), and 25 μM (G and H) of Rac1 inhibitor NSC23766. I: quantification of fold change in number of buds (no. of buds at t = 48 h/no. of buds at t = 0 h). Data are represented as means ± SE of at samples from at least 4 independent litters; n = 5 at least; *P = 0.0281; ***P = 0.0005.
Fig. 3.
Rac1 inhibitor EHop-016 impairs embryonic mouse lung branching. Whole-mount view of E12.5 mouse lung explants at 0 h (A, C, E) and following 48 h in culture (B, D, F) in the absence (A and B) or presence of 8 μM (C and D) and 10 μM (E and F) of Rac1 inhibitor EHop-016. G: quantification of fold change in number of buds in explants treated with 8 μM EHop-016 vs. control DMSO (no. of buds at t = 48 h/no. of buds at t = 0 h). H: fold change in Axin2 expression in explants treated with 8 μM EHop-016 compared with controls. Data are represented as means ± SE of at samples from at least 4 independent litters. *P ≤ 0.01.
Rac1 inhibition increases mesenchymal cell death and cell proliferation in lung explants.
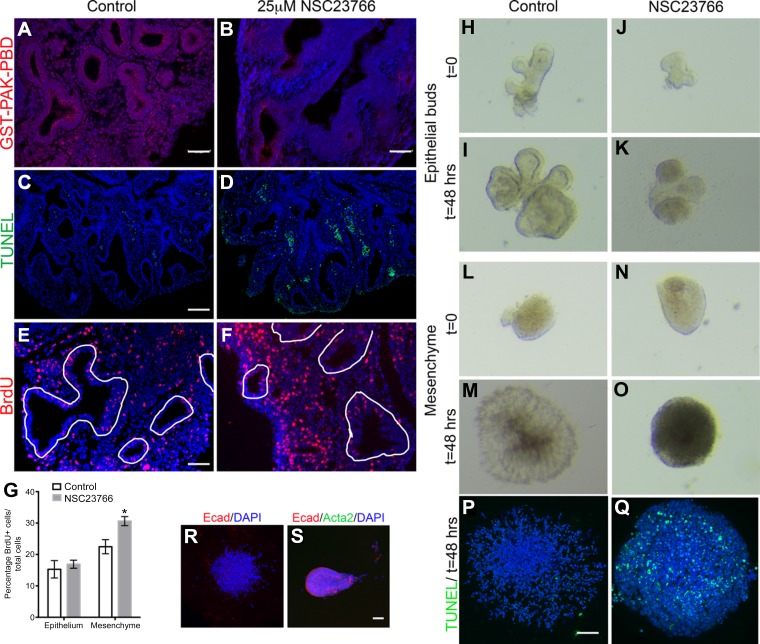
To confirm that Rac1 was inhibited in the lung explants, staining for GST-PAK-PBD was performed on the explant cultures following 48 h treatment with 25 μM NSC23766. A strong but not complete inhibition in Rac1 activity was observed in the explants treated with NSC23766 compared with controls (Fig. 4, B vs. A). Decreased branching is often associated with decreased cell proliferation or increased cell death. Therefore, we next assessed whether Rac1 inhibition modulates cell proliferation and/or cell death in the explants treated with 25 μM of NSC23766 compared with nontreated explants. Detection of apoptosis by TUNEL assay showed that cell death is increased in the inhibitor-treated explants compared with control explants (Fig. 4, D vs. C). The apoptotic cells in the treated explants were mostly mesenchymal, whereas the epithelial cells displayed no change in cell death compared with control explants (Fig. 4, D vs. C).
Fig. 4.
Rac1 inhibition leads to increased cell proliferation and cell death in the lung mesenchyme. A and B: GST-PAK-PBD staining on sections treated with 25 μM NSC23766 (B) vs. controls (A). C and D: TUNEL staining on E12.5 mouse lung sections cultured for 48 h in absence (C) or presence of 25 μM Rac1 inhibitor (D). E and F: BrdU staining on E12.5 mouse lung sections cultured for 48 h in absence (E) or presence of 25 μM Rac1 inhibitor (F). White lines delineate epithelial structures. G: quantification of BrdU-positive cells in either the epithelium or mesenchyme as percentage to the total number of cells within the appropriate each compartment in controls (white bars) and treated explants (gray bars). Data are represented as means ± SE of at least 6 independent samples, P = 0.002. H–K: cultured isolated epithelial buds from E11.5 mouse lungs at t = 0 (H and J) and t = 48 h (I and K) in presence of FGF10 alone (H and I) or FGF10 + NSC23766 (J and K). L–O: cultured isolated mesenchymal explants from E11.5 mouse lungs at t = 0 (L and N) and t = 48 h (M and O) in presence of FGF9 alone (L and M) or FGF9 + NSC23766 (N and O); n = 4 independent samples. P and Q: TUNEL staining on cultured isolated mesenchymal explants from E11.5 mouse lungs cultured for 48 h treated with FGF9 (P) or FGF9 + NSC23766 (Q). R: Ecad staining on cultured isolated mesenchymal explant from E11.5 mouse lung cultured for 48 h. S: Ecad and Acta2 staining on cultured isolated epithelial bud from E11.5 mouse lung at t = 0. Scale bars are 100 μm in A, C, P, and S and 50 μm in E.
Cell proliferation was assessed through the incorporation of BrdU for 3 h before the end of the culture period. This allows for halogenated derivatives of thymidine to become incorporated into the cells' nuclei during S-phase. The BrdU stain demonstrated no change in BrdU+ cells in the epithelium, but an increase in BrdU+ cells in the mesenchyme of the treated explants compared with controls (Fig. 4, F vs. E). In the mesenchyme of treated explants, 30.5 ± 1.4% of cells incorporated BrdU compared with 22.6 ± 2.2% in control explants, demonstrating a significant increase in proliferation (Fig. 4G, n = 6, P = 0.004), while no significant change was observed in the epithelium (15.3 ± 2.8% in treated explants vs. 16.9 ± 1.3% in controls, P = 0.555; Fig. 4G).
To determine whether Rac1 inhibition affects primarily the epithelium or the mesenchyme, we treated mesenchymal and epithelial-only explant cultures with NSC23766. The mesenchyme of E11.5 mouse lungs was separated from the epithelial tips by microdissection, and explants of either mesenchyme or epithelial tips were separately embedded in Matrigel and cultured as previously described (15) for up to 48 h. The epithelial tips grown with FGF10 alone or both FGF10 and Rac1 inhibitor displayed comparable growth at 48 h (Fig. 4, K vs. I). Epithelial tips do not survive in culture in the absence of FGF10 (15). Although not apparent within the first 24 h of Rac1 inhibition, by 48 h it was evident that inhibition of Rac1 altered lung mesenchyme growth, where dense aggregation of cells was seen in the center of the tissue (Fig. 4, O vs. M), and the tissue had increased cell death as assessed by TUNEL staining (Fig. 4Q) compared with controls (Fig. 4P). The mesenchymal explants did not contain any epithelial contaminations as assessed by E-cadherin staining (Fig. 4R). Epithelial explants at t = 0 did not show any Acta2 staining at t = 0, suggesting absence of smooth muscle cells, and all the rest of the cells stained positive for E-cadherin (Fig. 4S). These data suggest that Rac1 inhibition primarily impairs mesenchymal growth and prevents proper signaling to the epithelium, thus preventing adequate branching.
Rac1 inhibition impairs smooth muscle differentiation and Fgf10 expression.
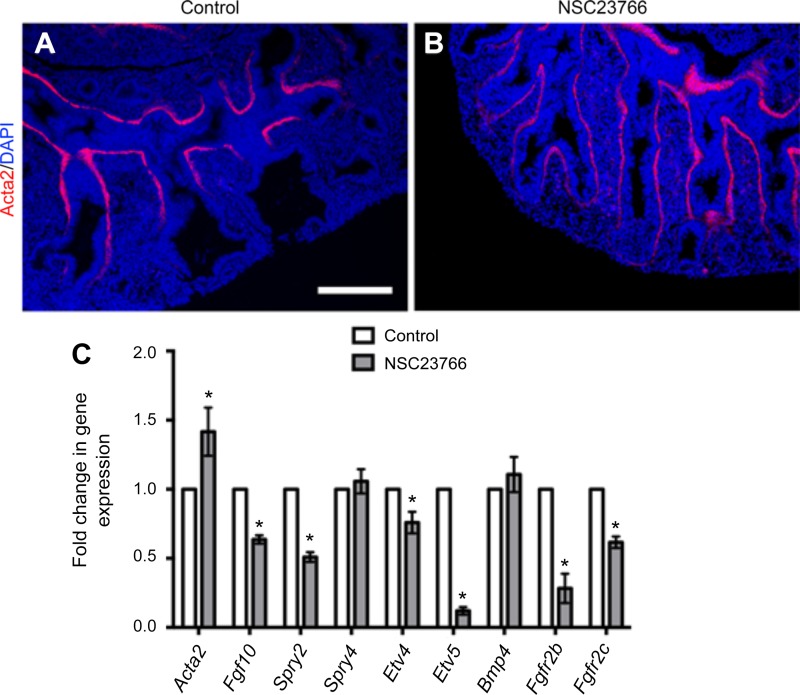
To further investigate the mesenchymal defects resulting from Rac1 inhibition, we assessed the expression and distribution of smooth muscle cells in the cultured lung explants. Immunofluorescent staining with Acta2 showed expansion of the smooth muscle cell domain to the very distal edge of the lung as shown in Fig. 5B compared with controls (Fig. 5A). Fgf10 signaling is known to play an important role in lung branching and smooth muscle cell differentiation. Moreover, the lack of Fgfr2b, the main receptor for Fgf10, results in elongation of the epithelial lung buds (1), similar to what is observed in the explants treated with the Rac1 inhibitor. Therefore, we analyzed the expression of Fgf10, many of its target genes, as well as its receptors Fgfr2b and Fgfr2c. Quantification by qRT-PCR validated that the expression of Fgf10 had significantly decreased following Rac1 inhibition (Fig. 5C, n = 7, P = 0.0006). Fgfr2b and Fgfr2c levels were also decreased in the Rac1 inhibitor-treated group (P = 0.0758). Spry4 showed no significant change in expression, whereas Spry2 expression significantly decreased in the lungs treated with NSC23766 (P = 0.0065). Transcription factors Etv4 and Etv5, direct targets of Fgf signaling, were both significantly decreased in the treated lungs compared with the controls (Etv4 P = 0.0241; Etv5 P < 0.0001), with a more pronounced effect on Etv5 expression, which is located in the distal epithelium of the lung (17).
Fig. 5.
Rac1 regulates smooth muscle cells and Fgf10 expression. A and B: Acta2 staining in control (A) and NSC23766-treated (B) explants following 48 h in culture. C: quantification by qRT-PCR of Acta2, Fgf10, Spry2, Spry4, Etv4, Etv5, Fgfr2b, and Fgfr2c of control and treated explants following 48 h in culture. Data are represented as means ± SE of at least 7 independent samples; *P ≤ 0.05. Scale bar is 50 μm.
Rac1 inhibition impairs vasculogenesis in the lung explants.
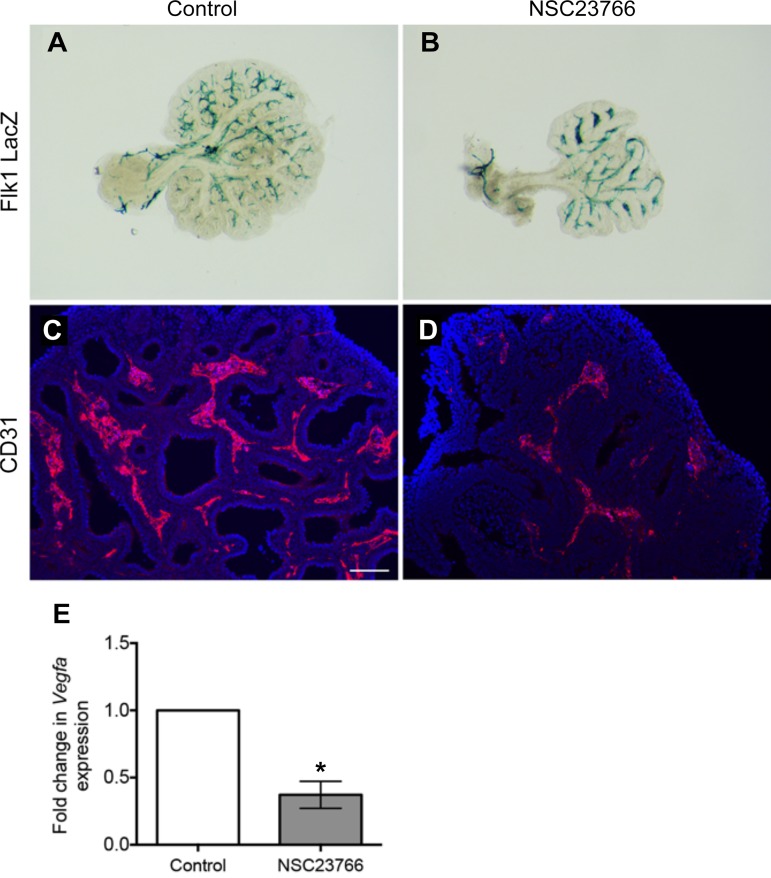
Branching and vascular development are tightly regulated during lung development. Given the pronounced mesenchymal defects, we next sought to determine whether Rac1 inhibition affects vasculogenesis. The use of lung explants from Flk1-LacZ reporter mice demonstrated compaction and accumulation of LacZ signal between the elongated epithelial branches in the presence of Rac1 inhibitor (Fig. 6B) compared with controls (Fig. 6A). Similarly, immunofluorescence staining for CD31 showed a decrease in CD31 expression following Rac1 inhibition (Fig. 6, D vs. C). Moreover, qRT-PCR analysis showed a significant decrease in expression of vascular endothelial growth factor a (Vegfa), the main ligand for the Flk1 receptor (n = 7, P = 0.0007) in Rac1 inhibitor-treated lungs compared with controls (Fig. 6E). These results suggest that Rac1 is required for proper vascular formation in the developing lung.
Fig. 6.
Rac1 inhibition alters lung vasculogenesis ex vivo. A and B: LacZ staining on Flk1-LacZ reporter explants cultured in absence (A) or presence (B) of Rac1 inhibitor. C and D: immunofluorescence for CD31 (red) of untreated (C) and Rac1 inhibitor treated (D) lung explants; blue is DAPI. E: quantification of Vegfa by qRT-PCR in control and treated lung explants. Data are represented as means ± SE of at least 7 independent experiments; *P = 0.0007. Scale bar is 50 μm.
Rac1 inhibition represses Wnt signaling during lung development.
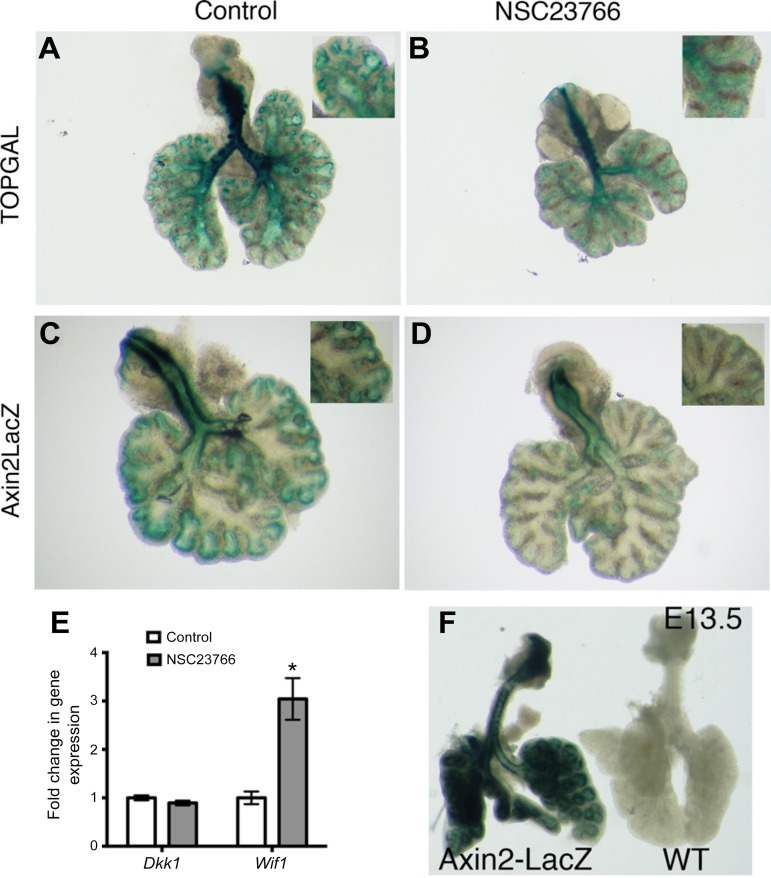
To determine whether Rac1 activity is mediated by Wnt signaling, we examined Wnt activity using TOPGAL and Axin2-LacZ lung explants. These lung explants were cultured in the presence of 25 μM NSC23766 for 48 h and were then stained with LacZ to evaluate Wnt signaling. TOPGAL expression is mostly detected in the epithelium and parabronchial smooth muscle cells of embryonic lung, whereas Axin2-LacZ expression is used as a readout in both the epithelium and the mesenchyme (2).
Whole-mount TOPGAL lungs demonstrated that there was a decrease in Wnt activity in the distal lungs as well as the main bronchi (Fig. 7, B vs. A). The trachea in both treated and control TOPGAL lungs displayed similar high levels of Wnt activity. The distal airways of the control lungs demonstrated a very strong specific staining that is clearly outlining the smooth muscle cells (Fig. 7A), whereas the treated lungs displayed a low diffuse signal (Fig. 7B).
Fig. 7.
Rac1 inhibition decreases canonical Wnt signaling. A and B: lung explants from TOPGAL reporter mice cultured in absence (A) or presence (B) of Rac1 inhibitor. Lung explants from Axin2-LacZ reporter mice cultured in absence (C) or presence (D) of Rac1 inhibitor. E: quantification of Wnt inhibitors Dkk1 and Wif1 by qRT-PCR in control and treated lungs. Data are represented as means ± SE of 7 independent experiments; *P = 0.0018. F: LacZ staining of E13.5 Axin2-LacZ lung and its WT littermate.
Comparable to TOPGAL explants, treated Axin2-LacZ lungs displayed no change in staining of the trachea; however, the lung had little to no detectable LacZ staining (Fig. 7D) compared with controls (Fig. 7C). Both Wnt reporters showed that the canonical Wnt pathway was decreased due to Rac1 inhibition. Moreover, EHop-016, another Rac1 inhibitor, significantly decreased the expression of Axin2 (n = 4, P = 0.003) in cultured explants compared with controls (Fig. 3H). To validate the specificity of the Lac-Z staining, lungs from E13.5 Axin2-LacZ-positive and WT littermate embryos were stained with LacZ. Littermate controls did not show any LacZ staining, while Axin2-LacZ lungs were strongly stained (Fig. 7F).
We next sought to determine whether Rac1 acts directly on Wnt inhibitors. qRT-PCR analyses for Dkk1 showed no significant difference between treated explants and controls (Fig. 7E), whereas the expression of Wnt inhibitory factor 1, Wif1, was significantly increased threefold following Rac1 inhibition (Fig. 7E, n = 7, P = 0.0018).
Canonical Wnt activation partially rescues branching defects caused by Rac1 inhibition.
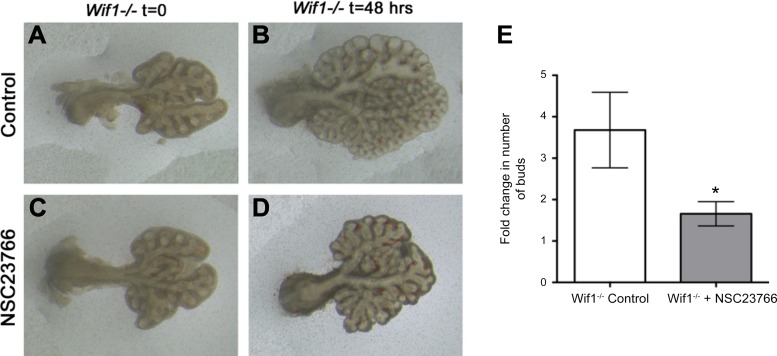
Given the strong decrease in canonical Wnt and increase in Wif1 expression, we aimed to determine whether upregulation of Wnt signaling could prevent or reverse the branching defects resulting form Rac1 inhibition. Two approaches were used to test this. First, we used Wif1−/− animals to determine whether decreasing Wif1 would abolish the effect of Rac1 inhibition. E12.5 Wif1−/− lungs were cultured in the absence (Fig. 8, A and B) or presence of NSC23766 (Fig. 8, C and D) for 48 h. In the absence of NSC23766, Wif1−/− lung explants branched normally (Fig. 8B), whereas in the presence of NSC23766 (Fig. 8D) branching was severely impaired, and we observed a similar elongation pattern in the epithelial buds as seen in the treated WT explants in Fig. 2. In control lungs, we observed a 3.68-fold increase in the number of buds at 48 h compared with t = 0, as opposed to an only 1.65-fold increase in the number of buds in the Wif1-null explants treated with NSC23766 (Fig. 8E, n = 8, P = 0.0001). Therefore, deletion of Wif1 did not reverse or rescue the effect of Rac1 inhibition.
Fig. 8.
Wif1 inactivation is not sufficient for rescuing lung branching due to Rac1 inhibition. A–D: E12.5 lung explants from Wif1−/− mice cultured in absence (A and B) or presence (C and D) of NSC23766 at t = 0 (A and C) and t = 48 h (B and D). E: quantification of the fold change in number of buds between 48 h and 0 h of control and NSC23766-treated lungs. Data are represented as means ± SE of 8 independent experiments. *P ≤ 0.05.
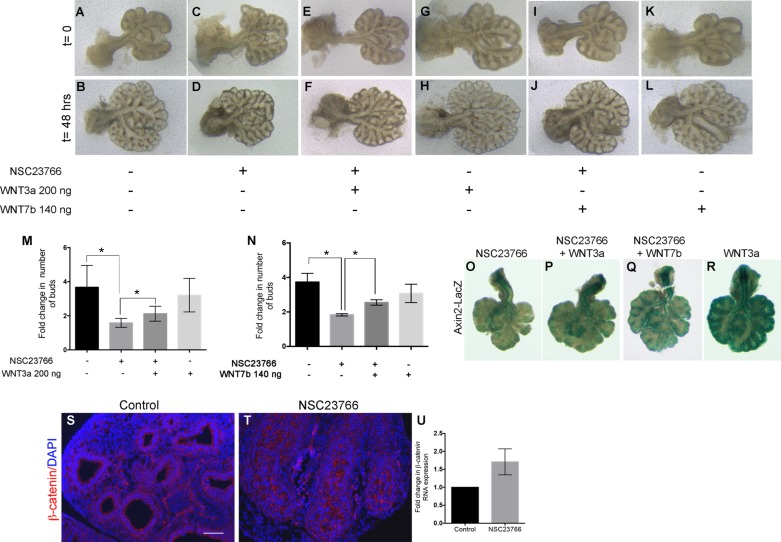
We then sought to activate Wnt signaling using WNT3a or WNT7b. E12.5 mouse lung explants were treated with recombinant WNT3a or WNT7b protein for 48 h in presence or absence of 25 μM NSC23766 (Fig. 9, A–L). Control explants branched as expected (Fig. 9, B vs. A), while explants treated with NSC23766 showed impaired branching (Fig. 9, D vs. C). Lung explants treated with NSC23766 in the presence of WNT3a showed slightly improved branching (Fig. 9F), with less elongation and a few new branches appearing at 48 h compared with NSC23766 treatment alone (Fig. 9D). Similarly, treatment of the explants with NSC23766 in presence of 140 ng of WNT7b resulted in a slight rescue and increase in branching compared with NSC23766 alone (Fig. 9, J vs. D). Explants treated with WNT3a or WNT7b alone had a similar branching pattern as the controls (Fig. 9, H and L, respectively). Quantification of the increase in branching showed a modest but significant increase in the number of buds in the explants treated with NSC23766 in the presence of WNT3a compared with NSC23766 alone (Fig. 9M, 2.12 ± 0.18 vs. 1.59 ± 0.09, respectively, n = 6, P = 0.0198). Explants treated with WNT3a alone or WNT7b alone had a similar branching pattern as that of control explants (Fig. 9, M and N, respectively). Quantification of the branching also demonstrated a slight but significant increase in branches in presence of WNT7b and NSC23766 compared with NSC23766 alone (Fig. 9N, 2.55 ± 0.16 vs. 1.83 ± 0.06, respectively, n = 6, P = 0.0019). To validate this result, we treated Axin2-LacZ reporter explants with NSC23766 in the presence or absence of WNT3a. LacZ staining following 48 h in culture showed a slight increase in Wnt activity in the explants treated with both NSC23766 and WNT3a (Fig. 9P), as well as explants treated with NSC23766 and WNT7b (Fig. 9Q) compared with the explants treated solely with NSC23766 (Fig. 9O). Explants treated with WNT3a alone had strong LacZ staining, indicating robust Wnt activity (Fig. 9R).
Fig. 9.
Wnt activation partially rescues lung branching defects caused by Rac1 inhibition. A–L: E12.5 WT lung explants at t = 0 (A, C, E, G, I, and K) and t = 48 h (B, D, F, H, J, and L) cultured in absence of NSC23766 (A and B), in presence of NSC23766 (C and D), in presence of NSC23766 and 200 ng WNT3a (E and F), in presence of 200 ng of WNT3a alone (G and H), in presence of NSC23766 and 140 ng WNT7b (I and J), and in presence of 140 ng of WNT7b alone (K and L). M: quantification of the fold change in number of buds in the explants after 48 h in culture in presence or absence of NSC23766 with or without WNT3a. Data are represented as means ± SE of 6 independent experiments; *P ≤ 0.05. N: quantification of the fold change in number of buds in the explants after 48 h in culture in presence or absence of NSC23766 with or without WNT7b. Data are represented as means ± SE of 6 independent experiments; *P ≤ 0.05. O–R: LacZ stain of Axin2-LacZ lung explants cultured for 48 h in presence of NSC23766 (O), NSC23766 and WNT3a (P), NSC23766 and WNT7b (Q), or WNT3a alone (R). S and T: immunofluorescence staining for β-catenin (red) on control (S) and NSC23766-treated explants (T). U: relative expression of β-catenin in control and treated explants as assessed by qRT-PCR. Results are expressed as means ± SE of 6 independent experiments normalized to control values. Scale bar in R is 50 μm.
Canonical Wnt signaling is mediated by β-catenin. Staining for β-catenin did not show altered expression of β-catenin in the treated explants compared with controls (Fig. 9, T vs. S). Similarly, the expression of β-catenin assessed by qRT-PCR was not significantly changed in the treated explants (Fig. 9U).
Inhibition of Rac1 inhibits human fetal lung branching likely via Wnt signaling.
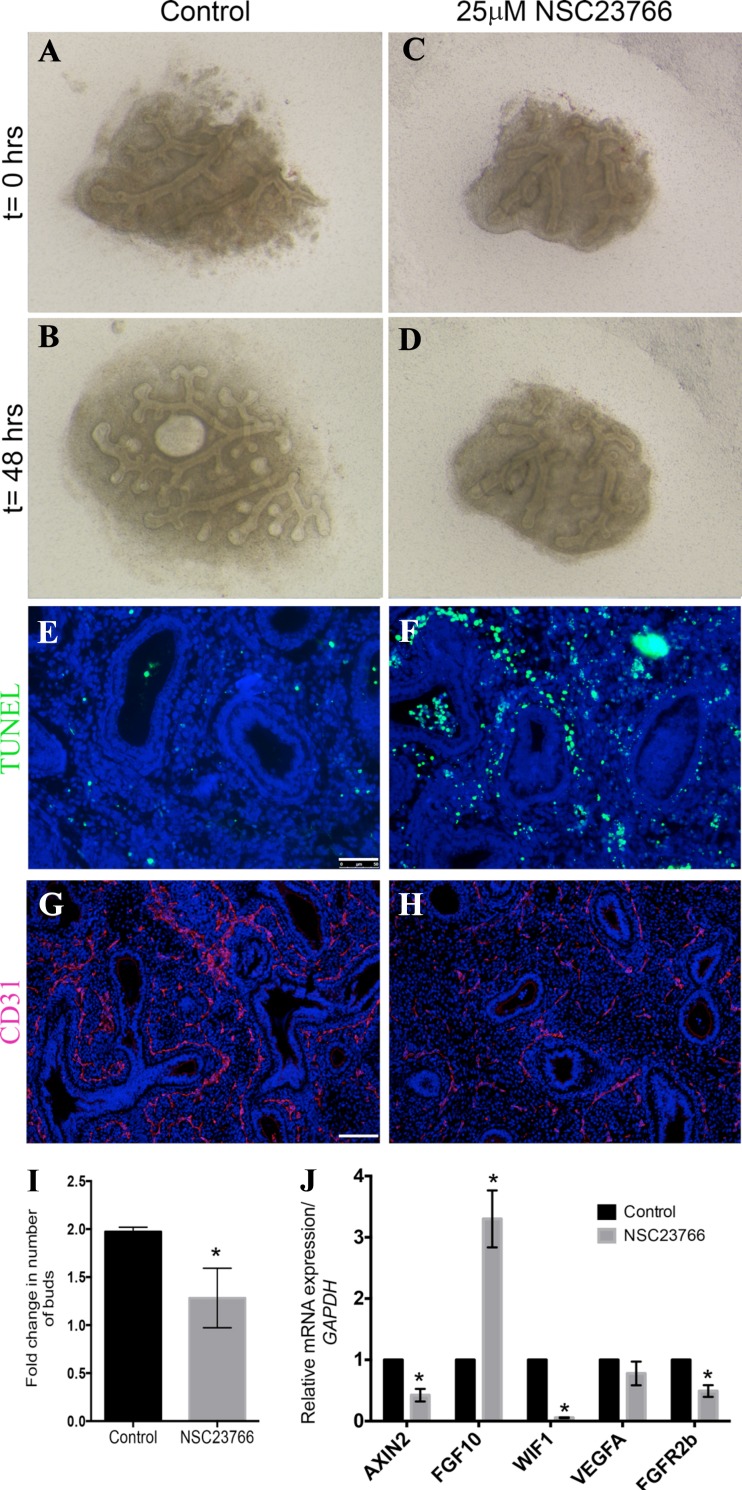
As active Rac1 is highly expressed in the epithelium of the human fetal lung, we attempted to determine whether our data in mouse translate to humans. Distal segments between 3 and 4 mm2 were dissected from human fetal lungs between gestational ages 10–12.5 wk. Dissection was carried out to preserve the integrity of the distal epithelial tips and the mesenchyme surrounding them. The explants were then cultured in an air-liquid interface system similar to that used for the mouse explant cultures. Human lung explants were treated with 25 μM NSC23766 for 48 h, after which branching was evaluated. Control human lung explants grew and expanded following 48 h in culture (Fig. 10, B vs. A); however, in the presence of the Rac1 inhibitor (NSC23766), these explants failed to grow and branch (Fig. 10, D vs. C). Quantification of the branch number between 48 h and t = 0 showed a twofold increase in the branching of the control group (Fig. 10I) compared with a 1.28 ± 0.15-fold increase in the NSC23766-treated explants (n = 4, P = 0.0136). TUNEL staining, used to assess cell death, showed very little cell death in the control explants (Fig. 10E), while there was an increase in cell death in the mesenchyme of the NSC23766-treated explants (Fig. 10F). Moreover, Rac1 inhibition altered vascular formation in the human fetal lung explants as shown by CD31 staining (Fig. 10, H vs. G). Finally, Rac1 inhibition resulted in a significant decrease in AXIN2 and FGFR2b expression in the human lung explants (n = 4, P = 0.01 and 0.0061, respectively), suggesting a decrease in Wnt signaling and FGFR2b signaling (Fig. 10J). However, the expression of FGF10 significantly increased following Rac1 inhibition (n = 4, P = 0.032) whereas WIF1 was decreased (n = 4, P = 0.0001, Fig. 10J). No change in VEGFA expression was observed (Fig. 10J). These results suggest that Rac1 controls branching in both mouse and human; however, the underlying molecular pathways are somewhat different.
Fig. 10.
Rac1 inhibition impairs human lung branching in vitro. A–D: Dissected 11.1-wk gestational age distal edges of 11 wk gestational age human lung explants at t = 0 (A, C) cultured on air liquid interface in absence (A, B) or presence of NSC23766 (C, D) for 48 h (B, D). E and F: TUNEL staining on sections from paraffin-embedded control explants (E) and NSC23766-treated explants (F) after 48 h in culture. G and H: immunofluorescence for CD31 on control (G) and treated sections (H). I: graph showing fold change in number of branches at 48 h compared with t = 0 h. J: graph illustrating the fold change in AXIN2, FGF10, WIF1, VEGFA, and FGFR2b expression in treated explants normalized to controls. Results in I and J are expressed as means ± SE of explants from 4 independent samples. Scale bars are 50 μm. *P ≤ 0.05.
DISCUSSION
This study describes the role of the small RhoGTPase Rac1 in both murine and human lung branching morphogenesis. Since Rac1 controls a multitude of cellular events, all of which are important for proper organogenesis, we hypothesized that Rac1 activity is essential for proper lung development. While Rac1 has been shown to play an important role in lung cancer (27, 44), its role in lung development has yet to be investigated.
Rac1 is expressed in various adult tissues including the lung (29). It is upregulated in lung carcinomas and correlates with poor outcome in non-small-cell lung cancer patients (27, 44). We first demonstrated that Rac1 is expressed in the developing lung of both human and mouse. In mammary gland epithelial buds, active Rac1 is expressed at the tip of the elongated branches (45). Using similar staining approach as well as active Rac1 antibody staining, we showed that in mouse and human lung active Rac1 was present throughout the epithelial buds but also in the mesenchyme. In the human lung, active Rac1 decreased at 18 wk gestation. Previous studies reported that Rac1 inhibition impairs branching elongation in mammary epithelial buds (21, 45). In our study, we showed that Rac1 inhibition inhibits new branch formation while epithelial buds continue to elongate. The discrepancy between our results and the previous studies on mammary epithelia could be due to the fact that these studies used epithelial-only culture, while in our explants we preserve the epithelial mesenchymal cross talk. The use of EHop-016, another Rac1 inhibitor and a potent inhibitor of Rac3, showed a slightly different phenotype than that observed with NSC23766, where distal bud dilation was observed in addition to impaired branching. This could be due to off-target effects of the inhibitors, hence presenting these differences in phenotype. Since Rac1−/− animals die in utero around midgestation (37), conditional Rac1 deletion in the epithelium or mesenchyme will be required to further analyze the effect of Rac1 on lung development in vivo.
It had been previously shown that a dominant-negative form of RAC1 leads to the inhibition of migration in many different cell types, including epithelial cells and fibroblasts (34). This suggests that Rac1 inhibition in the lung has the ability to affect either tissue compartment, which could ultimately result in the inhibition of epithelial lung branching. As previously demonstrated by numerous studies, a continuous cross talk between the epithelium and mesenchyme is essential to lung growth (4, 18, 31, 40). Increased cell turnover, shown by increased cell proliferation and cell death in the mesenchyme of the treated explants as well as necrosis of isolated mesenchymal explants, suggests that Rac1 has a greater impact on the mesenchyme. This was validated by demonstrating the downregulation of Fgf10, one of the growth factors that is released from lung mesenchyme to act upon the epithelium via Fgfr2(III)b, a signaling pathway necessary for lung bud outgrowth (1, 4). Interestingly, the resulting bud elongation following Rac1 inhibition resembles the phenotype obtained when Fgfr2b signaling is impaired, which is consistent with the decrease of Fgfr2b expression in the explants treated with Rac1. The expression of Fgfr2c was also decreased along with Etv4 and Etv5, targets of FGF signaling. Moreover, we observed an expansion of the smooth muscle cell domain to the very distal edge of the epithelial buds, similar to previously published findings in E12.5 Fgf9-null lungs as well as lungs lacking Fgfr1 and Fgfr2 expression (43). In addition, deletion of mesenchymal β-catenin results in similar findings (11). Although the mechanism is still not completely understood, FGF10 signaling via FGFR2(III)b controls the proliferation of pulmonary epithelial progenitors partially through the regulation of epithelial β-catenin signaling, thus indicative of canonical Wnt signaling (33). Therefore, with the downregulation of Fgf10 due to an incompetent mesenchyme, a decrease in canonical Wnt signaling would also be expected, which was confirmed through the use of Wnt reporter explants.
Alongside the other Rho GTPases, Cdc42, and RhoA, Rac1 has been described in numerous signaling pathways, including MAPK and PI3K. However, Rac1 is the only Rho GTPAse shown to be involved in stimulating β-catenin/T cell factor-mediated transcription (16). Using Wnt reporter explants, we demonstrated that canonical Wnt signaling was downregulated in the explants treated with the Rac1 inhibitor. Whereas previous studies reported genetic interaction between Dkk1 and Rac1, we did not observe any change in the levels of Dkk1 following Rac1 inhibition. However, another Wnt inhibitor, Wif1 was significantly upregulated. Wif1 negatively regulates Wnt activity by binding to Wnt proteins, thus preventing them from binding their Frizzled receptor (19). Although Wif1 expression has been shown to be highest in adult mouse heart and lung (19), its role in lung development is still unclear. It was demonstrated that increased Wnt activity due to deficient Smad-Wif1 regulation in fetal lung epithelial cells results in distal epithelial cell proliferation (42). This suggests that Wif1 regulates canonical Wnt signaling in distal lung morphogenesis and plays an important role in lung development. However, no major lung defects have been described in the Wif1-null animals, and these animals survive normally through adulthood (23). The use of Wif1−/− explants did not reverse the effects of Rac1 inhibition. Though silencing of Wif1 results in increased β-catenin and Lef-1 expression (24), this approach was not sufficient to block the effect of Rac1 inhibition in our explant model. This could perhaps be due to other compensatory mechanisms taking place. We also demonstrated that there was no change in β-catenin expression upon Rac1 inhibition, suggesting that Rac1 inhibition did not affect β-catenin expression per se. In contrast, using recombinant WNT3a and WNT7b proteins, widely used canonical Wnt activators, resulted in a modest rescue of the branching defects. However, this rescue was not complete as evidenced by the number of new branches and the LacZ activity in Axin2-LacZ explants treated with both Rac1 inhibitor and WNT3a or WNT7b. Wu et al. (41) demonstrated that WNT3a activates Rac1 in several cell lines, thus leading to activation of β-catenin signaling, whereas, in absence of Rac1 activity, Wnt7b is unable to activate canonical Wnt signaling. Since the effect of Rac1 inhibition on branching is dose dependent, and WNT3a activates Rac1, it is likely that the inhibition of Rac1 is less severe in presence of WNT3a. In the case of WNT7b, we stipulate that because Rac1 is not completely inhibited, WNT7b is still able to activate to some extent canonical Wnt signaling in our model. Nonetheless, the mechanism through which Rac1 interacts with canonical Wnt pathway remains very controversial. While Wu et al. reported that Rac1 regulates β-catenin expression and nuclear localization (41), other studies showed that Rac1 does not affect the nuclear localization of β-catenin (22). The latter showed that Rac1 enhances β-catenin phosphorylation at serine sites S191A and S605A, sites required for the binding of β-catenin to LEF1 (22). Further studies are needed to determine how Rac1 modulates β-catenin in our model.
To investigate whether this Rac1/Wnt signaling axis is conserved in human lung branching, we used human lung explant culture and successfully showed that Rac1 inhibition has similar effects in the human fetal lung, and it likely acts through Wnt signaling as we observed a decrease in AXIN2 expression. Rac1 has also been shown to be involved in vascular development, whether through its association with polarizing microtubule growth in endothelial cells (5) or through the regulation of vascular smooth muscle cell migration (26). Moreover, conditional deletion of endothelial Rac1 using Tie2-Cre driver results in embryonic death around midgestation. Herein, we showed that inhibition of Rac1 results in decreased Flk1-LacZ expression in the mouse lung as well as CD31 expression in both human and mouse explants, confirming an important role for Rac1 in vasculogenesis. Rac1 inhibition also decreased Vegfa expression in mouse explants, an important factor for branching morphogenesis (14). A decrease in FGFR2b signaling was also seen in both human and mouse explants. However, WIF1 and FGF10 expression varied in opposing ways in human and mouse. While lung branching in mouse has been extensively studied, limited knowledge is available on the mechanisms underlying human lung branching.
Altogether, our studies suggest that Rac1 is critical to adequate lung epithelial branching in both mouse and human via the canonical Wnt signaling pathway. Further studies are needed to elucidate how Rac1-β-catenin-LEF1 interact to result in proper lung branching morphogenesis. Divergences in the molecular pathways controlling human and mouse lung development are starting to emerge (6). Understanding the molecular differences in major pathways between mouse and human will be crucial to gaining more insight into the potential role of these pathways, e.g., Rac1/canonical Wnt in diseases such as pulmonary hypoplasia and bronchopulmonary dysplasia, and may hence provide a therapeutic target.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.D., B.G., and D.A.A. conceived and designed research; S.D., M.K., O.T., M.T., and D.A.A. performed experiments; S.D. and D.A.A. analyzed data; S.D., M.K., B.G., and D.A.A. interpreted results of experiments; S.D. and D.A.A. prepared figures; S.D. and D.A.A. drafted manuscript; S.D., B.G., and D.A.A. edited and revised manuscript; S.D., M.K., O.T., M.T., B.G., and D.A.A. approved final version of manuscript.
ACKNOWLEDGMENTS
S. Danopoulos acknowledges the support of NIH training grant (5T90DE021982-03). D. Al Alam acknowledges the support of the Saban Research Institute and the American Heart Association. We thank Dr. Igor Dawid from NIH for providing the Wif1−/− animals. We also thank Drs. Melissa L. Wilson (Department of Preventive Medicine, University of Southern California) and Rachel Steward (Family Planning Associates) for coordinating the efforts to obtain human fetal lungs.
REFERENCES
- 1.Abler LL, Mansour SL, Sun X. Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev Dyn 238: 1999–2013, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Alam D, Green M, Tabatabai Irani R, Parsa S, Danopoulos S, Sala FG, Branch J, El Agha E, Tiozzo C, Voswinckel R, Jesudason EC, Warburton D, Bellusci S. Contrasting expression of canonical Wnt signaling reporters TOPGAL, BATGAL and Axin2(LacZ) during murine lung development and repair. PLoS One 6: e23139, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagci H, Laurin M, Huber J, Muller WJ, Cote JF. Impaired cell death and mammary gland involution in the absence of Dock1 and Rac1 signaling. Cell Death Dis 5: e1375, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development 124: 53–63, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Braun A, Dang K, Buslig F, Baird MA, Davidson MW, Waterman CM, Myers KA. Rac1 and Aurora A regulate MCAK to polarize microtubule growth in migrating endothelial cells. J Cell Biol 206: 97–112, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan SC, Wilkinson WJ, Tseng HE, Finney B, Monk B, Dibble H, Quilliam S, Warburton D, Galietta LJ, Kemp PJ, Riccardi D. The extracellular calcium-sensing receptor regulates human fetal lung development via CFTR. Sci Rep 6: 21975, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo-Pichardo L, Humphries-Bickley T, De La Parra C, Forestier-Roman I, Martinez-Ferrer M, Hernandez E, Vlaar C, Ferrer-Acosta Y, Washington AV, Cubano LA, Rodriguez-Orengo J, Dharmawardhane S. The rac inhibitor EHop-016 inhibits mammary tumor growth and metastasis in a nude mouse model. Transl Oncol 7: 546–555, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornett B, Snowball J, Varisco BM, Lang R, Whitsett J, Sinner D. Wntless is required for peripheral lung differentiation and pulmonary vascular development. Dev Biol 379: 38–52, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danopoulos S, Parsa S, Al Alam D, Tabatabai R, Baptista S, Tiozzo C, Carraro G, Wheeler M, Barreto G, Braun T, Li X, Hajihosseini MK, Bellusci S. transient inhibition of FGFR2b-ligands signaling leads to irreversible loss of cellular beta-catenin organization and signaling in AER during mouse limb development. PLoS One 8: e76248, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126: 4557–4568, 1999. [DOI] [PubMed] [Google Scholar]
- 11.De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y, Minoo P, Hajihosseini MK, Drouin J, Kaartinen V, Bellusci S. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS One 3: e1516, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Langhe SP, Reynolds SD. Wnt signaling in lung organogenesis. Organogenesis 4: 100–108, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean CH, Miller LA, Smith AN, Dufort D, Lang RA, Niswander LA. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol 286: 270–286, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Del Moral PM, Sala FG, Tefft D, Shi W, Keshet E, Bellusci S, Warburton D. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol 290: 177–188, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Del Moral PM, Warburton D. Explant culture of mouse embryonic whole lung, isolated epithelium, or mesenchyme under chemically defined conditions as a system to evaluate the molecular mechanism of branching morphogenesis and cellular differentiation. Methods Mol Biol 633: 71–79, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esufali S, Charames GS, Bapat B. Suppression of nuclear Wnt signaling leads to stabilization of Rac1 isoforms. FEBS Lett 581: 4850–4856, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Herriges JC, Yi L, Hines EA, Harvey JF, Xu G, Gray PA, Ma Q, Sun X. Genome-scale study of transcription factor expression in the branching mouse lung. Dev Dyn 241: 1432–1453, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hines EA, Sun X. Tissue crosstalk in lung development. J Cell Biochem 115: 1469–1477, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398: 431–436, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Huang Z, Kim J, Lacruz RS, Bringas P Jr, Glogauer M, Bromage TG, Kaartinen VM, Snead ML. Epithelial-specific knockout of the Rac1 gene leads to enamel defects. Eur J Oral Sci 119, Suppl 1: 168–176, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huebner RJ, Neumann NM, Ewald AJ. Mammary epithelial tubes elongate through MAPK-dependent coordination of cell migration. Development 143: 983–993, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamieson C, Lui C, Brocardo MG, Martino-Echarri E, Henderson BR. Rac1 augments Wnt signaling by stimulating beta-catenin-lymphoid enhancer factor-1 complex assembly independent of beta-catenin nuclear import. J Cell Sci 128: 3933–3946, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons PJ, Dawid IB, Thomas DM. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest 119: 837–851, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MJ, Kim EJ, Li L, Jung HS. Roles of Wnt inhibitory factor 1 during tooth morphogenesis. Cell Tissue Res 362: 61–68, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Li A, Li M, Xing Y, Chen H, Hu L, Tiozzo C, Anderson S, Taketo MM, Minoo P. Stabilized beta-catenin in lung epithelial cells changes cell fate and leads to tracheal and bronchial polyposis. Dev Biol 334: 97–108, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin YC, Chen LH, Varadharajan T, Tsai MJ, Chia YC, Yuan TC, Sung PJ, Weng CF. Resveratrol inhibits glucose-induced migration of vascular smooth muscle cells mediated by focal adhesion kinase. Mol Nutr Food Res 58: 1389–1401, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Wang Y, Zhang Y, Miao Y, Zhao Y, Zhang PX, Jiang GY, Zhang JY, Han Y, Lin XY, Yang LH, Li QC, Zhao C, Wang EH. Abnormal expression of p120-catenin, E-cadherin, and small GTPases is significantly associated with malignant phenotype of human lung cancer. Lung Cancer 63: 375–382, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 22: 1184–1193, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matos P, Skaug J, Marques B, Beck S, Verissimo F, Gespach C, Boavida MG, Scherer SW, Jordan P. Small GTPase Rac1: structure, localization, and expression of the human gene. Biochem Biophys Res Commun 277: 741–751, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 278: 40231–40238, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Park WY, Miranda B, Lebeche D, Hashimoto G, Cardoso WV. FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Dev Biol 201: 125–134, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Pirraglia C, Jattani R, Myat MM. Rac function in epithelial tube morphogenesis. Dev Biol 290: 435–446, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM, Del Moral PM, De Langhe S, Parsa S, Kelly LK, Kelly R, Shia W, Keshet E, Minoo P, Warburton D, Bellusci S. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol 307: 237–247, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridley AJ. Rho GTPases and cell migration. J Cell Sci 114: 2713–2722, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol 283: 226–239, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Sollier K, Gaude HM, Chartier FJ, Laprise P. Rac1 controls epithelial tube length through the apical secretion and polarity pathways. Biol Open 5: 49–54, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugihara K, Nakatsuji N, Nakamura K, Nakao K, Hashimoto R, Otani H, Sakagami H, Kondo H, Nozawa S, Aiba A, Katsuki M. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene 17: 3427–3433, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Takayasu H, Nakazawa N, Montedonico S, Puri P. Down-regulation of Wnt signal pathway in nitrofen-induced hypoplastic lung. J Pediatr Surg 42: 426–430, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Curr Top Dev Biol 90: 73–158, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development 127: 2695–2704, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell 133: 340–353, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu B, Chen C, Chen H, Zheng SG, Bringas P Jr, Xu M, Zhou X, Chen D, Umans L, Zwijsen A, Shi W. Smad1 and its target gene Wif1 coordinate BMP and Wnt signaling activities to regulate fetal lung development. Development 138: 925–935, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi L, Domyan ET, Lewandoski M, Sun X. Fibroblast growth factor 9 signaling inhibits airway smooth muscle differentiation in mouse lung. Dev Dyn 238: 123–137, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan K, Qian C, Zheng R. Prognostic significance of immunohistochemical Rac1 expression in survival in early operable non-small cell lung cancer. Med Sci Monit 15: BR313–BR319, 2009. [PubMed] [Google Scholar]
- 45.Zhu W, Nelson CM. PI3K regulates branch initiation and extension of cultured mammary epithelia via Akt and Rac1, respectively. Dev Biol 379: 235–245, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]