Abstract
The interaction between natural killer (NK) cell and dendritic cell (DC), two important cellular components of innate immunity, started to be elucidated in the last years. The crosstalk between NK cells and DC, which leads to NK cell activation, DC maturation, or apoptosis, involves cell-cell contacts and soluble factors. This interaction either in the periphery or in the secondary lymphoid organs acts as a key player linking innate and adaptive immune responses to microbial stimuli. This review focuses on the mechanisms of NK-DC interaction and their relevance in antimicrobial responses. We specifically aim to emphasize the ability of various microbial infections to differently influence NK-DC crosstalk thereby contributing to distinct adaptive immune response.
1. Introduction
The implementation of an effective immune response requires recognition of pathogen and consequent induction of innate and adaptive immune systems. Even though adaptive immune system provides a more versatile means of defense by ultimate protection and memory against the pathogen, innate immune system is crucial in the initiation and subsequent direction of adaptive immune responses. NK cells and dendritic cells represent two central components of the innate immune system, both of which play a key role in combating early infection. NK cells provide the first line of defense against a variety of tumors and microbial pathogens. Morphologically they are characterized as large granular bone marrow derived lymphocytes, which represent 10% of peripheral blood lymphocytes. In humans, NK cells are divided, based on their functional and phenotypic properties, into two main subsets, namely, CD56dim and CD56bright. CD56dim subset shows enhanced cytotoxic activity and expresses CD16, KIRs (killer cell immunoglobulin-like receptors), and perforin whereas CD56bright subset secretes enormous amounts of cytokines and expresses low levels of perforin and CD16 [1]. Upon stimulation, NK cells secrete large amounts of cytokines and chemokines such as IFN-γ, TNF-α, GM-CSF, CCL3, CCL4, and CCL5 [2]. NK cells identify their targets through a number of activating and inhibitory receptors on their surface and the balance between these signals controls NK cell activation [3].
DCs are the major antigen-presentation cells (APCs) of the immune system and have a crucial role in both sensing pathogens and tuning the immune responses [4]. They consist of different subtypes and are classified based on their phenotype, location, and function [5]. Conventional DCs (cDCs) mainly reside in the lymphoid tissues such as spleen, thymus, and secondary lymph nodes (LNs). These conventional DCs express higher levels of MHC-II and CD11c and can be further divided into CD8α+ and CD8α− DCs in mice. When compared with CD8α+ DCs which more often induce Th0 cells to elicit Th1 response, CD8α− DCs more likely induce Th2 responses [6]. In addition, cDCs in the nonlymphoid tissues such as the intestine and the lung consist of two major subsets: CD103+ and CD11bhi DCs. Interestingly, CD103+ DC in the nonlymphoid organs including lung, gut, and skin form a unified subset, which is developmentally related to the CD8+ cDC in lymphoid organs [7]. This correlation is demonstrated by their shared dependence on certain transcriptional factors such as Batf3 and Irf8 and functional characteristics of antigen cross-presentation. The linkage between CD8+ DC and CD103+ DC was further strengthened by the reports showing unique common expression of XCR1, a chemokine receptor, by these DC subsets [8, 9]. Since XCR1 are also expressed in human BDCA3+ DC and sheep CD26+ DC (the equivalents of mouse CD8+ DC), the term “XCR1+ DC” could be designated to “CD8+ type DC” in both lymphoid and peripheral tissues across all mammalian species. Plasmacytoid DCs (pDCs) represent a small subset of DCs that enter the lymph nodes through the blood circulation. Upon activation through Toll-like receptor (TLR)-7 and TLR9 stimulations, pDCs secrete profound amounts of IFN-α and several chemokines (CCL3, CCL5, and CXCL10) [10]. In humans, DCs express high levels of MHC II and lack markers such as CD3, CD19/20 and CD56. They can be classified as either myeloid or plasmacytoid [11]. Myeloid DCs (mDCs) correspond to mouse cDCs and express myeloid antigens such as CD11c, CD13, CD33, and CD11b. They are divided into CD1c+ and CD141+ DCs, which share homology with mouse CD11b+ DC and CD8/CD103+ DC, respectively. CD14+ DCs, originally described as interstitial DCs, are a third subset CD11c+ myeloid DC found in tissues and lymph nodes. Human plasmacytoid DCs lack myeloid antigens and express CD123, CD303, and CD304 [11]. DCs reside in an immature form at various portals of pathogen entry. Under steady state conditions, DCs express low levels of MHC and costimulatory molecules. On exposure to pathogens, TLRs and other receptors on surface of DCs recognize molecular patterns associated with microbes, which initiates DC maturation, upregulation of CCR7, and consequent migration to the local draining lymph nodes where interaction with naive T cell occurs. Mature DCs express high levels of MHC and costimulatory molecules which enable them to activate naive T cells in T cell areas of secondary lymphoid organs [12]. Priming and modulation of T cells by DCs involves the interaction of CD80 (B7-1)/CD86 (B7.2) and CD40 with CD28/CTLA4 (CD152) and CD40L on T cells, respectively [13]. In addition, activated DCs produce proinflammatory and immunomodulatory cytokines and chemokines, which shape the pattern of immune responses [14].
2. NK-DC Interaction
The bidirectional crosstalk between DCs and NK cells can occur in the periphery or in secondary lymphoid tissues where they interact with each other through cell–cell contact and soluble factors. Interaction of NK cells with DC results in maturation, activation, and cytokine production by both cells.
2.1. DCs Induce NK Activation
TLR mediated recognition of pathogen by DC stimulates their maturation and secretion of several cytokines, which can activate NK cells. DC promotes NK cell proliferation, cytokine production, and cytolytic activity mainly through the release of cytokines and cell-cell contacts. In vitro studies have demonstrated a central role for DC-derived IL-12 in the induction of IFN-γ producing NK cells. IL-18 produced by DC can further induce the expression of IL-12 receptor on NK cells [15]. IL-15 is another relevant cytokine produced by DC which can stimulate NK cell proliferation, survival, and priming of protective NK cell response [1]. In addition, pDCs secrete profound amounts of type 1 interferon (IFN-α/β) which induce NK cell cytotoxicity [16]. Furthermore, it is found that upon TLR stimulation, IFN-β produced by DC induces IL-15 production by DCs as well as NK cells. This IL-15 can be transpresented by DCs to NK cells as well as cispresented by an NK cell to itself for efficient NK cell activation [17, 18]. It has also been shown that TLR-9 stimulated pDCs promote a selective proliferation of CD56bright NK cell subset [19]. Other soluble factors, such as prostaglandin E2 (PGE2) produced by DC have emerged as a potential regulator of NK-DC crosstalk. It can modulate secretion of the chemokines and cytokines that are involved in NK cell recruitment [20]. NK cell activation by DCs also requires direct cell-to-cell contacts. Even though there are controversial reports regarding formation of stable or transient NK-DC interactions in vivo, it is evident that cell-cell contact is required for the confined secretion of IL-18 at the immunological synapse [21, 22]. In fact, the formation of stimulatory synapses, between DCs and NK cells, promotes DC to secrete preassembled stores of IL-12 towards the NK cell. This synaptic delivery of IL-12 by DCs is required for IFN-γ secretion by NK cells [23]. Other interactions that promote NK cell IFN-γ production include CXC3CL1 expressed on DCs with its receptor on NK cells and triggering of activation receptors NKp46 and NKG2D [24, 25].
2.2. NK Cells Promote DC Activation
It is well known from in vitro studies that activated NK cells release profound amounts of IFN-γ and TNF which promote DC maturation [1]. Mature DCs secrete cytokines such as IL-12, IL-6, IL-27, IL-21, IL-23, and TGF-β [26]. These cytokine signals provided by NK cell-activated DCs in turn control the adaptive immune responses against infections. On the other hand, activated NK cells also have the ability to kill DCs that fail to undergo proper maturation (“DC editing”) through engagement of the activating receptor NKp30 [27]. NK cells discriminate between mature and immature DCs (iDC) by recognizing low amount of class 1 MHC molecules on the surface of immature DCs. In vitro studies have demonstrated that culture of activated human NK cells with iDCs at low NK/DC ratios promotes DC maturation, whereas a higher NK/DC ratio can result in NK cell-mediated killing of DCs [28].
3. NK-DC Crosstalk in Viral Infection
The role of NK-DC crosstalk in controlling viral infection has long been recognized. Studies in human immunodeficiency virus (HIV), hepatitis C virus (HCV) and mouse cytomegalovirus (MCMV) infection provide a strong body of evidence to implicate NK-DC interaction in fine-tuning the adaptive immune response to viral infections.
3.1. NK-DC Crosstalk in HIV Infection
Recent evidence suggests that the crosstalk between NK cells and DCs is impaired during HIV-1 infection. In vitro studies by Mavilio et al. have shown that there was a marked impairment in the interactions between CD56neg NK subset and autologous DCs from HIV 1-infected viremic but not aviremic individuals. Defective interaction includes abnormalities in the process of reciprocal NK–DC activation, maturation as well as a defect in the NK cell–mediated editing of immature DCs (iDCs) [29]. This defect in DC editing is mediated by an increase in CD56neg NK cells with impaired NKp30 function. In addition, DCs infected with HIV-1 are resistant to NK-induced TNF-alpha-related-apoptosis-inducing-ligand- (TRAIL-) mediated apoptosis. This resistance occurs due to High-mobility group box 1 (HMGB1) mediated upregulation of two anti-apoptotic molecules, the cellular-Flice like inhibitory protein (c-FLIP) and the cellular inhibitor of apoptosis 2 (c-IAP2) [30]. In fact, in vitro studies suggest that HIV infection induces IL-10 production of DC, which promotes resistance of immature DCs to NK cell-mediated elimination [31]. Moreover, pDCs from HIV-infected individuals are found to have deficiency for producing IFN-α and TNF [32]. Similarly, cDCs from HIV-1-infected individuals show impaired secretion of IL-12, IL-15, and IL-18, leading to decreased NK cell activation [33].
3.2. NK-DC Crosstalk in HCV Infection
In vitro studies have demonstrated that NK cells from chronic hepatitis C virus infected donors (HCV-NK) express higher levels of NK inhibitory receptor, CD94/NKG2A. On binding of CD94/NKG2A with HLA-E expressed on hepatic cells, HCV-NK cells produce IL-10 and transforming growth factor-β (TGFβ) [34]. IL-10 and TGFβ are found to act as suppressive factors of DC activation. Studies suggest that IL-10/TGFβ-modified DCs can induce IL-10-producing T cells as well as CD25+CD4+ regulatory T cells [35]. The blockade of NKG2A also stimulated NK cells to generate Th1-polarized CD4+ T cells. The results suggest that NK cell modulation of DC through NKG2A interferes with the ability of DCs to generate HCV-specific adaptive immune responses. In addition, chronic hepatitis C virus (HCV) infected DC virtually lost the ability of type I IFN-mediated production of IL-15. This defect in IL-15 production leads to reduced expression of MHC class I-related chains A and B (MICA/B) on DCs. MIC-A and MIC-B are ligands for NKG2D, which transduce positive intracellular signals in NK cells to promote NK activation [36]. This explains the reduced frequency of NK cells in individuals with chronic HCV infection [37].
3.3. NK-DC Crosstalk in Mouse Cytomegalovirus (MCMV) Infection
During early stage of MCMV infection, pDCs produce IFN-α/β and IL-12 as a consequence of recognition of viral CpG DNA sequences through the TLR9/MyD88 pathway. The IFN-α/β and IL-12 cytokines produced by pDCs promote the capacity of NK cells in cytotoxicity, IFN-γ production, and functional maturation of cDC for effective antiviral CD8+ T cell response [38]. Moreover, CD11b+ myeloid DCs also activate NK cells through NKG2D-NKG2D ligand interactions as well as the production of IFN-α/β, IL-18, and IL-12 [39]. This effect was independent of TLR9 but requires TLR2 and/or TLR3 [40]. In addition, MCMV infected CD8α+ DCs produce very high levels of IL-12 and IL-18, which specifically expand Ly49H+ subset of NK cells [41]. It has been reported that MCMV can induce high IFN-α/β to promote its own survival by inhibiting cDCs and delaying the response of antiviral effector, CD8+ T cells. Interestingly, Ly49H+ NK cell−mediated functions during early stage of MCMV infection prevent release of immunosuppressive levels of IFN-α/β to protect against MCMV-induced loss of splenic CD8α+ DCs [42]. Altogether, these studies suggest that NK cell and DC interact with each other to create a balance between the positive and negative effects of IFN-α/β and other cytokines for the optimal control of MCMV infection.
4. NK-DC Crosstalk in Parasite Infection
In response to Plasmodium chabaudi AS infection in mice, NK cells stimulated DC to mature and produce IL-12 by cell-cell contact and NK cell derived IFN-γ [43]. In vitro studies suggest that during infection with Toxoplasma gondii, NK-DC interaction leads to elevated IL-12 production by DC which in turn increases their ability to prime CD8+ T cell response against the parasite. This effect was due to TLA (Toxoplasma lysate Ag) mediated upregulation of NKG2D receptors such as MULT-1 and RAE-1 on DC. Neutralization of NK activating receptor, NKG2D, in T. gondii infected mice led to defect in parasite clearance [44]. Moreover, adoptive transfer studies of preactivated NK cells into Leishmania amazonensis-infected mice significantly increased DC and T cell activation as well as reduced tissue parasite loads [45]. Similarly, it is found that, in response to P. berghei ANKA infection, NK cells stimulated DC-mediated priming of CD8+ T cells. On the other hand, both DC-depletion and genetic deletion of IL-12 in mice almost completely abrogated NK cell-mediated IFN-γ responses to P. berghei ANKA infection [46]. Moreover, IL-12 has been shown to be essential for the in vitro NK cell-derived IFN-γ response to P. falciparum [47], P. chabaudi [43], T. gondii [48], and in vivo NK cell responses to Leishmania major [49]. In addition, studies in P. falciparum infected RBC [50] and Leishmania major infection in mice [51] suggest that NK cell activation also depends on IL-2 production from antigen-specific CD4+ T cells apart from DC derived stimuli. These studies highlight the concept that stimulation of adaptive immune response by NK-DC crosstalk enhances NK cell activation and cytotoxicity for optimal immune response.
5. NK-DC Crosstalk in Bacterial Infection
Recent studies suggest that the cooperation between NK cells and DC is also crucial in several antibacterial responses. Initial studies using in vitro systems demonstrated that stimulation of human peripheral NK cells with LPS treated mature DC augmented the cytolytic activity of NK cells. Reciprocally, coculture of NK cells with immature DC induced DC maturation and IL-12 production and promoted ability of DCs to activate allogenic naive CD4+ T cells. This effect on DC maturation was mainly dependent on cell-cell contact although NK produced IFN-γ and TNF also made contribution [26]. The studies on effect of DC on NK cells by Van Elssen et al. demonstrated that DCs triggered by membrane fraction of Klebsiella pneumoniae have the ability to induce CCR7 expression on CD56dimCD16+ NK cells which promoted their migration in response to lymph node associated chemokine, CCL19. Interestingly, bacterial fragment–matured DC also promoted NK cell activation resulting in higher production of IFN-γ and Th1 polarisation [52]. Similarly, chemokines (CXCL10) released from DCs infected with Mycobacterium tuberculosis (Mtb) enhanced the migration of NK cells to the site of infection where they can directly destroy Mtb-infected cells or produce IFN-γ to stimulate macrophage activation [53]. Moreover, acute systemic infections with L. monocytogenes and Yersinia pestis identified the regulatory role of NK cells in NK-DC crosstalk. It is found that NK cells are the major source of IL-10, which suppresses IL-12 secretion of DC, thereby preventing further NK cell activation and tissue damage [54].
Our laboratory has studied the modulating effect of NK cells on the function of DCs using an in vivo model of chlamydial lung infection [55, 56]. Chlamydia is an obligate intracellular bacterium causing various human diseases. The most important protective mechanism in host defense against chlamydial infection is Th1 mediated immunity [57, 58]. Chlamydial infection induces strong NK and NKT cell responses [59–62]. We examined NK and DCs collected from the spleen and lung of Chlamydiae-infected mice and analyzed DC phenotype and function by flow cytometry, primary cell culture, and adoptive transfer experiments. We found that depletion of NK cells altered the phenotypic and functional maturation of DCs and promoted infection and pathological reaction in the lung. In line, adoptive transfer of DCs from Chlamydiae-infected NK-depleted mice (NK-DC) in contrast to DC from the infected mice without NK depletion (NK+DC), failed to induce type 1 protective immunity in recipient mice after challenge infection. Moreover, NK cells from Chlamydiae-infected mice showed enhancing effect on IL-12 production by DC. This effect was depending on NKG2D receptor signaling and IFN γ production by NK cells [55]. Furthermore, cytokine analysis of the local tissues of the recipient mice receiving NK-DC compared with those receiving NK+DC exhibited lower levels of Th1 (IFN-γ) and Th17 (IL-17) but higher levels of Th2 (IL-4) cytokines. Consistently, NK-DCs were less efficient in directing Chlamydiae-specific Th1 and Th17 responses than NK+DCs when co-cultured with CD4+ T cells [56]. These studies taken together demonstrate that NK cells modulate DC function to elicit Th1/Th17 immunity during intracellular bacterial infection (Figure 1).
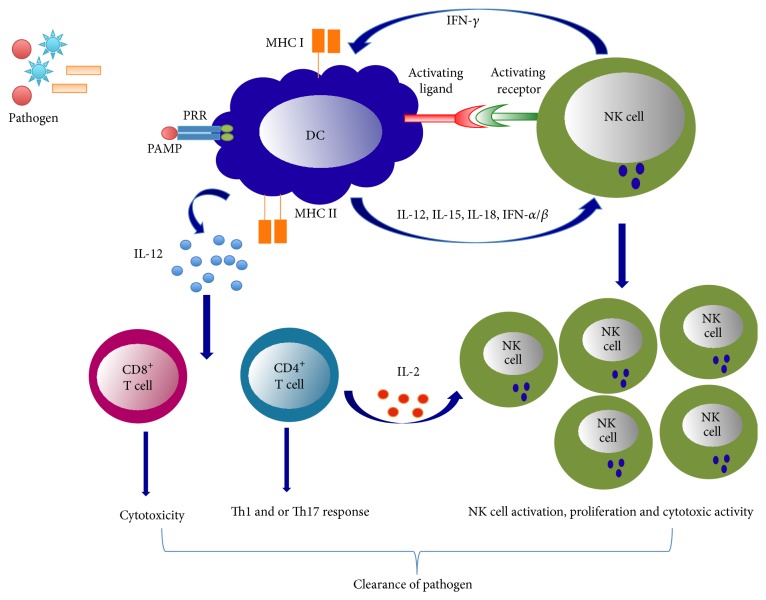
Figure 1.
Bidirectional activation of NK cells and DCs for optimal immune response to microbial infection. DCs recognize pathogen-associated molecular patterns (PAMPs) found on the surface of pathogen through the expression of pattern recognition receptor (PRR). Following recognition of pathogen, DCs release cytokines such as IL-12, IL-15, IL-18, and IFN-α/β. These cytokines produced by DCs as well as the interactions of DC with NK cell activation receptors promote NK cell activation, proliferation, and cytotoxicity. Reciprocally, NK cells release IFN-γ to promote DC maturation and release of IL-12. IL-12 produced by DC further promotes CD8+ T cell and/or CD4+ T cell activation depending upon the nature of pathogen. IL-2 produced by CD4+ T cell can also stimulate NK cell activation for optimal immune response.
6. Conclusion
The generation of pathogen specific adaptive immune response requires efficient interaction between NK cells and DC in the periphery or secondary lymphoid organs. This crosstalk is evident in several models of viral, parasite, and bacterial infection and provides a strong rationale for the combined use of NK cells and DCs in the immunotherapy of chronic infections. The development of therapeutic interventions aimed at enhancing the immune response against infections will require a complete understanding of the molecular mechanisms involved in NK-DC crosstalk and how it becomes disrupted during chronic infection.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and Manitoba Health Research Council (MHRC) to Xi Yang. Rony Thomas is a recipient of graduate studentship of Research Manitoba. The authors thank Bency Thomas and Alpho Rose K Thomas for their assistance with proofreading the manuscript.
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
References
- 1.Ferlazzo G., Morandi B. Cross-talks between natural killer cells and distinct subsets of dendritic cells. Frontiers in Immunology. 2014;5, article 159 doi: 10.3389/fimmu.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walzer T., Dalod M., Vivier E., Zitvogel L. Natural killer cell-dendritic cell crosstalk in the initiation of immune responses. Expert Opinion on Biological Therapy. 2005;5, supplement 1:S49–S59. doi: 10.1517/14712598.5.1.s49. [DOI] [PubMed] [Google Scholar]
- 3.Tran J., Mahmood S., Carlyle J. R., Kung S. K. P. Altering the specificity of NK:target cell interactions by genetic manipulation of NK receptor expression on primary mouse NK cells. Vaccine. 2010;28(22):3767–3772. doi: 10.1016/j.vaccine.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Pulendran B., Palucka K., Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293(5528):253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 5.Liu K., Nussenzweig M. C. Origin and development of dendritic cells. Immunological Reviews. 2010;234(1):45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 6.Shortman K., Heath W. R. The CD8+ dendritic cell subset. Immunological Reviews. 2010;234(1):18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 7.Edelson B. T., Wumesh K. C., Juang R., et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. The Journal of Experimental Medicine. 2010;207(4):823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crozat K., Tamoutounour S., Vu Manh T.-P., et al. Cutting edge: expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8α+ type. Journal of Immunology. 2011;187(9):4411–4415. doi: 10.4049/jimmunol.1101717. [DOI] [PubMed] [Google Scholar]
- 9.Crozat K., Guiton R., Contreras V., et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8α+ dendritic cells. Journal of Experimental Medicine. 2010;207(6):1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persson C. M., Chambers B. J. Plasmacytoid dendritic cell-induced migration and activation of NK cells in vivo. European Journal of Immunology. 2010;40(8):2155–2164. doi: 10.1002/eji.200940098. [DOI] [PubMed] [Google Scholar]
- 11.Collin M., Mcgovern N., Haniffa M. Human dendritic cell subsets. Immunology. 2013;140(1):22–30. doi: 10.1111/imm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nature Reviews Immunology. 2002;2(12):957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J., Briere F., Caux C., et al. Immunobiology of dendritic cells. Annual Review of Immunology. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 14.Marcenaro E., Carlomagno S., Pesce S., Moretta A., Sivori S. NK/DC crosstalk in anti-viral response. Advances in Experimental Medicine and Biology. 2012;946:295–308. doi: 10.1007/978-1-4614-0106-3_17. [DOI] [PubMed] [Google Scholar]
- 15.Walzer T., Dalod M., Robbins S. H., Zitvogel L., Vivier E. Natural-killer cells and dendritic cells: ‘l'union fait la force’. Blood. 2005;106(7):2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 16.Biron C. A., Nguyen K. B., Pien G. C., Cousens L. P., Salazar-Mather T. P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annual Review of Immunology. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 17.Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26(4):503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanoni I., Spreafico R., Bodio C., et al. IL-15 cis presentation is required for optimal NK cell activation in lipopolysaccharide-mediated inflammatory conditions. Cell Reports. 2013;4(6):1235–1249. doi: 10.1016/j.celrep.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Romagnani C., Della Chiesa M., Kohler S., et al. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. European Journal of Immunology. 2005;35(8):2452–2458. doi: 10.1002/eji.200526069. [DOI] [PubMed] [Google Scholar]
- 20.Gualde N., Harizi H. Prostanoids and their receptors that modulate dendritic cell-mediated immunity. Immunology and Cell Biology. 2004;82(4):353–360. doi: 10.1111/j.0818-9641.2004.01251.x. [DOI] [PubMed] [Google Scholar]
- 21.Mingozzi F., Spreafico R., Gorletta T., et al. Prolonged contact with dendritic cells turns lymph node‐resident NK cells into anti‐tumor effectors. EMBO Molecular Medicine. 2016;8(9):1039–1051. doi: 10.15252/emmm.201506164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beuneu H., Deguine J., Breart B., Mandelboim O., Di Santo J. P., Bousso P. Dynamic behavior of NK cells during activation in lymph nodes. Blood. 2009;114(15):3227–3234. doi: 10.1182/blood-2009-06-228759. [DOI] [PubMed] [Google Scholar]
- 23.Borg C., Jalil A., Laderach D., et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104(10):3267–3275. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 24.Pallandre J. R., Krzewski K., Bedel R., et al. Dendritic cell and natural killer cell cross-talk: a pivotal role of CX3CL1 in NK cytoskeleton organization and activation. Blood. 2008;112(12):4420–4424. doi: 10.1182/blood-2007-12-126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Draghi M., Pashine A., Sanjanwala B., et al. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. Journal of Immunology. 2007;178(5):2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 26.Gerosa F., Baldani-Guerra B., Nisii C., Marchesini V., Carra G., Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. The Journal of Experimental Medicine. 2002;195(3):327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moretta L., Ferlazzo G., Bottino C., et al. Effector and regulatory events during natural killer-dendritic cell interactions. Immunological Reviews. 2006;214(1):219–228. doi: 10.1111/j.1600-065X.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 28.Piccioli D., Sbrana S., Melandri E., Valiante N. M. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. Journal of Experimental Medicine. 2002;195(3):335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mavilio D., Lombardo G., Kinter A., et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. Journal of Experimental Medicine. 2006;203(10):2339–2350. doi: 10.1084/jem.20060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melki M.-T., Saïdi H., Dufour A., Olivo-Marin J.-C., Gougeon M.-L. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk—a pivotal role of HMGB1. PLoS Pathogens. 2010;6(4):1–15. doi: 10.1371/journal.ppat.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alter G., Kavanagh D., Rihn S., et al. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. Journal of Clinical Investigation. 2010;120(6):1905–1913. doi: 10.1172/JCI40913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reitano K. N., Kottilil S., Gille C. M., et al. Defective plasmacytoid dendritic cell-NK cell cross-talk in HIV infection. AIDS Research and Human Retroviruses. 2009;25(10):1029–1037. doi: 10.1089/aid.2008.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altfeld M., Fadda L., Frleta D., Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nature Reviews Immunology. 2011;11(3):176–186. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jinushi M., Takehara T., Tatsumi T., et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. Journal of Immunology. 2004;173(10):6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 35.Steinman R. M., Hawiger D., Nussenzweig M. C. Tolerogenic dendritic cells. Annual Review of Immunology. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 36.Jinushi M., Takehara T., Tatsumi T., et al. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. Journal of Immunology. 2003;171(10):5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 37.Dessouki O., Kamiya Y., Nagahama H., et al. Chronic hepatitis C viral infection reduces NK cell frequency and suppresses cytokine secretion: reversion by anti-viral treatment. Biochemical and Biophysical Research Communications. 2010;393(2):331–337. doi: 10.1016/j.bbrc.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Swiecki M., Gilfillan S., Vermi W., Wang Y., Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8+ T cell accrual. Immunity. 2010;33(6):955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andoniou C. E., van Dommelen S. L. H., Voigt V., et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nature Immunology. 2005;6(10):1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 40.Horowitz A., Stegmann K. A., Riley E. M. Activation of natural killer cells during microbial infections. Frontiers in Immunology. 2012;2, article 88 doi: 10.3389/fimmu.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrews D. M., Scalzo A. A., Yokoyama W. M., Smyth M. J., Degli-Esposti M. A. Functional interactions between dendritic cells and NK cells during viral infection. Nature Immunology. 2003;4(2):175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 42.Robbins S. H., Bessou G., Cornillon A., et al. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS pathogens. 2007;3(8, article e123) doi: 10.1371/journal.ppat.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ing R., Stevenson M. M. Dendritic cell and NK cell reciprocal cross talk promotes gamma interferon-dependent immunity to blood-stage Plasmodium chabaudi AS infection in mice. Infection and Immunity. 2009;77(2):770–782. doi: 10.1128/IAI.00994-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guan H., Moretto M., Bzik D. J., Gigley J., Khan I. A. NK cells enhance dendritic cell response against parasite antigens via NKG2D pathway. Journal of Immunology. 2007;179(1):590–596. doi: 10.4049/jimmunol.179.1.590. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez Sanabria M. X., Vargas-Inchaustegui D. A., Xin L., Soong L. Role of natural killer cells in modulating dendritic cell responses to Lishmania amazonensis infection. Infection and Immunity. 2008;76(11):5100–5109. doi: 10.1128/IAI.00438-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryg-Cornejo V., Nie C. Q., Bernard N. J., et al. NK cells and conventional dendritic cells engage in reciprocal activation for the induction of inflammatory responses during Plasmodium berghei ANKA infection. Immunobiology. 2013;218(2):263–271. doi: 10.1016/j.imbio.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Artavanis-Tsakonas K., Riley E. M. Innate immune response to malaria: rapid induction of IFN-γ from human NK cells by live Plasmodium falciparum-infected erythrocytes. Journal of Immunology. 2002;169(6):2956–2963. doi: 10.4049/jimmunol.169.6.2956. [DOI] [PubMed] [Google Scholar]
- 48.Gazzinelli R. T., Hieny S., Wynn T. A., Wolf S., Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell- deficient hosts. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(13):6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scharton-Kersten T., Afonso L. C. C., Wysocka M., Trinchieri G., Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental Leishmaniasis. Journal of Immunology. 1995;154(10):5320–5330. [PubMed] [Google Scholar]
- 50.Horowitz A., Newman K. C., Evans J. H., Korbel D. S., Davis D. M., Riley E. M. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. Journal of Immunology. 2010;184(11):6043–6052. doi: 10.4049/jimmunol.1000106. [DOI] [PubMed] [Google Scholar]
- 51.Bihl F., Pecheur J., Bréart B., et al. Primed antigen-specific CD4+ T cells are required for NK cell activation in vivo upon Leishmania major infection. Journal of Immunology. 2010;185(4):2174–2181. doi: 10.4049/jimmunol.1001486. [DOI] [PubMed] [Google Scholar]
- 52.Van Elssen C. H. M. J., Vanderlocht J., Frings P. W. H., et al. Klebsiella pneumoniae-triggered DC recruit human NK cells in a CCR5-dependent manner leading to increased CCL19-responsiveness and activation of NK cells. European Journal of Immunology. 2010;40(11):3138–3149. doi: 10.1002/eji.201040496. [DOI] [PubMed] [Google Scholar]
- 53.Lande R., Giacomini E., Grassi T., et al. IFN-αβ released by Mycobacterium tuberculosis-infected human dendritic cells induces the expression of CXCL10: selective recruitment of NK and activated T cells. Journal of Immunology. 2003;170(3):1174–1182. doi: 10.4049/jimmunol.170.3.1174. [DOI] [PubMed] [Google Scholar]
- 54.Perona-Wright G., Mohrs K., Szaba F. M., et al. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host and Microbe. 2009;6(6):503–512. doi: 10.1016/j.chom.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiao L., Gao X., Joyee A. G., et al. NK cells promote type 1 T cell immunity through modulating the function of dendritic cells during intracellular bacterial infection. Journal of Immunology. 2011;187(1):401–411. doi: 10.4049/jimmunol.1002519. [DOI] [PubMed] [Google Scholar]
- 56.Shekhar S., Peng Y., Gao X., et al. NK cells modulate the lung dendritic cell-mediated Th1/Th17 immunity during intracellular bacterial infection. European Journal of Immunology. 2015;45(10):2810–2820. doi: 10.1002/eji.201445390. [DOI] [PubMed] [Google Scholar]
- 57.Yang X., Brunham R. C. T lymphocyte immunity in host defence against Chlamydia trachomatis and its implication for vaccine development. Canadian Journal of Infectious Diseases. 1998;9(2):99–109. doi: 10.1155/1998/395297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X. Role of cytokines in Chlamydia trachomatis protective immunity and immonopathology. Current Pharmaceutical Design. 2003;9(1):67–73. doi: 10.2174/1381612033392486. [DOI] [PubMed] [Google Scholar]
- 59.Han X., Fan Y., Wang S., Jiao L., Qiu H., Yang X. NK cells contribute to intracellular bacterial infection-mediated inhibition of allergic responses. The Journal of Immunology. 2008;180(7):4621–4628. doi: 10.4049/jimmunol.180.7.4621. [DOI] [PubMed] [Google Scholar]
- 60.Joyee A. G., Uzonna J., Yang X. Invariant NKT cells preferentially modulate the function of CD8α+ dendritic cell subset in inducing type 1 immunity against infection. Journal of Immunology. 2010;184(4):2095–2106. doi: 10.4049/jimmunol.0901348. [DOI] [PubMed] [Google Scholar]
- 61.Hook C. E., Telyatnikova N., Goodall J. C., et al. Effects of Chlamydia trachomatis infection on the expression of natural killer (NK) cell ligands and susceptibility to NK cell lysis. Clinical and Experimental Immunology. 2004;138(1):54–60. doi: 10.1111/j.1365-2249.2004.02596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tseng C.-T. K., Rank R. G. Role of NK cells in early host response to chlamydial genital infection. Infection and Immunity. 1998;66(12):5867–5875. doi: 10.1128/iai.66.12.5867-5875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]