Abstract
Epithelial ovarian cancer (EOC) is the most lethal gynecologic malignancy. Despite the anticancer capabilities of emodin observed in many cancers, including EOC, the underlying molecular mechanism remains to be elucidated. A crucial link has been discovered between the acquisition of metastatic traits and the epithelial-mesenchymal transition (EMT). The present study aimed to determine whether emodin could inhibit the EMT of EOC cells and explore the underlying mechanism. The CCK-8 assay and transwell assay showed that emodin effectively repressed the abilities of proliferation, invasion, and migration in A2780 and SK-OV-3 cells. The Western blot showed that emodin upregulated epithelial markers (E-cadherin and Claudin) while it downregulated mesenchymal markers (N-cadherin and Vimentin) and transcription factor (Slug) in a dose-dependent fashion. After transfection of siRNA-Slug, both Slug and N-cadherin were downregulated in EOC cells while E-cadherin was upregulated, which was intensified by emodin. Besides, emodin decreased the expression of ILK, p-GSK-3β, β-catenin, and Slug. Transfection of siRNA-ILK also achieved the same effects, which was further strengthened by following emodin treatment. Nevertheless, SB216763, an inhibitor of GSK-3β, could reverse the effects of emodin except for ILK expression. These findings suggest that emodin inhibited the EMT of EOC cells via ILK/GSK-3β/Slug signaling pathway.
1. Introduction
Epithelial ovarian cancer (EOC) has the highest mortality rate among the female genital malignant tumors [1, 2]. The lack of effective early detection markers, coupled with the vague, nonspecific symptoms of EOC [3], often results in the late diagnosis of the disease with widespread metastases [4]. Mortality of EOC is directly related to the prevalence of metastatic diseases; however, the underlying molecular mechanisms of metastases are still not fully elucidated.
Epithelial-mesenchymal transition (EMT), an important process, has been linked to cell metastasis in kinds of cancers. A typical characteristic of EMT is the loss of the cell-cell adhesion and gain of mesenchymal traits [5]. Transcription factors, such as Snail, Slug, ZEB1/2, and TWIST1, regulate EMT and are correlated to cancer metastasis [6]. Recent work suggests that the transcriptional factors, Snail and Slug, are important effectors of the process of metastasis and cell survival [7]. The fruitful work demonstrated that Slug attributed to promoting the EMT of human ovarian cancer [8].
Integrin-linked kinase (ILK) is a serine/threonine protein kinase, which was initially discovered through its interactions with the β1 and β3 integrin subunits [9]. ILK has a fundamental role in the regulation of cell survival, proliferation, and migration by mediating integrin signaling in diverse cell types [10]. Once activated, ILK directly phosphorylates several key signaling molecules, including protein kinase B/Akt (PKB/Akt) at Ser473 and glycogen synthase kinase 3β (GSK3β) at Ser9, to affect cell survival, cycle, adhesion, and extracellular matrix (ECM) modification [11, 12]. The expression of ILK is often upregulated in various human malignancies and correlated with advanced tumor stage and grade [10]. Moreover, ILK expression is enhanced in malignant ovarian tumors compared to the benign ones or normal epithelium [13]. Furthermore, ILK has been reported to promote cancer cell migration and invasion by inducing EMT process [14, 15].
Emodin (1,3,8-trihydroxy-6-methylanthraquinone), an anthraquinone, isolated from rhizomes of Rhubarb, aloes, and other plants is known to have antiproliferative, antitumorigenic, antimetastatic and antiangiogenic effects on various cancers [16]. Recent studies have shown that emodin repressed the EMT of cells through WNT/β-catenin pathway in several cancer cells [17–19]. Meanwhile, previous studies demonstrate that emodin inhibited the migration and invasion abilities of human endometrial stromal cells by facilitating the mesenchymal-epithelial transition (MET) through targeting ILK [20].
This study aimed to investigate whether emodin could inhibit EOC cells invasion and migration by suppressing EMT via targeting ILK and then to explore the underlying mechanism.
2. Materials and Methods
2.1. Cell Lines and Culture
A2780 and SK-OV-3 cells, the human epithelial ovarian cancer cell lines, were purchased from the Cell Bank of China Academy of Sciences (Shanghai, China). A2780 cells were cultured in RPMI-1640 medium modified (GE Healthcare Life Sciences, HyClone Laboratories, USA), supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified incubator at 37°C containing 5% carbon dioxide. SK-OV-3 cells were cultured in McCoy'S 5A medium (M&C Gene Technology, China), supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified incubator at 37°C containing 5% carbon dioxide. The medium was replaced every 3 days. The cells were checked routinely and trypsinized until they reached about 90% confluence.
2.2. Antibodies and Chemicals
Emodin and GSK-3β inhibitor (SB216763) were purchased from Sigma Aldrich (St. Louis, MO, USA). Dimethylsulfoxide (DMSO) was obtained from Solarbio (Beijing, China). The emodin or SB216763 was diluted in corresponding medium, and the DMSO's final concentration was under 0.1% (v/v). The antibodies E-cadherin, N-cadherin, Vimentin, Claudin-1, Slug, ILK, Akt, phosphorylated Akt (p-Akt), GSK-3β, β-catenin, and GAPDH were purchased from Cell Signaling Technology (MA, USA). And phosphorylated GSK-3β (p-GSK-3β) was obtained from Santa Cruz Biotechnology (CA, USA).
2.3. Cell Viability Assay
Influence of emodin on the cell viability of the human epithelial ovarian cancer cell lines, A2780 and SK-OV-3, was assessed by the cell counting kit-8 (CCK-8 kit), which was purchased from BestBio (Shanghai, China). The SK-OV-3 and A2780 cells were seeded in a 96-well plate at a density of 2500 cells/well and 3500 cells/well, respectively. After incubation overnight, the cells were exposed to various concentrations of emodin (0–80 μM) for 24 hours, 48 hours, and 72 hours, respectively. At each time point, 100 μL of fresh medium containing 10 μL CCK-8 solution was added to each well and incubated at 37°C for 2 hours. The absorbance at 450 nm was measured by a plate reader (Bio-Rad, CA, USA).
2.4. Transwell Migration and Invasion Assays
Cell migration was measured using 24-well Boyden chambers (8 μm pore size; Corning Costar, Cambridge, MA, USA). 3 × 104 SK-OV-3 cells or 6 × 104 A2780 cells were loaded onto the top of a 24-well migration chamber in 100 ul serum-free medium. The lower chamber was filled with 0.75 mL of the medium containing 10% FBS. After 24 hours of incubation, cells that had migrated into the lower surface of the filter were fixed with methanol solution for 5 minutes and 3.7% paraformaldehyde for 5 minutes and then stained with Giemsa stain. Pictures (200x) were taken by the Olympus IX51 inverted microscope. Cells were counted in five random fields per insert. The same sterile Boyden chambers were used to perform the invasion assay. Before commencing the experiment, the BD Matrigel (BD Biosciences, San Jose, CA, USA) was placed in a −4°C refrigerator for 24 hours. The BD Matrigel would turn into liquid. It was diluted to 1 : 9 ratios with serum-free medium. When the plates were already precoated with Matrigel, the similar steps were performed as the migration assay.
2.5. Western Blot Analysis
The protein of cells was extracted with RIPA Lysis Buffer (Beyotime, Jiangsu, China) containing 1% phenylmethanesulfonyl fluoride (PMSF) on ice. The protein concentration was detected according to BCA Kits. We mixed proteins with 5x SDS-PAGE sample loading buffer at a ratio of 1 : 4. Following heating at 95°C for 5 min, denatured proteins were subjected to 10% Tris-glycine gel and transferred electrophoretically to polyvinylidene difluoride membranes. Then 5% nonfat milk in Tris-buffered saline tween (TBST) was used to block nonspecific sites at room temperature for 1 h. The membranes were incubated with the primary antibodies (E-cadherin, N-cadherin, Vimentin, Claudin-1, Slug, ILK, Akt, p-Akt GSK-3β, p-GSK-3β, β-catenin, and GAPDH, 1 : 1000) overnight at 4°C and washed with TBST three times. Secondary HRP-conjugated antibodies (1 : 3000) were incubated with the membranes for 1 hour at 37°C. Antibody-positive bands were visualized using Image Quant LAS 4000. Data were analyzed by ImageJ software.
2.6. Small Interfering RNA (siRNA) Transfection
Slug and ILK specific siRNA and their control were purchased from GenePharma Company (Shanghai, China). For siRNA transfection, EOC cells were seeded in 6-well plates without antibiotics treated with 50%–60% confluence before transfection. Cell transfection was carried out using Lipofectamine 2000 (Invitrogen Life Technologies). Six hours after incubation, the culture medium was changed into fresh medium with 10% FBS. After 48 hours of transfection, cells were harvested for the following cell experiments. For A2780 and SK-OV-3 the sequence for Slug specific siRNA is 5′-CCCAUUCUGAUGUAAAGAATT-3′, 5′-GGACCACAGUGGCUCAGAATT-3′, respectively. Besides, the sequence for ILK specific siRNA is 5′-GACCCAAAUUUGACAUGAUTT-3′, 5′-UGGACACCGUGAUAUUGUATT-3′, respectively, in A2780 and SK-OV-3 cells.
2.7. Statistical Analysis
All statistical analyses were carried out using GraphPad Prism Version 5.01. The data was expressed as a mean ± standard deviation (SD) of three independent experiments. Data were analyzed by Student's t-test between any two groups. One-way ANOVA analysis of variance was used to assess the difference of means among groups. For all analyses, values of P < 0.05 were considered statistically significant.
3. Results
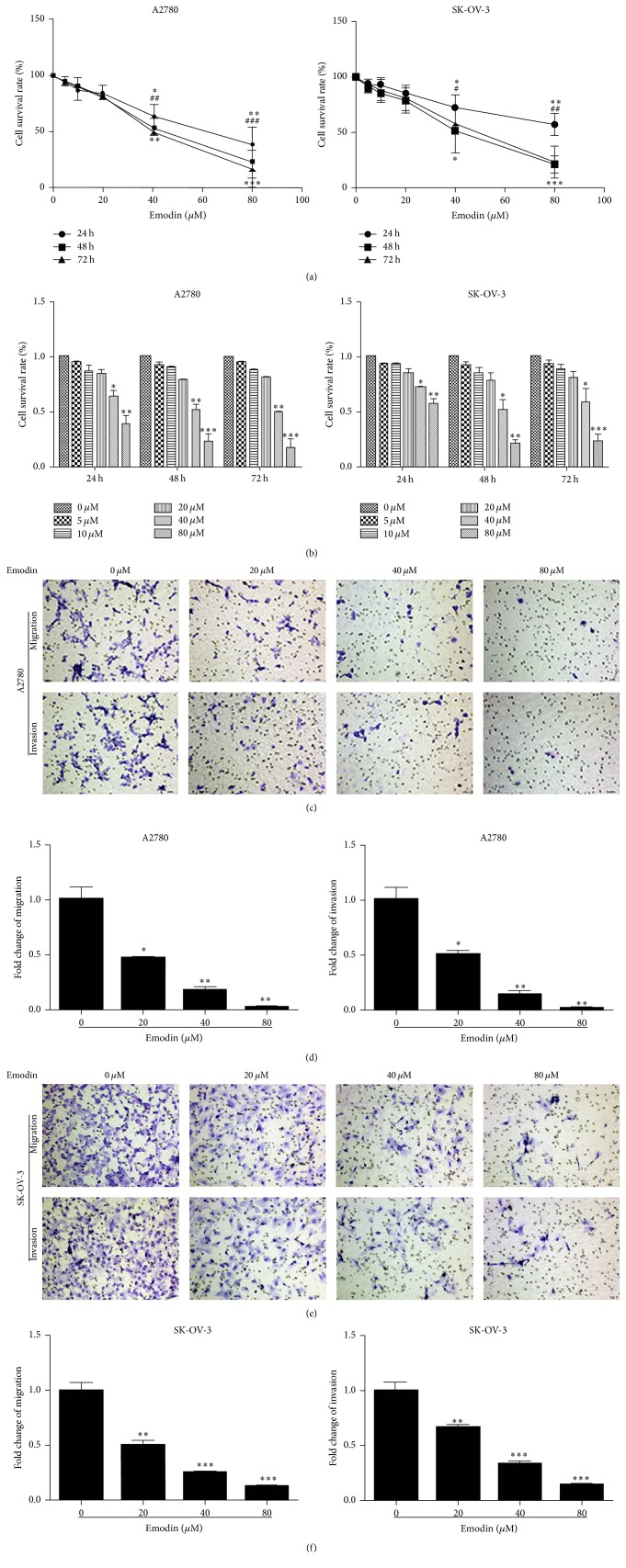
Emodin suppressed the proliferation, migration, and invasion property of A2780 and SK-OV-3 cells. To detect the effect of emodin on the proliferation of human EOC cells, SK-OV-3 and A2780 cells were treated with a range of concentrations of emodin for up to 24 hours, 48 hours, and 72 hours to examine the cell viability. We found that emodin inhibited the proliferation of SK-OV-3 cells in a dose- and time-dependent fashion, with significant inhibition at 40 μM and 80 μM (Figures 1(a) and 1(b)). Similar results were also found in A2780 cells (Figures 1(a) and 1(b)). Transwell migration and invasion assays were used to test the effect of emodin on the migration and invasion abilities of A2780 and SK-OV-3 cells. As expected, the migration and invasion abilities of A2780 and SK-OV-3 cells were significantly decreased after treatment with emodin for 48 hours (Figures 1(c)–1(f)). All these results suggested that emodin could effectively inhibit the proliferation, migration, and invasion abilities of A2780 and SK-OV-3 cells.
Figure 1.
Emodin suppressed the proliferation, migration, and invasion property of A2780 and SK-OV-3cells. (a) SK-OV-3 cells and A2780 cells were treated with increased concentrations of emodin for up to 72 hours to examine the cell viability. 24 h: ∗ P < 0.05, ∗∗ P < 0.005; 48 h: # P < 0.05, ## P < 0.005, and ### P < 0.001; 72 h: ∗ P < 0.05, ∗∗ P < 0.005, and ∗∗∗ P < 0.001. (b) Emodin inhibited A2780 and SK-OV-3 cells growth in the dose- and time-dependent fashion. ∗ P < 0.05, ∗∗ P < 0.005, and ∗∗∗ P < 0.001. (c) Representative transwell migration and invasion assay of SK-OV-3 cells and A2780 cells after treatment with emodin (×200 magnification). (d) Emodin weakened migration and invasion capacity of A2780 and SK-OV-3 cells. ∗ P < 0.05, ∗∗ P < 0.005, and ∗∗∗ P < 0.001.
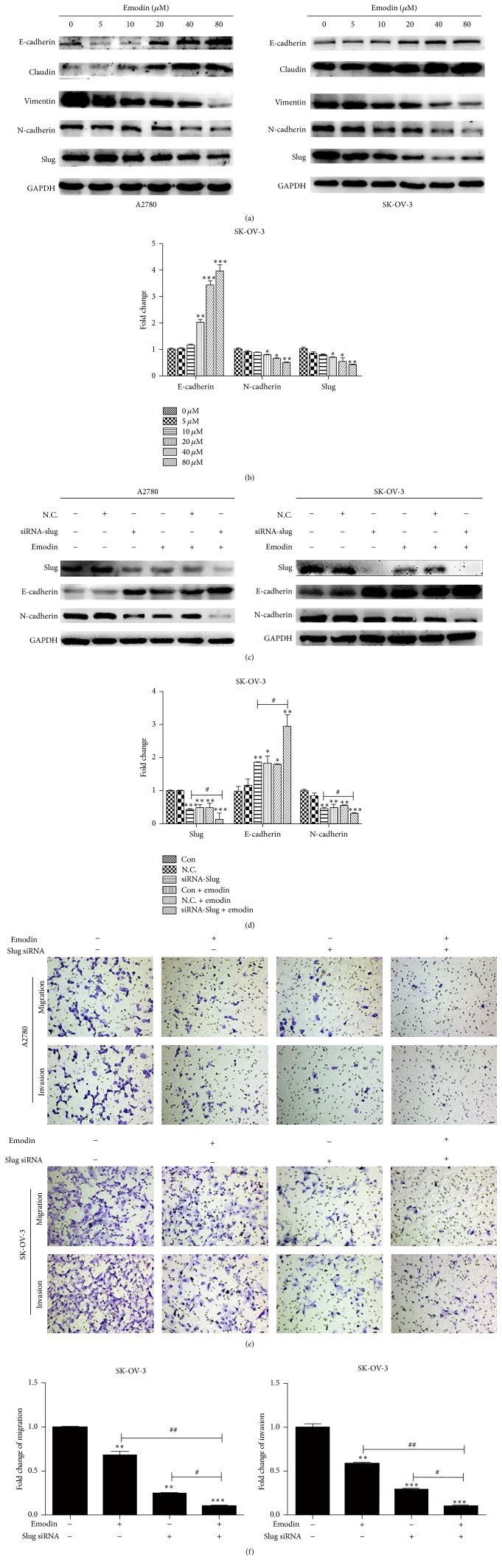
3.1. Emodin Inhibited EMT in A2780 and SK-OV-3 Cells through Targeting Slug
Given that emodin could significantly inhibit the migration and invasion abilities of A2780 and SK-OV-3 cells, we tested the expression of EMT-related factors by Western blot analysis to evaluate whether emodin could affect EMT in EOC cells. As shown in Figures 2(a) and 2(b), the epithelial markers, E-cadherin and Claudin, were upregulated after the treatment with emodin for 48 hours in both A2780 and SK-OV-3 cells. Meanwhile, the mesenchymal markers, N-cadherin and Vimentin, were downregulated. Additionally, the level of transcription factor, Slug, was downregulated in dose-dependent manner. After transfection of siRNA-Slug, the levels of Slug and N-cadherin were both downregulated in A2780 and SK-OV-3 cells while the level of E-cadherin was upregulated, which were intensified by emodin (Figures 2(c) and 2(d)). Furthermore, we investigated the effects of Slug knockdown on cell migration and invasion followed by emodin treatment. As expected, the migration and invasion abilities of A2780 and SK-OV-3 cells were lowest in Slug knockdown cells followed by treating with emodin (Figures 2(e) and 2(f)).
Figure 2.
Emodin inhibited EMT in A2780 and SK-OV-3 cells through targeting Slug. (a) Different concentration of emodin exposure for 48 hours upregulated the protein expression of E-cadherin and Claudin and downregulated protein expression of N-cadherin, Vimentin, and Slug in A2780 and SK-OV-3 cells. (b) Quantitative analyses of E-cadherin, N-cadherin, and Slug in SK-OV-3 cells shown in (a). ∗ P < 0.05, ∗∗ P < 0.005, and ∗∗∗ P < 0.001. (c) A2780 and SK-OV-3 cells were transfected with siRNA of Slug and cultured for an additional 48 h treated with emodin 20 μM or without emodin. Western blot analysis results of Slug, E-cadherin, and N-cadherin protein levels were shown. (d) Quantitative analyses of markers in SK-OV-3 cells shown in (c). ∗ P < 0.05, ∗∗ P < 0.005, and ∗∗∗ P < 0.001. # P < 0.05. (e) Representative transwell migration and invasion assay of A2780 and SK-OV-3 cells after transfection with siRNA of Slug with or without emodin treatment. (f) Quantification of migration and invasion capacity of SK-OV-3 cells in (e). ∗∗ P < 0.005 and ∗∗∗ P < 0.001. # P < 0.05 and ## P < 0.005. N.C. represents transfection of siRNA-control.
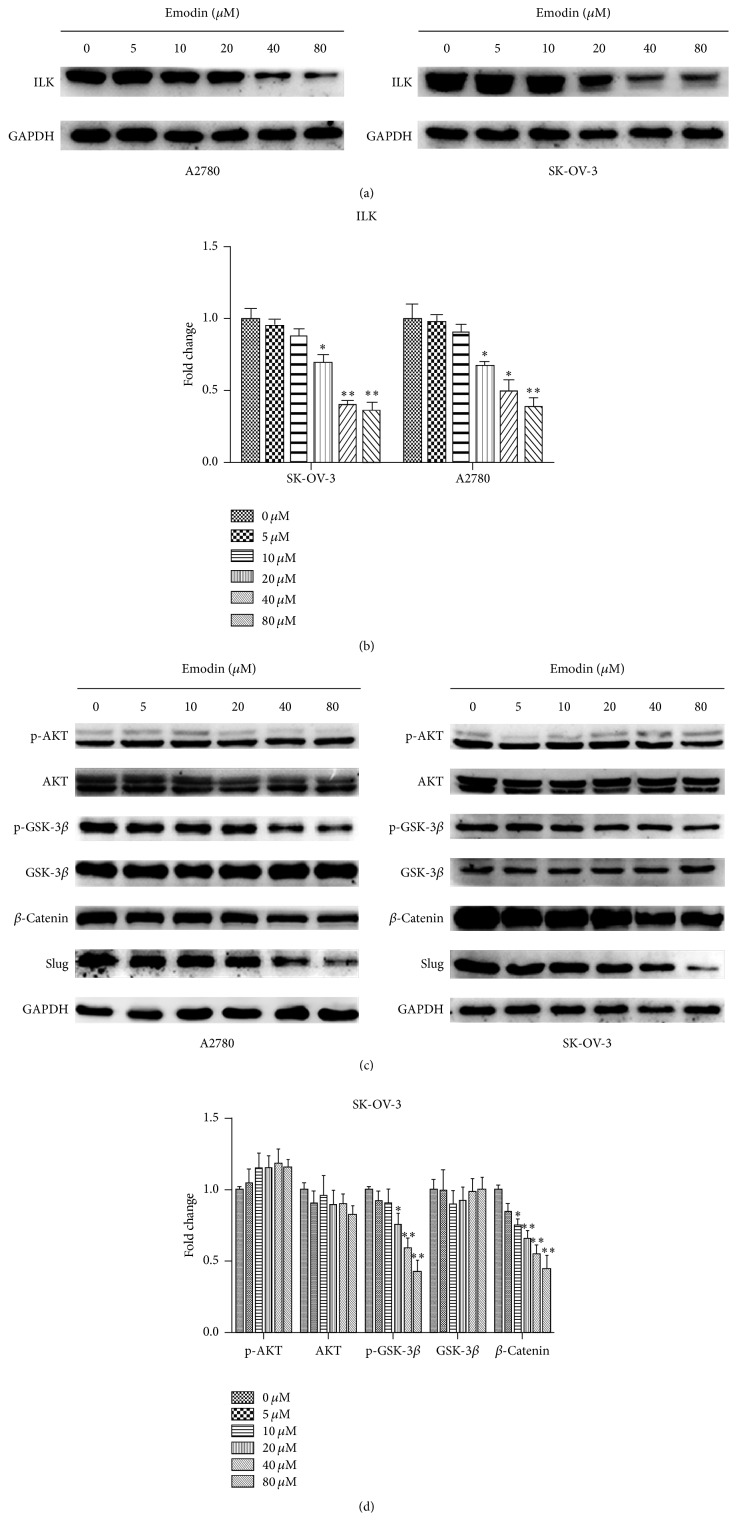
3.2. Emodin Decreased the Expression of ILK, p-GSK-3β, β-Catenin, and Slug in A2780 and SK-OV-3 Cells
Western blot was used to detect the expression of ILK and its downstream targets, p-Akt and p-GSK-3β, in A2780 and SK-OV-3 cells after treatment with emodin. Dose-dependent decrease of ILK expression was observed after treatment with emodin for 48 hours (Figures 3(a) and 3(b)). The expression of p-GSK-3β, one of the ILK downstream targets, was also decreased in a dose-dependent manner, while p-Akt failed to participate in the above process (Figures 3(c) and 3(d)). Additionally, the levels of β-catenin and Slug were both downregulated in dose-dependent manner (Figures 3(c) and 3(d)).
Figure 3.
Emodin decreased the expression of ILK, p-GSK-3β, β-catenin, and Slug in A2780 and SK-OV-3 cells. (a, c) The expression of ILK, p-Akt, Akt, p-GSK-3β, GSK-3β, β-catenin, and Slug detected by Western blot analysis after treatment with emodin for 48 hours were shown. (b) Quantitative analyses of ILK protein expression in A2780 and SK-OV-3 cells shown in (a). ∗ P < 0.05, ∗∗ P < 0.01. (d) Quantitative analyses of factors in SK-OV-3 cells shown in (c). ∗ P < 0.05, ∗∗ P < 0.01.
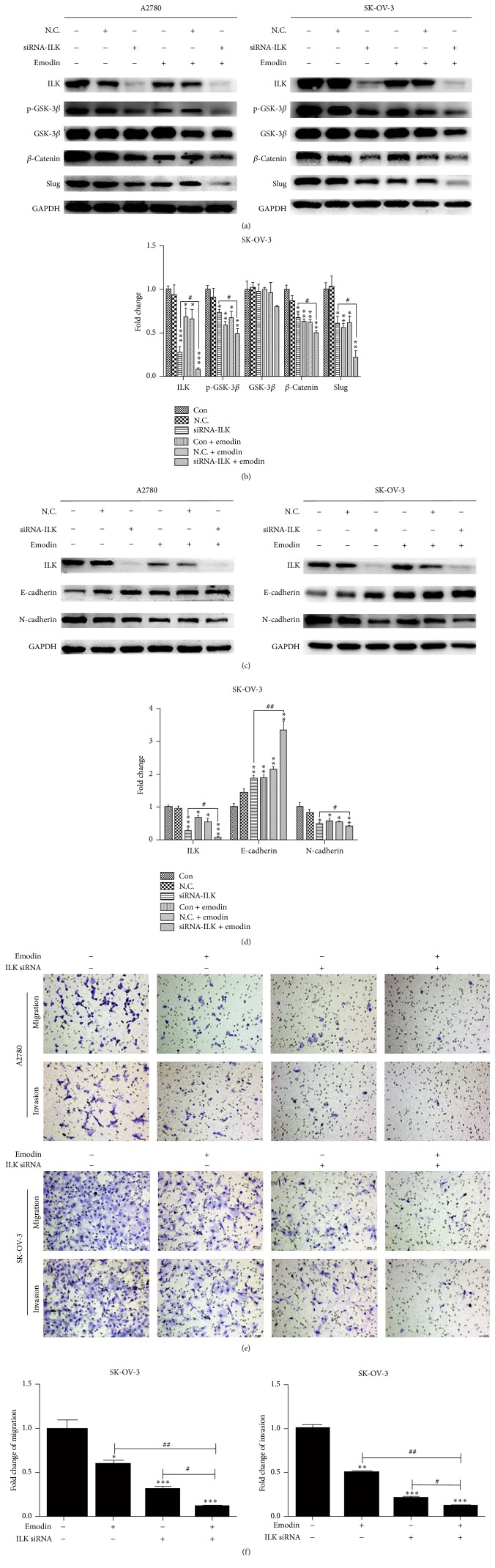
3.3. Emodin Repressed EMT by Targeting ILK in A2780 and SK-OV-3 Cells
To verify whether emodin inhibited the EMT of EOC cells by targeting ILK, we knocked down the ILK in A2780 and SK-OV-3 cells by transfecting with siRNA-ILK. After transfection of siRNA-ILK, the levels of ILK, p-GSK-3β, β-catenin, and Slug were all decreased in A2780 and SK-OV-3 cells, which were reinforced by emodin (Figures 4(a) and 4(b)). When it came to EMT hallmarks, the level of epithelial markers, E-cadherin, was increased while the level of mesenchymal markers, N-cadherin, was decreased in A2780 and SK-OV-3cells, which can also be intensified by additional emodin (Figures 4(c) and 4(d)). Furthermore, we investigated the effects of emodin on cell migration and invasion followed by ILK knockdown. The migration and invasion abilities of A2780 and SK-OV-3 cells were weakened after the transfection of siRNA-ILK, which could be further reduced by emodin treatment (Figures 4(e) and 4(f)). Therefore, emodin decreased the migration and invasion abilities by suppressed EMT via targeting ILK in A2780 and SK-OV-3 cells.
Figure 4.
Emodin repressed EMT by targeting ILK in A2780 and SK-OV-3 cells. (a, c) A2780 and SK-OV-3 cells were transfected with siRNA-ILK and treated with or without emodin 20 μM for 48 hours. Protein levels of ILK, p-GSK-3β, GSK-3β, β-catenin, Slug, E-cadherin, and N-cadherin were shown. (b, d) Quantitative analyses of factors in SK-OV-3 cells shown in (a) and (c). ∗ P < 0.05, ∗∗ P < 0.005, and ∗∗∗ P < 0.001. # P < 0.05, ## P < 0.005. (e) Representative transwell migration and invasion assay of A2780 and SK-OV-3 cells after transfection with siRNA of ILK with or without emodin treatment. (f) Quantification of migration and invasion capacity of SK-OV-3 cells in (e). ∗ P < 0.05, ∗∗ P < 0.005, and ∗∗∗ P < 0.001. # P < 0.05, ## P < 0.005. N.C. represents transfection of siRNA-control.
3.4. Emodin Inhibited the EMT of EOC Cells by ILK/GSK-3β Signaling Pathway
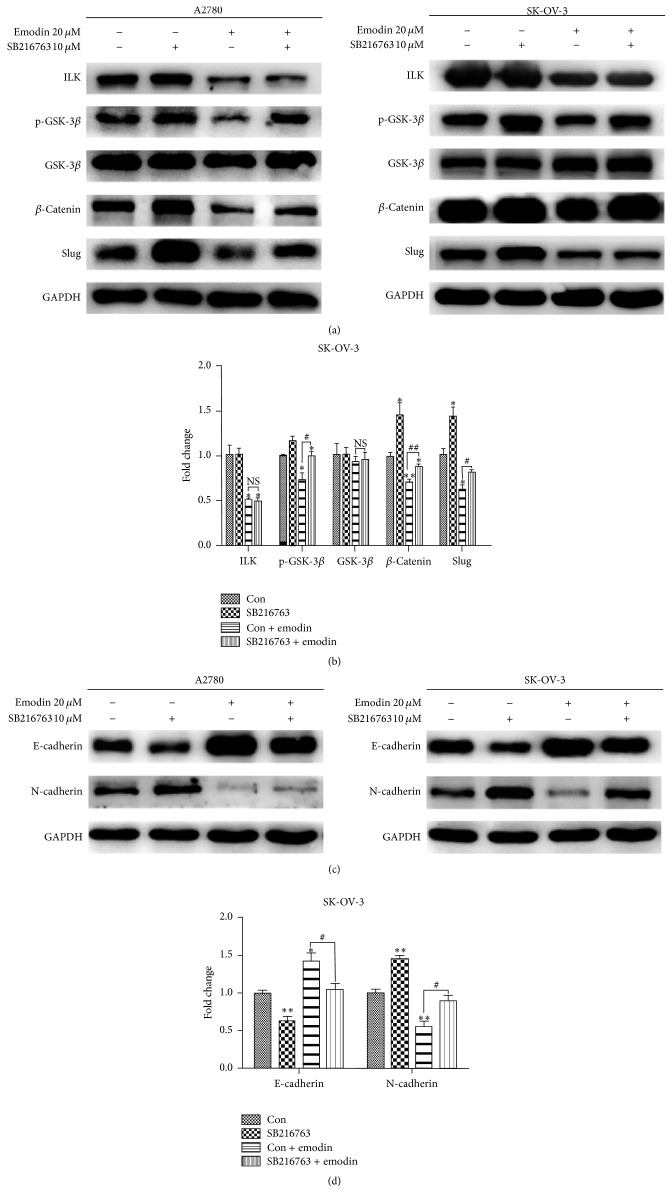
To further clarify that whether ILK targeted GSK-3β to suppress EMT of EOC cells, we used GSK-3β inhibitor (SB216763) to treat A2780 and SK-OV-3 cells for 6 hours. SB216763 restored emodin-induced downregulation of p-GSK-3β, β-catenin, and Slug expression except for ILK (Figures 5(a) and 5(b)). Furthermore the level of epithelial markers, E-cadherin, was downregulated while the level of mesenchymal markers, N-cadherin, was upregulated in A2780 and SK-OV-3 cells by SB216763 (Figures 5(c) and 5(d)). Our findings verified that emodin could inhibit the EMT via ILK/GSK-3β signaling pathway in EOC cells.
Figure 5.
Emodin inhibited the EMT of EOC cells by ILK/GSK-3β signaling pathway. (a, c) A2780 and SK-OV-3 cells were treated with 10 μM SB216763 and exposed with or without emodin 20 μM for 48 hours. Protein levels of ILK, p-GSK-3β, GSK-3β, β-catenin, Slug, E-cadherin, and N-cadherin were shown. (b, d) Quantitative analyses of factors in SK-OV-3 cells shown in (a) and (c). ∗ P < 0.05, ∗∗ P < 0.005. # P < 0.05, ## P < 0.005.
4. Discussion
EOC is the most lethal gynecologic malignancy and most of the patients are diagnosed at advanced stage with widespread metastasis [1]. What is more, the mortality of EOC is directly related to the prevalence of metastatic diseases [21]. EMT is an important process that culminates with loss of epithelial traits and gain of mesenchymal traits [22]. In recent studies, a crucial link has been discovered between the acquisition of metastatic traits in cancer cells and the EMT. EMT promotes cellular and microenvironmental changes, which resulted in cells invading and migrating to distant sites [23–25]. Emodin has been found to suppress proliferation, inhibit angiogenesis, induce apoptosis, repress metastasis, and synergize chemotherapeutic response in various cancers such as human colorectal cancer and human lung adenocarcinoma [19, 26, 27]. Previous reviews showed that emodin inhibited EMT in manifold cancer, including EOC [17, 18]. However, the underlying mechanism was not fully understood.
A number of transcription factors, such as Snail, Slug, ZEB1/2, and TWIST1, regulate EMT and correlate with cancer aggressiveness [6]. Among them, Slug is closely associated with tumor metastasis in ovarian cancer [28]. In the present study, Slug has identified specific mechanisms controlled during EMT in the A2780 and SK-OV-3 cells. After treatment with emodin, the migration and invasion abilities of A2780 and SK-OV-3 cells were significantly decreased. Emodin induced downregulation of E-cadherin and Claudin expression accompanied by an upregulation of N-cadherin and Vimentin expression. We showed that exposure to emodin could inhibit EOC cells invasion and metastasis by inhibiting EMT. At the same time, emodin could suppress the expression of Slug. Furthermore, silence of Slug suppressed EMT, which was validated by molecular and functional experiments. Therefore, we have reason to think that emodin inhibits EMT in A2780 and SK-OV-3 cells through targeting Slug.
It has been shown that ILK is expressed in ovarian cancers and ILK expression increases with ovarian cancer progression [29–31]. ILK expression is an initial early event in EOC metastasis, which suggested that further evaluation of ILK inhibitors in EOC is warranted [32]. Previous study demonstrated that emodin could inhibit glucose-induced EMT through ILK inhibition in diabetic kidney disease [33]. Besides, emodin repressed the EMT of endometrial stromal cells by inhibiting the ILK expression [20]. What is more, emodin suppressed EMT through the ILK/Akt/mTOR signaling pathway in breast cancer cells [34]. But whether emodin could inhibit the expression of ILK in ovarian cancer is still unclear. In our study, dose-dependent decrease of ILK expression was observed after treatment with emodin. To show the significance of ILK in the regulation of EMT and cell migration and invasive abilities, ILK expression was downregulated by siRNA-ILK in EOC cells. Emodin strengthened siRNA-ILK-induced E-cadherin expression and N-cadherin expression. At the same time, the migration and invasion abilities were reduced by treating with siRNA-ILK in EOC cells. The current study verified that emodin weakened the migration and invasion abilities of A2780 and SK-OV-3 by inhibiting EMT via targeting ILK. This result is consistent with a previous study which confirmed that ILK overexpression promotes epithelial-mesenchymal transition in colorectal cancer cells [35].
As a vital regulator of the phosphoinositide 3-kinase (PI3K) signaling pathway, ILK phosphorylates downstream targets such as PKB/Akt and GSK-3β to regulate their activities [36, 37]. In oral squamous cell carcinoma cells, knockdown of ILK inhibited EMT by suppressing the phosphorylation of downstream signaling targets Akt and GSK-3β [38]. In the present study, we have verified that emodin inhibited EMT by targeting ILK. However, only the expression of p-GSK-3β was decreased along with ILK in a dose-dependent manner, while p-Akt failed to participate in the process. It is well known that decrease of the expression of p-GSK-3β led to the increase of GSK-3β kinase activity, followed by phosphorylation of β-catenin [39, 40]. Similarly, our present results showed downregulated β-catenin after treatment with emodin. Furthermore, ILK knockdown significantly decreased the levels of ILK, p-GSK-3β, β-catenin, and Slug in A2780 and SK-OV-3 cells, which were reinforced by emodin. However, GSK-3β inhibitor treatment restored emodin-induced effects except for ILK. So, our data confirmed that emodin inhibited the EMT of EOC cells through ILK/GSK-3β/Slug pathway (Figure 6).
Figure 6.
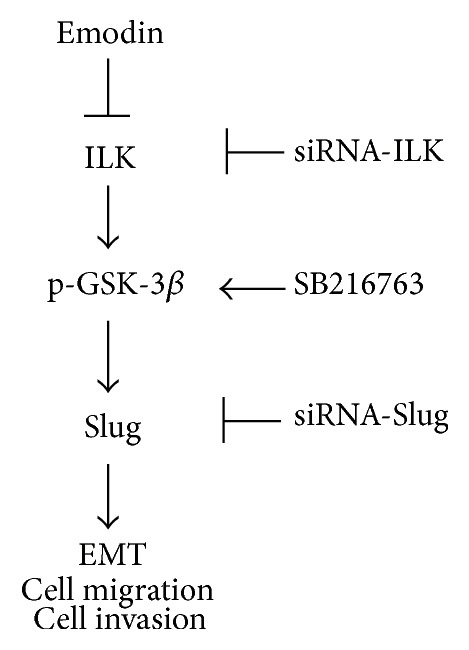
A schematic of ILK/p-GSK-3β/Slug pathway. Emodin inhibited the ILK/p-GSK-3β/Slug signaling pathway. ILK/GSK-3β/Slug axis contributes to EMT program, cellular migration, and cellular invasion.
5. Conclusions
Our studies demonstrated that emodin could suppress the proliferation, invasion, and migration capabilities of A2780 and SK-OV-3 cells. The latter two might be mediated by repressing the EMT through targeting Slug. Besides, we showed for the first time that emodin inhibited EMT via targeting ILK in EOC cells. Furthermore, we demonstrated that emodin inhibited the migration and invasion abilities of EOC cells by suppressing the EMT through ILK/GSK-3β/Slug signaling pathway. These results suggest that emodin might be a potential candidate for metastasis targeted therapy of EOC. However, further studies are required before introducing emodin into the clinical regimen.
Acknowledgments
This study was supported by the Science and Technology Development Project of Shandong Province grants (2008GG2NS02017).
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contributions
Jingjing Lu and Ying Xu contributed equally to this paper.
References
- 1.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2016. CA Cancer Journal for Clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Grabowski J. P., Sehouli J. Current management of ovarian cancer. Minerva Medica. 2015;106:151–161. [PubMed] [Google Scholar]
- 4.Banerjee S., Kaye S. B. New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential. Clinical Cancer Research. 2013;19(5):961–968. doi: 10.1158/1078-0432.ccr-12-2243. [DOI] [PubMed] [Google Scholar]
- 5.Yilmaz M., Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer and Metastasis Reviews. 2009;28(1-2):15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 6.Sun L., Fang J. Epigenetic regulation of epithelial-mesenchymal transition. Cellular and Molecular Life Sciences. 2016;73(23):4493–4515. doi: 10.1007/s00018-016-2303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurrey N. K., Amit K., Bapat S. A. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecologic Oncology. 2005;97(1):155–165. doi: 10.1016/j.ygyno.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 8.Peng J., Zhang G., Wang Q., et al. ROCK cooperated with ET-1 to induce epithelial to mesenchymal transition through SLUG in human ovarian cancer cells. Bioscience, Biotechnology and Biochemistry. 2012;76(1):42–47. doi: 10.1271/bbb.110411. [DOI] [PubMed] [Google Scholar]
- 9.Hannigan G. E., Leung-Hagesteijn C., Fitz-Gibbon L., et al. Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature. 1996;379(6560):91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 10.McDonald P. C., Fielding A. B., Dedhar S. Integrin-linked kinase—essential roles in physiology and cancer biology. Journal of Cell Science. 2008;121(19):3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 11.Wu C., Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. The Journal of Cell Biology. 2001;155(4):505–510. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dedhar S., Williams B., Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends in Cell Biology. 1999;9(8):319–323. doi: 10.1016/s0962-8924(99)01612-8. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed N., Oliva K., Rice G. E., Quinn M. A. Cell-free 59 kDa immunoreactive integrin-linked kinase: a novel marker for ovarian carcinoma. Clinical Cancer Research. 2004;10(7):2415–2420. doi: 10.1158/1078-0432.ccr-03-0042. [DOI] [PubMed] [Google Scholar]
- 14.Chen D., Zhang Y., Zhang X., et al. Overexpression of integrin-linked kinase correlates with malignant phenotype in non-small cell lung cancer and promotes lung cancer cell invasion and migration via regulating epithelial-mesenchymal transition (EMT)-related genes. Acta Histochemica. 2013;115(2):128–136. doi: 10.1016/j.acthis.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Becker-Santos D. D., Guo Y., Ghaffari M., et al. Integrin-linked kinase as a target for ERG-mediated invasive properties in prostate cancer models. Carcinogenesis. 2012;33(12):2558–2567. doi: 10.1093/carcin/bgs285.bgs285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demirezer L. Ö., Kuruüzüm-Uz A., Bergere I., Schiewe H.-J., Zeeck A. The structures of antioxidant and cytotoxic agents from natural source: anthraquinones and tannins from roots of Rumex patientia . Phytochemistry. 2001;58(8):1213–1217. doi: 10.1016/s0031-9422(01)00337-5. [DOI] [PubMed] [Google Scholar]
- 17.Hu C., Dong T., Li R., Lu J., Wei X., Liu P. Emodin inhibits epithelial to mesenchymal transition in epithelial ovarian cancer cells by regulation of GSK-3beta/beta-catenin/ZEB1 signaling pathway. Oncology Reports. 2016;35(4):2027–2034. doi: 10.3892/or.2016.4591. [DOI] [PubMed] [Google Scholar]
- 18.Way T.-D., Huang J.-T., Chou C.-H., Huang C.-H., Yang M.-H., Ho C.-T. Emodin represses TWIST1-induced epithelial-mesenchymal transitions in head and neck squamous cell carcinoma cells by inhibiting the β-catenin and Akt pathways. European Journal of Cancer. 2014;50(2):366–378. doi: 10.1016/j.ejca.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Pooja T., Karunagaran D. Emodin suppresses Wnt signaling in human colorectal cancer cells SW480 and SW620. European Journal of Pharmacology. 2014;742:55–64. doi: 10.1016/j.ejphar.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Q., Xu Y., Lu J., Zhao J., Wei X., Liu P. Emodin inhibits migration and invasion of human endometrial stromal cells by facilitating the mesenchymal-epithelial transition through targeting ILK. Reproductive Sciences. 2016;23(11):1526–1535. doi: 10.1177/1933719116645192. [DOI] [PubMed] [Google Scholar]
- 21.Grassi M. L., Palma C. S., Thome C. H., Lanfredi G. P., Poersch A., Faca V. M. Proteomic analysis of ovarian cancer cells during epithelial-mesenchymal transition (EMT) induced by epidermal growth factor (EGF) reveals mechanisms of cell cycle control. Journal of Proteomics. 2017;151:2–11. doi: 10.1016/j.jprot.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y., Sun X., Feng J., et al. MT2-MMP induces proteolysis and leads to EMT in carcinomas. Oncotarget. 2016;7(30):48193–48205. doi: 10.18632/oncotarget.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J. M., Dedhar S., Kalluri R., Thompson E. W. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. The Journal of Cell Biology. 2006;172(7):973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernaudo S., Salem M., Qi X., et al. Cyclin G2 inhibits epithelial-to-mesenchymal transition by disrupting Wnt/β-catenin signaling. Oncogene. 2016;35:4816–4827. doi: 10.1038/onc.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelfand R., Vernet D., Bruhn K., Vadgama J., Gonzalez-Cadavid N. Long-term exposure of MCF-12A normal human breast epithelial cells to ethanol induces epithelial mesenchymal transition and oncogenic features. International Journal of Oncology. 2016;48(6):2399–2414. doi: 10.3892/ijo.2016.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Q., Lu G., Shen H.-M., Chung M. C. M., Ong C. N. Anti-cancer properties of anthraquinones from rhubarb. Medicinal Research Reviews. 2007;27(5):609–630. doi: 10.1002/med.20094. [DOI] [PubMed] [Google Scholar]
- 27.Su Y.-T., Chang H.-L., Shyue S.-K., Hsu S.-L. Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochemical Pharmacology. 2005;70(2):229–241. doi: 10.1016/j.bcp.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Gu A., Jie Y., Yao Q., Zhang Y., Mingyan E. Slug is associated with tumor metastasis and angiogenesis in ovarian cancer. Reproductive Sciences. 2016 doi: 10.1177/1933719116654989. [DOI] [PubMed] [Google Scholar]
- 29.Bruney L., Liu Y., Grisoli A., Ravosa M. J., Stack M. S. Integrin-linked kinase activity modulates the pro-metastatic behavior of ovarian cancer cells. Oncotarget. 2016;7(16):21968–21981. doi: 10.18632/oncotarget.7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed N., Riley C., Oliva K., Stutt E., Rice G. E., Quinn M. A. Integrin-linked kinase expression increases with ovarian tumour grade and is sustained by peritoneal tumour fluid. The Journal of Pathology. 2003;201(2):229–237. doi: 10.1002/path.1441. [DOI] [PubMed] [Google Scholar]
- 31.Choi Y. P., Kim B. G., Gao M.-Q., Kang S., Cho N. H. Targeting ILK and β4 integrin abrogates the invasive potential of ovarian cancer. Biochemical and Biophysical Research Communications. 2012;427(3):642–648. doi: 10.1016/j.bbrc.2012.09.114. [DOI] [PubMed] [Google Scholar]
- 32.Hannigan G., Troussard A. A., Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nature Reviews Cancer. 2005;5(1):51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 33.Chen T., Zheng L. Y., Xiao W., Gui D., Wang X., Wang N. Emodin ameliorates high glucose induced-podocyte epithelial-mesenchymal transition in-vitro and in-vivo. Cellular Physiology and Biochemistry. 2015;35(4):1425–1436. doi: 10.1159/000373963. [DOI] [PubMed] [Google Scholar]
- 34.Ma J. W., Hung C. M., Lin Y. C., Ho C. T., Kao J. Y., Way T. D. Aloe-emodin inhibits HER-2 expression through the downregulation of Y-box binding protein-1 in HER-2-overexpressing human breast cancer cells. Oncotarget. 2016;7(37):58915–58930. doi: 10.18632/oncotarget.10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen H., Ma J. L., Zhang Y., et al. Integrin-linked kinase overexpression promotes epithelial-mesenchymal transition via nuclear factor-kappaB signaling in colorectal cancer cells. World Journal of Gastroenterology. 2016;22(15):3969–3977. doi: 10.3748/wjg.v22.i15.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delcommenne M., Tan C., Gray V., Rue L., Woodgett J., Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(19):11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C. Integrin-linked kinase and PINCH: partners in regulation of cell-extracellular matrix interaction and signal transduction. Journal of Cell Science. 1999;112(24):4485–4489. doi: 10.1242/jcs.112.24.4485. [DOI] [PubMed] [Google Scholar]
- 38.Que L., Zhao D., Tang X.-F., et al. Effects of lentivirus-mediated shRNA targeting integrin-linked kinase on oral squamous cell carcinoma in vitro and in vivo. Oncology Reports. 2016;35(1):89–98. doi: 10.3892/or.2015.4374. [DOI] [PubMed] [Google Scholar]
- 39.Naspi A., Zingariello M., Sancillo L., et al. IGFBP-3 inhibits Wnt signaling in metastatic melanoma cells. Molecular Carcinogenesis. 2016 doi: 10.1002/mc.22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potz B. A., Sabe A. A., Elmadhun N. Y., et al. Glycogen synthase kinase 3β inhibition improves myocardial angiogenesis and perfusion in a swine model of metabolic syndrome. Journal of the American Heart Association. 2016;5(7) doi: 10.1161/jaha.116.003694.e003694 [DOI] [PMC free article] [PubMed] [Google Scholar]