Significance
Humans possess the remarkable ability to recombine details of divergent memories into imaginings of future events. Such imaginings are useful, for example, because they foster planning and motivate farsighted decisions. Importantly, recurrently imagining feared situations can also undermine our well-being and may even contribute to the development of anxiety. Here, we demonstrate that fearful imaginings about the future can be inhibited by neural mechanisms that help to suppress the past. Importantly, suppression reduces later apprehensiveness about the feared events, a benefit that was diminished in individuals with greater trait anxiety. This pattern suggests that the observed inhibition mechanism serves to control people’s future fears and its disruption may foster psychological disorders characterized by intrusive prospective thoughts.
Keywords: suppression, episodic simulation, anxiety, cognitive control, prefrontal cortex
Abstract
Imagining future events conveys adaptive benefits, yet recurrent simulations of feared situations may help to maintain anxiety. In two studies, we tested the hypothesis that people can attenuate future fears by suppressing anticipatory simulations of dreaded events. Participants repeatedly imagined upsetting episodes that they feared might happen to them and suppressed imaginings of other such events. Suppressing imagination engaged the right dorsolateral prefrontal cortex, which modulated activation in the hippocampus and in the ventromedial prefrontal cortex (vmPFC). Consistent with the role of the vmPFC in providing access to details that are typical for an event, stronger inhibition of this region was associated with greater forgetting of such details. Suppression further hindered participants’ ability to later freely envision suppressed episodes. Critically, it also reduced feelings of apprehensiveness about the feared scenario, and individuals who were particularly successful at down-regulating fears were also less trait-anxious. Attenuating apprehensiveness by suppressing simulations of feared events may thus be an effective coping strategy, suggesting that a deficiency in this mechanism could contribute to the development of anxiety.
We spend a significant part of our everyday life contemplating the future (1, 2), often vividly imagining anticipated episodes (3). Such episodic simulation conveys great adaptive value by eliciting the emotional impact that a future episode might hold (4–6). However, dwelling on the future can also be detrimental to our well-being.
Thinking about past and future affairs—rather than about current activities—can induce unhappiness (7), and exaggerated future simulation may even be integral to psychological disorders such as bipolar disorder (8) and anxiety (9–11). Highly anxious individuals think of more negative future experiences than healthy people (10, 12), their imagination of those episodes is more vivid (12), and anticipatory processing of distressing situations aggravates symptoms (13). Recurrent simulations of feared situations may moreover help to maintain anxiety, because imagined episodes are deemed as more likely to occur (14–16). Thus, sometimes it may be beneficial to stop persistent simulations of feared prospective episodes.
This study tests the hypothesis that people can inhibit recurring imaginings of the future by engaging a control mechanism that has been shown to suppress memories of the past. Our hypothesis is based on two lines of research. First, memory and future simulation are mediated by a common core network of brain regions, including the hippocampus (HC) and ventromedial prefrontal cortex (vmPFC) (17–19). The HC is critical for the recombination of stored episodic details into imaginings of any future scenes (18), as evidenced by the profound simulation impairments caused by lesions to this structure (e.g., refs. 20−21; but see also ref. 22). The vmPFC, by comparison, may particularly support simulations of recurrent themes. Accumulating evidence suggests that this region comes to represent features that are shared across similar episodes, thus creating a schematic model of the respective scenario (23, 24). Reactivation of such a model, in turn, can facilitate simulations by providing the details that are typical for the imagined situation (25, 26). Accordingly, the vmPFC may especially contribute to simulations that share recurring features such as when someone persistently reimagines particular fears they have about their future.
Second, people can exert remarkable mnemonic control. When confronted with an unwelcome reminder, people can suppress retrieval of the associated unwanted memory (27–29). Such suppression is thought to be achieved by down-regulating brain regions supporting memory storage and retrieval, via modulatory influence of the right dorsolateral prefrontal cortex (dlPFC) (28–35). This mechanism suppresses episodic details, impairs their access, and eventually causes forgetting (36, 37).
Together, these two lines of research raise the possibility that people may stop recurring imaginings of feared future events by engaging a control mechanism that suppresses persistent episodic simulations. Such “future suppression” may require the down-regulation of the HC, given its fundamental contribution to both the retrieval of past episodes and the mental construction of future events (20, 21). Critically, however, unlike the suppression of recently acquired and unique memories, which has been the focus of most pertinent studies, the suppression of recurrent imaginings may also involve down-regulation of the vmPFC, given this region’s putative involvement in facilitating access to typical, repeated event details that are part of a schematic model of the dreaded situation (23, 24, 26).
To examine future suppression, we adapted the “Think/No-Think” procedure, used to study the suppression of past events (38), to create the new “Imagine/No-Imagine” paradigm (Fig. 1). The procedure first asked participants to describe their fears (see Fig. 1, stage 1, for examples). Importantly, they only provided recurrent future fears—that is, those that they had already worried might happen before entering the experiment. Participants then gave one key detail for each fear that was typical to their recurring imaginings of it. (These typical event details served as a dependent measure; see below.) Afterward, they entered the critical Imagine/No-Imagine phase, which was composed of trials that presented reminders to these fears (stage 2 in Fig. 1). For some trials, participants were asked to imagine the feared event as vividly as possible in response to the reminder (Imagine condition); for others, participants were asked to suppress their imagining of the event, upon seeing the reminder (Suppress condition). (A third of the originally provided episodes, the Baseline items, were set aside and were not cued during this phase.) Over the course of the Imagine/No-Imagine phase, participants either imagined or suppressed a feared event 12 times. Following this phase, we gave participants each reminder again and asked them to recall the typical feature of its corresponding fear (stage 3 in Fig. 1). Once all typical details were tested, participants were then asked to freely imagine each episode aloud in detail for 2 min (stage 4 in Fig. 1). Finally, we assessed the impact of suppression on participants’ apprehensiveness toward these future events (stage 5 in Fig. 1).
Fig. 1.
Illustration of the Imagine/No-Imagine procedure. (Upper) Participants provided episodes that they had already previously feared might happen to them. (The pictures illustrate some of these provided episodes.) For each episode, they also gave a reminder word as a strong cue for the respective fear and a key detail word that denotes a detail typical of their imagination of the respective fear. (This detail was used as a dependent measure as described below.) (Middle) In the Imagine/No-Imagine phase, participants repeatedly imagined events cued with green reminder words while trying to suppress all thoughts and images from coming to mind of episodes cued with red reminders. (Lower) Finally, we tested participants’ ability to recall typical details of their imagination in the form of the key detail words, asked them to freely imagine each episode out loud, and assessed their apprehensiveness toward the events.
We used this Imagine/No-Imagine procedure in two studies to test key predictions of the hypothesis that persistent future fears can be controlled by a similar mechanism to the one that mediates the suppression of unwanted memories. As detailed in the following, these predictions concerned (i) the behavioral consequences of suppression, (ii) the relationship between the efficacy of suppression and trait anxiety, and (iii) in the second study, the neural basis of the future suppression process.
If suppressing recurring fears engages a control mechanism that inhibits typical episodic details, three behavioral findings should emerge: First, participants should have greater difficulty recalling such details for suppressed than for baseline episodes. Second, given that these details constitute the building blocks of imagination (18), suppression also should make it harder for people to freely imagine suppressed episodes, even when they later simulate them on purpose. Third, if participants suppress typical details of a future episode that otherwise would evoke anxious feelings, these details should be less accessible when they then judge how apprehensive they feel about the episode. Because the availability of pertinent details influences how people evaluate a situation (39, 40), the reduced accessibility may make people feel less apprehensive about the event.
The predicted behavioral consequences of suppression would suggest that suppression constitutes a coping mechanism for dealing with intrusive imaginings of future fears. Given that such imaginings may be prevalent in anxious individuals (10, 15) and indeed can amplify fears (41), this raises the intriguing possibility that deficient suppression contributes to the development of anxiety. This hypothesis is consistent with the proposal that anxiety is characterized by a general deficiency in inhibiting threat-related information (42), presumably resulting from an underrecruitment of the dlPFC (42–44). It is also consistent with more specific observations that anxious individuals and people with lower control over intrusive thoughts suffer deficits in their ability to suppress aversive memories (45–49). Given our hypothesis, such impaired memory control may extrapolate to the control of prospective thoughts. Thus, individuals exhibiting high trait anxiety may be less efficient at reducing their future fears by suppression.
Finally, if future suppression is indeed achieved by a similar mechanism as memory suppression, then it should be mediated by similar neural processes. That is, it should entail a modulatory top-down influence of the right dlPFC on regions supporting the construction of recurrent imaginings. We addressed this part of our hypothesis in the second study using fMRI and dynamic causal modeling (DCM) (50, 51).
The hypothesis makes three key predictions: First, the influence of the dlPFC should be particularly pronounced when participants suppress their imaginings. Second, this context-dependent modulation should affect the effective connectivity of dlPFC with both the HC and the vmPFC (rather than with just the HC), given the proposed role of the vmPFC in recurrent simulations (23, 24, 26). Third, given the presumed inhibitory nature of the dlPFC signal, the top-down connectivity should be negative, so that activation in the dlPFC causes deactivation in the target regions. Critically, if the vmPFC supports the retrieval of details that are typical for specific, recurring episodes, strong inhibition of this region may render those typical features inaccessible. In this case, the effective top-down connectivity from the dlPFC to the vmPFC may be more negative for individuals who later also show greater forgetting of typical details of suppressed fears.
Together, the two studies thus elucidate a critical mechanism of suppressing intrusive imaginings of future fears and gauge whether deficits of this mechanism may contribute to persisting fears associated with anxiety.
Results
We first report whether future suppression causes inhibitory aftereffects that attenuate persistent fears before exploring whether additional imaginings also had an impact on already established fears. We then examine the complementary neural prediction that future suppression is based on modulatory top-down mechanisms similar to those engaged during memory suppression. To test our behavioral predictions, we compared the respective dependent variables in the Suppress and Baseline conditions. By this, we compare the effects of suppression with a condition that did not require participants to engage with the feared episodes at all. Moreover, to verify the consistency of our findings, the reported ANOVAs always included a between-subject factor of experiment (study 1, study 2) in addition to the within-subject factor of suppression status (suppression, baseline). All of the comparisons were qualitatively identical in the two studies, and accordingly, none of the interactions were significant (all Fs < 1.34, all Ps > 0.25).
Suppression Induces Forgetting of Typical Details of Recurring Fears.
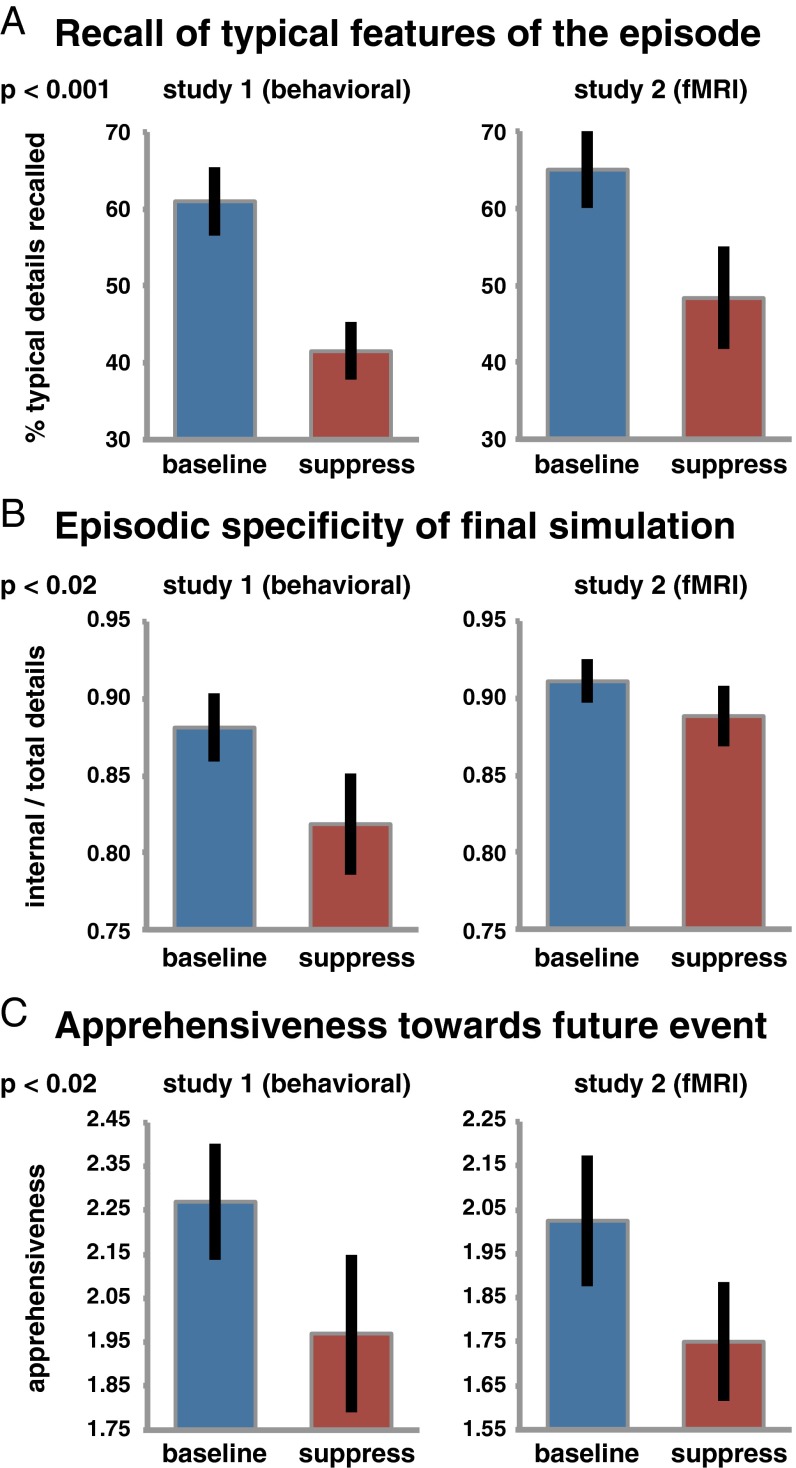
We predicted that suppressing imaginings of feared future events would impair the later recall of typical details of those episodes, similar to the forgetting observed after suppressing unwanted memories (e.g., refs. 36, 48). In both studies, participants had provided a single word for each feared event that signified a typical recurring detail of their imagination (Materials and Methods and Fig. 1). Critically, the recall rate for those typical details was indeed significantly worse for the fears that people had suppressed compared with baseline episodes that they had not suppressed, F(1, 38) = 18.27, P < 0.001 (Fig. 2A).
Fig. 2.
Effects of suppressing recurrent simulations of feared future episodes. (A) Participants were impaired at recalling features of their typical imaginations, as revealed by a worse recall rate for key detail words. (B) This impairment extrapolated to a lesser episodic specificity of subsequent free simulations, as indicated by a reduced proportion of internal (i.e., episodic) details included in the imaginations of the previously suppressed episodes. (C) Suppressing feared future events also led to a reduction in apprehensiveness. Error bars indicate the SE. We obtained no interaction with study.
Suppression Hinders People’s Ability to Imagine the Feared Future Event.
The foregoing finding indicates that suppression reduces the ability to access details that are typically part of participants’ imagination of the respective event. If details such as these are building blocks of episodic future simulations (18), then prior suppressions of a feared event should also hinder the ability to freely imagine it on later occasions.
To test this prediction, we asked participants to freshly imagine each feared episode out loud following the suppression phase. In this task, participants were encouraged to imagine the event in detail for 2 min, including whatever details they wished, and there was no requirement to remember or include any details previously listed. We scored these final free simulations with the Autobiographical Interview (52, 53), which quantifies the internal (i.e., episodic; directly relating to the event) versus external (i.e., nonepisodic information; e.g., general facts) details of the narrative (Table S1). The resulting proportion of internal details indicates the imagination’s episodic specificity. As predicted, episodic specificity was significantly lower for previously suppressed fears than it was for baseline episodes that were not suppressed, F(1, 38) = 6.77, P < 0.05 (Fig. 2B). However, one participant yielded a particularly strong reduction in episodic specificity (i.e., Baseline–Suppress more than 2.5 SDs above the study mean). To mitigate the influence of this bivariate outlier on the inferential statistics, we also compared the Baseline and Suppress conditions using a Wilcoxon signed-rank test that is not sensitive to the magnitude of the effect. This test corroborated the reduction in episodic specificity (z = 1.98, P < 0.05). Thus, participants’ ability to freshly imagine future happenings was hindered as a consequence of previous suppressions, compared with episodes that they had not at all been cued to think about in the interim.
Table S1.
Internal vs. external details
| Internal details | External details | ||||||
| Study | Mean ± SEM | Baseline | Imagine | Suppress | Baseline | Imagine | Suppress |
| 1 | Mean | 20.7 | 22.8 | 19.0 | 2.8 | 3.0 | 3.0 |
| SEM | 1.8 | 2.1 | 1.5 | 0.6 | 0.6 | 0.6 | |
| 2 | Mean | 23.3 | 25.1 | 23.7 | 2.3 | 2.7 | 2.9 |
| SEM | 2.5 | 2.9 | 2.7 | 0.4 | 0.7 | 0.5 | |
These results support the hypothesis that suppressing imagination of feared future events interrupts episodic simulation processes, inhibiting typical episodic details underlying the imagination of the event. This hypothesis may further predict a relationship between impaired recall of typical details and diminished episodic specificity. We tested for this relationship by normalizing the respective below-baseline reductions (i.e., Baseline–Suppress) within the two studies to then compute a robust correlation (54) across all participants. However, this correlation was not significant (r = –0.11, 95% confidence interval = [–0.47; 0.22]), suggesting that individual differences in the episodic-specificity effect were also based on factors other than just the diminished accessibility of the most typical details. In the next section, we tested whether suppression further reduced apprehensiveness toward future events.
Suppression Attenuates Apprehensiveness About the Future.
Evaluating our feelings toward possible future situations involves judgments that are likely influenced by information that is readily available at the time (39, 40). Impoverished imagery of a feared situation may thus lead to a less anxious outlook. To test this possibility, we asked participants to rate how apprehensive they felt toward the future events they provided and indeed observed that suppressing imagination for these feared future events attenuated apprehensiveness compared with Baseline, F(1, 37) = 6.58, P < 0.05 (Fig. 2C).
The Impact of Suppression on Apprehensiveness Predicts Trait Anxiety.
The observation that suppression can attenuate apprehensiveness about the future suggests that it may function as an effective coping mechanism for some individuals. However, given that anxious people experience intrusive fears (12) and are also significantly impaired at suppressing negative memories (46, 47), this raises the possibility that these individuals may be less efficient also at down-regulating their fears of the future by suppression.
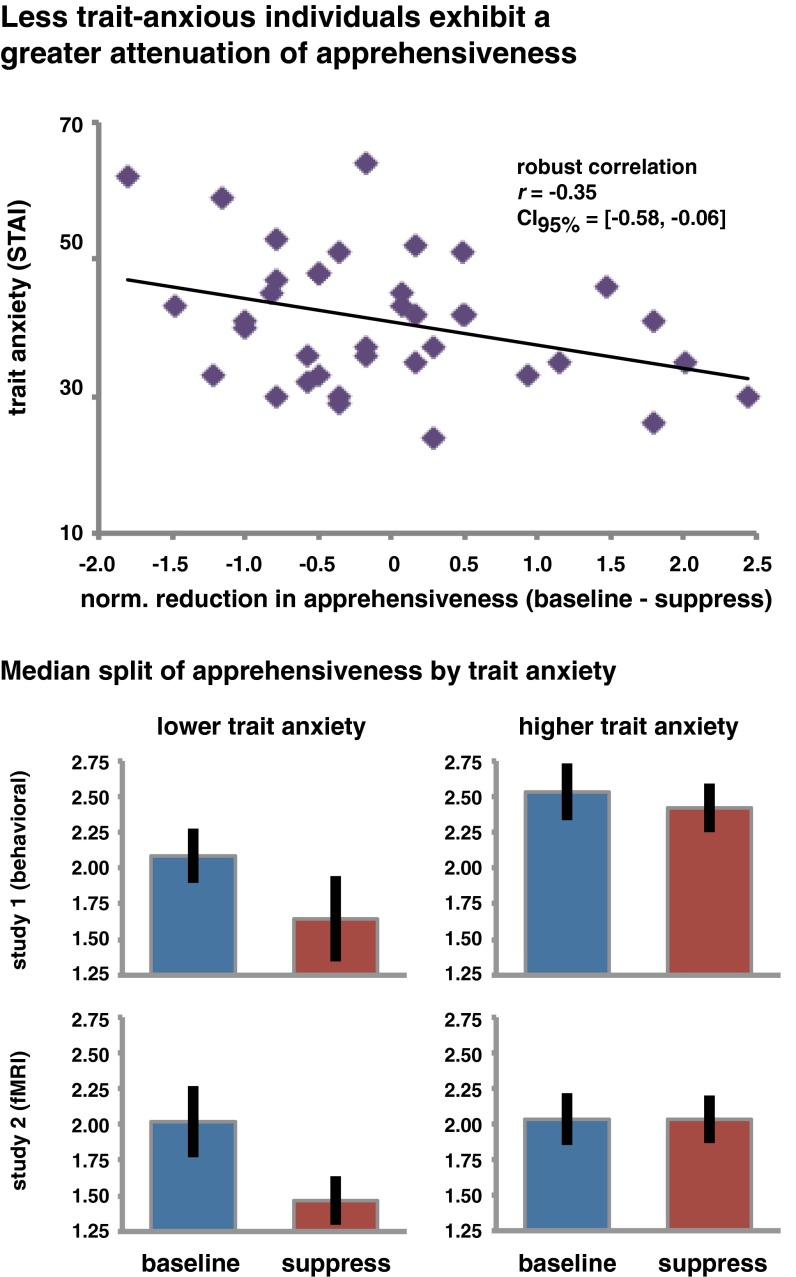
To test this possibility, we examined whether the observed effects of suppression on future apprehensiveness were related to individual differences in trait anxiety. Specifically, for each participant, we subtracted the apprehensiveness scores given for items of the Suppress condition from the Baseline condition, yielding values that express the efficiency in reducing feelings of fear about the future events. We normalized these values within each study to then compute a robust correlation (54) with trait anxiety (as measured by the State-Trait Anxiety Inventory Form Y, STAI-Y) across both experiments. (Of study 1, this analysis includes only the 18 participants who completed the STAI-Y.) The correlation between these scores and trait anxiety was significantly negative (r = –0.35, 95% confidence interval = [–0.58; –0.06]) (Fig. 3), supporting the hypothesis that more anxious people are less effective in reducing their recurring fears by suppression.
Fig. 3.
Individuals who were less trait anxious in everyday life were more successful in reducing their future apprehensiveness by suppression. (Upper) A robust correlation analysis revealed a negative relationship between trait anxiety and reduction in apprehensiveness (normalized within study). (Lower) Median splits illustrating the consistency of this relationship across studies. Note that we did not compute any inferential statistics on these splits. Error bars indicate the SE.
Do Additional Imaginings Change Already Established Fears?
Before turning to the complementary neural predictions, we explore whether additional imaginings further change the representations of established fear episodes. That is, in the Imagine condition, participants simulated episodes that they had already previously feared might happen to them. They were thus asked to imagine episodes that constituted recurrent fears. As such, we did not have strong predictions of whether additional simulations during the Imagine/No-Imagine phase would affect the dependent measures relative to Baseline.
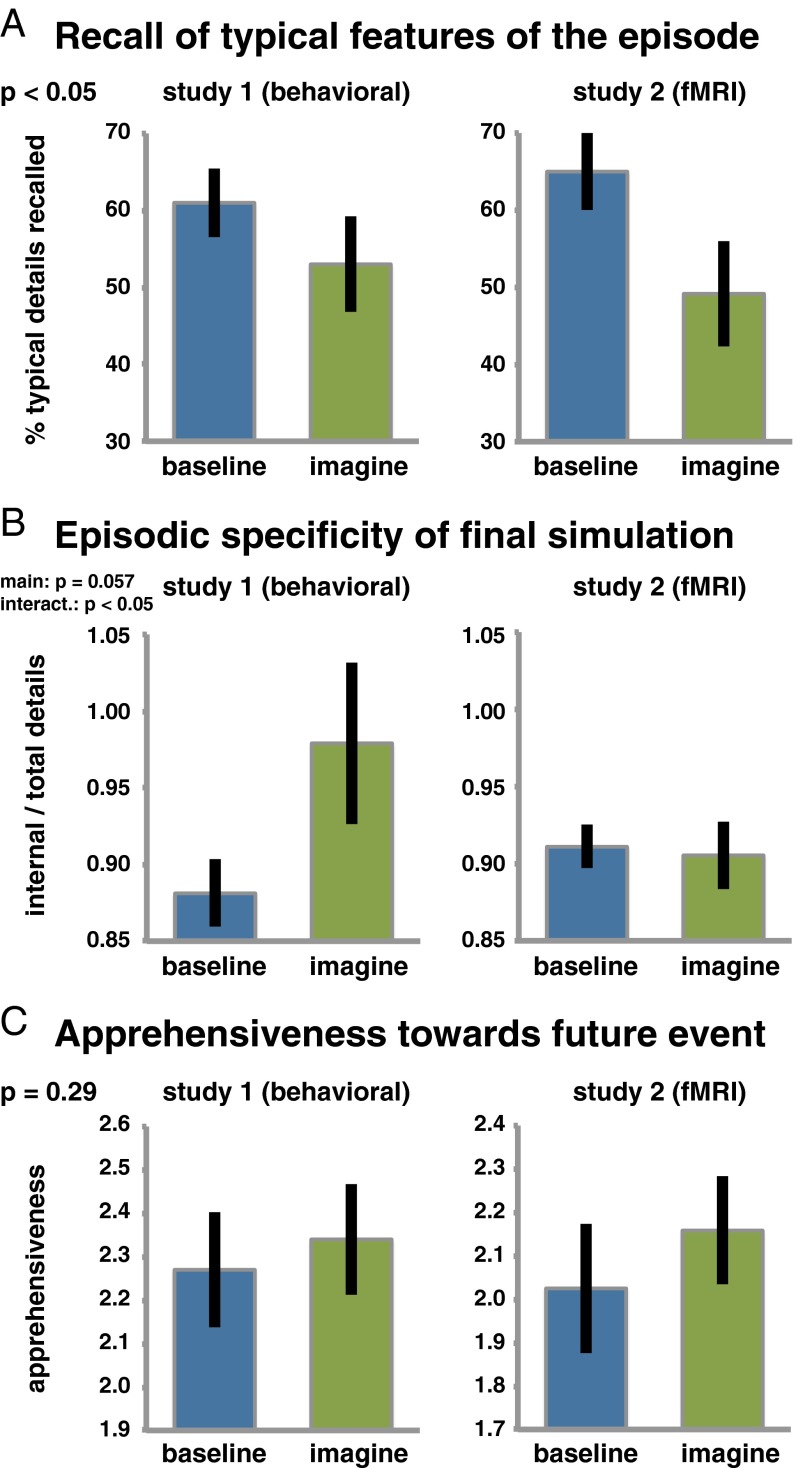
Indeed, the difference between Imagine and Baseline varied across dependent measures and was not as consistent across the two studies as for the comparison of Suppress and Baseline (Fig. 4). In the following, we examine each dependent variable in turn, reporting the respective results from an Imagination Status (imagine, baseline) × Study (study 1, study 2) ANOVA.
Fig. 4.
Effects of further simulating recurrent imaginings of feared future episodes. In contrast to suppression, additional simulations of already established fears did not have a consistent impact across all dependent measures [i.e., (A) detail recall, (B) episodic specificity, and (C) apprehensiveness]. Error bars indicate the SE.
The typical details yielded an unexpected pattern of greater recall in the Baseline than in the Imagine condition, F(1, 38) = 5.02, P < 0.05. However, unlike the recall impairment caused by suppression, this effect was not associated with neural markers of inhibitory top-down modulation (see dlPFC and the Down-Regulation of Hippocampal and vmPFC Activation and Discussion), suggesting that the effect was caused by a different mechanism.
Turning to the episodic specificity scores, these yielded a trend for the effect of imagination status, F(1, 38) = 3.84, P = 0.057, and the significant interaction of imagination status and study, F(1, 38) = 4.7, P < 0.05. Follow-up tests indicated that the Imagine condition exhibited a greater episodic specificity than Baseline in study 1 only, t(19) = –2.2, P < 0.05.
Finally, we analyzed changes in apprehensiveness and observed no significant effect, both Fs(1, 38) < 1.12, all Ps > 0.29. Thus, additional simulations during the Imagine/No-Imagine phase did not further increase participants’ fears of the future.
Future Suppression Engages Brain Systems Involved in Retrieval Suppression.
Suppressing imagination of feared future events diminishes the accessibility of episodic details in a manner akin to the inhibitory aftereffects caused by memory suppression (37). Study 2 tested the complementary hypothesis that suppressing recurring imaginings of the future is also supported by neural mechanisms similar to those engaged during the suppression of past events. Retrieval suppression is typically associated with modulation of hippocampal activity. We tested the further idea that suppressing recurring imaginings involves an additional modulation of vmPFC activation, consistent with this region’s putative contribution to the facilitation of typical details of simulated scenarios (25, 26).
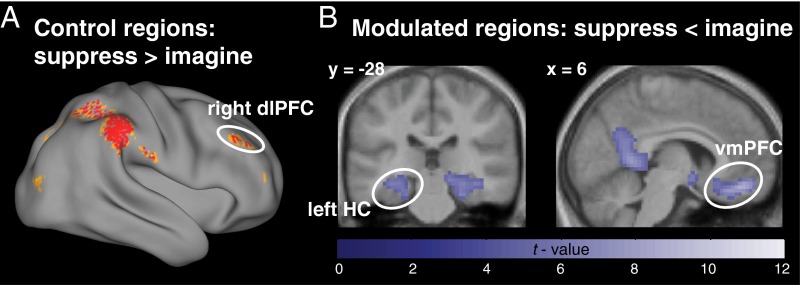
The findings strongly support these hypotheses. Comparing activation for Suppress versus Imagine trials confirmed that suppressing future imaginings does indeed recruit brain regions typically associated with memory suppression (28–34) (Fig. 5A). (For full exploratory results, see Tables S2 and S3.) Future suppression engaged the dlPFC, particularly a cluster within the right middle frontal gyrus. The peak of the cluster was in close proximity to the dlPFC peak previously associated with direct memory suppression (i.e., within a 10-mm distance) (28), suggesting that overlapping prefrontal regions exert inhibitory control over both episodic retrieval and simulation.
Fig. 5.
(A) Regions exhibiting greater activation during Suppress than Imagine trials included the right dlPFC, whereas (B) the hippocampi (with a maximum in the left hemisphere) and the vmPFC showed reduced activation during Suppress compared with Imagine trials. For display purposes, the images are thresholded at P < 1*10−4, uncorrected.
Table S2.
Suppress > Imagine
| MNI coordinates | ||||||
| Region | Hemisphere | Cluster size | t value | x | Y | Z |
| dlPFC | r | 16 | 8.56 | 39 | 38 | 40 |
| pPC | r | 127 | 8.60 | 51 | −34 | 46 |
| iOC | l | 36 | 8.87 | −39 | −67 | −5 |
| mOC | l | 65 | 10.96 | −33 | −85 | 13 |
dlPFC, dorsolateral prefrontal cortex; iOC, inferior occipital cortex; l, left; MNI, Montreal Neurological Institute; mOC, middle occipital cortex; pPC, posterior parietal cortex; r, right; thresholded at P < 0.05, whole-brain FWE-corrected, with at least 15 voxels.
Table S3.
Imagine > Suppress
| MNI coordinates | ||||||
| Region | Hemisphere | Cluster size | t value | x | Y | z |
| vmPFC | b | 128 | 8.71 | 6 | 41 | −17 |
| lTC | l | 18 | 7.99 | −57 | −13 | −17 |
| Prec/PCC | b | 404 | 12.92 | −12 | −58 | 19 |
| 8.99 | 18 | −52 | 19 | |||
| mOC/mTC | l | 102 | 10.57 | −39 | −82 | 31 |
| 7.78 | −42 | −64 | 19 | |||
| MTL/HC | l | 23* | 6.35 | −33 | −28 | −14 |
| MTL/HC | r | 18* | 5.72 | 33 | −28 | −14 |
| 5.29 | 27 | −19 | −14 | |||
b, bilateral; HC, hippocampus; iTC, inferior temporal cortex; l, left; MNI, Montreal Neurological Institute; mOC, middle occipital cortex; mTC, middle temporal cortex; MTL, medial temporal lobe; PCC, posterior cingulate cortex; Prec, precuneus; r, right; vmPFC, ventromedial prefrontal cortex; thresholded at P < 0.05, whole-brain FWE-corrected, with at least 15 voxels; *P < 0.05, small-volume FWE-corrected for the hippocampal volume.
To explore whether the engagement of right dlPFC varied with individual differences in trait anxiety, we extracted the corresponding contrast estimates from a spherical region-of-interest (ROI) (r = 6 mm), centered on the peak in this region. However, these estimates did not significantly correlate with STAI scores (robust correlation: r = 0.12, 95% confidence interval = [–0.35; 0.61]).
Critically, we did observe the complementary reduction of activation in the regions predicted to be targets of inhibitory control by the dlPFC (Fig. 5B): the vmPFC and, using small-volume correction, also bilateral hippocampal clusters with the strongest effect in the left hemisphere (left: XYZ, –33, –28, –14; z = 4.6; right: XYZ, 33, –28, –14; z = 4.31). (The cluster also extended into the broader medial temporal lobes and amygdala.)
The foregoing findings are consistent with the possibility that, as in studies of retrieval suppression, the dlPFC originates a top-down inhibitory control signal that modulates activation in medial temporal lobe regions, in this instance, to suppress processes necessary for prospective simulation. The results moreover suggest that inhibitory control of future events also affects vmPFC, consistent with the possibility that episodic simulation is stopped, in part, by modulating activity in this structure to suppress access to recurring details. From these patterns alone, however, it is not possible to conclude whether dlPFC dynamically interacts with these two regions in support of future suppression. To address this issue, the next section reports effective connectivity analyses testing the hypothesis that the dlPFC down-regulated activation in HC and vmPFC.
dlPFC and the Down-Regulation of Hippocampal and vmPFC Activation.
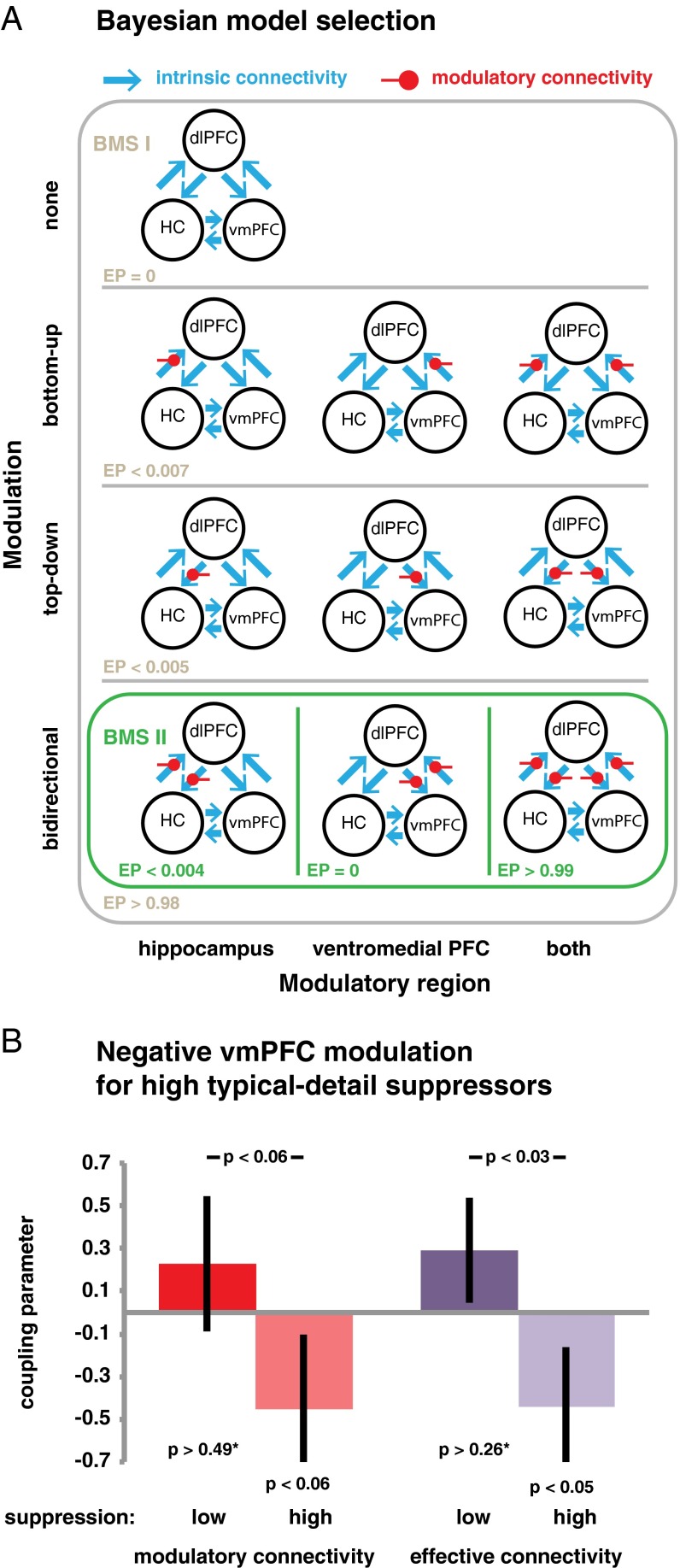
We used DCM to determine whether dlPFC modulated activity in the HC and vmPFC during suppression. This method explains regional activation in terms of changing patterns of connectivity during experimentally induced contextual modulations (50, 51). DCM requires the specification of a set of models that include the ROIs as well as connections between the regions. Different models can then be estimated that systematically vary key features of the model architecture, such as whether connections are invariant or whether they can be modulated during experimental conditions—for example, when participants attempt to suppress simulations of recurrent future fears. Using Bayesian Model Selection (BMS), it is then possible to compare the evidence for those models, which enables inferences about the presence and direction of modulatory connections.
We created three basic models that comprised the three nodes (dlPFC, vmPFC, and HC), bilateral intrinsic connections and inhibitory autoconnections. The basic models differed in the location of the driving input—that is, the activation of the network elicited by any reminder. Specifically, this input could enter the model either via the dlPFC, the vmPFC and HC, or all three nodes. We then configured four model families (each entailing all three basic models) that systematically varied the connections that could be modulated during suppression (similar to refs. 28, 29, 33) (Fig. 6A). Family I did not entail any modulatory component, whereas family II included bottom-up modulatory connections (i.e., from the putatively modulated regions to the dlPFC). These families are thus inconsistent with the hypothesized suppression mechanism. By comparison, family III did feature top-down modulatory connections (i.e., from dlPFC to the modulated regions), and family IV exhibited bidirectional modulatory connections. These families are thus consistent with a change in connectivity from the dlPFC to the modulated regions during suppression. Finally, within families II to IV, we further specified three versions of each model, which differed in the regions that exhibited modulatory connections with the dlPFC (i.e., the HC, vmPFC, or both). This approach allowed us to examine whether the putative modulation of the dlPFC was restricted to either the HC or the vmPFC, or extended to both.
Fig. 6.
(A) All estimated models included the presumed control node dlPFC and the putatively modulated nodes HC and vmPFC. BMS first yielded evidence in favor of those models that exhibited a change in connectivity during suppression for both top-down and bottom-up connections. Within this family, the models could account best for the data that included modulations of the connections between the dlPFC and both HC and vmPFC. (B) As predicted, the top-down influence on the vmPFC was more negative for those participants who also suppressed more typical features of the avoided episodes as assessed by the recall of those details. The average modulatory connectivity is shown in red, whereas the resulting effective connectivity (i.e., the sum of the intrinsic and modulatory components) is shown in purple. Error bars indicate the SE. An asterisk denotes two-tailed tests.
On the estimated models, we ran BMS in a random-effects approach (55), which reports the exceedance probability (EP) to which any of the families is more likely to have generated the data than the other included families. Echoing research on memory suppression, family IV was the clear winner, with an EP greater than 0.98. The winning model family thus entailed a modulation of the connection from the dlPFC to regions likely involved in episodic simulation. We then compared the models within that family and thus examined whether the dlPFC modulated the HC (as previously shown during suppression of simple verbal and visual memories) (28, 29, 33) or whether it also modulated the vmPFC. Critically, BMS deemed those models to be overwhelmingly superior that featured modulation of both HC and vmPFC targets rather than just a modulation of single sites (EP > 0.99). (Of these models, in turn, the one receiving driving input via all nodes was the clear winner; EP > 0.97.)
The best models thus shared a structure consistent with the hypothesized modulation of regions involved in recurrent simulations of future episodes. For the impact of the dlPFC to be inhibitory, top-down connections would be expected to be negative. We examined the respective connectivity parameters using Bayesian Model Averaging (BMA) of the winning models within family IV (i.e., the ones including modulations of HC and vmPFC). BMA computes weighted averages of the parameters, where the weighting is determined by the posterior probability of the models. We focus on two measures: the modulatory top-down connectivity, indicating the change in coupling during suppression, and the sum of the modulatory and intrinsic (i.e., average) connectivity—that is, the effective connectivity during suppression. Given the clear theoretical direction of our prediction, we used one-tailed statistical tests.
The modulatory connectivity to the HC was significantly negative, t(19) = –2.56, P < 0.01, which resulted in a trend for a negative effective connectivity, t(19) = –1.51, P = 0.07. Thus, we observed some evidence for inhibitory modulation of the HC during suppression.
By contrast, overall, the analogous coupling estimates for the vmPFC were numerically negative but were not significant: modulatory connectivity, t(19) = –0.52, P = 0.3; effective connectivity, t(19) = –0.42, P = 0.34. Importantly, we had hypothesized that down-regulating activation in this region would inhibit representations of typical, recurrent event details, impairing their subsequent accessibility. As such, we expected the top-down connectivity to be more negative for people who then found it harder to retrieve the typical details of suppressed episodes (cf. refs. 28 and 29). We therefore split our sample into stronger versus weaker suppressors, based on below-baseline forgetting of the typical details (i.e., Baseline–Suppress). The modulatory component showed a trend for a more negative modulation in the stronger suppressors, t(18) = 1.65, P = 0.058 (Fig. 6B). Critically, this difference in modulatory connectivity contributed to a significant group difference in effective connectivity, t(18) = 2.17, P < 0.05, which rendered the effective top-down modulation of vmPFC negative for the stronger suppressors, t(9) = –1.91, P < 0.05. By contrast, an analogous median split based on differences in recall between the Baseline and Imagine episodes did not yield any connectivity differences: modulatory connectivity, t(18) = 0.39, P = 0.71; effective connectivity, t(18) = 0.55, P = 0.59. Thus, the down-regulation of vmPFC activity during suppression was selectively associated with a subsequent impairment in recalling suppressed event details.
Discussion
Recurrently imagining dreaded future situations potentiates fears and can even support the development and maintenance of anxiety disorders (8, 11, 13). The reported studies tested the hypothesis that such simulations can be suppressed with the opposite effect of down-regulating apprehensiveness. Our data indicate that future suppression is based on a brain mechanism that is remarkably similar to a system implicated in the voluntary suppression of past experiences. This mechanism recruits right dlPFC, which originates an inhibitory signal that down-regulates activation in brain regions supporting both retrieval and episode-construction processes. Paralleling the suppression of recently acquired memories (28–34), the regions targeted by future suppression included the HC, a structure that is fundamental for the retrieval of past episodes and the construction of coherent future and fictitious events (20, 21).
Critically, the suppression of recurring fears of the future differs from suppressing past events in that it also involved modulating the vmPFC. The mPFC fosters the integration of overlapping memories into a common representation (56, 57), presumably by representing past experiences during new learning (57) and by interacting with distributed brain regions that code for the shared elements (26). Over time, the mPFC thus binds typical features of similar episodes into a schematic model of the respective scenario (23, 24, 58). The vmPFC may further integrate such models with associated affective information, thereby contributing to the emergence of personally relevant concepts (59, 60). On the one hand, these representations then foster the encoding and retrieval of information consistent with a given model (24). On the other hand, they support simulation by providing access to typical event details (25, 26).
As such, the vmPFC would particularly support recurrent imaginings by facilitating the retrieval of details typical of the feared situation. In turn, a targeted suppression of the vmPFC might weaken the representation of the feared situation and render its typical details less accessible. Supporting this prediction, effective connectivity analysis revealed inhibitory modulation of the vmPFC especially for those people who were particularly disrupted at recalling suppressed typical details. By contrast, we did not observe any relationship between vmPFC modulation and retrieval of typical details from repeatedly imagined events. Taken together, these results thus selectively link negative top-down modulation of vmPFC to future suppression. [At the same time, they suggest that the impairment in retrieving event details of Imagine episodes was caused by a different mechanism, such as retrieval interference (61; see SI Discussion).]
Moreover, suppression did not merely lead to an inability to recall typical features of past imaginings but also hindered the ability to subsequently simulate the episodes, however they came to mind. Thus, suppression may reduce access to the necessary building blocks of the imagined events, an effect that was not overcome even within the 2 min allowed for each of the final simulations. However, the absence of a significant correlation between diminished detail recall and hindered episodic simulation suggests that individual differences in the latter effect may be influenced by further factors such as baseline differences in narrative style (see ref. 62).
We had moreover hypothesized that hindered imagery of suppressed scenarios would also weaken participants’ feelings of fear about the future event. When probed about their feelings toward those possible future events, participants should thus report less apprehensiveness. This prediction was indeed supported by the data, suggesting that suppressing recurring future imaginings may constitute an effective coping mechanism for dealing with persistent fears for many people. Given that we assessed apprehensiveness via subjective ratings, one may be concerned whether this observation simply reflects a demand effect. For two reasons, we think that this interpretation is unlikely to account for the data. First, if participants tried to align their judgments with assumed researcher expectations, it seems likely that they also would have reported increased anxiety for imagined episodes in addition to decreased anxiety for suppressed episodes. Although there was a numerical difference, this was not reliably the case. Second, and critically, we not only obtained a main effect of reduced apprehensiveness but also observed that the magnitude of this reduction correlated with individual differences in trait anxiety. It seems difficult to explain why less anxious individuals would exhibit a greater demand effect.
Alternatively, reduced apprehensiveness may not arise from a specific inhibitory process but may have been caused by any task that requires an effortful engagement with the feared episode. We think that this interpretation is unlikely. First, in a test of the difficulty hypothesis, recent data have demonstrated that simply engaging in a highly difficult task (even if it closely mimics a suppression condition) does not lead to inhibitory effects typically caused by suppression (63). Second, if merely performing any effortful task reduces apprehensiveness, then one would expect the Imagine task to also cause such a reduction. That is, both suppressing and imagining should reduce apprehensiveness compared with Baseline. In contrast to this prediction, numerically, participants reported greater apprehensiveness for Imagine than Baseline episodes (although the direct comparison was not significant). Critically, a contrast analysis corroborated a linear trend across the three conditions, F(1, 38) = 6.58, P < 0.05, reflecting a rank order (Suppress < Baseline < Imagine) that is hard to reconcile with a high-engagement account.
The observed reduction in future apprehensiveness highlights the hypothesized adaptiveness of stopping recurrent simulations. By contrast, the opposite tendency to spontaneously dwell on past and future concerns may constitute a driving force of neuroticism (64) and thus increase the susceptibility for anxiety disorders (65). The mPFC, in particular, may mediate the contribution of recurrent imaginings to the development and maintenance of anxiety (cf., refs. 9–11). The repeated simulation of feared events, similar to the actual experience of overlapping episodes (23–34, 58), may build up a schematic representation of the dreaded scenario in the mPFC. (This process might have already established schemata of the participants’ recurrent fears before they entered the study.) Such a representation, in turn, would facilitate future simulations of the scenario, thereby enhancing accessibility of the event (10, 15) and increasing its perceived plausibility (14–16). It thus may be why the mPFC is particularly engaged when people worry more strongly about possible future events (66).
This is not to say, however, that representations of feared situations always promote anxiety. Indeed, vmPFC activation also increases during extinction and reversal learning—that is, in situations when new representations of previously aversive conditioned-stimuli are being established (67). These observations thus indicate that anxiety can be controlled not only by the suppression of fear representations but also by the acquisition of novel safety representations in the vmPFC (67, 68). Importantly, there is notable evidence for functional specialization within this region (see ref. 69), suggesting that it is particularly the posterior vmPFC that is involved in negative mood and anticipatory anxiety, whereas more anterior parts may support positive affect and fear extinction. Although parts of the cluster observed in the current study appear to cover the posterior vmPFC, direct comparisons are necessary to determine the extent to which the regions affected by future suppression colocalize with those involved in anticipatory anxiety or extinction.
In addition to having stronger schematic models of feared situations, anxious individuals may also be impaired at suppressing fear imagery. Evidence from Stroop (70), antisaccade (71), and flanker tasks (72) demonstrates an inhibitory deficit in anxiety, with individuals showing a stronger deficiency also experiencing more negative intrusive thoughts (72). Consistently, we observed that anxious individuals were worse at attenuating their fears by suppression, a finding that mirrors their impairment in suppressing negative memories (45, 47–49). Anxiety may thus be characterized by a diminished control over the contents of one’s awareness, a function in part mediated by processes controlling the retrieval of past happenings and the construction of future imaginings (see ref. 9).
The similarities between the suppression of the future and the past further suggest that the same core mechanism may not just be recruited for coping with emotionally negative episodes but could also help controlling any intrusive imagery, irrespective of valence. That is, in the memory domain, essentially the same neural network is engaged during the suppression of negative (31, 34) and neutral content (e.g., refs. 28–30). Similarly, suppression causes forgetting of not only negative (e.g., refs. 48, 49, 73–75) but also neutral memories (e.g., refs. 28–30), and in both cases, the efficiency of this process is inversely related to trait anxiety (46, 47) and rumination (76). More broadly, these findings echo the related literature on emotion regulation (see ref. 34), which indicates that some lateral prefrontal regions are engaged irrespective of the exact goal of the regulation effort (e.g., both up- and down-regulation) (e.g., refs. 77, 78).
Although a general inhibitory control process may suppress future imaginings of any valence, there may also be valence-specific effects akin to emotion regulation (79). A recent study, for example, showed that anxious individuals are generally impaired at suppressing unwanted memories of any valence but that this deficiency is particularly pronounced for negative material (47). The efficiency of the inhibitory control process may thus vary with the valence of the to-be-suppressed content. Moreover, even if the same inhibitory control process operates on both negative and positive imaginings, reducing the accessibility of the events’ respective typical details, it may cause opposite changes in people’s outlook toward the future: As suppressing negative details of dreaded situations can reduce apprehensiveness, suppressing positive details of welcome events may decrease pleasant anticipation (39, 40). The reported procedure allows prospective studies to determine the exact interactions between future suppression and valence.
However, we do not take the data to suggest that it is always beneficial to suppress feared imaginings. It may, for example, be maladaptive to merely replace concrete imagery with abstract worrying thoughts of a more verbal nature (80). From a construal-level perspective, focusing on more abstract features may shift participants’ outlook toward other high-level features of the event, such as its putative causes (81). This shift, in turn, has been shown to increase anxiety (82). Accordingly, we carefully instructed participants to suppress any pertinent image or thought (i.e., whether concrete or abstract) from coming to mind. Indeed, some established therapeutic interventions for anxiety disorders, including imaginal exposure and imagery rescripting, successfully reduce anxiety by fostering the mental engagement with the feared situation (for a review, see ref. 83). It thus can be adaptive to simulate anticipated events, particularly if they are deemed to be controllable (84–86). With the described procedure, it will be possible to assess the relative benefits of suppressing versus imagining and to determine how their efficacy varies with features of the dreaded situation and with participants’ abilities. Nevertheless, the present data do suggest that, on average, people without anxiety disorders may often benefit from suppressing fearful imaginings, perhaps accounting for why suppressing unwelcome fears is such a common coping response in the general population.
To conclude, the reported studies facilitate our understanding of a mechanism supporting suppression of recurring, intrusive fears. Our findings suggest that future suppression is achieved by an inhibitory top-down modulation originating from the dlPFC akin to memory suppression. In case of future suppression, the dlPFC also exerts control over the vmPFC, consistent with this region’s role in supporting particularly recurrent simulations, in addition to the HC. Suppression can have a beneficial impact on our outlook toward the future. The observation that suppression is less effective in anxious individuals suggests that deficits in this process may be involved in sustaining psychological disorders that are characterized by intrusive prospective thoughts (8, 9).
SI Discussion
Here, we discuss the comparison of the Imagine vs. Baseline conditions: the recall of the typical details yielded an unexpected pattern of greater recall in the Baseline than in the Imagine condition. However, unlike the recall impairment caused by suppression, this effect was not associated with inhibitory top-down modulation of the vmPFC, suggesting that the effect was caused by a different mechanism. A plausible candidate would be retrieval interference (61). That is, in the Imagine task, participants repeatedly simulated the same episodes as vividly as possible, which would have strengthened the representations of details that were central to these particular imaginings. Given that the details were typical but not the only central elements of the scenario, they may have featured variably across repeated simulations, given the unconstrained nature of those simulations. When participants then attempted to retrieve the details during the recall phase, their access may have sometimes been blocked by interference of other details that had been strengthened during the Imagine/No-Imagine phase. By comparison, there would be no such interference for the typical details of the Baseline condition.
Materials and Methods
Participants.
Twenty volunteers participated in each study (study 1—7 male; age: M = 23.5 y, range = 19–31; study 2—8 male; age: M = 26.1 y, range = 19–35). They were not color-blind and reported no history of psychiatric disorder. All participants of study 2 were also right-handed with no contraindication for MRI. (Three further participants did not complete the procedure.) All gave written informed consent as approved by the Cambridge Psychology Research Ethics Committee.
Procedure.
The Imagine/No-Imagine procedure entails (i) a generation phase, during which participants provided recurrent feared future episodes; (ii) the Imagine/No-Imagine phase, during which they repeatedly simulated some of those episodes while suppressing others; (iii) a detail-recall phase, during which they recalled typical features of each episode; (iv) a final simulation phase, during which they freely imagined each episode; and (v) an apprehension assessment phase.
During generation (stage 1 in Fig. 1), participants provided 20 (22 in study 2) episodes that they might experience in the future (see Fig. 1 for examples). Each of these had to be negative—that is, unpleasant or a cause to worry—and had to be possible to happen within the next 2 y (although it did not need to be likely to happen). The events had to be specific instances, lasting between a few minutes and a day, which the participants could imagine through their own eyes. Critically, participants only provided events that were of genuine concern to them and that were recurrent fears—that is, which they had already worried about at least once within the last year. Participants provided a short description of the event, which was used to verify compliance with those rules. For each event, they also provided a reminder word, which served as an obvious cue to the event (often a word from the episode’s description), and a typical detail. The typical detail referred to a key feature of the participants’ imagination that would remind them of the respective episode. However, it could not be a part of its description.
Participants rated each episode on the following 5-point scales: vividness of typical imagination, emotional intensity, likelihood of occurrence, distance in the future, and frequency of thought. We randomly assigned 15 episodes (18 in study 2) to three conditions (Imagine, Suppress, and Baseline) that were matched on these ratings (study 1: all |t| < 1.64, P > 0.11; study 2: all |t| < 1.02, P > 0.32).
Participants then practiced the Imagine/No-Imagine task (stage 2 in Fig. 1). Reminder words for the Imagine condition were presented in green, and participants simulated the corresponding episode as vividly as possible. Each reminder was presented multiple times, and they were instructed to keep elaborating on their previous simulations. Reminders for the Suppress condition were recurrently presented in red, and for these items, participants received instructions similar to those shown to engage a mechanism of retrieval suppression (27, 28): They briefly recognized the associated episode, before trying to block all thoughts or imaginings. If any thought entered their awareness, participants were instructed to push it out. Moreover, they were carefully instructed to not distract themselves by generating substituting thoughts but to remain focused on the reminder.
During the critical Imagine/No-Imagine phase, participants encountered each reminder from the Imagine and Suppress conditions 12 times across four blocks. We presented each reminder for 5 s in a pseudorandom order, ensuring that (i) any word was only repeated once all of the others had been shown and (ii) conditions alternated after a maximum of four trials. In study 1, the interstimulus interval (ISI) was 600 ms; in study 2, it was jittered between 1.5 and 7.5 s (average, 2.5 s; SD, 1.2 s) to optimize the efficiency of the event-related design as determined by optseq2 (https://surfer.nmr.mgh.harvard.edu/optseq/).
Afterward, we assessed participants’ memory for the typical details of their imaginations (stage 3 in Fig. 1). They saw each reminder for a maximum of 4 s (ISI, 400 ms) and tried recalling the detail associated with the cued episode. The reminders were presented in pseudorandom order, matching the conditions for average serial position.
Participants then freely simulated each episode one more time while describing each image and thought out loud for maximally 2 min or until they had come to a natural end (stage 4 in Fig. 1). Following each trial, they indicated whether they had imagined the event through their own eyes and rated it according to its vividness and emotional intensity (and, in study 1, on the other scales). Apart from a reduced vividness for Suppress, t(19) = 3.94, P < 0.002, and Baseline, t(19) = 2.87, P < 0.02, compared with Imagine items in study 2, there were no differences between conditions (all other |t| < 1.92, P > 0.07).
All simulations were audio-recorded and later rated with the Autobiographical Interview procedure (52), which has been adapted for the examination of future simulations (e.g., ref. 53). It quantifies the internal (i.e., episodic; directly relating to the event) versus external (i.e., nonepisodic information; e.g., general facts) details of the simulation (see Table S1), where the proportion of internal details indicates the imagination’s episodic specificity. Scoring was performed, blind to the conditions, by D.J.D. We established interrater reliability based on 60 episodes of each study, which were also scored blindly by an independent rater. The reliability was very good for internal (Cronbach’s α = 0.89) and good for external details (0.73).
Participants then indicated how apprehensive they felt toward each event on a 5-point scale (0, no anxiety; 4, very anxious) (stage 5 in Fig. 1). Finally, all (but two participants in study 1) completed the trait part of the STAI-Y.
fMRI Analyses.
Data acquisition and preprocessing.
Using a 3T Siemens TIM Trio MRI scanner with a 32-channel head coil, we acquired T2*-weighted echoplanar images [repetition time (TR), 2 s; echo time (TE), 30 ms; flip angle, 78°; field-of-view (FOV), 192 mm × 192 mm; 3 × 3 x 3 mm3 voxels; interslice gap: 25%; 32 slices obtained in descending order; 139 volumes for each run, including five dummy volumes] and a magnetization-prepared rapid gradient-echo (MPRAGE) structural image (TR, 2,250 ms; TE, 2.99 ms; flip angle, 9°; FOV, 256 mm × 240 mm × 192 mm; 1 mm3 isotropic voxels).
Preprocessing and univariate analyses used SPM8 (www.fil.ion.ucl.ac.uk/spm). The functional images were realigned, corrected for slice acquisition times, and coregistered with the structural image. This image was spatially normalized, and the resulting parameters served to normalize the functional images into 3 × 3 × 3 mm3 cubic voxels by fourth degree B-spine interpolation (using the Montreal Neurological Institute reference brain). The images were then smoothed by an isotropic 8 mm FWHM Gaussian kernel.
Regional activation.
fMRI data of each participant were analyzed with a general linear model (GLM) that decomposed the BOLD time series separately for each run. The Imagine and No-Imagine conditions were modeled by separate regressors that coded for the respective 5 s of each trial and that were convolved with the canonical hemodynamic response function. In addition, we included regressors representing the mean over scans and residual movement artifacts. We applied a 1/128 Hz high pass filter to model and data, before estimating the model parameters from the least-square fit. Contrast estimates for Imagine versus No-Imagine were entered into a one-sample t test at the second level. The resulting statistical maps were thresholded at P < 0.05, family-wise error (FWE) corrected for the whole brain. The predicted effects in the HC were tested using small-volume FWE correction for a mask of this structure (87).
Effective connectivity analyses.
Effective connectivity between the dlPFC, HC, and vmPFC was estimated using DCM (50, 51) as implemented in SPM12 (www.fil.ion.ucl.ac.uk/spm/software/spm12/). We extracted the eigenvariate of each region’s time course, adjusted for effects of interest, from a 6-mm radius sphere centered on subject-specific peaks. These were identified as the individual peaks located within a 10-mm radius sphere centered on the respective group peaks and, for the HC, also within the anatomical mask of this region. Model fitting was based on these data and was achieved by adjusting the parameters to maximize the free-energy estimate of the model evidence (50). Specifically, as detailed in Results, we estimated 30 models that were variants of the same basic model (dlPFC, HC, and vmPFC as nodes; within-region inhibitory autoconnections; bidirectional intrinsic connections between all regions). Across models, we varied (i) the direction of the connectivity that could be modulated during the 5 s of No-Imagine trials (i.e., none, bottom-up, top-down, bidirectional), (ii) the putatively modulated region (i.e., HC, vmPFC, both), and (iii) the location of the driving-input (HC and vmPFC, dlPFC, and all) (Fig. 6). We parsed the estimated models into families and ran BMS in a random-effects approach. BMS reports the EP—that is, the probability to which a given family of models is more likely than the other families to have generated the data from a random participant (55). Note that BMS penalizes for model complexity. We then used BMA (55) to assess the modulatory influence and the effective connectivity (i.e., the sum of the intrinsic and modulatory connectivity) of the winning models. BMA computes weighted averages of each model parameter, where the weighting is determined by each model’s posterior probability.
Acknowledgments
We thank Nuttida Rungratsameetaweemana for scoring simulations. This work was supported by Medical Research Council Grant MC-A060-5PR00. R.G.B. is funded by the Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.S. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606604114/-/DCSupplemental.
References
- 1.Klinger E, Cox WM. Dimensions of thought flow in everyday life. Imagin Cogn Pers. 1987;7:105–128. [Google Scholar]
- 2.Jason LA, Schade J, Furo L, Reichler A, Brickman C. Time orientation: Past, present, and future perceptions. Psychol Rep. 1989;64:1199–1205. [Google Scholar]
- 3.D’Argembeau A, Renaud O, Van der Linden M. Frequency, characteristics and functions of future-oriented thoughts in daily life. Appl Cogn Psychol. 2011;25:96–103. [Google Scholar]
- 4.Boyer P. Evolutionary economics of mental time travel? Trends Cogn Sci. 2008;12:219–224. doi: 10.1016/j.tics.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J Neurosci. 2011;31:6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demblon J, D’Argembeau A. Networks of prospective thoughts: The organisational role of emotion and its impact on well-being. Cogn Emotion. 2016;30(3):582–591. doi: 10.1080/02699931.2015.1015967. [DOI] [PubMed] [Google Scholar]
- 7.Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- 8.Holmes EA, et al. Mood stability versus mood instability in bipolar disorder: A possible role for emotional mental imagery. Behav Res Ther. 2011;49:707–713. doi: 10.1016/j.brat.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg R, Liebowitz M, Hope DA, Schneier FR, editors. Social Phobia: Diagnosis, Assessment and Treatment. Guilford; New York: 1995. pp. 69–93. [Google Scholar]
- 10.MacLeod AK, Byrne A. Anxiety, depression, and the anticipation of future positive and negative experiences. J Abnorm Psychol. 1996;105:286–289. doi: 10.1037//0021-843x.105.2.286. [DOI] [PubMed] [Google Scholar]
- 11.Miloyan B, Pachana NA, Suddendorf T. The future is here: A review of foresight systems in anxiety and depression. Cogn Emotion. 2014;28:795–810. doi: 10.1080/02699931.2013.863179. [DOI] [PubMed] [Google Scholar]
- 12.Morina N, Deeprose C, Pusowski C, Schmid M, Holmes EA. Prospective mental imagery in patients with major depressive disorder or anxiety disorders. J Anxiety Disord. 2011;25:1032–1037. doi: 10.1016/j.janxdis.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong QJ, Moulds ML. Impact of anticipatory processing versus distraction on multiple indices of anxiety in socially anxious individuals. Behav Res Ther. 2011;49:700–706. doi: 10.1016/j.brat.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Gregory WL, Cialdini RB, Carpenter KM. Self-relevant scenarios as mediators of likelihood estimates and compliance: Does imagining make it so? J Pers Soc Psychol. 1982;43:89–99. [Google Scholar]
- 15.Muse K, McManus F, Hackmann A, Williams M, Williams M. Intrusive imagery in severe health anxiety: Prevalence, nature and links with memories and maintenance cycles. Behav Res Ther. 2010;48(8):792–798. doi: 10.1016/j.brat.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu JQ, Szpunar KK, Godovich SA, Schacter DL, Hofmann SG. Episodic future thinking in generalized anxiety disorder. J Anxiety Disord. 2015;36:1–8. doi: 10.1016/j.janxdis.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benoit RG, Schacter DL. Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia. 2015;75:450–457. doi: 10.1016/j.neuropsychologia.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schacter DL, et al. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA. 2007;104(5):1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Race E, Keane MM, Verfaellie M. Medial temporal lobe damage cause deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci. 2011;31:10262–10269. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Squire LR, et al. Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci USA. 2010;107(44):19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlichting ML, Preston AR. Hippocampal-medial prefrontal circuit supports memory updating during learning and post-encoding rest. Neurobiol Learn Mem. 2015 doi: 10.1016/j.nlm.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Kesteren MT, Ruiter DJ, Fernández G, Henson RN. How schema and novelty augment memory formation. Trends Neurosci. 2012;35(4):211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Barron HC, Dolan RJ, Behrens TE. Online evaluation of novel choices by simultaneous representation of multiple memories. Nat Neurosci. 2013;16(10):1492–1498. doi: 10.1038/nn.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benoit RG, Szpunar KK, Schacter DL. Ventromedial prefrontal cortex supports affective future simulation by integrating distributed knowledge. Proc Natl Acad Sci USA. 2014;111(46):16550–16555. doi: 10.1073/pnas.1419274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergström ZM, de Fockert JW, Richardson-Klavehn A. ERP and behavioural evidence for direct suppression of unwanted memories. Neuroimage. 2009;48:726–737. doi: 10.1016/j.neuroimage.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 28.Benoit RG, Anderson MC. Opposing mechanisms support the voluntary forgetting of unwanted memories. Neuron. 2012;76:450–460. doi: 10.1016/j.neuron.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gagnepain P, Henson RN, Anderson MC. Suppressing unwanted memories reduces their unconscious influence via targeted cortical inhibition. Proc Natl Acad Sci USA. 2014;111:E1310–E1319. doi: 10.1073/pnas.1311468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson MC, et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- 31.Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317:215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- 32.Paz-Alonso PM, Bunge SA, Anderson MC, Ghetti S. Strength of coupling within a mnemonic control network differentiates those who can and cannot suppress memory retrieval. J Neurosci. 2013;33:5017–5026. doi: 10.1523/JNEUROSCI.3459-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benoit RG, Hulbert JC, Huddleston E, Anderson MC. Adaptive top-down suppression of hippocampal activity and the purging of intrusive memories from consciousness. J Cogn Neurosci. 2015;27(1):96–111. doi: 10.1162/jocn_a_00696. [DOI] [PubMed] [Google Scholar]
- 34.Depue BE, Orr JM, Smolker HR, Naaz F, Banich MT. The organization of right prefrontal networks reveals common mechanisms of inhibitory regulation across cognitive, emotional, and motor processes. Cereb Cortex. 2016;26(4):1634–1646. doi: 10.1093/cercor/bhu324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson MC, Bunce JG, Barbas H. Prefrontal–hippocampal pathways underlying inhibitory control over memory. Neurobiol Learn Mem. 2015 doi: 10.1016/j.nlm.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noreen S, Macleod MD. It’s all in the detail: Intentional forgetting of autobiographical memories using the autobiographical think/no-think task. J Exp Psychol Learn Mem Cogn. 2013;39:375–393. doi: 10.1037/a0028888. [DOI] [PubMed] [Google Scholar]
- 37.Anderson MC, Hanslmayr S. Neural mechanisms of motivated forgetting. Trends Cogn Sci. 2014;18:279–292. doi: 10.1016/j.tics.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410:366–369. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- 39.Tversky A, Kahneman D. Availability: A heuristic for judging frequency and probability. Cognit Psychol. 1973;5:207–232. [Google Scholar]
- 40.Gilbert DT, Wilson T. Prospection: Experiencing the future. Science. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- 41.Holmes EA, Geddes JR, Colom F, Goodwin GM. Mental imagery as an emotional amplifier: Application to bipolar disorder. Behav Res Ther. 2008;46(12):1251–1258. doi: 10.1016/j.brat.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 43.Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12(1):92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- 44.Forster S, Nunez Elizalde AO, Castle E, Bishop SJ. Unraveling the anxious mind: Anxiety, worry, and frontal engagement in sustained attention versus off-task processing. Cereb Cortex. 2015;25(3):609–618. doi: 10.1093/cercor/bht248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hertel PT, Gerstle M. Depressive deficits in forgetting. Psychol Sci. 2003;14:573–578. doi: 10.1046/j.0956-7976.2003.psci_1467.x. [DOI] [PubMed] [Google Scholar]
- 46.Waldhauser GT, Johansson M, Bäckström M, Mecklinger A. Trait anxiety, working memory capacity, and the effectiveness of memory suppression. Scand J Psychol. 2010;52(1):21–27. doi: 10.1111/j.1467-9450.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 47.Marzi T, Regina A, Righi S. Emotions shape memory suppression in trait anxiety. Front Psychol. 2014;4:1001. doi: 10.3389/fpsyg.2013.01001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Küpper CS, Benoit RG, Dalgleish T, Anderson MC. Direct suppression as a mechanism for controlling unpleasant memories in daily life. J Exp Psychol Gen. 2014;143(4):1443–1449. doi: 10.1037/a0036518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Catarino A, Küpper CS, Werner-Seidler A, Dalgleish T, Anderson MC. Failing to forget inhibitory-control deficits compromise memory suppression in posttraumatic stress disorder. Psychol Sci. 2015;26(5):604–616. doi: 10.1177/0956797615569889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friston KJ, Harrison L, Penny WD. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 51.Stephan KE, et al. Ten simple rules for dynamic causal modeling. Neuroimage. 2010;49:3099–3109. doi: 10.1016/j.neuroimage.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychol Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- 53.Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychol Sci. 2008;19:33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- 54.Pernet CR, Wilcox R, Rousselet GA. Robust correlation analyses: False positive and power validation using a new open source matlab toolbox. Front Psychol. 2013;3:606. doi: 10.3389/fpsyg.2012.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penny WD, et al. Comparing families of dynamic causal models. PLOS Comput Biol. 2010;6(3):e1000709. doi: 10.1371/journal.pcbi.1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75(1):168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richter FR, Chanales AJ, Kuhl BA. Predicting the integration of overlapping memories by decoding mnemonic processing states during learning. Neuroimage. 2016;124(Pt A):323–335. doi: 10.1016/j.neuroimage.2015.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewis PA, Durrant SJ. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn Sci. 2011;15(8):343–351. doi: 10.1016/j.tics.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: The valuation hypothesis. Front Hum Neurosci. 2013;7:372. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson MC, Neely J. Interference and inhibition in memory retrieval. In: Bjork EL, Bjork RA, editors. Memory. A Volume in the Handbook of Perception and Cognition. Academic; New York: 1996. pp. 237–313. [Google Scholar]
- 62.Gaesser B, Sacchetti DC, Addis DR, Schacter DL. Characterizing age related changes in remembering the past and imagining the future. Psychol Aging. 2011;26:80–84. doi: 10.1037/a0021054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hulbert JC, Henson RN, Anderson MC. Inducing amnesia through systemic suppression. Nat Commun. 2016;7:11003. doi: 10.1038/ncomms11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perkins AM, Arnone D, Smallwood J, Mobbs D. Thinking too much: Self-generated thought as the engine of neuroticism. Trends Cogn Sci. 2015;19(9):492–498. doi: 10.1016/j.tics.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Bienvenu OJ, et al. Anxiety and depressive disorders and the five-factor model of personality: A higher-and lower-order personality trait investigation in a community sample. Depress Anxiety. 2004;20:92–97. doi: 10.1002/da.20026. [DOI] [PubMed] [Google Scholar]
- 66.Servaas MN, Riese H, Ormel J, Aleman A. The neural correlates of worry in association with individual differences in neuroticism. Hum Brain Mapp. 2014;35(9):4303–4315. doi: 10.1002/hbm.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14(6):268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fullana MA, et al. Neural signatures of human fear conditioning: An updated and extended meta-analysis of fMRI studies. Mol Psychiatry. 2016;21(4):500–508. doi: 10.1038/mp.2015.88. [DOI] [PubMed] [Google Scholar]
- 69.Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: Implications for mood and anxiety disorders. Mol Psychiatry. 2012;17(2):132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fox E. Attentional bias in anxiety: Selective or not? Behav Res Ther. 1993;31:487–493. doi: 10.1016/0005-7967(93)90129-i. [DOI] [PubMed] [Google Scholar]
- 71.Derakshan N, Ansari TL, Hansard M, Shoker L, Eysenck MW. Anxiety, inhibition, efficiency, and effectiveness: An investigation using the antisaccade task. J Exp Psychol. 2009;56:48–55. doi: 10.1027/1618-3169.56.1.48. [DOI] [PubMed] [Google Scholar]
- 72.Fox E, Dutton K, Yates A, Georgiou GA, Mouchlianitis E. Attentional control and suppressing negative thought intrusions in pathological worry. Clin Psychol Sci. 2015;3(4):593–606. doi: 10.1177/2167702615575878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joorman J, Hertel PT, Brozovich F, Gotlib IH. Remembering the good, forgetting the bad: Intentional forgetting of emotional material in depression. J Abnorm Psychol. 2005;114(4):640–648. doi: 10.1037/0021-843X.114.4.640. [DOI] [PubMed] [Google Scholar]
- 74.Joormann J, Hertel PT, LeMoult J, Gotlib IH. Training forgetting of negative material in depression. J Abnorm Psychol. 2009;118(1):34–43. doi: 10.1037/a0013794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Depue BE, Banich MT, Curran T. Suppression of emotional and nonemotional content in memory: Effects of repetition on cognitive control. Psychol Sci. 2006;17(5):441–447. doi: 10.1111/j.1467-9280.2006.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fawcett JM, et al. The origins of repetitive thought in rumination: Separating cognitive style from deficits in inhibitory control over memory. J Behav Ther Exp Psychiatry. 2015;47:1–8. doi: 10.1016/j.jbtep.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ochsner KN, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 78.Buhle JT, et al. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24(11):2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19(5):776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- 80.Borkovec TD, Lyonfields JD, Wiser SL, Diehl L. An examination of image and thought processes in generalized anxiety. Behav Res Ther. 1993;31(3):321–324. doi: 10.1016/0005-7967(93)90031-o. [DOI] [PubMed] [Google Scholar]
- 81.Trope Y, Liberman N. Construal-level theory of psychological distance. Psychol Rev. 2010;117(2):440. doi: 10.1037/a0018963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doré B, Ort L, Braverman O, Ochsner K. Sadness shifts to anxiety with time and distance from national tragedy in Newtown CT. Psychol Sci. 2015;26(4):363–373. doi: 10.1177/0956797614562218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holmes EA, Mathews A. Mental imagery in emotion and emotional disorders. Clin Psychol Rev. 2010;30(3):349–362. doi: 10.1016/j.cpr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Rivkin ID, Taylor SE. The effects of mental simulation on coping with controllable stressful events. Pers Soc Psychol Bull. 1999;25(12):1451–1462. [Google Scholar]
- 85.Brown GP, MacLeod AK, Tata P, Goddard L. Worry and the simulation of future outcomes. Anxiety Stress Coping. 2002;15(1):1–17. [Google Scholar]
- 86.Jing HG, Madore KP, Schacter DL. Worrying about the future: An episodic specificity induction impacts problem solving, reappraisal, and well-being. J Exp Psychol Gen. 145(4):402–418. doi: 10.1037/xge0000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]