Significance
The combination of emissions cuts and solar geoengineering could reduce climate risks in ways that cannot be achieved by emissions cuts alone: It could keep Earth under the 1.5-degree mark agreed at Paris, and it might stop sea level rise this century. However, this promise comes with many risks. Injection of sulfuric acid into the stratosphere, for example, would damage the ozone layer. Injection of calcite (or limestone) particles rather than sulfuric acid could counter ozone loss by neutralizing acids resulting from anthropogenic emissions, acids that contribute to the chemical cycles that destroy stratospheric ozone. Calcite aerosol geoengineering may cool the planet while simultaneously repairing the ozone layer.
Keywords: climate change, geoengineering, stratospheric ozone, climate engineering, atmospheric chemistry
Abstract
Injecting sulfate aerosol into the stratosphere, the most frequently analyzed proposal for solar geoengineering, may reduce some climate risks, but it would also entail new risks, including ozone loss and heating of the lower tropical stratosphere, which, in turn, would increase water vapor concentration causing additional ozone loss and surface warming. We propose a method for stratospheric aerosol climate modification that uses a solid aerosol composed of alkaline metal salts that will convert hydrogen halides and nitric and sulfuric acids into stable salts to enable stratospheric geoengineering while reducing or reversing ozone depletion. Rather than minimizing reactive effects by reducing surface area using high refractive index materials, this method tailors the chemical reactivity. Specifically, we calculate that injection of calcite (CaCO3) aerosol particles might reduce net radiative forcing while simultaneously increasing column ozone toward its preanthropogenic baseline. A radiative forcing of −1 W⋅m−2, for example, might be achieved with a simultaneous 3.8% increase in column ozone using 2.1 Tg⋅y−1 of 275-nm radius calcite aerosol. Moreover, the radiative heating of the lower stratosphere would be roughly 10-fold less than if that same radiative forcing had been produced using sulfate aerosol. Although solar geoengineering cannot substitute for emissions cuts, it may supplement them by reducing some of the risks of climate change. Further research on this and similar methods could lead to reductions in risks and improved efficacy of solar geoengineering methods.
Deliberate introduction of aerosol into the stratosphere, a form of solar geoengineering or Solar Radiation Management (SRM), may reduce impacts of climate change, including regional changes in precipitation and surface temperature (1, 2), by partially and temporarily (3) offsetting radiative forcing from greenhouse gases. The most salient direct risk of sulfate aerosol, the material most commonly proposed for SRM, is stratospheric ozone loss (4, 5). In a 2006 paper (5) that triggered recent interest in SRM, Crutzen suggested that soot particles could warm the polar stratosphere and “thereby hinder the formation of ozone holes.” Crutzen’s approach rests on manipulating radiative properties to indirectly influence chemistry, whereas here we propose that use of solid aerosol composed of alkaline metal salts that react with hydrogen halides and nitric and sulfuric acid to form stable salts may enable stratospheric SRM while allowing partially independent manipulation of the catalytic reactions that control stratospheric ozone. In contrast to Crutzen’s approach, this provides a direct chemical method that may restore ozone concentrations that are reduced by anthropogenic NOx and halogens while simultaneously providing SRM radiative forcing with minimal stratospheric heating.
Three acids, HNO3, HCl, and HBr, serve as reservoirs for the nitrogen, NOx, chlorine, ClOx, and bromine, BrOx, radical families that participate in coupled catalytic cycles that destroy ozone. Sulfuric acid, H2SO4, can accelerate ozone loss by forming aqueous aerosol that catalyzes reactions such as HCl + ClONO2 → Cl2 + HNO3, shifting halogens from reservoir species to reactive compounds and altering the NOx budget via hydrolysis of N2O5 to HNO3. Previous studies found that injection of sufficient SO2 or particulate sulfate to produce −2 W⋅m−2 of radiative forcing—a useful benchmark for SRM—reduced average column ozone by 1 to 13% (2, 6–8).
The use of solid, high refractive index, aerosol particles for SRM was first suggested (9) in the 1990s. That work and most subsequent analyses focused on the mass-specific scattering efficiency, with the implication that solid aerosol might be able to reduce the total mass required for SRM (9–12). In prior work (2), we explored the possibility that solid aerosol might reduce important environmental risks of SRM including (i) heating of the lower stratosphere, (ii) diffuse scattering of incident sunlight, and (iii) ozone loss. Using a model that included the microphysical interactions of solid aerosol with natural background sulfate aerosol, we found that, although some ozone impacts came from reactions such as HCl + ClONO2 on hydrophilic oxide surfaces, the dominant impact was from an increase in sulfuric acid surface as the preexisting background sulfuric acid was distributed over a large surface area of solid particles (2).
Here we consider the possibility of using alkaline (basic) salts of group 1 and 2 metals, such as Na and Ca, to neutralize the acids involved in the catalytic destruction of ozone. These metals might be introduced to the stratosphere in metallic form, as oxides, or as salts formed with weak acids such as carbonic acid. Gas-phase acids will then react to form neutral, solid salts such as NaCl, Ca(NO3)2, and Na2SO4 that are stable in the stratosphere. The surfaces of these salts have low rates for acid-catalyzed reactions, as they are neutral, and do not contribute to bulk hydrolysis reactions, as they are solid.
Particles composed of, or coated with, alkaline compounds might reduce ozone loss through reaction with the background sulfate aerosol or reaction with gas-phase acids. The rate of the first mechanism depends on the flux of H2SO4 onto particles by coalescence or condensation. The liquid−solid reaction will rapidly neutralize the acid to form a dry salt if there is sufficient base. The rate of the second mechanism will depend on the kinetics of the gas−solid reactions.
As a specific example, we model the use of calcite (CaCO3) aerosol for SRM using an extension of the model we developed for solid aerosols such as alumina and diamond (2). We simulate a monodisperse 275-nm-radius calcite aerosol injected uniformly between 20 km and 25 km altitude within 30 degrees of the equator. We use a 2D chemical transport and aerosol microphysics model that includes a prognostic size distribution for three categories of aerosol: liquid aerosol, solid aerosol, and liquid-coated solid aerosol (Methods). As we mention in Discussion, however, many of the rate constants have significant uncertainty. Radiative forcing is computed using a high-resolution band model (Methods).
Results
Sulfate aerosol warms the lower stratosphere, which would likely increase the flux of water vapor through the tropical tropopause. Once in the stratosphere, the additional water vapor can accelerate ozone loss and will add to the radiative forcing of climate, offsetting some of the intended benefit of adding the sulfates. Heating of the lower stratosphere is therefore a significant contributor to the risks of sulfate aerosol SRM. Calcite may reduce these risks because it causes less warming than either sulfates or solids such as titania or alumina that have been analyzed elsewhere (12–17). A high-accuracy radiative calculation using a column model with fixed dynamical heating shows that for a −2-W⋅m−2 radiative forcing using optimally sized particles, sulfate warms the lower stratosphere by 2.4 K, whereas warming is only 0.2 K for calcite (18).
The extent to which acids will react with calcite and be neutralized as calcium salts depends on their relative abundance, acidity, and vapor pressure. Although H2SO4 is the weakest of the four acids, formation of CaSO4 is favored due to the low vapor pressure of sulfuric acid, so H2SO4 aerosol will react with Ca(NO3)2 to release HNO3 gas unless unreacted calcite remains. A similar competition exists between HNO3 and HCl, with formation of Ca(NO3)2 being favored over CaCl2 due to the high vapor pressure of HCl. At low calcite loadings, the coagulation process with sulfuric acid aerosol will therefore reduce the effectiveness of removing gas-phase HNO3 and HCl. However, the resulting solid, nonacidic CaSO4 surfaces have much lower catalytic activity than liquid sulfuric acid aerosol for acid-catalyzed and liquid-phase reactions.
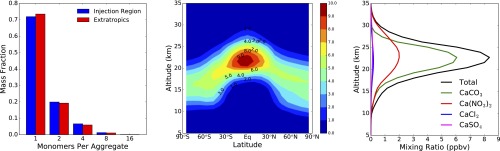
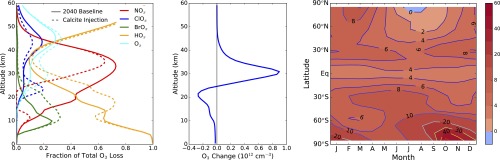
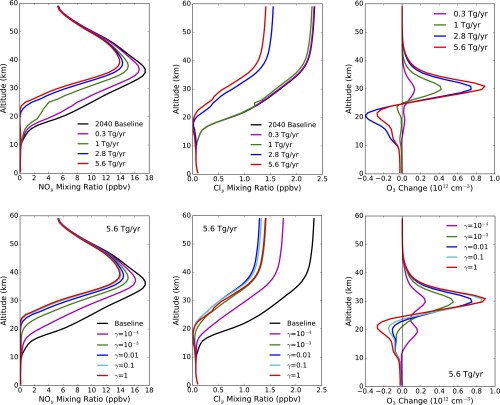
Fig. 1 shows the extent of particle aggregation along with the spatial distribution and composition of solid particles for a calcite injection rate of 5.6 Tg⋅y−1, the maximum injection rate we studied, which was chosen to produce approximately −2 W⋅m−2 of global average radiative forcing. The concentration of solid particles in the lower stratosphere ranges from about 4 cm−3 to 8 cm−3, and only about a third of the solid aerosol coalesces into aggregates. The dominant salt formed is Ca(NO3)2, consistent with the greater stratospheric abundance of HNO3 relative to HCl, HBr, and H2SO4. Fig. 2 shows the resulting changes in the individual catalytic cycles and the ozone distribution. The largest impact is the reduction in NOx in the lower stratosphere. The decrease in NOx shifts the halogens from reservoir species to ClOx and BrOx, which increases their relative importance along with the HOx cycle (Fig. 2, Left), but, because HCl and HBr are also removed by reaction with calcite, the overall impact is nevertheless a decrease in ozone loss rate. In addition, ozone destruction via the NOx catalytic cycle is greatly reduced below 35 km. Annual average column ozone is increased by 6.4%, although ozone concentration decreases in the lowermost stratosphere and upper troposphere. Figs. S1 and S2 show similar results for smaller injection rates.
Fig. 1.
Particle aggregation, spatial distribution and chemistry. All plots represent annual average conditions resulting from a 5.6-Tg⋅y−1 steady-state injection of calcite. (Left) The fraction of solid particle mass per sectional bin vs. number of monomers in the fractal aggregate. (Middle) Particle number density (cm−3) as a function of latitude and altitude. (Right) Composition of solid particles resulting from reaction with acids showing total (black line) and CaCO3, CaCl2, Ca(NO3)2, and CaSO4 mixing ratios (parts per billion by volume) averaged from 60°S to 60°N.
Fig. 2.
Changes in ozone chemistry and distribution. All plots show changes resulting from a 5.6-Tg⋅y−1 steady-state injection of calcite. (Left) Fraction of ozone loss caused by various catalytic cycles as a function of altitude, averaged from 60°S to 60°N for annual average conditions (see Table S1 for definitions of the catalytic cycles). (Middle) Annual average change in ozone (1012 molecules cm−3) as a function of latitude and altitude. (Right) Change in column ozone (percent) as a function of latitude and season.
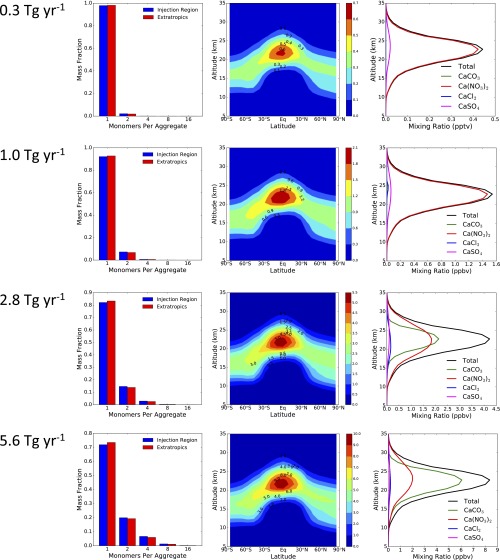
Fig. S1.
Particle aggregation, spatial distribution, and chemistry as a function of calcite injection rate. All plots represent annual average conditions. Rows show results from steady-state injection of calcite at rates of (from top to bottom) 0.3, 1, 2.8, and 5.6 Tg⋅y−1. (Left) Fraction of solid particle mass per sectional bin vs. number of monomers in the fractal aggregate. (Middle) Particle number density (cm−3) as a function of latitude and altitude. (Right) Composition of solid particles resulting from reaction with acids, showing total (black line) and CaCO3, CaCl2, Ca(NO3)2, and CaSO4 mixing ratios (parts per billion by volume) averaged annually and from 60°S to 60°N.
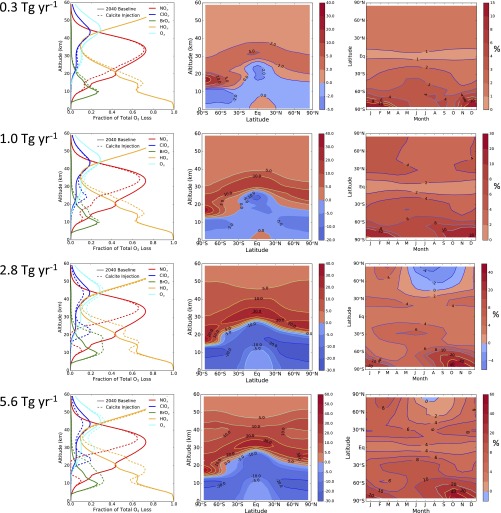
Fig. S2.
Changes in ozone chemistry and distribution. Rows show results from steady state injection of calcite at rates of (from top to bottom) 0.3, 1, 2.8, and 5.6 Tg⋅y−1. (Left) Fraction of ozone loss caused by various catalytic cycles as a function of altitude, averaged from 60°S to 60°N for annual average conditions (see Table S1 for definitions of the catalytic cycles). (Middle) Annual average change in ozone (percent) as a function of latitude and altitude. (Right) Change in column ozone (percent) as a function of latitude and season.
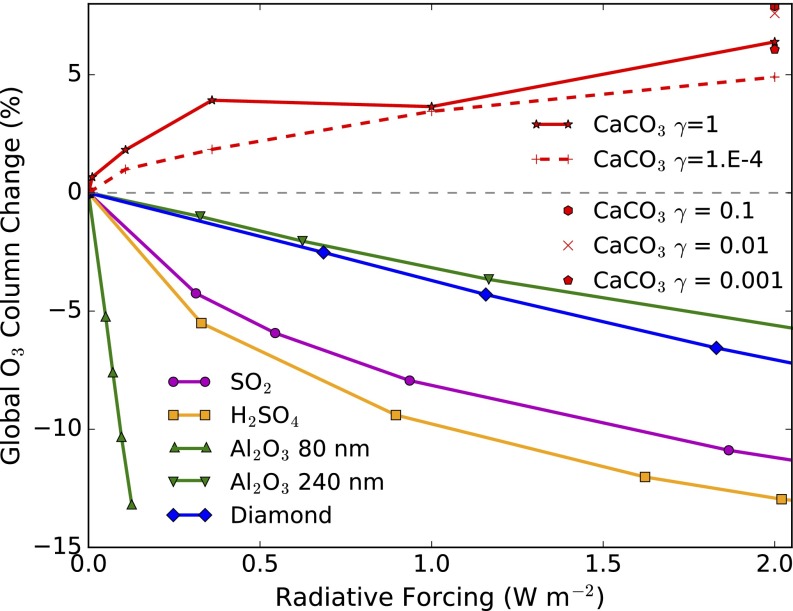
The resulting trade-off between radiative forcing and ozone loss is shown in Fig. 3 for a range of calcite injection rates. Note the strong nonlinearity in the ozone response to injection rates that arises, in part, from the competition between HNO3, HCl, and H2SO4 as the amount of CaCO3 is increased (Figs. S3 and S4). The response for calcite may be compared with prior results for sulfate, alumina, and diamond, which all reduce column ozone. As a crude sensitivity test, we scaled all of the gas−solid reaction rates from 10−1 to 10−4 and found that the column response (also shown in Fig. 3) is surprisingly robust although the dominant mechanism and vertical distributions of ozone change shift considerably (Fig. S4).
Fig. 3.
Trade-off between ozone loss and radiative forcing from geoengineering. Change in annual average global column ozone is plotted versus the computed aerosol radiative forcing. Changes in ozone are computed with respect to a 2040 baseline. Positive values represent an increase in ozone. Results for calcite injection rates ranging from 0.3 Tg⋅y−1 to 5.6 Tg⋅y−1 shown (red line and symbols) where the gas−solid reaction rates, , are varied to explore parameter uncertainty. For comparison, we show prior results for injection of alumina, diamond, and sulfate, either from injection of gas-phase SO2 or sulfuric acid (2).
Fig. S3.
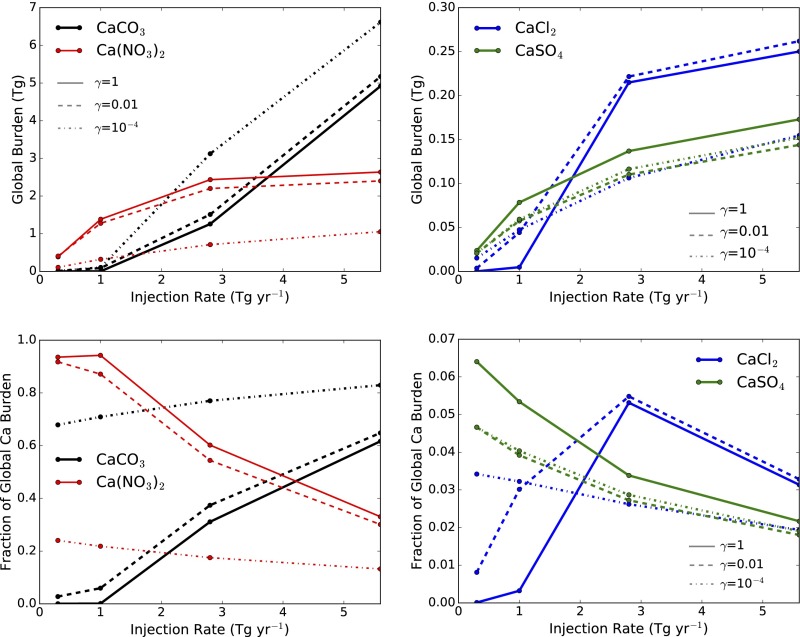
Change in particle composition as a function of injection rate. (Left) CaCO3 and Ca(NO3)2, which dominate particle composition. (Right) CaCl2 and CaSO4. (Top) The global burden (in teragrams) of each species as a function of injection rate. (Bottom) The percentage of total Ca in the given species. As a rough sensitivity test, results are shown for three different values of γ, the dimensionless reaction rate.
Fig. S4.
Sensitivity of NOy, Cly, and O3 to injection rate and reaction probabilities (γ) on calcite particles. (Top) Sensitivity to injection rate (with γ = 1.0 in all cases). (Bottom) Sensitivity to reaction probability (with injection rate of 5.6 Tg⋅y−1 in all cases). Vertical profiles of (Left) NOy and (Middle) Cly mixing ratios, and (Right) O3 number density change averaged annually from 60°S to 60°N.
Discussion
We note the conceptual similarities between our alkali addition and Cicerone et al.’s 1991 proposal (19) to add propane to the Antarctic polar vortex to limit ozone loss by converting ClOx into HCl, a proposal that was later found to have ignored a crucial HOx feedback. We cannot discount the possibility that we too have ignored some crucial feedback. Our specific numerical results depend on uncertain assumptions. Perhaps most importantly, the gas−solid reaction rates for many of the neutralization reactions are not known, especially under stratospheric conditions. We assume that the entire particle is available for reaction, with rates declining linearly to zero in proportion to the fraction of remaining reactant, e.g., CaCO3/Ca, but we do not know how uptake/neutralization rates change as calcite surfaces are transformed to salt coatings, although the range of gammas we explore, 1.0 to 10−4, encompasses the range of observations (20). Finally, the rate constants for heterogeneous halogen-activating reactions (e.g., HCl + ClONO2) are not known for the (mixed) salt surfaces, and refractive indices for such particles have not been measured. Nor do we know if the photochemistry of mixed Ca(NO3)2/CaCO3 particles is relevant.
Notwithstanding these uncertainties, we suggest that there is a nontrivial possibility that use of CaCO3, or a hybrid approach that employs reactive alkali metal salts in combination with high refractive index solid aerosol, could have significantly less environmental risk than sulfate aerosol for a given level of radiative forcing. We therefore suggest that research on stratospheric aerosol SRM needs to move beyond an exclusive focus on sulfate.
Any practical application of this idea should not, of course, proceed until uncertainties about the science and governance are substantially resolved. However, future effort to assess calcite aerosol for SRM is, in part, contingent on judgments about feasibility of implementation. Although analysis of the feasibility is far beyond the scope of this study, we note that (i) submicron calcite particles are available commercially, (ii) methods of preparing monodisperse calcite exist (21), and (iii) engineering studies have demonstrated that teragrams per year of material can be lofted to the lower stratosphere with relatively low cost and technical risk (22). The most obvious engineering unknown would seem to be the ability to disperse solid particles while avoiding agglomeration.
Calcium delivered to the stratosphere will eventually return to the surface, so further consideration of this idea must include studies of the environmental risks of calcium aerosol in the troposphere or its biological impact once deposited on the surface. Calcium is an important component in windblown aerosol “dust,” so a comparison of fluxes provides some indication of the impact of stratospheric Ca on the chemistry of the lower atmosphere and surface. A flux of 5.6 Tg⋅y−1 CaCO3, the largest value analyzed, corresponds to a global average Ca deposition rate of 0.005 g⋅m−2⋅y−1. In comparison, the lowest estimate of Ca deposition by Aeolian dust in areas remote from dust sources is of order 0.01 g⋅m−2⋅y−1, though deposition rates exceed 1 g Ca⋅m−2⋅y−1 over large areas of the continental land surface (23). In addition, speciation of the stratospheric nitrate transported to the surface will be shifted from HNO3 toward Ca(NO3)2, which may have consequences on rainwater acidity and nitrate bioavailablity.
Previous work has shown that solid aerosol can enable SRM with less heating of the lower stratosphere (18) and less ozone loss than sulfates, and that high refractive index particles such as alumina or diamond have lower forward scattering (2). Our work suggests that solid alkali aerosol might significantly reduce the risks of SRM compared with the use of sulfate to produce the same radiative forcing. The combination of solid high-index aerosol with alkali coatings or separate alkali aerosol might allow partially independent manipulation of radiative forcing and stratospheric chemistry and heating. In addition to reversing ozone loss caused by historical chlorofluorocarbon emissions, the injection of alkalis may provide a method to counter the steady growth of stratospheric NOx caused by anthropogenic N2O emissions (24). Laboratory and small-scale field experiments (using <1 kg of materials) (25) are, however, essential to reduce uncertainties in heterogeneous reaction rates and photochemistry of mixed salt aerosol, which are critical to predicting stratospheric ozone loss rates.
Materials and Methods
Chemical Transport Model.
We use the AER 2-D chemical transport−aerosol model (26–28), as modified to include both liquid sulfate particles and solid particles along with their interactions (2). The model uses a sectional representation of aerosol size distributions, with 40 logarithmically spaced bins for sulfate particles and eight bins for solid particles. Calcite particles are treated the same as alumina particles, forming fractal aggregates upon coagulation. We use a monomer radius of 275 nm and take the fractal dimension Df to be 2.1 (29). Compared with the alumina particles of 240 nm radius studied in Weisenstein et al. (2), pure calcite particles in this study sediment about 15% slower due to their larger radius but lower density of 2.7 g⋅cm−3.
We simulate emission of monodisperse calcite particles of 275 nm radius, selecting this radius for optimal radiative properties (18). Emissions are uniform in time and space between 30°S and 30°N and 20 km to 25 km altitude at rates of 0.3, 1, 2.8, and 5.6 Tg⋅y−1. Our calculations represent the 2040 atmosphere, with halogen and trace gas concentrations under representative concentration pathway (RCP) 6.0 (30) The model transport and temperature fields are prescribed to the 1978–2004 climatology (31) and are repeated annually. Chemistry and aerosol are interactive via the sulfur source gases and heterogeneous reactions affecting ozone concentration, as described in Weisenstein et al. (2). We integrate model calculations for about 10 y, to an annually repeating condition, and analyze results from the final year.
Calcite Chemistry.
Because CaCO3 is basic (alkaline), it is expected to react with gas-phase acid species such as HCl and HNO3 in the stratosphere, forming solid salts of CaCl2 and Ca(NO3)2; this will modify the composition of the solid particles while removing chlorine and nitrogen from the gas phase. Our model is not equipped to handle multicomponent solid particles and their interaction with gases, but we can easily model gas-phase interactions on a surface and assign a sedimentation rate to the resulting salts. Therefore, we added five pseudogas-phase compounds CaCO3, CaCl2, CaBr2, Ca(NO3)2, and CaSO4, to the model and applied sedimentation velocities obtained as the mass-weighted average over the solid particle bin sizes. The model injects the pseudogas CaCO3 at the same rate as particulate CaCO3, so we can track the unreacted fraction. The following additional reactions are added to the model:
| [1] |
| [2] |
| [3] |
| [4] |
where here and in Eqs. 6−10, all of the Ca-containing species are in solid phase and all other species are gas phase. These reactions result in HCl, HBr, HNO3, and H2SO4 being removed from the stratosphere, decreasing the concentrations of Cly, Bry, NOy, and H2SO4. We assume that reaction probabilities () for reactions 1−4 are 1.0 (we vary this assumption as a sensitivity test) and that their reaction rates, , depend on the surface area density () of the solid CaCO3 particles as well as the reaction probability () and the thermal speed () of the gas-phase reactant.
| [5] |
where [] is the concentration of the gas-phase reactant in molecules per cubic centimeter and is the fraction of the total Ca as CaCO3, assuming that the full particle volume may react with acidic species, not the surface layer alone. Goodman et al. (32) reported such behavior for the reaction of calcite with HNO3 at a relative humidity of 20%. However, the system has not been studied under stratospheric condition with respect to T and RH, although it has been shown that reactions of HNO3 with solids are faster in the presence of UV radiation (33). In this work, we scale the rate linearly to zero, via factor , as the unreacted calcite is used up.
In addition, we assume that coagulation between solid CaCO3 particles and liquid sulfate particles, or condensation of gas-phase H2SO4 onto CaCO3 particles, results in formation of solid CaSO4, as in reaction 4. Because H2SO4 has a much lower volatility in the stratosphere than HCl and HNO3, we make the further assumption that, if all CaCO3 has been reacted but H2SO4 remains as a liquid on the particles, then the chlorine in CaCl2 or the nitrate in Ca(NO3)2 can be displaced by sulfate, liberating gas-phase HCl or HNO3. These reactions between liquid sulfate and solid calcite or calcite salts are assumed instantaneous. Similar gas-phase reactions of H2SO4 and HNO3 may displace CaCl2 or Ca(NO3)2 from the solid particles (reactions 6−8). Reactions of ClONO2 and BrONO2 are also likely to displace CaCl2 in favor of Ca(NO3)2, in this case producing the gas-phase products Cl2 and BrCl, which would rapidly decompose into ozone-destroying monatomic Cl and Br (reactions 9 and 10). Again, we assume reaction probabilities of 1.0, which are likely overestimates. The additional gas−solid reactions are
| [6] |
| [7] |
| [8] |
| [9] |
| [10] |
where the reaction rates follow Eq. 5, except that is the fraction of the total Ca as solid reactant, either CaCl2 or Ca(NO3)2.
The treatment we have adopted of the solid salt components produced by reactions with calcite particles allows us to avoid modeling five components of the solid particles in each of eight size bins. Here we assume the same composition for all eight size bins and use the fractions of CaCO3 and derived salts in the reaction rate calculations. See Fig. S3 for the calcite particle composition as a function of injection rate. We find that the pseudogas-phase calcium species have a 9% larger stratospheric burden than the solid species, providing a rough estimate of the error committed by our use of the pseudogas species approximation.
Mechanisms of Ozone Change.
The consequences of reactions 1−3 are to remove chlorine, bromine, and nitrogen oxide gases from the stratosphere and convert them into solid salts that would not participate in catalytic reactions destroying ozone. The solid salts would be removed from the stratosphere by sedimentation and advective transport and subsequently removed from the troposphere by cloud processes and deposition. For an injection of 5.6 Tg⋅y−1 of CaCO3, we find that the stratospheric burdens of gaseous Cly, Bry, and NOy decrease by 77%, 4%, and 77%, respectively. In the lower stratosphere, Cly and NOy decrease by over 90% (Fig. S4). The liquid sulfate surface area density of the stratosphere also decreases, because any sulfate that contacts the calcite particles is converted to the stable salt CaSO4. Thus, the surface area for heterogeneous reactions that convert reservoir ClOx and NOx to active radical forms is reduced, and the surface of the calcite particles is unlikely to participate in catalytic reactions as long as the unreacted CaCO3 exceeds the adsorbed liquid acid. Table S1 lists the rate-limiting reactions of the ozone loss cycles important in the extrapolar stratosphere, and includes reactions 9 and 10 above.
Table S1.
Principal ozone loss reactions and their multipliers included in the cycles shown in Fig. 2 and Fig. S2
| Cycle | Multiplier | Reaction |
| HOx | 1 | OH+O3→HO2+O2 |
| 1 | HO2+O3→OH+2O2 | |
| 1 | H+O3→OH+O2 | |
| 1 | OH+O→H+O2 | |
| 1 | HO2+O→OH+O2 | |
| NOx | 2 | NO3+hν→NO+O2 |
| 2 | NO2+O→NO+O2 | |
| ClOx | 2 | HOCl+hν→Cl+OH |
| 2 | Cl2O2+hν→Cl+ClOO | |
| 1 | ClO+OH→HO2+Cl | |
| 2 | ClO+O→Cl+O2 | |
| 2 | ClONO2+CaCl2→Cl2+Ca(NO3)2 | |
| BrOx | 1 | HOBr+hν→Br+OH |
| 1 | BrO+OH→Br+HO2 | |
| 2 | BrO+BrO→2Br+O2 | |
| 2 | BrO+BrO→Br2+O2 | |
| 2 | BrO+ClO→Br+ClOO | |
| 2 | BrO+ClO→BrCl+O2 | |
| 2 | BrO+O→Br+O2 | |
| 2 | BrONO2+CaCl2→BrCl+Ca(NO3)2 | |
| Ox | 2 | O+O3→2O2 |
| 2 | O1D+O3→2O2 |
Radiative Transfer Calculations.
Radiative transfer calculations are performed in this study using the Rapid Radiative Transfer Model (RRTM) (34, 35) including absorption and scattering processes for 16 streams. RRTM separates shortwave and longwave calculations into two separate programs, spanning 820 cm−1 to 50,000 cm−1 in 14 bands and 10 cm−1 to 3,250 cm−1 in 19 bands. Fluxes are computed via the delta-Eddington approximation. The diurnal variability in the shortwave radiative calculations is taken into account by computing radiative quantities at 10 solar zenith angles, and averaging to compute the daily mean. Aerosol scattering parameters are calculated using tabulated complex refractive index data for calcite (36, 37) within a fractal scattering framework (38). Further details are found in Dykema et al. (18).
Acknowledgments
This work was funded by the Fund for Innovative Climate and Engineering Research and the Star Family Challenge for Promising Scientific Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615572113/-/DCSupplemental.
References
- 1.Kravitz B, et al. A multi-model assessment of regional climate disparities caused by solar geoengineering. Environ Res Lett. 2014;9(7):074013. [Google Scholar]
- 2.Weisenstein DK, Keith DW, Dykema J. Solar geoengineering using solid aerosol in the stratosphere. Atmos Chem Phys. 2015;15(20):11835–11859. [Google Scholar]
- 3.Keith DW, MacMartin DG. A temporary, moderate and responsive scenario for solar geoengineering. Nat Clim Change. 2015;5(3):201–206. [Google Scholar]
- 4.National Research Council . Climate Intervention: Reflecting Sunlight to Cool Earth. Natl Acad Press; Washington, DC: 2015. [Google Scholar]
- 5.Crutzen PJ. Albedo enhancement by stratospheric sulfur injections: A contribution to resolve a policy dilemma? Clim Change. 2006;77(3):211–220. [Google Scholar]
- 6.Heckendorn P, et al. The impact of geoengineering aerosols on stratospheric temperature and ozone. Environ Res Lett. 2009;4(4):045108. [Google Scholar]
- 7.Pitari G, et al. Stratospheric ozone response to sulfate geoengineering: Results from the Geoengineering Model Intercomparison Project (GeoMIP) J Geophys Res Atmos. 2014;119(5):2629–2653. [Google Scholar]
- 8.Tilmes S, Müller R, Salawitch R. The sensitivity of polar ozone depletion to proposed geoengineering schemes. Science. 2008;320(5880):1201–1204. doi: 10.1126/science.1153966. [DOI] [PubMed] [Google Scholar]
- 9.Teller E, Wood L, Hyde R. Global Warming and Ice Ages: I. Prospects for Physics-Based Modulation of Global Change. Lawrence Livermore Natl Lab; Livermore, CA: 1997. [Google Scholar]
- 10.Blackstock J, Battisti D, Caldeira K. Climate Engineering Responses to Climate Emergencies. Novim Study Group; Santa Barbara, CA: 2009. [Google Scholar]
- 11.Keith DW. Photophoretic levitation of engineered aerosols for geoengineering. Proc Natl Acad Sci USA. 2010;107(38):16428–16431. doi: 10.1073/pnas.1009519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pope F, et al. Stratospheric aerosol particles and solar-radiation management. Nat Clim Change. 2012;2(10):713–719. [Google Scholar]
- 13.Benduhn F, Lawrence M. An investigation of the role of sedimentation for stratospheric solar radiation management. J Geophys Res Atmos. 2013;118(14):7905–7921. [Google Scholar]
- 14.Maruyama S, Nagayama T, Gonome H, Okajima J. Possibility for controlling global warming by launching nanoparticles into the stratosphere. J Thern Sci Tech-Jpn. 2015;10(2):JTST0022. [Google Scholar]
- 15.Committee on Science Engineering and Public Policy . Policy Implications of Greenhouse Warming: Mitigation, Adaptation, and the Science Base. Natl Acad Press; Washington, DC: 1992. Appendix Q: Geoengineering options; pp. 817–835. [Google Scholar]
- 16.Ferraro AJ, Highwood EJ, Charlton‐Perez AJ. Stratospheric heating by potential geoengineering aerosols. Geophys Res Lett. 2011;38(24):L24706. [Google Scholar]
- 17.Jones AC, Haywood JM. Climatic impacts of stratospheric geoengineering with sulfate, black carbon and titania injection. Atmos Chem Phys. 2016;16(5):2843–2862. [Google Scholar]
- 18.Dykema JA, Keith DW, Keutsch FN. Improved aerosol radiative properties as a foundation for solar geoengineering risk assessment. Geophys Res Lett. 2016;43(14):7758–7766. [Google Scholar]
- 19.Cicerone RJ, Elliott S, Turco RP. Reduced antarctic ozone depletions in a model with hydrocarbon injections. Science. 1991;254(5035):1191–1194. doi: 10.1126/science.254.5035.1191. [DOI] [PubMed] [Google Scholar]
- 20.Santschi Ch, Rossi MJ. Uptake of CO2, SO2, HNO3 and HCl on calcite (CaCO3) at 300 K: Mechanism and the role of adsorbed water. J Phys Chem A. 2006;110(21):6789–6802. doi: 10.1021/jp056312b. [DOI] [PubMed] [Google Scholar]
- 21.Atchudan R, Na HB, Cheong IW, Jool J. Facile synthesis of monodispersed cubic and spherical calcite nanoparticles in the presence of cetyltrimethylammonium bromide. J Nanosci Nanotechnol. 2015;15(4):2702–2714. doi: 10.1166/jnn.2015.9154. [DOI] [PubMed] [Google Scholar]
- 22.McClellan J, Keith DW, Apt J. Cost analysis of stratospheric albedo modification delivery systems. Environ Res Lett. 2012;7(3):034019. [Google Scholar]
- 23.Lawrence CR, Neff JC. The contemporary physical and chemical flux of aeolian dust: A synthesis of direct measurements of dust deposition. Chem Geol. 2009;267(1):46–63. [Google Scholar]
- 24.Portmann RW, Daniel JS, Ravishankara AR. Stratospheric ozone depletion due to nitrous oxide: Influences of other gases. Philos Trans R Soc Lond B Biol Sci. 2012;367(1593):1256–1264. doi: 10.1098/rstb.2011.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dykema JA, Keith DW, Anderson JG, Weisenstein D. Stratospheric controlled perturbation experiment: a small-scale experiment to improve understanding of the risks of solar geoengineering. Philos Trans R Soc Lond A Phys Eng Sci. 2014;372(2031):20140059. doi: 10.1098/rsta.2014.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisenstein D, Penner J, Herzog M, Liu X. Global 2-D intercomparison of sectional and modal aerosol modules. Atmos Chem Phys. 2007;7(9):2339–2355. [Google Scholar]
- 27.Weisenstein DK, et al. Separating chemistry and transport effects in two‐dimensional models. J Geophys Res Atmos. 2004;109(D18):D18310. [Google Scholar]
- 28.Weisenstein DK, et al. A two‐dimensional model of sulfur species and aerosols. J Geophys Res Atmos. 1997;102(D11):13019–13035. [Google Scholar]
- 29.Farin D, Avnir D. Reactive fractal surfaces. J Phys Chem. 1987;91(22):5517–5521. [Google Scholar]
- 30.Hijioka Y, Matsuoka Y, Nishimoto H, Masui T, Kainuma M. Global GHG emission scenarios under GHG concentration stabilization targets. J Global Environ Eng. 2008;13:97–108. [Google Scholar]
- 31.Fleming EL, Jackman CH, Stolarski RS, Considine DB. Simulation of stratospheric tracers using an improved empirically based two‐dimensional model transport formulation. J Geophys Res Atmos. 1999;104(D19):23911–23934. [Google Scholar]
- 32.Goodman A, Underwood G, Grassian V. A laboratory study of the heterogeneous reaction of nitric acid on calcium carbonate particles. J Geophys Res Atmos. 2000;105(D23):29053–29064. [Google Scholar]
- 33.Usher CR, Michel AE, Grassian VH. Reactions on mineral dust. Chem Rev. 2003;103(12):4883–4940. doi: 10.1021/cr020657y. [DOI] [PubMed] [Google Scholar]
- 34.Clough SA, et al. Atmospheric radiative transfer modeling: A summary of the AER codes. J Quant Spectrosc Radiat Transfer. 2005;91(2):233–244. [Google Scholar]
- 35.Mlawer EJ, Taubman SJ, Brown PD, Iacono MJ, Clough SA. Radiative transfer for inhomogeneous atmospheres: RRTM, a validated correlated‐k model for the longwave. J Geophys Res Atmos. 1997;102(D14):16663–16682. [Google Scholar]
- 36.Ghosh G. Dispersion-equation coefficients for the refractive index and birefringence of calcite and quartz crystals. Opt Commun. 1999;163(1):95–102. [Google Scholar]
- 37.Long L, Querry M, Bell R, Alexander R. Optical properties of calcite and gypsum in crystalline and powdered form in the infrared and far-infrared. Infrared Phys. 1993;34(2):191–201. [Google Scholar]
- 38.Rannou P, McKay C, Botet R, Cabane M. Semi-empirical model of absorption and scattering by isotropic fractal aggregates of spheres. Planet Space Sci. 1999;47(3):385–396. [Google Scholar]