Abstract
Chronic epithelial injury triggers a TGF-β-mediated cellular transition from normal epithelium into a mesenchymal-like state that produces subepithelial fibrosis and airway remodeling. Here we examined how TGF-β induces the mesenchymal cell state and determined its mechanism. We observed that TGF-β stimulation activates an inflammatory gene program controlled by the NF-κB/RelA signaling pathway. In the mesenchymal state, NF-κB-dependent immediate-early genes accumulate euchromatin marks and processive RNA polymerase. This program of immediate-early genes is activated by enhanced expression, nuclear translocation, and activating phosphorylation of the NF-κB/RelA transcription factor on Ser276, mediated by a paracrine signal. Phospho-Ser276 RelA binds to the BRD4/CDK9 transcriptional elongation complex, activating the paused RNA Pol II by phosphorylation on Ser2 in its carboxy-terminal domain. RelA-initiated transcriptional elongation is required for expression of the core epithelial-mesenchymal transition transcriptional regulators SNAI1, TWIST1, and ZEB1 and mesenchymal genes. Finally, we observed that pharmacological inhibition of BRD4 can attenuate experimental lung fibrosis induced by repetitive TGF-β challenge in a mouse model. These data provide a detailed mechanism for how activated NF-κB and BRD4 control epithelial-mesenchymal transition initiation and transcriptional elongation in model airway epithelial cells in vitro and in a murine pulmonary fibrosis model in vivo. Our data validate BRD4 as an in vivo target for the treatment of pulmonary fibrosis associated with inflammation-coupled remodeling in chronic lung diseases.
Keywords: mesenchymal transition, nuclear factor-κB, BRD4, airway epithelial cells, fibrosis
chronic obstructive airway diseases, notably chronic obstructive pulmonary disease (COPD), asthma, and cystic fibrosis (CF), are highly prevalent conditions characterized by acute episodic decompensations linked to inexorable declines in pulmonary function. Although the etiologies of these obstructive diseases are distinct, a major cause of morbidity is acute respiratory virus infections that lead to irreversible airway remodeling (1, 4, 18, 30). Airway remodeling is a structural alteration of the respiratory tree produced by subepithelial fibrosis, myofibroblast hyperplasia, and smooth muscle hypertrophy (1). Although airway remodeling is the product of complex cell state changes and intercellular interactions, a major initiator of the process is epithelial injury (10, 14). Acute oxidative injury produced by innate inflammation (13) produces defects in epithelial barrier function and stimulates the release of growth factors and cytokines linked to airway remodeling (21, 45). In particular, chronic epithelial injury induces the secretion of transforming growth factor (TGF)-β to promote mucosal repair and renewal (31). The in vivo action of TGF-β in airway remodeling and obstruction has been shown (11, 26, 27, 52).
The action of TGF-β induces enhanced motility, resistance to reactive oxygen stress, and expression of fibrotic genes (42, 43). Ligand-activated TGF-β receptor type II (TGFβRII) signals through Smad-dependent “canonical” and Smad-independent “noncanonical” pathways (43, 73). In the canonical pathway, phosphorylated Smad2/3 binds to Smad4 and the complex translocates into the nucleus. The Smad2/3/4 complex regulates components of a “core set” of transcriptional regulators responsible for coordinate processes in the mesenchymal cell state change. These core epithelial-mesenchymal transition (EMT) regulators include snail family zinc finger 1 (SNAI1), a zinc finger-containing transcription factor responsible for repressing epithelial cadherin (CDH1) (48); twist family bHLH transcription factor 1 (TWIST1), a basic helix-loop-helix activator that induces the expression of FGF receptor 2 and periostin (63); and zinc finger E-box binding homeobox 1 (ZEB1), a homeobox protein that upregulates TGF-β1 and vimentin (VIM) (43, 48, 73). Collectively, these proteins produce coordinate activation and repression of genes important in EMT.
Through the action of SNAI1, ZEB1, and TWIST1, TGF-β stimulation induces epithelial cells to reprogram their transcriptome, altering expression of ∼3,000 genes, driven in part by epigenetic histone modifications (51, 70). This transcriptional reprogramming dedifferentiates highly specialized epithelial cells to become fibroblast-like cells with stem cell-like characteristics, a process referred to as type II EMT (42). At the cellular level, TGF-β-induced type II EMT leads to the loss of apical polarity and disruption of epithelial adherens junctions (38, 42, 73). In addition, type II EMT enables transformed epithelial cells to express α-SMA stress fibers and intermediate filament VIM and to produce extracellular matrix through the secretion of collagen, fibronectin (FN1), and matrix metalloproteinases, promoting extracellular matrix remodeling and airway fibrosis (38).
In cancer cells, it has been shown that the TGF-β-induced type III EMT program is influenced by inflammatory cytokines via the NF-κB/RelA transcription factor (35, 36, 44, 49). Recently, we first observed that TGF-β induced the expression of a core NF-κB/RelA transcription factor network during type II EMT (41, 70). NF-κB/RelA is an inactive cytoplasmic transcription factor in normal epithelial cells that functions as an integrator of innate inflammatory signals. Previous work from our group has shown that NF-κB/RelA activation is controlled by a two-step process involving liberation from its cytoplasmic IκB inhibitor followed by site-specific phosphorylation of RelA on Ser276. Relief from cytoplasmic inhibition is mediated by rate-limiting serine-directed IκB kinases (IKKs) that phosphorylate the amino terminus of IκB within minutes, triggering its degradation via the E3 ubiquitin ligase BTRC/βTrCP and subsequent proteolytic destruction through the 26S proteasome and calpain pathways (6). A separate activation step is mediated by RelA Ser phosphorylation by a family of ribosomal S6 kinases in a stimulus-dependent manner. Specifically, formation of phospho-Ser276 RelA is critical for activation of a subset of highly inducible, “immediate-early” inflammatory genes maintained in an open chromatin conformation (6). In this pathway, phospho-Ser276 RelA binds the cyclin-dependent kinase (CDK)9 kinase complex, responsible for phosphorylation of Ser2 of the carboxy-terminal domain (CTD) of RNA Pol II, licensing it to produce fully spliced transcripts, a process known as transcriptional elongation (6, 55). This work has further shown that NF-κB-dependent target genes are under a phosphorylation code, with immediate-early genes requiring Ser276 phosphorylation coupled to CDK9-mediated transcriptional elongation whereas antiapoptotic and NF-κB signaling pathway inhibitor genes are Ser276 phosphorylation and CDK9 independent (6). Through this phosphorylation code, NF-κB/RelA coordinates temporal waves of gene expression, controlling distinct functions in the evolution of the inflammatory response (66).
Although the role of NF-κB in inflammation-mediated signaling is well established, its role in mediating type II EMT is not understood. In our earlier systematic investigation of TGF-β signaling in immortalized primary human small airway epithelial cells (hSAECs), we observed that TGF-β induced the expression of a core NF-κB/RelA transcription factor network (41, 70). In this study, we sought to define the mechanism for NF-κB/RelA in the transcriptional reprogramming of the core EMT regulators in airway epithelial cells during mesenchymal transition. TGF-β stimulation results in time-dependent expression of a phospho-Ser276-dependent subnetwork of NF-κB-controlled immediate-early genes coincidentally with that of the core EMT regulators, SNAI1, ZEB1, and Twist1. In contrast to NF-κB's direct activation by TNFRI, occurring within minutes, activation by TGFBR occurs 3 days after stimulation in a paracrine-dependent manner. In the mesenchymal state, the NF-κB Ser276-induced immediate-early genes accumulated euchromatin H3Lys (K)4 trimethylation (Me3) marks and the processive form of RNA polymerase. TGF-β induced the formation of an NF-κB·BRD4 complex and recruitment of BRD4 to EMT core transcription regulators. Inhibition of BRD4 with small-molecule inhibitors or short hairpin RNA (shRNA)-mediated depletion blocked the EMT program and stable assembly of the transcriptional elongation complex. Inhibition of BRD4 with small-molecule inhibitors attenuated experimental lung fibrosis by repetitive TGF-β challenge in a mouse model. These data provide novel insights into the role of the NF-κB-BRD4 pathway in controlling transcriptional elongation of core EMT regulators essential for the mesenchymal transition of airway epithelium and pulmonary fibrosis. Our study has significant implications for the molecular pathogenesis of chronic airway disease by providing a unifying mechanistic link between inflammation and fibrosis.
MATERIALS AND METHODS
hSAEC culture and induction of mesenchymal transition.
An immortalized hSAEC line established by infecting primary hSAECs with human telomerase (hTERT) and CDK4 retrovirus constructs was obtained from Dr. John Minna (University of Texas Southwestern) (57). hSAECs were grown in SAGM small airway epithelial cell growth medium (Lonza, Walkersville, MD) in a humidified 5% CO2 atmosphere. For mesenchymal transition, hSAECs were TGF-β stimulated for 15 days (10 ng/ml; PeproTech, Rocky Hill, NJ) (70). The small-molecule BRD4 inhibitor JQ1 was purchased from Cayman Chemical (Ann Arbor, MI) and used at a final concentration of 10 μM in culture medium (23).
Immunostaining and confocal immunofluorescence microscopy.
hSAECs were incubated ± TGF-β (10 ng/ml) for 15 days, replated on glass coverslips pretreated with rat tail collagen (Roche Applied Sciences), and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS). Afterwards, the fixed cells were stained with Alexa Fluor 568 phalloidin (Life Technologies) for cytoplasmic distribution of F-actin (red color) and also counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear staining (blue color). The cells were visualized with a Nikon fluorescence confocal microscope at a magnification of ×63 (6, 69).
For immunofluorescence staining, hSAECs were plated on rat tail collagen-treated cover glasses and stimulated for the indicated times. The cells were fixed with 4% paraformaldehyde in PBS and incubated with 0.1 M ammonium chloride for 10 min. Cells were permeabilized with 0.5% Triton X-100, followed by incubation in blocking buffer (5% goat serum, 0.1% IGEPAL CA-630, 0.05% NaN3, and 1% BSA), and incubated with anti-SNAIL1, anti-E-cadherin, and anti-VIM antibodies (Abs) (Abcam, Santa Cruz, CA) in incubation buffer (0.1% IGEPAL CA-630, 0.05% NaN3, and 2% BSA) overnight at 4°C. After washing, cells were stained with Alexa Fluor 488-, 568-, and 647-conjugated goat anti-rabbit IgG (Life Technologies), respectively, in incubation buffer for 1 h and then visualized with a Nikon fluorescence confocal microscope at a magnification of ×63 (6, 69).
RelA shRNA and control shRNA stable hSAECs.
The TRIPZ Tet-on inducible lentiviral RelA shRNA of RelA and related constructs were obtained commercially (Dharmacon, ThermoFisher Scientific, Lafayette, CO). To generate viruses, these constructs along with a packaging construct were reverse-transfected into BOS23 cell lines per the vendor's instructions. The virus-containing conditioned medium (CM) was collected and later used to infect hSAECs. Seventy-two hours after virus infection, 4 μg/ml puromycin was added to the culture medium of infected hSAECs for antibiotic selection. Five to eight weeks after addition of puromycin, the puromycin-resistant hSAECs stably expressing RelA shRNA (and empty vector shRNA controls) were harvested and later characterized for inducible RelA depletion under 2 μg/ml doxycycline (Dox).
Reverse small interfering RNA transfection.
Control, RelA, and BRD4 small interfering RNAs (siRNAs) (Dharmacon, ThermoFisher Scientific) were reverse-transfected by plating trypsinized hSAECs into a dish containing 100 nM siRNA-TransIT-siQUEST complexes (Mirus Bio). At the indicated times, cells were washed with PBS twice and lysed in TRI Reagent (Sigma-Aldrich, St. Louis, MO) (6, 69).
Subcellular fractionation and Western immunoblot analyses.
For preparation of whole cell lysate (WCE), hSAECs were collected into Eppendorf tubes and washed twice with PBS. After washing, the cells were suspended in RIPA buffer with complete protease inhibitor cocktail (Sigma-Aldrich) and 0.1% IGEPAL CA-630 (MP Biomedicals) and incubated on ice for 30 min (6, 25, 32, 69, 70). After incubation, the cells were centrifuged at 13,000 rpm for 20 min. The supernatants were collected and their protein concentrations quantified.
Nuclear and cytoplasmic proteins were fractionated as previously described (6, 25, 32, 69, 70). For Western blots, equal amounts of nuclear protein were fractionated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were incubated with affinity-purified rabbit polyclonal Abs to RelA (Santa Cruz Biotechnology). Washed membranes were then incubated with IRDye 800-labeled anti-rabbit IgG Abs (Rockland Immunochemicals, Gilbertsville, PA), and immune complexes were quantified with the Odyssey Infrared Imaging system (LI-COR Biosciences, Lincoln, NE). All data from Western blots shown in the present study are means ± SD from n = 3 experiments.
Quantitative real-time reverse transcription-PCR.
Total RNA was extracted with acid guanidinium-phenol extraction (Tri Reagent; Sigma-Aldrich). For gene expression analyses, 1 μg of RNA was reverse-transcribed with SuperScript III in a 20-μl reaction mixture (6, 69, 70). The rest of the procedures were as described previously (6, 69, 70). The forward and reverse gene-specific quantitative real-time reverse transcription-PCR (Q-RT-PCR) primers are listed in Table 1; relative changes in gene expression were quantified by the ΔΔCT method (where CT is threshold cycle). All data for Q-RT-PCR shown in the present study are means ± SD from n = 3 experiments. Data shown are the fold change in mRNA abundance normalized to cyclophilin.
Table 1.
Sequences of PCR primers for Q-RT-PCR
| Sequence (5′ → 3′) |
||
|---|---|---|
| Primer Set | Forward | Reverse |
| hRelA | CCGGACCGCTGCATCCACAG | AGTCCCCACGCTGCTCTTCT |
| hBRD4 | ACCTCCAACCCTAACAAGCC | TTTCCATAGTGTCTTGAGCACC |
| hFN1 | CGGTGGCTGTCAGTCAAAG | AAACCTCGGCTTCCTCCATAA |
| hCOL1A | CCAGAAGAACTGGTACATCAGCA | CGCCATACTCGAACTGGAATC |
| hIL-6 | CTGGATTCAATGAGGAGACTTGC | TCAAATCTGTTCTGGAGGTACTCTAGG |
| hSNAI1 | GCGCTCTTTCCTCGTCAGG | GGGCTGCTGGAAGGTAAACTCT |
| hTWIST1 | TCTCGGTCTGGAGGATGGA | CAATGACATCTAGGTCTCCG |
| hVIM | GCTCAATGTTAAGATGGCCCTT | TGGAAGAGGCAGAGAAATCCTG |
| hZEB1 | GATGATGAATGCGAGTCAGATGC | GATGATGAATGCGAGTCAGATGC |
| hIL-8 | AAGACATACTCCAAACCTTTCCACC | CAATAATTTCTGTGTTGGCGCA |
| hGroβ | CACACTCAAGAATGGGCAGA | GCTTCCTCCTTCCTTCTGGT |
| hTNFAIP3 | TCCTCAGGCTTTGTATTTGAGC | TGTGTATCGGTGCATGGTTTTA |
| hNFKBIA | CTCCGAGACTTTCGAGGAAATAC | GCCATTGTAGTTGGTAGCCTTCA |
| hCDH1 | CGAGAGCTACACGTTCACGG | GGGTGTCGAGGGAAAAATAGG |
| hPPIA | CCCACCGTGTTCTTCGACATT | GGACCCGTATGCTTTAGGATGA |
| mIL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| mSNAI1 | CACACGCTGCCTTGTGTCT | GGTCAGCAAAAGCACGGTT |
| mZEB1 | ACCGCCGTCATTTATCCTGAG | CATCTGGTGTTCCGTTTTCATCA |
| mTWIST1 | GGACAAGCTGAGCAAGATTCA | CGGAGAAGGCGTAGCTGAG |
| mCOL1A1 | GCTCCTCTTAGGGGCCACT | CCACGTCTCACCATTGGGG |
| mVIM | CGTCCACACGCACCTACAG | GGGGGATGAGGAATAGAGGCT |
| mαSMA | GTCCCAGACATCAGGGAGTAA | TCGGATACTTCAGCGTCAGGA |
| mFN1 | ATGTGGACCCCTCCTGATAGT | GCCCAGTGATTTCAGCAAAGG |
| mPPIA | GAGCTGTTTGCAGACAAAGTTC | CCCTGGCACATGAATCCTGG |
Q-RT-PCR, quantitative real-time reverse transcription-PCR.
Dual cross-link chromatin immunoprecipitation.
Dual cross-link chromatin immunoprecipitation (XChIP) was performed as described previously (54, 68). hSAECs (4 × 106 to 6 × 106 per 100-mm dish) were washed twice with PBS. Protein-protein cross-linking was first performed with disuccinimidyl glutarate (2 mM; Pierce), followed by protein-DNA cross-linking with formaldehyde. Equal amounts of sheared chromatin were immunoprecipitated overnight at 4°C with 4 μg of the indicated Ab in ChIP dilution buffer (54). Anti-rabbit IgG was used as the negative control. Immunoprecipitates were collected with 40 μl of protein A magnetic beads (Dynal), washed, and eluted in 250 μl of elution buffer for 15 min at room temperature. Samples were de-cross-linked in 0.2 M NaCl at 65°C for 2 h. The precipitated DNA was phenol/chloroform-extracted, precipitated with 100% ethanol, and dried as previously described (54, 68).
Quantitative real-time genomic PCR.
Gene enrichment in XChIP was determined by quantitative real-time genomic PCR (Q-gPCR) as previously described (68, 69) with region-specific PCR primers shown in Table 2. Standard curves were generated with a dilution series of genomic DNA (from 1 ng to 100 ng) for each primer pair. The fold change of DNA in each immunoprecipitate was determined by normalizing the absolute amount to the input DNA reference and calculating the fold change relative to that amount in unstimulated cells. All data for Q-gPCR shown in the present study are means ± SD from n = 3 experiments.
Table 2.
Sequences of primer sets for XChIP
| Sequence (5′ → 3′) |
||
|---|---|---|
| Gene | Forward | Reverse |
| hSNAI1-5′κB | ACGTCAGCTGAAGGGAAACAAACA | CGGTTCAGGCAGCTGCACTCTT |
| hZEB1-5′κB | TGGTTCCCCTGAACTTTACTGT | TGGGCACCAGAGGCATGATA |
| hVIM-5′κB | CCAGGCATCTGCCACAATG | CACTCAAGAGCTTCCCAGCAA |
| hIL-6-5′κB | TCGTGGGGAAATGTGTCCAG | CTGGCCGAGTTCCAGCAG |
| hIL-8-5′κB | AGGTTTGCCCTGAGGGGATG | GGAGTGCTCCGGTGGCTTTT |
XChIP, dual cross-link chromatin immunoprecipitation.
Immunoprecipitation.
hSAECs (4 × 106 to 6 × 106 per 100-mm dish) were washed twice with PBS. Protein-protein cross-linking was first performed with disuccinimidyl glutarate. The cross-linked cells were then collected into Eppendorf tubes and washed twice with PBS. After washing, the cells were suspended in RIPA buffer with complete protease inhibitor cocktail (Sigma-Aldrich) and 0.1% IGEPAL CA-630 (MP Biomedicals) and incubated on ice for 30 min (6, 69). After incubation, the cells were sonicated four times and centrifuged at 13,000 rpm for 10 min. The supernatants were collected and their protein concentrations quantified. Equal volumes of whole cell lysates were immunoprecipitated overnight at 4°C with 4 μg of the Anti-RelA and Anti-BRD4 Abs (Santa Cruz) in ChIP dilution buffer (69). Immunoprecipitates were collected with 40 μl of protein A magnetic beads (Dynal) The samples were deassociated with beads and prepared for stable isotope dilution (SID)-selected reaction monitoring (SRM)-mass spectrometry (MS) analysis. All data for immunoprecipitation (IP)-SID-SRM-MS shown in the present study are means ± SD from n = 3 experiments.
TGF-β-induced pulmonary fibrosis in mice.
Animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch Animal Resource Center (protocol number 1312058), conforming to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male C57BL/6J mice aged 18 wk (The Jackson Laboratory, Bar Harbor, ME) were housed in the Animal Resource Center under pathogen-free conditions with food and water ad libitum. Mice were given repetitive challenges with TGF-β (1 μg/mouse intranasally) every other day for a total of 15 TGF-β treatments. Each of the four different experimental groups included five mice. A separate treatment arm was pretreated with JQ1 (50 mg/kg body wt ip) immediately before TGF-β administration. Ten days after the last TGF-β treatment, animals were anesthetized, bronchoalveolar lavage fluid was obtained, and the mice were killed. Lung tissues were taken for RNA extraction or fixed for histological examination. For histological examination, lungs were inflated under 25 cmH2O pressure with 10% (vol/vol) neutral buffered formalin through the tracheal cannula and immersed in formalin for at least 24 h. After being processed into paraffin blocks, the lungs were cut into 5-μm sections and stained with either Masson trichrome or hematoxylin and eosin to assess fibrotic changes.
Assessment of levels of pulmonary fibrosis.
Pulmonary fibrosis was graded with the Ashcroft scoring method as described previously (37). In brief, the entire left and right longitudinal lung sections were scored separately (score range 0–9) at ×100 magnification, and the scores were combined to create a total score (range 0–18) (37). Ashcroft scores shown are means ± SD from n = 5 experiments.
Hydroxyproline assay of whole lung tissue.
To estimate amount of collagen in the lung, hydroxyproline content was measured colorimetrically (28) with a hydroxyproline assay kit (Sigma-Aldrich) with minor modifications. Briefly, the lungs were weighed and homogenized in liquid nitrogen with 2 ml of PBS, after which 2.0 ml of 12 N HCl was added and the samples were hydrolyzed at 120°C within a polytetrafluoroethylene-lined capped pressure-tight vial for 6 h. Ten-microliter hydrolyzed samples were mixed with 100 μl of chloramine T-oxidation buffer at room temperature for 5 min and later incubated with 4-(dimethylamino)benzaldehyde (DMAB) reagent for 90 min at 60°C. The absorbance of oxidized hydroxyproline was determined by absorbance at 560 nm (Infinite M200 PRO multimode microplate reader; Tecan Instruments). Standard curves were generated for each assay with hydroxyproline standards. The amount of collagen was expressed in micrograms per milligram of lung tissue. The data shown are means ± SD from n = 5 experiments.
Immunohistochemistry and immunofluorescence staining.
Formalin-fixed, paraffin-embedded lung sections were rehydrated with serial concentrations of ethanol. For immunohistochemistry, antigen retrieval was performed with antigen unmasking solution (TE buffer, pH 9.0). Paraffin-embedded sections were blocked with 0.1% Triton X-5% normal goat serum and incubated with rabbit anti-SNAI (1:200; Abcam), rabbit anti-VIM (1:200; Abcam), rabbit anti-FN1 (1:200; Abcam), and rabbit anti-COL1A (1:200; Abcam) Abs overnight at 4°C. Normal anti-rabbit IgG was used as the controls of staining specificity. After washing, cells were stained with Alexa Fluor 488- or 568-conjugated goat anti-rabbit IgG (Life Technologies) in incubation buffer for 1 h and then visualized with a Nikon fluorescence confocal microscope at a magnification of ×63 (71).
Stable isotope dilution-selected reaction monitoring-mass spectrometry.
The selection of the signature peptides for targeted MS-based quantification of RelA, CDK9, and BRD4 used a published workflow (78–80). The signature peptides and SRM parameters are listed in Table 3. Stable isotope-labeled signature peptide standards were chemically synthesized incorporating isotopically labeled [13C615N4]arginine or [13C615N2]lysine to a 99% isotopic enrichment (Thermo Scientific, San Jose, CA).
Table 3.
IP-SRM-MS assays
| Gene Name | Swiss-Prot No | Sequence | Q1 m/z | Q3 m/z | Ion Type | CE, V |
|---|---|---|---|---|---|---|
| RelA | Q04206 | TPPYADPSLQAPVR | 756.396 | 867.504 | y4 | 30 |
| 756.396 | 982.531 | y5 | 24 | |||
| 756.396 | 1,053.568 | y6 | 26 | |||
| 756.396 | 1,313.684 | y7 | 26 | |||
| BRD4 | O60885 | AASVVQPQPLVVVK | 717.938 | 782.513 | y7 | 27 |
| 717.938 | 879.566 | y8 | 27 | |||
| 717.938 | 1,007.624 | y9 | 27 | |||
| 717.938 | 1,106.693 | y10 | 27 | |||
| CDK9 | P50750 | DPYALDLIDK | 581.803 | 603.334 | y5 | 23 |
| 581.803 | 716.418 | y6 | 23 | |||
| 581.803 | 787.455 | y7 | 23 | |||
| 581.803 | 950.519 | y8 | 23 |
Shown are gene name, Swiss-Prot identification number, sequence of the proteotypic peptide, m/z value of the quadrupole (Q)1 and Q3 ions, and collision energy (CE) for each target protein.
The proteins were immunoprecipitated with specific Abs, captured on protein A magnetic beads (Dynal), and digested with trypsin (78–80). Afterwards, an aliquot of 5 μl of stable isotope-labeled signature peptides was added to each tryptic digest. These samples were desalted with a ZipTip C18 cartridge before MS analysis. SRM assay parameters for RelA, BRD4, and CDK9 were as described previously (78–80). The signature peptide of phospho-Ser276 RelA, RPS [phosphoryl]DR (m/z 355.653) (20), was used for parallel reaction monitoring (PRM)-MS analyses. All peptide samples were separated on an online nanoflow Easy nLC1000 UHPLC system (Thermo Scientific) and analyzed on the Q Exactive Orbitrap mass spectrometer (Thermo Scientific). The acquisition employed an Orbitrap resolution of 35,000 (at m/z 200), a target AGC value of 2e5, and maximum fill times of 100 ms. PRM targeted the native and stable isotope-labeled signature peptide of RelA phospho-Ser276 (20). All data for IP-SID-SRM-MS shown in the present study are means ± SD from n = 3 experiments.
Statistical analysis.
One-way analysis of variance (ANOVA) was performed when looking for time differences, followed by Tukey's post hoc test to determine significance. Mann-Whitney tests were used for nonparametric data. All data subjected to statistical analysis are means ± SD from n = 3 experiments or n = 5 experiments (for in vivo experiments). A P value of <0.05 was considered significant (6, 69).
RESULTS
TGF-β program indirectly activates nuclear translocation of NF-κB/RelA.
We previously established a model of TGF-β-induced type II EMT using a continuously replicating line of hSAECs that maintains characteristics of primary airway epithelial cells (41, 70). We have selected hSAECs for these studies because they maintain a stable epithelial morphology, express differentiated airway epithelial cytokeratin isoforms after hundreds of population doublings, form pseudostratified columnar epithelium in air-liquid interfaces, and support the replication of mucosal-restricted viruses (15, 40, 57, 77). Moreover, unbiased whole genome studies show that these cells maintain gene expression patterns of primary cells (57, 70). Similarly, proteomics profiling indicated that these cells secrete cytokeratin patterns and viral-induced inflammatory protein patterns that faithfully reproduce explants of primary human small airway cells (77).
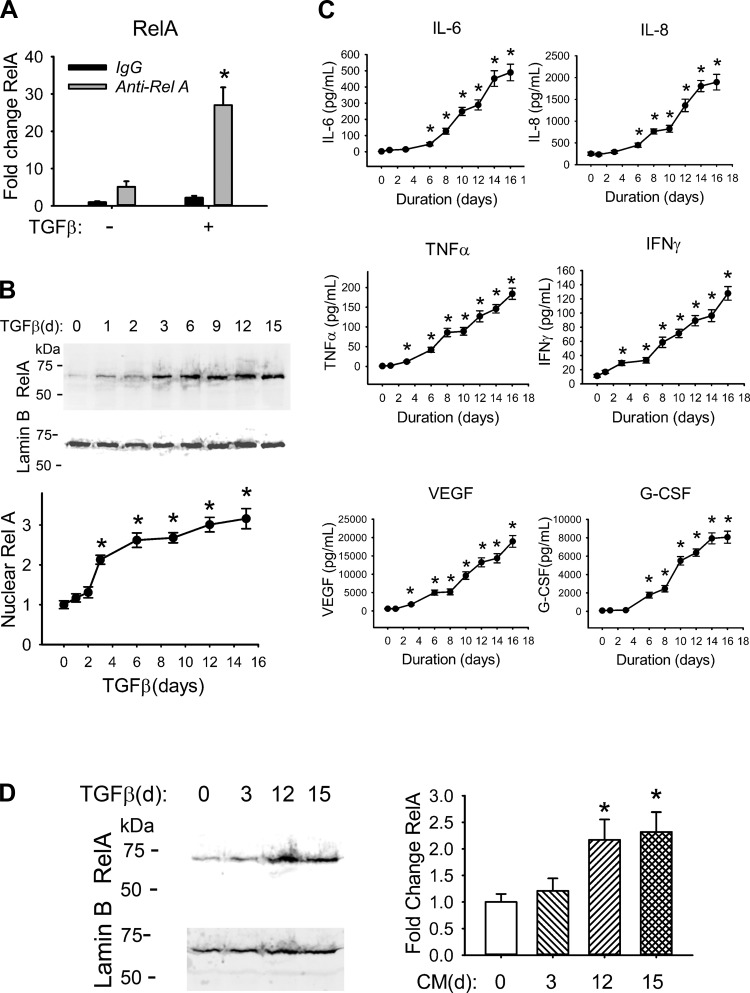
To better understand the mechanism for TGF-β-induced transcriptional reprogramming of the NF-κB gene program during type II EMT, we first measured the total cellular RelA abundance with a quantitative IP-selected reaction monitoring assay (SID-SRM-MS) (78–80). In this assay, whole cell extracts of control or EMT hSAECs were isolated and immunoprecipitated with control IgG or anti-RelA Ab and the immune complexes were analyzed by SID-SRM-MS, normalized by the input protein concentration. In each cell state, the RelA signal was significantly increased in the anti-RelA IP compared to that with IgG, indicating that RelA was enriched in the immune complex (Fig. 1A). Comparison of the RelA abundance in the anti-RelA IPs showed that RelA was fivefold enriched in the EMT state vs. untreated cells (Fig. 1A).
Fig. 1.
TGF-β activates the NF-κB signaling pathway. A: TGF-β increases total RelA abundance. hSAECs were incubated with TGF-β (10 ng/ml) for 15 days. Whole cell extracts of hSAECs were isolated and immunoprecipitated with primary anti-RelA Ab. RelA abundance was quantified by SID-SRM-MS. Shown is fold change abundance normalized to the input protein concentration. *P = 0.002 compared with mock treatment. B, top: TGF-β induces RelA nuclear translocation. hSAECs were incubated with a time series of TGF-β (10 ng/ml) for up to 15 days. One hundred fifty micrograms of nuclear extracts was processed for Western blot using anti-RelA Ab (top). Lamin B was detected as a loading control (bottom). Bottom: quantification of nuclear RelA. Shown is the fold change in protein abundance normalized to Lamin B. *P < 0.01 compared with mock treatment. C: TGF-β induces secretion of NF-κB-dependent cytokines/chemokines. hSAECs were incubated with a time series of TGF-β (10 ng/ml) up to 16 days. The conditioned medium was collected for cytokine determination by multiplex ELISA. Shown are the concentrations of IL-6, IL-8, TNF-α, IFN-γ, VEGF, and G-CSF. *P < 0.05 compared with control epithelial cells. D: conditioned medium from TGF-β-treated cells induces RelA nuclear translocation in naive hSAECs. Naive hSAECs were incubated for 1 h with 3-, 12-, or 15-day conditioned medium from TGF-β-treated hSAECs. Left: Western blot of 150 μg of nuclear extracts using anti-RelA Ab (top); the blot was probed with Lamin B as the loading control (bottom). Right: quantification of nuclear RelA. Shown is the fold change in protein abundance normalized to Lamin B. *P < 0.01 compared with mock treatment. All data shown are means ± SD from 3 independent experiments.
Previously, we demonstrated that TGF-β-induced the NF-κB/RelA pathway by promoting NF-κB/RelA nuclear translocation (70). To quantify the dynamic changes in NF-κB/RelA nuclear translocation during induction of type II EMT, RelA abundance was determined in nuclear extracts prepared from hSAECs stimulated with TGF-β over a time course experiment up to 15 days (Fig. 1B). We observed that TGF-β increased nuclear RelA levels 2.2-fold 3 days after TGF-β stimulation, plateauing at a 3.2-fold increase after 15 days of TGF-β stimulation (Fig. 1B). Together these data further confirm and extend our previous findings that TGF-β induces RelA abundance and enhances its nuclear translocation during induction of type II EMT (41, 70).
To explore the mechanism of TGF-β-induced NF-κB, CM from a time course of TGF-β-stimulated hSAECs was collected and cytokine secretion measured by multiplex ELISA. We observed that TGF-β induced secretion of IL-6, IL-8, TNF-α, IFN-γ, VEGF, and granulocyte colony-stimulating factor (G-CSF)—all NF-κB-dependent cytokine/chemokines (Fig. 1C). To examine whether NF-κB activation was direct or indirect (paracrine), we added CM from hSAECs treated with TGF-β for 3, 12, or 15 days to naive hSAECs. One hour later, we fractionated nuclei and measured RelA abundance by Western blot. We observed that nuclear RelA levels were 2.3-fold higher in hSAECs stimulated with 12-day and 15-day CM vs. control (Fig. 1D). We conclude that TGF-β induces a paracrine factor important for NF-κB translocation in the mesenchymal transition (46).
TGF-β induces epigenetic reprogramming of NF-κB-dependent immediate-early genes.
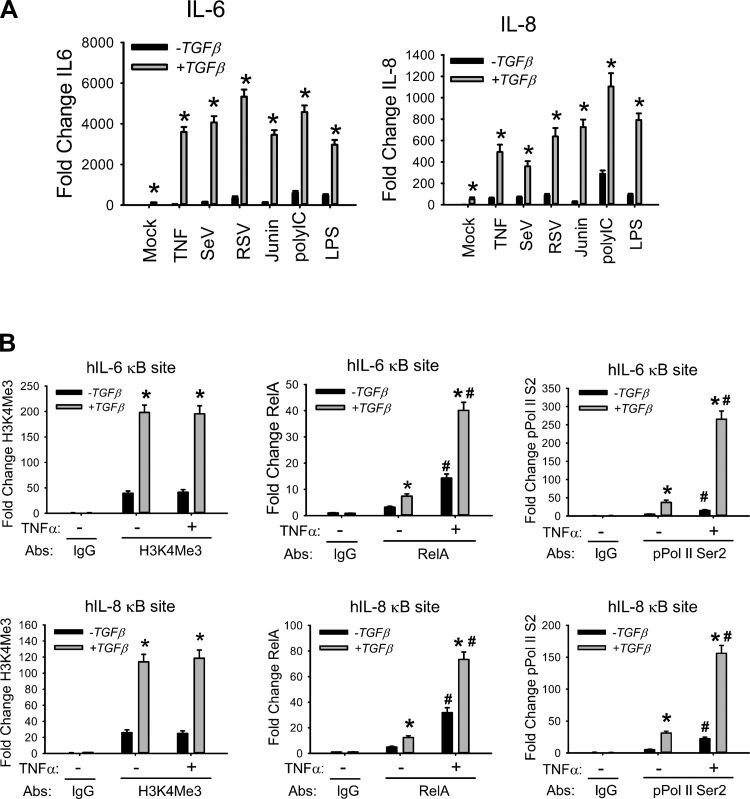
In systems modeling studies of the EMT, we observed that TGF-β promoted more efficient coupling of the NF-κB canonical and cross-talk pathways (41). To determine whether the NF-κB signaling response was functionally altered by the EMT, we challenged normal or EMT hSAECs with a battery of prototypical innate activators, including TNF-α, a ligand that activates NF-κB via TNFRI (67); Sendai (SeV), respiratory syncytial, and candid 1, RNA viruses that activate NF-κB via the RIG-I-MAVS pathway (6, 33, 50); poly(I:C), a pathogen-associated molecular pattern that activates NF-κB via Toll-like receptor (TLR)-3 (2); and lipopolysaccharide (LPS), a pathogen-associated molecular pattern that activates NF-κB via TLR4 (24). We observed that TNF induced IL-6 expression 50-fold in hSAECs but 3,600-fold in EMT-hSAECs (Fig. 2A). This extraordinarily high level of NF-κB-dependent gene induction was observed across all activators and for other members of the immediate-early gene network (Fig. 2A).
Fig. 2.
Enhanced innate immune responses in hSAECs with EMT. A: innate genes are hyperinducible in the mesenchymal state. hSAECs in the absence or presence of tonic TGF-β stimulation (15 days) were challenged with TNF-α, Sendai virus (SeV), respiratory syncytial virus (RSV), junin virus, poly(I:C), or LPS as shown. Expression of IL-6 and IL-8 mRNA was quantified by Q-RT-PCR. Shown is the fold change in mRNA abundance normalized to cyclophilin. *P < 0.001 compared with control (without TGF-β). Data are means ± SD from n = 3 experiments. B: accumulation of euchromatin marks and processive RNA polymerase in EMT. hSAECs were stimulated with TGF-β for 0 or 15 days, chromatin cross-linked, and immunoprecipitated with Abs specific for H3K4Me3, RelA, or phospho-Ser2 CTD RNA Pol II. Anti-rabbit IgG was used as the negative control. Binding of H3K4Me3, RelA, and phospho-Ser2 CTD RNA Pol II to the 5′ NF-κB site of the IL-6 (top) and IL-8 (bottom) promoters was determined by Q-gPCR. *P < 0.01 compared to without TGF-β; #P < 0.05 compared to without TNF-α. Data are means ± SD from 3 independent experiments.
Previous studies indicated that TGF-β-mediated EMT is associated with epigenetic modifications at specific genetic loci, including induction of the euchromatin H3K4Me3 mark (41, 51). To examine whether TGF-β induces epigenetic reprogramming of the immediate-early genes as a mechanism for their functional hyperinducibility, we quantitated H3K4Me3 binding with a highly quantitative two-step ChIP (XChIP) assay we have developed (54, 68) (Fig. 2B). We found that H3K4Me3 binds to the IL-6 promoter 5′ NF-κB site in unstimulated cells and was induced fivefold by TGF-β (Fig. 2B; primers are shown in Table 2). We similarly examined the binding of RelA and the hallmark of transcriptional elongation, the processive form of RNA Pol II, phospho-Ser2 CTD RNA Pol II. We observed enhanced RelA binding in EMT hSAECs over that in control hSAECs. More strikingly, we observed an 18-fold increase in TNF-α-induced phospho-Ser2 CTD RNA Pol II binding compared with that in control hSAECs (Fig. 2B). Similar observations were also found in the IL-8 promoter (Fig. 2B). The evidence above indicates that epigenetic reprogramming of NF-κB gene loci could be responsible for their hyperinduction in the mesenchymal state.
Requirement for NF-κB/RelA in type II EMT program.
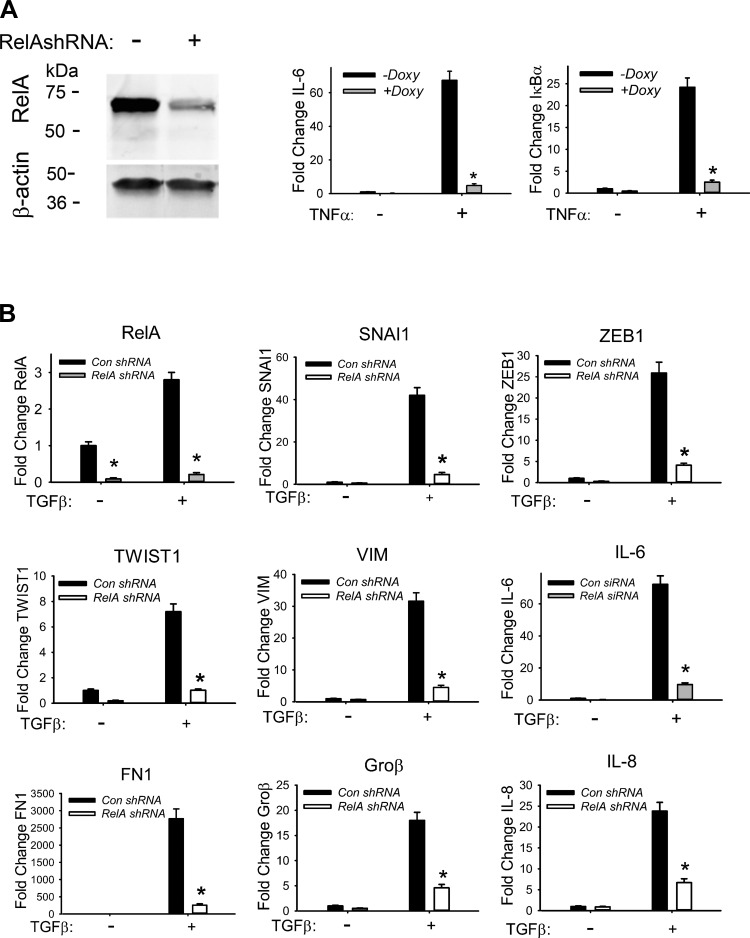
We previously observed a requirement for IKK in the TGF-β-induced EMT in hSAECs with a small-molecule inhibitor (9, 70). To extend these studies, we examined the effect of NF-κB/RelA-mediated silencing using Dox-inducible shRNA. hSAECs stably expressing control or RelA-directed shRNA were cultured for 5 days ± Dox (2 μg/ml) to induce shRNA expression. RelA abundance in WCEs was quantified by Western immunoblots and showed a dramatic 90% decrease in RelA protein abundance after RelA-shRNA induction (Fig. 3A, left). In these cells, the robust TNF-α-inducible expression of IL-6 and IκBα mRNA was almost completely blocked in the stable RelA-shRNA-expressing hSAECs (Fig. 3A, center and right). Together these data indicate that RelA shRNA functionally inhibits RelA signaling.
Fig. 3.
Requirement of NF-κB signaling for the TGF-β-induced type II EMT. A: efficiency of RelA depletion. hSAECs stably expressing doxycycline (Doxy)-inducible RelA shRNA were cultured for 5 days ± Doxy (2 μg/ml) to silence RelA. Left: protein levels of RelA in whole cell lysate by Western blots. Wild-type (WT) or RelA-silenced hSAECs were treated ± TNF (25 ng/ml), and expression of IL-6 and IκBα mRNAs was measured by Q-RT-PCR. Data are mean ± SD normalized fold change from n = 3 experiments. *P < 0.001 compared to without Doxy treatment. B: RelA depletion blocks TGF-β-induced EMT gene expression. RNA from WT and RelA-silenced SAECs were treated ± TGF-β (10 ng/ml) for 15 days and were quantified for the expression of RelA, IL-6, SNAI1, ZEB1, TWIST1, VIM, Groβ, IL-8, and FN1 mRNAs. *P < 0.01 compared to control siRNA. Data are means ± SD from 3 independent experiments.
To determine the role of NF-κB/RelA in the TGF-β-induced EMT, wild-type (WT) or RelA-silenced hSAECs were stimulated with or without TGF-β (10 ng/ml) for 15 days. RelA silencing significantly inhibited TGF-β-induced SNAI1 expression, from 42-fold in WT to ∼6-fold in RelA-silenced hSAECs (Fig. 3B). Similarly, TGF-β-induced ZEB1 was reduced from 25-fold in WT to ∼3.5-fold in RelA-silenced cells and TWIST1 from 8.5-fold to ∼1.5-fold, indicating that RelA is required for TGF-β-induced expression of core EMT transcriptional regulators. Similar RelA dependence was observed for mesenchymal genes VIM and FN1 and the NF-κB-dependent genes (IL-6, IL-8, and Groβ; Fig. 3B).
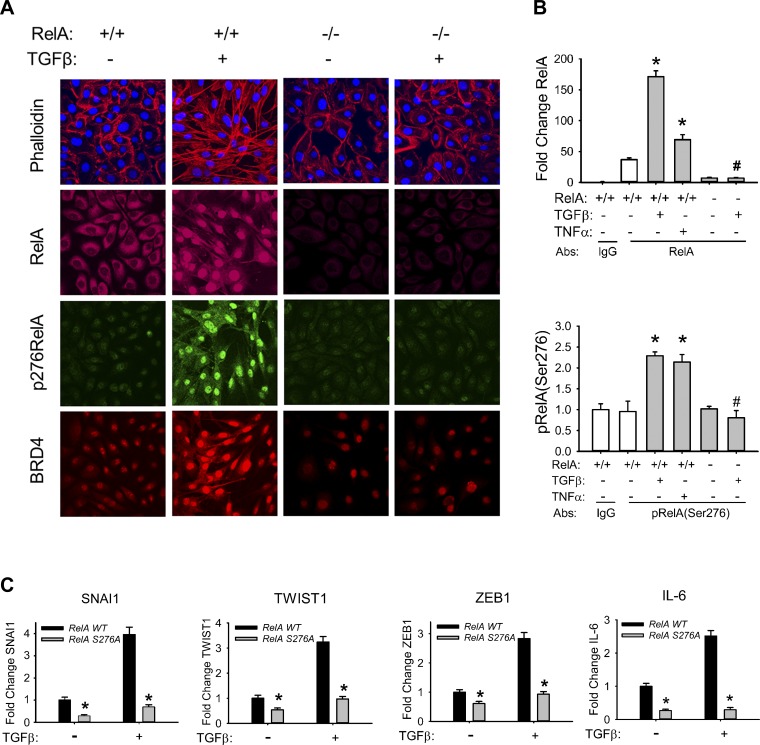
NF-κB/RelA Ser276 phosphorylation is required for activation of type II EMT program.
In light of our findings that TGF-β activates phospho-Ser276 RelA-dependent immediate-early genes (Fig. 2A), we next examined whether NF-κB RelA Ser276 phosphorylation is required for TGF-β-induced EMT. For this purpose, we first performed immunocytochemical staining of phospho-Ser276 RelA and found that chronic TGF-β treatment significantly elevated the level of RelA Ser276 phosphorylation (Fig. 4A). By contrast, RelA silencing significantly inhibited TGF-β-induced RelA Ser276 phosphorylation, along with the characteristic stress fiber formation (Fig. 4A) (34). Furthermore, RelA shRNA silencing significantly inhibited BRD4 redistribution, indicating that this epigenetic reader is also under NF-κB pathway control (Fig. 4A).
Fig. 4.
Requirement of RelA Ser276 phosphorylation for TGF-β-induced mesenchymal transition. A: TGF-β induced changes in RelA, BRD4, and phospho-Ser276 RelA. Both WT and RelA-silenced hSAECs were treated ± TGF-β (10 ng/ml) for 15 days. Cells were fixed, stained with Alexa Fluor 568-conjugated phalloidin (red) and DAPI (blue), and imaged by confocal microscopy. Separate coverslips were incubated with primary anti-RelA, BRD4, or phospho-Ser276 RelA Abs and stained with Alexa Fluor-conjugated secondary Abs (shown in green, red, and deep red, respectively). Cells were counterstained with DAPI (blue) and imaged via confocal fluorescence microscopy. B: induction of phospho-Ser276 RelA and total RelA in response to TGF-β stimulation. WCEs from WT or RelA-silenced hSAECs treated ± TGF-β (10 ng/ml) for 15 days were obtained. Equal amounts of WCE were immunoprecipitated with pan-anti-RelA Ab and subjected to SID-SRM-MS analysis for total (top) and phospho (p; bottom)-Ser276 RelA. Shown are fold changes in relative abundance relative to control cells. *P < 0.01 compared to without TGF-β; #P < 0.001 compared to RelA WT. C: RelA Ser276 phosphorylation is required for TGF-β-induced EMT gene program. RelA−/− MEFs stably transfected with either FLAG-EGFP-tagged RelA WT or a FLAG-EGFP-tagged nonphosphorylatable RelA with a Ser276-to-Ala mutation (RelA Ser276Ala) were treated with TGF-β for 3 days. The effects of TGF-β on mSNAI1, TWIST1, mZEB1, and mIL-6 gene expression were compared by Q-RT-PCR. Data are mean ± SD fold change from n = 3 experiments. *P < 0.05 compared with RelA WT.
The abundance of total and phospho-Ser276 RelA was quantitated by IP-SID-SRM-MS assay of WCEs in TGF-β-treated hSAECs (Fig. 4B). We found that chronic TGF-β treatment significantly enriched the levels of phospho-Ser276 RelA to levels of the positive control, TNF (Fig. 4B). As a measure of specificity, both signals were abolished in RelA-silenced cells (Fig. 4B).
Finally, as an independent confirmation, we examined previously characterized RelA−/− mouse embryonic fibroblasts (MEFs) that were stably transfected with either FLAG-EGFP-tagged wild-type RelA (RelA WT) or an FLAG-EGFP-tagged nonphosphorylatable Ser276-to-Ala mutation (RelA Ser276Ala; Ref. 55). The time course of mSNAI1, mTWIST1, mZEB1, and mIL-6 gene expression in response to TGF-β was examined in both cell types by Q-RT-PCR. We found that TGF-β induced a fourfold increase in mSNAI1 mRNA in WT MEFs; although this induction was lower than that seen in epithelial cells, both basal and TGF-β-inducible mSNAI1 expression were significantly inhibited in the RelA Ser276Ala-expressing MEFs (Fig. 4C). Similarly, the 3.5-fold induction of mTWIST1, 2.8-fold induction of mZEB1, and 2.8-fold induction of mIL-6 mRNA expression in TGF-β-treated RelA WT-expressing MEFs were completely abolished in the RelA Ser276Ala-expressing MEFs (Fig. 4C). All of these data strongly suggest that RelA Ser276 phosphorylation is required for the TGF-β-induced EMT.
NF-κB/RelA is required for recruitment of CDK9-BRD4 complex to core EMT transcription factor genes.
Our experimental data suggested that TGF-β-induced mesenchymal transition is absolutely NF-κB/RelA dependent and implicated that its mechanism involves nuclear translocation of phospho-Ser276 RelA. Building on our previous work showing that phospho-Ser276 RelA activates transcriptional elongation of immediate-early genes in innate signaling by recruitment of the BRD4·CDK9 complex to inducible promoters (6, 55), and that RelA silencing affects BRD4 subcellular distribution (Fig. 4A), we sought to test whether this mechanism was used in the TGF-β-induced type II EMT.
We therefore examined TGF-β-induced transcriptional elongation complex assembly in native chromatin in the absence or presence of RelA with XChIP assays (54, 68) with region-specific primers. Chromatin from WT or RelA-silenced hSAECs stimulated ± TGF-β was immunoprecipitated with anti-RelA, -CDK9, -BRD4, or -phospho-Ser2 CTD RNA Pol II Abs. We found that in control shRNA-expressing hSAECs RelA bound to the SNAI1 promoter and was further induced fourfold by TGF-β (Fig. 5A). In the RelA shRNA-expressing hSAECs, b oth the unstimulated and TGF-β-induced RelA binding were completely inhibited (Fig. 5A). We observed that TGF-β stimulation induced a fourfold increase in both CDK9 and BRD4 binding to the SNAI1 promoter; this induction was also significantly attenuated by RelA depletion (Fig. 5A). The 15-fold induction of phospho-Ser2 CTD RNA Pol II was also blocked by RelA depletion (Fig. 5A). These data suggest that TGF-β induces direct RelA binding to the SNAI1 promoter and facilitates formation of the transcriptional elongation complex in native chromatin.
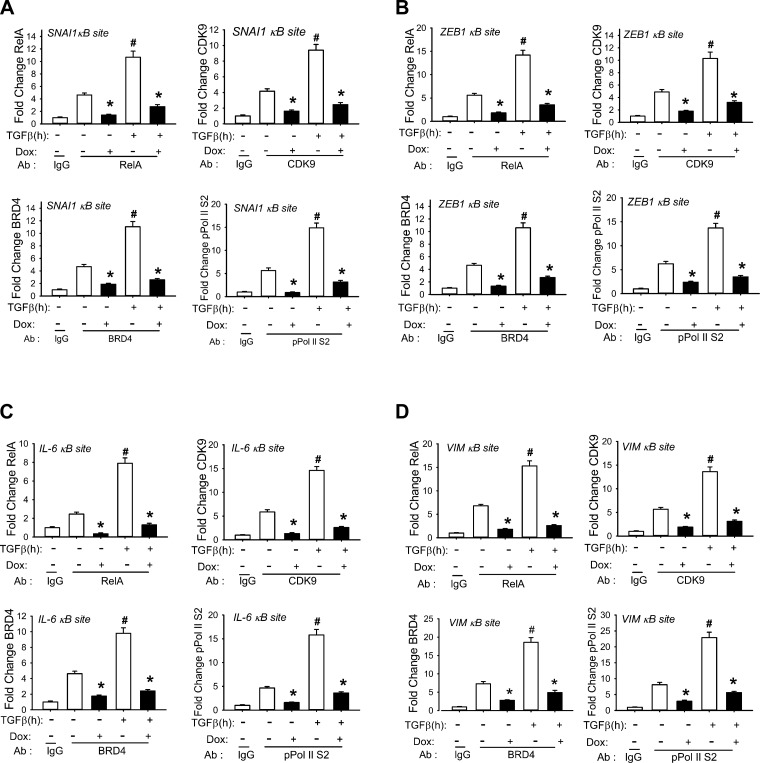
Fig. 5.
NF-κB binding is required for TGF-β-induced recruitment of the CDK9/BRD4 complex to EMT genes. Cross-linked chromatin from WT or RelA-silenced hSAECs treated ± TGF-β (10 ng/ml) was subjected to IP with Abs specific for RelA, CDK9, BRD4, or phospho-Ser2 CTD Pol II. Anti-rabbit IgG was used as the negative control. The recruitment of RelA, CDK9, BRD4, and pSer2 CTD RNA Pol II to the 5′ NF-κB site of the SNAI1 (A), ZEB1 (B), IL-6 (C), and VIM (D) promoters was determined by Q-gPCR using gene-specific primers. *P < 0.01 compared to without doxycycline (Dox); #P < 0.01 compared to mock treatment. Data are from n = 3 independent experiments.
We extended these findings to examine the effects of the TGF-β-induced NF-κB pathway on CDK9, BRD4, and phospho-Ser2 CTD RNA Pol II recruitment to the ZEB1, IL-6, and VIM promoters. We observed similar patterns of TGF-β-mediated induction. RelA depletion inhibited RelA, CDK9, BRD4, and phospho-Ser2 CTD RNA Pol II recruitment to all genes in a manner similar to that of SNAI1 (Fig. 5, B–D).
TGF-β induces nuclear complex formation of RelA with BRD4·CDK9.
BRD4 is a mammalian bromodomain protein that is a critical mediator of transcriptional elongation, functioning both to recruit activated CDK9 to the promoter and to activate it by phosphorylation (23, 39). Previous work has shown that BRD4 recruitment to target genes occurs via one of two known mechanisms: 1) by binding acetylated histone H4 and 2) by association with site-specific transcription factors (6, 39). To examine whether TGF-β stimulated formation of an active RelA·BRD4·CDK9 complex, we adapted the IP-SID-SRM-MS assay for quantification of RelA-associated BRD4 and vice versa. Nuclear extracts of control or TGF-β-stimulated hSAECs were prepared. RelA and BRD4 complexes were separately enriched by IP with anti-RelA or anti-BRD4 Abs and the immune complexes quantified for RelA, CDK9, and BRD4 by SID-SRM-MS, normalized to the input protein concentration. Relative to the IgG control, anti-RelA Ab increased abundance of RelA; this effect was further increased upon TGF-β stimulation, consistent with upregulation and nuclear translocation of RelA (Fig. 1A, Fig. 4B, and Fig. 6A, left). In the RelA IPs, both CDK9 and BRD4 signals were enriched by TGF-β stimulation (Fig. 6A, center and right).
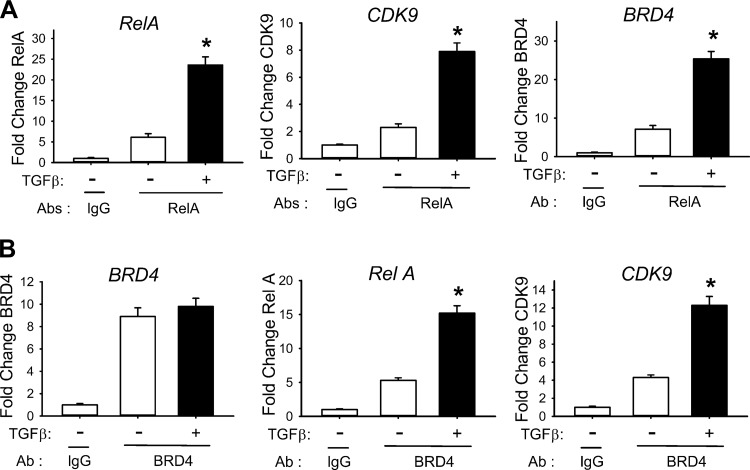
Fig. 6.
TGF-β induces RelA/BRD4/CDK9 nuclear complex formation. Nuclear extracts of hSAECs with or without TGF-β stimulation were enriched by IP with Abs to RelA or BRD4, and the abundance of RelA, CDK9, and BRD4 proteins was determined by SID-SRM-MS. Data are normalized by the input protein concentration and plotted as fold change over control. Controls represent samples immunoprecipitated with IgG. A: SID-SRM-MS analysis of nuclear RelA complexes. RelA, CDK9, and BRD4 protein levels were determined in the samples immunoprecipitated with anti-RelA or control IgG. *P < 0.01 compared with regular hSAECs IPed with anti-RelA. B: SID-SRM-MS analysis of nuclear BRD4 complexes. BRD4, RelA, and CDK9 protein levels were determined in the samples immunoprecipitated with anti-BRD4 or control IgG. *P < 0.01 compared with regular hSAECs immunoprecipitated with anti-BRD4. Data are means ± SD from n = 3 experiments.
Although there were similar levels of BRD4 in both control and TGF-treated cells compared with IgG control (Fig. 6B, left), RelA was significantly enriched in the BRD4 immune complexes from TGF-β stimulated hSAECs (Fig. 6B, center). We also observed that CDK9 was significantly enriched in the BRD4 complexes (Fig. 6B, right), indicating that TGF-β treatment induces formation of an active RelA·BRD4·CDK9 complex.
BRD4 is required for type II EMT.
To determine the functional role of BRD4, we first examined the effect of the small-molecule BRD4 inhibitor JQ1 on TGF-β-induced EMT. JQ1 binds the acetyl lysine recognition pocket, displacing it from chromatin-associated acetylated histones (23), providing a powerful tool to probe the role of the BRD4 bromodomain in cellular signaling. hSAECs were treated with solvent or JQ1 (10 μM) and TGF-β stimulated (15 days). In vehicle-treated cells we observed that TGF-β induced a typical response including elongated shape with stress fiber formation, redistribution of SNAI1 and VIM, and disappearance of CDH1 (Fig. 7A). By contrast, JQ1 treatment blocked TGF-β-induced stress fiber formation, acquisition of elongated shape, and upregulation and cytosolic redistribution of SNAI1 and VIM (Fig. 7A). JQ1 treatment also maintained the expression and cellular distribution of CDH1 (Fig. 7A).
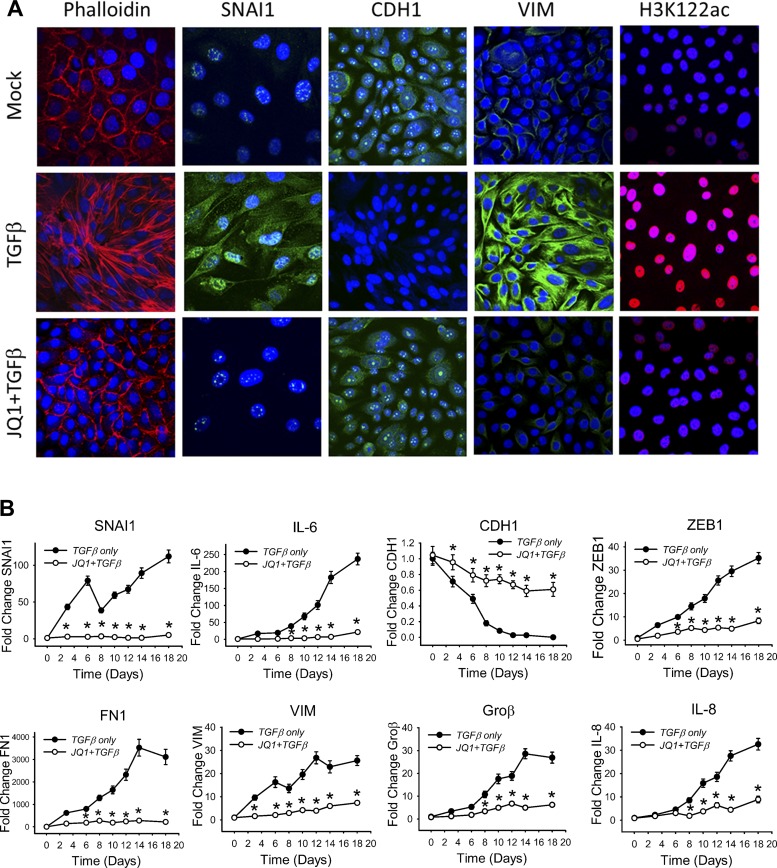
Fig. 7.
Requirement of BRD4 in mesenchymal transition. A: BRD4 inhibitor JQ1 blocks mesenchymal transition. hSAECs treated with vehicle (Mock), TGF-β (10 ng/ml, 15 days) or TGF-β + JQ1 (10 μM, 15 days) were fixed and stained with Alexa Fluor 568-conjugated phalloidin and DAPI. Cells were imaged by confocal microscopy. Separate coverslips were subjected to immunofluorescence by incubation with primary Abs for SNAI1, VIM, CDH1, and H3K122ac, then stained with Alexa Fluor-conjugated secondary Abs (green or red), counterstained with DAPI (blue), and imaged via confocal fluorescence microscopy. B: JQ1 blocks TGF-β-induced EMT gene expression program. hSAECs were pretreated with JQ1 (10 μM) ± TGF-β (10 ng/ml) for 15 days. Expression of SNAI1, CDH1, VIM, ZEB1, FN1, IL-6, IL-8, and Groβ mRNA was measured by Q-RT-PCR. *P < 0.01 compared with TGF-β only. Data are means ± SD from n = 3 experiments.
It has been recently reported that the histone acetyltransferase activity of BRD4 uniquely acetylates H3 K122, a process that promotes chromatin decompaction, resulting in increased transcriptional elongation (16). We found that TGF-β treatment induced a dramatic increase of nuclear H3K122ac staining that was completely blocked by JQ1 treatment (Fig. 7A). These data provide validation that H3K122ac formation is TGF-β induced and BRD4 dependent in cellulo.
To examine the role of BRD4 in TGF-β-induced gene transcription, we assayed the effect of JQ1 on expression of the core EMT transcription factors, mesenchymal proteins, and inflammatory genes. We found that JQ1 significantly blocked expression of SNAI1, ZEB1, FN1, and VIM, as well as that of NF-κB-dependent IL-6, IL-8, and Groβ mRNAs (Fig. 7B), suggesting that BRD4 mediates NF-κB-dependent type II EMT (Fig. 7B). By contrast, JQ1 partially maintained CDH1 expression, but not to levels seen in normal hSAECs (Fig. 7B).
As an independent approach, we evaluated the effect of siRNA-mediated BRD4 depletion on TGF-β-induced mesenchymal transition. Control or BRD4-specific siRNAs were reverse-transfected into hSAECs, producing an 85% inhibition of BRD4 expression in the absence or presence of TGF-β stimulation (data not shown). Consistent with the results with JQ1, we found that siRNA-mediated BRD4 depletion also significantly inhibited the TGF-β-induced enhancement of SNAI1, ZEB1, FN, VIM, and IL-6 expression (data not shown). Together, these results strongly suggest the requirement for BRD4 in the initiation and maintenance of NF-κB-dependent EMT in hSAECs.
BRD4 binding stabilizes NF-κB and transcriptional elongation complex assembly for initiation of type II EMT and transcriptional reprogramming.
BRD4 plays a major role in stabilizing CDK9 binding to acetylated histone H4-enriched chromatin. To determine whether BRD4 is involved in stabilizing the TGF-β-induced NF-κB complex, we examined the effect of JQ1 on RelA, CDK9, and phospho-Ser2 RNA Pol II recruitment to EMT core regulators by XChIP (54, 68). We found that the TGF-β-induced recruitment of BRD4 to the 5′ NF-κB site of SNAI1 was significantly attenuated, as expected (Fig. 8A). We also observed that binding of RelA, CDK9, and phospho-Ser2 CTD RNA Pol II to SNAI1 were significantly disrupted (Fig. 8A). Similar findings were observed for the ZEB1, IL-6, and VIM promoters (Fig. 8, B–D). Together, these results support the thesis that BRD4 recruitment stabilizes RelA-dependent assembly of the transcriptional elongation complex required for phospho-Ser2 CTD RNA Pol II formation, providing its mechanism for transcriptional reprogramming in type II EMT.
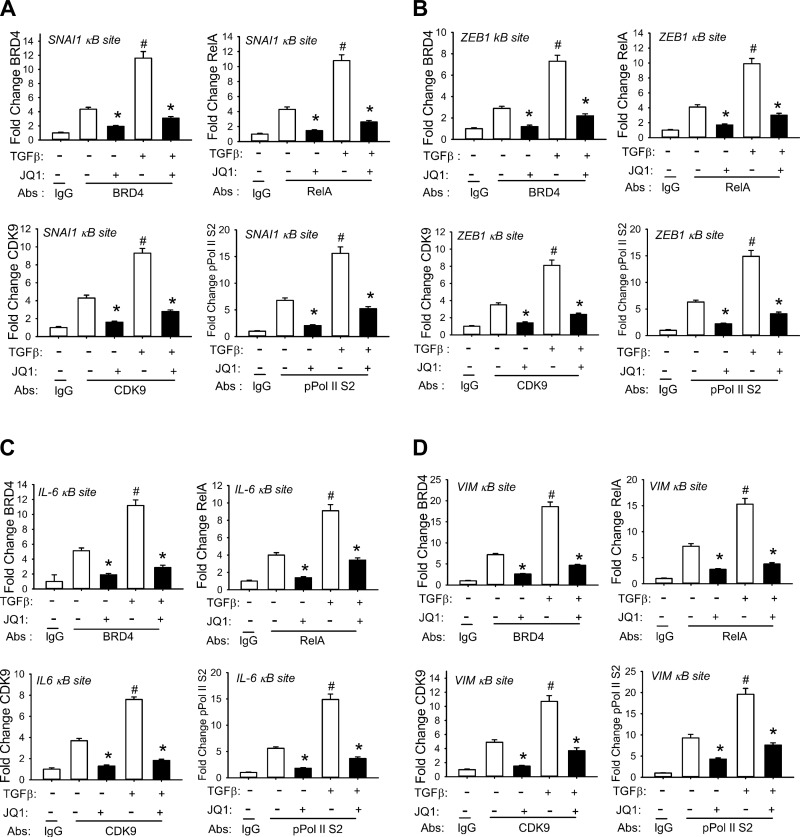
Fig. 8.
XChIP analysis of NF-κB binding sites of EMT genes under BRD4 inhibition. hSAECs were treated with TGF-β for 0 or 15 days ± JQ1 and chromatin cross-linked and subjected to IP with Abs specific for RelA, CDK9, BRD4, or phospho-Ser2 CTD RNA Pol II. Anti-rabbit IgG was used as the negative control. The recruitment of RelA, CDK9, BRD4, and phospho-Ser2 CTD RNA Pol II to the SNAI1 (A), ZEB1 (B), IL-6 (C), and VIM (D) promoters was determined by XChIP using gene-specific primers in Q-gPCR. *P < 0.01 compared to without JQ1; #P < 0.01 compared to without TGF-β. Data are means ± SD from n = 3 independent experiments.
BRD4 mediates chronic TGF-β treatment-induced pulmonary fibrosis in vivo.
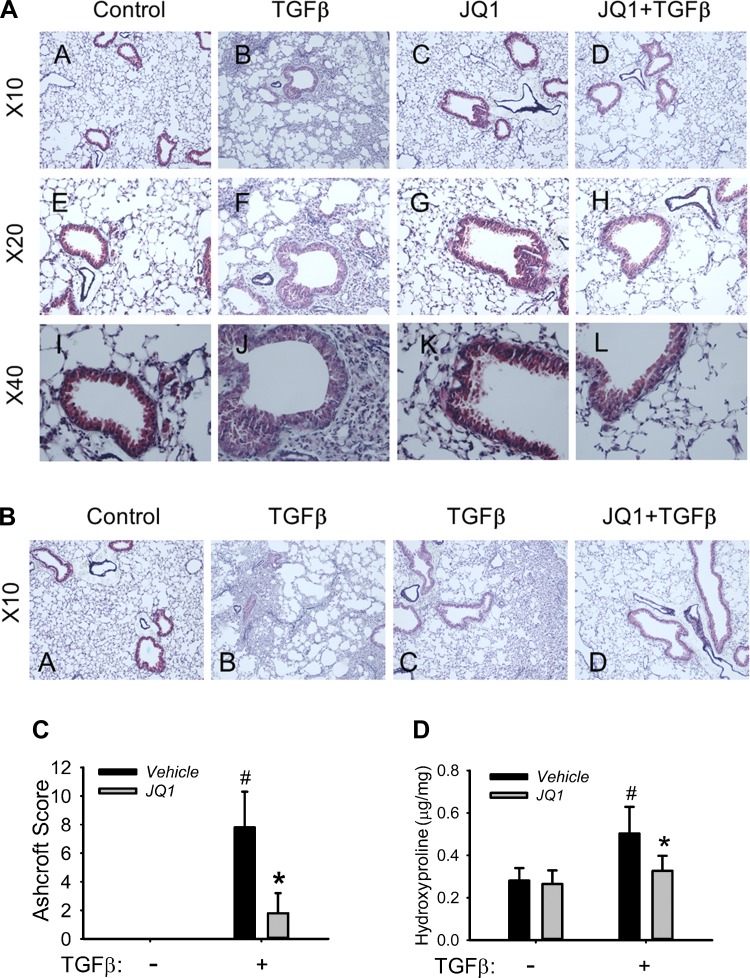
It is well established that TGF-β plays a central role in the development of pulmonary fibrosis in humans (7, 22), and its overexpression is sufficient to induce the formation of pulmonary fibrosis in rodents (62, 72). To establish the role of the NF-κB-BRD4 pathway in the development of airway fibrosis in vivo, we evaluated the effect of JQ1 in a mouse model of TGF-β-induced fibrosis. C57BL/6J mice were given repetitive intranasal challenge with TGF-β in the absence or presence of JQ1. Repetitive TGF-β treatment induced marked accumulation of subepithelial fibrosis with enhanced collagen distribution throughout the parenchyma and interstitial space surrounding the small and medium-sized airways (Fig. 9, AB, AF, AJ, BB, and BC; collagen in blue). The TGF-β-treated epithelium was flattened, with loss of its brush border, and enhanced numbers of glandular cells were apparent. At higher power, we observed expansion of mesenchymal supporting cells, subepithelial fibrosis, and thickening of the alveolar septae (Fig. 9AJ). All of the above are typical characteristic changes of human airway remodeling/fibrosis seen in COPD/asthma (1, 58, 59). The histology data of this pulmonary fibrosis model of mice suggest a mainly bronchiolocentric pattern of fibrosis with enhanced peribronchiolar and perivascular collagen fibril staining as well as the presence of epithelial brush border flattening. Meanwhile, thickening of the alveolar septae seen on some areas of lung also indicates intra-alveolar fibrosis (Fig. 9, AB, AF, AJ, BB, and BC; Fig. 10B). By contrast, the lungs of animals treated with JQ1 were histologically normal, indicating that JQ1 effectively blocks TGF-β-induced airway fibrosis (Fig. 9, AD, AH, AL, and BD). We also noted that JQ1 treatment restored the normal brush border appearance of the epithelium and reduced both the mesenchymal expansion and thickening of the alveolar septae (Fig. 9AL).
Fig. 9.
BRD4 mediates TGF-β-induced pulmonary fibrosis in mice. Eighteen-week-old C57BL/6 mice were pretreated ± JQ1 (50 mg/kg body wt ip; n = 5) and given repetitive intranasal challenges with TGF-β (1 μg/mouse every other day for 30 days). A: morphological changes. Masson trichrome staining of lungs from C57BL/6 mice chronically treated in the absence (AA and AC) or presence (AB and AD) of TGF-β; 2 groups of mice were treated with JQ1 (50 mg/kg, AC and AD). Images were taken at magnifications of ×10 (AA–AD), ×20 (AE–AH), and ×40 (AI–AL). B: additional histology. Images from different regions of lungs from mice chronically treated in the absence (BA) or presence (BB–BD) of TGF-β. Mice were also treated with JQ1 (50 mg/kg) in the presence of TGF-β (BD). All images were taken at ×10 magnification. C: Ashcroft scoring. Levels of lung fibrosis with assessed with the Ashcroft scoring method. *P = 0.002 compared to without JQ1 and #P < 0.001 compared to without TGF-β from n = 5 experiments. D: total collagen content. Whole lung hydroxyproline content was determined in all treatment groups. *P = 0.026 compared to without JQ1 and #P = 0.007 compared to without TGF-β from n = 5 experiments.
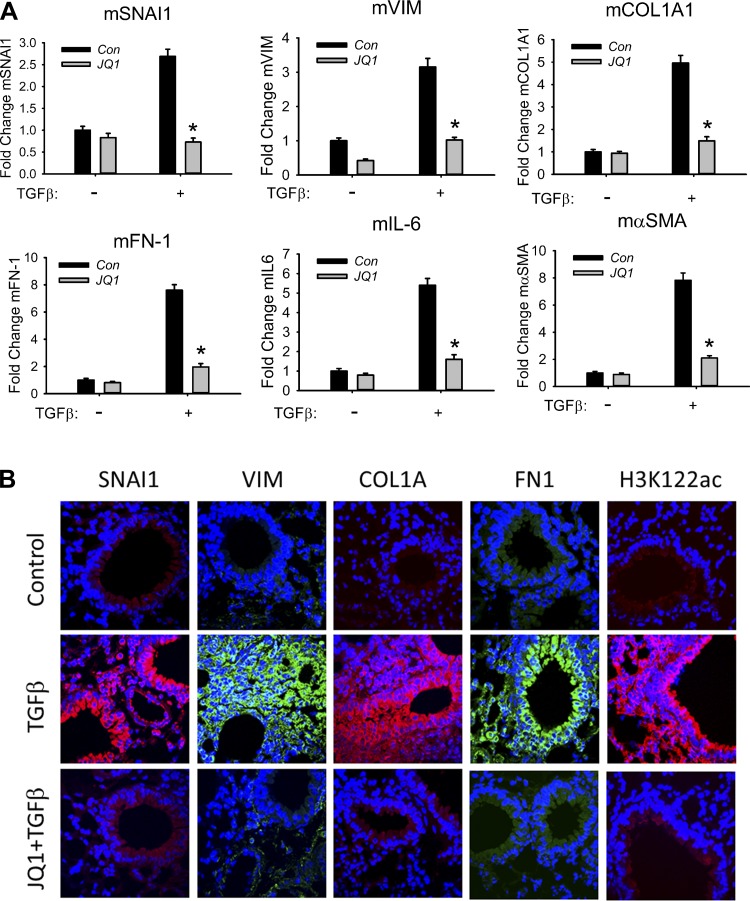
Fig. 10.
BRD4 controls TGF-β-induced pulmonary fibrotic program in vivo. Eighteen-week-old C57B6 mice were pretreated ± JQ1 (50 mg/kg body wt ip) and given repetitive intranasal challenges with TGF-β (1 μg/mouse every other day for 30 days). A: activation of the fibrotic program. Q-RT-PCR for mCol1A1, mFN1, mSNAI1, mVIM, mαSMA, and mIL-6 mRNAs was performed in total lung RNA. *P < 0.01 compared to without JQ1 from n = 5 experiments. B: expression of the mesenchymal transition. Paraffin-embedded lung sections were immunostained for mesenchymal markers SNAI1, VIM, FN1, and COL1A as well as BRD4 activation marker H3K122ac, detected with secondary Alexa Fluor 488 (green)- or 568 (red)-conjugated goat anti-rabbit IgG respectively, counterstained with DAPI, and imaged via confocal fluorescence microscopy. Images are at ×63 magnification. Data representative of n = 5 experiments.
To assess the levels of pulmonary fibrosis, pulmonary fibrosis was graded with the Ashcroft scoring method (Ref. 37; Fig. 9C). The average combined Ashcroft score in mice with chronic TGF-β treatment was 8, indicating a moderate level of pulmonary fibrosis, while the combined Ashcroft score in JQ1 mice with chronic TGF-β treatment was significantly lower (Fig. 9C). We further quantified whole lung collagen by measuring hydroxyproline content (28). We found that repetitive TGF-β stimulation induced a nearly 1.8-fold increase of hydroxyproline content (Fig. 9D) and JQ1 treatment significantly inhibited this increase to normal levels (Fig. 9D). These results not only demonstrate the role of BRD4 in the development of airway remodeling but also provide evidence that BRD4 is a valid target for inflammation-associated pulmonary fibrosis in chronic airway disease.
BRD4 controls TGF-β-induced pulmonary fibrotic program in vivo.
At the molecular level, repetitive TGF-β stimulation induced the expression of mSNAI1, mesenchymal genes (mFN-1, mαSMA, mCol1A1), and NF-κB/RelA-dependent mIL-6 genes (assessed by Q-RT-PCR; Fig. 10A). JQ1 treatment significantly inhibited this TGF-β-induced pulmonary fibrotic program (Fig. 10A). Additionally, lung sections of mice were subjected to immunofluorescence by staining with primary antibodies to the mesenchymal markers SNAI1, VIM, FN1, and COL1A as well as the BRD4 activation marker (H3K122ac). Only faint staining of SNAI1, VIM, FN1, COL1A, and H3K122ac was detected in airways of control mice. We found that chronic TGF-β treatment induced dramatically enhanced staining of SNAI1, VIM, FN1, COL1A, and H3K122ac in the airways (Fig. 10B) (16). By contrast, JQ1 treatment abolished TGF-β-induced SNAI1, VIM, FN1, and COL1A, providing strong evidence that JQ1 effectively blocks TGF-β-induced mesenchymal transition and the pulmonary fibrotic program (Fig. 10B). Also, our finding that JQ1 blocked TGF-β-induced formation of H3K122ac provides direct evidence that TGF-β activates BRD4 in vivo (Fig. 10B). Together our mechanistic studies have identified BRD4 as a key epigenetic regulator of TGF-β-induced airway remodeling downstream of NF-κB.
DISCUSSION
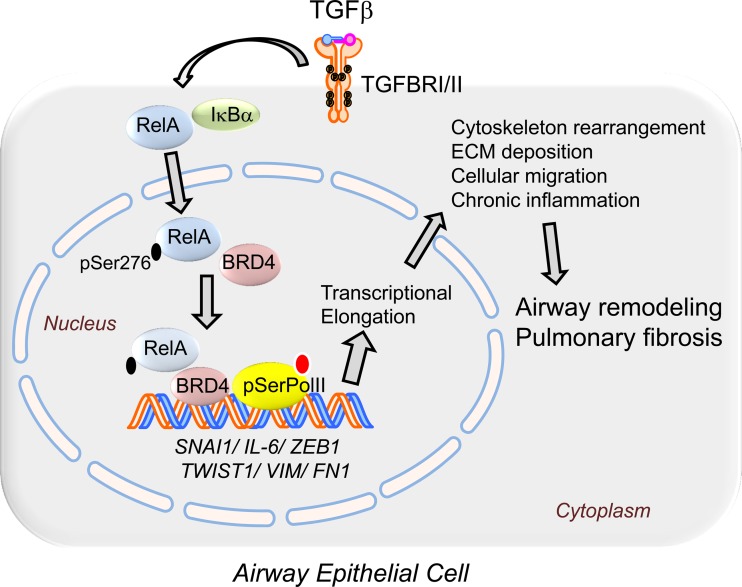
Airway remodeling is a major cause of irreversible decline in pulmonary function in patients with severe obstructive and interstitial lung diseases. Of relevance to this study, innate inflammation and chronic epithelial injury is a hallmark of severe asthma, COPD, and CF (11, 26, 27, 47). Similarly, non-immune-mediated chronic epithelial injury plays an important role in the progression of idiopathic pulmonary fibrosis (10, 53). Epithelial injury stimulates the liberation of morphogenic cytokines, most notably TGF-β, to promote epithelial-mesenchymal cross talk. Although the actions of TGF-β are initially protective to initiate reepithelialization and repair by inducing the cell state change known as EMT, chronic TGF-β signaling is associated with pathological fibrosis and irreversible restriction of pulmonary function. In this study we have mechanistically extended our earlier systems-level studies that suggested that the NF-κB gene program is a master regulator of the core EMT transcriptional regulators. Our studies indicate that TGF-β signaling both induces and activates NF-κB/RelA translocation, in part, via a paracrine mechanism that preferentially activates a subset of phospho-Ser276 RelA-dependent immediate-early genes. We demonstrate for the first time that TGF-β induces phospho-Ser276 RelA formation, a posttranslational modification required for the EMT. Our data show that phospho-Ser276 RelA complexes with CDK9 and BRD4, recruiting the elongation complex to the promoters of the core EMT transcriptional regulators, SNAI1/ZEB1/Twist1, whose actions are required for initiation of type II EMT and its fibrotic program. Furthermore, we have demonstrated that pharmacological inhibition of BRD4 can attenuate an experimental lung fibrosis program induced by repetitive TGF-β challenge. Our results strongly support the thesis that NF-κB-dependent BRD4 recruitment is a major determinant of the type II mesenchymal transition of airway epithelium and of pulmonary fibrosis (Fig. 11).
Fig. 11.
BRD4 mediates NF-κB-dependent EMT and pulmonary fibrosis via transcriptional elongation. TGF-β stimulation activates an NF-κB-dependent inflammatory gene program by enhanced expression, nuclear translocation, and phosphorylation of the NF-κB/RelA transcription factor on Ser276. Phospho-Ser276-activated RelA binds to the BRD4/CDK9 transcriptional elongation complex, targeting it to core mesenchymal transcriptional regulators activating phosphorylation of the paused RNA Pol II carboxy-terminal domain, whose actions are required for initiation of type II EMT and its fibrotic program. NF-κB-dependent BRD4 recruitment is a major determinant of the type II mesenchymal transition of airway epithelium and of pulmonary fibrosis.
Our previous work has shown that the TGF-β program includes a core of NF-κB-dependent genes (70). In that study we integrated the experimentally determined TGF-β transcriptional program with ENCODE ChIP-Seq studies to identify three major transcription factor clusters associated with a stable EMT. Of these, the NF-κB pathway was a prominent component whose functional importance was demonstrated by small-molecule IKK inhibition. Our work here, using selective shRNA-mediated knockdown of NF-κB/RelA, is the first direct demonstration that NF-κB/RelA mediates type II EMT in hSAECs. Although our studies are potentially limited by being based in TERT-immortalized cells, we believe these data are highly relevant to normal epithelial cells for the following reasons: 1) hSAECs form pseudostratified columnar epithelium in air-liquid interfaces (15, 40, 57); 2) hSAECs support the replication of mucosal-restricted viruses (77); 3) hSAECs maintain gene expression patterns of primary cells (57, 70); and 4) hSAECs secrete cytokeratin patterns and viral-induced inflammatory protein patterns that faithfully reproduce explants of primary human small airway cells (77). Nevertheless, in future studies we will seek to validate the role of NF-κB-BRD4 in EMT in primary epithelial isolates.
The detailed mechanism by which NF-κB/RelA controls EMT is incompletely understood. In studies of cancer-associated type III EMT, NF-κB is required for IGF-induced EMT by directly inducing SNAI1 (44). SNAI1 expression is important because our detailed time series studies indicate that SNAI1 is the earliest upregulated core transcription factor in type II EMT and its significant induction precedes that of ZEB1 and TWIST1, leading us to suggest that SNAI1 expression is an initial trigger of the EMT (41, 70).
In differentiated epithelial cells, NF-κB is an inducible transcription factor controlled at two levels—through cytoplasmic sequestration and activation by phosphorylation. In contrast to the very rapid activation of NF-κB within minutes by cytokines and toll ligands (6, 55, 67), TGF-β induces a delayed activation requiring 3 days of continuous TGF-β stimulation. We note that other studies in transformed cells have shown that NF-κB is directly activated by TGF-β via expression of the TGF-β-associated kinase (TAK) upstream of IKK (35, 36). However, our finding that CM from chronically TGF-β-stimulated cells rapidly activates NF-κB translocation in naive hSAECs suggests that NF-κB activation is indirect in primary airway epithelial cells. These findings further support the idea that oncogenic transformation has complex effects on intercellular signaling pathways. Further studies will be required to identify the paracrine factor involved.
Changes in the phenotype of epithelial cells in asthma may determine effects on innate signaling responses (41). Our earlier systems-level study observed that EMT had dramatic effects on the induction of the innate NF-κB pathway, by producing an exaggerated inflammatory response (41, 70). We observe here that the immediate-early NF-κB-dependent gene program is hyperinducible by all innate activators (Fig. 2A). It has been shown that type III EMT is associated with epigenetic modifications at specific genetic loci, including induction of the euchromatin mark H3K4Me3 (41, 70). Our ChIP experiments indicate that the type II EMT program induces the levels of this euchromatin mark as well as RelA and the processive form of the RNA polymerase, even in the absence of TNF-α stimulation (Fig. 2B). This suggests that innate immune genes are located in open chromatin domains in the mesenchymal state (75), enabling their hyperinduction. As a result, the dynamic inflammatory response of the epithelial cell is fundamentally changed by the EMT state.
Our time series experiments show that TGF-β preferentially triggers expression of the immediate-early stress genes IL-6, CXCL2/Groβ, and IL8, genes known to be phospho-Ser276 RelA dependent (6, 55). Consistently, both our immunofluorescence and quantitative IP-SID-SRM-MS assays demonstrated that chronic TGF-β treatment significantly increases the abundance of nuclear phospho-Ser276 RelA (Fig. 4, A and B). The functional requirement for this phosphorylation by the core EMT regulators is indicated by the inhibition of TGF-β-induced mSNAI1, mTWIST1, and mZEB1 expression in nonphosphorylatable RelA Ser276Ala-expressing MEFs (Fig. 4C). All of the above evidence demonstrates that NF-κB/RelA Ser276 phosphorylation is required for activation of the type II EMT program.
Recent signaling studies from our group have shown that phospho-Ser276 RelA is controlled by a nuclear reactive oxygen species (ROS) pathway mediated via the phosphoinositide 3-kinase ataxia telangiectasia mutated (ATM) (13, 19). In this signaling pathway, the nuclear ROS-activated ATM is upstream of a family of ribosomal S6 kinases that directly phosphorylate RelA on Ser276. RelA Ser276 phosphorylation is a rate-limiting posttranslational modification required for RelA to complex with the CDK9-BRD4 transcriptional elongation complex (6, 55). Although TGF-β is known to induce ROS via the inducible NADPH (NOX4) pathway, required for EMT (29), identification of the ribosomal S6 kinase required for EMT will require further investigation.
Although previous work in type III EMT has shown that SNAI1 and ZEB are regulated at a posttranscriptional level by reciprocal negative feedback loops with microRNA (miR)34a and miR200 (5, 61), this mechanism only explains how SNAI1 and ZEB1 are maintained at low levels in epithelial cells and high levels in mesenchymal cells. Although the regulation of miRs has not been examined in primary epithelial cells, our studies demonstrate that NF-κB/RelA activation is an upstream trigger of SNAI1 expression.
Extrapolating from the preferential activation of phospho-Ser276 RelA-dependent genes in response to TGF-β, and the requirement for phospho-Ser276 RelA in the EMT, we investigated the role of transcriptional elongation. We found that chronic TGF-β treatment induces an active RelA·BRD4·CDK9 nuclear complex (Fig. 6) and its recruitment to the core EMT regulators (Fig. 5, Fig. 8). Similar to our studies of TNF signaling, BRD4 recruitment to its target genes is mediated through its RelA association because TGF-β-induced CDK9 and BRD recruitment was abolished by RelA inhibition, as was the formation of phospho-Ser2 RNA Pol II (Fig. 5). These results strongly support the mechanism that TGF-β-induced type II EMT initiation is triggered by transcriptional elongation mediated by RelA translocation.
The bromodomain protein BRD4 is a chromatin-remodeling enzyme recognized as one of the most important regulators of immune responses (8, 23). The bromodomain and extraterminal domain (BET) family proteins (74), including BRD2, BRD3, BRD4, and BRDT, contain two bromodomains (74). Among ubiquitously expressed BET family proteins, BRD4 is unique in that it interacts with the CDK9-containing transcriptional elongation complex through the carboxy-terminal tail of BRD4 (3). BRD4 is a critical mediator of transcriptional elongation, functioning to recruit CDK9 to target promoters and activate its activity by phosphorylation (6, 39). In cancer cells, BRD4 promotes type III EMT and tumor growth (12, 60, 76). In this study, we reveal how activated NF-κB and BRD4 control EMT initiation and transcriptional elongation in an airway type II EMT model and in a murine pulmonary fibrosis model (Fig. 11). Our findings that BRD4 inhibition abolishes the initiation of the TGF-β-induced type II EMT program (Fig. 7) and disrupts the transcriptional elongation complexes on the EMT transcriptional regulators (Fig. 8) suggest that BRD4 recruitment is a major determinant of NF-κB-dependent initiation of EMT and transcriptional reprogramming. There is one report that BRD4 maintains constitutively active NF-κB by binding to acetylated RelA (81). It will be interesting to determine whether JQ1 disrupts BRD4 binding to acetylated RelA, downstream of Ser276 phosphorylation (6).
It has recently been reported that NF-κB coordinates rapid, BRD4-dependent remodeling of proinflammatory superenhancers in inflammation and atherogenesis. Superenhancer-bound BRD4 coactivates inflammatory genes, and chronic treatment with the BRD4 inhibitor JQ1 attenuates atherogenic responses and atherosclerosis (8). This work reveals new principles of enhancer dynamics and provides insights into the therapeutic modulation of enhancer function with BET bromodomain inhibitors, and targeting BRD4 exhibits promising specificity for inflammatory responses in vivo (8). It is currently unknown to us whether NF-κB- or BRD4-dependent superenhancers are formed during type II EMT.
To our knowledge, there are no studies linking the actions of BRD4 in the initiation or maintenance of type II EMT. We found that BRD4 inhibition blocks the TGF-β-induced EMT morphology and decreases protein levels and the cellular distribution pattern of the EMT genes induced by chronic TGF-β treatment. Interestingly, BRD4 does not completely restore expression of E-cadherin in TGF-β-treated hSAECs, suggesting that there are BRD4-independent signals contributing to the TGF-β-induced EMT. Our studies further implicate BRD4 in the development of airway fibrosis in a mouse model of TGF-β-induced fibrosis in vivo. Airway remodeling in obstructive lung disease is associated with disruption in epithelial barrier function and changes in innate immunity. Although the restoration of the epithelium to normal suggests improvement in epithelial barrier function, this question will require further investigation.
Although JQ1 attenuates bleomycin-induced lung fibrosis in mice (64, 65), the relevance of this model in understanding the EMT and airway remodeling responses may be limited. We note that the initial effect of bleomycin is through a toxic response mediated by non-immune-mediated epithelial injury with free radical formation and stromal expansion. Epithelial cell involvement is a late-stage phenomena, not the primary event in our experimental models. Similarly, BRD4 inhibition affects the profibrotic program in a mouse model of carbon tetrachloride-induced cirrhosis (17). We are also aware of the study implicating BRD4 in carbon tetrachloride-induced liver fibrosis. The mechanism for fibrosis in liver is mediated through stellate cell activation, and although BRD4 was implicated its formation of a complex with NF-κB was not examined. Collectively, the present study extends this prior work to demonstrate the role of epithelial NF-κB-BRD4 in growth factor-induced remodeling.
Standard treatments for COPD/asthma target acute bronchospasm/wheezing and reduce repeated episodes. Despite these therapies, 15–20% of patients with COPD have temporal declines in FEV1 and 10% of patients with asthma, those with glucocorticoid-dependent asthma, undergo progressive decline in FEV1 (56). Although these agents target acute bronchospasm/wheezing and reduce repeated episodes, they have no effect on the innate immune response and NF-κB-BRD4 pathway. The results of this study validate the NF-κB-BRD4 pathway as a target for reversing hyperactive innate immune response, reducing inflammation and preventing lung function decline in patients with active remodeling.
In conclusion, our studies are the first to provide a detailed mechanism for how activated NF-κB and BRD4 control EMT initiation and transcriptional elongation in an airway type II EMT model and in a murine pulmonary fibrosis model. Targeting the actions of BRD4 could represent new therapeutic approaches to the treatment of pulmonary fibrosis associated with chronic obstructive lung diseases such as COPD, severe asthma, CF, and bronchiectasis.
GRANTS
Research support was provided by the Sealy Center for Molecular Medicine, National Institute of Allergy and Infectious Diseases Signaling in Airway inflammation PO1 AI-068865 (A. R. Brasier), UTMB CTSA UL1TR-001439 (A. R. Brasier), National Institute of Environmental Health Sciences (NIEHS) P30 ES-006676 (A. R. Brasier), UTMB NIEHS CET Pilot Project 83232 (B. Tian), and DMS-1361411/DMS-1361318 (A. R. Brasier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.T. and A.R.B. conceived and designed research; B.T., Y. Zhao, H.S., Y. Zhang, J.Y., and A.R.B. performed experiments; B.T., Y. Zhao, Y. Zhang, and A.R.B. analyzed data; B.T. and Y. Zhao interpreted results of experiments; B.T. prepared figures; B.T. and Y. Zhao drafted manuscript; B.T. and A.R.B. edited and revised manuscript; B.T., Y. Zhao, H.S., Y. Zhang, J.Y., and A.R.B. approved final version of manuscript.
ACKNOWLEDGMENTS
University of Texas Medical Branch (UTMB) core laboratory support was provided by the Optical Microscopy Core and the Sealy Center for Molecular Medicine Selected Reaction Monitoring Facility. We thank Dr. David Konkel for critically editing the manuscript.
REFERENCES
- 1.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol 128: 451–462, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732–738, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bisgrove DA, Mahmoudi T, Henklein P, Verdin E. Conserved P-TEFb-interacting domain of BRD4 inhibits HIV transcription. Proc Natl Acad Sci USA 104: 13690–13695, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 161: 1720–1745, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO Rep 11: 670–677, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brasier AR, Tian B, Jamaluddin M, Kalita MK, Garofalo RP, Lu M. RelA Ser276 phosphorylation-coupled Lys310 acetylation controls transcriptional elongation of inflammatory cytokines in respiratory syncytial virus infection. J Virol 85: 11752–11769, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA 88: 6642–6646, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JD, Lin CY, Duan Q, Griffin G, Federation AJ, Paranal RM, Bair S, Newton G, Lichtman AH, Kung AL, Yang T, Wang H, Luscinskas FW, Croce KJ, Bradner JE, Plutzky J. NF-kappaB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell 56: 219–231, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke JR, Pattoli MA, Gregor KR, Brassil PJ, MacMaster JF, McIntyre KW, Yang X, Iotzova VS, Clarke W, Strnad J, Qiu Y, Zusi FC. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem 278: 1450–1456, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Camelo A, Dunmore R, Sleeman MA, Clarke DL. The epithelium in idiopathic pulmonary fibrosis: breaking the barrier. Front Pharmacol 4: 173, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol 111: 1293–1298, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Chang H, Liu Y, Xue M, Liu H, Du S, Zhang L, Wang P. Synergistic action of master transcription factors controls epithelial-to-mesenchymal transition. Nucleic Acids Res 44: 2514–2527, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhary S, Boldogh I, Brasier AR. Inside-out signaling pathways from nuclear reactive oxygen species control pulmonary innate immunity. J Innate Immun 8: 143–155, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies DE. The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc 6: 678–682, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgado O, Kaisani AA, Spinola M, Xie XJ, Batten KG, Minna JD, Wright WE, Shay JW. Multipotent capacity of immortalized human bronchial epithelial cells. PLoS One 6: e22023, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaiah BN, Case-Borden C, Gegonne A, Hsu CH, Chen Q, Meerzaman D, Dey A, Ozato K, Singer DS. BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat Struct Mol Biol 23: 540–548, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding N, Hah N, Yu RT, Sherman MH, Benner C, Leblanc M, He M, Liddle C, Downes M, Evans RM. BRD4 is a novel therapeutic target for liver fibrosis. Proc Natl Acad Sci USA 112: 15713–15718, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahy O, Hammad H, Senechal S, Pestel J, Tonnel AB, Wallaert B, Tsicopoulos A. Synergistic effect of diesel organic extracts and allergen Der p 1 on the release of chemokines by peripheral blood mononuclear cells from allergic subjects: involvement of the MAP kinase pathway. Am J Respir Cell Mol Biol 23: 247–254, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Fang L, Choudhary S, Zhao Y, Edeh CB, Yang C, Boldogh I, Brasier AR. ATM regulates NF-kappaB-dependent immediate-early genes via RelA Ser 276 phosphorylation coupled to CDK9 promoter recruitment. Nucleic Acids Res 42: 8416–8432, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang L, Choudhary S, Tian B, Boldogh I, Yang C, Ivanciuc T, Ma Y, Garofalo RP, Brasier AR. Ataxia telangiectasia mutated kinase mediates NF-kappaB serine 276 phosphorylation and interferon expression via the IRF7-RIG-I amplification loop in paramyxovirus infection. J Virol 89: 2628–2642, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorov IA, Wilson SJ, Davies DE, Holgate ST. Epithelial stress and structural remodelling in childhood asthma. Thorax 60: 389–394, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez IE, Eickelberg O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc 9: 111–116, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La TN, French CA, Wiest O, Kung AL, Knapp S, Bradner JE. Selective inhibition of BET bromodomains. Nature 468: 1067–1073, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med 198: 1043–1055, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbus J, Spratt H, Wiktorowicz J, Wu Z, Boldogh I, Denner L, Kurosky A, Brasier RC, Luxon B, Brasier AR. Functional analysis of the nuclear proteome of human A549 alveolar epithelial cells by HPLC-high resolution 2-D gel electrophoresis. Proteomics 6: 2656–2672, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, Knight DA. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med 180: 122–133, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Harris WT, Kelly DR, Zhou Y, Wang D, MacEwen M, Hagood JS, Clancy JP, Ambalavanan N, Sorscher EJ. Myofibroblast differentiation and enhanced TGF-B signaling in cystic fibrosis lung disease. PLoS One 8: e70196, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest 106: 1341–1350, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiraga R, Kato M, Miyagawa S, Kamata T. Nox4-derived ROS signaling contributes to TGF-beta-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res 33: 4431–4438, 2013. [PubMed] [Google Scholar]
- 30.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 364: 709–721, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc 1: 93–98, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Hu CP, Wu XR, Li QG, Sun ZW, Wang AP, Feng JT, Wang J. Proteomic analysis of NGF-induced transdifferentiation of adrenal medullary cells. Int J Mol Med 32: 347–354, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Huang C, Kolokoltsova OA, Yun NE, Seregin AV, Poussard AL, Walker AG, Brasier AR, Zhao Y, Tian B, de la Torre JC, Paessler S. Junin virus infection activates the type I interferon pathway in a RIG-I-dependent manner. PLoS Negl Trop Dis 6: e1659, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang RY, Guilford P, Thiery JP. Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J Cell Sci 125: 4417–4422, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest 114: 569–581, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huber MA, Beug H, Wirth T. Epithelial-mesenchymal transition: NF-kappaB takes center stage. Cell Cycle 3: 1477–1480, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Hubner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 44: 507–511, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Ijaz T, Pazdrak K, Kalita M, Konig R, Choudhary S, Tian B, Boldogh I, Brasier AR. Systems biology approaches to understanding epithelial mesenchymal transition (EMT) in mucosal remodeling and signaling in asthma. World Allergy Organ J 7: 13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell 19: 523–534, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Kaisani A, Delgado O, Fasciani G, Kim SB, Wright WE, Minna JD, Shay JW. Branching morphogenesis of immortalized human bronchial epithelial cells in three-dimensional culture. Differentiation 87: 119–126, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalita M, Tian B, Gao B, Choudhary S, Wood TG, Carmical JR, Boldogh I, Mitra S, Minna JD, Brasier AR. Systems approaches to modeling chronic mucosal inflammation. Biomed Res Int 2013: 505864, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol 25: 76–84, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol 27: 3165–3175, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knight DA, Ernst M, Anderson GP, Moodley YP, Mutsaers SE. The role of gp130/IL-6 cytokines in the development of pulmonary fibrosis: critical determinants of disease susceptibility and progression? Pharmacol Ther 99: 327–338, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK, Chung S, Luther T, Paholak HJ, Liu S, Hassan KA, Zen Q, Clouthier SG, Wicha MS. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell 47: 570–584, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 18: 684–692, 2012. [DOI] [PubMed] [Google Scholar]
- 48.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15: 178–196, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL, Chao CH, Yamaguchi H, Yang NK, Ding Q, Wang Y, Lai YJ, LaBaff AM, Wu TJ, Lin BR, Yang MH, Hortobagyi GN, Hung MC. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res 72: 1290–1300, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol 81: 1401–1411, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDonald OG, Wu H, Timp W, Doi A, Feinberg AP. Genome-scale epigenetic reprogramming during epithelial-to-mesenchymal transition. Nat Struct Mol Biol 18: 867–874, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 17: 326–333, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest 122: 2756–2762, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nowak DE, Tian B, Brasier AR. Two-step cross-linking method for identification of NF-kappaB gene network by chromatin immunoprecipitation. Biotechniques 39: 715–725, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Nowak DE, Tian B, Jamaluddin M, Boldogh I, Vergara LA, Choudhary S, Brasier AR. RelA Ser276 phosphorylation is required for activation of a subset of NF-kappaB-dependent genes by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Mol Cell Biol 28: 3623–3638, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med 179: 19–24, 2009. [DOI] [PubMed] [Google Scholar]
- 57.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, Milchgrub S, Smith AL, Souza RF, Gilbey L, Zhang X, Gandia K, Vaughan MB, Wright WE, Gazdar AF, Shay JW, Minna JD. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res 64: 9027–9034, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet 1: 520–524, 1989. [DOI] [PubMed] [Google Scholar]
- 59.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, Jeffery PK. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med 176: 858–864, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, Lin Y, Li J, Kang T, Tao M, Rusinova E, Zhang G, Wang C, Zhu H, Yao J, Zeng YX, Evers BM, Zhou MM, Zhou BP. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell 25: 210–225, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siemens H, Jackstadt R, Hunten S, Kaller M, Menssen A, Gotz U, Hermeking H. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 10: 4256–4271, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100: 768–776, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell 112: 169–180, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Tang X, Peng R, Ren Y, Apparsundaram S, Deguzman J, Bauer CM, Hoffman AF, Hamilton S, Liang Z, Zeng H, Fuentes ME, DeMartino JA, Kitson C, Stevenson CS, Budd DC. BET bromodomain proteins mediate downstream signaling events following growth factor stimulation in human lung fibroblasts and are involved in bleomycin-induced pulmonary fibrosis. Mol Pharmacol 83: 283–293, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Tang X, Peng R, Phillips JE, Deguzman J, Ren Y, Apparsundaram S, Luo Q, Bauer CM, Fuentes ME, DeMartino JA, Tyagi G, Garrido R, Hogaboam CM, Denton CP, Holmes AM, Kitson C, Stevenson CS, Budd DC. Assessment of Brd4 inhibition in idiopathic pulmonary fibrosis lung fibroblasts and in vivo models of lung fibrosis. Am J Pathol 183: 470–479, 2013. [DOI] [PubMed] [Google Scholar]
- 66.Tian B, Nowak DE, Brasier AR. A TNF-induced gene expression program under oscillatory NF-kappaB control. BMC Genomics 6: 137, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian B, Nowak DE, Jamaluddin M, Wang S, Brasier AR. Identification of direct genomic targets downstream of the nuclear factor-kappaB transcription factor mediating tumor necrosis factor signaling. J Biol Chem 280: 17435–17448, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Tian B, Yang J, Brasier AR. Two-step cross-linking for analysis of protein-chromatin interactions. Methods Mol Biol 809: 105–120, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian B, Zhao Y, Kalita M, Edeh CB, Paessler S, Casola A, Teng MN, Garofalo RP, Brasier AR. CDK9-dependent transcriptional elongation in the innate interferon-stimulated gene response to respiratory syncytial virus infection in airway epithelial cells. J Virol 87: 7075–7092, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tian B, Li X, Kalita M, Widen SG, Yang J, Bhavnani SK, Dang B, Kudlicki A, Sinha M, Kong F, Wood TG, Luxon BA, Brasier AR. Analysis of the TGFbeta-induced program in primary airway epithelial cells shows essential role of NF-kappaB/RelA signaling network in type II epithelial mesenchymal transition. BMC Genomics 16: 529, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest 119: 3637–3651, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warshamana GS, Pociask DA, Fisher KJ, Liu JY, Sime PJ, Brody AR. Titration of non-replicating adenovirus as a vector for transducing active TGF-beta1 gene expression causing inflammation and fibrogenesis in the lungs of C57BL/6 mice. Int J Exp Pathol 83: 183–201, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 166: 1321–1332, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem 282: 13141–13145, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Yang J, Mitra A, Dojer N, Fu S, Rowicka M, Brasier AR. A probabilistic approach to learn chromatin architecture and accurate inference of the NF-kappaB/RelA regulatory network using ChIP-Seq. Nucleic Acids Res 41: 7240–7259, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang P, Dong Z, Cai J, Zhang C, Shen Z, Ke A, Gao D, Fan J, Shi G. BRD4 promotes tumor growth and epithelial-mesenchymal transition in hepatocellular carcinoma. Int J Immunopathol Pharmacol 28: 36–44, 2015. [DOI] [PubMed] [Google Scholar]
- 77.Zhao Y, Jamaluddin M, Zhang Y, Sun H, Ivanucic T, Garofalo RP, Brasier AR. Systematic analysis of cell-type differences in the epithelial secretome reveals insights into pathogenesis of RSV-induced lower respiratory tract infections. J Immunol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao Y, Widen SG, Jamaluddin M, Tian B, Wood TG, Edeh CB, Brasier AR. Quantification of activated NF-kappaB/RelA complexes using ssDNA aptamer affinity-stable isotope dilution-selected reaction monitoring-mass spectrometry. Mol Cell Proteomics 10: M111.008771, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao Y, Brasier AR. Applications of selected reaction monitoring (SRM)-mass spectrometry (MS) for quantitative measurement of signaling pathways. Methods 61: 313–322, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Y, Tian B, Edeh CB, Brasier AR. Quantitation of the dynamic profiles of the innate immune response using multiplex selected reaction monitoring-mass spectrometry. Mol Cell Proteomics 12: 1513–1529, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zou Z, Huang B, Wu X, Zhang H, Qi J, Bradner J, Nair S, Chen LF. Brd4 maintains constitutively active NF-kappaB in cancer cells by binding to acetylated RelA. Oncogene 33: 2395–2404, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]