Significance
The sympathetic nervous system influences various immune cell functions, in particular via β2-adrenergic receptor (β2AR) signaling. Although immune cell recruitment is critical for cardiac repair following ischemia, the impact of β2AR on this process is unclear. We describe how immune cell-specific β2AR depletion ablates chemokine receptor 2 (CCR2) expression and leukocyte recruitment to the heart postischemia. Reciprocally, β2AR activation increases CCR2 expression and responsiveness in a β-arrestin–dependent manner, and expression of a β-arrestin–biased β2AR in β2AR-depleted immune cells restores CCR2 levels and leukocyte recruitment to the postischemic heart. These results highlight the potential utility of next-generation β-arrestin–biased β2AR ligands to selectively modulate leukocyte responsiveness, and suggest that β-blockers, used commonly in peri/postischemic patients, may impact leukocyte-mediated repair mechanisms.
Keywords: β2-adrenergic receptor, leukocyte, C-chemokine receptor 2, cardiac injury, β-arrestin
Abstract
Following cardiac injury, early immune cell responses are essential for initiating cardiac remodeling and tissue repair. We previously demonstrated the importance of β2-adrenergic receptors (β2ARs) in the regulation of immune cell localization following acute cardiac injury, with deficient leukocyte infiltration into the damaged heart. The purpose of this study was to investigate the mechanism by which immune cell-expressed β2ARs regulate leukocyte recruitment to the heart following acute cardiac injury. Chemokine receptor 2 (CCR2) expression and responsiveness to C-C motif chemokine ligand 2 (CCL2)-mediated migration were abolished in β2AR knockout (KO) bone marrow (BM), both of which were rescued by β2AR reexpression. Chimeric mice lacking immune cell-specific CCR2 expression, as well as wild-type mice administered a CCR2 antagonist, recapitulated the loss of monocyte/macrophage and neutrophil recruitment to the heart following myocardial infarction (MI) observed in mice with immune cell-specific β2AR deletion. Converse to β2AR ablation, β2AR stimulation increased CCR2 expression and migratory responsiveness to CCL2 in BM. Mechanistically, G protein-dependent β2AR signaling was dispensable for these effects, whereas β-arrestin2–biased β2AR signaling was required for the regulation of CCR2 expression. Additionally, activator protein 1 (AP-1) was shown to be essential in mediating CCR2 expression in response to β2AR stimulation in both murine BM and human monocytes. Finally, reconstitution of β2ARKO BM with rescued expression of a β-arrestin–biased β2AR in vivo restored BM CCR2 expression as well as cardiac leukocyte infiltration following MI. These results demonstrate the critical role of β-arrestin2/AP-1–dependent β2AR signaling in the regulation of CCR2 expression and recruitment of leukocytes to the heart following injury.
Healing following ischemic cardiac injury is highly regulated by immune responses, with impairments or exacerbations in inflammation leading to alterations in infarct expansion, remodeling, and ultimately cardiac function (1). Cells of the innate immune system including monocytes/macrophages, mast cells, and neutrophils play critical roles in infarct healing through tissue phagocytosis and activation of reparative responses. Recruitment and trafficking of these leukocytes to the heart following acute injury occur through the action of chemokines on their receptors to promote their migration to the site of injury (2), and have been the focus of much research in recent years (1, 3).
Sympathetic activity is important for regulating immune responses, primarily through the β2-adrenergic receptor (β2AR) subtype (4–6). Recently, we showed that immune cell-expressed β2AR is required for leukocyte recruitment to the heart following acute myocardial infarction (MI), without which the heart cannot mount a repair response, ultimately undergoing rupture (7). Because chemokine receptors play a critical role in migration and infiltration of leukocyte populations, we hypothesized that immune cell-expressed chemokine receptor activity and/or expression may be altered in the absence of β2AR, thereby impairing leukocyte migration to the injured heart.
The impact of immune cell-specific β2AR expression on chemokine receptor expression and leukocyte infiltration following MI was investigated through the use of chimeric mice, wherein bone marrow transplant (BMT) recipient mice received bone marrow from β2ARKO donor mice. Through the use of these chimeric mice, we demonstrate that β2AR is critical in regulating chemokine receptor 2 (CCR2) expression, and responsiveness to its ligand C-C motif chemokine ligand 2 (CCL2), via a β-arrestin2 (βARR2)–biased signaling pathway involving activator protein 1 (AP-1). These results highlight the importance of β2AR in regulating immune cell expression of CCR2, thereby impacting the ability of leukocytes to respond to acute cardiac injury.
Results
CCR2 Expression and Migratory Responsiveness Are Abolished in β2ARKO BM.
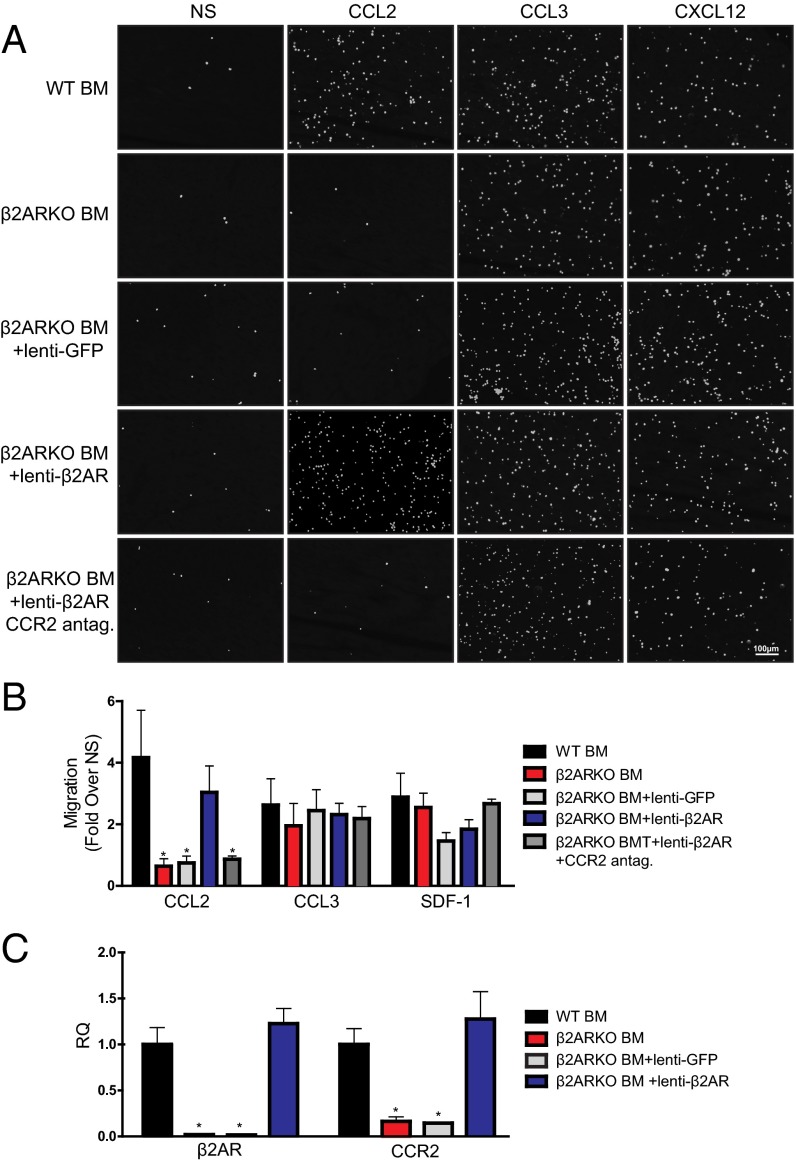
We recently observed decreased leukocyte infiltration into the hearts of chimeric mice lacking immune cell-expressed β2AR following MI (7). Chemokines produced following injury are important for recruitment of immune cells, through their action on chemokine receptors. Thus, to assess whether differences in chemokine receptor expression could contribute to alterations in leukocyte infiltration in β2ARKO BMT mouse hearts post-MI, reverse transcription–quantitative PCR (RT-qPCR) was used to examine those known to play an important role in immune cell migration following acute cardiac injury (Table 1 and Table S1). β2ARKO BM had significantly decreased expression of CCR2 and C-X-C motif chemokine receptor 4 (CXCR4) compared with WT BM. To test the impact of these altered chemokine receptor levels, we performed in vitro migration assays, wherein β2ARKO BM displayed decreased migration toward CCL2 (MCP-1), the ligand for CCR2, with no difference in migratory responses to CCL3 or C-X-C motif chemokine ligand 12 (CXCL12), a CXCR4 ligand (Fig. 1 A and B). Lentiviral-mediated restoration of β2AR expression in β2ARKO BM restored CCR2 expression to endogenous levels (Fig. 1C) as well as the migratory response to CCL2, without affecting migration to CCL3 or CXCL12 (Fig. 1 A and B). CCR2 antagonism blocked migration toward CCL2 following β2AR rescue but had no effect on CCL3 and CXCL12 responses, confirming that this response was CCR2-dependent.
Table 1.
Effects of β2ARKO on chemokine receptor expression
| Chemokine receptor | WT BMT | β2ARKO BMT |
| CCR1 | 1.00 ± 0.20 | 1.06 ± 0.13 |
| CCR2 | 1.00 ± 0.21 | 0.27 ± 0.07* |
| CCR5 | 1.00 ± 0.16 | 1.32 ± 0.18 |
| CXCR1 | 1.00 ± 0.54 | 1.71 ± 0.56 |
| CXCR2 | 1.00 ± 0.51 | 1.63 ± 0.62 |
| CXCR4 | 1.00 ± 0.06 | 0.32 ± 0.02* |
| CXCR7 | 1.00 ± 0.03 | 0.90 ± 0.04 |
| CXC3CR1 | 1.00 ± 0.12 | 1.09 ± 0.14 |
| CD45 | 1.00 ± 0.22 | 0.90 ± 0.30 |
RT-qPCR analysis of changes in expression of chemokine receptor transcripts in reconstituted WT or β2ARKO BM from transplanted mice. n = 4–8.
P < 0.001 vs. WT BMT, two-tailed unpaired t test.
Table S1.
Primer sequences used for RT-qPCR
| Gene name | Reference sequence* | Sequence, 5′-3′ |
| ADRB2 | NM_007420 | Forward AAG AAT AA GCC CGA GTG GT |
| Reverse GTA GGC CTG GTT CGT GAA GA | ||
| CCR1 | NM_009912 | Forward GCT ATG CAG GGA TCA TCA GAA T |
| Reverse GGT CCA GAG GAG GAA GAA TAG A | ||
| Mouse CCR2 | NM_009915 | Forward TTA CAC CTG TGG CCC TTA TTT |
| Reverse CTG AGT AGC AGA TGA CCA TGA C | ||
| Human CCR2 | NM_001123041 | Forward GGA TTG AAC AAG GAC GCA TTT C |
| Reverse CAC CGC TCT CGT TGG TAT TT | ||
| CCR5 | NM_009917 | Forward GCT CCA AGA GAT GAG GAA AGA G |
| Reverse GAA CAC AGA GAG CAG TCG TTA T | ||
| CD45 | NM_001111316 | Forward ACA TCA TCG CCA GCA TCT ATC |
| Reverse CTT GCC TCC ATC CAC TTC ATT A | ||
| CXCR1 | NM_178241 | Forward GCT GT GAT GCT GGT TAT CT |
| Reverse CAA GAA GGG CAG GGT CAA T | ||
| CXCR2 | NM_009909 | Forward GCC CTG CCC ATC TTA ATT CT |
| Reverse ACC CTC AAA CGG GAT GTA TTG | ||
| CXCR4 | NM_009911 | Forward CTG CCC ACC ATC TAC TTC ATC |
| Reverse CGT CAT GCT CCT TAG CTT CTT | ||
| CXCR7 | NM_001271607 | Forward CCT CTT TGG GAG CAT CTT CTT |
| Reverse CGG CGT ACC ATC TTC TTC TTA T | ||
| CX3CR1 | NM_009987 | Forward GAG AGA TGG CTC AGT GGT TAA G |
| Reverse CAC AGG AAC AGG GAG CTA TTT | ||
| Mouse GAPDH | NM_001289726 | Forward AAC AGC AAC TCC CAC TCT TC |
| Reverse CCT GTT GCT GTA GCC GTA TT | ||
| Human GAPDH | NM_001256799 | Forward GGT GTG AAC CAT GAG AAG TAT GA |
| Reverse GAG TCC TTC CAC GAT ACC AAA G | ||
| TPT1 | NM_009429 | Forward ATC ATC TAC CGG GAC CTC ATC |
| Reverse CCC TCT GTT CTA CTG ACC ATC T |
NCBI Reference Sequence (RefSeq) Database.
Fig. 1.
Effects of β2ARKO on BM migration in response to chemokines. (A) Representative Hoechst staining (white) from a 4-h migration assay of WT BM, β2ARKO BM, β2ARKO BM+lenti-GFP, and β2ARKO BM+lenti-β2AR in response to CCL2 (100 ng/mL), CCL3 (100 ng/mL), or CXCL12 (10 ng/mL). A 1-h pretreatment with a CCR2 antagonist (10 nM) was used to inhibit CCR2-mediated migration. (B) Quantification of migration assay results. Values are expressed as fold over vehicle-stimulated migration. n = 4–8; one-way ANOVA, *P < 0.05 vs. WT BMT. (C) RT-qPCR was used to measure β2AR and CCR2 expression in WT and β2ARKO BM and β2ARKO BM that had been transduced with either a GFP or β2AR lentivirus. n = 4–8; one-way ANOVA, *P < 0.05 vs. WT BMT. Data are expressed as mean ± SEM. NS, nonstimulated; RQ, relative quantitation.
Specific Ablation of CCR2 Reduces Leukocyte Recruitment to the Heart Following MI.
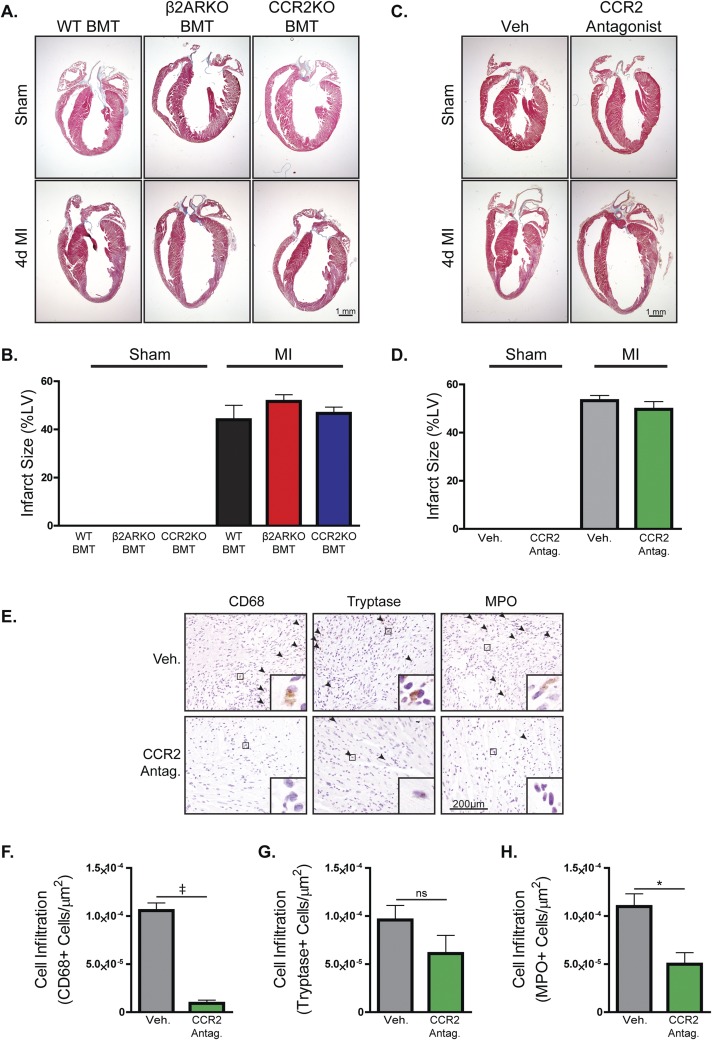
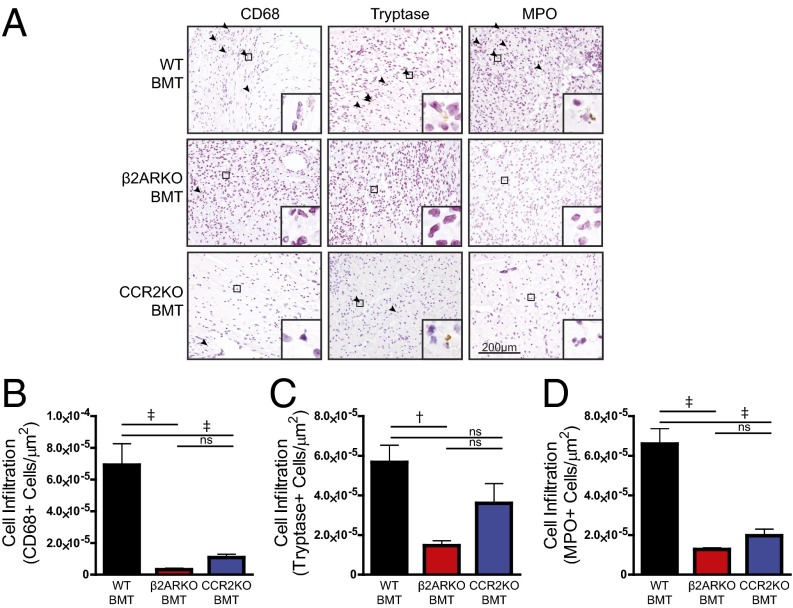
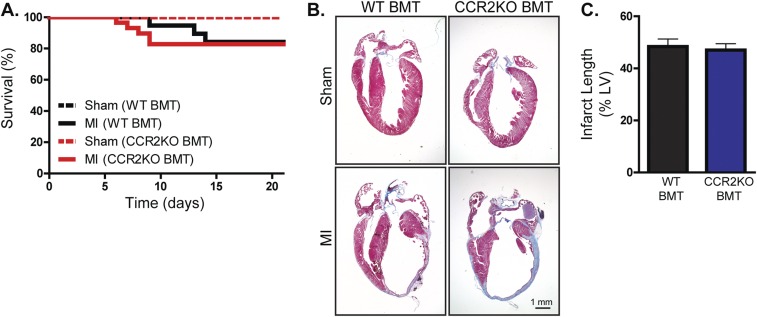
Based on our in vitro assessment of the impact of β2AR deletion on CCR2 expression and BM cell migration, we sought to determine whether CCR2 inhibition in vivo, either pharmacologically or genetically, could recapitulate the impaired leukocyte post-MI infiltration phenotype observed in β2ARKO BMT mice (7). To assess this, WT mice underwent sham or MI surgery followed by daily injections with vehicle or CCR2 antagonist (2 mg⋅kg−1⋅d−1), or underwent irradiation and received WT, β2ARKO, or CCR2KO BM 1 mo before surgery (Fig. S1). Analysis of infarct size 4 d post-MI showed no differences between groups, confirming similar surgical conditions for all groups of animals (Fig. S2). Immunohistochemistry was performed on heart sections 4 d postsurgery to quantify infiltration of immune cell populations in sham hearts and the remote (Fig. S3), border (Fig. 2 and Fig. S2 E–H), and infarct (Fig. S4) zones of MI hearts. Both pharmacological CCR2 antagonism (Fig. S2 E and F) and genetic CCR2 deletion (Fig. 2 A and B) significantly reduced the infiltration of monocytes/macrophages (CD68+ cells) and neutrophils [myeloperoxidase (MPO)+ cells; Fig. 2 A and D and Fig. S2 E and H] to the border and infarct (Fig. S4) zones of the heart following MI. These data recapitulate those attained in β2ARKO BMT mice (Fig. 2A), where decreased infiltration of monocytes/macrophages (Fig. 2B) and neutrophils (Fig. 2D) into the border and infarct (Fig. S4) zones were observed 4 d following MI. Interestingly, unlike in the β2ARKO BMT mice, CCR2 inhibition did not impact mast cell infiltration (tryptase+ cells; Fig. 2 A and C and Fig. S2 E and G). Further, post-MI survival, infarct size, and contractility did not differ between WT BMT and CCR2KO BMT mice (Fig. S5A and Table S2), although CCR2KO BMT mice had slightly less dilation following MI than WT BMT mice. These results suggest that altered CCR2 expression may be a major contributing, but not sole, factor to the decreased leukocyte recruitment response to the injured heart in mice lacking immune cell-expressed β2AR.
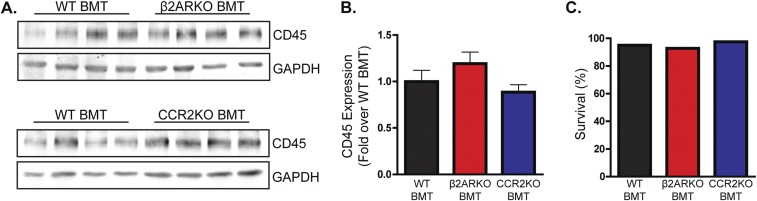
Fig. S1.
BM reconstitution. (A) Representative CD45 immunoblot for BM from WT C57BL/6 mice that underwent WT, β2ARKO, or CCR2KO BMT. GAPDH is shown as a loading control. (B) Quantification of CD45 immunoblot from WT, β2ARKO, and CCR2KO BMT mice. n = 4–8. (C) Survival of WT C57BL/6 mice following WT, β2ARKO, or CCR2KO BMT. n = 20–40. Data are expressed as mean ± SEM.
Fig. S2.
Infarct size and leukocyte infiltration with CCR2 inhibition and CCR2KO BMT. (A) Representative Masson’s trichrome staining for hearts from WT C57BL/6 mice that underwent MI surgery followed by administration (i.p.) of 2 mg⋅kg−1⋅d−1 of a CCR2 antagonist or vehicle (Veh) for 4 d. (B) Quantification of Masson’s trichrome staining for sham and 4-d post-MI hearts from Veh- and CCR2 antagonist-treated mice. n = 4–10. (C) Representative Masson’s trichrome staining for hearts from WT C57BL/6 mice receiving WT, β2ARKO, or CCR2KO BMT that underwent MI surgery. (D) Quantification of Masson’s trichrome staining for sham and 4-d post-MI hearts from WT, β2ARKO, or CCR2KO BMT mice. n = 4–10. (E) Representative CD68, tryptase, and MPO staining for the border zone of hearts from WT C57BL/6 mice that underwent MI surgery followed by administration (i.p.) of 2 mg⋅kg−1⋅d−1 of a CCR2 antagonist or Veh for 4 d. Arrowheads indicate positive staining. Insets show higher magnification at 250×. (F–H) Quantification of CD68 (F), tryptase (G), and MPO (H) staining for the border zone of 4-d post-MI hearts from Veh- and CCR2 antagonist-treated mice. n = 4–8; t test, *P < 0.05 vs. Veh, ‡P < 0.001 vs. Veh. Data are expressed as mean ± SEM.
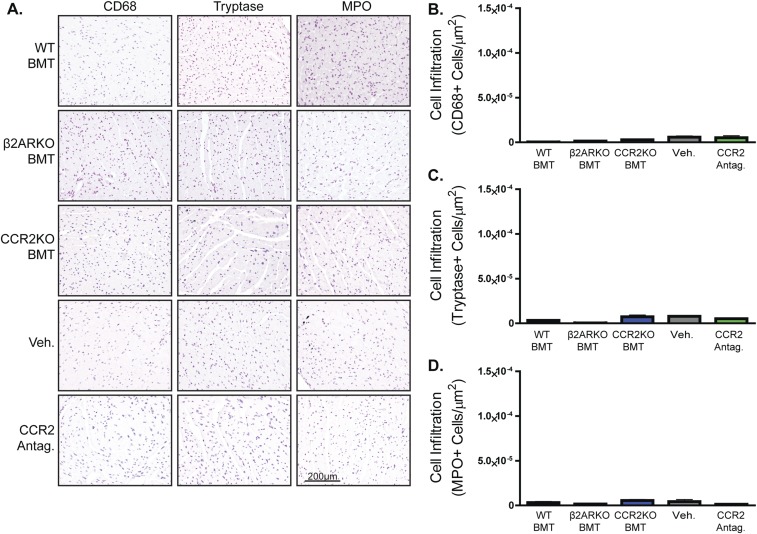
Fig. S3.
CCR2 inhibition and CCR2KO BMT reduce leukocyte infiltration into the heart following MI. (A) Representative CD68, tryptase, and MPO staining for the remote zone of hearts from WT C57BL/6 mice that underwent MI surgery followed by administration (i.p.) of 2 mg⋅kg−1⋅d−1 of a CCR2 antagonist or vehicle for 4 d or WT C57BL/6 mice receiving WT, β2ARKO, or CCR2KO BMT animals that underwent MI surgery. (B–D) Quantification of CD68 (B), tryptase (C), and MPO (D) staining for the remote zone of 4-d post-MI hearts from Veh- and CCR2 antagonist-treated mice or WT, β2ARKO, and CCR2KO BMT animals. n = 4–8. Data are expressed as mean ± SEM.
Fig. 2.
CCR2KO BMT reduces leukocyte infiltration into the heart following MI. (A) Representative CD68, tryptase, and MPO staining for the border zone of hearts from WT C57BL/6 mice receiving WT, β2ARKO, or CCR2KO BMT that underwent MI surgery. Arrowheads indicate positive staining. Insets show higher magnification at 250×. (B–D) Quantification of CD68 (B), tryptase (C), and MPO (D) staining for the border zone of 4-d post-MI hearts from WT, β2ARKO, and CCR2KO BMT mice. n = 4–8; one-way ANOVA, †P < 0.01 vs. WT BMT, ‡P < 0.001 vs. WT BMT; ns, not significant. Data are expressed as mean ± SEM.
Fig. S4.
CCR2 inhibition and CCR2KO BMT reduce leukocyte infiltration into the heart following MI. (A) Representative CD68, tryptase, and MPO staining for the infarct zone of hearts from WT C57BL/6 mice that underwent MI surgery followed by administration (i.p.) of 2 mg⋅kg−1⋅d−1 of a CCR2 antagonist or vehicle for 4 d or WT C57BL/6 mice receiving WT, β2ARKO, or CCR2KO BMT animals that underwent MI surgery. Arrowheads indicate positive staining. Insets show higher magnification at 250×. (B–D) Quantification of CD68 (B), tryptase (C), and MPO (D) staining for the infarct zone of 4-d post-MI hearts from Veh- and CCR2 antagonist-treated mice or WT, β2ARKO, and CCR2KO BMT animals. n = 4–8; t test or one-way ANOVA, *P < 0.05 vs. WT BMT or Veh, ‡P < 0.001 vs. Veh BMT. Data are expressed as mean ± SEM.
Fig. S5.
Heart failure progression in WT and CCR2KO BMT animals. (A) Survival was monitored daily in WT C57BL/6 mice receiving WT or CCR2KO BMT following sham or MI surgery. (B) Representative Masson’s trichrome staining for hearts from WT C57BL/6 mice receiving WT or CCR2KO BMT 21 d following sham or MI surgery. (C) Quantification of Masson’s trichrome staining for sham and 21-d post-MI hearts from WT or CCR2KO BMT mice. n = 16–19. Data are expressed as mean ± SEM.
Table S2.
Echocardiography measurements
| WT BMT sham (n = 7) | WT BMT MI (n = 16) | CCR2KO BMT sham (n = 5) | CCR2KO BMT MI (n = 19) | |||||||||||||
| Measurement | Baseline | 1 wk | 2 wk | 3 wk | Baseline | 1 wk | 2 wk | 3 wk | Baseline | 1 wk | 2 wk | 3 wk | Baseline | 1 wk | 2 wk | 3 wk |
| Fractional shortening, % | 30.6 ± 1.4 | 29.6 ± 1.9 | 29.6 ± 1.5 | 31.0 ± 1.1 | 33.4 ± 1.1 | 8.6 ± 0.4 | 8.6 ± 0.9 | 10.2 ± 2.4 | 32.0 ± 2.1 | 32.0 ± 2.0 | 31.4 ± 1.3 | 32.8 ± 2.1 | 32.3 ± 0.8 | 10.9 ± 0.9 | 10.7 ± 1.6 | 11.7 ± 1.9 |
| Ejection fraction, % | 57.7 ± 2.3 | 56.9 ± 2.4 | 57.8 ± 3.1 | 60.5 ± 2.7 | 62.8 ± 1.6 | 18.9 ± 1.0 | 18.8 ± 1.9 | 21.6 ± 4.6 | 60.1 ± 3.0 | 62.3 ± 3.2 | 59.9 ± 1.8 | 59.4 ± 2.7 | 61.1 ± 1.2 | 23.4 ± 1.9 | 22.8 ± 3.0 | 24.6 ± 3.4 |
| LV volume-diastolic, μL | 63.4 ± 6.5 | 77.0 ± 7.3 | 61.7 ± 3.7 | 45.6 ± 4.6 | 60.2 ± 2.9 | 150.7 ± 11.4 | 151.2 ± 15.7 | 141.2 ± 18.6 | 66.0 ± 2.3 | 59.7 ± 3.3 | 62.4 ± 3.7 | 64.3 ± 4.1 | 50.3 ± 1.7 | 124.2 ± 9.5 | 106.4 ± 9.8† | 107.7 ± 8.2 |
| LVAW-diastolic, mm | 0.69 ± 0.03 | 0.72 ± 0.03 | 0.73 ± 0.05 | 0.07 ± 0.04 | 0.70 ± 0.01 | 0.40 ± 0.02 | 0.36 ± 0.01 | 0.40 ± 0.02 | 0.71 ± 0.03 | 0.72 ± 0.03 | 0.75 ± 0.04 | 0.75 ± 0.04 | 0.68 ± 0.03 | 0.39 ± 0.02 | 0.38 ± 0.03 | 0.45 ± 0.04 |
| LVPW-diastolic, mm | 0.72 ± 0.05 | 0.68 ± 0.03 | 0.68 ± 0.03 | 0.72 ± 0.09 | 0.73 ± 0.03 | 0.36 ± 0.03 | 0.38 ± 0.03 | 0.38 ± 0.04 | 0.73 ± 0.06 | 0.75 ± 0.07 | 0.78 ± 0.06 | 0.74 ± 0.06 | 0.70 ± 0.02 | 0.44 ± 0.03 | 0.41 ± 0.05 | 0.40 ± 0.05 |
| LVID-diastolic, mm | 3.82 ± 0.16 | 4.14 ± 0.16 | 3.79 ± 0.09 | 3.33 ± 0.15 | 3.73 ± 0.08 | 5.48 ± 0.20 | 5.51 ± 0.26 | 5.33 ± 0.32 | 3.81 ± 0.08 | 3.76 ± 0.09 | 3.79 ± 0.10 | 3.85 ± 0.10 | 3.72 ± 0.0 | 5.04 ± 0.18 | 4.69 ± 0.20* | 4.75 ± 0.17 |
| LV volume-systolic, μL | 26.5 ± 3.7 | 35.9 ± 4.4 | 28.8 ± 1.7 | 25.3 ± 2.0 | 20.3 ± 1.8 | 123.5 ± 10.4 | 124.5 ± 14.7 | 114.6 ± 18.3 | 24.8 ± 2.8 | 21.1 ± 2.9 | 25.5 ± 2.1 | 25.0 ± 3.3 | 23.4 ± 1.5 | 96.6 ± 8.1* | 85.2 ± 9.3* | 83.0 ± 7.8 |
| LVAW-systolic, mm | 1.06 ± 0.04 | 1.06 ± 0.02 | 1.10 ± 0.05 | 1.06 ± 0.05 | 1.12 ± 0.02 | 0.55 ± 0.02 | 0.51 ± 0.03 | 0.59 ± 0.05 | 1.14 ± 0.05 | 1.25 ± 0.01 | 1.20 ± 0.09 | 1.31 ± 0.05 | 1.08 ± 0.04 | 0.61 ± 0.03 | 0.56 ± 0.04 | 0.69 ± 0.06 |
| LVPW-systolic, mm | 1.03 ± 0.06 | 1.01 ± 0.07 | 1.04 ± 0.08 | 1.17 ± 0.06 | 1.06 ± 0.03 | 0.44 ± 0.02 | 0.48 ± 0.02 | 0.47 ± 0.05 | 1.09 ± 0.05 | 1.10 ± 0.06 | 1.11 ± 0.07 | 1.02 ± 0.05 | 1.04 ± 0.03 | 0.62 ± 0.04* | 0.60 ± 0.06* | 0.54 ± 0.06 |
| LVID-systolic, mm | 2.65 ± 0.15 | 3.01 ± 0.14 | 2.77 ± 0.07 | 2.13 ± 0.17 | 2.50 ± 0.08 | 5.24 ± 0.19 | 5.05 ± 0.28 | 4.83 ± 0.37 | 2.59 ± 0.12 | 2.50 ± 0.16 | 2.61 ± 0.09 | 2.59 ± 0.13 | 2.52 ± 0.06 | 4.51 ± 0.19*† | 4.22 ± 0.23 | 4.21 ± 0.20 |
LV, left ventricular; LVAW, left ventricular anterior wall; LVID, left ventricular interior diameter; LVPW, left ventricular posterior wall.
P < 0.05 vs. WT BMT MI, two-way ANOVA.
P < 0.01 vs. WT BMT MI, two-way ANOVA.
β2AR Stimulation Alters CCR2 Expression in a β-Arrestin2–Dependent Manner.
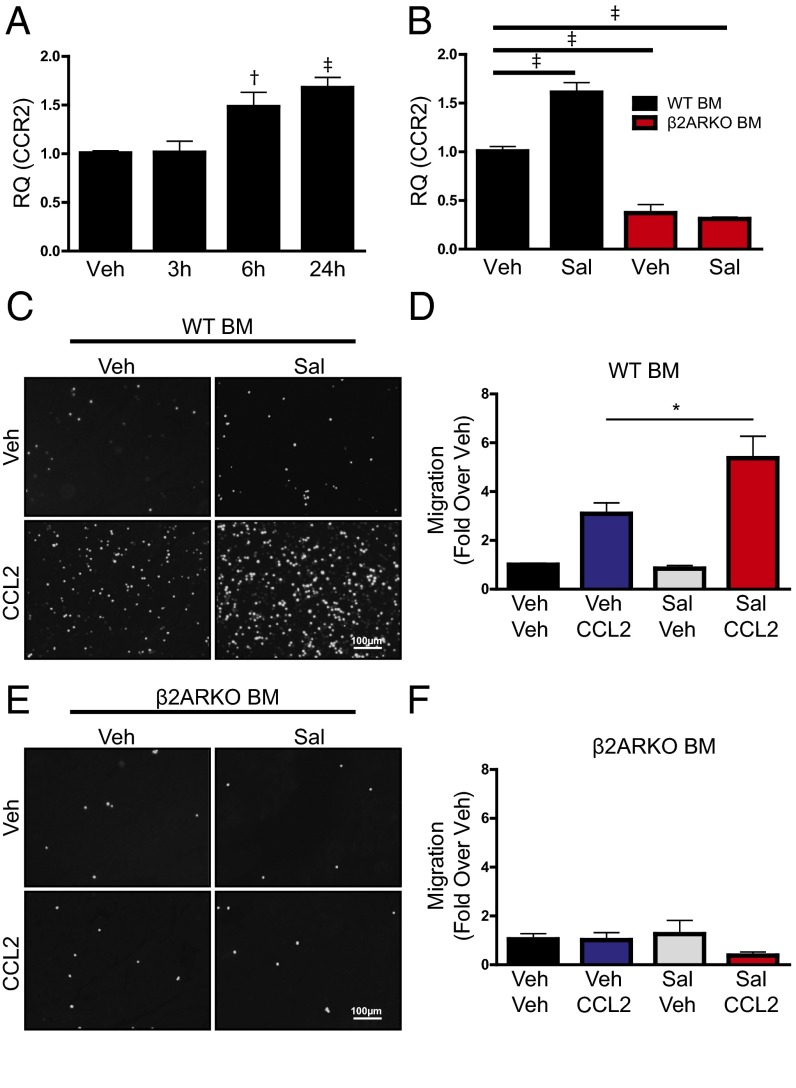
Because β2ARKO BM has decreased expression of CCR2 compared with WT, we next sought to determine whether pharmacological activation of β2AR reciprocally increases CCR2 expression. Thus, BM was isolated from WT C57BL/6 mice and treated with the β2AR-selective agonist salbutamol (Sal). Expression of CCR2 was quantified by RT-qPCR following Sal treatment over time. CCR2 levels were increased 6 and 24 h following β2AR activation, demonstrating the ability to pharmacologically alter CCR2 levels using β2AR ligands (Fig. 3A). Salbutamol-induced CCR2 expression observed in WT BM was not observed in β2ARKO BM (Fig. 3B), confirming the specificity of the response. Migration assays were performed to determine whether β2AR-dependent increases in CCR2 expression result in an enhanced functional response to CCL2-mediated migration. Indeed, CCL2-induced migration of WT BM was augmented with Sal pretreatment (Fig. 3 C and D), whereas β2ARKO BM did not migrate in response to CCL2, and Sal treatment had no effect on this response (Fig. 3 E and F).
Fig. 3.
β2AR stimulation increases CCR2 expression and migration. (A) RT-qPCR was used to measure CCR2 expression in WT BM treated with vehicle (Veh) control or 1 μM Sal over time (3 to 24 h). n = 3–8; one-way ANOVA, †P < 0.01, ‡P < 0.001 vs. Veh. (B) RT-qPCR was used to measure CCR2 expression in WT and β2ARKO BM treated with Sal. n = 6; one-way ANOVA, ‡P < 0.001 vs. Veh. (C) Representative Hoechst staining (white) from a 4-h migration assay of WT BM pretreated with vehicle or Sal and allowed to migrate in response to CCL2 (100 ng/mL). (D) Quantification of WT BM migration assay results. Values are expressed as fold over WT vehicle-stimulated migration. n = 7–8; one-way ANOVA, *P < 0.05. (E) Representative Hoechst staining (white) from a 4-h migration assay of β2ARKO BM pretreated with vehicle or Sal and allowed to migrate in response to CCL2 (100 ng/mL). (F) Quantification of β2ARKO BM migration assay results. Values are expressed as fold over WT vehicle-stimulated migration. n = 7–8. Data are expressed as mean ± SEM.
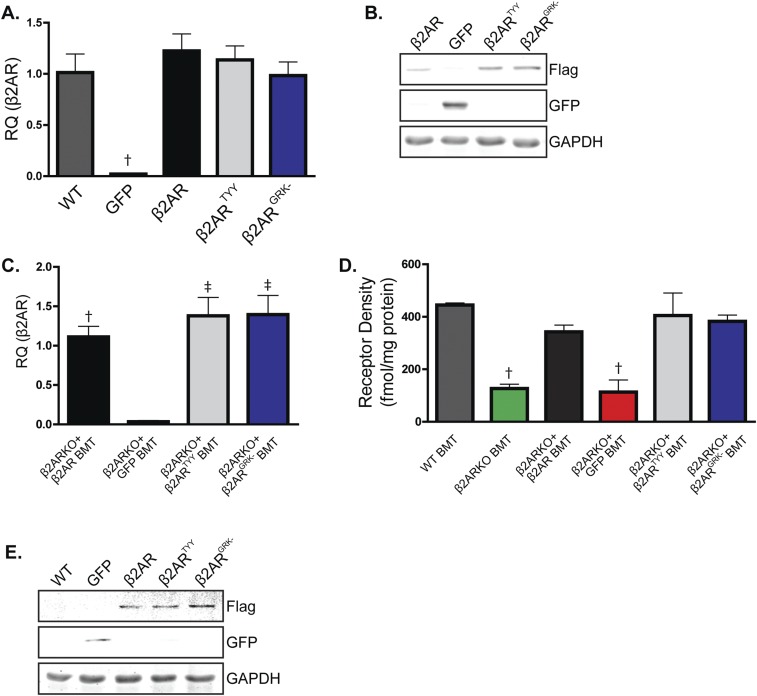
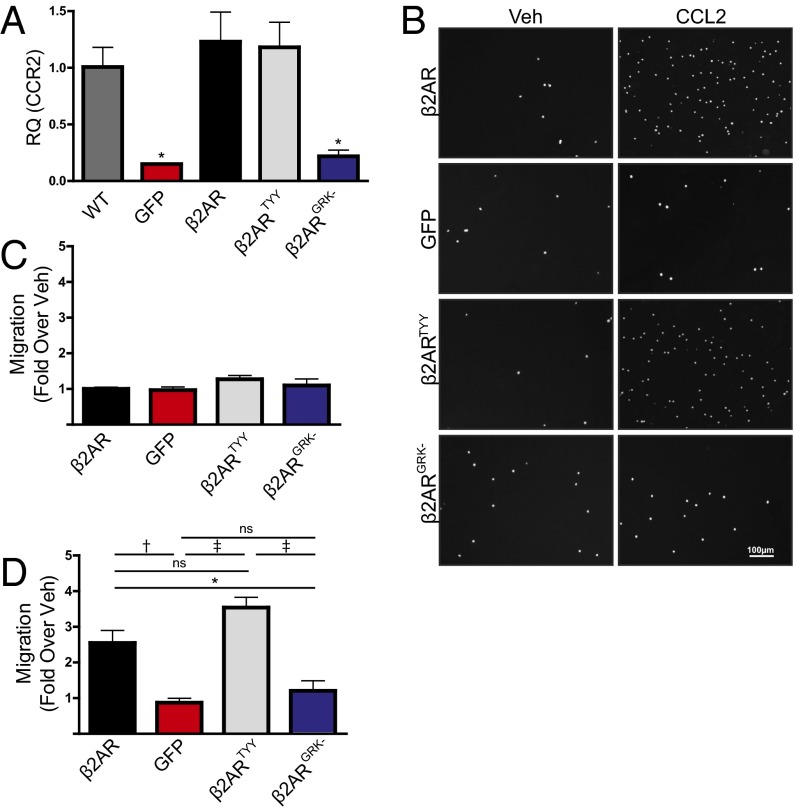
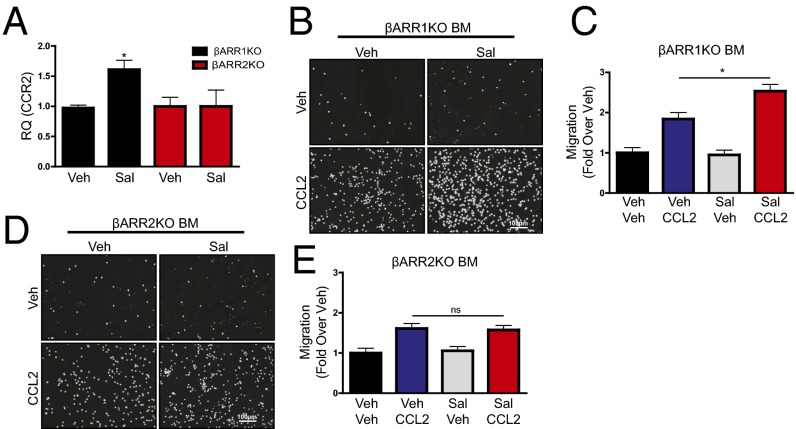
Because β2AR stimulation engages both G protein- and β-arrestin (βARR)–dependent signaling cascades, either of which may regulate downstream gene expression (8), we next sought to determine the proximal mechanism through which β2AR stimulation increases CCR2 expression. Thus, β2ARKO BM was infected with lentiviral constructs encoding either WT β2AR (versus a GFP control lentivirus), β2ARTYY [lacking stimulatory G alpha subunit (Gαs) coupling (9)], or β2ARGRK− [deficient in G protein-coupled receptor kinase (GRK)-mediated phosphorylation and βARR recruitment (10)], and CCR2 expression was measured. Each lentiviral construct induced β2AR expression in β2ARKO BM to levels similar to WT BM, whereas GFP had no effect (Fig. S6A). Flag and GFP expression was also assessed by immunoblot to confirm transgene expression (Fig. S6B). WT β2AR and β2ARTYY restored CCR2 expression in β2ARKO BM, whereas neither GFP nor β2ARGRK− altered CCR2 expression (Fig. 4A). Functionally, migration in response to vehicle was unchanged by expression of any β2AR construct (Fig. 4 B and C). However, corresponding to changes in CCR2 expression, β2ARGRK− did not alter CCL2-mediated migration, whereas both WT β2AR and β2ARTYY had enhanced migration in response to CCL2 (Fig. 4 D and F). These results indicate that β2AR-mediated changes in CCR2 are dependent proximally upon βARR-dependent signaling. To confirm these results, BM was isolated from βARR1KO and βARR2KO BM, and CCR2 expression and migration responses were examined following treatment with Sal. As was observed in WT BM, Sal treatment increased CCR2 expression in βARR1KO BM (Fig. 5A) and resulted in enhanced migration in response to CCL2 (Fig. 5 B and C). Conversely, Sal treatment of βARR2KO BM was unable to increase CCR2 expression (Fig. 5A) or CCL2-mediated migration (Fig. 5 D and E). Thus, βARR2-dependent β2AR signaling increases CCR2 expression, thereby enhancing immune cell responsiveness to CCL2-mediated migration.
Fig. S6.
Expression with lentiviral infection. (A) β2AR expression was measured by RT-qPCR in BM from WT or β2ARKO BM transduced with lentiviral constructs for GFP, β2AR, β2ARTYY, or β2ARGRK−. n = 4–8; one-way ANOVA, †P < 0.01 vs. WT. (B) Representative immunoblot for Flag and GFP of β2ARKO+β2AR-, β2ARKO+GFP-, β2ARKO+β2ARTYY-, or β2ARKO+β2ARGRK−-infected BM. GAPDH is shown as a loading control. (C) β2AR expression was measured by RT-qPCR in reconstituted BM from mice that had GFP, WT β2AR, β2ARTYY, or β2ARGRK− transduced into β2ARKO BM by lentivirus before transplantation. n = 4–8. Values are expressed relative to WT BMT. One-way ANOVA, †P < 0.01, ‡P < 0.001 vs. β2AR+GFP. (D) Radioligand binding was used to measure βAR expression. n = 3; one-way ANOVA, †P < 0.01 vs. WT BMT. (E) Representative immunoblot for Flag and GFP of reconstituted BM from WT, β2ARKO+GFP, β2ARKO+β2AR, β2ARKO+β2ARTYY, or β2ARKO+β2ARGRK− BMT mice. GAPDH is shown as a loading control. Data are expressed as mean ± SEM.
Fig. 4.
Restoration of β2AR expression reverses impairments in migration through βARR-dependent mechanisms. (A) RT-qPCR was used to measure CCR2 expression in BM from WT or β2ARKO BM transduced with GFP, β2AR, β2ARTYY, or β2ARGRK− lentivirus. n = 4–8; one-way ANOVA, *P < 0.05 vs. WT. (B) Representative Hoechst staining (white) from a 4-h migration assay in response to vehicle or CCL2 for β2ARKO BM transduced with lentiviral constructs for GFP, β2AR, β2ARTYY, or β2ARGRK−. (C and D) Quantification of migration assay results. Values are expressed as fold over β2ARKO+β2AR Veh. n = 4–8; one-way ANOVA, *P < 0.05, †P < 0.01, ‡P < 0.001. Data are expressed as mean ± SEM.
Fig. 5.
β2AR stimulation increases CCR2 expression and migration through βARR2. (A) RT-qPCR was used to measure CCR2 expression in βARR1KO and βARR2KO treated with vehicle control or Sal. One-way ANOVA, *P < 0.05 vs. Veh. (B–E) Representative Hoechst staining (white) and quantified results from a 4-h migration assay of βARR1KO (B and C) and βARR2KO BM (D and E) pretreated with Veh or Sal (1 μM; 24 h) before migration in response to Veh or CCL2 (100 ng/mL). Values are expressed as fold over Veh. n = 4–8; one-way ANOVA, *P < 0.05. Data are expressed as mean ± SEM.
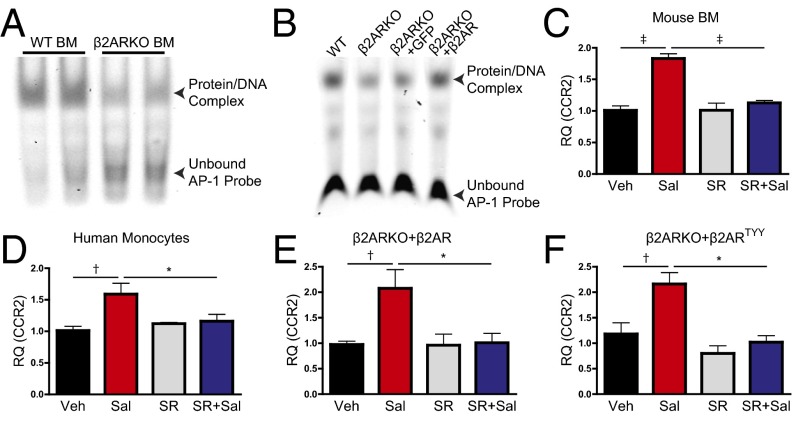
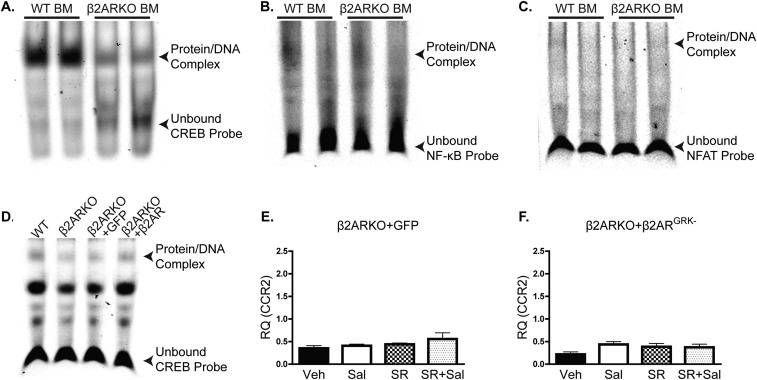
To further define the mechanism through which β2AR regulates CCR2 expression, transcription factor activation was examined using EMSAs. We assessed DNA binding of transcription factors reported to have putative binding sites in the CCR2 promoter and/or to regulate CCR2 expression [AP-1 (11), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (12), and nuclear factor of activated T cells (NFAT) (13)], as well as cAMP response element binding protein (CREB) as a positive control, because it is known to be regulated downstream of βAR but with minimal impact on CCR2 transcription (14–16). AP-1 (Fig. 6A) and CREB (Fig. S7A) transcription factor binding were both decreased in BM from β2ARKO mice when compared with WT BM, whereas NF-κB (Fig. S7B) and NFAT (Fig. S7C) binding were unaltered between groups. We subsequently tested whether rescue of β2AR expression would restore transcription factor binding in the β2ARKO BM and, as expected, canonical βAR-sensitive CREB DNA binding was restored upon reexpression of β2AR (Fig. S7D). Similarly, whereas GFP-infected β2ARKO BM still displayed reduced AP-1 DNA binding similar to β2ARKO alone (Fig. 6B), reexpression of WT β2AR restored AP-1 binding to WT levels. To determine the importance of AP-1 activation in the induction of CCR2 transcription, WT BM was pretreated with the AP-1 inhibitor SR11302 before Sal treatment. Increased CCR2 expression in response to Sal treatment was blocked by pretreatment with SR11302 (Fig. 6C). Human monocytes were also treated with Sal ± SR11302, yielding identical results to those attained in mouse BM cells, highlighting the potential human relevance of our findings (Fig. 6D).
Fig. 6.
AP-1 activation is decreased in β2ARKO BM. (A) Representative EMSAs from nuclear WT and β2ARKO BM extracts incubated with an AP-1–specific probe. (B) A representative EMSA from nuclear WT and β2ARKO BM extracts or β2ARKO BM infected with lentiviral-expressed GFP or β2AR and incubated with an AP-1–specific probe. (C) CCR2 expression was quantified in WT BM treated with vehicle, Sal ± SR11302, or SR11302 alone. n = 4–8; one-way ANOVA, ‡P < 0.001. (D) Human monocytes were treated with vehicle, Sal ± SR11302, or SR11302 alone. CCR2 expression was measured using RT-qPCR. n = 6–12; one-way ANOVA, *P < 0.05, †P < 0.01. (E and F) β2ARKO BM was infected with lentiviral-expressed WT β2AR (E) or lenti-β2ARTYY (F) and treated with vehicle, Sal ± SR11302, or SR11302 alone. CCR2 expression was examined by RT-qPCR. n = 4–6; one-way ANOVA, *P < 0.05, †P < 0.01. Data are expressed as mean ± SEM.
Fig. S7.
Alterations in transcription factor binding with β2ARKO. Representative EMSAs from nuclear WT and β2ARKO BM extracts incubated with CREB- (A), NF-κB– (B), or NFAT- (C) specific probes. (D) A representative EMSA from nuclear WT and β2ARKO BM extracts or β2ARKO BM infected with lentiviral-expressed GFP or β2AR and incubated with a CREB-specific probe. (E and F) β2ARKO BM was infected with lentiviral-expressed GFP (E) or lenti-β2ARGRK− (F) and treated with vehicle, Sal ± SR11302, or SR11302 alone. CCR2 expression was examined by RT-qPCR. n = 4–6; one-way ANOVA. Data are expressed as mean ± SEM.
To determine whether βARR-dependent β2AR signaling is involved in the control of AP-1–dependent CCR2 expression, as suggested from our results above, β2ARKO BM was infected with the WT β2AR, β2ARTYY, and β2ARGRK− lentiviral constructs, or GFP control, and CCR2 expression was assessed. With restoration of WT β2AR expression, Sal increased CCR2, as previously seen in WT BM, which could be prevented with SR11302 pretreatment (Fig. 6E). GFP-infected cells were unresponsive to Sal ± SR11302 (Fig. S7E). Similar to the WT β2AR rescue, β2ARTYY-infected β2ARKO BM had increased CCR2 expression following Sal treatment, which was blocked by SR11302 (Fig. 6F); however, β2ARGRK−-infected β2ARKO BM showed no alteration in CCR2 expression with either Sal or SR11302 (Fig. S7F). These findings confirm that βARR2-dependent β2AR signaling controls CCR2 expression via regulation of AP-1 in hematopoietic cells.
Restoration of β2AR-Mediated βARR Signaling in Vivo Reverses Leukocyte Dysfunction Following MI.
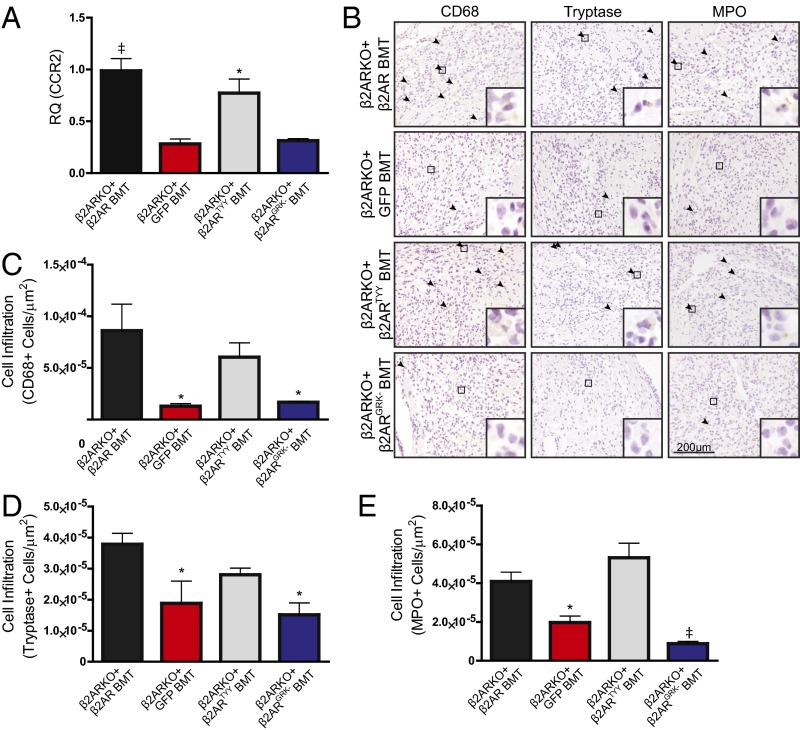
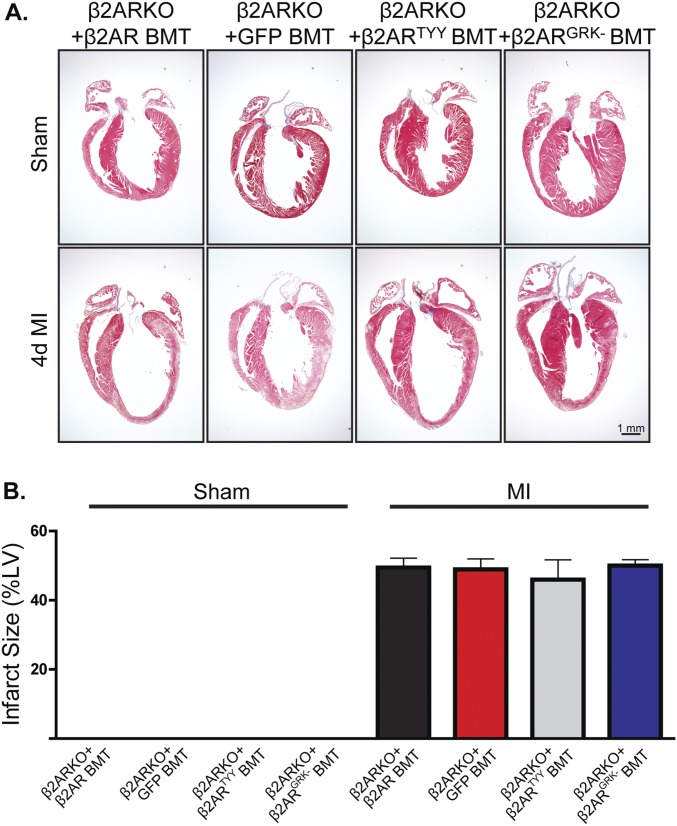
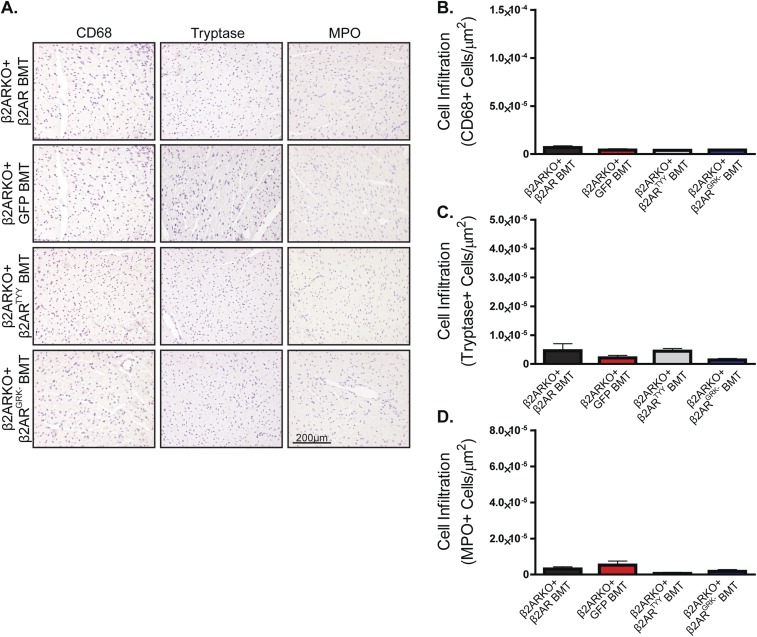
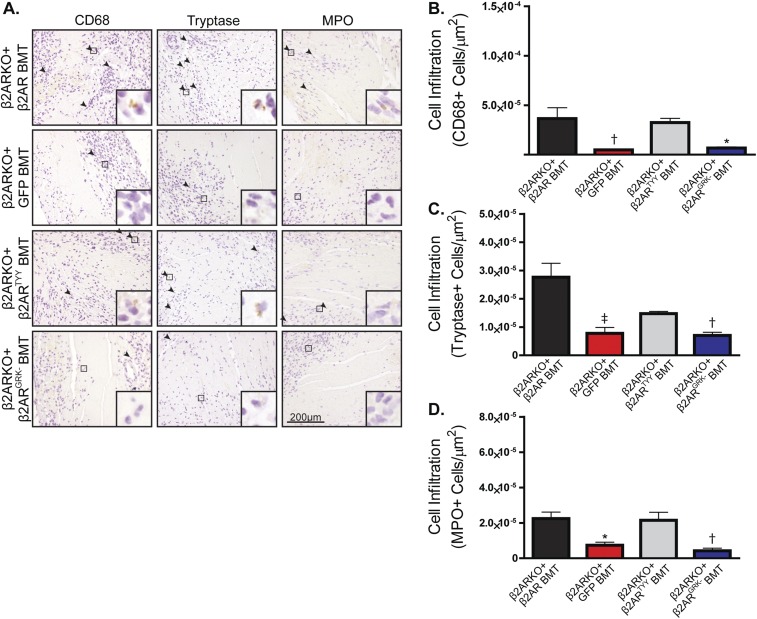
To determine whether restoration of β2AR expression, in particular β2AR-mediated βARR signaling, in β2ARKO BM could rescue the impaired cardiac leukocyte recruitment observed in post-MI β2AR BMT mice, β2ARKO BM was transduced with either the WT β2AR, β2ARTYY, β2ARGRK−, or GFP control lentiviral constructs before BMT. β2AR transcript expression in β2ARKO BM following reconstitution was approximately that of endogenous levels for WT and mutant β2AR (Fig. S6C), which corresponded to restoration of βAR membrane expression (Fig. S6D). Flag and GFP expression was assessed by immunoblot to confirm transgene expression in reconstituted BM (Fig. S6E). CCR2 expression was restored with the reexpression of WT β2AR as well as with β2ARTYY, but not in either GFP- or β2ARGRK−-transduced BM (Fig. 7A). Analysis of the infarct size of animals showed similar surgical conditions between groups (Fig. S8). Leukocyte levels in the remote region (Fig. S9) were unchanged following MI; however, correlating with restoration of CCR2 expression, immunohistochemistry for monocyte/macrophages, mast cell and neutrophil infiltration to the border zone (Fig. 7 B–E), and infarct (Fig. S10) of the injured myocardium of WT β2AR and β2ARTYY were rescued compared with those of β2ARKO+GFP and β2ARGRK− BMT mice.
Fig. 7.
Restoration of β2AR expression in β2ARKO BM reverses immune dysfunction following MI. (A) RT-qPCR was used to quantify CCR2 expression in reconstituted BM from mice that had GFP, WT β2AR, β2ARTYY, or β2ARGRK− transduced into β2ARKO BM by lentivirus before transplantation. Values are expressed relative to WT BMT. n = 4–8; one-way ANOVA, *P < 0.05, ‡P < 0.001 vs. β2AR+GFP. (B) Representative CD68, tryptase, and MPO staining for the border zone of hearts from WT C57BL/6 mice receiving β2ARKO BM with GFP, WT β2AR, β2ARTYY, or β2ARGRK− transduced before transplantation that underwent MI surgery. Arrowheads indicate positive staining. Insets show higher magnification at 250×. (C–E) Quantification of CD68 (C), tryptase (D), and MPO (E) staining for the border zone of 4-d post-MI hearts from GFP, WT β2AR, β2ARTYY, or β2ARGRK− BMT mice. Arrowheads indicate positive staining. n = 4–8; one-way ANOVA, *P < 0.05, ‡P < 0.001 vs. β2ARKO+β2AR BMT. Data are expressed as mean ± SEM.
Fig. S8.
Infarct size with restored β2AR expression. (A) Representative Masson’s trichrome staining for hearts from WT C57BL/6 mice receiving β2ARKO+β2AR, β2ARKO+GFP, β2ARKO+β2ARTYY, or β2ARKO+β2ARGRK− BMT that underwent MI surgery. (B) Quantification of Masson’s trichrome staining for sham and 4-d post-MI hearts from β2ARKO+β2AR, β2ARKO+GFP, β2ARKO+β2ARTYY, or β2ARKO+β2ARGRK− BMT mice. n = 5–10. Data are expressed as mean ± SEM.
Fig. S9.
Restoration of β2AR expression in β2ARKO BM reverses immune dysfunction following MI. (A) Representative CD68, tryptase, and MPO staining for the remote zone of hearts from WT C57BL/6 mice receiving β2ARKO BM with GFP, WT β2AR, β2ARTYY, or β2ARGRK− transduced before transplantation that underwent MI surgery. (B–D) Quantification of CD68 (B), tryptase (C), and MPO (D) staining for the remote zone of 4-d post-MI hearts from GFP, WT β2AR, β2ARTYY, or β2ARGRK− BMT mice. n = 4–8. Data are expressed as mean ± SEM.
Fig. S10.
Restoration of β2AR expression in β2ARKO BM reverses immune dysfunction following MI. (A) Representative CD68, tryptase, and MPO staining for the infarct zone of hearts from WT C57BL/6 mice receiving β2ARKO BM with GFP, WT β2AR, β2ARTYY, or β2ARGRK− transduced before transplantation that underwent MI surgery. Arrowheads indicate positive staining. Insets show higher magnification at 250×. (B–D) Quantification of CD68 (B), tryptase (C), and MPO (D) staining for the infarct zone of 4-d post-MI hearts from GFP, WT β2AR, β2ARTYY, or β2ARGRK− BMT mice. n = 4–8; one-way ANOVA, *P < 0.05, †P < 0.01, ‡P < 0.001 vs. β2ARKO + β2AR BMT. Data are expressed as mean ± SEM.
Discussion
Inflammatory processes are activated following acute cardiac injury, including chemokine-induced recruitment of immune cells to the site of injury, which are essential to mounting a reparative response and allowing subsequent healing (1). Sympathetic activity is known to regulate inflammation, with β2AR being the predominant adrenergic receptor subtype involved in immunomodulation (4–6). Recent findings from our laboratory have identified a critical role for hematopoietically expressed β2AR in survival following MI, wherein a lack of β2AR on immune cells resulted in decreased leukocyte infiltration to the heart, failed scar formation, and cardiac rupture (7). Although splenic retention of leukocytes played a role in the phenotype, whether the diminished leukocyte recruitment to the injured heart in the absence of immune cell-expressed β2AR involved an alteration in the response to promigratory chemokines was not determined. Thus, the purpose of this study was to determine whether immune cell-expressed β2AR plays a key role in regulating chemokine responsiveness and leukocyte infiltration following acute cardiac injury.
Because trafficking of immune cells to sites of inflammation occurs through various chemokine receptors (2), we initially surveyed the expression levels of a number of the receptors in WT versus β2ARKO BM, finding that CCR2 and CXCR4 levels in particular were significantly decreased. However, only CCR2 deficiency had a negative effect on BM cell migration in response to its ligand CCL2. This was in the absence of deficiencies in egress of cells from BM (7) that occur with global depletion of CCR2 (17, 18). Although the importance of CCL2 as a major chemoattractant of mononuclear cells to the ischemic heart has been extensively studied, the results are often in conflict, with both administration and inhibition of CCL2 showing improvements and detrimental effects in the remodeling following MI (19, 20). These discrepancies may be a result of the timing of administration or inhibition, because short-term elevations in CCL2 are protective whereas sustained elevations in CCL2 contribute to an enhanced progression toward heart failure (21–24). Indeed, studies examining the involvement of CCR2 following cardiac injury have shown beneficial effects with CCR2 inhibition (25–27), wherein both global and monocyte-directed RNAi-mediated deletion of CCR2 in mice that underwent MI surgery resulted in improved left ventricular remodeling (25, 26).
CCR2 is highly expressed on proinflammatory monocyte populations and, although β2AR has been shown to modulate chemotaxis, our findings are novel in that they directly link decreased hematopoietic cell β2AR expression with a corresponding reduction in CCR2 levels and CCL2-dependent migration. Previous studies have shown either no effect of sympathetic nervous system (SNS) stimulation on CCR2 expression in peripheral blood mononuclear cells (28) or decreased CCR2 expression in BM with SNS stimulation (29). These differences may be due to the lack of specificity of norepinephrine and epinephrine treatment and activation of multiple adrenergic receptor subtypes. Further, β2AR stimulation was previously shown in THP-1 human monocytic cells to increase CCR2 expression and migration through an undefined mechanism (30), whereas another study demonstrated that treatment of THP-1 cells with cAMP-elevating agents, including PDE3 inhibitors and dibutyryl cAMP, decreased both CCR2 expression and CCL2-mediated migration (31). These results may suggest that Gαs protein-dependent β2AR signaling acts to repress CCR2 expression.
Consistent with these reports, using β2AR mutants lacking the ability to either engage Gαs protein signaling (β2ARTYY) or to be phosphorylated by GRK (β2ARGRK−), we identified GRK-dependent β2AR signaling as the mechanism by which β2AR stimulation enhances CCR2 expression. Additionally, using BM cells from βARRKO mice, we demonstrated that βARR2, but not βARR1, is specifically required for this effect. βARR2 has been shown to regulate inflammatory responses in MI (32), as βARR2KO mice had decreased survival after MI and decreased infiltration of macrophages to the infarct following MI, similar to our findings. βARRs are known to be involved, either directly or indirectly, in the regulation of a number of transcription factors that could regulate CCR2 gene transcription, including AP-1 and NF-κB (33–37). Although the role of AP-1 has been minimally studied following MI (38, 39), it plays a well-established role in inflammation (40). We have demonstrated that βARR2-dependent β2AR signaling via AP-1 is required for CCR2 up-regulation in response to sympathetic stimulation, and that restoration of GRK/βARR-dependent β2AR signaling in immune cells rescues CCR2 expression, migration in response to CCL2, and cardiac infiltration following MI in vivo.
Although we show in our study that CCR2 deficiency in immune cells reduces their capacity for chemotaxis and infiltration to the heart post-MI, this deficiency was limited to the monocyte/macrophage and neutrophil populations in vivo, whereas mast cell infiltration was unchanged. This is in contrast to our previously reported study in which β2ARKO chimeric mice had impairment in cardiac recruitment of all three cell types (7). Further, in our current study, we did not observe an altered progression toward heart failure in CCR2KO BMT mice, which, coupled with the positive outcome of CCR2 inhibition in the aforementioned studies (25, 26) versus the catastrophic impact of immune cell-specific β2AR deletion on cardiac remodeling following MI we previously reported (7), suggests that β2ARKO chimeric mice likely have additional factors that contribute to their observed post-MI phenotype. For instance, we previously showed that enhanced vascular cell adhesion molecule 1 expression was associated with splenic retention of leukocytes in β2ARKO BMT mice and that splenectomy partially restored cardiac leukocyte infiltration following MI (7), demonstrating that even with diminished CCR2 expression and responsiveness, β2AR-deficient leukocytes retain some capacity to traffic to sites of injury in vivo. Thus, it may be beneficial to further investigate the role of CXCR4 expression, as well as a larger number of chemokine receptors and secreted factors from individual immune cell populations, to more fully elucidate how β2AR controls these processes. The potential existence of multiple β2AR-dependent mechanisms that could influence distinct immune cell populations suggests a widespread impact of β2AR modulation on the regulation of early immune responses that could be targeted to alter post-MI recovery.
In summary, we have identified a role for β2AR in the regulation of immune cell-specific CCR2 expression, where a lack of β2AR expression in leukocytes results in decreased CCR2 expression, impaired migration to CCL2 in vitro, and decreased monocyte/macrophage and neutrophil cardiac infiltration following MI in vivo. Lentiviral-mediated reexpression of β2AR in β2ARKO BM before transplantation restored CCR2 expression and BM migration through a βARR2-dependent pathway. These results demonstrate an immunomodulatory role for βARR-biased β2AR signaling in early immune responses following MI, which could be targeted to promote reparative processes while preventing chronic inflammatory events that are detrimental to healing. Further, because β-blockers are commonly used in patients around the time of acute cardiac ischemia, our results suggest they could impact the leukocyte-mediated repair response, warranting further investigation.
Materials and Methods
Surgery and Assays.
Detailed descriptions of coronary artery occlusion surgery, echocardiography, human monocyte cell culture, reverse transcription–quantitative PCR, migration assay, histological analysis, radioligand binding, immunoblot analysis, bone marrow transplant, bone marrow isolation, and lentiviral infection are provided in SI Materials and Methods. All animal procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at the Temple University School of Medicine.
Statistical Analysis.
Data presented are expressed as mean ± SEM. Statistical analysis was performed using unpaired Student t tests, one-way ANOVA with a Tukey’s multiple comparison test, or two-way repeated-measures ANOVA where appropriate using Prism 5.0 software (GraphPad Software), with P values indicated in the figure legends.
SI Materials and Methods
Bone Marrow Transplant.
WT C57BL/6 recipient mice (male, 8 wk) were lethally irradiated with 950 rads using X-ray irradiation to remove endogenous BM cells. Donor BM isolated from the femurs of β2ARKO, CCR2KO, or WT C57BL/6 mice was introduced by retroorbital injection (1 × 107 cells) within 24 h of irradiation. BM was allowed to reconstitute for 1 mo before MI surgery. Reconstitution was confirmed at the conclusion of the study for each mouse using reverse transcription–quantitative PCR (RT-qPCR) analysis for β2AR or CCR2 expression on recipient BM. CD45 expression, a marker of cells of the hematopoietic lineage, was assessed by immunoblot in BM from WT, β2ARKO, and CCR2KO BMT animals following reconstitution, and survival following BM reconstitution was assessed for each line before coronary artery occlusion surgery, demonstrating no differences in reconstitution between groups (Fig. S1). All animal procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at the Temple University School of Medicine.
Coronary Artery Occlusion Surgery.
Myocardial infarction was induced as previously described (7). In brief, mice were anesthetized with 2% (vol/vol) isoflurane inhalation. A small skin incision was made and the pectoral muscles were dissected and retracted to expose the fourth intercostal space. A small hole was made and the heart popped out. The left coronary artery was sutured ∼3 mm from its origin and the heart was placed back into the intrathoracic space and closure of muscle and skin. For CCR2 antagonist-treated mice, immediately following surgery, mice were injected (i.p.) with vehicle [8% (vol/vol) DMSO in PBS] or 2 mg/kg CCR2 antagonist (Millipore; 227016) was administered by daily injections until the termination of the study. Animals received a single dose (0.3 mg/kg) of buprenorphine immediately following surgery.
Echocardiography.
Cardiac function was assessed via transthoracic 2D echocardiography performed at baseline and at weekly intervals post-MI using a 12-mHz probe on mice anesthetized with isoflurane (1.5%). M-mode echocardiography was performed in the parasternal short-axis view to assess several cardiac parameters including left ventricular (LV) end-diastolic dimension, wall thickness, LV fractional shortening, and fractional shortening. Percent fractional shortening was calculated using the equation ((LVID;d − LVID;s)/LVID;s) × 100%, where LVID;d is left ventricular internal diameter diastolic and LVID;s is left ventricular internal diameter systolic. Percent ejection fraction was calculated using the equation ((LV vol;d − LV vol;s)/LV vol;d) × 100%, where LV vol;d is left ventricular volume diastolic and LV vol;s is left ventricular volume systolic.
Human Monocyte Cell Culture.
THP-1 cells (American Type Culture Collection), a human monocytic cell line, were cultured in modified RPMI-1640 media containing 2 mM l-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM Hepes, and 1 mM sodium pyruvate supplemented with 10% (vol/vol) FBS under standard cell-culture growth conditions (37 °C/5% CO2/95% humidified air).
Bone Marrow Isolation and Lentiviral Infection.
BM isolated from the femurs of WT or β2ARKO mice was treated for 24 h with the β2AR selective agonist salbutamol (Sal; 1 μM). Pretreatment with SR11302 (10 μM; 1 h) was used to inhibit AP-1. For lentiviral infection, isolated BM was transduced with lentiviral vectors for 3XFlag-β2AR-RFP, Flag-β2ARTYY-RFP, Flag-β2ARGRK−-RFP, or GFP (7). Transductions were performed in MEM + 10% (vol/vol) FBS in the presence of 5 μg/mL Polybrene (Sigma-Aldrich). For in vitro experiments, media were changed 24 h following infection to complete media [MEM + 10% (vol/vol) FBS] and incubated an additional 24 h prior to experiments. For in vivo experiments, BM was rinsed 1 h following infection and transplanted into irradiated mice via retroorbital injection. BM was allowed to reconstitute for 1 mo.
Reverse Transcription–Quantitative PCR.
cDNA was synthesized from the total RNA of BM samples using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). RT-qPCR was performed with SYBR Select Master Mix (Applied Biosystems) in triplicate for each sample using primers listed in Table S1 at an annealing temperature of 60.1 °C. All RT-qPCR data were analyzed using the Applied Biosystems Comparative CT Method (ΔΔCT). Gene expression analysis was normalized to GAPDH or translationally controlled tumor protein 1 (TPT1) and expressed as relative quantitation (RQ) and RQmin/max.
Migration Assay.
BM isolated from WT or β2ARKO mice was plated on 8-μm transmembrane inserts (Corning) in a 24-well plate containing vehicle (Veh), CCL2 (100 ng/mL), CCL3 (100 ng/mL), or CXCL12 (10 ng/mL). For agonist pretreatment, isolated BM was treated for 24 h with 1 μM Sal before plating with chemokine. To inhibit CCR2-mediated migration, a 1-h, 10 nM CCR2 antagonist treatment was used (Millipore). Cells were stained with NucBlue Live ReadyProbes Reagent (Hoechst 33342; Molecular Probes) and migration was imaged after 4 h. Cells were visualized in the blue channel using a Nikon Eclipse fluorescence microscope at 10× magnification and analyzed from 10 random fields per treatment using NIS Elements software. Representative images are shown in black and white at 20×.
Histological Analysis.
Excised hearts were fixed in 4% (vol/vol) paraformaldehyde, paraffin-embedded, and sectioned at 5-μm thickness. To quantify infarct size, hearts were stained for Masson trichrome (Sigma-Aldrich). NIS Elements software was used for recording images and image analysis. Infarct length was measured and expressed as percentage of total LV length.
Immunohistochemistry was performed on deparaffinized sections to examine infiltration of various immune cell types. Antigens were retrieved using a citrate-based antigen unmasking solution (Vector Laboratories). Hearts were blocked (10% FBS/PBS) and a 0.3% H2O2 solution was used to block endogenous peroxide activity. Hearts were incubated with antibodies against CD68 (1:100; Abcam), mast cell tryptase (1:100; Abcam), or myeloperoxidase (MPO; 1:100; Santa Cruz). Washed slides were incubated with the appropriate secondary antibodies anti-mouse HRP (1:1,000; GE Healthcare) or anti-goat HRP (1:1,000; Santa Cruz), followed by staining hematoxylin. Immunofluorescence-stained hearts were mounted using ProLong Gold Antifade Reagent (Invitrogen). Stained hearts were developed using a DAB Substrate Kit (Vector Laboratories) and mounted using Permount Mounting Medium (Thermo Scientific). Staining was visualized on a Nikon Eclipse microscope at 20× magnification, and NIS Elements software was used for recording images and image analysis. Images were quantified as the number of positive cells per area.
Membrane Preparation and Radioligand Binding.
Membrane preparations from BM were prepared by homogenization in ice-cold lysis buffer (25 mM Tris, pH 7.4, 5 mM EDTA, 1 μg/mL aprotinin, 1 μg/mL leupeptin) and centrifuged at 1,000 × g for 5 min at 4 °C. The supernatant was centrifuged at 30,000 × g, and the crude membrane pellet was resuspended in lysis buffer containing 10% glycerol and stored at −80 °C until use.
The density of βAR on membranes was determined by saturation binding experiments. Membrane preparations (25 μg of protein) were incubated with [125I]cyanopindolol ([125I]CYP; 200 pM; PerkinElmer) in binding buffer (75 mM Tris, pH 7.4, 2 mM EDTA, 12.5 mM MgCl2, 1 μg/mL aprotinin, 1 μg/mL leupeptin). Incubations were performed in the presence or absence of propranolol (10 μM) to determine nonspecific binding. The reactions were performed in a 250-μL volume and allowed to equilibrate at 37 °C for 1 h before filtering the membranes through a glass fiber filter (Whatman GF/C; Brandel). Each filter was washed five times with 5 mL of ice-cold wash buffer (10 mM Tris, pH 7.4, 10 mM EDTA) to remove unbound drug. The amount of total and nonspecific radiolabel bound to cell membranes was determined on a gamma counter. All assays were performed in triplicate. Receptor density was normalized to milligrams of membrane protein.
Immunoblot.
Bone marrow samples were homogenized in RIPA buffer containing 1× HALT protease inhibitor mixture (78437; Thermo Scientific) and phosphatase inhibitor mixture set IV (524628; Calbiochem). Equal amounts of lysates were resolved by SDS/polyacrylamide gel electrophoresis (10% gels) and transferred to Immobilon-PSQ polyvinylidene fluoride 0.2-μm-pore size membranes (Millipore). Odyssey Blocking Buffer (LI-COR Biosciences) was used to prevent nonspecific binding. Immunoblotting was performed overnight at 4 °C with diluted antibodies against CD45 (1:1,000; R&D Systems), Flag M2 (1:10,000; Sigma-Aldrich), GFP (1:1,000; Cell Signaling), or GAPDH (1:1,000; Cell Signaling). After washing with Tris-buffered saline + 0.1% Tween 20, membranes were incubated at room temperature for 60 min with the appropriate diluted secondary antibody [IRDye 680 donkey anti-rabbit IgG (H + L) (heavy chain + light chain) at 1:20,000; IRDye 800CW goat anti-mouse IgG (H + L) at 1:15,000; IRDye 680 donkey anti-goat IgG (H + L) at 1:20,000; LI-COR Biosciences]. Bound antibody was detected using the Odyssey System (LI-COR Biosciences). Intensities were normalized to corresponding GAPDH intensities.
Acknowledgments
This work was supported by NIH Grants HL105414 (to D.G.T.), HL091799 and HL085503 (to W.J.K.), and HL091804 (to C.J.T.) and an American Heart Association postdoctoral fellowship (to L.A.G.).
Footnotes
Conflict of interest statement: W.J.K. and D.G.T. have equity in Renovacor, Inc., which has neither funded this study nor has a relevant product related to this study.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611023114/-/DCSupplemental.
References
- 1.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843(11):2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Cavalera M, Frangogiannis NG. Targeting the chemokines in cardiac repair. Curr Pharm Des. 2014;20(12):1971–1979. doi: 10.2174/13816128113199990449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21(6):736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve—An integrative interface between two supersystems: The brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 6.Padro CJ, Sanders VM. Neuroendocrine regulation of inflammation. Semin Immunol. 2014;26(5):357–368. doi: 10.1016/j.smim.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grisanti LA, et al. Leukocyte-expressed β2-adrenergic receptors are essential for survival after acute myocardial injury. Circulation. 2016;134(2):153–167. doi: 10.1161/CIRCULATIONAHA.116.022304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorton D, Bellinger DL. Molecular mechanisms underlying β-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int J Mol Sci. 2015;16(3):5635–5665. doi: 10.3390/ijms16035635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luttrell LM, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA. 2001;98(5):2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shenoy SK, et al. Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281(2):1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 11.Phillips RJ, Lutz M, Premack B. Differential signaling mechanisms regulate expression of CC chemokine receptor-2 during monocyte maturation. J Inflamm (Lond) 2005;2:14. doi: 10.1186/1476-9255-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, et al. Novel NEMO/IkappaB kinase and NF-kappaB target genes at the pre-B to immature B cell transition. J Biol Chem. 2001;276(21):18579–18590. doi: 10.1074/jbc.M100846200. [DOI] [PubMed] [Google Scholar]
- 13.Jung H, Miller RJ. Activation of the nuclear factor of activated T-cells (NFAT) mediates upregulation of CCR2 chemokine receptors in dorsal root ganglion (DRG) neurons: A possible mechanism for activity-dependent transcription in DRG neurons in association with neuropathic pain. Mol Cell Neurosci. 2008;37(1):170–177. doi: 10.1016/j.mcn.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilley DG, Kim IM, Patel PA, Violin JD, Rockman HA. Beta-arrestin mediates beta1-adrenergic receptor-epidermal growth factor receptor interaction and downstream signaling. J Biol Chem. 2009;284(30):20375–20386. doi: 10.1074/jbc.M109.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda A, et al. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153(5):2052–2063. [PubMed] [Google Scholar]
- 16.Yamamoto K, et al. Cloning and functional characterization of the 5′-flanking region of the human monocyte chemoattractant protein-1 receptor (CCR2) gene. Essential role of 5′-untranslated region in tissue-specific expression. J Biol Chem. 1999;274(8):4646–4654. doi: 10.1074/jbc.274.8.4646. [DOI] [PubMed] [Google Scholar]
- 17.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J Exp Med. 2014;211(13):2583–2598. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono K, et al. Prevention of myocardial reperfusion injury in rats by an antibody against monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Lab Invest. 1999;79(2):195–203. [PubMed] [Google Scholar]
- 20.Birdsall HH, et al. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation. 1997;95(3):684–692. doi: 10.1161/01.cir.95.3.684. [DOI] [PubMed] [Google Scholar]
- 21.Wilson EM, Diwan A, Spinale FG, Mann DL. Duality of innate stress responses in cardiac injury, repair, and remodeling. J Mol Cell Cardiol. 2004;37(4):801–811. doi: 10.1016/j.yjmcc.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 22.Tarzami ST, Cheng R, Miao W, Kitsis RN, Berman JW. Chemokine expression in myocardial ischemia: MIP-2 dependent MCP-1 expression protects cardiomyocytes from cell death. J Mol Cell Cardiol. 2002;34(2):209–221. doi: 10.1006/jmcc.2001.1503. [DOI] [PubMed] [Google Scholar]
- 23.Tarzami ST, et al. MCP-1/CCL2 protects cardiac myocytes from hypoxia-induced apoptosis by a G(alphai)-independent pathway. Biochem Biophys Res Commun. 2005;335(4):1008–1016. doi: 10.1016/j.bbrc.2005.07.168. [DOI] [PubMed] [Google Scholar]
- 24.Dewald O, et al. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96(8):881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 25.Kaikita K, et al. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165(2):439–447. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majmudar MD, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127(20):2038–2046. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavine KJ, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA. 2014;111(45):16029–16034. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okutsu M, Suzuki K, Ishijima T, Peake J, Higuchi M. The effects of acute exercise-induced cortisol on CCR2 expression on human monocytes. Brain Behav Immun. 2008;22(7):1066–1071. doi: 10.1016/j.bbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Xiu F, Stanojcic M, Jeschke MG. Norepinephrine inhibits macrophage migration by decreasing CCR2 expression. PLoS One. 2013;8(7):e69167. doi: 10.1371/journal.pone.0069167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo YL, et al. Monocyte/macrophage β2-AR as a target of antisympathetic excitation-induced atherosclerotic progression. Genet Mol Res. 2014;13(4):8080–8088. doi: 10.4238/2014.October.7.2. [DOI] [PubMed] [Google Scholar]
- 31.Chuang SY, Yang SH, Pang JH. Cilostazol reduces MCP-1-induced chemotaxis and adhesion of THP-1 monocytes by inhibiting CCR2 gene expression. Biochem Biophys Res Commun. 2011;411(2):402–408. doi: 10.1016/j.bbrc.2011.06.163. [DOI] [PubMed] [Google Scholar]
- 32.Watari K, Nakaya M, Nishida M, Kim KM, Kurose H. β-Arrestin2 in infiltrated macrophages inhibits excessive inflammation after myocardial infarction. PLoS One. 2013;8(7):e68351. doi: 10.1371/journal.pone.0068351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. Beta-arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci USA. 2004;101(23):8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao H, et al. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell. 2004;14(3):303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 35.Kang J, et al. A nuclear function of beta-arrestin1 in GPCR signaling: Regulation of histone acetylation and gene transcription. Cell. 2005;123(5):833–847. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Kizaki T, et al. Beta2-adrenergic receptor regulates Toll-like receptor-4-induced nuclear factor-kappaB activation through beta-arrestin 2. Immunology. 2008;124(3):348–356. doi: 10.1111/j.1365-2567.2007.02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, et al. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7(2):139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida K, et al. Activation of mitogen-activated protein kinases in the non-ischemic myocardium of an acute myocardial infarction in rats. Jpn Circ J. 2001;65(9):808–814. doi: 10.1253/jcj.65.808. [DOI] [PubMed] [Google Scholar]
- 39.Luo X, et al. c-Jun DNAzymes inhibit myocardial inflammation, ROS generation, infarct size, and improve cardiac function after ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2009;29(11):1836–1842. doi: 10.1161/ATVBAHA.109.189753. [DOI] [PubMed] [Google Scholar]
- 40.Schonthaler HB, Guinea-Viniegra J, Wagner EF. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann Rheum Dis. 2011;70(Suppl 1):i109–i112. doi: 10.1136/ard.2010.140533. [DOI] [PubMed] [Google Scholar]