Significance
Nearly all eukaryotic physiological events are regulated by protein kinases. Aberrations in functions of kinases are linked to numerous diseases. Significant progress has been made in dissecting the role of many kinases. However, understanding how specific kinases orchestrate complex signaling events remains challenging. Here we present a method to control the timing and the duration of kinase activity. This system provides the ability to mimic physiologically relevant transient activation of a kinase. We demonstrate the specificity and efficiency of this method by regulating tyrosine kinase (c-Src) and serine/threonine kinase (p38α). By using this approach, we identify morphological changes induced by transient activation of Src and demonstrate the role of sequential Src-PI3K and Src-Rac1 signaling in regulation of cell morphology.
Keywords: kinase, signaling, phosphorylation, synthetic biology, Src
Abstract
Physiological stimuli activate protein kinases for finite periods of time, which is critical for specific biological outcomes. Mimicking this transient biological activity of kinases is challenging due to the limitations of existing methods. Here, we report a strategy enabling transient kinase activation in living cells. Using two protein-engineering approaches, we achieve independent control of kinase activation and inactivation. We show successful regulation of tyrosine kinase c-Src (Src) and Ser/Thr kinase p38α (p38), demonstrating broad applicability of the method. By activating Src for finite periods of time, we reveal how the duration of kinase activation affects secondary morphological changes that follow transient Src activation. This approach highlights distinct roles for sequential Src-Rac1– and Src-PI3K–signaling pathways at different stages during transient Src activation. Finally, we demonstrate that this method enables transient activation of Src and p38 in a specific signaling complex, providing a tool for targeted regulation of individual signaling pathways.
Many physiological and pathological processes are regulated by protein kinases. Therefore, dissection of kinase functions helps to uncover molecular mechanisms and guides the development of new therapeutic strategies (1). However, progress in our understanding of kinase-mediated signaling is often hampered by the limitations of available tools (2). Pharmacological inhibitors of kinases have been valuable for the study of kinase functions, but they often do not provide the desired specificity and they are not available for the majority of kinases (1, 3). Also, the application of inhibitors is limited to the identification of processes affected by the inactivation of the kinase. Activation of a kinase is often achieved by the treatment of cells with growth factors or other extracellular stimuli. However, this approach triggers a multitude of parallel signaling pathways, which significantly complicates the analysis of individual kinase functions (4). Specific activation or inactivation of a kinase can be achieved by genetic modifications. However, this method is susceptible to compensatory artifacts and does not allow us to control the level and timing of kinase activation. Thus, development of novel tools that combine high specificity and tight temporal control of kinase activity remains a necessity. Under physiological conditions, kinases are activated for a finite period, and the duration of this activation is critical for eliciting specific biological outcomes (5, 6). Therefore, to mimic the biological activity of a kinase we need to use methods that allow for transient activation of a kinase with precise temporal control. An optogenetic approach has been successfully used for transient regulation of Raf kinase by dimerization (7). However, this method relies on the fact that Raf dimerization is required for its activation and thus has limited applicability to other kinases. Therefore, development of broadly applicable methods for tightly controlled transient activation of a specific kinase remains an important goal.
To achieve efficient and specific control of kinase activation and inactivation, we combined two protein-engineering strategies. Kinase activation was regulated using a recently developed protein-engineering method that employs a rapamycin-regulated allosteric switch, the iFKBP (a portion of the FKBP12 protein) domain (8). This strategy provides high specificity and temporal control of kinase activation in living cells. However, this approach results in persistent activation of a kinase without the ability for defined inactivation. To achieve independent control of kinase inactivation, we used a strategy developed by Shokat and colleagues that employs a functionally silent mutation that sensitizes the kinase to an allele-specific inhibitor, 1-Naphthyl-PP1 CAS 221243–82-9 (1NA-PP1) (9–17). We hypothesized that the combination of these two methods will enable the transient activation of the engineered kinase. We used c-Src (Src) and p38α (p38) kinases as models to test this hypothesis, optimize the method, and demonstrate its applicability to different types of kinases. Using this approach, we dissected signaling mediated by transient activation of Src and found that the same pathways play different roles during persistent Src activation.
Results
Engineering Src Kinase with Independent Activation and Inactivation Controls.
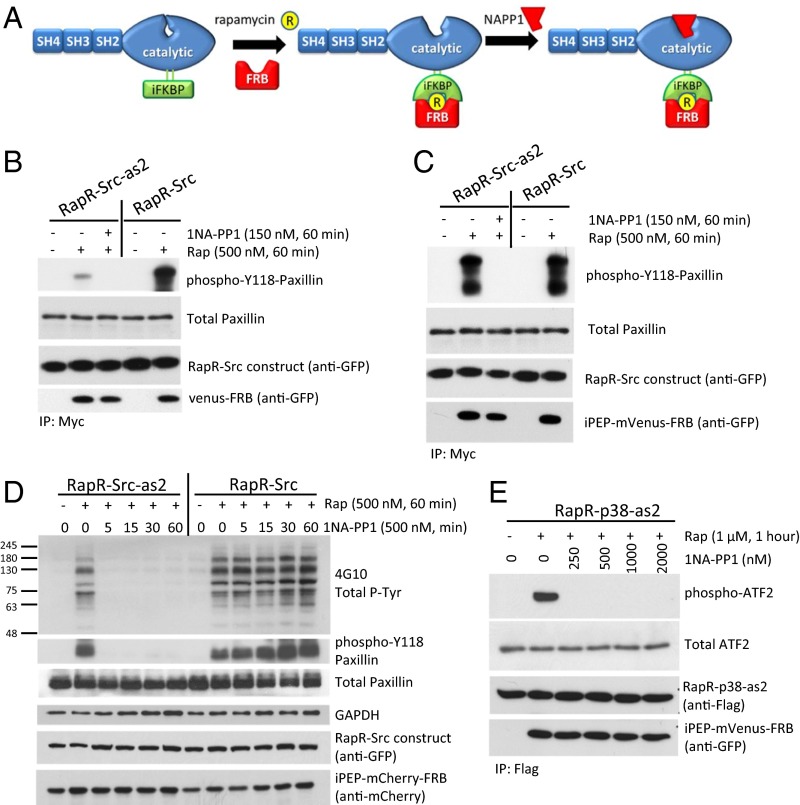
To activate Src, we used an engineered RapR-Src kinase construct that can be activated in living cells by addition of rapamycin or its nonimmunosuppressive analogs (8, 18, 19). RapR-Src contains a Tyr529-to-Phe mutation making it insensitive to inhibitory regulation by endogenous factors (8, 19). Introduction of this mutation in wild-type (WT) Src prevents inhibitory phosphorylation by Csk kinase, leading to generation of a constitutively active Src (20). In the context of RapR-Src, this property enables us to control Src activity only through engineered components. To gain independent control of kinase inactivation, we introduced a Thr338-to-Ala mutation in RapR-Src. The equivalent mutation (I338A) renders v-Src sensitive to 1NA-PP1 inhibitor (9, 15–17). Thus, we hypothesized that the T338A mutant of RapR-Src (RapR-Src-as2) would be sensitive to 1NA-PP1 inhibition and reverse rapamycin-induced activation (Fig. 1A). Indeed, RapR-Src-as2 is activated by rapamycin, and subsequent 1NA-PP1 treatment efficiently inhibits its activity (Fig. 1B).
Fig. 1.
Transient activation of engineered RapR-as2 kinases. (A) Schematic of RapR-Src-as2 regulation. (B and C) In vitro kinase assay of immunoprecipitated Src constructs. We cotransfected LinXE cells with either (B) mVenus-FRB or (C) iPEP-mVenus-FRB and indicated Src constructs bearing both a cerulean tag (recognized by GFP antibody) and a myc tag. Cells were treated with rapamycin (Rap, 500 nM) or ethanol (solvent) for 1 h and immunoprecipitated. Kinase assays were carried out in the presence of equivalent volumes of either DMSO (solvent) or 1NA-PP1 (150 nM). The purified N-terminal fragment of paxillin was used as a substrate. (D) Regulation of Src activity in living cells. LinXE cells were cotransfected with iPEP-mCherry-FRB and either RapR-Src-as2-cerulean-myc or RapR-Src-cerulean-myc. Transfected cells were treated with rapamycin (500 nM) or ethanol (solvent) for 1 h and subsequently with 1NA-PP1 (500 nM) for the designated amount of time. Cell lysates were analyzed for total tyrosine phosphorylation and endogenous paxillin phosphorylation. (E) LinXE cells transfected with Flag-RapR-p38-as2and iPEP-venus-FRB were activated with rapamycin (1 μM) for 1 h. Five minutes before collection cells were exposed to UV for 30 s. Flag-RapR-p38-as2 was immunoprecipitated and analyzed by an in vitro kinase assay using ATF-2 as a substrate. 1NA-PP1 or DMSO (solvent) was added at the indicated dose to the kinase reactions. (B–E) Data are representative of at least three independent experiments.
RapR-Src-as2 activation, however, was not as robust as activation of RapR-Src (Fig. 1B). This is consistent with previous observations where a similar reduction in catalytic activity was reported for analog-sensitive mutants of v-Src (21). We therefore sought to improve the catalytic activity of RapR-Src-as2 by modifying our approach for kinase activation. Prior studies suggested that iFKBP inactivates RapR-Src by increasing conformational dynamics of the catalytic domain, whereas the interaction with rapamycin and FRB (FKBP rapamycin binding domain) stabilizes RapR-Src and rescues its activity (8). Decreased activity of RapR-Src-as2 suggested that the T338A mutant may further destabilize the RapR-Src catalytic domain, thus reducing its affinity for rapamycin and/or FRB. We hypothesized that tighter binding of FRB to iFKBP would stabilize RapR-Src-as2 and improve its catalytic activity. The iFKBP domain was initially engineered by deletion of the first 15 amino acids of FKBP12 that comprise an additional β-strand in the structure (8) (SI Appendix, Fig. S1A). We predicted that the addition of these residues to the N terminus of FRB might enhance the interaction between FRB and iFKBP, leading to improved activation of RapR-Src and RapR-Src-as2 (SI Appendix, Fig. S1A). FRB bearing the first 15 amino acids of FKBP12 at the N terminus (iPEP-FRB) dramatically improved activation of RapR-Src-as2 (Fig. 1C). Addition of iPEP to FRB significantly improved binding to RapR-Src-as2 and resulted in higher kinase activity at lower rapamycin concentrations (SI Appendix, Fig. S1B), suggesting that T338A mutation affected RapR-Src-as2 activity by reducing affinity to rapamycin and/or FRB. In addition, iPEP-FRB was able to activate unmodified RapR-Src at lower doses of rapamycin than FRB without the iPEP, (SI Appendix, Fig. S1C), further supporting our hypothesis that iPEP improves interaction between FRB and iFKBP. Importantly, RapR-Src-as2 was still efficiently inhibited by 1NA-PP1 (Fig. 1C). Thus, we chose to use RapR-Src-as2 and iPEP-FRB for our studies because this newly engineered system enables both the robust activation and inactivation of Src signaling. We chose to use 500 nM rapamycin to ensure efficient activation of RapR-Src-as2 and to keep our experiments consistent with previously published studies (8, 19).
Next, we characterized the efficiency and the selectivity of RapR-Src-as2 regulation in vitro and in living cells. An in vitro analysis revealed complete inactivation of RapR-Src-as2 at concentrations of 1NA-PP1 as low as 50 nM (SI Appendix, Fig. S2A). Importantly, similar doses of 1NA-PP1 did not affect the activity of nonmodified RapR-Src or a constitutively active mutant of c-Src (SI Appendix, Fig. S2 B and C). Activation of RapR-Src-as2 in living cells led to a dramatic increase in phosphorylation of the endogenous Src substrate paxillin and an increase in the level of tyrosine phosphorylated proteins (Fig. 1D). RapR-Src-as2 was overexpressed at a two- to threefold higher level than endogenous Src (SI Appendix, Fig. S3A). A substantial reduction in phosphorylation was observed upon addition of 250 nM and 500 nM 1NA-PP1, indicating efficient inactivation of RapR-Src-as2 (Fig. 1D and SI Appendix, Fig. S3B). Importantly, similar concentrations did not reduce the activity of unmodified RapR-Src, verifying the selectivity of 1NA-PP1 toward the analog-specific mutant (Fig. 1D and SI Appendix, Fig. S3B). Inactivation of RapR-Src-as2 was fast. Treatment with 1NA-PP1 for only 5 min reduced protein phosphorylation to the levels preceding Src activation (Fig. 1D). Phosphorylation levels of paxillin and p130Cas and total protein phosphorylation remained at the same reduced levels for at least 1 h after addition of 1NA-PP1 (Fig. 1D and SI Appendix, Fig. S3C), indicating sustained inactivation of RapR-Src-as2. These data demonstrate efficient regulation of Src activity in vitro and in living cells using RapR-Src-as2 and iPEP-FRB.
Transient Regulation of Src Activity in Specific Signaling Complexes.
RapR-Src can be activated in specific protein complexes when the FRB domain is fused to a selected protein using the “rapamycin-regulated targeted activation of pathways” (RapRTAP) method (19), which enables activation of individual signaling pathways downstream of Src. However, subsequent inactivation of these pathways was not achievable with the RapRTAP approach. Therefore, we evaluated whether RapR-Src-as2 could be used to turn on and off Src activity in a specific complex. Targeted activation of Src in complex with its known binding partner and substrate, paxillin, was used as the model (SI Appendix, Fig. S4A). Association of Src with other proteins is primarily mediated by domains N-terminal to its catalytic domain (SH2, SH3, SH4) (22, 23). To prevent Src interaction with endogenous binding partners, we used only the catalytic domain of RapR-Src-as2 (RapR-SrcCat-as2). This approach limits Src signaling to only the paxillin complex. iPEP-FRB was added to the N terminus of paxillin to mediate binding and activation of RapR-SrcCat-as2 (SI Appendix, Fig. S4A). Targeted activation of RapR-SrcCat-as2 induced phosphorylation of iPEP-FRB-paxillin (SI Appendix, Fig. S4 B and C), and subsequent addition of 1NA-PP1 led to its dephosphorylation (SI Appendix, Fig. S4 B and C). Thus, RapR-SrcCat-as2 can be used for activation and inactivation of Src-mediated signaling in specific protein complexes.
Engineered Transient Activation of p38 Kinase.
To explore the general applicability of this method, we tested whether we could transiently activate a Ser/Thr kinase. We previously demonstrated activation of p38 using the RapR method (8). To achieve independent control of inactivation, we mutated residue Thr106 to an Ala in RapR-p38, generating RapR-p38-as2. To our knowledge, analog-sensitive p38 has not been generated previously, and its inhibition by 1NA-PP1 has not been demonstrated. Activation and inactivation of RapR-p38-as2 was tested in vitro using purified ATF-2, a known p38 substrate (8, 24). The in vitro kinase assay shows that RapR-p38-as2 activity was specifically inactivated by 1NA-PP1 (Fig. 1E). Importantly, activity of RapR-p38 and WT p38 was not affected using similar concentrations of 1NA-PP1 (SI Appendix, Fig. S5). Activation of RapR-p38-as2 in living cells led to elevated levels of phosphorylated ATF2, whereas subsequent inactivation reduced ATF2 phosphorylation (SI Appendix, Fig. S6A). These results demonstrate successful transient activation of engineered p38 in vitro and in living cells and suggest that this tool can be applied for regulation of both Tyr and Ser/Thr kinases.
To demonstrate targeted regulation of RapR-p38-as2 in living cells, we generated an ATF2 construct bearing iPEP-FRB at the N terminus (iPEP-mVenus-FRB-ATF2). Treatment of cells with rapamycin induced binding of RapR-p38-as2 to the engineered ATF2 and stimulated its phosphorylation. Addition of 1NA-PP1 resulted in a significant reduction in phospho-ATF2 levels (SI Appendix, Fig. S6B). These results demonstrate transient activation of p38 in complex with ATF2 in living cells.
Regulation of Morphological Changes Using Transient Stimulation of Src.
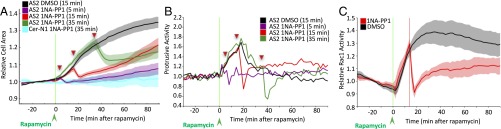
Previously developed technologies did not allow us to assess the effect of transient Src activation on cell morphological changes. We can overcome this limitation by using RapR-Src-as2. Therefore, we evaluated how activation and inactivation of RapR-Src-as2 affects Src-induced cell morphological changes. Prior studies demonstrated that RapR-Src localization is not perturbed by the engineered modification (19). RapR-Src-as2 also exhibited similar localization patterns to RapR-Src and WT Src. Inactive Src localized primarily to the perinuclear area, whereas activation of Src stimulated its translocation to the plasma membrane and focal adhesions (SI Appendix, Fig. S7). Sustained activation of RapR-Src-as2 in HeLa cells induced robust cell spreading and transient stimulation of protrusive activity (Fig. 2 A and B and SI Appendix, Figs. S8 and S9A) in agreement with our previously published results (8, 19). A substantial increase in paxillin phosphorylation was detected 5 min after addition of rapamycin, indicating fast activation of RapR-as2 in living cells (SI Appendix, Fig. S9B). Treatment with 1NA-PP1 after rapamycin resulted in an immediate reduction in cell area and inhibition of protrusive activity (Fig. 2 A and B and Movie S1). By adding 1NA-PP1 at different time points after Src activation, we were capable of inducing Src-mediated cell spreading and protrusive activity for different periods of time (Fig. 2 A and B and SI Appendix, Fig. S8 C–E). These results demonstrate the capability of our approach to regulate the extent and duration of Src-mediated morphological changes. Treatment of cells expressing RapR-Src-as2 with DMSO (solvent control) (SI Appendix, Fig. S8A) or treatment of cells expressing unmodified RapR-Src with 1NA-PP1 (SI Appendix, Fig. S8B) did not affect Src-induced cell-area changes, indicating that the reversal of morphology was specific to inactivation of RapR-Src-as2.
Fig. 2.
Regulation of Src-mediated morphological changes and Rac1 activity. (A and B) HeLa cells transfected with either cerulean-myc–tagged RapR-Src-as2 or cerulean-N1, iPEP-mCherry-FRB, and Stargazin-mVenus were imaged live. Stargazin-mVenus imaging was used to evaluate cell area and protrusive activity changes. Cells were treated with rapamycin (500 nM) (green arrowhead/line) and then treated with either DMSO (15 min) or 1NA-PP1 (250 nM) at the indicated time (red arrowhead/triangles). (A) Quantification of cell-area changes. (B) Quantification of changes in protrusive activity. Confidence intervals were omitted for clarity, but they are presented in SI Appendix, Fig. S8. (C) HeLa cells transfected with RapR-Src-as2-mCherry-myc and Rac1 FLARE biosensor and infected with adenovirus expressing iPEP-GFP(Y66S)-FRB (nonfluorescent GFP mutant) were imaged live. Cells were treated with rapamycin (500 nM) (green line), and 15 min later cells were treated with either DMSO (solvent) or 1NA-PP1 (250 nM) (red line). Quantification of Rac1 activity over time. Rac1 activity was assessed using Rac1 FLARE biosensor (FRET/CFP signal) in living cells. (A and C) Shading represents 90% confidence intervals.
Inactivation of RapR-Src-as2 by 1NA-PP1 reversed Src-induced phenotypes. However, initial reduction in cell area and protrusive activity was followed by a slow and steady cell spreading and increased protrusive activity (Fig. 2 A and B and SI Appendix, Figs. S8D and S9A). These secondary morphological changes were not caused by restoration of RapR-Src-as2 activity after inactivation (Fig. 1D and SI Appendix, Fig. S3C). Additionally, the extent of secondary changes depended on the duration of Src activation. Activation of RapR-Src-as2 for 15 min was followed by significantly faster cell spreading and greater protrusive activity than stimulation for 5 or 35 min (SI Appendix, Figs. S8 C–E and S9 C and D). Thus, using this methodology for controlling the duration of kinase signaling, we identified optimal conditions for the induction of specific morphological changes independent of continuous kinase activation.
Dissecting Src-Mediated Signaling Pathways Using Engineered Transient Activation of Src.
Our results suggest that transient Src activation induces additional signaling pathways capable of stimulating slow cell spreading and protrusive activity independently of Src activity. Slow spreading that follows transient Src activation could be mediated by an increased formation of membrane protrusions. One of the key pathways for the stimulation of membrane protrusions is signaling through small GTPase Rac1 (25, 26). To assess changes in Rac1 activity mediated by regulation of RapR-Src-as2, we used a previously described biosensor for Rac1 (27–29). Our studies reveal that activation of RapR-Src-as2 leads to robust stimulation of Rac1 at the cell periphery (SI Appendix, Fig. S10 and Movie S2). Inactivation of RapR-Src-as2 caused fast down-regulation of Rac1 activity that was partially restored at the later time points (Fig. 2C; SI Appendix, Fig. S10 and Movie S3). Importantly, the secondary increase in Rac1 activity following 1NA-PP1 treatment occurred concurrently with the increase in protrusive activity (Fig. 2C and SI Appendix, Fig. S8D). These data demonstrate regulation of Rac1 by RapR-Src-as2 and show a dynamic correlation between Src-induced changes in Rac1 activity and cell morphodynamics.
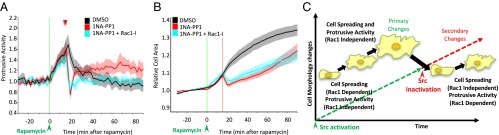
To test the role of Rac1-induced protrusive activity, we treated cells with an inhibitor of Rac1 activation, CAS 1177865–17-6 (30). Rac1 inhibition at the time of Src inactivation suppressed secondary protrusive activity (Fig. 3A and SI Appendix, Fig. S11). However, inhibition of protrusive activity did not affect secondary cell spreading that followed transient Src activation (Fig. 3B). Strikingly, inhibition of Rac1 at the time of Src activation had the opposite effect: protrusive activity was not affected, whereas cell spreading was significantly inhibited (SI Appendix, Fig. S12). Inhibition of Rac1 15 min after activation of Src did not have a significant effect on either cell spreading or protrusive activity, suggesting that, during Src activation, Rac1 is needed only to maintain the initial rate of spreading (SI Appendix, Fig. S12 A and B). These data demonstrate that Rac1 plays different roles during Src activation and following transient stimulation of Src (Fig. 3C).
Fig. 3.
Dissecting the role of Rac1 in the regulation of Src mediated morphological changes. Effect of Rac1 inhibition on Src mediated (A) protrusive activity and (B) cell spreading. HeLa cells transfected with: RapR-Src-as2-cerulean-myc, iPEP-mCherry-FRB, and Stargazin-mVenus were imaged live. Stargazin-mVenus images were used to assess cell area and protrusive activity. (A and B) Cells were treated with rapamycin (500 nM) (green line), and 15 min later cells were treated with either DMSO (solvent), 1NA-PP1 (250 nM), or 1NA-PP1+Rac1-I (1NA-PP1+CAS 1177865–17-6, 250 nM + 100 μM) as indicated by red triangle or line. All error bars/shading represent 90% confidence intervals. (C) Schematic depicting different roles for Rac1 signaling at different stages upon transient activation of Src.
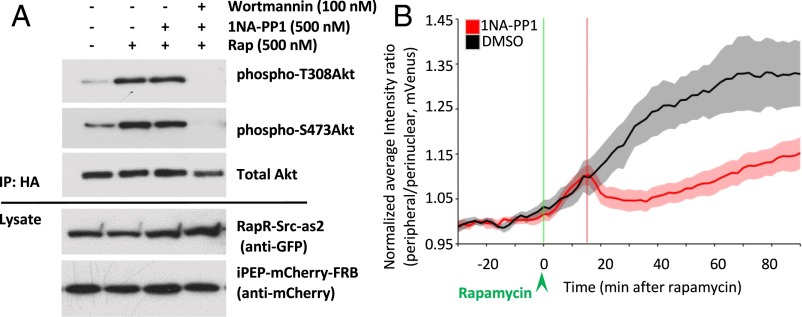
Our data suggest that signaling events upstream of Rac1 control the cell spreading following transient activation of Src. The PI3-kinase/Akt pathway is known to be stimulated by Src and plays an important role in the regulation of cell morphology (31, 32). PI3-kinase (PI3K) also stimulates Rac1 and membrane protrusions (33–35). Furthermore, the PI3K/Akt pathway can induce cell spreading independently of Src (32). We detected a significant increase in Akt phosphorylation following activation of RapR-Src-as2, indicating stimulation of PI3K/Akt signaling (36–38) (Fig. 4A). Cells lacking RapR-Src-as2 did not exhibit changes in Akt phosphorylation after rapamycin treatment, verifying that PI3K/Akt stimulation was specific to the activation of Src (SI Appendix, Fig. S13). Akt phosphorylation was not affected by treatment with 1NA-PP1, suggesting that transient activation of Src induces sustained PI3K/Akt signaling following Src inactivation (Fig. 4A). To elucidate dynamic changes in PI3K activity in living cells, we monitored distribution of the Akt pleckstrin homology (PH) domain upon activation and inactivation of RapR-Src-as2. Previous studies demonstrated that translocation of the Akt PH domain from a cytoplasmic/perinuclear to a peripheral/plasma membrane location indicate the production of phosphatidylinositol (3–5) trisphosphate [PI(3,4,5)P3] generated by activated PI3K (39). Our results show that activation of RapR-Src-as2 stimulates redistribution of the Akt PH domain from a perinuclear to a peripheral location, supporting our biochemical evidence of PI3K activation (SI Appendix, Fig. S14 and Movie S4). Subsequent inactivation of RapR-Src-as2 leads to transient perinuclear accumulation of the Akt PH domain that is followed by redistribution to the cell periphery (SI Appendix, Fig. S14 and Movie S5). Quantitative analysis of Akt PH domain localization supports this observation (Fig. 4B) and suggests that transient activation of RapR-Src-as2 is followed by partial reduction in PI(3,4,5)P3 levels that is later restored. Interestingly, dynamics of Akt PH domain localization mirrors changes in cell spreading (Figs. 2A and 4B), demonstrating correlation between PI3K signaling and Src-induced morphological changes.
Fig. 4.
Regulation of PI3K signaling by RapR-Src-as2. (A) Akt phosphorylation. LinXE cells coexpressing cerulean-myc–tagged RapR-Src-as2, iPEP-mCherry-FRB, and HA-Akt were treated with rapamycin (500 nM) or ethanol (solvent) for 35 min and then with 1NA-PP1 (500 nM), 1NA-PP1 + Wortmannin (500 nM, 100 nM), or DMSO for 30 min. HA-Akt was immunoprecipitated and analyzed for phosphorylation at Thr308 and Ser473. Data are representative of three independent experiments. (B) Changes in peripheral/perinuclear mVenus-PH-AKT localization over time. HeLa cells, expressing RapR-Src-as2-cerulean-myc (adenovirus infection), mVenus-PH-AKT, and iPEP-mCherry-FRB (transfection) were imaged live. Cells were treated with rapamycin (500 nM) (green line), and 15 min later cells were treated with either DMSO (solvent) or 1NA-PP1 (250 nM) (red line). Shading represents 90% confidence intervals.
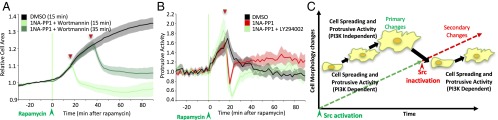
To test if the PI3K/Akt pathway mediates cell spreading after Src inhibition, HeLa cells were treated with the PI3K inhibitors Wortmannin and LY294002 (40, 41). Addition of these inhibitors at the same time as 1NA-PP1 dramatically reduced Akt phosphorylation, abolished cell spreading, and reduced protrusive activity after Src inactivation (Figs. 4A and 5 A and B; SI Appendix, Fig. S15). These data demonstrate that transient activation of Src induces downstream PI3K/Akt signaling, which mediates cell spreading after Src inactivation.
Fig. 5.
Dissecting the role of PI3K in the regulation of Src-mediated morphological changes. (A and B) Effect of PI3K inhibitor on cell spreading (A) and protrusive activity (B). HeLa cells transfected with RapR-Src-as2-cerulean-myc, iPEP-mCherry-FRB, and Stargazin-mVenus were imaged live before and after the addition of rapamycin (green line). Stargazin-mVenus images were used to evaluate changes in cell area and protrusive activity. (A) Cells were treated with DMSO (solvent) or 1NA-PP1+Wortmannin (250 nM, 100 nM) at the indicated time points (red triangles) after addition of rapamycin. (B) Cells were treated with the DMSO (solvent), 1NA-PP1 (250 nM), or 1NA-PP1+LY294002 (250 nM + 50 μM) (red triangle) 15 min after rapamycin treatment. (C) Schematic depicting different roles for PI3K signaling at different stages upon transient activation of Src.
We also found that PI3K plays different roles during Src activation. Inhibition of PI3K at the time of Src activation prevented Src-mediated cell spreading and delayed stimulation of protrusive activity (SI Appendix, Fig. S16). Addition of PI3K inhibitor 15 min after Src activation had no effect on either cell spreading or protrusive activity (SI Appendix, Fig. S16). Combined, our data suggest that PI3K plays different roles at three different stages during transient Src activation (Fig. 5C). At the time of Src activation PI3K is required for stimulation of cell spreading and protrusive activity. Once Src has been active for at least 15 min, PI3K becomes dispensable for Src-mediated morphological changes. Following Src inactivation, PI3K takes control of secondary morphological changes.
Discussion
Our study describes an approach for transient activation of kinases in living cells. This method enables the precise temporal regulation of kinase activation and inactivation. Furthermore, this method allows for control of the duration of kinase activation and identification of optimal conditions for specific kinase-induced morphological effects. Using this approach, we identified distinct roles for Rac1 and PI3K signaling in mediating morphological changes during Src activation and following its inactivation. By regulating the catalytic activity of Src in complex with paxillin and p38 activity in complex with ATF2, we demonstrated that this approach can be used to induce transient activation of specific signaling complexes.
Successful regulation of Tyr kinase Src and Ser/Thr kinase p38 and previous studies suggest that this strategy could be broadly applicable for the regulation of different kinases in living cells. The analog-sensitive approach has been successfully used for more than 80 different kinases from different classes and different species (17). RapR analogs of six different Tyr and Ser/Thr kinases have been reported (8). Importantly, activation and inactivation directly targets the catalytic domain without affecting other functions of the protein (8, 18, 19). The main components of this system are genetically encoded, and regulation is achieved by using cell-permeable compounds. Our previous studies show that activation of RapR kinases can be achieved using nonimmunosuppressive analogs of rapamycin (8, 18). Because 1NA-PP1 has very low affinity toward endogenous kinases, this approach will have minimal off-target effects on endogenous signaling pathways (9–17).
We demonstrate that this approach for regulation of kinases can be used to dissect dynamics of signaling processes mediated by transient kinase activation. Our studies show that the same signaling component can play different roles at different stages during kinase activation and following its inactivation. Upon Src activation, Rac1 signaling preferentially affects cell spreading but not protrusive activity, suggesting that, at the initial stage of Src activation, Rac1 is more important for establishing cell contacts with the extracellular matrix. This result correlates with previously reported observations of Rac1-independent protrusion formation in MTLn3 and Rac1-dependent focal adhesion formation and migration (42–44). Our results show that the function of PI3K is important for Src-induced cell spreading and protrusive activity upon Src activation and following its inactivation. However, neither Rac1 nor PI3K activity was critical at later stages during Src activation (15 min), suggesting that prolonged activation can drive morphological transformation independently of Rac1 and PI3K. This is in agreement with previously reported studies showing that inhibition of PI3K signaling does not block v-Src–induced transformation (45). A different report suggests that Rac1 activity is required for v-Src–mediated transformation (46). However, in these experiments dominant negative Rac1 was cotransfected together with v-Src, supporting our evidence that inhibition of Rac1 at the time of Src activation suppresses Src-induced morphological changes. Overall, our studies show that engineered control of kinase activity for finite periods of time provides opportunities for dissection of phosphorylation-mediated signaling in living cells.
Materials and Methods
DNA Constructs.
RapR-Src-cerulean-myc (19), GFP-FRB (8), and RapR-p38α (8) have already been described. Rac1-FLARE and the control plasmids expressing Y-PET and Turq (29) were gifts from Klaus Hahn, University of North Carolina, Chapel Hill, NC. HA-Akt1 construct was a gift from John O’Bryan, University of Illinois-Chicago, Chicago. PH-AKT-mVenus was generated from PH-Akt-GFP, a gift from Wonhwa Cho, University of Illinois, Chicago. Flag-ATF2 (a gift from Bruce Cuevas, Loyola University, Chicago). The cerulean-N1 vector is from AddGene (#54742). Adenoviruses for expression of RapR-Src-as2-Cerulean and iPEP-GFP(Y66S)-FRB were generated at Vector Development Core, Loyola University. Generation of all other DNA constructs is described in detail in SI Appendix, SI Materials and Methods.
Cell Imaging and Image Analysis.
HeLa cells were plated on fibronectin-coated coverslips at 5 mg/L and incubated for 2–4 h. Before imaging, coverslips were placed into an Attufluor Cell Chamber (Invitrogen, catalog no. A78-16) with Leibovitz (L-15) medium (Sigma Aldrich, catalog no. L1518-500mL) supplemented with 5% (vol/vol) FBS. Live-cell imaging was done using an Olympus IX-83 microscope controlled by Metamorph software and equipped with a heated stage (Warner Instruments), Olympus UPlanSAPO 40× (oil, N.A. 1.25) objective, Xcite 120 LED (Lumen Dynamics) light source, and Image EMX2 CCD (Hamamatsu) camera. Analyses of cell area, protrusive activity, Rac1-FLARE biosensor, and localization of PH-Akt-mVenus are described in SI Appendix, SIl Materials and Methods.
Antibodies and reagents, cell culture, immunoprecipitation, DNA cloning, image analysis, and kinase assays are described in SI Appendix, SI Materials and Methods . The data analysis description and the numbers of cells imaged for each experiment are listed in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Jeff Klomp for technical assistance and advice and Dr. Jalees Rehman and Dr. John O’Bryan for discussions, advice, and comments on the manuscript. This work was funded by the NIH, the Chicago Biomedical Consortium (CBC), and the University of Illinois at Chicago Center for Clinical and Translational Science (UIC CCTS) for funding (R21CA159179 and a CBC Pilot Grant) (to A.V.K.), NIH Grant T32 HL007829-22 (to A.B.M.), and UIC CCTS Grant UL1TR000050 (to J.E.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609675114/-/DCSupplemental.
References
- 1.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garske AL, Peters U, Cortesi AT, Perez JL, Shokat KM. Chemical genetic strategy for targeting protein kinases based on covalent complementarity. Proc Natl Acad Sci USA. 2011;108(37):15046–15052. doi: 10.1073/pnas.1111239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lampson MA, Kapoor TM. Unraveling cell division mechanisms with small-molecule inhibitors. Nat Chem Biol. 2006;2(1):19–27. doi: 10.1038/nchembio757. [DOI] [PubMed] [Google Scholar]
- 4.Perona R. Cell signalling: Growth factors and tyrosine kinase receptors. Clin Transl Oncol. 2006;8(2):77–82. doi: 10.1007/s12094-006-0162-1. [DOI] [PubMed] [Google Scholar]
- 5.Hunter T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell. 1995;80(2):225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 6.Marshall CJ. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 7.Wend S, et al. Optogenetic control of protein kinase activity in mammalian cells. ACS Synth Biol. 2014;3(5):280–285. doi: 10.1021/sb400090s. [DOI] [PubMed] [Google Scholar]
- 8.Karginov AV, Ding F, Kota P, Dokholyan NV, Hahn KM. Engineered allosteric activation of kinases in living cells. Nat Biotechnol. 2010;28(7):743–747. doi: 10.1038/nbt.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop AC, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407(6802):395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 10.Weiss EL, Bishop AC, Shokat KM, Drubin DG. Chemical genetic analysis of the budding-yeast p21-activated kinase Cla4p. Nat Cell Biol. 2000;2(10):677–685. doi: 10.1038/35036300. [DOI] [PubMed] [Google Scholar]
- 11.Fan Q-W, Zhang C, Shokat KM, Weiss WA. Chemical genetic blockade of transformation reveals dependence on aberrant oncogenic signaling. Curr Biol. 2002;12(16):1386–1394. doi: 10.1016/s0960-9822(02)01070-9. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin KR, Zhang C, Shokat KM, Herskowitz I. Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev. 2003;17(12):1524–1539. doi: 10.1101/gad.1101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okuzumi T, et al. Inhibitor hijacking of Akt activation. Nat Chem Biol. 2009;5(7):484–493. doi: 10.1038/nchembio.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Au-Yeung BB, et al. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat Immunol. 2010;11(12):1085–1092. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop AC, et al. Design of allele-specific inhibitors to probe protein kinase signaling. Curr Biol. 1998;8(5):257–266. doi: 10.1016/s0960-9822(98)70198-8. [DOI] [PubMed] [Google Scholar]
- 16.Bishop AC, et al. Generation of monospecific nanomolar tyrosine kinase inhibitors via a chemical genetic approach. J Am Chem Soc. 1999;121(4):627–631. [Google Scholar]
- 17.Zhang C, et al. Structure-guided inhibitor design expands the scope of analog-sensitive kinase technology. ACS Chem Biol. 2013;8(9):1931–1938. doi: 10.1021/cb400376p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karginov AV, Hahn KM. 2011. Allosteric activation of kinases: Design and application of RapR kinases. Curr Protoc Cell Biol Chapter 14:Unit 14.13.
- 19.Karginov AV, et al. Dissecting motility signaling through activation of specific Src-effector complexes. Nat Chem Biol. 2014;10(4):286–290. doi: 10.1038/nchembio.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kmiecik TE, Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- 21.Kraybill BC, Elkin LL, Blethrow JD, Morgan DO, Shokat KM. Inhibitor scaffolds as new allele specific kinase substrates. J Am Chem Soc. 2002;124(41):12118–12128. doi: 10.1021/ja0264798. [DOI] [PubMed] [Google Scholar]
- 22.Cooper JA, Howell B. The when and how of Src regulation. Cell. 1993;73(6):1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- 23.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 24.Gerwins P, Blank JL, Johnson GL. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J Biol Chem. 1997;272(13):8288–8295. doi: 10.1074/jbc.272.13.8288. [DOI] [PubMed] [Google Scholar]
- 25.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 26.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114(Pt 15):2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 27.Kraynov VS, et al. Localized Rac activation dynamics visualized in living cells. Science. 2000;290(5490):333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- 28.Machacek M, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461(7260):99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacNevin CJ, et al. Ratiometric imaging using a single dye enables simultaneous visualization of Rac1 and Cdc42 activation. J Am Chem Soc. 2016;138(8):2571–2575. doi: 10.1021/jacs.5b09764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dwivedi S, Pandey D, Khandoga AL, Brandl R, Siess W. Rac1-mediated signaling plays a central role in secretion-dependent platelet aggregation in human blood stimulated by atherosclerotic plaque. J Transl Med. 2010;8:128. doi: 10.1186/1479-5876-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantley LC, et al. Oncogenes and signal transduction. Cell. 1991;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- 32.Zeller KS, et al. PI3-kinase p110α mediates β1 integrin-induced Akt activation and membrane protrusion during cell attachment and initial spreading. Cell Signal. 2010;22(12):1838–1848. doi: 10.1016/j.cellsig.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Hawkins PT, et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5(4):393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 34.Ma AD, Metjian A, Bagrodia S, Taylor S, Abrams CS. Cytoskeletal reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinase gamma, a Rac guanosine exchange factor, and Rac. Mol Cell Biol. 1998;18(8):4744–4751. doi: 10.1128/mcb.18.8.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welch HCE, Coadwell WJ, Stephens LR, Hawkins PT. Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 2003;546(1):93–97. doi: 10.1016/s0014-5793(03)00454-x. [DOI] [PubMed] [Google Scholar]
- 36.Alessi DR, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 37.Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279(39):41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 38.Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4(9):a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao X, et al. PI3K/Akt signaling requires spatial compartmentalization in plasma membrane microdomains. Proc Natl Acad Sci USA. 2011;108(35):14509–14514. doi: 10.1073/pnas.1019386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269(7):5241–5248. [PubMed] [Google Scholar]
- 41.Wymann MP, et al. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16(4):1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yip S-C, et al. The distinct roles of Ras and Rac in PI 3-kinase-dependent protrusion during EGF-stimulated cell migration. J Cell Sci. 2007;120(Pt 17):3138–3146. doi: 10.1242/jcs.005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Sibai M, et al. RhoA/ROCK-mediated switching between Cdc42- and Rac1-dependent protrusion in MTLn3 carcinoma cells. Exp Cell Res. 2008;314(7):1540–1552. doi: 10.1016/j.yexcr.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li A, Machesky LM. Melanoblasts on the move: Rac1 sets the pace. Small GTPases. 2012;3(2):115–119. doi: 10.4161/sgtp.19494. [DOI] [PubMed] [Google Scholar]
- 45.Penuel E, Martin GS. Transformation by v-Src: Ras-MAPK and PI3K-mTOR mediate parallel pathways. Mol Biol Cell. 1999;10(6):1693–1703. doi: 10.1091/mbc.10.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Servitja J-M, Marinissen MJ, Sodhi A, Bustelo XR, Gutkind JS. Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src. J Biol Chem. 2003;278(36):34339–34346. doi: 10.1074/jbc.M302960200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.