Significance
This work resonates with the intense, rapidly broadening interest in macrocycle synthesis, enabling the preparation of collections of size-congener macrocycles in a single reaction. More specifically, we have outlined the use of the Mitsunobu reaction to forge ester linkages between hydroxy acid monomers for depsipeptides in an oligomerization/macrocyclization mode. Additionally, we disclose that salt additives, which are normally contraindicated in a Mitsunobu reaction, are able to either enhance ring-size diversity (collections) or optimize the production of one macrocycle (selection) in an ion-templated fashion. Combination of this strategy and contemporary chemical methods allows rapid access to cyclodepsipeptide natural products and their unnatural isomers.
Keywords: Mitsunobu, total synthesis, collective, macrocycle, oligomerization
Abstract
Macrocyclic small molecules are attractive tools in the development of sensors, new materials, and therapeutics. Within early-stage drug discovery, they are increasingly sought for their potential to interact with broad surfaces of peptidic receptors rather than within their narrow folds and pockets. Cyclization of linear small molecule precursors is a straightforward strategy to constrain conformationally mobile motifs, but forging a macrocycle bond typically becomes more difficult at larger ring sizes. We report the development of a general approach to discrete collections of oligomeric macrocyclic depsipeptides using an oligomerization/macrocyclization process governed by a series of Mitsunobu reactions of hydroxy acid monomers. Ring sizes of 18, 24, 30, and 36 are formed in a single reaction from a didepsipeptide, whereas sizes of 24, 36, and 60 result from a tetradepsipeptide. The ring-size selectivity inherent to the approach can be modulated by salt additives that enhance the formation of specific ring sizes. Use of chemical synthesis to prepare the monomers suggests broad access to functionally and stereochemically diverse collections of natural product-like oligodepsipeptide macrocycles. Two cyclodepsipeptide natural products were prepared along with numerous unnatural oligomeric congeners to provide rapid access to discrete collections of complex macrocyclic small molecules from medium (18) to large (60) ring sizes.
Interest in the preparation and use of small molecules exhibiting macrocyclic rings is growing at a tremendous pace, driving the development of new approaches, new chemical reactions, and the underlying chemistry and tools (catalysts) to support them. Historically, studies of macrocyclization reactions have been guided by natural products bearing unusually large carbocycles or medium- and large-ring lactones, amides, and ethers. Fragmentation reactions of polycyclic molecules have long played a role in access to medium-sized macrocycles, whereas macrocyclizations to lactones and amides are often accomplished from linear precursors at high dilution. Insofar as these efforts were typically driven by the goal to prepare a specific macrocycle, often in the form relevant to a natural product synthesis or an engineered constraint to a peptide, absent from the field is an approach that provides rapid access to collections (1) of natural product-like macrocycles, particularly from stereochemically complex, functionally rich, linear precursors (2–6).
Pioneers in this area have recorded some success (Fig. 1). Esterification reactions of activated hydroxy acids have led predominantly to diolides, (7–10) and in a single case, oligolides (11). Amidation reactions of activated polypeptides were central to the preparations of macrocyclic peptides by Rothe and Kreiss (12, 13) and Wipf and coworkers (14, 15), but they were low yielding and size nonselective. Burke and coworkers (16, 17) have shown that the efficiency of an 18-membered oligolide macrocyclization can be enhanced by templation effects, but to the best of our knowledge, there is no evidence of the successful use of templation to control oligomerization/macrocyclization to produce collections of small molecules. The impact of such an approach would be further magnified by the ability to manipulate the relative amounts of ring-size congeners within collections or even bias the process toward a single cyclic oligomer.
Fig. 1.
(A) Combined inter-/intramolecular esterification or amidation approaches to prepare macrocycles and functional group synthesis options for depsipeptide synthesis for both (B) linear and (C) macrocycle construction.
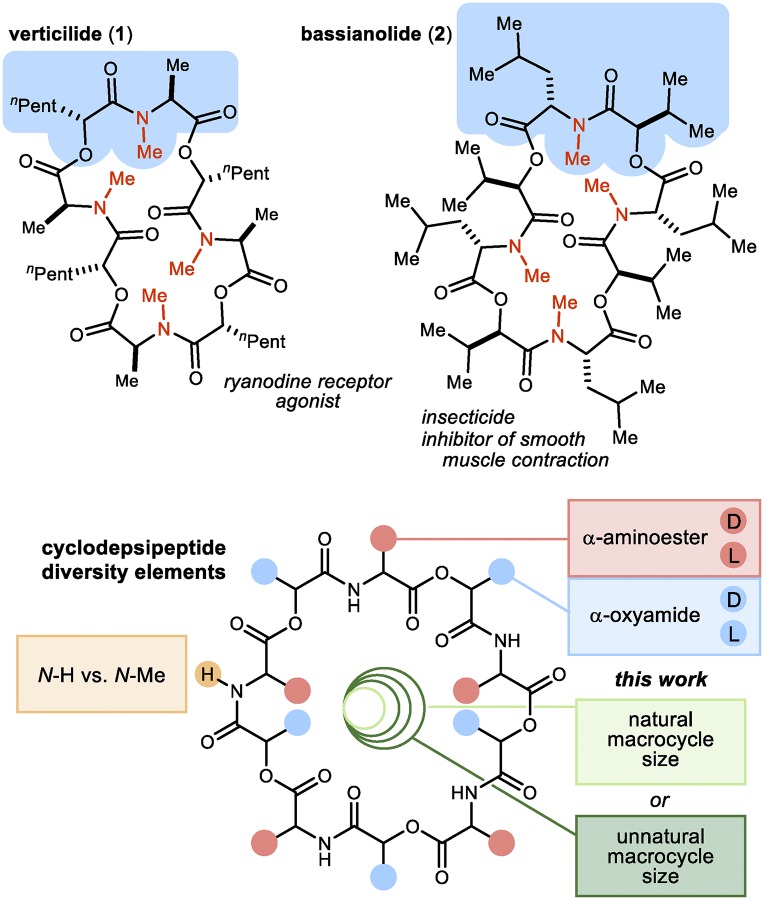
We sought an approach to small molecules that provides a platform for control over elements of (i) structural diversity, (ii) stereochemical diversity, and (iii) macrocycle size distribution sampled across a diverse size range. To achieve all three of these goals without compromising synthetic efficiency or relevance to complex natural products, we were attracted to cyclodepsipeptides, in which biological activity is quite diverse (18–23). Herein, we report rapid access to size-diverse collections of cyclooligodepsipeptides using a controlled oligomerization/macrocyclization sequence. A strategic combination of contemporary amide and ester preparations provides a platform for control of structural and stereochemical diversity elements, and modulation of macrocycle size distribution is possible through the use of salt additives as cyclization templates. A six-step synthesis of the natural product (−)-verticilide (1), a 24-membered macrocyclic oligodepsipeptide, in addition to its analogs ranging from 18- to 60-ring atoms displays the efficiency and simplicity with which collections of size congeners can be prepared. This approach allows access to macrocycle size congeners within cyclodepsipeptide natural product-like collections to a degree not achieved in the pioneering work with macrolides and peptidic libraries developed using dimerization and oligomerization techniques (7–17).
Results and Discussion
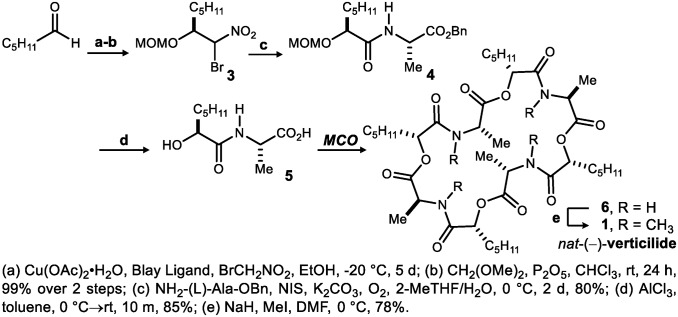
Verticilide (1) (Fig. 2) is a 24-membered cyclodepsipeptide isolated by Omura and coworkers in 2006 (24, 25). Its ability to selectively inhibit insect RyR led to a 14-step synthesis (24) from the (R)-oxazolidone chiral auxiliary and N-Me-N-Boc alanine. Construction of the acyclic oligomer used PyBrop-mediated N-Me amide synthesis, and the final step used PyBop-mediated macrolactamization at low concentration (0.005 M) to form the remaining N-Me amide (Fig. 1C, amidation). An esterification approach is feasible in theory (26–29), but a drawback of esterification by nucleophilic acyl substitution is the diminished reactivity inherent to alcohol nucleophiles, especially when secondary hindered alcohols are prescribed (30) (Fig. 1C, esterification I). We instead turned to a Mitsunobu-based esterification to form the C(sp3)-O ester bond (Fig. 1C, esterification II) (31, 32) and a combination of the enantioselective Henry reaction and Umpolung Amide Synthesis (UmAS) (33) to prepare hydroxy acid didepsipeptide 5 (Scheme 1). Blay’s ligand (34), conveniently synthesized in two steps from (+)-camphor, was found to provide optimal levels of enantioselectivity and yield for the copper(II) acetate-catalyzed Henry addition (35) of bromonitromethane to hexanal. Protection of the β-hydroxy-α-bromonitroalkane as its methoxymethylene (MOM) ether (3) was followed by UmAS with l-alanine benzyl ester and deprotection to hydroxy acid 5 (AlCl3; toluene). The procedure could be performed on gram scale in 67% overall yield from hexanal.
Fig. 2.
Representative macrocyclic oligomeric depsipeptide natural products and diagram summarizing cyclooligomeric depsipeptide diversity elements.
Scheme 1.
Total chemical synthesis of (natural) (−)-verticilide using MCO.
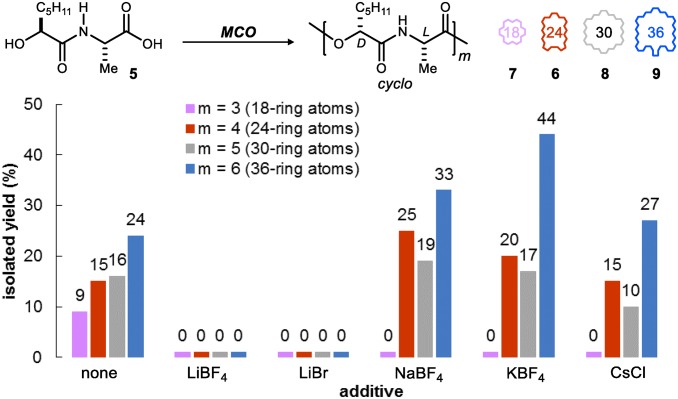
A 20 mM solution—intentionally not high dilution—of hydroxy acid 5 in benzene was subjected to typical Mitsunobu conditions [5 equivalents (equiv) diisopropyl azodicarboxylate (DIAD) and 6 equiv triphenyl phosphine (PPh3)] at room temperature to explore a macrocyclooligomerization (MCO) approach to verticilide. Careful analysis and purification of the reaction mixture (SI Appendix discusses initial findings in more detail) identified four major products, identified as cyclodepsipeptide oligomers ranging in yield from 9 to 24%. NMR analysis of these cyclooligomers revealed very similar spectra (SI Appendix discusses stacked NMR spectra comparison), differing primarily in the chemical shift rather than coupling pattern of each proton and reinforcing the expectation that small collections of constitutionally identical but size-distinct molecules could be prepared and purified with relative ease. The major constituent was determined to be the 36-membered congener formed in 24% yield, whereas the 24-membered macrocycle was formed in 15% yield. The 24-membered oligomer (6) was readily converted to natural (−)-verticilide (1) by straightforward amide permethylation (Scheme 1). The natural product exists as a mixture of averaged conformational isomers in solution (1H NMR), but the N-H precursor displays a single averaged conformer.
Although this route constituted a synthesis of verticilide in six steps with 8% overall yield, an improved yield of verticilide by MCO was sought based on the hypothesis that the growing oligomers might bind to salt additives (16, 17, 36–41), which in turn, might lead to depsipeptide–salt complexes with a conformationally enhanced rate of macrocyclization relative to chain elongation. Known applications of this phenomenon to depsipeptide macrocycle synthesis are macrolactamization (38, 39) reactions, which are conducted in a polar solvent that can readily solubilize the salt additive. However, the use of polar solvents and salt additives is contraindicated in a Mitsunobu reaction because of their ability to disrupt the reactive ion pairs (42–44), potentially slowing or stopping the coupling reaction entirely. Therefore, the unprecedented solubility and reactivity that we observed with the addition of various alkali metal salts (2.5 equiv) to the reaction conditions is hypothesized to be caused by an ion-templating effect between the linear depsipeptide chain and the metal ions in solution.
Fig. 3 summarizes the effect of a subset of alkali metal salts examined, with attention to those reported to bind peptides and depsipeptides in solution (45–50). Weakly coordinating counteranions were chosen for their respective alkali metal cation to promote solubility of the salt additive and interaction of the metal cation with the growing depsipeptide chain. Bromide, chloride, and tetrafluoroborate anions were chosen in particular based on their likelihood to remain inert in the reaction conditions, unlike other noncoordinating counterions (PF6− and ClO4−), which are known to form stable and insoluble complexes with arylphosphonium cations (51). Compared with the standard conditions, use of LiBF4 and LiBr additives arrested the production of any cyclodepsipeptide products. Lithium salts are known chaotropes, and therefore, it is not surprising that they interacted with the ionic Mitsunobu intermediates at such a level that no reactivity was observed. However, a shift in the size distribution of macrocyclic products was observed in the presence of NaBF4, KBF4, and CsCl. In the presence of NaBF, KBF4, or CsCl, production of the 18-membered ring was completely suppressed. These three salts also provided increased amounts of 24-membered ring relative to 30-membered ring compared with providing them in nearly equal amounts without a salt additive. This effect was most significant with NaBF4, which provided the 24-membered ring in 25% yield as opposed to only 15% without the salt. Ring-size enhancement in the presence of a salt additive was most significant for the 36-membered ring, which was retrieved in 44% isolated yield with KBF4, nearly double the yield of salt-free conditions. Additionally, KBF4 increased the overall isolated yield of macrocycles from 64 to 81% along with evidence that less material was converted into linear DIAD substrate acylation by-products. This type of by-product is common (52–54) in Mitsunobu reactions, especially in the presence of excess coupling reagents, which are generally required for a macrolactonization to occur. However, an ion template would allow the linear chain to reside in a conformation that increases the rate of cyclization relative to acylation, and the latter would be inherently slower as the linear chain increases in size. This observation supports the hypothesis that enhanced ring-size selection and overall production of macrocycles are occurring through an ion-templating effect. More notably, these results document the unprecedented ability of alkali metal salts to enhance formation of macrocycles in Mitsunobu MCO conditions.
Fig. 3.
Effect of salts on the production of cyclooligomers using a Mitsunobu-based oligomerization/cyclization approach and didepsipeptide 5.
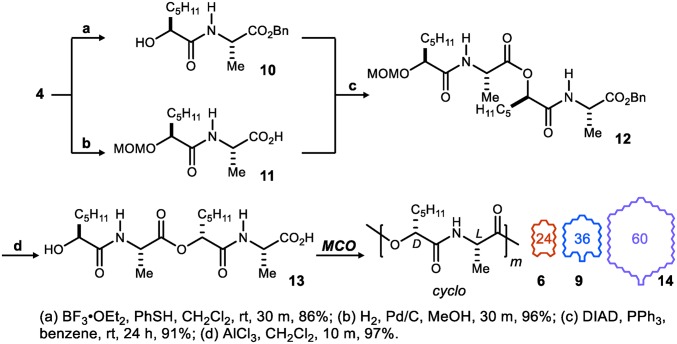
Control over cyclodepsipeptide size (Fig. 2) can also be modulated through judicious choice of monomer length. Although didepsipeptide 5 provides access to oligomers that are multiples of 6-atom lengths, tetradepsipeptide 13 limits the possibilities to multiples of 12. Tetradepsipeptide 13 was prepared using a similar approach, requiring only selective deprotection of the MOM ether to 10 and the benzyl ester to produce 11 (Scheme 2). Mitsunobu coupling of these units followed by deprotection to the hydroxy acid provided 13 in 60% yield over six steps. Use of a similar oligomerization protocol led to a very different profile (SI Appendix discusses reaction profile in more detail) of macrocyclic oligomers (Fig. 4). Additive-free conditions led to three major products of two, three, and five units in size, corresponding to 24- (6), 36- (9), and 60-membered (14) rings. The 24-membered ring 6 was formed in 63% yield, whereas 36- and 60-membered cyclooligomers were formed in 10% and 5% yield, respectively. This result was particularly notable, because previously reported cyclooligomerizations of large monomers faced significant challenges with direct monomer cyclization and low overall conversion to macrocyclic products (11, 13). Because of the inherent propensity of 13 to cyclodimerize, we wondered if salt effects could make formation of the 24-membered (6) precursor to verticilide selective. Our previous experiments with didepsipeptide monomer 5 showed that this was possible to some extent when the yield of the 36-membered ring was doubled in the presence of KBF4.
Scheme 2.
Synthesis of tetradepsipeptide 13 for Mitsunobu-based MCOs.
Fig. 4.
Mitsunobu-based MCO of d/l-macrocycle progenitor tetradepsipeptide 13: effect of salt additives on yield of 24-, 36-, and 60-membered ring formation.
A similar exploration of salt effects was pursued as shown in Fig. 4, but this time, the chloride versions of the tetrafluoroborate salts were tested to further examine counteranion effects. NaCl, KCl, and CsCl did not increase 24-membered ring formation. However, with increasing metal cation size, the amounts of 36- and 60-membered rings were increased. Most notably, CsCl tripled the yield of the 60-membered ring, and nearly 40% of total isolated macrocycles were larger than 24-ring atoms—results much different from salt-free conditions, where only 20% of total isolated macrocycles were greater than 24-ring atoms. As in the previous study, the most drastic templating effects were observed with tetrafluoroborate salts. Sodium tetrafluoroborate, which produced the most 24-membered ring out of all of the salt MCO conditions with monomer 5, dramatically enhanced the formation of the 24-membered macrocycle (89% yield) and completely suppressed formation of other ring sizes with monomer 13. Collectively, these additive effects provide a range of control that either (i) enhances diversity of oligomer size in a single reaction or (ii) enhances the formation of one macrocycle size relative to others. Contrasting approaches, the shorter preparation of verticilide results in several cyclooligomers while leveraging the didepsipeptide; use of tetradepsipeptide 13 and a salt effect that favors dimerization/macrocyclization to 6 is only slightly longer (eight steps; 36% yield).
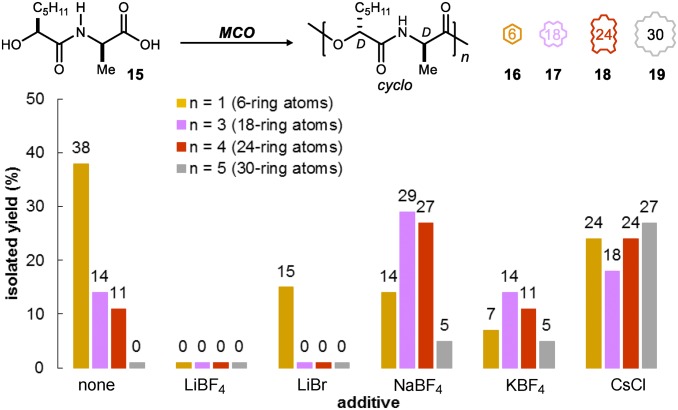
These studies delineated the key variables that might provide controlled access to size complexity in a short step count. Insofar as enantioselective catalysis is the basis for didepsipeptide synthesis here, a broad range of precursors can be prepared. Among these precursors is the d-Ala epimer of 5, because it is the progenitor to the d,d-cyclodepsipeptides. Monomer 15 was, therefore, prepared, and its behavior was compared with that of 5 to probe the extent to which this configuration determined the array of selectivity observed in Fig. 3. In the event, the behaviors generally paralleled those observed using the d,l-progenitor (5) (compare Fig. 3 with Fig. 5), but some differences in the cyclization of diastereomers 5 and 15 were apparent. Direct cyclization to the six-membered diketomorpholine product (38% yield) was dominant in the additive-free MCO. In general, the smaller ring sizes were most favored, forming the 18-membered macrocycle in 14% yield and the 24-membered macrocycle in 11% yield. Added salts again modified both size distribution and efficiency of MCO. Sodium tetrafluoroborate clearly enhanced the production of the higher congeners at the expense of direct cyclization to the six-membered ring. The effect of cesium chloride was also notable, because it produced a rather even distribution of four ring sizes, including a 30-membered congener that was not observed in salt-free conditions, in a combined isolated chemical yield of 93%.
Fig. 5.
Mitsunobu-based MCO of 15: effect of salt additives on yield of 6-, 18-, 24-, and 30-membered ring formation.
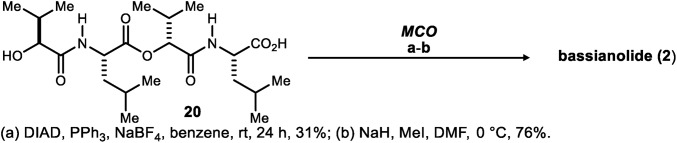
The extension of this approach (Scheme 3) to a synthesis of bassianolide (55, 56) (2) was also pursued as a means to estimate the level of generality that might be inherent to the approach and methods. Tetradepsipeptide 20 was prepared in a manner analogous to 13 and then, subjected to Mitsunobu conditions [DIAD (2 equiv), PPh3 (3 equiv), benzene (5 mM)]. These salt-free conditions led to N-H bassianolide but in only 11% yield. In their synthesis of N-H bassianolide, Suzuki and coworkers attributed the low yield (2.8%) of their macrolactamization (PCl5 and Et3N) to 310-helical intramolecular hydrogen bonding (57, 58) in their amino acid octadepsipeptide precursor (59). As a substrate for Mitsunobu esterification, 20 is a more hindered alcohol than 13. Notwithstanding this challenge, the macrocyclodimerization of 20 was improved to 31% yield by adding NaBF4 (5 equiv) (see SI Appendix for more details). Permethylation resulted in bassianolide in 9% overall yield, with an eight-step longest linear sequence.
Scheme 3.
Synthesis of bassianolide (2).
Conclusion
In summary, a combination of enantioselective α-oxy amide synthesis and Mitsunobu-based depsipeptide synthesis provides rapid access to macrocyclic depsipeptides. Straightforward modifications of the Mitsunobu reaction through the use of salt additives can enhance the formation of size-diverse collections of macrocycles or selectivity for a single macrocycle size. A concise synthesis of the natural product verticilide grounds the work in a biologically active natural product, while providing equally straightforward access to its enantiomer and macrocyclic oligomers not yet identified in nature. Finally, bassianolide was prepared in eight steps to establish the generality of approach while highlighting the advantage of a Mitsunobu reaction-based synthesis. Attempts to develop cyclooligomerization have historically suffered from direct cyclization (12–14, 56), favoring medium ring sizes (e.g., 12-membered depsipeptide) and competitive epimerization (15, 60) during amide formation. The Mitsunobu substitution strategy used here provides the possibility for either the synthesis of collections of oligodepsipeptide macrocycle size congeners or the synthesis of a select ring size in high yield as well as the ability to frame-shift macrocycle size distribution by the judicious choice of monomer size (didepsipeptide vs. tetradepsipeptide). Moreover, the hybridization of enantioselective methods and UmAS provides broad access to α-oxyamide side chains without risk of epimerization during amide synthesis. The additive effects described here illustrate the potential for achieving orthogonal aims of diversity and selectivity in separate experiments and evidence of reagent control over oligomerization/cyclization. Future studies will explore this tool further, perhaps enabling higher levels of selectivity favoring large macrocycles. In this vein, the existence of large macrocyclic natural products is notable (61, 62) as is the dearth of methods that provide rapid access to this size regime.
Supplementary Information
Procedures, complete experimental details, and analytical data for all reported compounds are provided. The supplementary data associated with this article can be found in SI Appendix.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by National Institute of General Medical Sciences Grant NIH GM 063557.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616462114/-/DCSupplemental.
References
- 1.Jones SB, Simmons B, Mastracchio A, MacMillan DWC. Collective synthesis of natural products by means of organocascade catalysis. Nature. 2011;475(7355):183–188. doi: 10.1038/nature10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodward RB, et al. Asymmetric total synthesis of erythromycin. 2. Synthesis of an erythronolide A lactone system. J Am Chem Soc. 1981;103(11):3213–3215. [Google Scholar]
- 3.Paterson I, Laffan DDP, Rawson DJ. Studies in macrolide synthesis: A concise asymmetric synthesis of a macrolide intermediate for the erythronolides. Tetrahedron Lett. 1988;29(12):1461–1464. [Google Scholar]
- 4.Evans DA, Dinsmore CJ, Evrard DA, DeVries KM. Oxidative coupling of arylglycine-containing peptides. A biomimetic approach to the synthesis of the macrocyclic actinoidinic-containing vancomycin subunit. J Am Chem Soc. 1993;115(14):6426–6427. [Google Scholar]
- 5.Boger DL, Nomoto Y, Teegarden BR. Vancomycin and ristocetin models: Synthesis via the Ullmann macrocyclization reaction. J Org Chem. 1993;58(6):1425–1433. [Google Scholar]
- 6.Clark JS, Xu C. Total synthesis of (-)-nakadomarin A. Angew Chem Int Ed Engl. 2016;55(13):4332–4335. doi: 10.1002/anie.201600990. [DOI] [PubMed] [Google Scholar]
- 7.Su Q, Beeler AB, Lobkovsky E, Porco JA, Jr, Panek JS. Stereochemical diversity through cyclodimerization: Synthesis of polyketide-like macrodiolides. Org Lett. 2003;5(12):2149–2152. doi: 10.1021/ol034608z. [DOI] [PubMed] [Google Scholar]
- 8.Beeler AB, et al. Synthesis of a library of complex macrodiolides employing cyclodimerization of hydroxy esters. J Comb Chem. 2005;7(5):673–681. doi: 10.1021/cc050064b. [DOI] [PubMed] [Google Scholar]
- 9.Mulzer J, Berger M. Total synthesis of the boron-containing ion carrier antibiotic macrodiolide tartrolon B. J Org Chem. 2004;69(3):891–898. doi: 10.1021/jo035391p. [DOI] [PubMed] [Google Scholar]
- 10.Barth R, Mulzer J. Total synthesis of efomycine M. Angew Chem Int Ed Engl. 2007;46(30):5791–5794. doi: 10.1002/anie.200701065. [DOI] [PubMed] [Google Scholar]
- 11.Burke SD, Heap CR, Porter WJ, Song Y. Cyclic hydropyran oligolides as preorganized ligand arrays: Modular assembly of 18-, 24-, 36-, 48-, 54- and 72-membered rings via iteration and cyclooligomerization. Tetrahedron Lett. 1996;37(3):343–346. [Google Scholar]
- 12.Rothe M, Kreiss W. Synthesis of valinomycin by the phosphite method. Angew Chem Int Ed Engl. 1973;12(12):1012–1013. doi: 10.1002/anie.197310121. [DOI] [PubMed] [Google Scholar]
- 13.Rothe M, Kreiss W. Synthesis of cyclodepsipeptides by cyclooligomerization. Angew Chem Int Ed Engl. 1977;16(2):113–114. [Google Scholar]
- 14.Wipf P, Miller CP, Grant CM. Synthesis of cyclopeptide alkaloids by cyclooligomerization of dipeptidyl oxazolines. Tetrahedron. 2000;56(46):9143–9150. [Google Scholar]
- 15.Wipf P, Miller CP. Total synthesis of westiellamide. J Am Chem Soc. 1992;114(27):10975–10977. [Google Scholar]
- 16.Burke SD, O’Donnell CJ, Porter WJ, Song Y. Cyclic hydropyran oligolides as preorganized ligand arrays: Cumulative effects of structural elements on shape and cation binding. J Am Chem Soc. 1995;117(50):12649–12650. [Google Scholar]
- 17.Burke SD, McDermott TS, O’Donnell CJ. Template macrolactonization of trichloroethyl ester derivatives catalyzed by potassium salts. J Org Chem. 1998;63(8):2715–2718. doi: 10.1021/jo970942v. [DOI] [PubMed] [Google Scholar]
- 18.Sivanathan S, Scherkenbeck J. Cyclodepsipeptides: A rich source of biologically active compounds for drug research. Molecules. 2014;19(8):12368–12420. doi: 10.3390/molecules190812368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen CM, Clausen MH. Biologically active macrocyclic compounds – from natural products to diversity-oriented synthesis. European J Org Chem. 2011;2011(17):3107–3115. [Google Scholar]
- 20.Marsault E, Peterson ML. Macrocycles are great cycles: Applications, opportunities, and challenges of synthetic macrocycles in drug discovery. J Med Chem. 2011;54(7):1961–2004. doi: 10.1021/jm1012374. [DOI] [PubMed] [Google Scholar]
- 21.Süssmuth R, Müller J, von Döhren H, Molnár I. Fungal cyclooligomer depsipeptides: From classical biochemistry to combinatorial biosynthesis. Nat Prod Rep. 2011;28(1):99–124. doi: 10.1039/c001463j. [DOI] [PubMed] [Google Scholar]
- 22.Gao M, Cheng K, Yin H. Targeting protein-protein interfaces using macrocyclic peptides. Biopolymers. 2015;104(4):310–316. doi: 10.1002/bip.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villar EA, et al. How proteins bind macrocycles. Nat Chem Biol. 2014;10(9):723–731. doi: 10.1038/nchembio.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monma S, et al. Verticilide: Elucidation of absolute configuration and total synthesis. Org Lett. 2006;8(24):5601–5604. doi: 10.1021/ol0623365. [DOI] [PubMed] [Google Scholar]
- 25.Shiomi K, et al. Verticilide, a new ryanodine-binding inhibitor, produced by Verticillium sp. FKI-1033. J Antibiot (Tokyo) 2010;63(2):77–82. doi: 10.1038/ja.2009.126. [DOI] [PubMed] [Google Scholar]
- 26.Shiina I, Kubota M, Oshiumi H, Hashizume M. An effective use of benzoic anhydride and its derivatives for the synthesis of carboxylic esters and lactones: A powerful and convenient mixed anhydride method promoted by basic catalysts. J Org Chem. 2004;69(6):1822–1830. doi: 10.1021/jo030367x. [DOI] [PubMed] [Google Scholar]
- 27.Shiina I, Kubota M, Ibuka R. A novel and efficient macrolactonization of ω-hydroxycarboxylic acids using 2-methyl-6-nitrobenzoic anhydride (MNBA) Tetrahedron Lett. 2002;43(42):7535. [Google Scholar]
- 28.Shiina I, Fujisawa H, Ishii T, Fukada Y. Stereoselective total synthesis of cephalosporolide D. Heterocycles. 2000;52(3):1105–1123. [Google Scholar]
- 29.Pettit GR, Hu S, Knight JC, Chapuis J-C. Antineoplastic agents. 571. Total synthesis of bacillistatin 2. J Nat Prod. 2009;72(3):372–379. doi: 10.1021/np800607x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakkar A, Trinh TB, Pei D. Global analysis of peptide cyclization efficiency. ACS Comb Sci. 2013;15(2):120–129. doi: 10.1021/co300136j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emmer G, Grassberger MA, Meingassner JG, Schulz G, Schaude M. Derivatives of a novel cyclopeptolide. 1. Synthesis, antifungal activity, and structure-activity relationships. J Med Chem. 1994;37(13):1908–1917. doi: 10.1021/jm00039a002. [DOI] [PubMed] [Google Scholar]
- 32.Li KW, Wu J, Xing W, Simon JA. Total synthesis of the antitumor depsipeptide FR-901,228. J Am Chem Soc. 1996;118(30):7237–7238. [Google Scholar]
- 33.Leighty MW, Shen B, Johnston JN. Enantioselective synthesis of α-oxy amides via Umpolung amide synthesis. J Am Chem Soc. 2012;134(37):15233–15236. doi: 10.1021/ja306225u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blay G, Domingo LR, Hernández-Olmos V, Pedro JR. New highly asymmetric Henry reaction catalyzed by Cu(II) and a C(1)-symmetric aminopyridine ligand, and its application to the synthesis of miconazole. Chemistry. 2008;14(15):4725–4730. doi: 10.1002/chem.200800069. [DOI] [PubMed] [Google Scholar]
- 35.Blay G, Hernández-Olmos V, Pedro JR. A catalytic highly enantioselective direct synthesis of 2-bromo-2-nitroalkan-1-ols through a Henry reaction. Chem Commun (Camb) 2008;39:4840–4842. doi: 10.1039/b809739a. [DOI] [PubMed] [Google Scholar]
- 36.Martí-Centelles V, Pandey MD, Burguete MI, Luis SV. Macrocyclization reactions: The importance of conformational, configurational, and template-induced preorganization. Chem Rev. 2015;115(16):8736–8834. doi: 10.1021/acs.chemrev.5b00056. [DOI] [PubMed] [Google Scholar]
- 37.Dale J, Daasvatn K. Selective preparation of cation-complexing cyclic oligoethers from ethylene oxide by a template effect. J Chem Soc Chem Commun. 1976;8:295–296. [Google Scholar]
- 38.Yep Y-h, Gao X-m, Liu M, Tang Y-c, Tian G-l. Studies on the synthetic methodology of head to tail cyclization of linear peptides. Int J Pept Res Ther. 2003;10:571–579. [Google Scholar]
- 39.Liu M, et al. Cyclization of several linear penta- and heptapeptides with different metal ions studied by CD spectroscopy. J Pept Res. 2005;65(1):55–64. doi: 10.1111/j.1399-3011.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 40.Chisholm MH, Gallucci JC, Yin H. 18-Membered cyclic esters derived from glycolide and lactide: Preparations, structures and coordination to sodium ions. Dalton Trans. 2007;42:4811–4821. doi: 10.1039/b709081a. [DOI] [PubMed] [Google Scholar]
- 41.Chisholm MH. Catalytic formation of cyclic-esters and -depsipeptides and chemical amplification by complexation with sodium ions. J Organomet Chem. 2008;693(5):808–818. [Google Scholar]
- 42.Hughes DL, Reamer RA, Bergan JJ, Grabowski EJJ. A mechanistic study of the Mitsunobu esterification reaction. J Am Chem Soc. 1988;110(19):6487–6491. [Google Scholar]
- 43.Elson KE, Jenkins ID, Loughlin WA. The Hendrickson reagent and the Mitsunobu reaction: A mechanistic study. Org Biomol Chem. 2003;1(16):2958–2965. doi: 10.1039/b305375j. [DOI] [PubMed] [Google Scholar]
- 44.But TYS, Toy PH. The Mitsunobu reaction: Origin, mechanism, improvements, and applications. Chem Asian J. 2007;2(11):1340–1355. doi: 10.1002/asia.200700182. [DOI] [PubMed] [Google Scholar]
- 45.Heitz F, Kaddari F, Heitz A, Raniriseheno H, Lazaro R. Conformations, cation binding, and transmembrane ion transfer properties of a cyclooctapeptide built by an alternation of D and L residues. Int J Pept Protein Res. 1989;34(5):387–393. doi: 10.1111/j.1399-3011.1989.tb00707.x. [DOI] [PubMed] [Google Scholar]
- 46.Pitchayawasin S, et al. Complexation of cyclic dodecadepsipeptide, cereulide with ammonium salts. Bioorg Med Chem Lett. 2003;13(20):3507–3512. doi: 10.1016/s0960-894x(03)00731-5. [DOI] [PubMed] [Google Scholar]
- 47.Kimura S, Imanishi Y. Complex formation with alkali and alkaline earth metal ions of cyclic octapeptides, cyclo(Phe-Pro)4, cyclo(Leu-Pro)4, and cyclo[Lys(Z)-Pro]4. Biopolymers. 1983;22(11):2383–2395. [Google Scholar]
- 48.Grell E, Funck T. Carbon- 13 nuclear-magnetic-resonance and infrared-absorption spectroscopy of valinomycin and its alkali-ion complexes. Eur J Biochem. 1973;34(3):415–424. doi: 10.1111/j.1432-1033.1973.tb02774.x. [DOI] [PubMed] [Google Scholar]
- 49.Patel DJ. Carbon framework of valinomycin and its metal ion complex in solution. Biochemistry. 1973;12(3):496–501. doi: 10.1021/bi00727a021. [DOI] [PubMed] [Google Scholar]
- 50.Ovchinnikov YA. Second FEBS-Ferdinand Springer lecture: Membrane active complexones. Chemistry and biological function. FEBS Lett. 1974;44(1):1–21. doi: 10.1016/0014-5793(74)80296-6. [DOI] [PubMed] [Google Scholar]
- 51.Poupon J-C, Boezio AA, Charette AB. Tetraarylphosphonium salts as solubility-control groups: Phosphonium-supported triphenylphosphine and azodicarboxylate reagents. Angew Chem Int Ed Engl. 2006;45(9):1415–1420. doi: 10.1002/anie.200503599. [DOI] [PubMed] [Google Scholar]
- 52.Evans DA, Ratz AM, Huff BE, Sheppard GS. Total synthesis of the polyether antibiotic lonomycin A (emericid) J Am Chem Soc. 1995;117(12):3448–3467. [Google Scholar]
- 53.Nicolaou KC, Sun Y-P, Guduru R, Banerji B, Chen DYK. Total synthesis of the originally proposed and revised structures of palmerolide A and isomers thereof. J Am Chem Soc. 2008;130(11):3633–3644. doi: 10.1021/ja710485n. [DOI] [PubMed] [Google Scholar]
- 54.Mukherjee S, Bordawekar S, Nere N. Investigation of variable impurity profile from a mitsunobu reaction using insights from kinetic modeling, multi-phase interactions, and computational fluid dynamics. Ind Eng Chem Res. 2016;55(17):4867–4877. [Google Scholar]
- 55.Kanaoka M, Isogai A, Suzuki A. Synthesis of bassianolide. Tetrahedron Lett. 1977;18(46):4049–4050. [Google Scholar]
- 56.Lücke D, Dalton T, Ley SV, Wilson ZE. Synthesis of natural and unnatural cyclooligomeric depsipeptides enabled by flow chemistry. Chemistry. 2016;22(12):4206–4217. doi: 10.1002/chem.201504457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naganawa H, et al. Conformational studies of destruxins, insecticidal cyclodepsipeptides. Agric Biol Chem. 1976;40(11):2223–2229. [Google Scholar]
- 58.Arndt JR, Brown RJ, Burke KA, Legleiter J, Valentine SJ. Lysine residues in the N-terminal huntingtin amphipathic α-helix play a key role in peptide aggregation. J Mass Spectrom. 2015;50(1):117–126. doi: 10.1002/jms.3504. [DOI] [PubMed] [Google Scholar]
- 59.Isogai A, Kanaoka M, Suzuki A. Bassianolide: Syntheses of its analogs and NMR studies. Pept Chem. 1979;16:165–170. [Google Scholar]
- 60.Chisholm MH, Gallucci JC, Yin H. Cyclic esters and cyclodepsipeptides derived from lactide and 2,5-morpholinediones. Proc Natl Acad Sci USA. 2006;103(42):15315–15320. doi: 10.1073/pnas.0602662103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Onodera K, Nakamura H, Oba Y, Ohizumi Y, Ojika M. Zooxanthellamide Cs: Vasoconstrictive polyhydroxylated macrolides with the largest lactone ring size from a marine dinoflagellate of Symbiodinium sp. J Am Chem Soc. 2005;127(29):10406–10411. doi: 10.1021/ja050810g. [DOI] [PubMed] [Google Scholar]
- 62.Ványolós A, et al. Gymnopeptides A and B, cyclic octadecapeptides from the mushroom Gymnopus fusipes. Org Lett. 2016;18(11):2688–2691. doi: 10.1021/acs.orglett.6b01158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.