Significance
Erickson convincingly inferred a pre-European floodplain fishery unlike any present-day system he knew, illustrating the principle that archaeological inference should not be constrained by the range of cultural variation observed today. Our comparison of these inferences with observations from a present-day fishery in a similar environment suggests strong convergence in both the ecology of fish communities and the cultural means people have devised to exploit them, providing support for a predictive model of cultural niche construction. This conceptual framework emphasizes the synergistic action of human agency and environmental constraint in shaping patterns in the human use and management of ecosystems.
Keywords: convergent evolution, cultural evolution, earthworks, historical ecology, tropical stream ecology
Abstract
Erickson [Erickson CL (2000) Nature 408 (6809):190–193] interpreted features in seasonal floodplains in Bolivia’s Beni savannas as vestiges of pre-European earthen fish weirs, postulating that they supported a productive, sustainable fishery that warranted cooperation in the construction and maintenance of perennial structures. His inferences were bold, because no close ethnographic analogues were known. A similar present-day Zambian fishery, documented here, appears strikingly convergent. The Zambian fishery supports Erickson’s key inferences about the pre-European fishery: It allows sustained high harvest levels; weir construction and operation require cooperation; and weirs are inherited across generations. However, our comparison suggests that the pre-European system may not have entailed intensive management, as Erickson postulated. The Zambian fishery’s sustainability is based on exploiting an assemblage dominated by species with life histories combining high fecundity, multiple reproductive cycles, and seasonal use of floodplains. As water rises, adults migrate from permanent watercourses into floodplains, through gaps in weirs, to feed and spawn. Juveniles grow and then migrate back to dry-season refuges as water falls. At that moment fishermen set traps in the gaps, harvesting large numbers of fish, mostly juveniles. In nature, most juveniles die during the first dry season, so that their harvest just before migration has limited impact on future populations, facilitating sustainability and the adoption of a fishery based on inherited perennial structures. South American floodplain fishes with similar life histories were the likely targets of the pre-European fishery. Convergence in floodplain fish strategies in these two regions in turn drove convergence in cultural niche construction.
Fifteen years ago, in his studies of human-modified landscapes in the Beni savannas of Bolivia, Erickson (1) identified a particular form of zigzag earthwork in a 525-km2 area of seasonal floodplain in the Baures region as vestiges of fish weirs. “A fishweir is … any structure constructed in water and acting as a funnel or barrier to direct fish into a trap or enclosure or to entrap fish behind it, where they can be easily harvested” (2, p. 5). Fish weirs are “… usually built in a flowing stream to funnel fish into a trap or built in a tidal flat to trap fish behind it as the tide goes out” (2, p. xv). The structures Erickson identified as vestiges of fish weirs are linear ridges of raised earth (now 1–2 m wide and 20–50 cm tall) that cross savanna floodplains from one forest island to another for distances up to 3.5 km, changing direction every 10–30 m. Erickson stated that “funnel-like openings, 1–3 m long and 1–2 m wide, are present where the structures form a sharp angle.” Erickson identified these gaps as passages where fish-catching devices were placed. Erickson’s conclusions, based on the form of these structures and on their orientation, location, and association with other hydraulic earthworks, were quite bold, because he lacked direct archaeological evidence of their function and because the ethnographic analogues he cited in support of his inferences all differ in important ways from the structures he described. Furthermore, examination of Connaway’s world review of fish weirs (2) also turned up no close analogues (SI Text). This paucity of evidence leaves room for skepticism, and the only archaeological study of fish consumption so far conducted in the Beni llanos found assemblages to be dominated by species dwelling in slow-moving water (swamps, marshes, or ponds), with few or no remains of fish species that exploit flooding cycles and thus are likely to have been trapped in large numbers by weirs (3). However, weir-like structures appear to be absent from the area these authors studied, which is about 150 km southwest of Erickson’s study area. Furthermore, no plausible alternative function of the features described by Erickson, and particularly of the funnel-shaped openings he described, has been suggested. That Erickson was able to make a compelling case despite the absence of close ethnographic analogues illustrates the validity of Binford’s (4) statement that ascribing too much importance to ethnographic analogy prevents archaeologists from admitting “the possibility of dealing with forms of cultural adaptation outside the range of variation known ethnographically” (p. 13). However, although archaeologists should not allow their interpretive scope to be limited by the bounds of the ethnographic present, neither can they afford to ignore this invaluable source of information, which often offers clues to aspects of culture inaccessible to archaeologists.
We present data on a present-day fishery based on earthen weirs in the Bangweulu floodplain, Zambia. Although this system was first described in the 1940s (5), it is not widely known, and Connaway’s review (2) failed to note it. We give a detailed description of this system, which appears to be a close ecological and cultural analogue of the putative pre-European fishery in Bolivia. The prehistoric fishery in Amazonia and the present-day fishery in Africa produce strikingly similar landscapes seen from the air (Fig. 1). This ethnographic example provides a fresh comparative perspective for evaluating Erickson’s inferences.
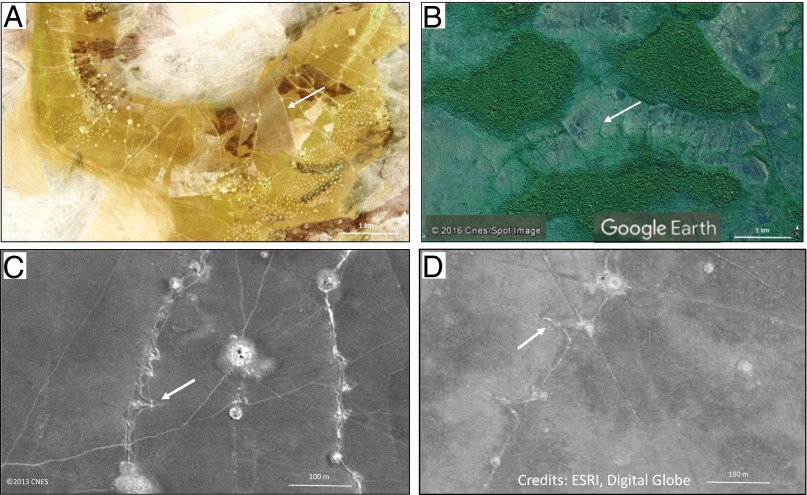
Fig. 1.
Seasonal floodplain landscapes bearing large numbers of earthen fish weirs (white arrows). (A) The present-day fishery in the Bangweulu basin, Zambia, described in this study. Multispectral satellite image by the Pléiades sensor, 12°00′S, 29°42′E, September 17, 2013 (Copyright 2013, CNES, Distribution Airbus DS, all rights reserved). (B) The archaeological fishery in Bolivian Amazonia described by Erickson (1). Image from Google Earth V7.1.5.1557; 13°51′S, 63°19′W. (C) Fishways (white arrow) in weirs in the Bangweulu basin, Zambia. Multispectral satellite image by the Pléiades sensor, 12°00′S, 29°42′E, September 17, 2013 (Copyright 2013, CNES, Distribution Airbus DS, all rights reserved). (D) Vestiges of V-shaped structures (white arrow) in the archaeological fishery in Bolivia. Image (13°45′30′′, 63°18′’52′′W) from “World Imagery” layer of ArcGis. Source: ESRI, Digital Globe, satellite WorldView1, June 2008.
We analyze the functioning of these fisheries within the conceptual framework of niche construction (6), the process whereby organisms modify their environment, changing their living conditions. We are not concerned with the merits, or lack thereof, of niche construction as a new paradigm in evolutionary theory (7); we use it as a heuristic concept for examining reciprocal feedbacks between actions of organisms and their environmental conditions (8). Culture amplifies the niche-constructing capacity of humans, who adapt to environments in large part by modifying the environments to suit their needs (9). The making of fish weirs has been treated explicitly as one of the principal niche-construction activities by which small-scale preindustrial societies manage wild plant and animal resources (10).
Niche-construction activities influence the environment of the constructors’ descendants. Long-lived structures (social-insect nests, beaver dams, or perennial earthen fish weirs) create an “ecological inheritance,” a legacy that can confer advantages across multiple generations. People are more likely to invest labor and time in building and maintaining structures if these structures are inherited and used by their descendants. Thus, the long-term sustainability or resilience of a fishery based on earthen weirs is key to the system’s functioning. For this reason, we explore the ecological and cultural mechanisms that favor sustainability in the present-day system and ask whether the convergence in human niche construction in similar floodplain systems of Africa and South America is itself based on convergence in the evolutionary ecology of fishes in the two regions.
Results and Discussion
Drawing on a recent report (11) and a Master of Science thesis (12), we describe in detail a present-day floodplain fishery in Zambia based on fish weirs that closely resembles the Baures fishery in terms of the kind of environment exploited, the dimensions of the weirs, the materials used in their construction, and the earth-moving operations that modified the landscape (Fig. 1). We compare Erickson’s inferences about the structure and functioning of the archaeological Baures fishery with our observations of the present-day ethnographic analogue.
Fish Weirs in the Two Landscapes: Their Distribution, Extent, Linear Density, and Structure.
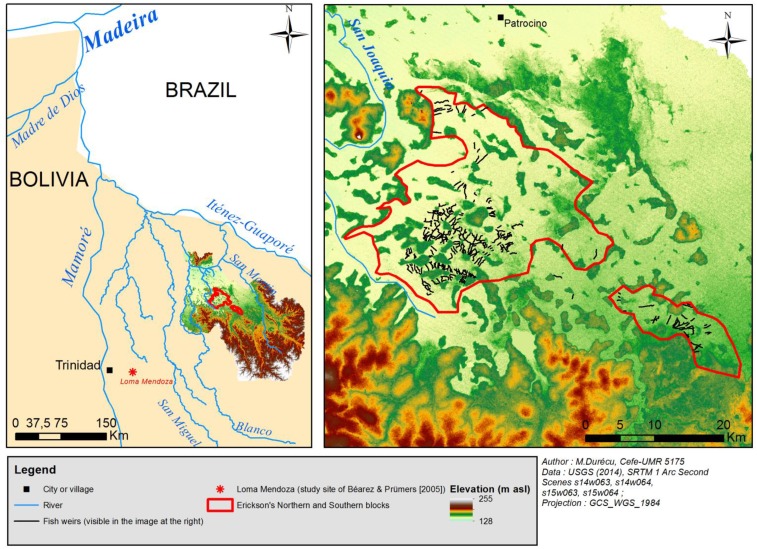
In the archaeological fishery, Erickson (1) indicated the perimeters of two areas containing fish weirs (Figs. S1 and S2). These are within the San Joaquin floodplains, within the Blanco-San Martin basin. Erickson reported a total area of 524 km2 for the two blocks he delineated. Based on inspection of unspecified aerial photographs, he estimated there were 1,515 linear km of weirs in this total area, giving a linear density of 2.89 km/km2. Radiocarbon dating of burned wood at the base of a causeway directly associated with a weir gave a calibrated date of 1490–1630 AD, well before the beginning of Spanish control of the region in 1708 (1).
Fig. S1.
Fine-scale distribution of fish weirs in a zone within the Northern block described by Erickson (1). (A–C) Comparison of Erickson’s original figure 4 based on aerial photographs (A) on a World Imagery (ArcGis) image of the identical area (B) and our photo-interpretation of this image (C). (A) Erickson’s figure 4 (1), reproduced with the original legend. Reprinted from ref. 1 with permission from MacMillan Publishers Ltd. (B) World Imagery including the area depicted in A (within the white-bordered rectangle). Image available in the World Imagery layer of ArcGis; 13°48′S, 63°19′W; source: ESRI, Digital Globe, satellite WorldView1, June 2008. (C) Map of fish weirs and causeways, digitized from the image in B and based on our photo-interpretation. The image is presented at the same scale as the image in A, but note the difference in the scale given by Erickson in A and the scale in B and C based on World Imagery.
Fig. S2.
Localization of the archaeological fish weirs described by Erickson (1). (Left) The location of the San Joaquin floodplain in relation to that of the study site of Béarez and Prümers (3). (Right) A larger-scale image showing the location of fish weirs (nonexhaustive mapping using Google Earth imagery) in relation to topography. The mapped weirs are superimposed on an SRTM (Shuttle Radar Topography Mission, National Aeronautics and Space Administration) 1 Arc Second image of the region (49). The map shows that weirs are found in the lowest-lying parts of the two blocks.
Erickson’s succinct description of fishways, the diagnostic traits of weirs, was accompanied by schematic line drawings of V-shaped fishways but not by aerial photographs on which the fishways are discernible. We present an image (Fig. 1D) that clearly shows the vestiges of these structures, which, as expected given their supposed function, are all oriented in the same direction, downstream with respect to water flowing out of the basin.
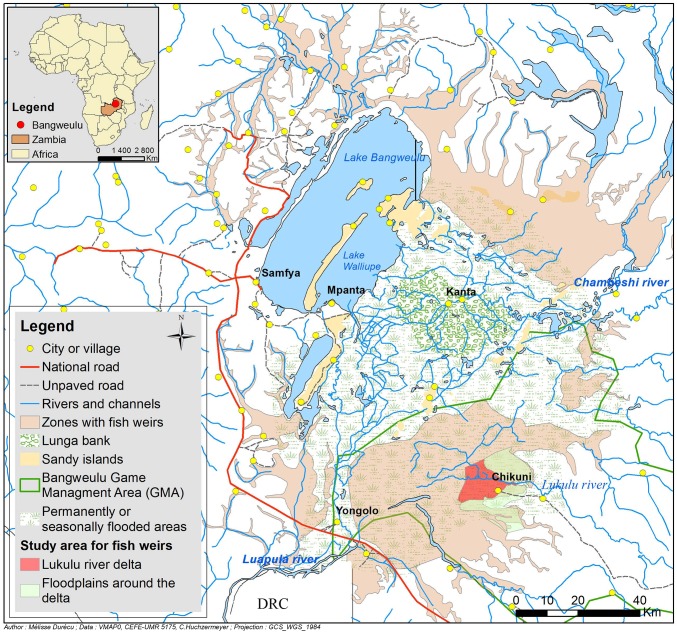
In the Bangweulu basin, active fish weirs occur throughout the vast area of seasonal floodplains that occupy almost half (7,100 km2) of the basin’s 15,000 km2 (Fig. 2) (13). The area in which earthen weirs are present in the Bangweulu basin is thus more than 13 times larger than the area in which vestiges of weirs are known to occur in Bolivia (about 524 km2). During the high-water season, the water level in this area varies from 0.5 m up to 1.5 m in the deepest parts of the floodplain. The height of the weirs depends on the depth of flooding, with low weirs in termite savanna where water is shallow and higher weirs in deeper parts of the floodplain. Deeper in the swamps, weirs of even greater height are built from vegetation.
Fig. 2.
Distribution of fish weirs in the seasonal floodplains of the Bangweulu basin, Zambia. See Methods and SI Text for sources and methods used to prepare the map.
The Zambian weirs can be up to 5 km long, traversing broad floodplains. Weirs sometimes zigzag but usually are more or less straight or curvilinear, running from one termitarium island to another. They are easily recognized by the presence of the frequent (every 2–10 m) fishways, which may be simple gaps or V-shaped structures (Fig. 3). Funnel-like openings (always oriented downstream with respect to the direction of water flow out of the floodplain) may be 1–8 m long and 1–2 m wide; simple gap openings are 1–2 m wide. Larger weirs are flanked by canals resulting from excavation of the earth to make the weirs and used for access by boat when nets and baskets are set, checked, and harvested. The excavation channels alongside the weirs also offer a slightly deeper habitat for fish and a route along which they can swim.
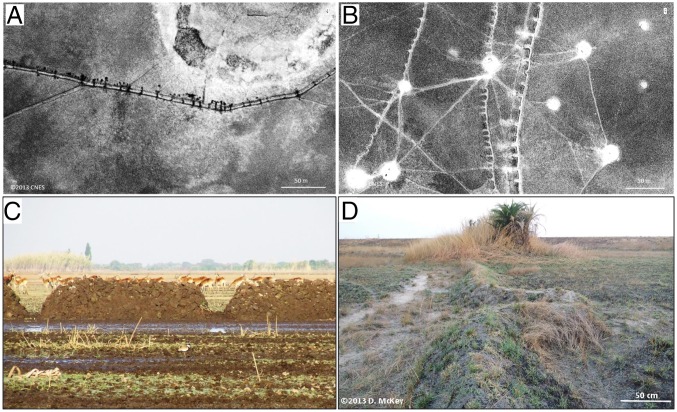
Fig. 3.
Fishways, the simple gaps or V-shaped structures that are the characteristic features of earthen fish weirs in Bangweulu basin, Zambia, seen from the air (A and B) and on the ground (C and D. (A and C) Simple gap fishways. (A) Simple gap fishways seen from the air. Panchromatic satellite image by the Pléiades sensor, 12°02′S, 30°05′E, September 17, 2013 (Copyright 2013, CNES, Distribution Airbus DS, all rights reserved). (C) Simple gap fishways in a newly constructed weir in the Bangweulu basin. Behind the weir is a herd of black lechwe (Kobus leche smithemani). (Photograph copyright 2011, C.F.H.) (B and D) V-shaped fishways. (B) V-shaped fishways seen from the air. Panchromatic satellite image by the Pléiades sensor, 12°02′S, 30°09′E, September 17, 2013 (Copyright 2013, CNES, Distribution Airbus DS, all rights reserved). (D) V-shaped fishways seen from the ground. (Copyright 2013, D.M.)
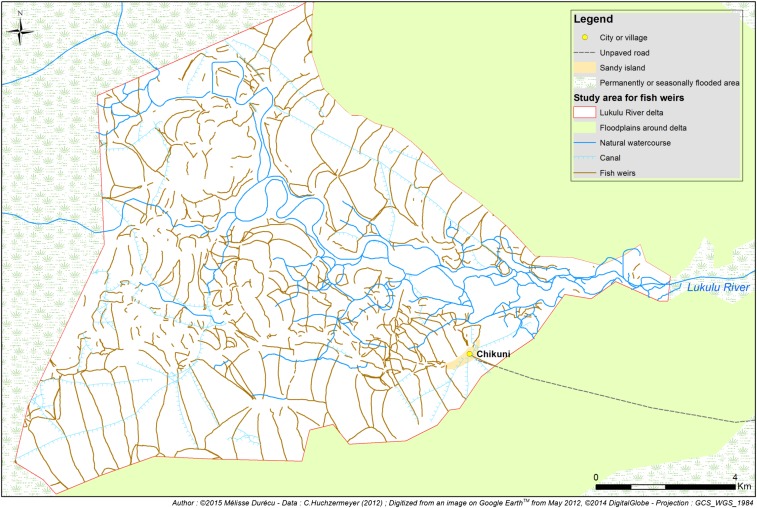
In the part of the Lukulu delta where most fieldwork was done (red area in Fig. 2), covering 129 km2 of floodplain, exhaustive mapping based on a satellite image taken in May 2012 (Fig. S3) yielded a total of 328 km of fish weirs and thus a linear density of 2.55 km/km2. Thus, although some uncertainty is associated with Erickson’s estimates in Bolivia (see SI Text), the density of weirs (or their vestiges, in Bolivia) appears to be roughly comparable in the two sites (2.89 vs. 2.55 km/km2 in the prehistoric and present-day fisheries, respectively).
Fig. S3.
Detailed map showing the distribution of fish weirs in a small part of the area shown in Fig. 2, the Lukulu delta, digitized from a Google Earth image taken in May 2012.
Functioning of the Weir Fishery.
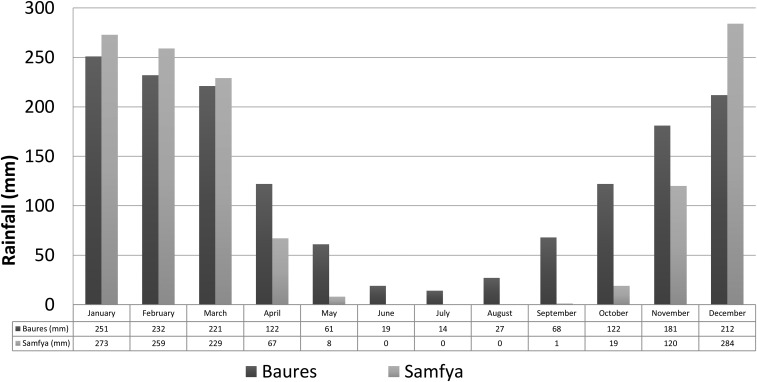
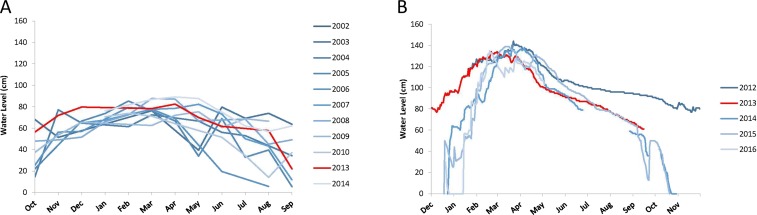
For the archaeological system, Erickson’s most fundamental inference—that the structures he observed were in fact fish weirs—was based on their form (particularly of the funnel-shaped structures), location, orientation, association with other earthworks, and (imperfect) ethnographic analogy. He excluded transport or water retention for flood-recessional farming as possible functions of the zigzag structures but considered that weirs, together with other hydraulic earthworks, could have been used to extend the period of flooding in some areas. Weirs are situated across areas that today are shallowly flooded in the wet season and functioned, he inferred, to trap fish that migrate from permanent water into seasonal floodplains to spawn and feed and then migrate back out of the floodplain to permanent water when flood waters recede (14, 15). Today, floodplains in the Baures region are flooded for a period of 4–5 mo, from January/February to June (16). The duration of flooding is similar to that in the Bangweulu basin, reflecting the similar amount and seasonal distribution of annual rainfall in the two areas (Fig. S4). Depth of flooding is also similar, currently not greatly exceeding 1 m in the areas where fish weirs (or in Bolivia, their vestiges) are found (Fig. S5). Building, maintaining, and using earthen weirs would likely be prohibitively time-consuming and impractical if flooding were frequently much deeper than 1 m.
Fig. S4.
Rainfall seasonality in the areas occupied by the archaeological weir-based fishery (Baures region of Bolivia) and the present-day analogue (Bangweulu basin, Zambia). Data are from the Global Precipitation Climatology Centre (GPCC) database (www.dwd.de/EN/ourservices/gpcc/gpcc.htm).
Fig. S5.
Seasonal and interannual variation in flood level. (A) Baures region, Bolivia. Two sources of data were used to compile the graph. Data from the RA-2 altimeter aboard the ENVISAT satellite launched in 2002 [ENVISAT/RA-2 Geophysical Data Records (GDR) version V 2.1] were obtained from the Center for Topographic Studies of the Ocean and Hydrosphere (CTOH; ctoh.legos.obs-mip.fr). Data from the Atika altimeter aboard the Satellite with Argos and Altika (SARAL) satellite launched in 2013 (SARAL data, version IGDR-T) were obtained from the AVISO (Archiving, Validation and Interpretation of Satellite Oceanographic data; www.aviso.altimetry.fr/en/home). We used Virtual Altimetry Station (VALS) software (50) to process the data. (B) Lukulu delta, Bangweulu basin, Zambia. Data are from gauges established and observed by C.F.H.
Erickson postulated that the fishery he described was highly productive, sustaining large populations in a seemingly marginal environment that today harbors few people. His claim of high productivity was based on a few reports of high fish biomass in river channels or ponds in seasonally flooded tropical savannas (17, 18). He also speculated that Pomacea snails, which are abundant in the area, could have been harvested in large numbers and that palms, also frequent around weirs today, provided both edible fruits and insect larvae. Nearby ponds, which Erickson assumed to be of artificial origin, could have held drinking water year-round, attracted game, and enabled dry-season persistence of fish and snails.
Erickson (1) considered the complex of weirs, ponds, and causeways to be “a form of intensive aquaculture” (p. 191) used to regulate water levels, extending the period of flooding by capturing the first rains and holding water longer into the dry season. As a perennial food-producing infrastructure, weirs must have been valuable and protected real estate, owned and inherited by clans and chiefly lineages. Like causeways, canals, raised fields, and other earthworks, they were durable investments in land created by modification of the landscape (19). Sustained high productivity justified the investment of labor required to build and maintain weirs. He also postulated that the system required social coordination. Landscape patterns suggested that both intercommunity cooperation and tension over fisheries and other resources may have occurred.
Considerable ethnographic information from the present-day Zambian weir fishery (SI Text and Fig. S6) can help us evaluate Erickson’s inferences and speculations about the functioning of the pre-European Bolivian system.
Fig. S6.
Construction, maintenance, and use of earthen weirs in the Bangweulu weir fishery. (A and B) Weirs in action. (A) A long weir set with mosquito-net traps, seen from the air. (B) Weir showing the slightly deeper passageway for canoes on one side. (C and D) Types of traps placed at fishways. (C) A mono basket trap (traditional) and a mosquito-net trap (modern gear). (D) Mono baskets before the addition of trap valve. (E and F) Construction and maintenance of weirs. (E) A new weir, showing a newly constructed simple-gap fishway and the adjacent excavation channel. The height of new weirs can be up to 1.5 m. (F) An old weir maintained by the addition of new sods. (G) Fish harvested using weirs. Large numbers of fish, mostly juveniles, are captured during falling water in flooded savanna. (H) Cooperation and social coordination. A chipupila (center, with foot raised on an old weir) giving a “pep talk” to villagers preparing to begin work repairing an old weir. (All photographs Copyright 2011–2016, C.F.H.)
First, the present-day fishery is highly productive, and high harvest levels appear to be sustainable, because overall yield, although fluctuating, shows an increasing trend (13). Based on data gathered for an intensively studied area of 15 × 15 km around Chukuni, Huchzermeyer (11) estimated the annual yield of the weir fishery for local fishermen using a 22,500-ha floodplain to be 800,000 kg fresh mass of fish, or 35.5 kg⋅ha−1⋅y−1.
For the Bolivian fishery, estimates of yield are of course unavailable, and the figures Erickson (1) cites for potential productivity are expressed in a form that does not allow comparison with Huchzermeyer’s (11) estimate for Zambia. Citing a reference (17), Erickson (1) writes that “yields of 1,000 kg per hectare per year have been recorded for shallow ponds in tropical savannas.” This very high figure certainly represents the concentration in dry-season refugia of fishes from a much larger (but unknown) area of rainy-season floodplain and cannot be compared with Huchzermeyer’s (11) estimate based on total floodplain area.
Secondly, weirs are indeed valuable real estate, perennial food-producing infrastructures that are owned and inherited. The perennial nature of earthen weirs warrants the considerable labor investment needed to construct and maintain them (SI Text). A person who builds an earthen weir when young can benefit from this initial investment throughout his life. The effort required to maintain a perennial earthen weir is probably much less than the effort that would be needed to build a new short-lived structure made of vegetation every year, a task that gets more difficult as the individual ages. Furthermore, a weir (or a section of weir) is inherited by the living descendants of the person who first built it and cannot easily be taken away from the lineage, because the “ancestor spirits” would keep that weir from having good catches unless it is used by their relatives (5). Weirs can remain operational for many decades, and there is no physical reason why they should ever be abandoned. Soils of the parts of the floodplain where earthen weirs occur are loamy in texture (20). Friable enough to allow easy working, they also are sufficiently clay-rich that weirs, held together by plant roots, are resistant to erosion. Furthermore, weirs are repaired regularly (Fig. S6F). The only cases known to us of weirs being abandoned are explained by conflict over ownership. In these cases, each feuding party is given a new area in which to build a weir.
Construction, maintenance, and use of weirs all require cooperation and coordination; access rights are regulated, and, as noted above, conflicts occur. Areas important for fishing are divided into tracts of land, each under the control of a traditional fishing chief known as a “chipupila” (Fig. S6H). These men are descended from notable people, and inheritance of the position is matrilineal. The chipupila determines where new weirs can be constructed, allocates fishing rights in the areas of water that drain through a weir, and mediates conflicts. Long fish weirs have multiple owners. Every owner assists with the construction and maintenance of a fish weir, and the gaps left in a weir for trap placement are individually owned. Formerly, the start of the fishing season and opening of new areas were surrounded by ritual, and the chipupila played a spiritual role [“fishing priests” (5)].
Thus, information about the present-day fishery supports Erickson’s view of the archaeological fishery as capable of sustained high productivity and of weirs as structures inherited across generations, whose construction and use required social coordination. However, analysis of the present-day system suggests a technological/ecological basis for the system’s sustainability that is different from, and simpler than, that proposed by Erickson.
The Ecological Basis of the Sustainability of the Present-Day Weir-Based Fishery.
Erickson postulated that the archaeological fishery involved practices such as the management of water by using weirs, ponds, and causeways to extend the period of inundation. Analysis of the present-day analogue suggests that sustained high harvest levels could have been achieved without such intensive management. In Bangweulu, weirs serve more to direct the movements of the fish across the floodplain than to control the hydrology.
Fish communities in Bangweulu include a large number of species that migrate seasonally into the vast floodplains for spawning and feeding (SI Text). Of 44 species recorded in the basin by Huchzermeyer (11), 25 occur in floodplain habitat. These species dominate assemblages and are the principal targets of the weir fishery. Different aspects of the fishery are illustrated in Fig. S6. At the start of the rains (December to February), fishways are left open to allow passage of in-migrating fish. Fish spawn, feed, and grow in the flooded plain, and weirs are tended and maintained. As soon as water levels begin to drop (April; see Fig. S5), the weirs are closed, and harvest begins, timed to coincide with massive out-migration of fish which are caught in large numbers in traps set in the fishways (May/June). As one area dries out, another becomes shallow enough to operate the weirs. Thus there is a gradual shift in the location of active weirs from March through to July/August, fishing on the same stock of juveniles as water draws through the various levels of plain and weir. The traditional high-current gear is a large funnel basket, without a valve, called “kansa” in the Bemba language (Fig. S6 C and D). When fishermen observe that flow rate is reduced, they place at the fishways smaller basket traps with a valve called “imono.” Larger fish are smoke-dried, and smaller ones are usually sun-dried. Fish thus preserved can be stored for long periods.
The Bangweulu fishery is characterized by great resilience in the face of high harvest levels. The key to this resilience appears to be the life-history adaptations of fishes to seasonal floodplain environments. A large proportion of the individuals caught in weirs are juveniles (11, 12). Management discourses tend sweepingly to condemn the catching of under-sized and immature fish as wasteful and unsustainable, and the weir technique is considered illegal in the Bangweulu basin [although regulations are not enforced (21)]. However, the “growth-overfishing” concept often makes little sense, given the age–survivorship curve typical of most fishes (22) and particularly of seasonally breeding floodplain fishes. Warm, shallow, well-oxygenated, and relatively nutrient-rich water favors breeding in the high-water season, but severe competition and predation lead to high mortality of juveniles in the first dry season. The boom-and-bust conditions favor fish species that can mature after 1 y of life, start breeding at a small size, and have multiple reproductive cycles. Juveniles of the species captured by weirs at the end of the high-water season would suffer high mortality shortly thereafter, in any case (11). The harvest of this age class thus may have a limited effect on the reproductive potential of the population (23–25). Also, because the age distribution of fishing mortality with the weir technique mimics that of natural mortality in fishes of seasonal floodplains, selective pressures acting on the population are unchanged, conferring long-term resilience (cf. ref. 22).
There are limits to the sustainability of any harvest regime, and many questions about the limits of the weir fishery remain open. Although the harvesting of juveniles of fish species adapted to periodic breeding in floodplains should be sustainable at higher levels than for most fish species, the threshold level beyond which harvest no longer allows adult cohort renewal is unknown. Sustainability also requires relatively high adult survival. If too many adults, particularly large, highly fecund individuals, are harvested by weirs or other gear, the sustainability of the weir fishery would be compromised. We have no indication that fishermen adopt any conservation-motivated practices to spare adult fish; such practices would be unlikely to appear if resources are abundant. However, several factors may give larger fish a higher probability of surviving passage through the weirs. Because larger fish need deeper water to avoid predation, adults likely leave the spawning ground before juveniles, at a time when higher water level allows them to swim (or jump, or in the case of Clarias spp., crawl) over weirs or through fishways not yet set with traps. Data on size-class–specific survivorship and the other open questions raised here are required to model the system’s sustainability.
Evolutionary Convergence in Floodplain Fish Life Histories in Africa and South America.
Lateral seasonal migrations such as those observed in Bangweulu are documented for many fish species in tropical floodplain rivers (26), and the combination of life-history traits described by Huchzermeyer (11, 12) is in fact typical of seasonally breeding floodplain fish species throughout the tropics, including those in South American floodplains [“seasonal strategists” (27) or “periodic” (P) strategies (28, 29)]. Of 71 fish species studied by Winemiller (27) in the Orinoco Llanos of Venezuela, 48 exhibited a P strategy, characterized by cyclic (often annual) reproduction, relatively long generation times, large clutches, and small investment per offspring. As in the Bangweulu floodplains, fishes identified as P strategists exhibit a characteristic burst of reproduction with the early rains, followed by gradual reductions in population size caused largely by predation on immature fish during the early dry season. In the Bangweulu wetlands, the reproduction of virtually all fish species is affected by the periodicity of this highly seasonal environment (Table S1). Following the classification of Winemiller and Rose (28), fish species with P strategies accounted for 57% of total biomass in that site (Table S2). Species with strategies intermediate between P and either “opportunist” (O) or “equilibrium” (E) strategies accounted for a further 38%. No pure E strategist species was present.
Table S1.
Classification of life-history strategies of fish species from the two compared sites
| Order | Family | Genus, species | LHS† | Source‡ | %B§ |
| Baures region¶ | |||||
| Characiformes | Erythrinidae | Hoplias malabaricus | E (E) | 27 | 9.3 |
| Characiformes | Serrasalmidae | Pygocentrus nattereri | P; I-E/P (I-E/P) | 27, 29 | 9.3 |
| Characiformes | Prochilodontidae | Semaprochilodus insignis | P (P) | 51 | 7.9 |
| Perciformes | Cichlidae | Cichla monoculus | E (E) | 27 | 7.0 |
| Characiformes | Acestrorhynchidae | Acestrorhynchus (7 spp.) | P (P) | 29 | 6.3 |
| Characiformes | Characidae | Colossoma macropomum | P (P) | 27 | 5.1 |
| Siluriformes | Pimelodidae | Pseudoplatystoma (2 spp.) | P (P) | 27, 52 | 5.0 |
| Characiformes | Curimatidae | Psectrogaster (4 spp.) | P (P) | 53 | 4.6 |
| Perciformes | Sciaenidae | Plagioscion squamosissimus | P (P) | 54, 55 | 4.4 |
| Myliobatiformes | Potamotrygonidae | Potamotrygon motoro | E (E) | 27 | 4.1 |
| Characiformes | Characidae | Serrasalmus (8 spp.) | P; E; I-E/O (I-E/P) | 27, 29, 52 | 4.0 |
| Clupeiformes | Pristigasteridae | Pellona (2 spp.) | P (P) | * | 3.6 |
| Siluriformes | Auchenipteridae | Ageneiosus (8 spp.) | P; E (I-E/P) | 27, 51 | 3.4 |
| Siluriformes | Loricariidae | Pterygoplichthys (5 spp.) | E (E) | 27 | 3.2 |
| Characiformes | Anostomidae | Schizodon fasciatum | P (P) | 27, 52 | 1.5 |
| Characiformes | Characidae | Triportheus (3 spp.) | P (P) | 27 | 1.3 |
| Characiformes | Curimatidae | Potamorhina (2 spp.) | P (P) | * | 1.1 |
| Characiformes | Characidae | Metynnis (2 spp.) | P (P) | * | 1.1 |
| Siluriformes | Doradidae | Doras (2 spp.) | O (O) | * | 1.0 |
| Characiformes | Cynodontidae | Rhaphiodon vulpinus | P (P) | 52 | 0.9 |
| Characiformes | Characidae | Bryconops (5 spp.) | P (P) | 29 | 0.8 |
| Siluriformes | Auchenipteridae | Auchenipterus (2 spp.) | E (E) | 27 | 0.8 |
| Siluriformes | Loricariidae | Loricariichthys (3 spp.) | E (E) | 27, 29, 52 | 0.8 |
| Siluriformes | Pimelodidae | Pinirampus pinirampu | P (P) | 52 | 0.7 |
| Characiformes | Characidae | Pristobrycon eigenmanni | P; E; I-E/O (I-E/P) | * | 0.6 |
| Characiformes | Characidae | Moenkhausia (6 spp.) | P; I-O/P (I-O/P) | 29, 52 | 0.6 |
| Siluriformes | Pimelodidae | Sorubim lima | P (P) | 52 | 0.6 |
| Siluriformes | Doradidae | Oxydoras niger | O (O) | * | 0.6 |
| Characiformes | Characidae | Mylossoma (2 spp.) | P (P) | 56 | 0.6 |
| Characiformes | Characidae | Myleus (2 spp.) | P (P) | 29 | 0.5 |
| Characiformes | Hemiodontidae | Hemiodus (4 spp.) | P (P) | * | 0.5 |
| Characiformes | Prochilodontidae | Prochilodus nigricans | P (P) | 27, 29, 52 | 0.5 |
| Siluriformes | Auchenipteridae | Auchenipterichthys thoracatus | E (E) | * | 0.5 |
| Characiformes | Hemiodontidae | Anodus elongatus | P (P) | * | 0.5 |
| Siluriformes | Loricariidae | Loricaria (3 spp.) | E (E) | 27, 29 | 0.4 |
| Characiformes | Curimatidae | Curimatella (2 spp.) | P (P) | * | 0.4 |
| Siluriformes | Loricariidae | Hypoptopoma (3 spp.) | P (P) | 27 | 0.4 |
| Characiformes | Curimatidae | Cyphocharax (6 spp.) | P (P) | * | 0.4 |
| Characiformes | Anostomidae | Laemolyta (3 spp.) | P (P) | * | 0.4 |
| Siluriformes | Pimelodidae | Pimelodus (2 spp.) | I-O/P (I-O/P) | 52 | 0.3 |
| Siluriformes | Pimelodidae | Hypophthalmus (3 spp.) | O (O) | 52 | 0.3 |
| Characiformes | Anostomidae | Leporinus (3 spp.) | P (P) | 27, 29, 52 | 0.3 |
| Perciformes | Cichlidae | Geophagus cf megasema | E (E) | 27, 29 | 0.3 |
| Bangweulu basin# | |||||
| Siluriformes | Clariidae | Clarias (4 spp.) | P; I-E/P (P) | 29, 43 | 34.7 |
| Perciformes | Cichlidae | Tilapia rendalli | E (I-E/P) | ** | 22.4 |
| Osteoglossiformes | Mormyridae | Marcusenius macrolepidotus | P; I (P) | 29, 43 | 18.0 |
| Perciformes | Cichlidae | Serranochromis (2 spp.) | E (I-E/P) | 29 | 5.0 |
| Osteoglossiformes | Mormyridae | Pollimyrus castelnaui | I-E/P (I-O/P) | * | 4.9 |
| Cypriniformes | Cyprinidae | Barbus trimaculatus | P (P) | *** | 4.7 |
| Cypriniformes | Cyprinidae | Barbus spp. (small) | O (I-O/P) | 29 | 4.3 |
| Cypriniformes | Cyprinidae | Barbus paludinosus | P (O) | *** | 3.0 |
| Perciformes | Cichlidae | Tilapia (2 spp.) | I-E/O (I-O/P) | **** | 2.2 |
The categorization of life-history strategy (LHS) for the species (or in some cases for other congeners) according to the literature is given first, followed by (in bold and between parentheses) the life-history strategy assessed based on our observations of species present in our sites. The latter categorization was used to calculate the contribution of each strategy to total biomass. Classification of life-history strategies follows Winemiller (27) and Winemiller and Rose (28): E, equilibrium; I, intermediate; O, opportunistic; P, periodic.
Bibliographic references. When no information was available from the literature on the species or the genus, information was used following that available for the family (*), for other large Tilapia species (**), for other medium-sized Barbus species (***), and for other small Tilapia species (****), respectively.
Relative percentage of biomass (%B) in the fish community. See Supplementary Information for methods used to estimate relative contributions to biomass in the two sites.
Baures region: 43 taxa, representing 96% of the total biomass, of the total of 111 taxa recorded.
Bangweulu basin: nine taxa, 99% of the total biomass, of a total of 20 taxa recorded. Six genera (two of which show considerable interspecific variation in life-history strategies) represented more than 99% of total biomass.
Table S2.
Comparison of the composition of fish communities in relation to life-history strategies in the Baures region (Bolivia, South America) and the Bangweulu basin (Zambia, Africa)
| Bangweulu | Baures | |||
| Life-history strategy | Genus, n | %B | Genus, n | %B |
| E | 9 | 26 | ||
| I-E/P | 2 | 27 | 4 | 17 |
| I-O/P | 3 | 11 | 2 | 1 |
| O | 1 | 3 | 3 | 2 |
| P | 3 | 57 | 25 | 49 |
| Total | 9 | 99 | 43 | 96 |
%B, percentage of biomass.
In the Iténez-Guaporé watershed (the names are those applied to the same river in Bolivia and Brazil, respectively), within which Erickson’s study area lies, experimental fishing conducted over a 2-y period (SI Text) (30) found that 43 fish genera together accounted for 96% of the fish biomass (Table S1). P strategists comprised 24 of the 43 genera and 49% of the biomass, followed by E strategists (eight genera, 26% of the biomass) and by species with strategies intermediate between E and P (I-E/P) (four genera, 17% of the biomass) (Table S2). Thus, as in the Bangweulu basin, a large proportion of the assemblage in this region, in terms of both richness and biomass, is comprised of fish that could have been targeted by the weir fishery.
Niche Construction, Ecological Inheritance, and Sustainability.
Because the investment required in niche construction and maintenance is favored when the constructed environment remains productive over long periods and is inherited by the constructors’ descendants, sustainability of the weir fishery is key to the system’s functioning. We identify several features of the social regulation of weir use that enhance sustainability in the present-day Bangweulu fishery; these features are quite similar to those posited by Erickson (1) for the archaeological Bolivian fishery. We also identify ecological factors favoring sustained high harvest levels in the Bangweulu fishery. We propose that, in the two cases we compare here, sustainability is assured by the match between the way weirs are used and the life-history strategies of the fish they capture.
In both regions, similar physical environments and similar floodplain fish life histories drove convergence in the construction and use of weirs. Flooding regimes are similar in the two regions, with a large expanse of floodplain being predictably covered each year (Fig. S5) with water that remains shallow enough to permit the operation of earthen weirs. In both regions, loamy or clay-rich soils (20, 31) and the organisms living in them contribute to making these structures resistant against erosion. Finally, in both environments many fish species respond to seasonal flooding in very similar ways, migrating into seasonal floodplains to spawn and feed and then migrating out as the water recedes. These similarities in ecosystem structure and ecological convergences, driven by natural selection in fish life histories and the functioning of fish populations, led to similar decisions by people in the two regions about how to exploit them. Thus, environmental similarities, natural selection, and human agency combined to drive strong convergence in how humans in these two regions constructed the fishing dimension of their ecological niche.
Methods
Our photo-interpretation of archaeological vestiges of fish weirs in the Baures region was based on images available through Google Earth and the World Imagery layer of ArcGis and were validated by comparison with Erickson’s (1) mapping (SI Text and Figs. S1 and S2).
To map the distribution of habitats and land uses in the Bangweulu basin, we combined field observations, photo-interpretation of high-resolution multispectral images (Landsat 8, scene ID LC81710682014135LGN00; pansharped to yield an image of 15-m resolution), and analysis of a digital terrain model (data uploaded from the site EarthExplorer and assembled using ArcGis 10.2.2; 30-m resolution), using supervised classification applied to predefined classes based on field observations. We then used very high-resolution images available through Google Earth to map the distribution of fish weirs within the region (Fig. 2). Within the area of the Lukulu delta where most field observations were conducted, we used Google Earth imagery, in addition to an image from the Pléiades sensor taken on September 17, 2013 [copyright 2013, Centre National d’Études Spatiales (CNES)], to map all fish weirs (Fig. S3) and calculate their linear density (kilometers of fish weirs per square kilometer). To examine changes over time, the distribution of weirs in this area was mapped on images taken on two different dates, May 9, 2012 and September 17, 2013. No earlier images of sufficiently high resolution are available for this area.
Observations of the construction, maintenance, and functioning of fish weirs in Bangweulu were conducted by C.F.H. during 5 y of fieldwork (11, 12). During this time, fish communities of the region were characterized by participatory observation of fishermen, experimental fishing, and opportunistic collection. Fish communities of the Baures region were characterized by similar methods (see SI Text for details).
SI Text
Bibliographic Survey of Ethnographic and Archaeological Examples of Weir Fisheries.
There are important differences between the putative Baures fishery and the previously known ethnographic examples of fisheries based on fish weirs, both those Erickson cited and others. Furthermore, although there are many known archaeological examples of fish weirs, these examples also differ greatly from the fishery Erickson described.
Offered in the absence of direct archaeological evidence for their function, Erickson’s (1) hypothesis that the features he described were vestiges of fish weirs went boldly beyond the ethnographic examples known to him. Still, his arguments were compelling, and since then no plausible alternative hypothesis has been proposed for the function of the most distinctive features, the funnel-shaped openings he described (Fig. 1D). Since Erickson’s account a comprehensive compilation of literature on fish weirs has appeared (2), documenting many other examples, both archaeological and present-day (see also refs. 32 and 33). Virtually all these examples also differ in at least one important way from the Baures fishery described by Erickson (1). First, the Baures fishery was in a seasonal tropical floodplain, whereas ethnographic examples (and other published archaeological examples) concern fisheries in stream or tidal environments. The Baures fishery must have differed greatly from known present-day analogues in both the spatial and temporal patterns of its use and in the kinds of fish it captured. Secondly, the materials used to construct weirs differed between the Baures fishery and almost all other known ethnographic and archaeological fisheries. The present-day and historical weirs cited by Erickson are mostly ephemeral structures made with plant materials and rebuilt each season, whereas the Baures fish weirs were perennial earthworks. We now know of many other examples of perennial fish weirs in many parts of the world (2, 32, 33), including South America (2), but virtually all are constructed of stone or coral, not of earth, and are located in rivers or tidal areas, not in tropical floodplains.
Examination of Connaway’s (2) annotated bibliography of fish weirs, in which we found 59 references that record the use of fish weirs in the Neotropics, confirms Erickson’s conclusion, based on a smaller number of examples (none of them cited by Connaway), that the Baures fishery differed in important ways from all the others documented in the Neotropics. Like Erickson’s examples, all those cited by Connaway concern Native Americans, all are ethnographic or historical rather than archaeological, and most concern South America. As in the examples cited by Erickson, the weirs in studies cited by Connaway all concerned stream, lakeshore, or tidal environments. They were constructed of stone or plant materials, not earth, and none was in a floodplain environment.
The only examples we have been able to uncover that resemble the Baures fishery, in terms of material structures, are found in similar seasonal floodplain environments in Africa and were made by human societies with histories very different from the pre-European occupants of Bolivian savannas. These examples are from two regions in Zambia, the Barotse floodplain in northwestern Zambia (34, 35) [both references were briefly cited by Connaway (2)] and the Bangweulu basin in the northeastern part of the country (5, 13) (neither reference was cited by Connaway). Our field study was conducted in the latter site.
Mapping of Vestiges of Fish Weirs in the Baures Region.
Erickson (1) used unspecified aerial photographs to prepare his map presenting the Northern and Southern blocks. We first assessed our ability to detect the landscape features he reported with the images available to us. We located on the World Imagery layer of ArcGis the area presented in Erickson’s figure 4 (reproduced herein as Fig. S1A), on which he mapped all fish weirs and causeways in a small part of the hydraulic complex he discovered. He recorded 48.43 linear km of weirs in an area he stated as comprising 16.78 km2. Then, without consulting Erickson’s map, we used imagery of the same area from World Imagery (Fig. S1B) to map these features independently based on our photo-interpretation and on Erickson’s descriptions (straight-line causeways, zigzag fish weirs) of the images. The map based on our independent photo-interpretation (Fig. S1C) shows that we detected all the features mapped by Erickson. Our mapping yielded an estimate of 44.36 linear km (compared with 48.43 km mapped by Erickson).
There are discrepancies between the figures Erickson (1) gave for the surface areas of the two blocks and the zone he exhaustively surveyed, on the one hand, and our own calculation of these areas using World Imagery. These discrepancies lead to some uncertainty about Erickson’s estimates of the density of weirs. Erickson (1) stated that areas of the Northern and Southern Blocks were 447 km2 and 77 km2, respectively, a total of 524 km2. However, our calculations based on polygons identical to those presented in his figure 2 gave substantially larger areas (575 km2 and 100 km2, respectively, for a total area of 675 km2) (Fig. S2). Erickson’s figure for the exhaustively surveyed zone (16.78 km2) is considerably smaller than our calculation of the area of the identical zone (21.54 km2). This latter discrepancy appears to be reflected in (but not completely explained by) an apparent error in the scale bar presented by Erickson (compare the scale bars in Fig. S1 A and C).
Fishways in the Pre-European System.
Erickson (1) presented drawings of V-shaped fishways based on aerial photographs but published no photographs in which these structures are discernible. In images available under the ArcGis layer World Imagery (e.g., Fig. 1D), V-shaped structures can be seen with a great level of detail. They are indeed very similar in form to (but often substantially larger than) the V-shaped fishways of the Bangweulu system. It is impossible to judge whether there also existed simple-gap fishways such as those we observed in Bangweulu (Fig. 3). In satellite images of the Baures region, we did observe some apparent simple gaps in the vestiges of weirs, but these gaps might just reflect the natural deterioration of the vestiges over time.
Likely Differences Between the Present-Day and the Pre-European Fishery.
There is of course no expectation of anything approaching a complete convergence of cultural patterns, even in the most similar environments, between two societies separated so far in space and time. Just as we cannot ignore environment and general principles, neither can we ignore history and uniqueness (36). Weirs are just one technique in the multigear multispecies fishery in the Bangweulu basin (13, 21), and fishing is part of a multiactivity subsistence system including farming, collecting, hunting, and commerce. Other components of the subsistence systems in the two regions may differ in important respects. Large aquatic snails and palms with edible fruits are abundant in both Bangweulu and the Baures floodplains. That present-day Bangweulu fishermen do not harvest these potential resources says nothing about Erickson’s (1) speculation that Pomacea snails and fruits of Mauritia flexuosa were important resources in the Baures region. In Bangweulu, people do harvest other organisms attracted to dammed floodplains, including antelopes, waterbirds, terrapins, and rodents, and the pre-European inhabitants of the Baures region likely did so as well.
Similarly, that present-day Bangweulu fishermen do not construct artificial ponds near weirs says nothing about Erickson’s supposition that Baures fishermen did so. In Bangweulu, megafauna wallows (11, 12) may play similar roles as dry-season refugia for fish. Finally, although weirs in the Bangweulu basin are not used in water management (e.g., to prolong the flooding period in certain areas), Erickson’s suggestion that they may have been used in this way in the archaeological Baures fishery is worth consideration, despite the lack of independent evidence for such management.
Environments are never identical. Although seasonal floodplains are considered in general to be relatively nutrient-rich habitats for the fish that spawn in them, there may have been differences in fish productivity between the prehistoric and the present-day fisheries compared here. A major ecological difference between African and South American savannas today is the greater importance of large mammals in the former. In the Bangweulu basin, the abundant feces deposited in floodplains by antelopes (and formerly other megaherbivores) that use them as dry-season grazing lawns are suspected to be important in the food webs that fuel fish productivity (11, 12). Before late Pleistocene–early Holocene extinctions, the megafauna of South American savannas may have played similar roles in nutrient biogeochemistry (37), but these animals had long since disappeared when the Baures fishery was active. It would be interesting to compare the production ecology of fish populations in the two regions today, to assess whether the productivity of the prehistoric South American fishery may have been lower than that of the present-day African fishery.
Additional Information on the Functioning of the Present-Day Weir-Based Fishery in the Bangweulu Basin.
In this seasonally flooded environment, earthen fish weirs (“amaamba” in the Bemba language), the iconic fishing gear of the Bangweulu floodplains, are highly effective in harvesting large quantities of fish. For some groups of fishermen, weir fishing is the only technique they use. Huchzermeyer (11, 12) provides information on other fishing techniques used in the Bangweulu basin.
Construction and maintenance of weirs.
New weirs are constructed in the dry season (Fig. S6E). Weirs are levees made of turfs cut from the grassy topsoil, gathered from 1–2 m on either side of the weir and packed up to a height of 0.7–1.5 m depending on the water depth. The base of the weir is usually about 1 m wide. The grass roots and rapidly regrowing vegetation hold the structure together, enabling it to resist water flow. Soil below a depth of 20 cm is very clay-rich, making construction more difficult, and is not used. A large hoe with a blade about 250 mm wide and 300 mm long, attached to a heavy wooden handle, is used for building the weirs. A turf can be cut in a single stroke and is tossed up onto the weir with the lifting stroke. This maneuver takes considerable skill but, once mastered, enables surprisingly rapid construction. Weirs can be built at a rate of about 20–40 m per day per person (i.e., 1 linear km in 25–50 person-days), depending on the height of the weir. For purposes of comparison, Erickson (1), basing his estimate on figures from the construction of experimental raised fields, calculated that the 1,515 linear km of weirs he estimated to exist in the Baures region could have been constructed in 300,000 person-days (equivalent to 1 linear km in 198 person-days). Thus they could have been constructed by small groups of kin or communities working over a quite short period (e.g., 1,000 people over 10 y). Our estimate of the labor cost of weir construction for the Bangweulu fishery is thus about four times lower than Erickson’s (1) estimate for the Baures fishery. Weirs in Bangweulu are constructed using metal hoes. If Erickson’s estimate is based on use of the wooden tools that would have been used by pre-European Amazonians, the difference in tools available could easily account for the difference in labor cost.
Each weir bears one or more boating gaps that allow passage of dugout canoes, the main source of transport around the floodplains during the peak of the floods. Reed mats or other barriers are devised that allow passage of canoes while impeding passage of fish.
Repair and maintenance are year-round activities.
Once the weir has been used for a season, it reduces considerably as the turfs compact. Maintenance in subsequent years (Fig. S6 F and H) involves patching areas that have subsided below the water level. Weirs are constructed and maintained as soon as water levels have receded enough for the surrounding ground to become firm but not hard, usually from July to October. Maintenance is also an important activity at peak water levels (March/early April), when weirs can be eroded by water flow, trampled by large animals, or damaged by catfish (Clarias) that burrow into them. When maintaining weirs in deeper water, a wooden “spade,” shaped roughly like a boat oar or paddle, but thick and heavy, is used to cut turfs in the water, where a hoe would splash too much. Reed stakes are sometimes used to add strength to weak parts of a weir. These reeds may take root, eventually creating impenetrable reedbeds that necessitate the digging of a new weir nearby.
The seasonal cycle driving the weir fishery.
During the high-water period from January to April, juvenile fish encounter ideal conditions for growth: warm, shallow, well-oxygenated, and relatively nutrient-rich water, with low competition and risk of predation. Fish biomass thus increases rapidly. A particular set of weather conditions called “apumbwe,” a steady, strong wind from the southeast, low cloud cover, greater fluctuation in temperatures between day and night, and an accelerated drop in water levels, signaling the onset of the dry season, triggers the mass out-migration of fish. As water levels fall in the cold, dry season (May–August), conditions change, and fish are crowded, resources are limiting, and predation by waterbirds and by larger fish is high. By the hot dry season (September–December) fish retreat into small refuge areas that retain their water or into the main swamp basin. These fish mature and are ready to renew the cycle with the onset of the rains in January.
Yield of the weir fishery.
Huchzermeyer’s (11) rough estimate of the annual yield of the weir fishery for local fishermen—800,000 kg fresh mass of fish, or 35.5 kg⋅ha−1⋅y−1 (see main text)—was based on data on the length of the fishing season, the number of bags of dried fish produced per fishing group, the relationship between the mass of dried fish and their fresh mass, the number of fishing groups in the area, and the surface area of floodplain used by these groups (22,500 ha). The mix of traditional (reed basket traps) and modern gear (mosquito-net traps) used today probably allows more effective harvesting than achieved using only traditional methods.
Capture gear used in the weirs.
The principal types of capture gear are shown in Fig. S6 C and D. Early in the season, fine-mesh nets made from mosquito netting are used where current is strong, because they offer little resistance to water flow. The use of mosquito nets, a recently adopted (<10 y ago) and now illegal innovation, is declining.
Social regulation of the fishery.
A particular fisherman can own gaps anywhere along a weir, and rarely are they all contiguous. When a new weir is constructed, ownership of each gap is indicated by branches of different types of bushes, palm fronds, or reeds, so that each owner can recognize his gaps by the marking code he has chosen. Once a weir has been used for a season, there is little risk of uncertainty about where individuals may place their traps. A person wishing to fish in a certain area must approach the chipupila controlling the area and pay a fee. The chipupila decides where the applicant may fish, and the size of the area (and hence density of fishers within it) is determined by its perceived productivity and yield.
Recent changes in the weir fishery.
According to fishermen, harvesting levels and yields appear to have been sustainable, at least until recently. Kolding et al. (13) found no evidence for overfishing. However, the introduction of new, more effective types of fishing gear and the growing importance of commercial fishing, as roads and transport systems are improved, are causes for concern. Adult fish are threatened by increasing pressure through other techniques, such as the spearing of spawning fish (despite a government ban on fishing during the main spawning season from December through February) and the selective capture of large fish using large-mesh gillnets and seine nets. As explained below, the sustainability of weir fishing depends on high adult survival.
There is no clear evidence that yield is decreasing; however, the number of fishermen is currently increasing, especially with the increasing importance of commercial fishing in the last two to three decades, and the catch per fishing group is decreasing. According to fishermen, the number of weirs is increasing because of the increasing commercial fishery and population growth. This increase in the number of weirs is borne out by analysis of satellite imagery. In the 129 km2 of the Lukulu delta where we recorded 328 km of weirs in May 2012 (Fig. S3), a Pléiades image of the same area taken on September 17, 2013 showed that new weirs had been constructed, with a net addition of 19 km of weirs. Linear density thus increased from 2.55 to 2.69 km/km2, a 5.5% increase in 16 mo.
The weir fishery as part of a multispecies, multigear fishery.
Weir fishery is only one of several techniques used by fishermen in Bangweulu. By not focusing pressures on a single resource, the very diversity of fishing techniques and of species caught contributes to the resilience of the Bangweulu fishery (11–13, 21). The catch, preserved by smoke- and sun-drying, supports subsistence and commerce throughout the year. Given the large numbers of fishermen, the resilience of the species caught by weirs to high harvest levels of young age classes is probably crucial to the sustainability of the overall fishery. Interestingly, in the Bangweulu basin, increasing linear density of weirs is correlated with increased harvest intensity, and the density of weirs has increased over time (SI Text, Recent Changes in the Weir Fishery). The fact that the linear density of weirs in the Baures region is similar to that in the Bangweulu basin indicates that harvest intensity may have been roughly comparable in the archaeological fishery and the life-history characteristics of the fish species captured by weirs thus were equally important to sustainability.
Fish Assemblages and Life-History Strategies in the Two Regions.
Iténez-Guaporé River and its tributaries (including the Baures region).
Experimental fishing was conducted during a 2-y period from August 2005 to July 2007 in several rivers of the Iténez-Guaporé watershed (30, 38), among them the San Martin River, which falls within Erickson’s study area. We thus have good information on fish assemblages for the region. As in all sampling methodologies, some biases are unavoidable. Our methodology focused on medium-sized species from lakes and rivers and certainly yielded underestimates of biomass for species living in habitats such as swampy areas (for example, no Synbranchus were captured) and for large migratory catfish. However, the assemblage sampled by this method probably corresponds quite closely to that which would be targeted by floodplain fish weirs and the small ponds associated with them. The standardized methodology used permits assessment not only of occurrence but also of relative abundance and biomass.
The Iténez-Guaporé watershed hosts high fish diversity, probably more than 500 species (39, 40) of the 3,000–3,500 species in the Amazon watershed (41) of which it forms a part. Camacho (38) listed 261 species from 136 genera for the lakes and rivers of the Bolivian part of the Iténez watershed. To compile the data for the Iténez watershed presented in Table S1, we used the information at the generic level, selecting the genera caught in the transparent waters, similar to those of rivers near Erickson’s study area (three rivers, 20 sites, 27 sampling dates) and extracting the subset of the 43 most common genera, which together accounted for 96% of the biomass.
Although life-history strategies in fish communities in the Beni floodplains have not been studied in any detail, some examples of the P strategists of Winemiller and Rose (28) are well documented. For example, Prochilodus nigricans is characterized by high fecundity, rapid growth, and seasonal reproduction and spawns in the vast floodplains along the Rio Mamoré in the high-water season, migrating into the river’s upper basin in the dry season (42). Many genera found in the Orinoco Llanos also occur in the Beni floodplains, and because life-history strategies in fishes show a strong phylogenetic signal (27), similar life-history patterns are expected. We ascribed to fishes of each genus the life-history classification [equilibrium (E), periodic (P), opportunist (O), and intermediates) established by Winemiller and Rose (28).
Characiformes (24 genera) and Siluriformes (14 genera) dominate the 43 genera, accounting for 58% and 18% of the biomass, respectively, followed by Perciformes (three genera), which accounted for 12%. Two other orders, each represented by a single species, together accounted for 7% of biomass. The assemblage is dominated by P strategists (25 genera; 49% of biomass), followed by E strategists (nine genera; 26%) and genera with intermediate E/P strategies (four genera; 17%) (Table S1). P strategists, which optimize their life cycle around seasonal variation, generally correspond to taxa that temporarily use the floodplain and are the most likely targets of capture by weirs during the falling-water period.
Bangweulu wetlands.
Fish communities in the Bangweulu wetlands were systematically sampled by counting and weighing fish caught using six different capture gears from April 2011 to May 2012 (12). For each capture gear, we calculated the proportion contributed by each species to the total biomass of fish caught by that gear. To estimate the contribution of each species to the overall total biomass captured by all gears, we calculated the mean value of the species’ contributions over all six gears. To characterize the life-history strategy of each species in the Winemiller and Rose (28) classification, we consulted genus-level information in the literature (29, 43).
Siluriformes (three genera, six species) and Perciformes (six genera, nine species) comprised more than half of the 17 genera found in this site and accounted for 35% and 30% of the biomass, respectively. The pantropical order Osteoglossiformes, represented by four genera (and four species) of the endemic African family Mormyridae, by far the largest family in this order, accounted for 23% of total biomass. Cypriniformes, represented by nine species of the single genus Barbus, accounted for 12% of biomass. Characiformes (much less diverse in Africa than in the Neotropics) and Cyprinodontiformes were each represented by a single genus and species, but together these accounted for less than 0.1% of total biomass. The assemblage is dominated by P strategists (belonging to three genera; 57% of biomass), followed by species with intermediate strategies (I-E/P: two genera, 27% of biomass; I-O/P: three genera, 11% of biomass). In two important genera in Bangweulu, there is variation among the species’ life-history strategy. These are the cyprinid genus Barbus, with two medium-sized species (one P, one O) and several smaller species intermediate between these two strategies, and the cichlid genus Tilapia, with one I-E/P and two I-O/P species. For each of these genera, species with different strategies are listed separately in Table S1. Opportunistic species are somewhat better represented (four genera, 3.6% of biomass) than in the Iténez-Guaporé watershed, but E strategists are lacking. These differences in the representation of life-history strategies (and in overall fish diversity) probably reflect the circumscription of the study area in Zambia to the dynamic habitats of the floodplains, whereas in the Iténez-Guaporé watershed a much larger area that included the permanent habitats of large rivers was sampled. In Bangweulu, in addition to the six relatively abundant genera listed above, 11 other genera were present, including seven that could not be placed with confidence into life-history categories, but together these 11 genera accounted for less than one-tenth of one percent of total biomass.
Other similarities in the structure and functioning of fish assemblages would likely be uncovered by further ecological research. Tropical floodplain fish assemblages tend to be dominated by small individuals (both small-sized species and juveniles of larger species), but variation in water depth is a factor in diversification, with the smallest species occurring in the shallowest water and some species being restricted to deeper waters (44). The ponds and canals associated with weirs not only allow fish to persist longer into the dry season but likely add to the total pool of species that can be exploited in the floodplain fishery.
Reconciling Narratives About the Historical Ecology of Amazonia.
Anthropology and archaeology are characterized by ever-present tension between nomothetic and particularist goals and research strategies (36) or, in their present avatars, evolutionary and interpretive approaches (45). Evolutionary approaches aim to find general “laws” through comparative study, whereas interpretive approaches—which emerged as a reaction against perceived reductionism (45)—emphasize the role and perception of acting individuals and are more particularist. The stance that Erickson and colleagues (e.g., ref. 46) have taken on the historical ecology of Amazonia is an illustrative example. Reacting to the cultural ecology school founded by Julian Steward and its position that external environmental imperatives drive human cultural evolution (47), these authors reject what they perceive as environmental determinism and adopt the position that intelligent human agents can overcome environmental constraints, transforming them into “negligible analytic phenomena” (46, p. 4) trumped by human agency. “Agency” indicates the capacity for intentional, conscious choice; the world presents people with options, and the choices they make alter the world (48). This statement aptly describes cultural niche construction. Ascribing importance to human agency is thus completely compatible with a view of adaptation to environment based on cultural niche construction. Instead of ascribing paramount importance to the external environment or to human agency, this framework emphasizes the interaction between the two; humans adapt in part by modifying environments to suit their needs (36). Far from becoming negligible phenomena, environmental constraints shape the contours, the limits, and the diversity of human cultural adaptation. Rather than overcoming environmental constraints, intelligent human choice provides a mechanism that acts synergistically with these constraints to produce more-or-less predictable matches between environments and the cultural patterns adapted to them, such as the convergence in cultural niche construction that we document here.
Acknowledgments
We thank the weir-fishing community of Chiundaponde Chiefdom for their help and cooperation in C.F.H.’s fieldwork in Zambia. D.B.M., M.D., and M.K. gratefully acknowledge the guidance and the help with fieldwork provided by the late Sesele Sokotela (Zambia Agricultural Research Institute), who died before this study could be completed. Heiko Prümers (German Archaeological Institute) alerted us to the images available under World Imagery, including that reproduced in Fig. 1D. Finn Kjellberg and Rumsaïs Blatrix (both of the Centre d’Ecologie Fonctionelle et Evolutive), Eric Garine (University of Paris West), Renée Borges (Centre for Ecological Sciences, Indian Institute of Science), and Oliver Coomes (McGill University) critically read the manuscript. This research was funded by grants from the Institut Universitaire de France, l’Institut Ecologie et Environnement (INEE)/CNRS (Projets Exploratoires Pluridisciplinaires program), and the Earth, Ocean, Continental Surfaces and Atmosphere committee of the CNES (to D.B.M.). Fieldwork by D.B.M., M.D., and M.K. in Zambia was funded by grants from the INEE and from the Groupement de Recherche (GDR) Mosaïque (GDR 3353), both of the CNRS (to D.B.M.). Fieldwork by M.P., P.B., and A.O. in Bolivia was conducted through a collaborative project of San Simón University (Cochabamba, Bolivia), the Autonomous University of the Beni (Trinidad, Bolivia), and the Institut de Recherche pour le Développement (IRD), Département Soutien et Formation and was supported by the IRD program Jeunes Équipes Associées à l’IRD.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613169114/-/DCSupplemental.
References
- 1.Erickson CL. An artificial landscape-scale fishery in the Bolivian Amazon. Nature. 2000;408(6809):190–193. doi: 10.1038/35041555. [DOI] [PubMed] [Google Scholar]
- 2.Connaway JM. 2007. Fishweirs: A World Perspective with Emphasis on the Fishweirs of Mississippi. (Mississippi Department of Archives and History, Jackson, MS) Archaeological report no. 33.
- 3.Béarez P, Prümers H. Prehispanic fishing at Loma Mendoza, Llanos de Moxos, Bolivia. In: Hüster Plogmann H, editor. The Role of Fish in Ancient Time. Proceedings of the 13th Meeting of the ICAZ Fish Remains Working Group. Marie Leidorf GmbH; Basel: 2007. pp. 3–10. [Google Scholar]
- 4.Binford LR. Archeological perspectives. In: Binford SR, Binford LR, editors. New Perspectives in Archeology. Aldine; Chicago: 1968. pp. 5–32. [Google Scholar]
- 5.Brelsford WV. Fishermen of the Bangweulu Swamps: A Study of the Fishing Activities of the Unga Tribe. The Rhodes-Livingstone Institute; Livingstone, Northern Rhodesia: 1946. [Google Scholar]
- 6.Odling-Smee J, Erwin DH, Palkovacs EP, Feldman MW, Laland KN. Niche construction theory: A practical guide for ecologists. Q Rev Biol. 2013;88(1):4–28. doi: 10.1086/669266. [DOI] [PubMed] [Google Scholar]
- 7.Scott-Phillips TC, Laland KN, Shuker DM, Dickins TE, West SA. The niche construction perspective: A critical appraisal. Evolution. 2014;68(5):1231–1243. doi: 10.1111/evo.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallach E. Niche construction theory as an explanatory framework for human phenomena. Synthese. 2015;193(8):2595–2618. [Google Scholar]
- 9.Laland KN, O’Brien MJ. Niche construction theory and archaeology. J Archaeol Method Theory. 2010;17(4):303–322. [Google Scholar]
- 10.Smith BD. General patterns of niche construction and the management of ‘wild’ plant and animal resources by small-scale pre-industrial societies. Philos Trans R Soc Lond B Biol Sci. 2011;366(1566):836–848. doi: 10.1098/rstb.2010.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huchzermeyer CF. 2012. Fish and Fisheries of the Bangweulu Wetlands and Lavushi Manda National Park (South African Institute for Aquatic Biodiversity, Grahamstown, South Africa). Available at https://bangweulufish.files.wordpress.com/2012/08/bangweulu-wetlands-fishes-and-fisheries-with-additions-and-changes-aug-2012.pdf. Accessed November 26, 2016.
- 12.Huchzermeyer CF. 2013. Fish and Fisheries of Bangweulu Wetlands, Zambia. MSc. thesis (Rhodes University, Grahamstown, South Africa)
- 13.Kolding J, Ticheler H, Chanda B. The Bangweulu swamps-A balanced small-scale multispecies fishery. FAO Fish Tech Pap. 2003;426(2):34–66. [Google Scholar]
- 14.Goulding M. The Fishes and the Forest: Explorations in Amazonian Natural History. Univ of California Press; Berkeley, CA: 1980. [Google Scholar]
- 15.Lowe-McConnell RH. Ecological Studies in Tropical Fish Communities. Cambridge Univ Press; Cambridge, UK: 1987. [Google Scholar]
- 16.Torrente-Vilara G, Doria CRC. Categorização e duração dos períodos hidrológicos do rio Guaporé. In: Van Damme PA, Maldonado M, Pouilly M, Doria CRC, editors. Aguas del Iténez-Guaporé. Recursos Hidrobiológicos de un Patrimonio Binacional (Bolivia y Brasil) Editorial INIA; Cochabamba, Bolivia: 2012. pp. 27–38. [Google Scholar]
- 17.Garson AG. Comment upon the economic potential of fish utilization in riverine environments and potential archaeological biases. Am Antiq. 1980;45(3):562–567. [Google Scholar]
- 18.Hanagarth W. Acerca de la Geoecología de las Sabanas del Beni en el Noreste de Bolivia. Instituto de Ecología; La Paz, Peru: 1993. [Google Scholar]
- 19.Erickson C, Walker J. Precolumbian causeways and canals as landesque capital. In: Snead JE, Erickson CL, Darling JA, editors. Landscapes of Movement. Trails, Paths, and Roads in Anthropological Perspective. University of Pennsylvania Museum of Archaeology and Anthropology; Philadelphia: 2009. pp. 232–252. [Google Scholar]
- 20.Government of the Republic of Zambia . Exploratory Soil Map of Zambia (1:1.000, 000). Soil Survey Research Branch, Ministry of Agriculture and Cooperatives; Lusaka, Zambia: 1991. [Google Scholar]
- 21.Kolding J, van Zwieten PA. Sustainable fishing of inland waters. J Limnol. 2014;73(1):132–148. [Google Scholar]
- 22.Kolding J, van Zwieten PA. The tragedy of our legacy: How do global management discourses affect small-scale fisheries in the south? Forum Dev Stud. 2011;38(3):267–297. [Google Scholar]
- 23.Caswell H. Matrix Population Models. Construction, Analysis and Interpretation. 2nd Ed Sinauer; Sunderland, MA: 2001. [Google Scholar]
- 24.Mac Arthur RH. On the relation between reproductive value and optimal predation. Proc Natl Acad Sci USA. 1960;46(1):143–145. doi: 10.1073/pnas.46.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law R. Harvest optimization in populations with age distributions. Am Nat. 1979;114(2):250–259. [Google Scholar]
- 26.Welcomme R. 1985. River Fisheries. (Food and Agricultural Organization of the United Nations, Rome) FAO Fisheries technical paper 262.
- 27.Winemiller KO. Patterns of variation in life history among South American fishes in seasonal environments. Oecologia. 1989;81(2):225–241. doi: 10.1007/BF00379810. [DOI] [PubMed] [Google Scholar]
- 28.Winemiller KO, Rose KA. Patterns of life-history diversification in North American fishes: Implications for population regulation. Can J Fish Aquat Sci. 1992;49(10):2196–2218. [Google Scholar]
- 29.Winemiller KO, Agostinho AA, Caramaschi ÉP. Fish ecology in tropical streams. In: Dudgeon D, editor. Tropical Stream Ecology. Academic; Amsterdam: 2008. pp. 107–146. [Google Scholar]
- 30.Pouilly M, Camacho J. Composición de la comunidad de peces en la cuenca del río Iténez (Bolivia) In: Van Damme PA, Maldonado M, Pouilly M, Doria CRC, editors. Aguas del Iténez o Guaporé. Recursos Hidrobiológicos de un Patrimonio Binacional (Bolivia y Brasil) Editorial INIA; Cochabamba, Bolivia: 2012. pp. 157–172. [Google Scholar]
- 31.Boixadera J, Poch RM, Garcí MT, Vizcayno C. Hydromorphic and clay-related processes in soils from the Llanos de Moxos (northern Bolivia) Catena. 2003;54(3):403–424. [Google Scholar]
- 32.Gabriel O, Lange K, Dahm E, Wendt T, editors. Von Brandt’s Fish Catching Methods of the World. 4th Ed Blackwell; Oxford: 2005. [Google Scholar]
- 33.Jeffery B. Reviving community spirit: Furthering the sustainable, historical and economic role of fish weirs and traps. Journal of Maritime Archaeology. 2013;8(1):29–57. [Google Scholar]
- 34.FAO/UN 1970. Report to the Government of Zambia on the Fishery Development of the Central Barotse Floodplain (Food and Agriculture Organization of the United Nations, Rome) FAO/UN Development Program technical assistance report 2816 based on the work of G. F. Weiss.
- 35.Bell-Cross G. Weir fishing on the central Barotse flood plain in Zambia. Fisheries Research Bulletin. 1971;5:331–340. [Google Scholar]
- 36.Spencer CS. Homology, analogy, and comparative research in archaeology. Cross-Cultural Res. 1992;26(1-4):163–168. [Google Scholar]
- 37.Doughty CE, Faurby S, Svenning JC. The impact of the megafauna extinctions on savanna woody cover in South America. Ecography. 2016;39(2):213–222. [Google Scholar]
- 38.Camacho J. 2008. Estructura de las Comunidades de Peces en Diferentes Tipos de Agua y Habitat en la Cuenca Iténez (Bolivia). MSc thesis (Universidad Mayor de San Simón, Cochabamba, Bolivia), 60 p.
- 39.Jégu M, et al. Catálogo de los peces de la cuenca Iténez-Guaporé (Bolivia y Brasil) In: Van Damme PA, Maldonado M, Pouilly M, Doria CRC, editors. Aguas del Iténez o Guaporé. Recursos Hidrobiológicos de un Patrimonio Binacional (Bolivia y Brasil) Editorial INIA; Cochabamba, Bolivia: 2012. pp. 111–156. [Google Scholar]
- 40.Carvajal-Vallejos FM, et al. Fish-AMAZBOL: A database on freshwater fishes of the Bolivian Amazon. Hydrobiologia. 2014;732(1):19–27. [Google Scholar]
- 41.Lévêque C, Oberdorff T, Paugy D, Stiassny MLJ, Tedesco PA. Global diversity of fish (Pisces) in freshwater. Hydrobiologia. 2008;595(1):545–567. [Google Scholar]
- 42.Loubens G, Panfili J. Biologie de Prochilodus nigricans (Teleostei: Prochilodontidae) dans le bassin du Mamoré (Amazonie bolivienne) Ichthyol Explor Freshwat. 1995;6(1):17–32. [Google Scholar]
- 43.Tedesco P, Hugueny B. Life history strategies affect climate based spatial synchrony in population dynamics of West African freshwater fishes. Oikos. 2006;115(1):117–127. [Google Scholar]
- 44.Fernandes IM, Machado FA, Penha J. Spatial pattern of a fish assemblage in a seasonal tropical wetland: Effects of habitat, herbaceous plant biomass, water depth, and distance from species sources. Neotrop Ichthyol. 2010;8(2):289–298. [Google Scholar]
- 45.Cochrane E, Gardner A, editors. Evolutionary and Interpretive Archaeologies: A Dialogue. Left Coast Press; Walnut Creek, CA: 2011. [Google Scholar]
- 46.Balée W, Erickson CL. Time, complexity and historical ecology. In: Balée W, Erickson CL, editors. Time and Complexity in Historical Ecology. Studies in the Neotropical Lowlands. Columbia Univ Press; New York: 2006. pp. 1–17. [Google Scholar]
- 47.Steward JH. Theory of Culture Change. The Methodology of Multilinear Evolution. Univ of Illinois Press; Urbana, IL: 1955. [Google Scholar]
- 48.Smith EA. Agency and adaptation: New directions in evolutionary anthropology. Annu Rev Anthropol. 2013;42:103–120. [Google Scholar]
- 49.USGS 2014. Shuttle radar topography mission, 1 arc second scenes SRTM1S14W063V3, SRTM1S14W064V3, SRTM1S155W063V3, SRTM1S5W064V3 February 2000 (US Geological Survey Earth Resources Observations and Science Center, Sioux Falls, SD)
- 50.Cochonneau G, Calmant S. 2011 VALS, Virtual ALtimetry Station, Version 1.0.3, 05/2011. Available at www.ore-hybam.org/index.php/eng/Software. Accessed November 26, 2016.
- 51.Barletta M, et al. Fish and aquatic habitat conservation in South America: A continental overview with emphasis on neotropical systems. J Fish Biol. 2010;76(9):2118–2176. doi: 10.1111/j.1095-8649.2010.02684.x. [DOI] [PubMed] [Google Scholar]
- 52.Lima DP, Junior, Hoeinghaus DJ, Bini LM, Agostinho AA. Are non-native species larger in their invaded range? A test with tropical floodplain fish assemblages following inundation of a biogeographic barrier. Biol Invasions. 2015;17(11):3263–3274. [Google Scholar]
- 53.García‐Vásquez A, Vargas G, Sánchez H, Tello S, Duponchelle F. Periodic life history strategy of Psectrogaster rutiloides, Kner 1858, in the Iquitos region, Peruvian Amazon. J Appl Ichthyology. 2015;31(S4):31–39. [Google Scholar]
- 54.De Merona B, Vigouroux R. The role of ecological strategies in the colonization success of pelagic fish in a large tropical reservoir (Petit-Saut Reservoir, French Guiana) Aquat Living Resour. 2012;25(1):41–54. [Google Scholar]
- 55.Sousa MM, Lopes SIM, da Costa RS, Novaes JL. Population structure and reproductive period of two introduced fish species in a Brazilian semiarid region reservoir. Rev Biol Trop. 2015;63(3):727–739. doi: 10.15517/rbt.v63i3.15669. [DOI] [PubMed] [Google Scholar]
- 56.Machado-Allison A. Larval ecology of fish of the Orinoco Basin. In: Hamlett WC, editor. Reproductive Biology of South American Vertebrates. Springer; New York: 1992. pp. 45–59. [Google Scholar]