Significance
We developed an MS-based method to determine kinetic isotope effects and binding isotope effects on protein lysine methyltransferase SET8-catalyzed monomethylation. These parameters, coupled with steady-state kinetics and molecular modeling, outlined the reaction path of SET8-catalyzed methylation. Upon the formation of the S-adenosyl-l-methionine–SET8–histone 4 lysine 20 intermediate complex followed by lysine deprotonation, the reaction goes through an early, asymmetrical transition state (TS) with the small engagement of the C-N bond and the partial dissociation of the C-S bond. This TS structure is distinct from the known TS structures of other protein lysine methyltransferases (PKMTs) and thus presents the feasibility to design selective TS analog inhibitors against PKMTs. The developed techniques can also be generally applicable to examining other protein methylation and posttranslational modifications.
Keywords: PMT, PKMT, KIE, BIE, methylation
Abstract
Protein lysine methyltransferases (PKMTs) catalyze the methylation of protein substrates, and their dysregulation has been linked to many diseases, including cancer. Accumulated evidence suggests that the reaction path of PKMT-catalyzed methylation consists of the formation of a cofactor(cosubstrate)–PKMT–substrate complex, lysine deprotonation through dynamic water channels, and a nucleophilic substitution (SN2) transition state for transmethylation. However, the molecular characters of the proposed process remain to be elucidated experimentally. Here we developed a matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) method and corresponding mathematic matrix to determine precisely the ratios of isotopically methylated peptides. This approach may be generally applicable for examining the kinetic isotope effects (KIEs) of posttranslational modifying enzymes. Protein lysine methyltransferase SET8 is the sole PKMT to monomethylate histone 4 lysine 20 (H4K20) and its function has been implicated in normal cell cycle progression and cancer metastasis. We therefore implemented the MS-based method to measure KIEs and binding isotope effects (BIEs) of the cofactor S-adenosyl-l-methionine (SAM) for SET8-catalyzed H4K20 monomethylation. A primary intrinsic 13C KIE of 1.04, an inverse intrinsic α-secondary CD3 KIE of 0.90, and a small but statistically significant inverse CD3 BIE of 0.96, in combination with computational modeling, revealed that SET8-catalyzed methylation proceeds through an early, asymmetrical SN2 transition state with the C-N and C-S distances of 2.35–2.40 Å and 2.00–2.05 Å, respectively. This transition state is further supported by the KIEs, BIEs, and steady-state kinetics with the SAM analog Se-adenosyl-l-selenomethionine (SeAM) as a cofactor surrogate. The distinct transition states between protein methyltransferases present the opportunity to design selective transition-state analog inhibitors.
Stepwise progression of an enzyme-catalyzed chemical reaction is accompanied by changes of bond orders and vibrational modes involved with specific atoms of the reactant(s) (1, 2). Such changes can be traced experimentally by measuring the ratios of turnover rates [kinetic isotope effects (KIEs)] or binding affinities [binding isotope effects (BIEs)] of the reactant(s) when the relevant atoms are replaced by heavy isotopes (3, 4). KIEs and BIEs are thus useful parameters for elucidating transition-state (TS) structures and catalytic mechanisms, which sometimes cannot be elucidated readily through sole measurement of steady-state kinetics (5–9). A sufficient set of KIEs and BIEs at the positions involved with bond motions can afford electrostatic and geometric constraints, when combined with computational modeling, to define an enzymatic TS (10–12). This information provides not only the atomic resolution of the transient structure at the highest energy summit along the reaction path, but also structural guidance for designing tight-binding TS analog inhibitors (13, 14).
Multiple approaches have been documented to measure KIEs and BIEs of enzymatic reactions (3, 15–17). A conventional method is to determine individual steady-state kinetic parameters (kcat and Km) with a pair of isotopic substrates and then calculate their ratios (18). This method is straightforward and can afford kinetic parameters and thus noncompetitive KIEs beyond ±10% from unity (2). Given that the values of many KIEs and BIEs of enzymatic reactions are within a range of a few percent from unity, more precise measurement requires enzymatic reactions to be carried out competitively with mixed isotopic substrates. This competitive condition is expected to minimize the errors originating from sample-to-sample variation (19, 20). The isotopic ratios of prereacted substrates vs. bound substrates or depleted substrates (or unconsumed substrates) are then quantified for the calculation of BIEs or KIEs, respectively. Several approaches that can precisely determine isotopic ratios of two mixed isomers are remote radioactive labeling, 1D/2D NMR spectroscopy, and isotope ratio MS (16, 21–23).
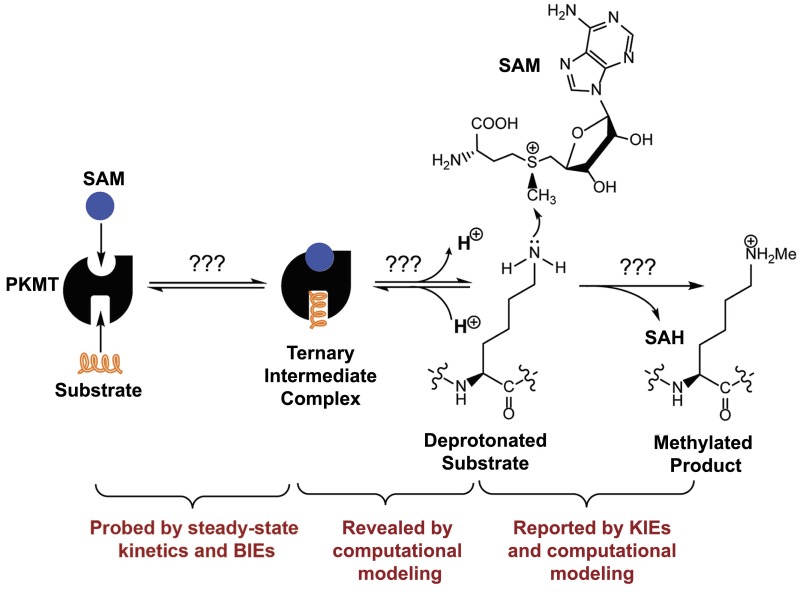
Protein lysine methyltransferases (PKMTs) belong to a subfamily of posttranslational modifying enzymes (24, 25). PKMTs catalyze the methylation of histone and nonhistone protein substrates at targeted lysine residues (26–28). All PKMTs use S-adenosyl-l-methionine (SAM) as a ubiquitous methyl donor (cosubstrate or cofactor with the latter used thereafter), but diverge in terms of their degree of substrate methylation (mono-, di-, and tri-) (29). PKMTs can play essential roles in modulating gene transcription, cellular differentiation, and organ development, and their dysregulation has been frequently implicated in diseases, including cancer (26, 30, 31). Most PKMTs are characterized by their canonical ∼130-aa SET domain (>50 aa encoded by the human genome) (24, 25). Several PKMTs have been examined previously under steady-state conditions and shown to follow a bisubstrate random or ordered sequential mechanism to bind SAM and substrates (Fig. 1) (32, 33). Computational modeling suggests that the target lysine residues of substrates are subject to deprotonation through dynamic water channels, followed by a putative nucleophilic substitution (SN2) TS for the transmethylation reaction (Fig. 1) (34, 35). However, limited efforts have been made to explore experimentally the (SN2) TS of PKMT-catalyzed methylation. Recent evidence from static PKMT structures and dynamic NMR chemical shifts suggests the existence of equatorial noncanonical CH–O interaction between the sulfonium methyl moiety of SAM and the oxygens of active-site amino acid residues of PKMTs (36–38), although it remains to examine how the equatorial CH–O interaction participates in the formation of the TS of PKMT-catalyzed methylation reaction.
Fig. 1.
A hypothetical reaction path of PKMT-catalyzed lysine monomethylation and relevant biochemical methods to examine each aspect of the process.
Protein lysine methyltransferase SET8 is the sole PKMT to monomethylate histone 4 lysine 20 (H4K20) and its functions have been implicated for normal cell cycle progression and cancer metastasis (39–41). The biological relevance of SET8, as well as our general interest in PKMTs, inspired us to leverage BIE and KIE studies of SET8 to explore bond motions along the reaction path of the PKMT-catalyzed methylation. Given the convenience of MS in characterizing protein methylation (42, 43), we developed a matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) method and corresponding mathematic matrix to determine precisely the ratios of isotopically labeled H4K20 peptide. With this approach, BIEs and KIEs of SAM and SeAM (Se-adenosyl-L-selenomethionine, a more reactive SAM surrogate) were determined for SET8-catalzyed H4K20 methylation. An inverse α-secondary (α-2°-CD3) CD3 KIE and a normal primary 13C KIE (1°-13C KIE) of SAM, together with computational modeling, indicate that SET8 adapts an early, asymmetrical SN2 TS for H4K20 methylation. In contrast, a small inverse BIE of [S-CD3]-SAM, together with structural evidence, argues that the large inverse intrinsic CD3 KIE originates from the structural changes at the TS rather than upon the formation of the SAM–SET8 complex. The small inverse BIE of [S-CD3]-SAM could arise from the noncanonical CH–O interaction upon the formation of the SAM–SET8 complex. These results are also consistent with BIE and steady-state kinetics of SeAM as a cofactor surrogate. Collectively, the characteristic KIEs and BIEs, together with structural and computational evidence, delineate the TS of SET8-catalyzed H4K20 monomethylation. The current work further serves as a starting point to leverage the MALDI-TOF-MS method to measure KIEs and BIEs of other PKMTs for characterizing their TS structures. Knowing the differences between these TS structures can be a key step toward developing selective TS analog inhibitors.
Results and Discussion
Kinetics of SET8-Catalyzed H4K20 Monomethylation.
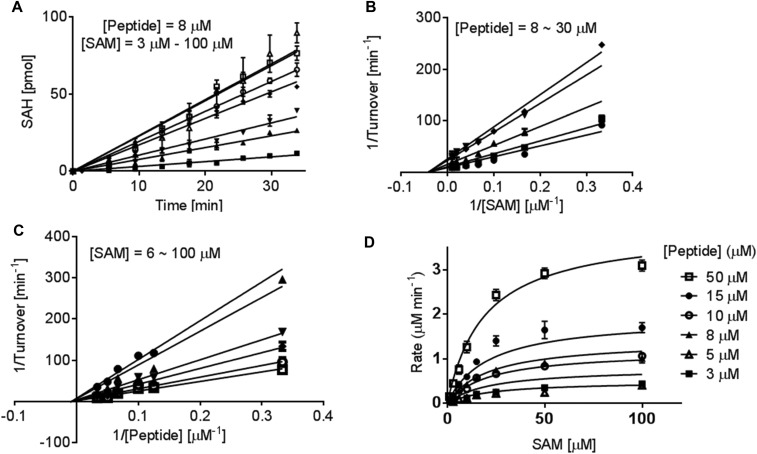
To use SET8-catalyzed H4K20 monomethylation as a model for studying KIEs and dissecting reaction paths of PKMTs, we first examined steady-state kinetics of SET8 with the SAM cofactor and H4K20 peptide substrate (Fig. S1). We previously developed an enzyme-coupled luciferase assay for PKMTs and demonstrated its use to characterize the steady-state kinetics of SET7/9 (44). In the assay, the reaction progress was monitored with a luciferin–luciferase kit by converting enzymatically the reaction byproduct S-adenosyl-l-homocysteine (SAH) into ATP. This assay was implemented to characterize the steady-state kinetics of SET8-catalyzed H4K20 methylation. By systematically altering the concentrations of the SAM cofactor and H4K20 peptide substrate, the initial velocities of SET8-catalyzed methylation were obtained by plotting the initial linear increase of luminescence vs. reaction time (10–20 min, depending on the concentrations of SAM and H4K20 peptide) (Fig. S1A). The initial velocities were then plotted against the concentrations of SAM and H4K20 to generate double-reciprocal curves (Fig. S1 B and C) (44, 45). Here, the linear regression converged in the second quadrant rather than on the y axis and thus excluded a ping-pong mechanism and a rapid-equilibrium ordered sequential mechanism (46). Because four possible mechanisms (rapid-equilibrium random, steady-state random, steady-state ordered, and Theorell–Chance) can fit the current kinetic data and dissecting the exact kinetic mechanism is not the focus of this work, we globally fitted the experimental data, using Eqs. S1 and S2 (SI Materials and Methods). The enzymatic turnover kcat of 7.0 ± 0.8 min−1, Km,SAM of 16 ± 6 µM, Km,H4K20 of 40 ± 8 µM, and α of 1.4 were obtained (Fig. S1D and Table 1).
Fig. S1.
Steady-state kinetics and reaction mechanism of SET8-catalyzed H4K20 monomethylation. The kinetic parameters of SET8 methylation were examined using 1 µM SET8 by systematically varying the amount of the H4K20 substrate peptide (3–50 µM) and SAM (3–100 µM). The product of the methylation reaction was quantified with the enzyme-coupled luciferase. (A) Representative initial-rate kinetics for SET8-catalyzed H4K20 methylation with a fixed amount of peptide H4 substrate (8 µM) and varied amounts of SAM cofactor: (■, 3 μM; ▲, 6 μM; ▼, 10 μM; ◆, 15 μM;  , 25 μM;
, 25 μM;  , 50 μM; and ∆, 100 μM). The initial linear range varies with cofactor and substrate concentrations. (B and C) Lineweaver–Burk analysis of the initial velocities vs. varied concentrations of the H4K20 substrate (●, 30 μM; ■, 20 μM; ▲, 15 μM; ▼, 10 μM; and ◆, 8 μM) and SAM (●, 6 μM; ▼,15 μM; ◆, 25 μM;
, 50 μM; and ∆, 100 μM). The initial linear range varies with cofactor and substrate concentrations. (B and C) Lineweaver–Burk analysis of the initial velocities vs. varied concentrations of the H4K20 substrate (●, 30 μM; ■, 20 μM; ▲, 15 μM; ▼, 10 μM; and ◆, 8 μM) and SAM (●, 6 μM; ▼,15 μM; ◆, 25 μM;  , 50 μM; and
, 50 μM; and  , 100 μM). All of the initial rates converge in the secondary quadrant. (D) Global fit of the initial velocities to a general, bisubstrate kinetic mechanism against SAM concentration to afford kcat, Km,SAM, Km,H4K20 and α according to Eqs. S1 and S2 with kcat = 7.0 ± 0.8 min−1, Km,peptide = 40 ± 8 µM, Km,cofactor = 16 ± 6 µM, and α = 1.4 ± 0.8 (45).
, 100 μM). All of the initial rates converge in the secondary quadrant. (D) Global fit of the initial velocities to a general, bisubstrate kinetic mechanism against SAM concentration to afford kcat, Km,SAM, Km,H4K20 and α according to Eqs. S1 and S2 with kcat = 7.0 ± 0.8 min−1, Km,peptide = 40 ± 8 µM, Km,cofactor = 16 ± 6 µM, and α = 1.4 ± 0.8 (45).
Table 1.
Comparison of apparent steady-state kinetics (kcat and Km), BIEs, forward commitment factors, KIEs, and BIEs with SAM and SeAM as cofactors (cosubstrates) in SET8-catalyzed H4K20 monomethylation
| Parameters | SAM | SeAM |
| kcat, min−1 | 7.0 ± 0.8 | 13 ± 2* |
| Km, μM | 16 ± 6 | 16 ± 6* |
| CD3 or 13CD3 BIE | 0.959 ± 0.006 | 0.962 ± 0.004 |
| CT3 or 14CT3 BIE | 0.979 ± 0.009 | NA |
| Cf | 0.10 ± 0.01 | 0.10 ± 0.01 |
| 0.878 ± 0.008 | 0.99 ± 0.02 | |
| 0.90 ± 0.01 | 1.02 ± 0.02 | |
| calculated | 0.896† | 1.018‡ |
| 0.907 ± 0.007 | 1.04 ± 0.01 | |
| 0.94 ± 0.02 | 1.09 ± 0.02 | |
| 1.04 ± 0.02 | 1.06 ± 0.02 | |
| calculated | 1.058† | 1.061‡ |
| 0.785 ± 0.009 | NA | |
| 0.78 ± 0.01 | NA | |
| calculated | 0.773† | NA |
NA, not applicable.
These values were calculated on the basis of kcat and Km values of SAM, and (1.94 and 1.03 for the latter two, respectively).
The experimental SAM KIEs match TS structures with dC-S = 2.00–2.05 Å and dC-N = 2.35–2.40 Å. The calculated KIEs are listed for a representative TS structure with dC-S = 2.05 Å (bond order 0.845), and dC-N = 2.38 Å (bond order 0.117).
The experimental SeAM KIEs match a wide range of TS structures with dC-Se + dC-N = 4.8–4.9 Å. The calculated KIEs are listed for a representative TS structure with dC-Se = 2.50 Å and dC-N = 2.35 Å.
Quantification of Isotopic Ratios by Deconvoluted MALDI-TOF MS.
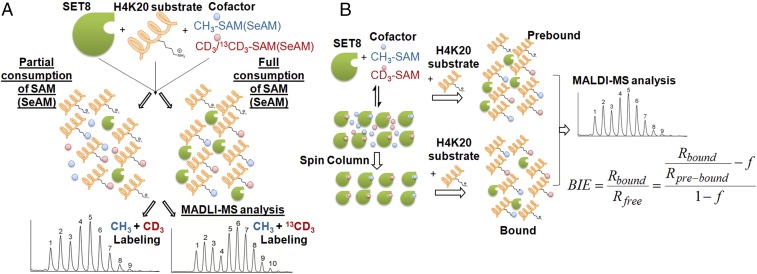
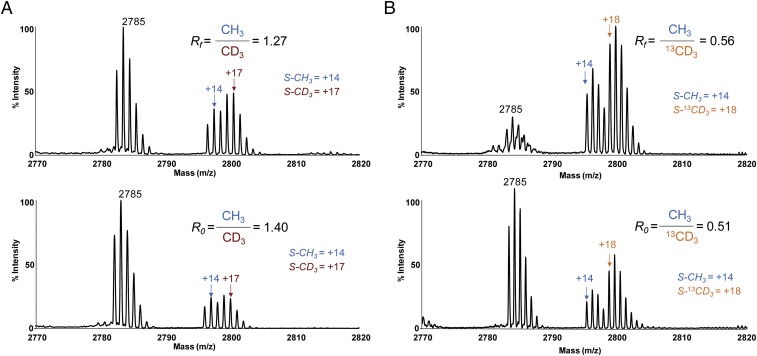
Whereas several previously established methods show capability for precise measurement of KIEs, MALDI-TOF MS was implemented here because of the generality and convenience of this method to probe protein posttranslational modifications with isotopically labeled cofactors (2, 3, 15, 21, 47). Unmodified H4K20 peptide displays a dominant characteristic set of six mass multiplets, which maintain fixed ratios of individual peak areas and arise from natural isotope abundance of 13C and other heteroatoms (Figs. 2A, 3, and 4). Modifying this peptide by one equivalent of [S-CH3]-SAM or [S-CD3]-SAM causes +14-Da () or +17-Da () mass shifts, respectively, without causing a significant change of the peak ratios among the six multiplets (Figs. 3A and 4A). Modification of H4K20 peptide by a mixture of [S-CH3]-SAM and [S-CD3]-SAM results in nine mass multiplets, of which the first three peaks ( i = 1,2,3) and the last three peaks ( i = 7,8,9) arise from [S-CH3]-SAM and [S-CD3]-SAM, respectively (Fig. 3A). The three central peaks ( j = 4,5,6) are the sum of the overlapping modification by both [S-CH3]-SAM and [S-CD3]-SAM. Given the fixed ratios for individual sets of six multiplets, the contribution of [S-CH3]-SAM () and [S-CD3]-SAM () to their sum ( j = 4,5,6) was deconvoluted with either the first three ( i = 1,2,3) or the last three ( i = 7,8,9) nonoverlapping peaks as independent variables (SI Materials and Methods). The resulting two sets of peak areas and were then averaged according to their relative weights ( vs. ) (Fig. 3A). Similarly, modifying the H4K20 peptide by one equivalent of [S-CH3]-SAM or [S-13CD3]-SAM causes +14-Da () or +18-Da () mass shift, respectively (Figs. 3B and 4B). The resultant isotopic ratios can be deconvoluted in a similar manner (SI Materials and Methods). The robustness of our MS-based deconvolution method to distinguish small changes of isotopic ratios was validated by its reproducible readouts (100% ± 0.3% precision) with isotopically modified peptides (Table 1) and the consistence of the resultant BIEs and KIEs with those obtained with the conventional radiometric method (see BIE and KIE of the [S-CT3]/[S-14CH3]-SAM cofactor pair). The MS-based deconvolution method was thus implemented in the current work to examine CD3 BIE, CD3 KIE, and 13CD3 KIE of SET8-catalyzed H4K20 monomethylation. This MS-based method is expected to be generally applicable to examining BIEs and KIEs of PKMTs as well as other posttranslational modifications.
Fig. 2.
Overview of KIE measurement under competitive conditions. (A) Schematic description of a competition reaction with a pair of isotopic cofactors and the resultant overlapping MS data with CH3/CD3 and CH3/13CD3 cofactor pairs. The reaction sample was split into two portions for partial consumption and full consumption of the pair of isotopic cofactors, respectively. Their light-to-heavy isotope ratios (blue vs. red) are quantified by MS as described in Materials and Methods. (B) Schematic description of BIE measurement under competitive conditions. Here a pair of prebound or SET8-bound CH3/CD3 isotopic cofactors were converted to label H4K20 peptide substrate under competitive conditions. The resultant methylated H4K20 peptide was subjected to MS analysis to quantify the isotopic ratios as described in Materials and Methods.
Fig. 3.
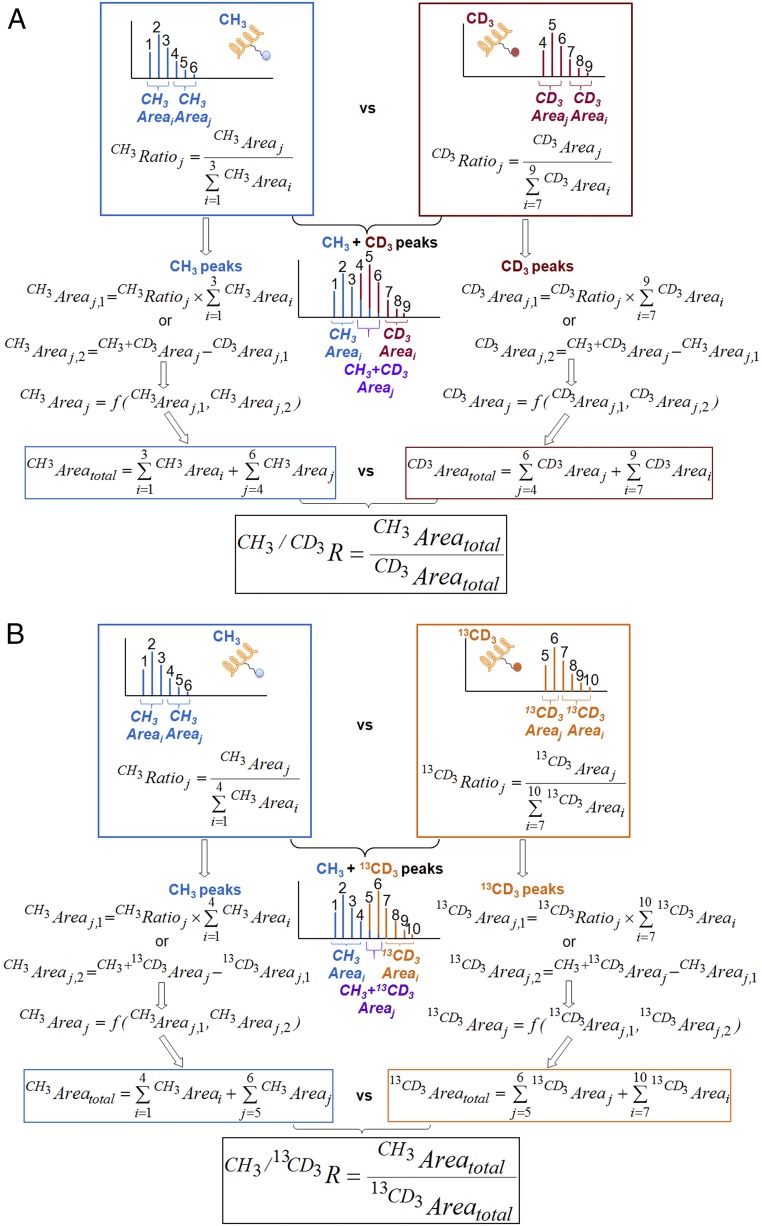
Overview of mathematical matrix to deconvolute the MS data and calculate light-to-heavy isotope ratios according to Eqs. S3–S7. (A) -CH3 vs. -CD3 (blue vs. red) (B) -CH3 vs. -13CD3 (blue vs. orange).
Fig. 4.
(A and B) Representative MALDI-TOF-MS data for partial consumption (Top) and 100% consumption (Bottom) of (A) [S-CH3]-SAM and [S-CD3]-SAM and (B) [S-CH3]-SeAM and [S-13CD3]-SeAM as a pair of isotopic cofactors. The ratios of [CH3] vs. [CD3] before initializing the reaction (R0) and after reaching partial completion (Rf) were determined by the MS-deconvolution method (Fig. 3 and Materials and Methods). Their ratio R0/Rf was used to determine the inverse KIEs according to Eq. 1.
Inverse BIEs of [S-CD3]-SAM and [S-CT3]-SAM.
Several prior works have shown that significant KIEs can arise from BIEs during the formation of a substrate–enzyme complex rather than the bond-order or conformational changes at the enzymatic TS (6, 48). The BIEs can either positively or negatively affect observed KIEs (49). Before obtaining we therefore evaluated the potential contribution of the BIE of [S-CD3]-SAM along the path of the SET8-catalyzed methylation reaction (Fig. 2B). A small but significant inverse CD3 BIE of 0.959 ± 0.006 was obtained upon calculating the difference of the isotopic ratios between prebound and SET8-bound SAM cofactors (Table 1). Given the small magnitude of the inverse CD3 BIE revealed by the MS-based method, we further validated the approach and the resultant inverse CT3 BIE with a radiometric method, using the [S-CT3]/[S-14CH3]-SAM cofactor pair. Both [S-CD3]-SAM and [S-CT3]-SAM show small but significantly inverse BIEs (Table 1). These results indicate an increased vibrational stiffness of the sulfonium-methyl group of SAM upon its binding by SET8.
Inverse α-2°-CD3 KIE for SET8-Catalyzed H4K20 Methylation with [S-CD3]-SAM as Cofactor.
The three hydrogen atoms of the sulfonium methyl group of SAM are one bond away from the electrophilic sulfonium-methyl reaction center. of [S-CD3]-SAM is expected to reflect the accumulated changes of bond orders and vibration modes involved with the deuterium atoms of the SAM cofactor from its unbound ground state to the enzymatic TS (1). Given the ability of the MS deconvolution method to distinguish the small changes of the isotopic ratios of the modified peptide product, we carried out internal competition experiments with a mixture of [S-CH3]-SAM and [S-CD3]-SAM cofactors and measured the α-2°-CD3 KIE of SET8-catalyzed H4K20 methylation (Figs. 3A and 4A). In these experiments, [S-CD3]-SAM displayed of 0.878 ± 0.008 (Table 1). In comparison with the intrinsic α-2°-CD3 KIE, the obtained above under the competitive conditions could be suppressed by forward commitment factor (Cf) and reverse commitment factor (Cr), respectively (19). We then measured the Cf of the cofactor in SET8-catalyzed H4K20 methylation with an isotope-trapping method (SI Materials and Methods). SET8 displays a small Cf (10%) for SAM cofactor (Table 1), suggesting that SAM is subject to a rapid exchange between its unbound and SET8-bound form within the timescale of the progression of the SET8-bound SAM toward the reaction product. The Cr of SET8 for SAM is negligible because of the formation of the stronger N-C bond and thus the irreversible character of SET8-catalyzed methylation reaction. Upon correction for Cf and BIE (Materials and Methods, Eqs. 1, 2a, and 2b), of 0.90 ± 0.01 of [S-CD3]-SAM was obtained for SET8-catalyzed methylation (Table 1). The significantly inverse α-2 °CD3 KIE of around 10%, together with the small inverse CD3 BIE of around 4% (Table 1), argues that that the vibrational stiffness of the sulfonium-methyl group of SAM is subject to a small increase upon the formation of the SAM–SET8 binary complex (a modest inverse BIE of 0.96) and then a more dramatic increase along the reaction path toward its TS (a significant inverse intrinsic α-2°-CD3 KIE of 0.90) (Table 1).
Normal 1°-13C KIE for SET8-Catalyzed H4K20 Methylation.
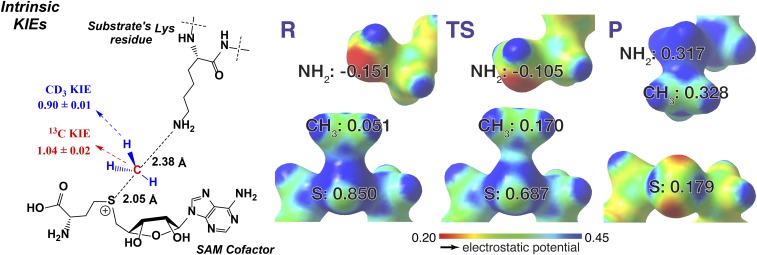
Because the magnitude of is sensitive to many factors besides the change of bond orders at the TS, we determined the intrinsic 1°-13C KIE () as another constraint to uncover the TS of SET8-catalyzed H4K20 methylation. Here and were obtained similarly with the MS-based method with [S-CH3]-SAM and [S-13CD3]-SAM as a cofactor pair (Figs. 3B and 4B). Here the use of [S-CH3]-SAM paired with [S-13CD3]-SAM rather than [S-13CH3]-SAM is expected to give better separation and thus precise isotopic quantification of the MALDI multiples of the products. The intrinsic 1°-13C KIE was obtained after correcting from according to Based on the rule of the geometric mean (50), the free energy contributions from two-isotope labeling in a molecule are additive; thus, the dual-labeling intrinsic KIE is the product of individual intrinsic KIEs; e.g., It is important to point out that the rule of geometric mean generally holds very well except in some hydrogen transfer reactions involving significant quantum-mechanic (QM) tunneling effects (51). The normal primary of 1.04 ± 0.02 indicates that there is a small decrease of the overall bond order of the methyl carbon of SAM from the SET8-bound ground state to the enzymatic TS (Table 1).
Computational Modeling of Transition State of SET8-Catalyzed H4K20 Methylation.
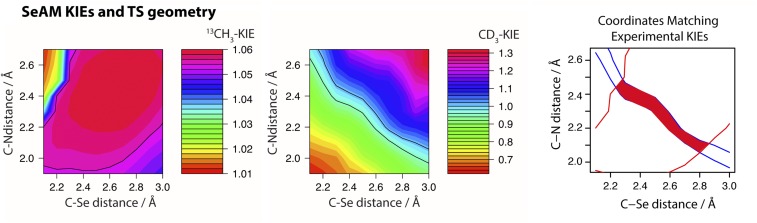
Molecular dynamic simulation suggested that SET8 catalyzes H4K20 monomethylation through an early SN2 TS with the C-N distance of 2.46 ± 0.15 Å, the C-S bond distance of 2.01 ± 0.12 Å, and the N-C-S bond angle of 172° (34). This coordinate is also in agreement with the C-S distance extracted from the crystal structure of the ternary complex of SET8 with the reaction products SAH and H4K20me (PDB ID: 2BQZ). Starting from the atomic coordinates of the SAH-SET8-H4K20me complex, we gradually altered the C-N and C-S distances (Materials and Methods) to search for the TS candidates with the experimental KIEs as geometrical constraints ( = 0.90 ± 0.01 and = 1.04 ± 0.02). Geometry optimizations and frequency calculations were performed by Gaussian09 (52) with a density functional theory with an M062X functional and 6-31+G(d,p) basis set (53), and KIEs were calculated using ISOEFF (54). The only TS geometry that matches the experimental KIEs shows early, asymmetrical SN2 TS characters with the C-N distance of 2.35–2.40 Å (bond order ∼0.12) and the C-S bond distance of 2.00–2.05 Å (bond order ∼0.85, Table 1 and Fig. 5). The modestly inverse of 0.90 is a key parameter to define the distinct TS geometry because late, asymmetrical SN2 TS geometries, although with the matched of 1.04, were calculated to show a higher magnitude of inverse ( < 0.7). The TS uncovered by KIEs is in remarkable agreement with the TS structure proposed by molecular dynamics simulations (2.35–2.40 Å vs. 2.46 ± 0.15 Å for C-N distances; 2.00–2.05 Å vs. 2.01 ± 0.12 for C-S distances). In contrast, the TS geometry without KIE constraints showed nearly symmetric characters with the C-N distance of 2.1 Å (bond order ∼0.32) and the C-S bond distance of 2.2 Å (bond order ∼0.62). These results delineated that SETD8 catalyzes the H4K20 monomethylation through an early, asymmetrical SN2 TS with the N-C distance around 2.4 Å, the N-S distance of 4.4 Å, and the N-C-S bond angle of 178° (Fig. 5). This KIE-constrained TS is enzymatically facilitated and is distinct from the TS obtained upon relaxing the C-N and C-S distances.
Fig. 5.
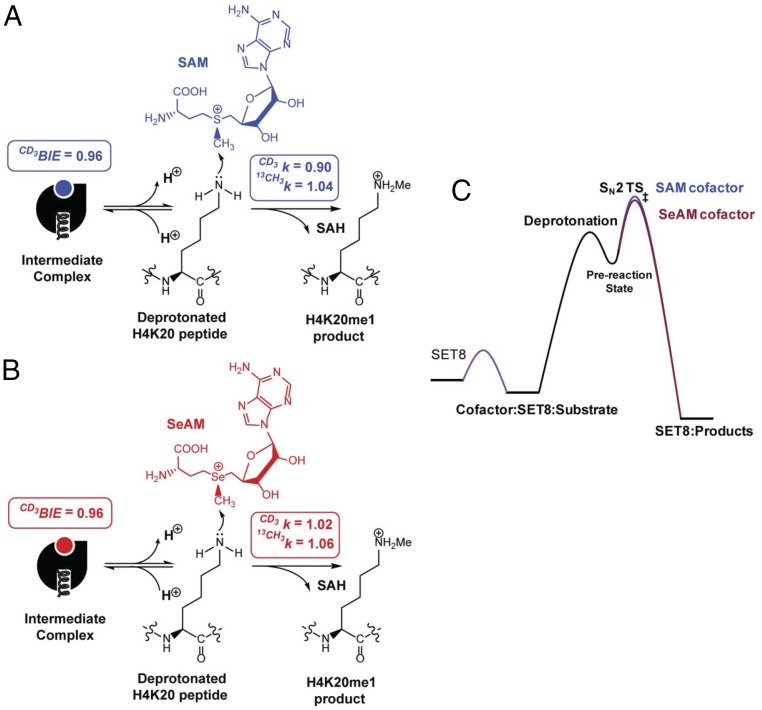
TS structure of SET8-catalyzed H4K20 monomethylation with SAM as the cofactors. α-2°-CD3 KIE and 1-13C KIE (intrinsic KIEs, Left) were used as computational constraints to solve the TS structures. The TS (Center) shows an early, asymmetrical character with the long C-N and short C-S distances of 2.35–2.40 Å and 2.00–2.05 Å, respectively. The structures of reactants (R, Left) and products (P, Right) are shown for comparison. The numbers in R, TS, and P are NBO charges for S, -CH3, and -NH2 with red for electron rich and blue for electron deficient in ESPS maps.
Additional Confirmation of Transition State of SET8-Catalyzed H4K20 Methylation Using .
To validate the accuracy of KIEs obtained by the MALDI-TOF-MS method, we determined with the [S-CT3]-SAM and [S-14CH3]-SAM as the paired cofactors, using an established radiometric method. The of 0.78 ± 0.01 is in agreement with the theoretical = 0.773 obtained for the TS structure with the MS-based KIEs as experimental constraints (Table 1). Such consistency further validates the accuracy of the MALDI-TOF-MS method to determine KIEs as well as the KIE-constrained TS structure.
Origins of KIEs and Their Implication for an SN2 Transition State of SET8-Catalyzed Methylation with SAM as a Cofactor.
The large inverse of 0.878 and of 0.90 for [S-CD3]-SAM for the SET8-catalyzed methylation argues for a more constrained TS around the methyl group of SAM in comparison with its unbound and SET8-bound ground states. A significant inverse CD3 BIE of 0.959 suggests that the binding of SAM to SET8 is accompanied by a certain degree of the stiffness of the methyl group of SAM. Along the reaction path, the stiffness of the methyl group is further enhanced from SET8-bound SAM to the TS as reflected by a larger inverse of 0.90. Several previous studies have reported inverse CD3 KIEs for SAM-dependent methyltransferases and proposed that the S-N motions tighten the vibrational modes of the sulfonium-methyl moiety at the SN2 TS (18, 55, 56). Our KIE measurements together with QM modeling suggest that SET8-catalyzed methylation adopts an early, asymmetrical SN2 mechanism with more axial compression between the methyl donor and acceptor at the TS relative to the unbound and SET8-bound ground states of SAM (Fig. 5), in agreement with previous molecular dynamics simulations (34). This motion of S-N at the TS leads to further stiffness of the vibrational modes of the C-H bonds of the methyl group. In contrast, the normal intrinsic 1°-13C KIE of 1.04 is largely due to the net loss of the S-C bond order, which has not been compensated by the weak formation of the N-C bond at the early TS.
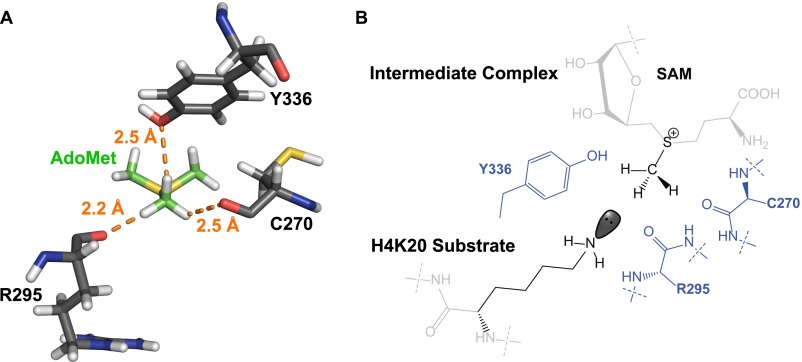
The recent elucidation of noncanonical CH–O interaction could shed light on an alternative, but mutually nonexclusive mechanism for the constrained TS of protein methyltransferases (36–38). In the context of the TS of SET8-catalyzed H4K20 monomethylation, the sulfonium-methyl group of SAM is expected to form three equatorial noncanonical CH–O interactions with the two backbone amide oxygens of Cys270 and Arg295 and the phenolic oxygen of the side chain of Tyr336 (Fig. S2) (57). These interactions can position the methyl group of SAM in a linear SN2 trajectory and thus lower the energetic barrier for catalysis. These interactions may rationalize the small inverse CD3 BIE of 0.959 and were thus corrected upon calculating intrinsic KIEs and TS structures (Eqs. 2a and 2b). In contrast, an inverse intrinsic α-2°-CD3 KIE for the SAM cofactor is expected to arise from the compressed axial S-N distance at the TS. These results are consistent with the computational data of a model SN2 transmethylation reaction, in which axial donor–acceptor compression and equatorial CH–O interaction contribute to inverse isotope effects (58).
Fig. S2.
Noncanonical CH–O interaction of SET8-catalyzed H4K20 monomethylation. (A) Static distances of the amide oxygens of Cys270 and Arg295 and the phenolic oxygen of Tyr336 to the sulfonium–methyl moiety of SAM upon the formation of the SAM–SET8 intermediate complex. The structure was extracted from PDB file 4IJ8. Hydrogen atoms were added to the PDB file using MolProbity online software. Their bond distances were measured with the measurement wizard in PyMol. (B) Two-dimensional representative interactions between SAM and Cys270/Arg295/Tyr336 upon the formation of the SAM–SET8–peptide intermediate complex.
SeAM, a SAM mimic containing a weaker methyl-chalcogen bond, showed the CD3 BIE, forward commitment factor, and Km,cofactor comparable with those of SAM, as well as only a 1.94-fold difference of steady-state kinetics [ = 1.94] (Table 1 and SI Materials and Methods). Therefore, the two cofactors may interact with SET8 in a similar manner for catalysis. Whereas SeAM has a larger intrinsic 1°-13C KIE of 1.06 ± 0.02 and a small normal α-2°-CD3 KIE of 1.02 ± 0.02 (Table 1 and SI Materials and Methods), computational modeling indicates that the experimental and of the SeAM-dependent H4K20 methylation match a suite of TS geometries with a relatively fixed Se-N distance around 4.9 Å but the altered position of the methyl group between the methyl donor and acceptor (Fig. S3). We thus cannot definitively conclude the TS geometry on the basis of and of the SeAM cofactor. The less compressed character of the SeAM-associated TS (the Se-N axial distance of 4.9 Å compared with the S-N axial distance of 4.4 Å at the TS) is consistent with the smaller magnitude of α-2°-CD3 KIE (1.02 ± 0.02 for SeAM vs. 0.90 ± 0.01 for SAM). The longer Se-N axial distance of 4.9 Å at the TS may associate with the intrinsic chemical properties of the chalcogen atom (i.e., the slightly weaker Se-C bond with selenonium as a leaving group or the different length of the ground-state chalcogen–carbon bond of 1.95 Å for SeAM vs. 1.82 Å for SAM). Here the KIEs and BIEs of SeAM do not conflict with the early SN2 TS of SET8-catalyzed H4K20 methylation with SAM as a cofactor.
Fig. S3.
TS geometries matched with KIEs of SeAM. Constrained TS geometries with varied distances of C-Se and C-N were optimized for the frequency calculation by Gaussian09. KIEs ( and ) were calculated using the ISOEFF program and then scaled by 0.967 to match experimental vibrational frequencies. The matched TS geometrical landscapes were highlighted for and The combined experimental KIEs ( and ) of the SeAM-dependent H4K20 methylation match a suite of TS geometries with a relatively fixed Se-N distance around 4.9 Å but the altered position of the methyl group between the methyl donor and acceptor.
Assembling the Reaction Pathway of SET8-Catalyzed H4K20 Monomethylation.
The collective data of steady-state kinetics, CD3 BIE, forward commitment factors, and as well as computational evidence, allow us to delineate SET8-mediated H4K20 methylation (Fig. 6). Along this reaction path, SET8 first binds SAM and H4K20 peptide with the apparent Km,SAM = 16 µM and Km,peptide = 40 µM to form a ternary SAM–SET8–H4K20 intermediate complex (Fig. 6 and Fig. S1). The small inverse CD3 BIE of [S-CD3]-SAM indicates that there is the equatorial CH–O interaction of the sulfonium-methyl moiety with Cys270, Arg295, and Tyr336 upon the formation of the intermediate (Fig. 6 and Fig. S2). The small forward commitment factor (Cf = 0.10) suggests a rapid exchange between unbound and SET8-bound SAM. Thereafter, the substrate is subjected to deprotonation, likely through dynamic water channels (34), to convert the positively charged ε-ammonium of the target lysine into a neutral and reactive nucleophile. The subsequent transmethylation through an early, asymmetrical SN2 TS stands as the highest energy barrier along the reaction path (Fig. 6). At the TS, equatorial noncanonical CH–O interactions of the sulfonium-methyl hydrogens with Cys270, Arg295, and Tyr336 may further contribute to the stabilization of the TS and project readily the amine nucleophile for an SN2 reaction as reported by the inverse (Fig. S2). Axial compression of the methylation donor and acceptor relative to the ground state is expected at the TS. This change is accompanied by the slight loss of the net bond order of the methyl carbon at the TS as reported by the small normal (Fig. 6). SET8 is then recycled for the next turnover after releasing the methylated H4K20 and SAH as products. This observation is consistent with the early SN2 character of the SET8-catalyzed methylation for which the TS has greater demand on the formation of the C-N bond rather than the breaking of the methyl-chalcogen bond (Fig. 5).
Fig. 6.
Reaction path of SET8-catalyzed H4K20 monomethylation. (A) Reaction mechanism of SET8-catalyzed H4K20 monomethylation with the SAM cofactor. After the formation of a ternary SAM–SET8–H4K20 intermediate complex and the subsequent deprotonation, the rate-determining step of the catalysis is the formation of an early, asymmetrical SN2 transition state for methyl transferring, reflected by an inverse CD3 BIE of 0.96, an inverse intrinsic α-2°-CD3 KIE of 0.90, and a normal 1°-13C KIE of 1.04. (B) Reaction mechanism of SET8-catalyzed H4K20 monomethylation with the SeAM cofactor. The reaction path for the SeAM cofactor is similar to that for SAM with CD3 BIE of 0.96, α-2°-CD3 KIE of 1.02, and 1-13C KIE of 1.06. (C) Relative energy barriers in SET8-catalyzed H4K20 monomethylation with SAM and SeAM as cofactors. SAM and SeAM are similar in terms of their affinity to SET8 and CD3 BIE and show only 1.94-fold difference of their kcat values along with the reaction path.
Comparison of TS Structures of SET8, NSD2, and Other PKMTs.
PKMTs are expected to catalyze the methyl transfer from SAM to a target lysine residue of their substrates through an SN2 mechanism. Our experimental KIEs and BIEs revealed that the SET8-catalyzed methylation goes through an early, asymmetrical SN2 TS with the long N-C distance of 2.35–2.40 Å and the short S-C distance of 2.00–2.05 Å. Poulin et al. (59) recently reported KIEs of NSD2 and solved its TS structure. In comparison with the early SN2 TS of SET8, the TS structure of NSD2 shows the later, asymmetrical SN2 characters with a short N-C distance of 2.02–2.10 Å and a long S-C distance of 2.5 Å. This TS structure is reflected by a large inverse α-2°-CD3 KIE of 0.83 and a large normal 1°-14C KIE of 1.11. Their data showed that the large inverse α-2°-CD3 KIE is mainly associated with the N-S axial compression at the TS of NSD2 because the comparable magnitude of α-2°-CD3 KIE can be modeled regardless of the presence of active-site residues of NSD2.
Besides the TS structures of SET8 and NSD2 solved with KIEs as experimental constraints, the TS structures of the SET-domain PKMTs including SET8, the viral histone methyltransferases (vSET), Rubisco large subunit methyltransferase (LSMT), and SET7/9 have been proposed solely based on computational modeling (34, 35, 60–62). The experimentally derived TS of SET8 is consistent with previous computational TS models with the N-C and S-C distances of 2.4 Å and 2.1 Å vs. 2.5 Å and 2.0 Å, respectively (34). Among the revealed TS structures of PKMTs, SET8 shows the distinct early, asymmetrical SN2 characters as reflected by the long N-C distance and the short S-C distance at the TS. The modeled TS structures of SET7/9 are substrate dependent with a symmetric SN2 TS (comparable N-C and S-C distances of 2.2−2.4 Å) for histone 3 lysine 4 peptide substrate and a late, asymmetric SN2 TS (N-C and S-C distances of 2.0 Å and 2.6 Å, respectively) for p53 lysine 372 peptide substrate (35, 60). In contrast, vSET like NSD2 adapts a late, asymmetric SN2 TS with the N-C distance of 2.0 Å and the S-C distance of 2.6 Å; Rubisco LSMT adapts a symmetric SN2 TS with the comparable N-C and S-C distances of 2.2−2.4 Å (59, 60). Interestingly, all of the revealed TS structures of PKMTs show a relatively fixed distance between the methyl donor and acceptor (the leaving group and nucleophile) with S-N distances around 4.4−4.6 Å but differ in the position of the methyl group (early, symmetric, or late SN2 TS). Such a difference may present the opportunity to design selective TS analog inhibitors against PKMTs.
SI Materials and Methods
Competition Assays with SAM and SeAM as Cofactors of SET8.
SeAM is expected to be similar to SAM in terms of overall structure, but is more reactive because of its weaker methyl–selenium bond (72). Given the early SN2 character of the TS of SET8-catalyzed H4K20 methylation with essential involvement of the formation of the C-N bond, we envision that the selenium–carbon bond of SeAM, although weaker than the sulfonium–carbon bond in SAM, may not have significant impact on the TS of SET8-catalyzed H4K20 methylation. We therefore explored with isotopic competition of SeAM vs. SAM as a means to quantify accurately their relative kcat/Km,cofactor and Km,cofactor values for SET8-catalyzed H4K20 methylation. Here, SET8 was mixed with [Se-CH3]-SeAM and [S-CD3]-SAM in the presence of H4K20 peptide substrate. The use of the [Se-CH3]-SeAM and [S-CD3]-SAM pair is critical for deconvoluting the relative amount of the consumed SAM vs. SeAM during the reaction course and thus allowing the comparison of a small difference of steady-state kinetics of the two cofactors under competitive conditions. The resulting ratios of the [CH3]/[CD3]-modified H4K20 peptides were dissected by MALDI-TOF MS to determine the relative kcat/Km,cofactor values of SeAM vs. SAM, which reflect all steps from SAM binding through irreversible catalysis. Although SeAM, which contains a weaker carbon–chalcogen bond, is a more reactive electrophile than SAM under a nonenzymatic setting (72), SeAM is only a slightly better cofactor ( = 1.94 ± 0.04) in SET8-catalzyed methylation (Table 1). Here, the comparable Km,cofactor values for SeAM and SAM ( = 1.03 ± 0.02) argue that the 1.94-fold difference in kcat/Km,cofactor values largely arises from the change of their kcat values (Table 1, main text).
CD3 BIE, α-2°-CD3 KIE, and 1°-13C KIE for SET8-Catalyzed H4K20 Methylation with SeAM as Cofactor.
To solve the TS structure with SeAM as a cofactor, we measured CD3 BIE, Cf, α-2°-CD3 KIE, and 13CD3 KIE of the SET8-catalyzed H4K20 methylation with [Se-CH3]/[Se-CD3]-SeAM and [Se-CH3]/[Se-13CD3]-SeAM as isotopic cofactor pairs. The forward commitment factor and CD3 BIE of SeAM (Cf = 0.10 ± 0.01 and = 0.962 ± 0.004) are nearly identical to those with SAM as a cofactor (Table 1, main text). In contrast with = 0.90 ± 0.01 and = 1.04 ± 0.020 for the SAM cofactor, SeAM-dependent H4K20 methylation by SET8 shows a larger intrinsic 1°-13C KIE of 1.06 ± 0.02 and a small normal α-2 °CD3 KIE of 1.02 ± 0.02 (Table 1, main text). Computational modeling indicates that the experimental and of the SeAM-dependent H4K20 methylation match a suite of TS geometries with a relatively fixed Se-N distance around 4.9 Å but with the altered position of the methyl group between the methyl donor and acceptor. Although we cannot definitively conclude the TS geometry with SeAM as a cofactor, the KIEs and BIEs of SeAM do not conflict with the early SN2 TS of SET8-catalyzed H4K20 methylation with SAM as a cofactor.
KIEs and BIEs in SET8 Transmethylation Reaction with SeAM as a Cofactor.
Given the comparable CD3 BIE values, forward commitment factors, and Km,cofactor between SAM and SeAM, as well as only a 1.94-fold difference of their steady-state kinetics (Table 1, main text), the two cofactors may interact with SET8 in a similar manner for catalysis. The small, significant CD3 BIE of 0.96 of SeAM may arise from three noncanonical CH–O interactions as proposed for the SAM cofactor. The normal of 1.02 for SeAM vs. the significantly inverse of 0.90 for SAM (Table 1, main text) can be rationalized by the longer axial Se-N distance (4.9 Å for SeAM vs. 4.4 Å for SAM) and thus the relaxation of the donor–acceptor compression at the TS for the SeAM cofactor. The longer Se-N axial distance of 4.9 Å at the TS is also consistent with a more dissociative character and thus slightly weaker Se-C bond with selenonium as a leaving group. The overall outcome is well reflected by the slightly larger of the SeAM cofactor (1.06 for SeAM vs. 1.04 for SAM; Table 1, main text). The and values may associate with the intrinsic chemical properties of the two chalcogen atoms (i.e., the distance of the ground-state chalcogen–carbon bond of 1.95 Å for SeAM vs. 1.82 Å for SAM).
Synthesis and Characterization of [S-CD3]-SAM, [S-13CD3]-SAM, SeAM, [Se-CD3]-SeAM, and [Se-13CD3]-SeAM.
SeAM and isotopically labeled SAM/SeAM were prepared according to previous methods with some modification (73). Briefly, SAH (Aldrich Chemical; 8 mg, 20.8 µmol) or Se-adenosyl-l-selenohomocysteine (SeAH, 8 mg, 18.5 µmol) was dissolved in 600 µL of a 1:1 mixture of formic acid and acetic acid. Into the stirred mixture was added 100 eq of iodomethane (CH3I) or deuterium-labeled iodomethane (CD3I) (2.08 ∼ 1.85 mmol). AgClO4 (8.62 ∼ 7.67 mg, 2.0 eq) was dissolved in 500 µL of 1:1 formic acid and acetic acid and then added slowly. After allowing the reaction to proceed in the absence of light at ambient temperature (22 °C) for 16−20 h, 5 mL of ddH2O containing 0.1% TFA (vol/vol) was added and the aqueous supernatant was washed three times with diethyl ether (3 × 5 mL). The resultant aqueous phase was centrifuged to remove precipitate and then filtered with a Nalgene 0.22-µm syringe filter. The crude products were injected into a preparative reversed-phase HPLC (XBridge Prep C18 5-µm OBD 19 × 150 mm) and eluted by acetonitrile in 0.1% aqueous trifluoroacetic acid (linear gradients from 0% to 10% in 20 min and then to 70% in 5 min) at a flow rate of 10 mL/min. Because the chalcogen−R/S diastereomers could not be resolved readily under the current HPLC conditions, the fractions containing both of the isomers were collected (HPLC retention time: 4.0 min for SAM and 5.5 min for SeAM) and lyophilized overnight. The HPLC-isolated yields for [S-CD3]-SAM and [Se-CH3/CD3]-SeAM were 50% and 30%, respectively. The dried products were redissolved in ddH2O containing 0.1% TFA (vol/vol) and stored at −80 °C before use. The concentrations of these stock solutions were determined by their UV absorption at 260 nm (ε260 = 15,400 L·mol−1·cm−1) for a ∼1:1 ratio of inactive R- and bio-active S-isomers. The quality of these compounds was confirmed by the expected isotopic mass from LC-MS and by their activities as the cofactors of SET8 to label H4K20 peptide.
A similar approach was applied to prepare [S-13CD3]-SAM and [Se-13CD3]-SeAM except that SAH (17.9 mg, 46.6 µmol) or SeAH (20.0 mg, 46.6 µmol) (74), iodomethane (13CD3I) (4.66 mmol, 100 eq), and AgClO4 (9.7 mg, 1.0 eq) were mixed to react for 3 h. For workup conditions, 10 mL of ddH2O containing 0.1% TFA (vol/vol) was added and the aqueous supernatant was washed three times with diethyl ether (3 × 10 mL). The resultant aqueous phase was processed, characterized, and restored in the same manner as described above. The HPLC-isolated yields for [S-13CD3]-SAM and [Se-13CD3]-SeAM were 80%.
It is worth noting that different reaction conditions have been documented for synthesis of SAM with slightly altered racemic product ratios of bioactive S-isomer and inactive R-isomer (72, 75–78). It should also be pointed out that the racemic ratios of the isolated S/R-isomers also depend upon the complete collection of the two not–well-resolved HPLC peaks, which could show batch-to-batch variation. It has also been reported that the inactive R-isomer can inhibit certain methyltransferases such as catechol O-methyltransferase (COMT), phenylethanolamine N-methyltransferase (PNMT), histamine N-methyltransferase (HMT), and hydroxyindole o-methyltransferase (HIOMT) (79). Whereas these variants may complicate the kinetics of SET8-catalyzed methylation, they have minimal effect on the measurement of KIE and BIE under competitive conditions because only active S-isomer can be processed for product quantification. Whereas S/R-isomers can slowly racemize with the first-order rate constant of 1.8 × 10−6 s−1 (76), only a negligible degree of racemization would occur in the time frame of our experiments (0.4−0.6% within 40 min). Such an effect is expected to be even less for commercially available SAM, which is synthesized enzymatically and predominantly contains active S-isomer (>97%), and SeAM given its negligible rate of racemization (76).
Preparation of SAM-Free SET8 for Enzymatic Assays.
SET8 (residues 191–352) containing an N-terminal 6×His tag was expressed and purified as described previously (63). Here we noticed that 0.3 ∼ 0.5 M eq of SAM is tightly bound to SET8 and coeluted with SET8 during purification. The degree of SAM contamination was estimated by the level of H4K20 methylation, as determined by MALDI-MS, when the SAM-contaminated SET8 was mixed with an equivalent of H4K20 peptide without addition of cofactor. The contaminated SAM causes errors in the subsequent measurement of BIEs and KIEs. To remove the coeluted SAM, the SAM-contaminated SET8 sample was incubated with a buffer containing 25 mM Tris⋅HCl (pH 10.0), 35 mM KCl, 5% glycerol, and 1:1 charcoal (wt/wt ratio of protein vs. charcoal) for 10 min. The charcoal-treated sample was then centrifuged and filtered to afford SAM-free SET8. To ensure that SAM is fully removed after the step of charcoal stripping, the absorbance of the treated enzyme was measured and the SAM-free SET8 was characterized by a smooth peak at 280 nm and without obvious shoulder absorbance at 260 nm. Furthermore, 10 µM of the stripped enzyme was incubated with 10 µM H4K20 peptide without the addition of SAM. No methylated peptide was observed, indicating that the batch of the treated enzyme was free of SAM. This SAM-free SET8 sample was flash frozen in liquid nitrogen and stored at −80 °C before use. The SAM-free preparation of SET8 was used for all SET8-involved experiments unless mentioned otherwise.
Biochemical Assays and Kinetics of SET8-Catalyzed H4K20 Methylation.
An enzyme-coupled luciferase assay was used to measure steady-state kinetics of SET8-catalyzed H4K20 methylation (Fig. 2) as described previously (44). Briefly, after SET8 processed SAM to label H4K20 peptide substrate (nonbiotinylated), the byproduct SAH was enzymatically converted into adenine, adenosine monophosphate, and then adenosine triphosphate (ATP) by 5′-methylthio-adenosine/SAH nucleosidase, adenine phosphoribosyl transferase, and pyruvate orthophosphate dikinase (PPDK), respectively. The resultant ATP, reflecting the turnover of the SAM cofactor, was quantified by a luciferin–luciferase kit. Here the reactions for examining steady-state kinetics (10 µL for each condition) were carried out at ambient temperature (22 °C) in a reaction buffer containing 50 mM Hepes (pH 8.0) buffer with 0.005% Tween 20, 0.0005% BSA, 1 mM TCEP buffer, 1 µM SET8, and various concentrations of the H4K20 peptide substrate (8−50 µM) and the SAM cofactor (6−100 µM). The linear increase of the luminescence signal (luminescence readouts after subtracting the background at time 0) vs. reaction time (initial 10−20 min depending on the concentrations of the SAM cofactor and the H4K20 substrate) was plotted to determine initial reaction velocities (ν in Eq. S1). Initial velocities were first plotted against the concentrations of the SAM cofactor and the peptide substrate to generate double-reciprocal curves. Because the two sets of double-reciprocal curves interact in the second quadrant, we excluded a ping-pong mechanism and rapid-equilibrium ordered sequential mechanism for the SET8-catalyzed methylation reaction. Our experimental data were then fitted globally to Eqs. S1 and S2 to obtain the Kd value for the formation of SAM-SET8 intermediate complex. Here [cofactor] and [peptide] are the concentrations of the SAM cofactor and the H4K20 peptide substrate, Km,cofactor and Km,substrate are their apparent binding constants for the formation of the ternary cofactor–SET8–substrate intermediate complex, Vmax is the maximal reaction velocity, and α is the factor to adjust the difference between Kd for the formation of the binary SET8 complex with the cofactor or substrate and Km for the formation of the ternary cofactor–SET8–substrate complex:
| [S1] |
| [S2] |
Deconvolution of MALDI-TOF-MS Data.
The MALDI-TOF mass spectrum of the unmodified H4K20 peptide contains 6 main mass multiplets for which the area under each multiplet maintains a fixed ratio relative to other peaks (Fig. 3A). Peptide modification by [S-CH3]-SAM and [S-CD3]-SAM gives +14-Da () and +17-Da () mass shift, respectively, with relative peak ratios nearly unchanged ( in Eq. S3a and in Eq. S3b) (Fig. 3A). The H4K20 peptide modified by a mixture of [S-CH3]-SAM:[S-CD3]-SAM results in 9 mass multiplets with the first three peaks ( i = 1,2,3) and the last three peaks ( i = 7,8,9) originating from [CH3]- and [CD3]-modified H4K20 peptide, respectively (Fig. 3 A and B):
| [S3a] |
| [S3b] |
For the three overlapping central peaks ( j = 4,5,6), the peak areas originating from [CH3]-cofactor ( j = 4,5,6) can be deconvoluted either directly on the basis of their fixed ratios to the first three peaks ( i = 1,2,3) to give (j = 4,5,6) according to Eq. S4a or indirectly by subtracting the peak areas arising from [CD3]-cofactor ( j = 4,5,6) from the overlapping peaks ( j = 4,5,6) to give (j = 4,5,6) according to Eqs. S4b and S5a (Fig. 3A). Similarly, the peak areas originating from the [CD3]-cofactor ( j = 4,5,6) can be deconvoluted either directly from the last three peaks ( i = 7,8,9) to give (j = 4,5,6) according to Eq. S5a or indirectly by subtracting the [CH3]-originating peak areas ( j = 4,5,6) from the overlapping peak areas ( j = 4,5,6) to give (j = 4,5,6) according to Eqs. S4a and S5b. With the two sets of data, the peak areas for the labeling of each cofactor ([CH3]- or [CD3]-) within the overlapping multiplets ( or j = 4,5,6) were then weighted on the basis of the contributions of and according to Eqs. S6a–S6d. Here “y” and “z” are the weights of the first three peaks ( i = 1,2,3) and the last three peaks ( i = 7,8,9) divided by the sum of all of the nonoverlapping peak areas (), respectively (Fig. 3A). In Eqs. S6c and S6d, and were weighted more than and because of their higher intensity and thus more reliable readouts. After dissecting the peak areas of the overlapping region ( j = 4,5,6), the sum of the peak areas originating from the [CH3]-cofactor and the [CD3]-cofactor were obtained according to Eqs. S7a and S7b, respectively. The same mathematic matrix was also applied to deconvolute the H4K20 products modified by the cofactor pairs of [Se-CH3]-SeAM/[Se-CD3]-SeAM and [Se-CH3]-SeAM/[S-CD3]-SAM under internal competition conditions:
| [S4a] |
| [S4b] |
| [S5a] |
| [S5b] |
| [S6a] |
| [S6b] |
| [S6c] |
| [S6d] |
| [S7a] |
| [S7b] |
A similar mathematic matrix was developed to quantify the isotopic ratios of the peptide modified by the cofactor pairs [S-CH3]/[S-13CD3]-SAM and [Se-CH3]/[Se-13CD3]-SeAM (Fig. 3 A and C). Here the modifications by CH3 and 13CD3 give +14-Da () and +18-Da () mass shift, respectively. Eqs. S3a–S7b were therefore modified into Eqs. S3c–S7d (Fig. 3B). Here the modified H4K20 peptide contains 10 main mass multiplets with the first four peaks ( i = 1,2,3,4) and the last four peaks ( i = 7,8,9,10) originating from the labeling of CH3 and 13CD3, respectively. For the two overlapping central peaks ( j = 5,6), the peak areas originating from [CH3]-cofactor ( j = 5,6) can be deconvoluted on the basis of their relative fixed ratios to the first four peaks ( i = 1,2,3,4) to give (j = 5,6) according to Eq. S4c or by subtracting the peak areas arising from [13CD3]-cofactor ( j = 5,6) from the overlapping peaks ( j = 5,6) to give (j = 5,6) according to Eqs. S4d and S5c. Similarly, the peak areas originating from the [13CD3]-cofactor ( j = 5,6) can be deconvoluted either from the last four peaks ( i = 7,8,9,10) to give (j = 5,6) according to Eq. S5c or by subtracting the [CH3]-originating peak areas ( j = 5,6) from the overlapping peak areas ( j = 5,6) to give (j = 5,6) according to Eqs. S4c and S5d. With the two sets of data, the peak areas for the labeling ([CH3]- or [13CD3]) of each cofactor within the overlapping multiplets ( or j = 5,6) were then weighted on the basis of the contributions of and according to Eqs. S6e–S6h. Here “a” and “b” are the weights, derived by the ratios of the first four peaks ( i = 1,2,3,4) and the last four peaks ( i = 7,8,9,10) to the sum of all of the nonoverlapping peak areas (), respectively (Fig. 3B):
| [S3c] |
| [S3d] |
| [S4c] |
| [S4d] |
| [S5c] |
| [S5d] |
| [S6e] |
| [S6f] |
| [S6g] |
| [S6h] |
| [S7c] |
| [S7d] |
Measurement of Forward Commitment Factors and Intrinsic KIEs.
Forward commitment factors for SET8 with SAM and SeAM as cofactors were determined by an isotope-trapping method as described previously with slight modification (65). Briefly, 20 µM SET8 was incubated with 100 µM [CD3]-cofactor ([S-CD3]-SAM or [Se-CD3]-SAM) for 5 min to allow the binding equilibrium of SET8 and the cofactor. The reaction was then initiated by adding 20 µM H4K20 peptide, together with a 10-fold excess of [CH3]-cofactor (final concentration of 1 mM). Aliquots of 20 µL were collected at various time intervals and then rapidly quenched by 2 µL trifluoroacetic acid within the reaction time period of 1.5 min during the consumption of 1 eq of the H4K20 peptide. The amount of [CD3]-modified H4K20 peptide was quantified by dissecting its peak areas from the [CD3]-modified H4K20 peptide by MALDI-TOF MS as described above. The amount of [CD3]-bound SET8 before the addition of [CH3]-cofactor was calculated on the basis of the concentrations of SET8 and [CD3]-cofactor and the Kd value of the cofactor (Eqs. S1 and S2). The forward commitment factors Cf of SET8 with SAM or SeAM as the cofactor were obtained from the ratios (Rc) of [CD3]-modified H4K20 peptide to [CD3]-cofactor–bound SET8 according to Cf = Rc/(1 − Rc) (11).
Determination of and BIEs with Radiometric Method.
All SET8-catalyzed H4K20 methylation reactions were examined under internal competition conditions containing the isotopic pairs of [S-14CH3]-SAM and [S-CT3]-SAM by a conventional radiometric method at ambient temperature (22 °C) in the aforementioned reaction buffer. To measure the prereacted or prebound isotopic ratio of [S-CT3]-SAM to [S-14CH3]-SAM, the premixed [S-14CH3]-SAM and [S-CT3]-SAM contain at least 105 cpm for [S-14CH3]-SAM and around 3:1 cpm ratio of [S-CT3]-SAM to [S-14CH3]-SAM. This cofactor mixture was added into the reaction buffer to reach the final concentrations of 1.2 μM [S-CT3]-SAM and 8.6 μM [S-14CH3]-SAM. This resultant mixture was incubated with 20 μM H4K20 peptide and 100 μM SET8 (the final concentration in a total volume of 110 μL mixture) for 3 h to reach the full conversion to quantify the ratio of [S-CT3]-SAM to [S-14CH3]-SAM. For the measurement of [S-CT3]/[S-14CH3]-SAM were added into the reaction mixture to reach the final concentrations of 1.2 μM [S-CT3]-SAM, 8.6 μM [S-14CH3]-SAM, and 20 μM H4K20 peptide. The reaction was initiated by adding 0.5 μM SET8 (the final concentration in a total volume of 110 μL reaction mixture) and allowed to proceed to reach 20% ∼ 30% partial conversion of the cofactors within 45 min. For the measurement of BIE, 10 μM SET8 was incubated with the premixed cofactors containing 1.2 μM [S-CT3]-SAM and 8.6 μM [S-14CH3]-SAM (a total volume of 110 μL) at ambient temperature (22 °C) for 5 min. The mixture was subjected to a 0.5-mL PD SpinTrap G-25 (GE Healthcare) spin column (preequilibrated with the reaction buffer and maintained at 4 °C) at 1,000 × g for 1 min to remove the unbound cofactors according to the spin protocol of the manufacturer. The recovered mixture of SET8 and the cofactors was subjected to the reaction initiated by adding 20 μM peptide for 3 h.
Each reaction mixture containing radiolabeled H4K20 peptide was split into five aliquots and then quenched by adding 2 μL trifluoroacetic acid. These samples were spotted on phosphocellulose (P-81) filter paper (3 cm × 3 cm) and allowed to dry for 2 h. The dried filter paper was then washed five times with 50 mM sodium bicarbonate (pH 9.2) and allowed to dry overnight. These filter paper samples were transferred into scintillation vials containing 1 mL ddH2O and 10 mL of scintillation fluid (Ultima Gold; Perkin-Elmer) and analyzed by a liquid scintillation analyzer (Perkin-Elmer Tri-Carb 2910 TR) in a dual-channel mode with >99% 3H signal appearing in channel 1. The windows for the radioactive signal from 3H (channel 1) and 14C (channel 2) were optimized to be 0 KeV ∼ 18 KeV and 18 KeV ∼ 156 KeV, respectively, to minimize the leakage of 3H signal into the 14C window. The radioactive signals of the two channels were determined from the average of six cycles of scintillation counting at 10 min of each sample per cycle. The ratio of the 14C cpm in channel 1 vs. channel 2 (“A” in Eq. S8a) and the ratio of the 3H cpm in channel 1 vs. channel 2 (“B” in Eq. S8b), although negligible for the latter, were determined by using the standards containing only 14C- or 3H-labeled peptide. These radioactive peptides were prepared by mixing [S-CT3]-SAM or [S-14CH3]-SAM, H4K20 peptide, and SET8 as described above in the KIE experiments. The 3H and 14C signals were then calculated according to Eqs. S8a and S8b, respectively (80, 81). The 3H:14C ratios of the products for the partial and complete conversation were determined and the KIEs were obtained according to Eq. 1 (80, 81). The 3H:14C ratios of prebound and SET8-bound cofactors were determined to derive the corresponding BIE according to Eq. 3. The 14C BIE is expected to be unity given the negligible contribution of 14C in the binding step. The 14CT3 BIE is thus equivalent to CT3 BIE:
| [S8a] |
| [S8b] |
Internal Competition Assays for Comparison of Km,cofactor and kcat/Km,cofactor of SAM and SeAM for SET8-Catalyzed H4K20 Methylation.
An internal competition method was used to obtain the relative ratios of kcat/Km,cofactor and Km,cofactor values of SAM and SeAM for SET8-catalyzed H4K20 methylation in a similar manner to that for obtaining KIEs and BIEs. Here the SeAM/SAM-cofactor pair was treated equivalently to the [CH3]/[CD3] light/heavy isotopic pairs of SeAM or SAM upon calculating KIEs or BIEs. The ratio of kcat/Km,cofactor values of SeAM vs. SAM [SeAM/SAM(kcat/Km,cofactor)] is analogous to KIEs; the ratio of 1/Km,cofactor values of SeAM vs. SAM [SeAM/SAM(1/Km,cofactor)] is analogous to BIEs. Briefly, similar to the internal competition assays for measuring [Se-CH3]-SeAM and [S-CD3]-SAM were premixed at the ratio of around 1.5:1 in a standard reaction buffer to the approximate final concentrations of around 45 µM [Se-CH3]-SeAM and 30 µM [S-CD3]-SAM (bioactive isomers). The competition reaction was initiated at ambient temperature (22 °C) by adding 500 nM SET8 and 20 µM H4K20 peptide as described for measuring KIEs. The ratios of [CH3]:[CD3]-modified H4K20 peptide for the partial completion (20% ∼ 30%, analogous to Rf in Eq. 1) and for full completion (analogous to R0 in Eq. 1) were obtained by the MS deconvolution method as described for measuring KIEs (at least five replicates). Because SeAM is more reactive than SAM under the current setting, the ratios of kcat/Km,cofactor values of [Se-CH3]-SeAM and [S-CD3]-SAM [SeAM/SAM(kcat/Km,cofactor)] were obtained from the isotopic ratio of 100% completion vs. 20% ∼ 30% completion (R0 vs. Rf) according to Eq. S9 (82), in which Rf and R0 are the light-to-heavy ratios at the partial and 100% completions, respectively; f is the reacted fraction of the overall cofactors (∼20% ∼ 30% in these cases) and was obtained by quantifying the corresponding modified vs. unmodified H4K20 peptide by MALDI-TOF MS; XH and XD are initial mole fractions of [Se-CH3]-SeAM and [S-CD3]-SAM, respectively; of SAM is used to correct the difference of kcat/Km,cofactor between [S-CD3]-SAM and [S-CH3]-SAM:
| [S9] |
The ratio of 1/Km,cofactor values of SeAM vs. SAM [SeAM/SAM(1/Km,cofactor)] was obtained with the internal competition assay as applied for measuring BIEs. Briefly, a 100-µM mixture of [Se-CH3]-SeAM:[S-CD3]-SAM was premixed at the molar ratio of around 1.5:1 in the reaction buffer with 10 µM SET8 to the final concentrations of around 45 µM [Se-CH3]-SeAM and 30 µM [S-CD3]-SAM (active isomers in a final volume of 110 µL). The mixture was preequilibrated at ambient temperature (22 °C) for 5 min. As described for measuring BIEs, a 10-µL portion of this mixture was saved and diluted 10-fold with the reaction buffer. The diluted sample was mixed with 15 µM of the H4K20 peptide (the final concentration) for 20 min to consume fully the cofactor mixture. This resultant sample was subjected to MALDI-TOF MS as described above to calculate the light-to-heavy ratio of prebound [CH3]:[CD3] cofactors (Rprebound, Eq. 3, five replicates). The remaining mixture (100 µL) was subjected to a PD SpinTrap G-25 (GE Healthcare) spin column (preequilibrated with the reaction buffer) to remove the unbound cofactors. Into the flow-through fraction of SET8-bound cofactors, 15 µM of the H4K20 peptide (the final concentration) was added. From the reaction mixture, five aliquots were split and incubated at ambient temperature (22 °C) for 20 min to consume fully the SET8-bound cofactor. MALDI-TOF MS was implemented to calculate the light-to-heavy ratio of bound [CH3]:[CD3] cofactors (Rbound) according to the MS-based deconvolution method described above for [CH3]/[CD3]-modified H4K20 peptide (at least five replicates). The ratios of 1/Km,cofactor values of [Se-CH3]-SeAM and [S-CD3]-SAM [SeAM/SAM(1/Km,cofactor)] were obtained on the basis of Rbound/Rprebound according to Eq. S10, in which Rbound and Rprebound are the MALDI-MS–derived light-to-heavy ratios of the bound (the samples after PD SpinTrap G-25) and prebound cofactors (the samples before PD SpinTrap G-25), respectively; f is the SET8-bound fraction of the [S-CD3] cofactor estimated on the basis of the concentrations of SET8 and the cofactor and the Kd value of the cofactor; the of SAM is used to correct the difference of the binding affinity between [S-CD3]-SAM and [S-CH3]-SAM. Here the Km,cofactor values for SAM and SeAM are comparable as reflected by the close-to-unity value of
| [S10] |
Materials and Methods
General Materials and Methods.
Chemicals for synthesis were purchased from Sigma Aldrich and used without purification. [S-CT3]-SAM (79.5 Ci/mmol as a fully tritiated product) and [S-14CH3]-SAM (58 mCi/mmol) were purchased from Perkin-Elmer. Other reagents were purchased from Fisher Scientific unless mentioned otherwise. Solvents for HPLC were degassed before use. HPLC purification of synthesized cofactors (SI Materials and Methods) was carried out with a Waters 600 Controller HPLC equipped with a 2,998-diode array detector and an XBridge Prep C18 5-µm OBD 19 × 150-mm reverse phase column. After collecting the desired HPLC fractions, residual solvents were removed by a Savant Sc210A SpeedVac Concentrator (Thermo), followed by lyophilization with a Flexi-Dry µP Freeze-Dryer. H4K20 peptide (residues 10–30) ([H2N-LGKGGAKRHRKVLRDNIQGIT-GG(K-Biotin)-OH] and [H2N-LGKGGAKRHRKVLRDNIQGIT-OH]) were prepared according to standard Fmoc-protected solid-phase peptide synthesis at the Proteomics Resource Center of Rockefeller University and purified by a reverse-phase preparative HPLC with a 0−40% acetonitrile gradient in 0.1% trifluoroacetic acid (TFA)/ddH2O to >90% purity. The quality of the peptide was confirmed by MALDI-MS. The two H4K20 peptides were shown previously to be active substrates of SET8 with the biotinylated peptide used in the present work unless specified (63). SeAM and isotopically labeled SAM/SeAM were prepared according to previous methods (SI Materials and Methods).
MALDI-TOF MS.
MALDI-TOF MS was implemented to quantify the ratios of isotopically labeled H4K20 peptide (CH3- vs. CD3- and CH3- vs. 13CD3- labeling), from which KIEs, BIEs, and commitment factors were obtained as detailed below. Here a mixture of SAM or SeAM and their isotopic analogs was incubated with SET8 and H4K20 peptide. After processing the reaction at ambient temperature (22 °C) for specific time intervals, the peptide sample was enriched by C18 ZipTip (Millipore), washed with 0.1% TFA/ddH2O (vol/vol) 10 times, and eluted out with 50% acetonitrile/0.1% TFA, according to the manufacturer’s instructions. The resultant peptide products were subjected to MALDI-MS as described previously (42). Briefly, to prepare MALDI-TOF-MS samples, 2 μL of the purified peptide was mixed with 1 μL of saturated α-cyano-hydroxy-cinnamic acid solution (50% acetonitrile/0.1% TFA) on a MALDI sample plate and allowed to dry at ambient temperature (22 °C). The dried samples were then subjected to MALDI-TOF-MS analysis (Voyager-DE STR; Applied Biosystems). Desorption/ionization was obtained using a delayed-extraction, positive ion mode with a 337-nm nitrogen laser (3-ns pulse width). Laser power was adjusted slightly above threshold to obtain good resolution and signal/background ratios. Spectra were gathered for at least 300 shots per position to ensure that the peak ratios were normalized over the course of data collection.
Determination of and by MALDI-TOF MS.
All SET8-catalyzed H4K20 methylation reactions were carried out at ambient temperature (22 °C) in the reaction buffer containing 50 mM Hepes (pH 8.5), 0.005% Tween 20, 0.0005% BSA, and 1 mM tris(2-carboxyethyl)phosphine (TCEP), unless otherwise specified. was obtained with MALDI-TOF MS under internal competition conditions containing isotopic pairs of [S-CH3]/[S-CD3]-SAM or [Se-CH3]/[Se-CD3]-SAM and H4K20 peptide substrate. Individual reactions were performed in a total volume of 115 µL containing 500 nM SET8 and 20 µM H4K20 peptide substrate. For the SAM cofactor, [S-CH3]-SAM and [S-CD3]-SAM were premixed at a molar ratio of around 1.5:1 and then added to the reaction mixtures, resulting in around 30 µM [S-CH3]-SAM and 20 µM [S-CD3]-SAM, respectively (final concentrations for active isomers). The reaction mixture was split into two portions with one for 100% consumption and the other for partial consumption of 20% ∼ 30% (100% and 20% ∼ 30% consumption were calculated on the basis of the amount of the consumed cofactor and were obtained by quantifying modified vs. unmodified H4K20 peptide with MALDI-TOF MS). For the portion of 100% consumption, 10 µL of the mixture above was saved to quantify the ratio of [S-CH3]-SAM to [S-CD3]-SAM as prereacted substrates. Here, the 10-µL aliquot was diluted 10-fold with the reaction buffer to afford a 100-µL reaction mixture containing ∼3 µM [S-CH3]-SAM and 2 µM [S-CD3]-SAM. This mixture was split into five aliquots and the consumption of [S-CH3]/[S-CD3]-SAM was driven to completion within 10 min by adding 10 µM SAM-free SET8 and 10 µM H4K20 peptide (the final concentrations). For the portion of 20−30% consumption, around 100 µL of the left reaction mixture was split into five aliquots. After processing the reaction to 20−30% completion within 40 min, individual aliquots were quenched by the addition of 2 µL trifluoroacetic acid. These samples were stored at –20 °C before MS analysis. For the isotopic pair of [Se-CH3]/[Se-CD3] cofactors, a similar protocol was used except that around 40 µM [Se-CH3]-SeAM and 30 µM [Se-CD3]-SeAM (final concentrations for active isomers) were used to initiate the reaction.
The ratios of the [CH3]:[CD3]-modified H4K20 peptide products were determined with MALDI-TOF MS as described above for the conditions of partial and 100% conversion (Rf and R0). The ratio of the two [CH3]:[CD3] isotopic ratios (partial conversion Rf vs. 100% conversion R0) afforded apparent KIEs (Rf/R0) under the condition of the partial conversion (at least five replicates). Given that inverse KIEs were observed under these settings, KIEs on were calculated according to Eq. 1 and used for small isotope effects, in which (x = CD3 or 13CD3), Rf, and R0 are the light-to-heavy ratios at the partial and 100% completion, respectively, and f is the reacted fraction of the cofactors (∼20–30% in these cases) (64). was obtained similarly as described above except that the isotopic pairs of [S-CH3]/[S-13CD3]-SAM or [Se-CH3]/[Se-13CD3]-SAM with the ratio around 1:1.5 were used according to the corresponding mathematic matrix (Eqs. S3–S7):
| [1] |
Forward commitment factors for SET8 with SAM and SeAM as cofactors were determined by an isotope-trapping method as described previously with slight modification (SI Materials and Methods) (65). The forward commitment factors Cf of SET8 with SAM or SeAM as the cofactor were obtained from the ratios (Rc) of [CD3]-modified H4K20 peptide to [CD3]-cofactor–bound SET8 according to Cf = Rc/(1 − Rc) (11). Given that SET8-catalyzed methylation is irreversible, ( or ) was calculated on the basis of Eqs. 2a and 2b (66), in which [ or ] is the experimental KIE obtained via Eq. 1, BIEs (CD3 and CT3 BIEs) are obtained according to Eq. 3 (the contribution of 13C/14C to BIE is expected to be negligible), and Cf is the forward commitment factor obtained via Cf = Rc/(1 − Rc) (19, 64):
| [2a] |
| [2b] |
Determination of BIEs by MALDI-TOF MS.
To measure the CD3 BIE of the [CH3]:[CD3]-cofactor pair (SAM or SeAM) with the MS-based method (Fig. 2B), 10 µM SET8 in the reaction buffer was incubated with a 100-µM mixture of ∼1:1 [CH3]:[CD3] cofactors (molar ratio) for 5 min at ambient temperature (22 °C) (110 µL as the final volume). A 10-µL portion of the mixture was saved and diluted by 10-fold with the reaction buffer. The diluted sample was mixed with 15 µM of H4K20 peptide (the final concentration) for 20 min to consume all of the cofactors. The resultant sample was subjected to MALDI-TOF MS as described above to calculate the light-to-heavy ratio of prebound [CH3]:[CD3] cofactors (Rprebound, five replicates). The remaining mixture (∼100 µL) was subjected to a 0.5-mL PD SpinTrap G-25 (GE Healthcare) spin column (preequilibrated with the reaction buffer and maintained at 4 °C) at 800 × g for 1 min according to the spin protocol of the manufacturer to remove the unbound cofactors. Into the flow-through fraction of SET8-bound cofactors, 15 µM of the H4K20 peptide was added. From the reaction mixture, five aliquots were split and incubated at ambient temperature (22 °C) for 20 min to drive the reaction to completion. MALDI-TOF MS was implemented to calculate the light-to-heavy ratio of bound [CH3]:[CD3] cofactors (Rbound) according to the deconvolution method described for [CH3]/[CD3]-modified H4K20 peptide (SI Materials and Methods).
The values of BIEs were obtained on the basis of Rprebound /Rbound according to Eq. 3 (67, 68), in which Rbound and Rprebound are the MALDI-MS–derived light-to-heavy ratios of the bound (the samples after PD SpinTrap G-25) and prebound (the samples before PD SpinTrap G-25) cofactors, respectively; Rfree is the light-to-heavy ratios of the unbound cofactors equilibrated with the cofactor–SET8 complex; f is the SET8-bound fraction of the light cofactors estimated on the basis of the concentration of SET8 and the cofactors and the Kd values of the cofactors (Eq. S2). The Kd values for the [CH3]:[CD3]-SAM and -SeAM cofactor pairs are comparable as determined below. Here apo- and cofactor-bound SET8 will be separated gradually from the unbound cofactor by the PD SpinTrap G-25 column upon centrifugation. During this process, a portion of the bound cofactor may dissociate from SET8, given the gradual depletion of the unbound cofactor pool. The resultant reequilibration of SET8-bound cofactor may increase the magnitude of BIEs in Eq. 3 if BIEs are not unity. However, such an effect is negligible because 80% ∼ 90% of the estimated SET8-bound cofactors can be recovered after the centrifugation step of the PD SpinTrap G-25 column, indicating that the rate of this reequilibrium in the spin column at 4 °C is slow in comparison with the timescale for the separation of the SET8-bound cofactor vs. unbound cofactor. Here CD3 BIE was used as 13CD3 BIE, given the negligible contribution of 13C to the binding step:
| [3] |
Computational Modeling and Calculation of Theoretic KIEs of SAM and SeAM.
We performed theoretical TS analysis of SET8-catalyzed methyl transfer following the procedures established by the Schramm Laboratory (59, 69). The methyl transfer reaction from SAM or SeAM to a target lysine was modeled by QM simulations, using a density functional theory with an M062X functional and a 6-31+G(d,p) basis set (53). Geometry optimization and frequency calculation were performed by Gaussian09. KIEs were calculated using the ISOEFF program at the experimental temperature of 22 °C (54). The theoretical vibrational frequencies were scaled by 0.967 (70) to match experimental vibrational frequencies during KIE calculation. The structures of SAM, SeAM, and lysine were modeled as S+CH3(CH2CH3)2, Se+CH3(CH2CH3)2, and CH3CH2NH2. Intrinsic KIEs of respective cofactors were obtained from experimental KIEs on after correcting for Cf and BIEs according to Eq. 2b. The starting atomic coordinates of the SET8-bound states of SAM and SeAM were extracted from the crystal structure of a SAM–SET8 complex (PDB 4IJ8) and optimized as local minima on the potential energy surfaces. The frequency calculations performed on the optimized structures showed no imaginary frequency.
The starting geometry of the TS was based upon the crystal structure of a SAH–SET8–H4K20me1 ternary complex (PDB ID: 2BQZ). The C-N and C-S distances were fixed at 1.6–2.5 Å and 1.9–2.8 Å with 0.1-Å increments to optimize a grid of the potential TS candidates of SET8-catalyzed H4K20 monomethylation. KIEs were predicted for these candidate TS structures and compared with experimental KIEs. The best-matched range of structures was explored with a finer grid at 0.01-Å increments in the C-N and C-S interatomic distances. Most of the constrained TS structures that matched the experimental SAM KIEs showed a small second imaginary frequency (10–20 cm−1) in addition to the major imaginary frequency (100–200 cm−1) that corresponds to the reaction coordinate of S–CH3–N transfer from SAM to lysine. The small second imaginary frequency is due to geometric constraints on the C-S and C-N distances and causes only marginal errors in TS predictions based on KIEs (71). The TS structures matching experimental SAM KIEs are characterized by dC-S = 2.00–2.05 Å and dC-N 2.35–2.40 Å, indicating a very early TS character for SET8-catalyzed methylation reaction. The geometry of TS without constraining the C-N and C-S distances was also calculated. The optimized TS shows a symmetric TS character with dC-S = 2.2 Å and dC-N 2.1 Å.
Similarly, the C-N and C-Se distances were fixed at 1.9–2.7 Å in and 2.1–3.0 Å with 0.1-Å increments to optimize a grid of the potential TS candidates of SET8-catalyzed H4K20 monomethylation, using SeAM as the cofactor. A range of TS structures matched experimental SeAM KIEs, as long as dC-S + dC-N = 4.8–4.9 Å with dC-S of 2.2–2.7 Å and dC-N of 2.2–2.6 Å (Fig. S3). All those constrained TS structures had a single large imaginary frequency corresponding to Se–CH3–N transfer.
Natural bond orbital (NBO) analysis and single-point energy calculations were then performed on the representative TS structure (dC-S = 2.05 Å and dC-N = 2.38 Å) for SAM methyl transfer, as well as the structures of corresponding reactants and products, using the NBO3.0 program available in Gaussian09. The electrostatic potential surfaces (ESPSs) were visualized in GaussView5.0 (isovalue = 0.06) from the electron density and potential cubes acquired from the checkpoint files of single-point energy calculations performed in Gaussian09.
Acknowledgments
We thank Dr. V. L. Schramm and Dr. M. Poulin for scientific discussion. We thank National Institute of General Medical Sciences (Grants R01GM096056, R01GM120570, and P01GM068036), National Cancer Institute Cancer Center (Support Grant 5P30 CA008748-44), Starr Cancer Consortium, Mr. William H. Goodwin and Mrs. Alice Goodwin Commonwealth Foundation for Cancer Research, the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center, Tri-Institutional Therapeutic Discovery Initiative, and Sohn Conference Foundation for financial support of this research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. F.M.R. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609032114/-/DCSupplemental.
References
- 1.Schramm VL. Enzymatic transition states, transition-state analogs, dynamics, thermodynamics, and lifetimes. Annu Rev Biochem. 2011;80:703–732. doi: 10.1146/annurev-biochem-061809-100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cleland WW. The use of isotope effects to determine enzyme mechanisms. Arch Biochem Biophys. 2005;433(1):2–12. doi: 10.1016/j.abb.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Cleland WW. Isotope effects: Determination of enzyme transition state structure. Methods Enzymol. 1995;249:341–373. doi: 10.1016/0076-6879(95)49041-8. [DOI] [PubMed] [Google Scholar]
- 4.Świderek K, Paneth P. Binding isotope effects. Chem Rev. 2013;113(10):7851–7879. doi: 10.1021/cr300515x. [DOI] [PubMed] [Google Scholar]
- 5.Northrop DB. Steady-state analysis of kinetic isotope effects in enzymic reactions. Biochemistry. 1975;14(12):2644–2651. doi: 10.1021/bi00683a013. [DOI] [PubMed] [Google Scholar]
- 6.Schramm VL. Binding isotope effects: Boon and bane. Curr Opin Chem Biol. 2007;11(5):529–536. doi: 10.1016/j.cbpa.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northrop DB. Uses of isotope effects in the study of enzymes. Methods. 2001;24(2):117–124. doi: 10.1006/meth.2001.1173. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Luo M, Ghanem M, Taylor EA, Schramm VL. Second-sphere amino acids contribute to transition-state structure in bovine purine nucleoside phosphorylase. Biochemistry. 2008;47(8):2577–2583. doi: 10.1021/bi7021365. [DOI] [PubMed] [Google Scholar]
- 9.Luo M, Li L, Schramm VL. Remote mutations alter transition-state structure of human purine nucleoside phosphorylase. Biochemistry. 2008;47(8):2565–2576. doi: 10.1021/bi702133x. [DOI] [PubMed] [Google Scholar]
- 10.Schramm VL. Enzymatic transition states: Thermodynamics, dynamics and analogue design. Arch Biochem Biophys. 2005;433(1):13–26. doi: 10.1016/j.abb.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Luo M, Singh V, Taylor EA, Schramm VL. Transition-state variation in human, bovine, and Plasmodium falciparum adenosine deaminases. J Am Chem Soc. 2007;129(25):8008–8017. doi: 10.1021/ja072122y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz SD, Schramm VL. Enzymatic transition states and dynamic motion in barrier crossing. Nat Chem Biol. 2009;5(8):551–558. doi: 10.1038/nchembio.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schramm VL. Transition states, analogues, and drug development. ACS Chem Biol. 2013;8(1):71–81. doi: 10.1021/cb300631k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schramm VL, Horenstein BA, Kline PC. Transition state analysis and inhibitor design for enzymatic reactions. J Biol Chem. 1994;269(28):18259–18262. [PubMed] [Google Scholar]
- 15.Bennet AJ. Kinetic isotope effects for studying post-translational modifying enzymes. Curr Opin Chem Biol. 2012;16(5-6):472–478. doi: 10.1016/j.cbpa.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Chan J, Lewis AR, Gilbert M, Karwaski MF, Bennet AJ. A direct NMR method for the measurement of competitive kinetic isotope effects. Nat Chem Biol. 2010;6(6):405–407. doi: 10.1038/nchembio.352. [DOI] [PubMed] [Google Scholar]
- 17.Bates C, Kendrick Z, McDonald N, Kline PC. Transition state analysis of adenosine nucleosidase from yellow lupin (Lupinus luteus) Phytochemistry. 2006;67(1):5–12. doi: 10.1016/j.phytochem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Hegazi MF, Borchardt RT, Schowen RL. alpha.-Deuterium and carbon-13 isotope effects for methyl transfer catalyzed by catechol O-methyltransferase. SN2-like transition state. J Am Chem Soc. 1979;101(15):4359–4365. doi: 10.1021/ja00426a079. [DOI] [PubMed] [Google Scholar]
- 19.Northrop DB. The expression of isotope effects on enzyme-catalyzed reactions. Annu Rev Biochem. 1981;50:103–131. doi: 10.1146/annurev.bi.50.070181.000535. [DOI] [PubMed] [Google Scholar]
- 20.Cleland WW. Use of isotope effects to elucidate enzyme mechanisms. CRC Crit Rev Biochem. 1982;13(4):385–428. doi: 10.3109/10409238209108715. [DOI] [PubMed] [Google Scholar]
- 21.Manning KA, Sathyamoorthy B, Eletsky A, Szyperski T, Murkin AS. Highly precise measurement of kinetic isotope effects using 1H-detected 2D [13C,1H]-HSQC NMR spectroscopy. J Am Chem Soc. 2012;134(51):20589–20592. doi: 10.1021/ja310353c. [DOI] [PubMed] [Google Scholar]
- 22.Liuni P, Olkhov-Mitsel E, Orellana A, Wilson DJ. Measuring kinetic isotope effects in enzyme reactions using time-resolved electrospray mass spectrometry. Anal Chem. 2013;85(7):3758–3764. doi: 10.1021/ac400191t. [DOI] [PubMed] [Google Scholar]
- 23.Horenstein BA, Parkin DW, Estupiñán B, Schramm VL. Transition-state analysis of nucleoside hydrolase from Crithidia fasciculata. Biochemistry. 1991;30(44):10788–10795. doi: 10.1021/bi00108a026. [DOI] [PubMed] [Google Scholar]
- 24.Dillon SC, Zhang X, Trievel RC, Cheng X. The SET-domain protein superfamily: Protein lysine methyltransferases. Genome Biol. 2005;6(8):227. doi: 10.1186/gb-2005-6-8-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richon VM, et al. Chemogenetic analysis of human protein methyltransferases. Chem Biol Drug Des. 2011;78(2):199–210. doi: 10.1111/j.1747-0285.2011.01135.x. [DOI] [PubMed] [Google Scholar]
- 26.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6(11):838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Berger SL. The emerging field of dynamic lysine methylation of non-histone proteins. Curr Opin Genet Dev. 2008;18(2):152–158. doi: 10.1016/j.gde.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Wen H, Shi X. Lysine methylation: Beyond histones. Acta Biochim Biophys Sin. 2012;44(1):14–27. doi: 10.1093/abbs/gmr100. [DOI] [PubMed] [Google Scholar]
- 29.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Varier RA, Timmers HT. Histone lysine methylation and demethylation pathways in cancer. Biochim Biophys Acta. 2011;1815(1):75–89. doi: 10.1016/j.bbcan.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Greer EL, Shi Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13(5):343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J, et al. Biochemical characterization of human SET and MYND domain-containing protein 2 methyltransferase. Biochemistry. 2011;50(29):6488–6497. doi: 10.1021/bi200725p. [DOI] [PubMed] [Google Scholar]
- 33.Patnaik D, et al. Substrate specificity and kinetic mechanism of mammalian G9a histone H3 methyltransferase. J Biol Chem. 2004;279(51):53248–53258. doi: 10.1074/jbc.M409604200. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Bruice TC. Product specificity and mechanism of protein lysine methyltransferases: Insights from the histone lysine methyltransferase SET8. Biochemistry. 2008;47(25):6671–6677. doi: 10.1021/bi800244s. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Bruice TC. Mechanism of product specificity of AdoMet methylation catalyzed by lysine methyltransferases: Transcriptional factor p53 methylation by histone lysine methyltransferase SET7/9. Biochemistry. 2008;47(9):2743–2748. doi: 10.1021/bi702370p. [DOI] [PubMed] [Google Scholar]
- 36.Couture JF, Hauk G, Thompson MJ, Blackburn GM, Trievel RC. Catalytic roles for carbon-oxygen hydrogen bonding in SET domain lysine methyltransferases. J Biol Chem. 2006;281(28):19280–19287. doi: 10.1074/jbc.M602257200. [DOI] [PubMed] [Google Scholar]
- 37.Horowitz S, Yesselman JD, Al-Hashimi HM, Trievel RC. Direct evidence for methyl group coordination by carbon-oxygen hydrogen bonds in the lysine methyltransferase SET7/9. J Biol Chem. 2011;286(21):18658–18663. doi: 10.1074/jbc.M111.232876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horowitz S, et al. Conservation and functional importance of carbon-oxygen hydrogen bonding in AdoMet-dependent methyltransferases. J Am Chem Soc. 2013;135(41):15536–15548. doi: 10.1021/ja407140k. [DOI] [PubMed] [Google Scholar]
- 39.Houston SI, et al. Catalytic function of the PR-Set7 histone H4 lysine 20 monomethyltransferase is essential for mitotic entry and genomic stability. J Biol Chem. 2008;283(28):19478–19488. doi: 10.1074/jbc.M710579200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tardat M, et al. The histone H4 Lys 20 methyltransferase PR-Set7 regulates replication origins in mammalian cells. Nat Cell Biol. 2010;12(11):1086–1093. doi: 10.1038/ncb2113. [DOI] [PubMed] [Google Scholar]
- 41.Beck DB, Oda H, Shen SS, Reinberg D. PR-Set7 and H4K20me1: At the crossroads of genome integrity, cell cycle, chromosome condensation, and transcription. Genes Dev. 2012;26(4):325–337. doi: 10.1101/gad.177444.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Islam K, Zheng W, Yu H, Deng H, Luo M. Expanding cofactor repertoire of protein lysine methyltransferase for substrate labeling. ACS Chem Biol. 2011;6(7):679–684. doi: 10.1021/cb2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo M. Current chemical biology approaches to interrogate protein methyltransferases. ACS Chem Biol. 2012;7(3):443–463. doi: 10.1021/cb200519y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibáñez G, McBean JL, Astudillo YM, Luo M. An enzyme-coupled ultrasensitive luminescence assay for protein methyltransferases. Anal Biochem. 2010;401(2):203–210. doi: 10.1016/j.ab.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Zheng W, et al. Sinefungin derivatives as inhibitors and structure probes of protein lysine methyltransferase SETD2. J Am Chem Soc. 2012;134(43):18004–18014. doi: 10.1021/ja307060p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segel IH. 1993. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems, Wiley Classics Library Edition (Wiley, New York)
- 47.Bahnson BJ, Anderson VE. Crotonase-catalyzed beta-elimination is concerted: A double isotope effect study. Biochemistry. 1991;30(24):5894–5906. doi: 10.1021/bi00238a013. [DOI] [PubMed] [Google Scholar]
- 48.Anderson VE. Quantifying energetic contributions to ground state destabilization. Arch Biochem Biophys. 2005;433(1):27–33. doi: 10.1016/j.abb.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 49.Silva RG, Kipp DR, Schramm VL. Constrained bonding environment in the Michaelis complex of Trypanosoma cruzi uridine phosphorylase. Biochemistry. 2012;51(34):6715–6717. doi: 10.1021/bi300914q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bigeleisen J. Statistical mechanics of isotopic systems with small quantum corrections. I. General considerations and the rule of the geometric mean. J Chem Phys. 1955;23(12):2264–2267. [Google Scholar]
- 51.Amin M, Price RC, Saunders WH. Isotope effects on isotope effects - failure of the rule of the geometric mean as evidence for tunneling. J Am Chem Soc. 1988;110(12):4085–4086. [Google Scholar]
- 52.Frisch MJ, et al. Gaussian 09. Gaussian, Inc.; Wallingford, CT: 2009. [Google Scholar]
- 53.Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc. 2008;120(1-3):215–241. [Google Scholar]
- 54.Anisimov V, Paneth P. ISOEFF98. A program for studies of isotope effects using Hessian modifications. J Math Chem. 1999;26(1-3):75–86. [Google Scholar]
- 55.Zhang J, Klinman JP. Enzymatic methyl transfer: Role of an active site residue in generating active site compaction that correlates with catalytic efficiency. J Am Chem Soc. 2011;133(43):17134–17137. doi: 10.1021/ja207467d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwig DF, Grippe AT, McIntyre TA, Booker SJ. Isotope and elemental effects indicate a rate-limiting methyl transfer as the initial step in the reaction catalyzed by Escherichia coli cyclopropane fatty acid synthase. Biochemistry. 2004;43(42):13510–13524. doi: 10.1021/bi048692h. [DOI] [PubMed] [Google Scholar]
- 57.Couture JF, Dirk LM, Brunzelle JS, Houtz RL, Trievel RC. Structural origins for the product specificity of SET domain protein methyltransferases. Proc Natl Acad Sci USA. 2008;105(52):20659–20664. doi: 10.1073/pnas.0806712105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson PB, Williams IH. Influence of equatorial CH⋅⋅⋅O interactions on secondary kinetic isotope effects for methyl transfer. Angew Chem Int Ed Engl. 2016;55(9):3192–3195. doi: 10.1002/anie.201511708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poulin MB, et al. Transition state for the NSD2-catalyzed methylation of histone H3 lysine 36. Proc Natl Acad Sci USA. 2016;113(5):1197–1201. doi: 10.1073/pnas.1521036113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, Bruice TC. Enzymatic mechanism and product specificity of SET-domain protein lysine methyltransferases. Proc Natl Acad Sci USA. 2008;105(15):5728–5732. doi: 10.1073/pnas.0801788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S, Hu P, Zhang Y. Ab initio quantum mechanical/molecular mechanical molecular dynamics simulation of enzyme catalysis: The case of histone lysine methyltransferase SET7/9. J Phys Chem B. 2007;111(14):3758–3764. doi: 10.1021/jp067147i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu P, Zhang Y. Catalytic mechanism and product specificity of the histone lysine methyltransferase SET7/9: An ab initio QM/MM-FE study with multiple initial structures. J Am Chem Soc. 2006;128(4):1272–1278. doi: 10.1021/ja056153+. [DOI] [PubMed] [Google Scholar]
- 63.Ibanez G, et al. A high throughput scintillation proximity imaging assay for protein methyltransferases. Comb Chem High Throughput Screen. 2012;15(5):359–371. doi: 10.2174/138620712800194468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birck MR, Schramm VL. Nucleophilic participation in the transition state for human thymidine phosphorylase. J Am Chem Soc. 2004;126(8):2447–2453. doi: 10.1021/ja039260h. [DOI] [PubMed] [Google Scholar]
- 65.Singh V, Lee JE, Núñez S, Howell PL, Schramm VL. Transition state structure of 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli and its similarity to transition state analogues. Biochemistry. 2005;44(35):11647–11659. doi: 10.1021/bi050863a. [DOI] [PubMed] [Google Scholar]
- 66.Purich DL, Schramm VL. Methods in Enzymology, Enzyme Kinetics and Mechanism: Part E Energetics of Enzyme Catalysis. Academic; New York: 1999. pp. 301–355. [Google Scholar]
- 67.Lewis BE, Schramm VL. Binding equilibrium isotope effects for glucose at the catalytic domain of human brain hexokinase. J Am Chem Soc. 2003;125(16):4785–4798. doi: 10.1021/ja0298242. [DOI] [PubMed] [Google Scholar]
- 68.LaReau RD, Wan W, Anderson VE. Isotope effects on binding of NAD+ to lactate dehydrogenase. Biochemistry. 1989;28(8):3619–3624. doi: 10.1021/bi00434a070. [DOI] [PubMed] [Google Scholar]
- 69.Du Q, Wang Z, Schramm VL. Human DNMT1 transition state structure. Proc Natl Acad Sci USA. 2016;113(11):2916–2921. doi: 10.1073/pnas.1522491113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alecu IM, Zheng J, Zhao Y, Truhlar DG. Computational thermochemistry: Scale factor databases and scale factors for vibrational frequencies obtained from electronic model chemistries. J Chem Theory Comput. 2010;6(9):2872–2887. doi: 10.1021/ct100326h. [DOI] [PubMed] [Google Scholar]
- 71.Hirschi JS, Takeya T, Hang C, Singleton DA. Transition-state geometry measurements from (13)c isotope effects. The experimental transition state for the epoxidation of alkenes with oxaziridines. J Am Chem Soc. 2009;131(6):2397–2403. doi: 10.1021/ja8088636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwig DF, Booker SJ. Insight into the polar reactivity of the onium chalcogen analogues of S-adenosyl-L-methionine. Biochemistry. 2004;43(42):13496–13509. doi: 10.1021/bi048693+. [DOI] [PubMed] [Google Scholar]
- 73.Bothwell IR, et al. Se-adenosyl-L-selenomethionine cofactor analogue as a reporter of protein methylation. J Am Chem Soc. 2012;134(36):14905–14912. doi: 10.1021/ja304782r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blum G, Bothwell IR, Islam K, Luo M. Profiling protein methylation with cofactor analog containing terminal alkyne functionality. Curr Protoc Chem Biol. 2013;5(1):67–88. doi: 10.1002/9780470559277.ch120241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kogami M, Koketsu M. An efficient method for the synthesis of selenium modified nucleosides: Its application in the synthesis of Se-adenosyl-L-selenomethionine (SeAM) Org Biomol Chem. 2015;13(36):9405–9417. doi: 10.1039/c5ob01316j. [DOI] [PubMed] [Google Scholar]
- 76.Hoffman JL. Chromatographic analysis of the chiral and covalent instability of S-adenosyl-L-methionine. Biochemistry. 1986;25(15):4444–4449. doi: 10.1021/bi00363a041. [DOI] [PubMed] [Google Scholar]
- 77.Stecher H, et al. Biocatalytic Friedel-Crafts alkylation using non-natural cofactors. Angew Chem Int Ed Engl. 2009;48(50):9546–9548. doi: 10.1002/anie.200905095. [DOI] [PubMed] [Google Scholar]
- 78.Dalhoff C, Lukinavicius G, Klimasăuskas S, Weinhold E. Direct transfer of extended groups from synthetic cofactors by DNA methyltransferases. Nat Chem Biol. 2006;2(1):31–32. doi: 10.1038/nchembio754. [DOI] [PubMed] [Google Scholar]
- 79.Borchardt RT, Wu YS. Potential inhibitors of S-adenosylmethionine-dependent methyltransferases. 5. Role of the asymmetric sulfonium pole in the enzymatic binding of S-adenosyl-L-methionine. J Med Chem. 1976;19(9):1099–1103. doi: 10.1021/jm00231a004. [DOI] [PubMed] [Google Scholar]
- 80.Luo M, Schramm VL. Ribosyl geometry in the transition state of Streptococcus pneumoniae methylthioadenosine nucleosidase from the 3′-(2)H kinetic isotope effect. J Am Chem Soc. 2008;130(35):11617–11619. doi: 10.1021/ja804578m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo M, Schramm VL. Transition state structure of E. coli tRNA-specific adenosine deaminase. J Am Chem Soc. 2008;130(8):2649–2655. doi: 10.1021/ja078008x. [DOI] [PubMed] [Google Scholar]
- 82.Cook PF, Cleland WW. Enzyme Kinetics and Mechanism. Garland Science; London: 2007. [Google Scholar]