Significance
Actomyosin networks are central to a broad range of cellular motile processes, including cell polarization and collective cell migration during morphogenesis and development. Myosin-IXa is critically involved in these processes. Using fluorescence spectroscopy, total internal reflection fluorescence, and electron microscopy, we demonstrate that myosin-IXa assembles actin filaments into highly ordered lattices. The actin filaments of parallel polarity are connected by myosin-IXa in distinct conformations and at a repeat distance of 36 nm across the network. The myosin-IXa–induced actin lattices introduce orientated actin tracks and a network of regularly spaced platforms for localized Rho-GTPase–activating protein activity in cell polarization and collective cell migration.
Keywords: electron microscopy, unconventional myosin, actin network
Abstract
The organization of actomyosin networks lies at the center of many types of cellular motility, including cell polarization and collective cell migration during development and morphogenesis. Myosin-IXa is critically involved in these processes. Using total internal reflection fluorescence microscopy, we resolved actin bundles assembled by myosin-IXa. Electron microscopic data revealed that the bundles consisted of highly ordered lattices with parallel actin polarity. The myosin-IXa motor domains aligned across the network, forming cross-links at a repeat distance of precisely 36 nm, matching the helical repeat of actin. Single-particle image processing resolved three distinct conformations of myosin-IXa in the absence of nucleotide. Using cross-correlation of a modeled actomyosin crystal structure, we identified sites of additional mass, which can only be accounted for by the large insert in loop 2 exclusively found in the motor domain of class IX myosins. We show that the large insert in loop 2 binds calmodulin and creates two coordinated actin-binding sites that constrain the actomyosin interactions generating the actin lattices. The actin lattices introduce orientated tracks at specific sites in the cell, which might install platforms allowing Rho-GTPase–activating protein (RhoGAP) activity to be focused at a definite locus. In addition, the lattices might introduce a myosin-related, force-sensing mechanism into the cytoskeleton in cell polarization and collective cell migration.
The actin-based cytoskeleton, composed of actin filaments and actin-binding partners, including a large variety of myosin motor proteins, is responsible for highly diverse forms of cellular motility. The actomyosin cytoskeleton continuously undergoes major structural reorganizations in the lamellipodium of migrating cells and during polarization in epithelium cells in morphogenesis (1–4). The molecular mechanical mechanisms of these local actomyosin networks, however, are still largely unknown.
Myosin class IX, a monomeric myosin with two mammalian isoforms, has been shown to play an important role in these processes. Whereas myosin-IXb is found in migrating cells of the immune system, myosin-IXa is abundantly expressed in the brain and testis and at lower levels in the kidney, adrenal gland, lung, and spleen and has been shown to play a critical role in epithelial differentiation and morphology (5–8). The N-terminal catalytic domain of myosin-IX binds actin and hydrolyses ATP, whereas the following light-chain binding-neck region acts as a lever arm to amplify the small conformational changes in the catalytic motor domain into nanometer displacements at the end of the lever. The C-terminal tail domain comprises one or two C1 zinc-binding domains and a Rho-GTPase–activating protein (RhoGAP) domain that inactivates the small GTPase Rho (5). Interestingly, the motor head of class IX myosins features a unique domain not found in any other class of myosin, namely a very large insertion of 100–200 aa of unknown structure into loop 2 (9, 10) (Fig. 1A). In other myosins this loop usually comprises only ∼17- to 20-aa bridging over the cleft between the upper and lower 50-kDa domain in the motor head and, in addition to other elements of the upper and lower 50-kDa catalytic domain, is involved in actin binding (11, 12). The large insertion in loop 2 was shown to enable the monomeric myosin-IX catalytic domain to move processively along single-actin filaments like an inchworm, possibly due to the presence of an additional actin-binding site (9, 10, 13–16). Intriguingly, however, the reported ATPase activity and velocity of processive movement was very slow compared with other processive myosin or kinesin motors (17), hinting at a more structural role of this motor molecule, which is supported by recent data indicating an important function of myosin-IXa in regulating the collective migration of epithelial cells via the assembly of actin bundles associated with nascent cell–cell adhesions (18, 19). The molecular mechanisms involved in myosins class IX organizing the actin cytoskeleton, however, are unknown.
Fig. 1.
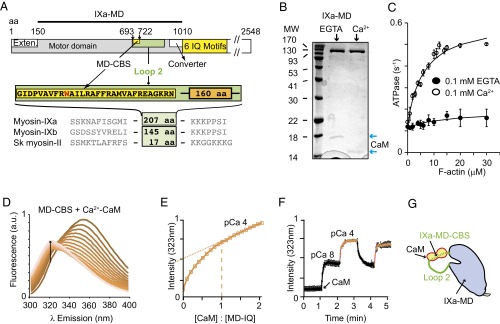
The IXa-MD binds a calmodulin in the presence and absence of calcium. (A) Domain structure of myosin-IXa, including the motor domain (gray), N-terminal extension (white), and CBS motif in loop 2 (MD-CBS) and the neck domain (yellow) with six calmodulin (CaM)-binding motifs, followed by the tail domain (white). The IXa-MD construct comprises the motor domain without the N-terminal extension. (B) SDS/PAGE of expressed IXa-MD confirms clean protein that copurified with CaM. Note the electrophoretic mobility shift of CaM in the presence of calcium (blue arrows). (C) Actin-activated ATPase of 100–200 nM IXa-MD at 2 mM ATP in the absence and presence of calcium. Fitting to Michaelis–Menten kinetics in EGTA: V0 = 0.12 ± 0.01 s−1, Vmax and Km not determined; in calcium: V0 = 0.13 ± 0.02 s−1, Vmax = 0.49 ± 0.03 s−1, and Km = 2.0 ± 1.3 μM. (D) Tryptophan fluorescence (λex, 290 nm) for 2 μM target peptide IXa-MD–CBS and increasing concentrations of CaM (0–4 μM) measured at pCa 4. (E) IXa-MD–CBS bound to CaM with a 1:1 stoichiometry and a Kd of 55 nM at pCa 4. The linear increase in fluorescence in the higher concentration range was due to increasing CaM dominating the fluorescence signal (dotted lines). (F) The change in tryptophan fluorescence (λex, 290 nm; λem, 323 nm) in response to a change in calcium concentration, measured with 2 μM IXa-MD–CBS and 2 μM CaM, showed that CaM binding to IXa-MD–CBS was fully reversible and calcium-dependent. Black line, pCa ≥8; red line, pCa 4. (G) The cartoon illustrates calcium–CaM binding to IXa-MD–CBS in loop 2 of the IXa-MD.
To investigate the collective motion and self-organization of the cytoskeleton at the physiologically high concentrations of the components in the cell and to develop theoretical models of the underlying molecular principles, in vitro minimal model systems have recently been developed that comprised actin filaments, class II myosin motors, and stabilizing actin-cross-linking molecules (20, 21). We now discovered that the widely expressed myosin-IXa uniquely incorporates the functions of both a molecular motor and a stabilizing actin cross-linker in the catalytic domain of a single molecule. This discovery reveals a mechanism of actin network formation in the cell. We found that the combined property of motor activity and cross-linking is sufficient to enable the catalytic domain of monomeric myosin-IXa to induce the formation of extended networks of actin filaments with parallel polarity, cross-linked at a repeat distance of 36 nm by myosin-IXa, both in the absence and in the presence of ATP. By inducing the formation of highly ordered actin lattices, the myosin-IXa motor domain (IXa-MD) might install platforms for localized RhoGAP activity and introduce specific tracks for other myosin motors into the actin cytoskeleton in cell polarization and collective cell migration.
Results
The Motor Domain of Myosin-IXa Binds Calmodulin.
The detailed structure of class IX myosins with their unique insert in the catalytic domain is unknown. To address this problem, we expressed the human IXa-MD, including the unique ∼207-aa insert into loop 2 (Fig. 1A, SI Text, and Fig. S1 A–D). Sequence analysis indicated a 1-8-14 calmodulin-binding site (CBS) at the N terminus of the loop 2 insert (MD-CBS) (Fig. S1E) (15). Consistent with this prediction, we found that the IXa-MD copurified with calmodulin at a 1:1 molar ratio in the absence and presence of calcium (Fig. 1B and Fig. S1C). Solution kinetics studies revealed that, in the presence of calcium, the ATPase of the motor domain was activated by a factor of 5 by actin, whereas the motor domain generated actin movement of ∼27 nm⋅s−1 in gliding-filament assays (Fig. S1F), which confirmed that the motor domain was enzymatically and mechanically active. The data also indicated that the activity of myosin-IXa was regulated by calcium–calmodulin binding to the motor domain (Fig. 1C). We then investigated the structural effect of calmodulin binding to the motor domain in further detail using the intrinsic tryptophan fluorescence of the target peptide MD-CBS in loop 2 (SI Text) (22). We chose calcium concentrations close to the physiological range (∼50 nM to ∼10 μM), which ensured homogeneous populations of the molecules (23–28). The blue shift of the emission spectrum and increase in tryptophan fluorescence intensity at λ emission (λem) 323 nm upon calcium–calmodulin binding to the peptide reached saturation at a 1:1 binding stoichiometry (dashed line) and yielded a binding constant Kd of 55 nM (Fig. 1 D and E). The binding studies indicated that calmodulin was binding the target peptide fully reversibly in different conformations in the presence and absence of calcium, consistent with calmodulin remaining bound to IXa-MD (Fig. 1F). Given that calmodulin was binding to the N-terminal part of loop 2, a region previously shown to contribute to the actin-binding site on the myosin motor domain (11, 12), the question arose whether myosin-IXa binding to actin was substantially different from that of other classes of myosin, which would have strong implications on its molecular mechanisms (Fig. 1G).
Fig. S1.
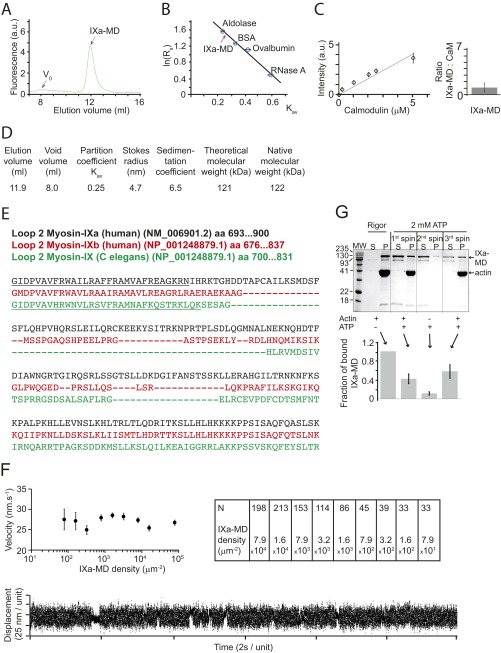
The IXa-MD binds calmodulin and binds to actin in the presence and absence of ATP. (A) Superdex-200 gel filtration showed that IXa-MD expressed in the presence of calmodulin eluted at 11.9 mL. (B) The Stokes radius of IXa-MD was determined from calibration curves using standard proteins of known Stokes radii as described (Stokes radii Rs; partition coefficient Kav) (40, 48). All experiments were performed at least three times. (C) Densitometry calibration using increasing calmodulin concentrations and determination of the molar ratio of IXa-MD to calmodulin in the purified complex in EGTA (Fig. 1B). (D) Using gel filtration and sucrose-density gradients, the myosin’s hydrodynamic properties were characterized as described (40, 48). The 122-kDa native molecular mass determined for IXa-MD was consistent with the theoretical value for monomeric IXa-MD. (E) Sequence analysis of loop 2 in IXa-MD indicated a 1-8-14 calmodulin-binding motif (underlined), as had been reported for other myosin-IX isoforms (C. elegans) (15). (F) Actin-gliding velocity at increasing densities of IXa-MD immobilized via a biotin–streptavidin linker on the surface of the experimental chamber (2 mM ATP; 22 °C). The table denotes the number of filaments analyzed in each experimental condition. In a single-molecule mechanical experiment using optical tweezers, IXa-MD produced single, intermittent interactions with actin, consistent with a nonprocessive motor. (G) Two forms of IXa-MD with different actin affinities interconverted. The SDS/PAGE gels and IXa-MD pellet (P)-band intensities were obtained by mixing 1.5 μM IXa-MD with 10 μM F-actin in the absence (rigor) or presence of 2 mM ATP, followed by a 20-min centrifugation at 435000 × g at 4 °C (first spin). In rigor, virtually all (and in the presence of ATP, 42 ± 10%) of IXa-MD pelleted with F-actin. From the IXa-MD remaining in the supernatant (S), 11 ± 4% pelleted following a spin performed without F-actin (second spin); 58 ± 14% of the IXa-MD remaining in S after the second spin pelleted with F-actin following a third spin. The fraction of bound IXa-MD is shown in percentage of the total IXa-MD (sum of S- and P-band intensities).
IXa-MD Cosediments with Actin in the Absence and Presence of ATP.
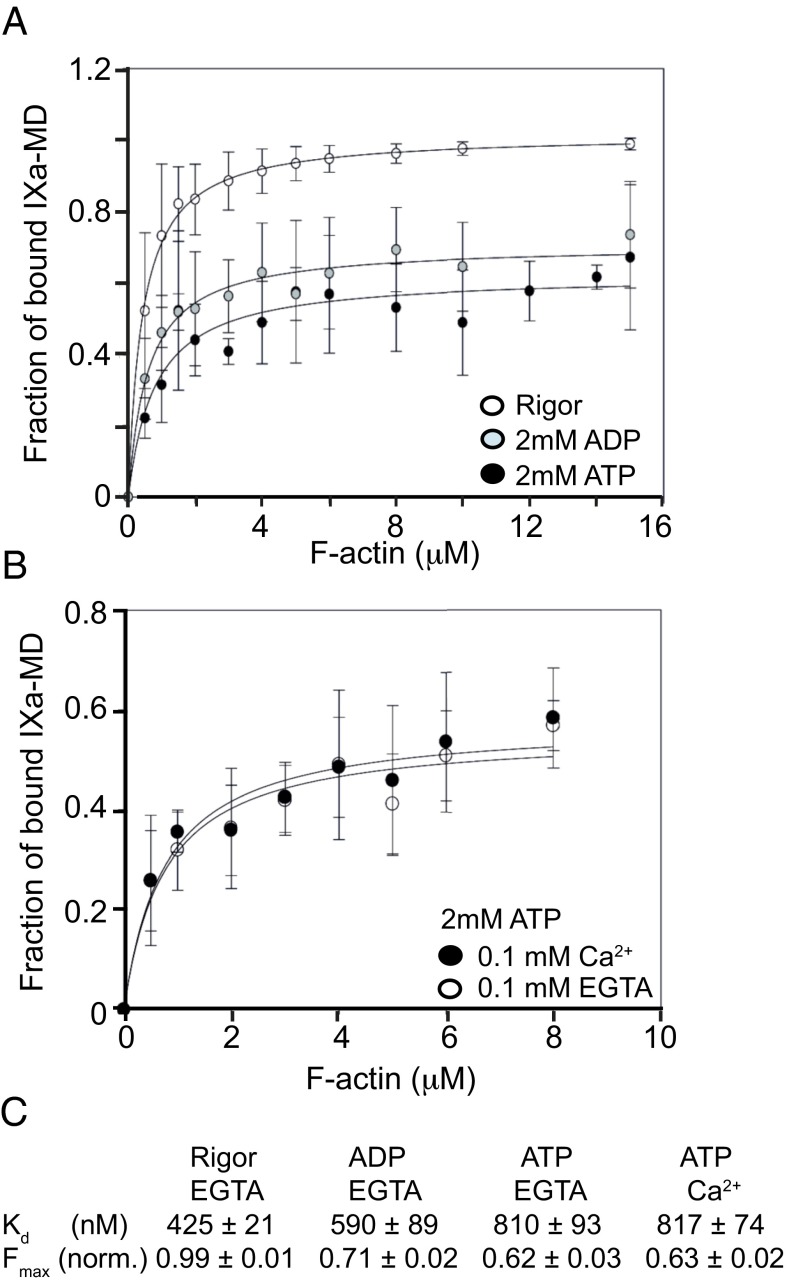
We investigated the ability of the IXa-MD to bind to actin filaments in different nucleotide conditions and performed cosedimentation experiments in which IXa-MD was mixed with increasing actin concentrations in the presence and absence of nucleotide. A complete binding of IXa-MD was observed in the nucleotide-free state. In the presence of ADP and ATP, however, the affinity for actin dropped only by a factor of ∼2, independent of calcium, which—in particular in the ATP-bound state—indicated a remarkably high actin affinity for a myosin motor (Fig. 2 A–C), as reported previously for other myosin-IX isoforms (17). The fact that both ADP and ATP only reduced the fraction of IXa-MD bound to actin by ∼50% implied that there was an equilibrium between a fraction of myosin-IXa that still bound to actin and another one that did not. When the IXa-MD population from the supernatant obtained in the presence of ATP was mixed with filamentous (F)-actin and resedimented, the two subpopulations of IXa-MD were observed again, which indicated that the two subpopulations interconverted on the time scale of the cosedimentation experiments (Fig. S1G). The cosedimentation with actin in the presence of ATP, combined with the reported processivity of single motor domains of other myosin-IX isoforms (9, 10, 13–16), leads to the following question: Does myosin-IXa contain a second actin-binding site in the motor domain which is able to cross-link actin filaments?
Fig. 2.
IXa-MD binds to actin filaments in the absence and presence of nucleotide. (A) The fractions of actin-bound IXa-MD versus actin concentration were obtained from cosedimentation assays using IXa-MD and increasing actin concentrations in the absence or presence of nucleotide (Materials and Methods). Mean values ± SD from three experiments in each condition are shown; the concentration of IX-MD used was between 0.2 and 0.5 μM, dependent on the preparation. (B) The fractions of actin-bound IXa-MD versus actin concentration were obtained from cosedimentation assays in the absence or presence of calcium. Mean values ± SD from three experiments in each condition are shown. (C) The affinities obtained from the experiments in A and B are summarized in the table, with the Kd dissociation constant and Fmax fraction of myosin bound to F-actin.
Formation of Actin Bundles Is Induced by IXa-MD.
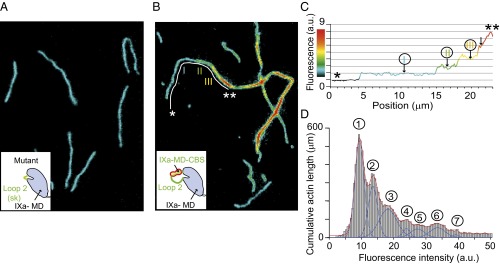
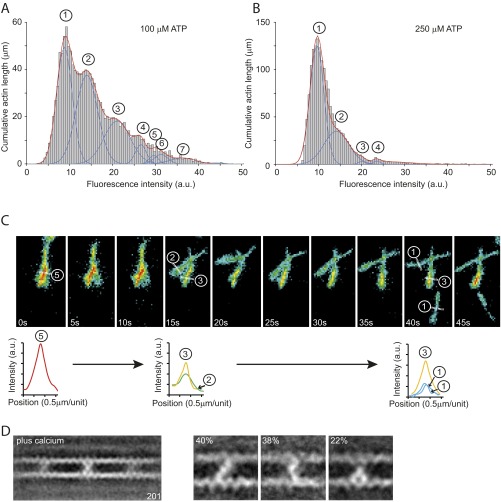
We tested the cross-linking hypothesis by quantitatively analyzing total internal-reflection fluorescence (TIRF) microscopy data, using Alexa-Fluor488–labeled actin filaments and mutant or wild-type myosin-IXa (Fig. 3). We first investigated the effect of a mutant construct with the myosin-IXa–specific loop 2 exchanged for a human skeletal muscle loop 2. Even at a 1:1 molar ratio of mutant IXa-MD to actin and in the absence of ATP, no bundling was observed (Fig. 3A). In contrast, at the same molar ratio, wild-type IXa-MD induced the formation of actin bundles in the absence and presence of ATP (Fig. 3B and Fig. S2). The bundles were characterized by analyzing the fluorescence intensity along single-actin bundles (Fig. 3C) and by analyzing the total pixel intensity across 100 fields of view (600 × 600 μm2 in total) (Fig. 3D). The stepwise increase in fluorescence intensity along individual actin bundles and the quantized distribution of total fluorescence intensity across 100 fields of view were consistent with up to 7 or more actin filaments forming bundles in the absence and presence of ATP. The formation of bundles was fully reversible and dependent on actin, myosin, and ATP concentrations. At 1 μM actin and a 1:1 molar ratio of actin to IXa-MD bundling was still observed at 0.25 mM ATP (Fig. S2 A and B). To understand the molecular mechanisms that enabled the IXa-MD to induce the dynamic formation of actin bundles, we investigated the structures of these myosin-IXa cross-links using EM.
Fig. 3.
IXa-MD induces the formation of actin bundles. Alexa-Fluor488–phalloidin–labeled actin filaments in the presence of the mutant IXa-MD (A) or wild-type IXa-MD (B) at a molar ratio of 1:1 imaged in an assay buffer in the absence of ATP (Materials and Methods). The color code in B and C indicates fluorescence intensity and actin bundling. (C) Stepwise increase in fluorescence intensity along the filament section marked * to ** indicated more than three filaments in the actin bundle. (D) An automated analysis of the pixel intensity values of 100 images (each 81 × 81 μm2) was used to determine the accumulated length of actin filaments and actin bundles. Up to seven filaments in the bundles could be resolved.
Fig. S2.
IXa-MD induced actin-bundle formation in the presence of ATP. The formation of actin bundles by IXa-MD at different nucleotide concentrations was analyzed using TIRF microscopy. Using an automated analysis procedure, the total number of pixels at different fluorescence intensities was determined and used to calculate the total length of actin bundles comprising two to seven actin filaments. (A) Distribution of fluorescence intensities across 100 fields of view (60 × 60 μm2) in the presence of 100 μM ATP. Up to seven filaments in a bundle could be resolved. (B) At 250 μM ATP, the number of filaments in the bundles was reduced, indicating that the formation of the bundles was fully reversible and dependent upon the concentrations of actin, IXa-MD, and ATP. (C) Disassembly of an actin bundle consisting of five filaments into smaller bundles and individual filaments over a period of 45 s in the presence of 250 μM ATP. The analysis of fluorescence intensity indicated that the formation of the bundle consisting of five filaments (0 s) was fully reversible. The bundle disassembled first into two bundles of two and three filaments (after 15 s) before single filaments detached from the bundle and reattached to the surface (40 s). (D) Ensemble average of 201 images in the presence of calcium (pCa 4.0). The cross-links can be divided into a diagonal-, bent-, and inchworm-type class.
IXa-MD Cross-Links Actin at a Repeat Distance of 36 nm to Form Lattices.
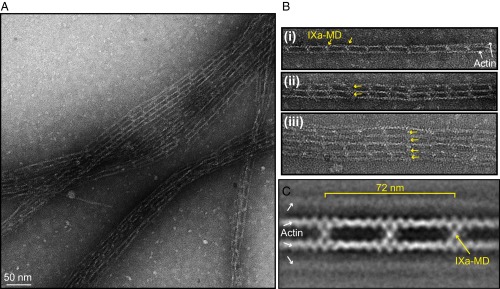
Negative stain EM of the actin bundles showed that the bundles consisted of regular networks formed by myosin-IXa cross-linking up to five actin filaments (Fig. 4A). The bundle size was limited in these experiments by the necessity to dilute the sample for EM. Remarkably, the myosin cross-links aligned across the network to generate regular lattice structures (Fig. 4B, yellow arrows). The class average of 981 images in Fig. 4C revealed that IXa-MD formed cross-links between actin filaments with a periodicity of 36 nm. IXa-MD binding to noncross-linked single-actin filaments was never observed, nor did we find extra molecules in between the 36-nm pattern, strongly suggesting that binding was highly cooperative between the two actin-binding sites on the IXa-MD.
Fig. 4.
Negative-stain EM revealed that IXa-MD induced the formation of cross-linked actin networks. (A) Actin networks induced by mixing F-actin and IXa-MD at a 0.5:1 molar ratio, here in the absence of ATP. (B) Examples of actin bundles consisting of two to five actin filaments. IXa-MD formed cross-links with a periodicity of 36 nm that aligned across the actin network (yellow arrows). (C) Ensemble average of 981 images of 2 or more actin filaments cross-linked by IXa-MD. Here, the polarity of the actin filaments was not taken into account.
IXa-MD Adopts Three Distinct Conformations and Binds to Actin Filaments with Parallel Polarity.
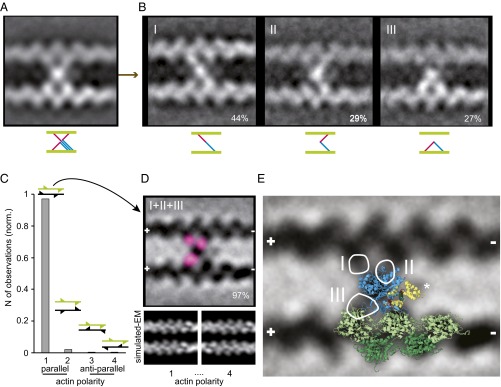
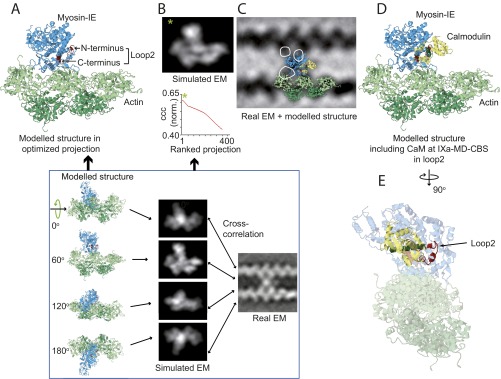
The shape of the averaged actin cross-link comprised a central mass connected to a total of 4 actin monomers, including two adjacent monomers on either actin filament (Fig. 5A and SI Text). Classification of the 981 images of IXa-MD according to the myosin connections to the four actin monomers unveiled three distinct conformations, two of which formed a cross-link between the actin filaments (Fig. 5B and Fig. S3). At 44%, the diagonally shaped cross-link (conformation I) was the most frequently observed, followed by the bent cross-link (conformation II) at 29%. In the remaining inchworm conformation (27%), the myosin molecules did not cross-link but formed a bridge between two adjacent actin monomers on the same actin filament (conformation III). To resolve the polarity of the actin filaments cross-linked by myosin-IXa, we generated model structures of two actin filaments with either parallel or antiparallel polarity and with the actin pitch either in phase or out of phase (Fig. 5C and SI Text). Cross-correlation of the real-EM images with the low pass-filtered actin models divulged that 97% of the myosin cross-links were formed between two parallel actin filaments aligned in phase (Fig. 5 C and D). Analysis of the variance between the images localized the hotspots of variability at the connection between the central myosin mass—bound to one actin monomer—and a second actin monomer, either on the same or on the other actin filament, which was consistent with the image classification into three myosin-IXa conformations [i.e., into the diagonal (I), the bent (II), and the inchworm conformation (III)] (Fig. 5D, pink spots, and Fig. 5E, white circles). To interpret the EM data of the actin cross-links, we fitted a modeled crystal structure of an actomyosin-IE complex to the data (29) (Fig. S4 A–D). Fig. 5E shows that the actomyosin-IE complex, overlaid onto the averaged real EM in an optimized projection [cross-correlation coefficient (ccc): 0.63; Fig. S4], could account for both the actin monomers on the bottom actin filament (green) and for the central mass of the myosin-IXa cross-links (blue). The three hot spots of variability in the EM average, linking the central myosin mass to each of the three actin-binding sites, were not covered by the model structure and therefore probably belong to the remaining part of the loop 2 insert in myosin-IXa. To include the calmodulin on loop 2, we fitted a calmodulin–peptide complex by extending the α-helix preceding the N terminus of loop 2 in the myosin-IE structure (Fig. 5E, asterisk). The inserted calmodulin was consistent with the additional mass next to the myosin catalytic domain in the EM data not directly connected to the actin filaments. The cross-linking connections to the second actin-binding sites in conformation I and II and the inchworm connection in conformation III (white circular outlines), however, were not accounted for by the model structure and are therefore probably due to the additional ∼200 aa present in loop 2 of the myosin-IXa head domain.
Fig. 5.
Interpretation of IXa-MD–induced actin cross-links using a modeled crystal structure. (A) Class average of 981 actomyosin-IXa cross-links. The polarity of the actin filaments was not taken into account. (B) The classification revealed three different conformations; the frequency of observation is given in percentage. (C) Cross-correlation with low pass-filtered parallel and antiparallel in and out of phase actin models showed that 97% of the cross-links were formed between parallel actin filaments, aligned in phase (polarity 1, 2.2% polarity 2, 0.4% polarity 3 and 4). The fins in the cartoons of two actin filaments indicate the polarity and phase. (D) Realigned class average (inverted) of the 221 cross-links between parallel actin filaments aligned in phase. The variance analysis between the realigned cross-links resulted in three hotspots (pink), indicating three secondary myosin-binding sites on actin, consistent with the three myosin conformations identified in B. (E) The IXa-MD cross-links were interpreted using an actomyosin-IE structure, including a calmodulin modeled onto loop 2. The optimized projection of the model was overlaid onto the real-EM class average (actin monomers, green; myosin motor domain, blue; calmodulin bound to loop 2, yellow). The circles mark the hotspots of variance seen in D, and the roman numerals denote the different conformations shown in B.
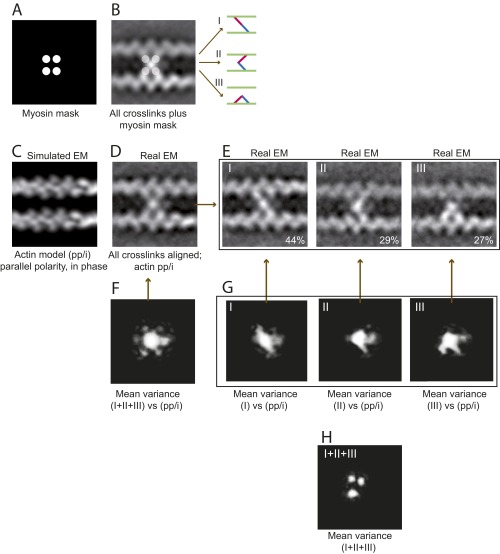
Fig. S3.
EM and image analysis of IXa-MD–induced actin cross-links. All 981 images of actomyosin cross-links were reference free-aligned to generate the first averaged image; actin polarity and phase were not taken into account. (A and B) The EM average indicated that IXa-MD bound to four actin monomers, providing a mask for image classification using HAC and uncovering three distinct binding conformations (I–III). (C) Cross-correlation of the real-EM images with the four actin models (SI Text) showed that 97% of the cross-links were formed between two actin filaments with parallel polarity, aligned in phase (pp/i). (D) Based on this model, 451 EM images with sufficient resolution were realigned and averaged. (E) Classification according to the mask in A was applied once more to the realigned dataset confirming the three distinct conformations. (F) The variance in the global EM average (white intensities) identified the central myosin mass, all four connections to actin, and an extra mass possibly due to the loop 2 calmodulin as parts of myosin-IXa. (G) The variance in the datasets of the three conformations reflected the diagonal, inchworm, and bent myosin conformation and confirmed that IXa-MD attached to only two actin monomers in each conformation. (H) The variance between the class averages confirmed the three distinct actin monomers as the varying second actin-binding site for myosin-IXa.
Fig. S4.
Modeling of the actomyosin-IXa cross-links. To interpret the actomyosin-IXa cross-links, we compared the EM averages with a model of the actomyosin complex [PDB ID code 4A7F (29)]. (A) A set of 360 low pass-filtered 2D projections of the model, related by 1° rotations around the actin filament axis, was calculated. (B) The projection that best matched the real-EM data was determined by comparing the ccc. The highest ccc value (*) was obtained with a model comprising five actin monomers and a myosin-IE motor domain attached to the middle binding position (PDB ID code 4A7F). (C) The model was complemented by a calmodulin bound to the N terminus of loop 2. This structure in the optimized projection was overlaid onto the real-EM global average of myosin-IXa cross-links. (D) The beginning and end of loop 2 are shown in red, calmodulin in yellow, and the binding target α-helix in dark green. (E) To accommodate calmodulin into loop 2, we extended the α-helix preceding the N terminus of loop 2 (black arrow) in myosin-IE by a calmodulin–peptide complex [PDB ID code 3GN4 (50)]. In this position, calmodulin could partly account for the additional mass on the right hand side of the IXa-MD in the EM average (C).
Discussion
A central feature of many cellular motile processes lies in the local organization of actomyosin networks. The molecular mechanisms of chemomechanical energy transduction of several myosin motors with very diverse mechanical and kinetic properties have been investigated at the single-molecule level (30, 31). However, the mechanisms organizing the local actomyosin networks, including those involved in cell polarization and during collective cell migration, remain unclear. Here, we discovered that the catalytic domain of myosin-IXa self-organizes regular actin networks in the shape of extended lattices. We discovered that this finding was due to the presence of a large insert of ∼207 aa in loop 2 of the catalytic domain of the myosin-IXa motor.
We found that a single calmodulin bound to the CBS motif at the N terminus of loop 2 in the IXa-MD, which is consistent with recent reports for other myosin-IX isoforms (10, 15). Calmodulin binding was observed in the absence and presence of calcium, albeit in two different conformations, which suggests that calcium–calmodulin on the motor domain might play a regulatory role for the myosin-IXa motor molecule. The switch from a high-affinity (Kd 55 nM) 1:1 calmodulin to peptide stoichiometry at high calcium to an undetermined stoichiometry at low calcium suggests that at low calcium, additional CBSs located in other regions of the loop 2 insert or even outside loop 2 might become involved to restore the correct configuration (23). A regulatory effect of calcium–calmodulin was consistent with the myosin-IXa ATPase, which was actin activated in the presence of calcium by a factor of 5. The maximum rate of ∼0.5 s−1 was similar to previous reports on motor domain constructs of another myosin-IX isoform in Caenorhabditis elegans (15). The fact that actin activation by a factor of 5 was observed for the myosin-IXa construct in the presence and for the C. elegans isoform in the absence of calcium (15) might be explained by differences in the sequence of the CBS motif and in the remainder of the loop 2 insert in these myosin-IX isoforms, which are considerably different in size (Fig. 1A and Fig. S1E).
The large ∼100- to 200-aa insert into loop 2, including the CBS motif, is a distinguishing feature of class IX myosins (15). We show that the modification of loop 2, which in other classes of myosin is involved in actin binding (11), not only features a CBS in myosin-IXa but also retains an unusually high actin affinity, specifically in the ATP-bound state of this myosin motor. A fraction of 0.6 of the IXa-MD cosedimented with F-actin at millimolar ATP concentrations, consistent with fractions of 0.24–0.9 reported previously for other myosin-IX isoforms, depending on the specific constructs and conditions used (10, 16, 17). This result indicated that the unusually high affinity for actin in the presence of ATP is a feature of the loop 2 insert of myosin-IXa and does not require the presence of other structural parts of the molecule, such as an N-terminal extension. Previous studies on other myosin-IX isoforms suggested that the high affinity for actin enables the loop 2 insert to act as an actin tether that prevents monomeric myosin-IX from dissociating from actin in the ATP-bound state during processive movement (10, 13–16, 32). We discovered that the insert’s high affinity for actin enables myosin-IXa to cross-link and bundle actin filaments.
The fluorescence studies showed that the loop 2 insert in myosin-IXa was in fact the molecular basis for myosin’s actin–cross-linking properties and the formation of large actomyosin bundles consisting of seven or more actin filaments. We found that this feature of a myosin catalytic domain comprises the fully reversible assembly and disassembly of actin bundles, dependent on the concentration of available actin filaments and on ATP. At higher ATP concentrations, smaller bundles were observed. Technical reasons required the actin to be kept at 1 μM, two orders of magnitude below the physiological intracellular concentration range [on average, ∼100 μM (33)], which limited the availability of actin filaments for dynamic assembly/disassembly by myosin-IXa into bundles, in particular, in the presence of ATP. Nevertheless, actin bundles still formed in the presence of up to 0.25 mM ATP (Fig. S2), confirming the high propensity of this motor to induce actin bundles.
It remains to be clarified how the assembly and disassembly of the actin lattices by myosin-IXa are regulated. The ability to discern bundle thickness using quantitation of the fluorescence signal enabled us to monitor the disassembly of larger bundles into smaller ones and the detachment of single-actin filaments from bundles (Fig. S2C). These observations were consistent with a mechanism in which preformed actin filaments are reversibly zipped up into bundles, regulated by the local availability and recruitment of myosin-IXa. The pull-down experiments at higher actin concentrations, closer to the physiological range, showed 40–80% of IXa-MD bound to actin at millimolar ATP or ADP concentrations, consistent with a regulatory role of nucleotide for the actin-bundle formation in the cell. As described for myosin-V (34, 35) the nucleotide states of myosin-IXa themselves might be affected by load regulating the structure of the actin bundles in a force-dependent fashion. Another regulatory candidate might be calcium–calmodulin bound to the insert in loop 2. In the pull-down experiments, the propensity of IXa-MD to induce actin bundles was unaffected by calcium, consistent with the EM data that confirmed the formation of IXa-MD cross-links at the repeat distance of 36 nm at pCa 4.0 (Fig. S2D). These cross-links were again consistent with the three IXa-MD conformations—diagonal, bent, and inchworm—as observed at low calcium conditions (pCa 8.0). However, the dynamic equilibrium between the different conformations in different regulatory calcium and nucleotide conditions remains to be established. Finally, for full-length myosin-IXa, a further regulatory candidate might be the Rho-GAP domain in the tail, possibly affecting both actin filament formation and the structure of the actin bundles.
The novelty for myosin-IXa is the presence of two separate actin-binding sites in the catalytic domain that enable this myosin motor to cross-link and orientate actin filaments and to induce the formation of highly regular actin lattice structures. The cross-linking property of the catalytic domain is based on a remarkable structural flexibility that enables the loop 2 insert to attach to three different actin-binding sites, forming either two types of cross-links bridging across two different actin monomers or forming an inchworm conformation on the same actin filament. The detailed structure of the loop 2 insert is currently unknown. However, studies on the myosin-IXb loop 2 have reported it to contain a substantial amount of secondary structure but to remain flexible with elastic properties and a high affinity for actin (10). In striking contrast to the cross-links observed with single-headed myosin I isoforms, which contain a second actin-binding site in the tail domain of the motor molecule (36, 37), we found that myosin-IXa induced actin bundles by forming highly regular binding patterns, cross-linking the actin filaments at a repeat distance of 36 nm matching the actin helical repeat, and leading to an alignment of myosin cross-links throughout the actin network. Intriguingly, we did not observe single-actin filaments decorated with IXa-MDs. This result suggests that the myosin-IXa binding to actin is highly cooperative and involves two actin-binding sites on the motor domain and a dynamic equilibrium between the inchworm (III) and the two cross-linking [diagonal (I) and bent (II)] conformations. Binding positions corresponding to the 36-nm actin helical repeat where myosin-IXa can adopt a maximum number of binding conformations seem to be strongly favored. ATP-dependent inchworm-type processive movement of myosin-IXa along single-actin filaments would not be required to generate actin lattices in this model. However, we propose that in the inchworm conformation, myosin-IXa might be able to proceed processively along a single-actin filament, as had been reported for other myosin-IX isoforms (9, 10, 13–16), to seek out the next preferred binding position, where a second actin filament comes into reach, so that both the inchworm and the cross-linking conformations can be adopted; otherwise, the actin binding of this particular motor might not be sustained. So far, we did not observe processive movement of single IXa-MDs along single-actin filaments, which we investigated using motility assays and single-molecule mechanical experiments (SI Text). This result may be due to either very short processive run lengths or some unfavorable interactions of IXa-MD with its larger insert compared with other myosin-IX isoforms, or the result might reflect differences in the structure of the loop 2 insert and possibly the distribution and function of the myosin-IXa isoform.
The three different myosin-IXa conformations obtained in the absence of ATP do not seem to represent different nucleotide states but rather three different states in nucleotide-free rigor at the end of the chemomechanical cycle. The difference in the frequency of observation indicated different binding affinities in the different conformations, possibly due to intramolecular strain, with bent cross-links experiencing a larger strain than diagonal ones. Future experiments could resolve the effect of nucleotide on the different conformations.
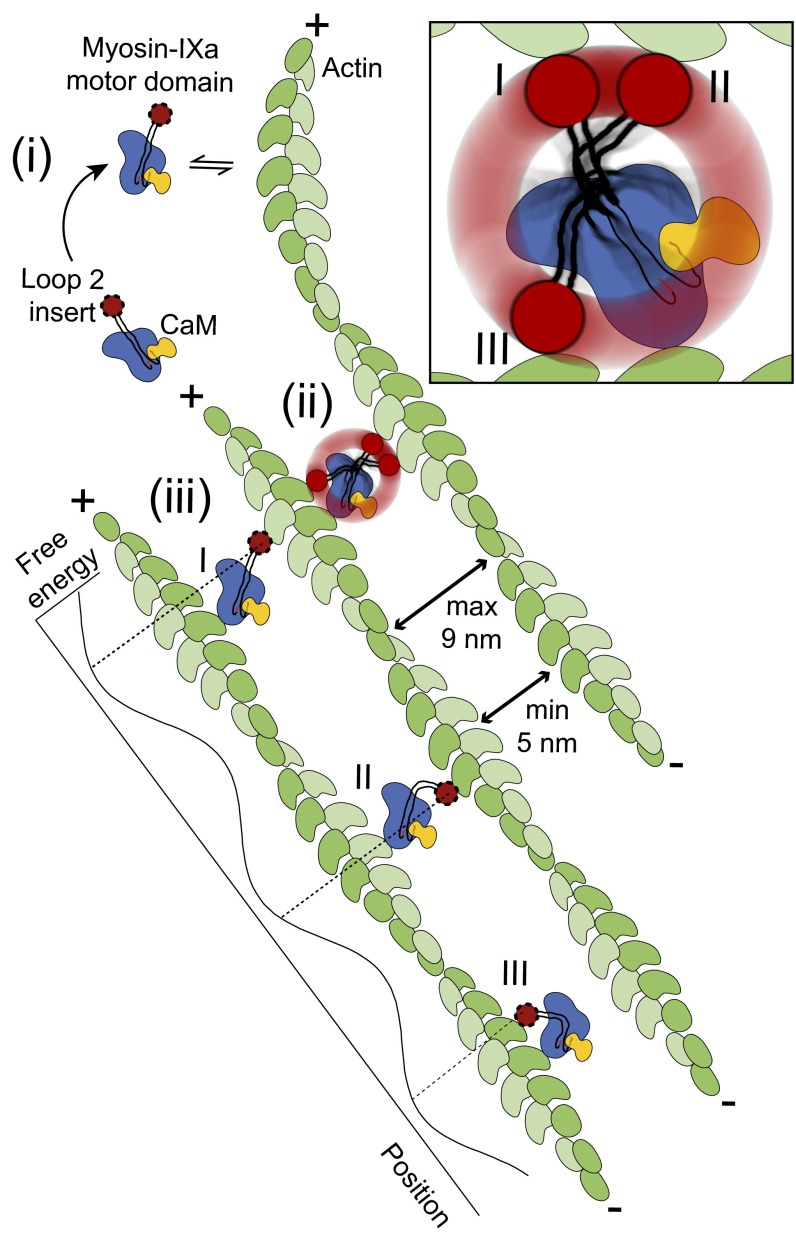
We propose the following model for the formation of lattice-like actomyosin networks induced by myosin-IXa (Fig. 6): (i) the IXa-MD binds to an actin filament with one of the two actin-binding sites in loop 2; (ii) if a second actin filament of the same polarity comes within reach, the IXa-MD adopts a diagonal or a bent cross-link (conformations I and II); the cross-link aligns the two actin filaments in parallel polarity and in phase; and (iii) additional IXa-MDs are now recruited at the preferred binding positions along actin, determined by the 36-nm periodicity and by the spacing in between the actin filaments; at the preferred binding positions, myosin-XIa can adopt a maximum number of binding conformations (inchworm as well as diagonal and bent cross-links), which enforces the formation of an actin lattice structure. ATP-dependent inchworm-type processive movement of myosin-IXa along single-actin filaments would not be required to generate actin lattices in this model. However, processive movement might promote myosin-IXa seeking out the next preferred binding position with the option to cross-link actin filaments. Future experiments could investigate the mechanical properties of myosin-IXa in these networks in further detail. By inducing the formation of highly ordered actin lattices, myosin-IXa might provide specific tracks for other myosin motors and install platforms, allowing RhoGAP activity at the myosin-IX tail to be focused at definite loci. In addition, the regularly spaced, cross-linking myosin motors in the lattice of oriented tracks might introduce a novel, cooperative force-sensing mechanism into the cytoskeleton in cell polarization and collective cell migration.
Fig. 6.
Model. Actin filaments with parallel polarity are cross-linked by the motor domains of single myosin-IXa molecules adopting three interchangeable conformations (I, II, and III) (Inset) that can be obtained at binding sites with free-energy minima. IXa-MD does not remain bound to single-actin filaments, and the precise 36-nm spacing is obtained due to the constraints set by the length and flexibility of loop 2 and the distances between actin filaments specified by their geometries.
Materials and Methods
Molecular Biology.
Full-length human myosin-IXa (7,647 bp) cDNA (National Center of Biotechnology accession no. AAI40870.1) was codon-optimized, chemically synthesized, and cloned into plasmid University of California 57 (pUC57) (GenScript). This construct was used as a template to subclone the IXa-MD (base pairs 445–3,057) construct using standard PCR methods and oligonucleotides via BamHI/XbaI sites into the pFBNX vector, with MD9a-forward (fwd) (AGGATCCATGGATGATTTATGTAGTTTA CCTGATTTG) and 1019-reverse (rev) FLAG (TTCTAGACTACTTGTCATCGTCATCCTTGTAA TCCACCTCTTGGTGAGCAGTC). The reverse primer contained a sequence encoding the FLAG-tag (DYKDDDK) upstream of the stop codon and XbaI site. Introduction of an AviTag between the end of the myosin-IXa sequence and the FLAG-tag was achieved via a ligation at the NotI site using chemically synthesized oligonucleotides with free phosphate groups at the 3′-end Avi-tag-fwd (GGCCGCAGGTGGCGGTCTGAACGAC ATCTTCGAGGCTCAGAAAATCGAATGGCACGAAGC) and Avi-tag-rev (GGCCG CTTCGTGCCATTCGATTTTCTGAGCCTCGAAGATGTCGTTCAGACCGCCACCTGC). Recombinant baculovirus (Bac) DNA was generated by the Bac-to-Bac (Invitrogen) method according to the manufacturer’s instructions and transferred into SF21 cells. Viruses were amplified to a P3 stock, and virus titer was determined before infection of cells for protein expression.
Tryptophan Fluorescence.
The tryptophan fluorescence studies were performed with the target peptide sequence from human myosin-IXa [National Center of Biotechnology accession no. NM_006901.2; the peptide sequence shown in Fig. S1E (green) was synthesized by GenScript] and human calmodulin. The titrations of the predicted target peptide (38, 39) with calmodulin were performed and analyzed at 20 °C in the following buffer: 25 mM Tris (pH 8.0), 100 mM KCl, 1 mM DTT, supplemented with either 1 mM CaCl2 or 0.2 mM EDTA, using a Varian Cary Eclipse fluorescence spectrophotometer [λ excitation (λex), 290 nm; λem, 323 nm], as described (22, 40). The dissociation constants Kd for the tryptophan-containing peptide were determined by direct titration, as described previously (22). The equations used to fit the data are described in SI Text.
Steady-State Mg-ATPase.
The experiments were performed in a buffer containing 10 mM 3-(N-morpholino)propanesulfonic acid (Mops) (pH 7.2–7.4), 50 mM KCl, 0.1 mM EGTA, 1 mM MgCl2, 1 mM DTT, and 2 μM calmodulin, using a NADH-coupled enzyme assay at 22 °C and a Varian Cary 50 spectrophotometer as described (17, 41). The buffer was supplemented with 1 mM phosphoenolpyruvate, 0.2 mM NADH, 18 U/mL lactate dehydrogenase, 12 U/mL pyruvate kinase, 2 mM ATP, and a range of phalloidin-stabilized F-actin concentrations. The reaction was started by adding 0.1–0.2 μM IXa-MD, and the decrease in the absorbance at 340 nm was recorded for ∼10 min. The Mg-ATPase rate at each actin concentration was determined from the change in absorbance at λ 340 nm (ΔA340), the myosin concentration in the reaction and the NADH extinction coefficient (e = 6220 M−1⋅cm−1) as described (17, 41). The ATPase rate of F-actin alone was subtracted from the actomyosin ATPase rate. The measurements were repeated with 3 different myosin preparations for each actin concentration and the data were fitted to the Michaelis–Menton equation (SI Text).
Cosedimentation of the IXa-MD with F-Actin.
Cosedimentation assays were performed with IXa-MD and increasing concentrations of actin in the presence and absence of nucleotides as described (17). In brief, actin was polymerized in 25 mM Mops (pH 7.2), 50 mM KCl, 2 mM MgCl2, 1 mM DTT, and 1 mM EGTA, and filaments were stabilized by adding phalloidin at a 1:1 molar ratio; 0.2–0.5 μM IXa-MD was incubated with 0–15 μM F-actin in the absence or presence of nucleotide in a buffer containing 10 mM Mops (pH 7.2), 100 mM KCl, 4 mM MgCl2, 1 mM DTT, and 0.1 mM EGTA. Apyrase was used to deplete the solution fully from nucleotide in experiments in the rigor condition. Following 20 min of centrifugation at 435000 × g and 4 °C, pellets were resuspended in an equal volume of phosphate buffer, and equal amounts of supernatants and pellets were analyzed by SDS/PAGE. For each experiment, myosin in the supernatants and in the pellets was quantified and normalized by densitometry. The dissociation constants Kd of myosin binding to actin were determined by fitting to the solution of the standard quadratic equation (17), as described in SI Text.
TIRF Microscopy.
TIRF imaging was performed on a Nikon (Eclipse TI) microscope. F-actin was stabilized with Alexa-Fluor488–phalloidin (Molecular Probes) at a 1:1 molar ratio and mixed with IXa-MD also at a 1:1 molar ratio. To image and analyze the IXa-MD–actin bundles, they were introduced into a flow cell coated with 10 μg/mL N-ethylmaleimide–modified myosin (42) in an assay buffer containing 10 mM Mops (pH 7.2), 250 mM NaCl, 0.1 mM EGTA, mM 10 DTT, 3 mg⋅mL−1 glucose, 0.1 mg⋅mL−1 glucose oxidase, 0.02 mg⋅mL−1 catalase, and ATP as indicated. For the automated image analysis, 100 adjacent fields of view covering a total area of 600 × 600 μm2 were recorded by systematically translocating the microscope stage. To ensure homogeneous conditions of fluorescence excitation and imaging, a central region of interest (ROI) (60 × 60 μm2) within the image was identified and the remaining inhomogeneity subtracted in each image by dividing by a reference image of the laser-intensity distribution. Based on the signal intensity of all pixels within the ROI, we calculated the total length of actin-bundle structures consisting of one to seven actin filaments.
Negative-Stain EM.
Nucleotide-free IXa-MD and F-actin were diluted to a final concentration of 1 μM and 0.5 μM, respectively, using a buffer containing 20 mM Mops (pH 7.2), 100 mM NaCl, 0.2 mM EGTA, and 1 mM DTT. The mixed protein sample was applied to hydrophilized (glow-discharge) carbon-coated copper grids (Science Services) and negatively stained with 2% (wt/vol) uranyl formate, as described (40, 43). Images of grids were recorded on a Phillips CM100 transmission electron microscope [Hendrick Dietz, Technical University of Munich (TU Munich)] operating at 100 kV and using a 4000- × 4000-pixel CCD camera at a resolution of 0.33 nm per pixel.
Single-Particle Analysis.
The micrographs were processed using Electron Micrograph Analysis 2 (EMAN2) (44) and System for Processing Image Data from Electron Microscopy (SPIDER) (45) software for particle picking, alignment, and classification. From 146 micrographs, 981 images of myosin cross-linking actin filaments were manually picked and windowed with a window size of 300 × 300 pixel, large enough to comprise three myosin cross-links and three actin pseudorepeats. These images were used to determine the distance between adjacent cross-links. For further processing, including alignment, classification, and actin-polarity determination, the images were windowed again to 90 × 90 pixels, covering only a single myosin cross-link and used for structural analysis of the cross-links.
SI Text
Gliding Filament Assays and Single-Molecule Mechanical Experiments.
To test whether the IXa-MD was mechanically functional, we used gliding-filament assay with IXa-MD immobilized on the surface of the experimental chamber and measured the gliding velocity of labeled actin filaments in the presence of ATP (Fig. S1F) (46). Using 0.2 mg⋅mL−1 biotinylated IXa-MD bound to the surface via a C-terminal biotin–streptavidin linker, the average gliding velocity was ∼27 nm⋅s−1, confirming that the motor domain was mechanically functional. Diluting the surface density of IXa-MD by three orders of magnitude down to eight molecules per square micron had no effect on the gliding velocity, consistent with processive movement of the individual motor molecules. To investigate whether IXa-MD interacted processively with single-actin filaments, we performed single-molecule mechanical experiments using optical tweezers in the three-bead configuration with IXa-MD immobilized on the surface of the experimental chamber (42). We observed only intermittent, single interactions of the motor with the suspended actin filament but no processive runs (Fig. S1F). The results do not exclude the possibility that the IXa-MD might be processive in different circumstances, but the results might also reflect differences in the structure of the loop 2 insert and possibly the distribution and function of this myosin-IXa isoform compared with other myosin-IX isoforms.
Protein Expression and Purification.
Protein expressions and purifications are based on previously published protocols (40, 47). Briefly, 1,000-mL cultures were resuspended in 80 mL of myosin extraction buffer [10 mM Mops (pH 7.4), 500 mM NaCl, 5 mM MgCl2, 1 mM EGTA] and sonicated for 5 min at 40% power (Bandelin HD2070). The cell extract was rotated in the presence of 2 mM ATP, 4 mM d-biotin (B4501; Sigma Aldrich) and 1 μM BirA ligase for 1 h at 4 °C (when the construct was required to have a C-terminal biotin attached), before being centrifuged at 48000 × g for 20 min at 4 °C. The supernatant was combined with 0.9 mL of Anti-FLAG M2 Affinity Gel (A2220; Sigma Aldrich) and nutated for 60 min at 4 °C. The resin was washed twice with buffer A [10 mM Mops (pH 7.4), 0.1 mM EGTA, and 100 mM NaCl] before the protein was eluted with 4 mL of 0.1 mg/mL FLAG peptide (F3290; Sigma Aldrich) in buffer A and loaded immediately onto 0.3 mL of the Q Sepharose Fast Flow (17-0510-10; GE Healthcare), equilibrated with buffer A. Finally, the protein was eluted at 250 mM and 500 mM NaCl in buffer A. Biotinylation was confirmed by Western blot.
Size-Exclusion Chromatography.
Purified proteins were loaded on a Superdex-200 (10/300 GL) analytical column (GE Healthcare). The native molecular mass of the proteins was calculated from their Stokes radius measured by size-exclusion chromatography, and their sedimentation coefficient was determined by sucrose-gradient centrifugation, as described previously (40, 48).
Steady-State Mg-ATPase Measurements.
The experiments were performed using a NADH-coupled enzyme assay at 22 °C and a Varian Cary 50 spectrophotometer, as described (17, 41). The buffer was supplemented with phosphoenolpyruvate, NADH, lactate dehydrogenase, pyruvate kinase, ATP, and varying concentrations of phalloidin-stabilized F-actin. The reaction was started by adding IXa-MD, and the decrease in the absorbance at 340 nm was recorded for ∼10 min. The Mg-ATPase rate at each actin concentration was determined from the change in absorbance at λ 340 nm (ΔA340), the myosin concentration in the reaction, and the NADH extinction coefficient (ε = 6,220 M−1⋅cm−1) according to
The ATPase rate of F-actin alone was subtracted from the actomyosin ATPase rate. The measurements were repeated with three different myosin preparations for each actin concentration, and the data were fitted to the Michaelis–Menton equation.
Quantitative Analysis of Actin Binding in Pull-Down Studies.
For analysis, the normalized band intensities of actin-bound myosin (Snorm) were calculated as the ratio of the myosin band intensity in the pellet to the sum of the band intensities of myosin in the pellet and in the supernatant (total myosin, [M0]). The normalized intensities were plotted as a function of total actin concentration [A0]. The dissociation constants K for myosin binding to actin at different nucleotide conditions were determined by fitting to the solution of the standard quadratic equation (17):
with [A • M] = [M0] Snorm; A, actin; M, myosin; N, nucleotide (either no nucleotide, ADP, or ATP).
Tryptophan Fluorescence.
Dissociation constants for tryptophan-containing peptides (W) and calmodulin (C) were determined by direct fluorometric titrations (λex, 290 nm; λem, 323 nm). As described previously (28), the direct fluorometric titrations were fitted to the following equation:
where F represents the molar fluorescence intensities. The dissociation constant Kd(W) was obtained from a nonlinear least-squares fit to this equation with concentrations calculated by solving the following equation, according to (28):
The subscript T denotes total concentrations. A factor XW was included in the fitting equation to correct for errors in the peptide concentration (i.e., the actual concentration was WTXW, and experiments with XW > 1.1 or XW < 0.9 were rejected). As described (28), the dissociation constant Kd(S) was obtained from a nonlinear least-squares fit to the following equation with concentrations calculated by solving
Kd(W) was fixed at the value determined from the direct titration. The titration experiments were performed with the construct described in Fig. 1A and Fig. S1E; the amino acid numbers refer to human myosin-IXa and C. elegans myosin-IX.
Motility Assay.
The procedures were adapted from motility assays described previously (23, 40, 46). Experiments were carried out using an assay buffer (containing 25 mM KCl, 25 mM imidazole, 4 mM MgCl2, 1 mM EGTA; pH 7.4) supplemented with an oxygen-scavenger system and ATP (2 mM creatine phosphate, 20 mM DTT, 2 mM ATP, 1 mg⋅mL−1 creatine phosphokinase, 0.5 mg⋅mL−1 BSA, 3 mg⋅mL−1 glucose, 0.1 mg⋅mL−1 glucose oxidase, and 0.02 mg⋅mL−1 catalase). Images of rhodamine–phalloidin–labeled actin filaments were recorded with a Nikon A1 TIRF microscope (90× magnification) every 10 s for a total period of 600 s. The data analysis included only filaments moving continuously for at least 20 frames. Biotinylated IXa-MD was immobilized on a streptavidin-coated nitrocellulose surface. The gliding velocity of actin filaments was calculated using the imaging motion analysis software GMimPro (www.mashanov.uk). All assays were carried out at 22 °C.
Optical-Tweezer Studies.
The optical-tweezer procedures and conditions using rhodamine–phalloidin–labeled rabbit F-actin were performed as described previously (42). IXa-MD was bound unspecifically to the coverslip surface, using 10–50 ng⋅mL−1 of protein in the assay buffer described in SI Text, Motility Assay. The experiments were carried out using the assay buffer supplemented with 20 μM ATP and an oxygen scavenger system as described in SI Text, Motility Assay. The stiffness of the optical was 0.015–0.03 pN⋅nm−1, and data were sampled at 5 kHz. The experiments were carried out at 22 °C.
Single-Particle Analysis of EM Data.
From 146 micrographs, 981 images of myosins cross-linking actin filaments were manually picked and windowed. The images of 300 × 300 pixels were large enough to cover three actin pseudorepeats and three consecutive myosin cross-links. For further processing, including alignment, classification, and actin-polarity determination, the images were windowed once more to 90 × 90 pixels covering only a single myosin cross-link.
Analysis of the Myosin-IXa Conformations in the EM Data.
As a first step, all 981 images were reference free-aligned. The average image of myosin-IXa obtained in the first alignment, which neglected the polarity and phase of the actin filaments, already indicated that IXa-MD could bind to four different actin monomers. The dataset was then classified according to these four binding positions using a custom mask, as shown in Fig. S3A. Classification was performed using the hierarchical ascendant classification (HAC) (45); 451 images with sufficient resolution indicated that IXa-MD did bind to only two actin monomers, either on the same or cross-linking two actin filaments, giving rise to three distinct conformations [i.e., the diagonal cross-link conformation (I), the inchworm conformation (III) with myosin binding to two adjacent actin monomers on the same filament, and the bent cross-link conformation (II)] (Fig. S3B). Each of these three conformations was observed in different, mirror-related appearances, depending on how the cross-link was lying on the grid.
Polarity of the Actin Filaments Cross-Linked by IXa-MD.
To analyze the polarity and phase of the cross-linked actin filaments, both filaments were aligned and cross-correlated with simulated EM images of filaments with parallel or antiparallel polarity, aligned in phase or out of phase, using a low pass-filtered actin filament model structure [Protein Data Bank (PDB) ID code 3B63; cartoon and simulated EM are in Fig. 5 C and D]. First, the 451 EM images of myosin cross-links were checked for consistency: only images were taken into account in which both filaments had sufficient resolution to identify the polarity and phase using cross-correlation of the real-EM image with the EM models. Using this approach, 228 EM images were identified as consistent, and 97% of these yielded two actin filaments cross-linked in parallel polarity and aligned in phase (for cartoon, see Fig. 5C). On the basis of this result, all 451 images were aligned once more using the model with two actin filaments of parallel polarity, aligned in phase. The previous classification with reference to the actin-binding positions was once more applied to the newly aligned dataset and yielded three classes of myosin conformation (i.e., diagonal, bent, and inchworm).
Variance Between the IXa-MD Conformations.
To probe which parts of the real EM belonged to actin and which ones to myosin, the variances between the real-EM images and the simulated EM of two actin filaments were calculated (Fig. S3). The variance between the images contributing to the global average and the best actin model [filaments with parallel polarity, aligned in phase (pp/i)] identified the central myosin mass, all four actin-binding positions and an additional mass, presumably the loop 2 calmodulin, as the myosin constituents of the IXa-MD cross-link (Fig. S3F, white intensities). The variances between the three myosin conformations and the actin model confirmed that in the two cross-linking conformations [diagonal (I) and bent (II)], as well as in the noncross-linking inchworm conformation (III), myosin-IXa was attached to two actin-binding sites. Finally, the variance between all class averages revealed three separate spots corresponding to the variable second actin-binding site for the three different myosin conformations.
Interpretation of the Structure of the IXa-MD Cross-Links.
To interpret the actomyosin-IXa cross-links, the EM global average was compared with a model of an actomyosin complex [PDB ID code 4A7F (29)]. This model comprised five actin monomers, tropomyosin, and three myosin-IE heavy chains, each bound to a different actin monomer. First, the tropomyosin was deleted and then three different models were generated, each of which contained only one myosin bound to one of the actin monomers. These actomyosin models were aligned with an actin model spanning 11 actin monomers (PDB ID code 3B63) using flexible structure alignment by chaining alignment fragment pairs allowing twist (FATCAT) rigid pairwise alignment (49). Six models, 3 of which covered 5 actin monomers, whereas the other 3 covered 11 monomers, were then used to analyze the EM averages by cross-correlation, as described previously (23). For each model, a set of 360 low pass-filtered 2D projections was calculated, obtained by 1° rotations of the model around the F-actin axis (Fig. S4A). To identify the best of the six models, we compared the cross-correlation coefficients with the real EM of the optimized projection for each model. The highest score was obtained for the model comprising five actin monomers and the myosin molecule bound to the middle position on the actin filament. Finally, this model was complemented by fitting a calmodulin to the calmodulin-binding motif at the N terminus of loop 2 using the second calmodulin of the myosin-VI structure [PDB ID code 3GN4 (50)].
Acknowledgments
We thank Joachim Rädler (Physics Department, Ludwig-Maximilians-Universität München) and Hendrick Dietz (Physics Department, TU Munich) for making their transmission electron microscopes available. We acknowledge the members of the Center for Nanosciences München and Steve Martin (The Crick Institute) for stimulating discussions. We also acknowledge Deutsche Forschungsgemeinschaft Grant SFB-863-B6, Friedrich-Baur-Stiftung, and Münchner Medizinische Wochenschrift for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612719113/-/DCSupplemental.
References
- 1.Nambiar R, McConnell RE, Tyska MJ. Myosin motor function: The ins and outs of actin-based membrane protrusions. Cell Mol Life Sci. 2010;67(8):1239–1254. doi: 10.1007/s00018-009-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buss F, Kendrick-Jones J. How are the cellular functions of myosin VI regulated within the cell? Biochem Biophys Res Commun. 2008;369(1):165–175. doi: 10.1016/j.bbrc.2007.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88(2):489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- 4.Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014;94(1):235–263. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- 5.Hanley PJ, et al. Motorized RhoGAP myosin IXb (Myo9b) controls cell shape and motility. Proc Natl Acad Sci USA. 2010;107(27):12145–12150. doi: 10.1073/pnas.0911986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thelen S, Abouhamed M, Ciarimboli G, Edemir B, Bähler M. Rho GAP myosin IXa is a regulator of kidney tubule function. Am J Physiol Renal Physiol. 2015;309(6):F501–F513. doi: 10.1152/ajprenal.00220.2014. [DOI] [PubMed] [Google Scholar]
- 7.Chieregatti E, Gärtner A, Stöffler HE, Bähler M. Myr 7 is a novel myosin IX-RhoGAP expressed in rat brain. J Cell Sci. 1998;111(Pt 24):3597–3608. doi: 10.1242/jcs.111.24.3597. [DOI] [PubMed] [Google Scholar]
- 8.Abouhamed M, et al. Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus. Mol Biol Cell. 2009;20(24):5074–5085. doi: 10.1091/mbc.E09-04-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elfrink K, Liao W, Pieper U, Oeding SJ, Bähler M. The loop2 insertion of type IX myosin acts as an electrostatic actin tether that permits processive movement. PLoS One. 2014;9(1):e84874. doi: 10.1371/journal.pone.0084874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Struchholz S, et al. Functional role of the extended loop 2 in the myosin 9b head for binding F-actin. J Biol Chem. 2009;284(6):3663–3671. doi: 10.1074/jbc.M808338200. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz M, Holmes KC. The actin-myosin interface. Proc Natl Acad Sci USA. 2010;107(28):12529–12534. doi: 10.1073/pnas.1003604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geeves MA, Holmes KC. The molecular mechanism of muscle contraction. Adv Protein Chem. 2005;71:161–193. doi: 10.1016/S0065-3233(04)71005-0. [DOI] [PubMed] [Google Scholar]
- 13.Inoue A, Saito J, Ikebe R, Ikebe M. Myosin IXb is a single-headed minus-end-directed processive motor. Nat Cell Biol. 2002;4(4):302–306. doi: 10.1038/ncb774. [DOI] [PubMed] [Google Scholar]
- 14.Post PL, et al. Myosin-IXb is a single-headed and processive motor. J Biol Chem. 2002;277(14):11679–11683. doi: 10.1074/jbc.M111173200. [DOI] [PubMed] [Google Scholar]
- 15.Liao W, Elfrink K, Bähler M. Head of myosin IX binds calmodulin and moves processively toward the plus-end of actin filaments. J Biol Chem. 2010;285(32):24933–24942. doi: 10.1074/jbc.M110.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kambara T, Ikebe M. A unique ATP hydrolysis mechanism of single-headed processive myosin, myosin IX. J Biol Chem. 2006;281(8):4949–4957. doi: 10.1074/jbc.M509141200. [DOI] [PubMed] [Google Scholar]
- 17.Nalavadi V, et al. Kinetic mechanism of myosin IXB and the contributions of two class IX-specific regions. J Biol Chem. 2005;280(47):38957–38968. doi: 10.1074/jbc.M507161200. [DOI] [PubMed] [Google Scholar]
- 18.Omelchenko T, Hall A. Myosin-IXA regulates collective epithelial cell migration by targeting RhoGAP activity to cell-cell junctions. Curr Biol. 2012;22(4):278–288. doi: 10.1016/j.cub.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood JM, Olson MF. Collective migration: Spatial tension relief. Curr Biol. 2012;22(4):R125–R127. doi: 10.1016/j.cub.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Schaller V, Weber C, Semmrich C, Frey E, Bausch AR. Polar patterns of driven filaments. Nature. 2010;467(7311):73–77. doi: 10.1038/nature09312. [DOI] [PubMed] [Google Scholar]
- 21.Weber CA, et al. Random bursts determine dynamics of active filaments. Proc Natl Acad Sci USA. 2015;112(34):10703–10707. doi: 10.1073/pnas.1421322112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin SR, Bayley PM. Calmodulin bridging of IQ motifs in myosin-V. FEBS Lett. 2004;567(2-3):166–170. doi: 10.1016/j.febslet.2004.04.053. [DOI] [PubMed] [Google Scholar]
- 23.Batters C, Brack D, Ellrich H, Averbeck B, Veigel C. Calcium can mobilize and activate myosin-VI. Proc Natl Acad Sci USA. 2016;113(9):E1162–E1169. doi: 10.1073/pnas.1519435113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malgaroli A, Milani D, Meldolesi J, Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987;105(5):2145–2155. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci USA. 1999;96(5):2135–2140. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridley AJ, et al. Cell migration: Integrating signals from front to back. Science. 2003;302(5651):1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 27.Wei C, et al. Calcium flickers steer cell migration. Nature. 2009;457(7231):901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin SR, Bayley PM. Regulatory implications of a novel mode of interaction of calmodulin with a double IQ-motif target sequence from murine dilute myosin V. Protein Sci. 2002;11(12):2909–2923. doi: 10.1110/ps.0210402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behrmann E, et al. Structure of the rigor actin-tropomyosin-myosin complex. Cell. 2012;150(2):327–338. doi: 10.1016/j.cell.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batters C, Veigel C. Mechanics and activation of unconventional myosins. Traffic. 2016;17(8):860–871. doi: 10.1111/tra.12400. [DOI] [PubMed] [Google Scholar]
- 31.Veigel C, Schmidt CF. Moving into the cell: Single-molecule studies of molecular motors in complex environments. Nat Rev Mol Cell Biol. 2011;12(3):163–176. doi: 10.1038/nrm3062. [DOI] [PubMed] [Google Scholar]
- 32.Nishikawa M, et al. A unique mechanism for the processive movement of single-headed myosin-IX. Biochem Biophys Res Commun. 2006;343(4):1159–1164. doi: 10.1016/j.bbrc.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 33.Kiuchi T, Nagai T, Ohashi K, Mizuno K. Measurements of spatiotemporal changes in G-actin concentration reveal its effect on stimulus-induced actin assembly and lamellipodium extension. J Cell Biol. 2011;193(2):365–380. doi: 10.1083/jcb.201101035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sellers JR, Veigel C. Direct observation of the myosin-Va power stroke and its reversal. Nat Struct Mol Biol. 2010;17(5):590–595. doi: 10.1038/nsmb.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veigel C, Schmitz S, Wang F, Sellers JR. Load-dependent kinetics of myosin-V can explain its high processivity. Nat Cell Biol. 2005;7(9):861–869. doi: 10.1038/ncb1287. [DOI] [PubMed] [Google Scholar]
- 36.Lynch TJ, et al. ATPase activities and actin-binding properties of subfragments of Acanthamoeba myosin IA. J Biol Chem. 1986;261(36):17156–17162. [PubMed] [Google Scholar]
- 37.Stafford WF, Walker ML, Trinick JA, Coluccio LM. Mammalian class I myosin, Myo1b, is monomeric and cross-links actin filaments as determined by hydrodynamic studies and electron microscopy. Biophys J. 2005;88(1):384–391. doi: 10.1529/biophysj.104.045245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheney RE, Mooseker MS. Unconventional myosins. Curr Opin Cell Biol. 1992;4(1):27–35. doi: 10.1016/0955-0674(92)90055-h. [DOI] [PubMed] [Google Scholar]
- 39.Rhoads AR, Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. 1997;11(5):331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- 40.Batters C, et al. Calmodulin regulates dimerization, motility, and lipid binding of Leishmania myosin XXI. Proc Natl Acad Sci USA. 2014;111(2):E227–E236. doi: 10.1073/pnas.1319285110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De La Cruz EM, Ostap EM. Kinetic and equilibrium analysis of the myosin ATPase. Methods Enzymol. 2009;455:157–192. doi: 10.1016/S0076-6879(08)04206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veigel C, Bartoo ML, White DCS, Sparrow JC, Molloy JE. The stiffness of rabbit skeletal actomyosin cross-bridges determined with an optical tweezers transducer. Biophys J. 1998;75(3):1424–1438. doi: 10.1016/S0006-3495(98)74061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohi M, Li Y, Cheng Y, Walz T. Negative staining and image classification - Powerful tools in modern electron microscopy. Biol Proced Online. 2004;6:23–34. doi: 10.1251/bpo70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang G, et al. EMAN2: An extensible image processing suite for electron microscopy. J Struct Biol. 2007;157(1):38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Frank J. Three-Dimensional Electron Microscopy of Macromolecular Assemblies: Visualization of Biological Molecules in Their Native State. Oxford Univ Press; New York: 2006. [Google Scholar]
- 46.Kron SJ, Toyoshima YY, Uyeda TQP, Spudich JA. Assays for actin sliding movement over myosin-coated surfaces. Methods Enzymol. 1991;196:399–416. doi: 10.1016/0076-6879(91)96035-p. [DOI] [PubMed] [Google Scholar]
- 47.Wang F, et al. Effect of ADP and ionic strength on the kinetic and motile properties of recombinant mouse myosin V. J Biol Chem. 2000;275(6):4329–4335. doi: 10.1074/jbc.275.6.4329. [DOI] [PubMed] [Google Scholar]
- 48.Batters C, Woodall KA, Toseland CP, Hundschell C, Veigel C. Cloning, expression, and characterization of a novel molecular motor, Leishmania myosin-XXI. J Biol Chem. 2012;287(33):27556–27566. doi: 10.1074/jbc.M112.381301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye Y, Godzik A. Flexible structure alignment by chaining aligned fragment pairs allowing twists. Bioinformatics. 2003;19(Suppl 2):ii246–ii255. doi: 10.1093/bioinformatics/btg1086. [DOI] [PubMed] [Google Scholar]
- 50.Mukherjea M, et al. Myosin VI dimerization triggers an unfolding of a three-helix bundle in order to extend its reach. Mol Cell. 2009;35(3):305–315. doi: 10.1016/j.molcel.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]