Abstract
We analysed 12 years of species-specific emergence dates of plants at a Low-Arctic site near Kangerlussuaq, Greenland to investigate associations with sea ice dynamics, a potential contributor to local temperature variation in near-coastal tundra. Species displayed highly variable rates of phenological advance, from a maximum of −2.55 ± 0.17 and −2.93 ± 0.51 d yr−1 among a graminoid and forb, respectively, to a minimum of −0.55 ± 0.19 d yr−1 or no advance at all in the two deciduous shrub species. Monthly Arctic-wide sea ice extent was a significant predictor of emergence timing in 10 of 14 species. Despite variation in rates of advance among species, these rates were generally greatest in the earliest emerging species, for which monthly sea ice extent was also the primary predictor of emergence. Variation among species in rates of phenological advance reshuffled the phenological community, with deciduous shrubs leafing out progressively later relative to forbs and graminoids. Because early species advanced more rapidly than late species, and because rates of advance were greatest in species for which emergence phenology was associated with sea ice dynamics, accelerating sea ice decline may contribute to further divergence between early- and late-emerging species in this community.
Keywords: climate change, plant phenology, warming
1. Introduction
Advancement of the annual timing of spring events, including the onset of plant growth, is one of the most prominent and consistent signals of ecological response to climate change [1,2]. In the Arctic, sea ice decline is a major abiotic consequence of and feedback to climatic warming [3,4]. However, its potential role in terrestrial vegetation responses to climate change is difficult to assess [5] and entirely lacking in some parts of the Arctic [6]. Sea ice extent and its variation may indirectly influence variation in primary productivity and the distribution of vegetation types in coastal regions of the Arctic [7,8], but potential associations between sea ice dynamics and plant phenology remain largely untested [5].
Where examined, thinning and earlier seasonal retreat of sea ice have been associated with earlier seasonal marine phytoplankton production [9,10] and earlier tundra green-up [11]. Such assessments have not, however, yet been made at the individual species level. If associated with sea ice dynamics, species-specific phenological dynamics might contribute to changes in plant community composition in a warming Arctic.
Our analyses coupled plant species-specific and community-level phenological trends to variation in local weather and Arctic-wide sea ice extent (ASIE) in a life-history context. Individual species' mean timing of emergence relative to other species with which they co-occur may be predictive of their responses to variation in abiotic conditions that drive phenology. Our analysis used 12 years of observations of species-specific emergence phenology at a long-term, Low-Arctic field site near Kangerlussuaq, Greenland [2].
2. Methods
Our site and methods were detailed previously [2,11,12]. The site is 20 km east of Kangerlussuaq, Greenland at approximately 67°6'41.31″ N and 50°20'25.22″ W, and lies approximately 150 km inland from the Baffin Bay/Davis Strait sea ice region. Since 2002, phenology has been recorded daily or near daily between early May and late June on randomly located, circular plots measuring 0.5 m2 distributed over an area encompassing 3 km2. Sampling methodology is described in the electronic supplemental material. Phenological stages recorded were, for shrubs ‘bud burst’ = leaf buds swollen and scales opened, and for graminoids and forbs, ‘emergent’ = at least 0.50 cm green tissue at the basal meristem or first presence of newly emergent tissue. ‘Emergence’ hereinafter represents the timing of both stages.
We estimated species' rates of advance in emergence as the slope of the regression of annual dates of emergence against year. Species' mean annual timing of emergence was estimated using ANOVAs for each species with ‘emergence date’ as the dependent variable, ‘year’ as a fixed factor and ‘site’ as a random blocking variable. The grand mean of a species' mean annual dates provided an index of that species' life-history strategy along a continuum from early- to late-emerging species. Abiotic correlates of species' emergence were identified with proc linear (SPSS Statistics v. 22; electronic supplementary material) with monthly mean temperature, total precipitation and ASIE (National Snow and Ice Data Center, Boulder, CO, USA) for January–June, the period previously identified as having the strongest statistical association with community phenology at the site [11]. The rationale for including ASIE rather than local sea ice extent is provided in the electronic supplementary material. To analyse species' rates of phenological advance in relation to the strength of their association with abiotic factors, we regressed species-specific slopes of the regression of emergence dates versus ‘year’ against the standardized regression coefficient of the abiotic factor identified in proc linear as the strongest statistically significant association with emergence timing.
Community-level emergence was estimated using a sigmoidal model of the mean daily proportion of the final number of species emergent on each plot
| 2.1 |
where Y = daily mean proportion of species emergent, X = ‘day of year’, and a and b are the intercept and slope, respectively [12]. Yearly estimates of a and b were used to solve equation (2.1) for annual dates of 10% (onset), 50% (mid-point) and 90% (end of) community emergence. Abiotic factors associated with community emergence were identified using proc linear with the same abiotic predictors above for January through May (onset) and January through June (mid-point and end) of emergence [11]. Data are archived online with the US National Science Foundation's Arctic Data Center.
3. Results and discussion
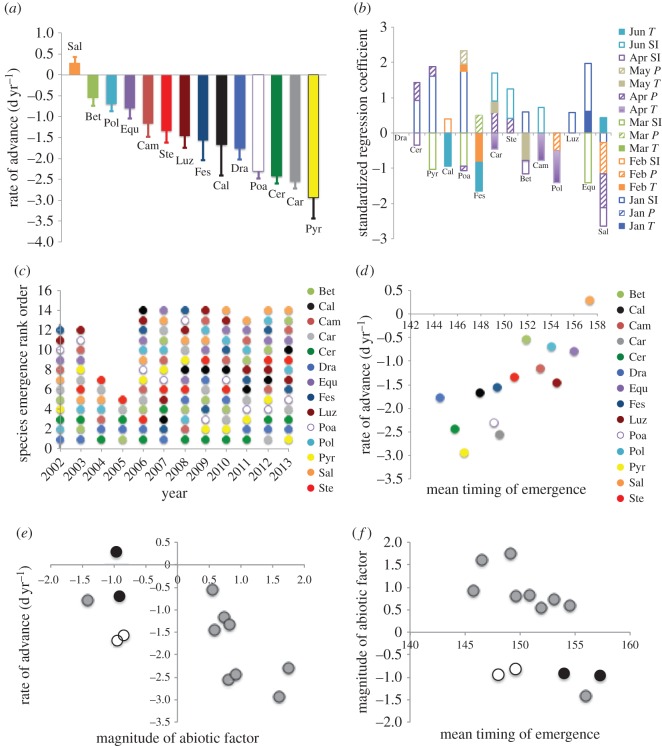
Species displayed highly individualistic trends in emergence timing (figure 1a). The two deciduous shrubs Salix glauca and Betula nana advanced not at all or at the lowest rate among all species (0.28 ± 0.21 and −0.55 ± 0.19 d yr−1, respectively; PSalix = 0.17, PBetula = 0.004), while Carex sp. and Pyrola grandiflora advanced most rapidly (−2.55 ± 0.17 and −2.93 ± 0.51 d yr−1, respectively; both p < 0.001). These latter rates are comparable to those reported for advances in flowering phenology at Zackenberg in northeast Greenland [13].
Figure 1.
(a) Rates of advance (d yr−1) in emergence phenology (2002–2013). All are significant (p < 0.05) except Sal. (b) Standardized regression coefficients of abiotic factors associated with emergence (see also electronic supplementary material, table S1). T = local mean temperature (solid bars), P = local total precipitation (hatched bars) and SI = sea ice extent (open bars). (c) Variation in species' rank order of emergence. Some species did not emerge in some years, and are thus absent in those years. (d) Relationship between rate of advance in emergence phenology and species' mean dates of emergence. (e) Relationship between species' rates of advance in emergence and the standardized regression coefficient of the primary abiotic factor associated with emergence. Open symbols are Calamagrostis sp. and Festuca sp. Grey denotes species for which sea ice extent was the primary abiotic factor. (f) Relationship between the magnitude of the primary abiotic factor associated with emergence, and species' mean dates of emergence. Open symbols and grey symbols as in panel (e). In (a–d) species abbreviations are the first three letters of genera in electronic supplementary material, table S1.
Abiotic factors associated with emergence varied among species (figure 1b; electronic supplementary material, table S1). However, the two most common were January ASIE and local April total precipitation. Sea ice extent was the primary abiotic factor associated with emergence timing in 8 of 14 species, and was a significant factor in 10 of 14 species (electronic supplementary material, table S1). In all but one species (S. glauca) for which it was significant, January ASIE positively associated with emergence, indicating earlier emergence in years with reduced sea ice extent [11]. Associations with local monthly late-winter or spring temperature, where significant, were negative, except for S. glauca (electronic supplementary material, table S1). Hence, declining ASIE and local late-winter and early-spring warming were associated with earlier emergence in 11 of 14 species.
Differential rates of advance altered the rank-order of species' emergence, re-organizing the phenological community (figure 1c). Betula nana, Polygonum viviparum, S. glauca and Stellaria longipes exhibited significant (p ≤ 0.05) positive trends in rank-order, assuming later positions in the emergence sequence. Such re-shuffling of the phenological community may eventually alter species interactions and community composition if more rapid advancement confers a competitive advantage for seasonally limited resources [14]. Also, ecosystem carbon dynamics could be altered if the relative abundance of graminoids versus deciduous shrubs is affected [15].
Life history predicted species' rates of phenological advance: early species advanced more than later species (figure 1d) (R2 = 0.63, p = 0.001). A similar relationship was documented across 66 phenophases over 30 years at the Poznań Botanical Garden in Poland [16]. Excluding the two species with the fewest years of data in our record, Calamagrostis sp. and Festuca sp. (see electronic supplementary material), did not alter this relationship (R2 = 0.64, p = 0.002).
The magnitude (linear coefficient) of each species' primary abiotic predictor of emergence scaled with its rate of phenological advance (r = −0.66, p = 0.02) (figure 1e). Excluding Calamagrostis sp. and Festuca sp. improved this relationship (r = −0.78, p = 0.005). The four species exhibiting the greatest rates of advance in emergence were associated primarily with monthly ASIE (figure 1e). The magnitude of the primary abiotic factor associated with species' emergence timing was greater for early than for late species when Calamagrostis sp. and Festuca sp. were excluded (figure 1f) (r = −0.81, p = 0.003).
Onset and progression of community emergence varied among years (figure 2a). The onset of community emergence advanced significantly by −2.36 ± 0.66 d yr−1 (R2 = 0.57, p = 0.005) (figure 2b), and in association with increasing April temperature (standardized beta = −0.76, p = 0.02). The mid-point of community emergence advanced −1.61 ± 0.45 d yr−1 (R2 = 0.56, p = 0.005), and in association with declining June ASIE (standardized beta = 0.73, p = 0.002). The end of community emergence did not advance significantly (p = 0.07), ostensibly because while it was positively associated with June ASIE (standardized beta = 0.92, p < 0.001), it was also positively associated with March temperature (standardized beta = 0.53, p = 0.007), and February precipitation (standardized beta = 0.44, p = 0.02) (total R2 = 0.86, p = 0.001). Hence, the onset of community emergence advanced with local spring warming while the end of community emergence was prolonged with local spring warming.
Figure 2.
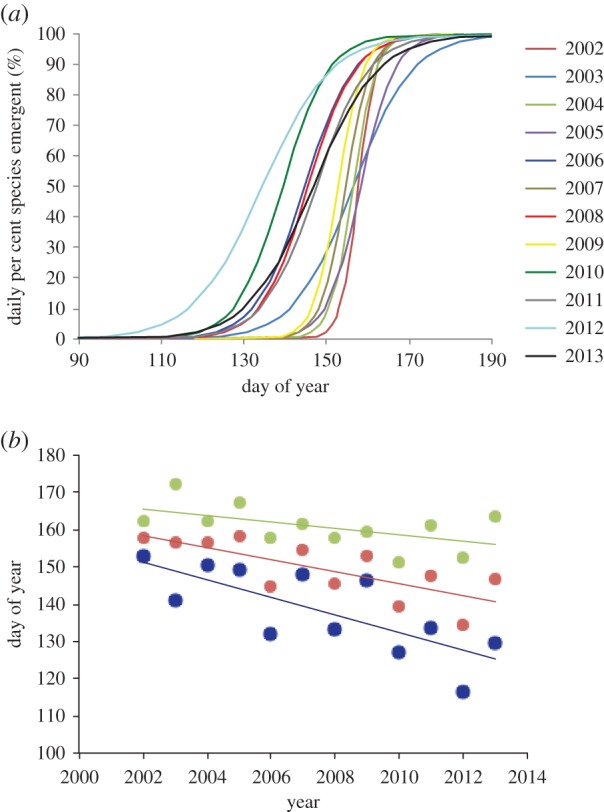
(a) Phenological progression during each year expressed as daily percentages of the total number of species emergent across all plots. (b) Annual dates of onset (10% emergence, blue), mid-point (50% emergence, red) and end (90% emergence, green) of community-level emergence (2002–2013).
Ascribing causality to associations between sea ice dynamics and terrestrial ecological dynamics is a major challenge [5], and impossible on the basis of correlations [11]. However, declining sea ice extent may contribute to local warming in some parts of the Arctic either by promoting greater heat flux from the ocean to the atmosphere [3,4,17], especially during winter months [17], or by altering sea-level pressure and atmospheric circulation patterns, producing negative North Atlantic Oscillation-like conditions over sea and land [18]. While we cannot address such mechanisms, analyses of temperature data from our site appear consistent with the hypothesis of local warming associated with sea ice decline. June temperature at our site is significantly negatively correlated with ASIE in each of the months from January through June (electronic supplementary material, table S2). Additionally, local monthly temperatures are significantly correlated at one- to two-month lags from January through June (electronic supplementary material, table S3). Hence, warming in winter months at the site is followed by warming in spring and early summer months. Finally, the number of growing days at the site, i.e. the number of days when temperature equalled or exceeded 10°C, increased significantly with May and June warming (electronic supplementary material, table S4). We regard these correlations as consistent with the hypothesis that declining sea ice extent contributes to local warming, which, in turn, results in more numerous early-spring days favourable for plant growth, promoting advanced phenology. While local monthly precipitation at our site does not correlate with sea ice extent from January to June (all p > 0.05), local precipitation in April–June increases with local temperature from February to April (electronic supplementary material, table S5).
Our analyses reveal three important insights. First, species respond highly individualistically to abiotic drivers [19]. Although variation in ASIE was most commonly associated with variation in emergence, significant associations also occurred with precipitation in four months and temperature in five months. Hence, an exclusive focus on any single environmental factor may compromise understanding of plant phenological responses to climate change [20].
Second, as noted previously [19], species' individualistic life-history strategies are strong predictors of their responsiveness to abiotic drivers of phenology and rates of phenological advance. Regardless of the abiotic factor involved, early species were more responsive to these factors than late species, and underwent more rapid rates of advance. This may, ultimately, have consequences for community composition and species interactions.
Finally, our results concur with the prediction that earlier-emerging species should segregate from later emerging species through more rapid advancement of the former compared with the latter under warming [21]. In near-coastal arctic plant communities, such divergence may be promoted by differential responsiveness to local abiotic changes associated with sea ice dynamics.
Supplementary Material
Acknowledgements
We thank CH2MHILL for logistical support, the US NSF for funding, and anonymous referees for constructive criticism. For field assistance, we thank Jesper Bahrenscheer, Pernille Bøving, Lorelei Carroll, Alison Donnelly, Toke Høye, Christian John, Syrena Johnson, Megan MacArthur, Ellorie McKnight, Mason Post, Taylor Rees and Henning Thing.
Data accessibility
Data are available online at NSF's Arctic Data Center (https://arcticdata.io).
Authors' contributions
All authors contributed equally to the conception and design of the paper. E.P., J.K. and C.P. collected and analysed the data, and all authors contributed to the interpretation of the analyses. All authors contributed to drafting and revising the manuscript, and approve of the final version for publication. All authors agree to be accountable for all aspects of the work.
Competing interests
The authors declare they have no competing interests.
Funding
This study was funded by US National Science Foundation (PLR0713917, PLR0902125, PLR1107381 and PLR1525636).
References
- 1.Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD. 2007. Shifting plant phenology in response to global change. Trends Ecol. Evol. 22, 357–365. ( 10.1016/j.tree.2007.04.003). [DOI] [PubMed] [Google Scholar]
- 2.Post E. 2013. Ecology of climate change: the importance of biotic interactions. Monogr. Pop. Biol., no. 52, Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Screen JA, Deser C, Simmonds I, Tomas R. 2014. Atmospheric impacts of Arctic sea-ice loss, 1979–2009: separating forced change from atmospheric internal variability. Clim. Dyn. 43, 333–344. ( 10.1007/s00382-013-1830-9) [DOI] [Google Scholar]
- 4.Vihma T. 2014. Effects of Arctic sea ice decline on weather and climate: a review. Surv. Geophys. 35, 1175–1214. ( 10.1007/s10712-014-9284-0) [DOI] [Google Scholar]
- 5.Post E, et al. 2013. Ecological consequences of sea ice decline. Science 6145, 519–524. ( 10.1126/science.1235225) [DOI] [PubMed] [Google Scholar]
- 6.Macias-Fauria M, Forbes BC, Zetterberg P, Kumpula T. 2012. Eurasian Arctic greening reveals teleconnections and the potential for structurally novel ecosystems. Nat. Clim. Change 2, 613–618. ( 10.1038/nclimate1558) [DOI] [Google Scholar]
- 7.Walker DA, Bhatt US, Epstein HE, Bieniek PA, Comiso JC, Frost GV, Pinzon J, Raynolds MK, Tucker CJ. 2012. Changing Arctic tundra vegetation biomass and greenness. Bull. Am. Meteorol. Soc. 93, S138–S139. ( 10.1175/2012BAMSStateoftheClimate.1) [DOI] [Google Scholar]
- 8.Bhatt US, et al. 2014. Implications of arctic sea ice decline for the Earth system. Annu. Rev. Environ. Resour. 39, 1211–12.33. ( 10.1146/annurev-environ-122012-094357) [DOI] [Google Scholar]
- 9.Arrigo KR, van Dijken G, Pabi S. 2008. Impact of a shrinking Arctic ice cover on marine primary production. Geophys. Res. Lett. 35, L19603 ( 10.1029/2008gl035028) [DOI] [Google Scholar]
- 10.Ji RB, Jin MB, Varpe O. 2013. Sea ice phenology and timing of primary production pulses in the Arctic Ocean. Glob. Change Biol. 19, 734–741. ( 10.1111/gcb.12074) [DOI] [PubMed] [Google Scholar]
- 11.Kerby JT, Post E. 2013. Advancing plant phenology and reduced herbivore production in a terrestrial system associated with sea ice decline. Nat. Commun. 4, 2514 ( 10.1038/ncomms3514) [DOI] [PubMed] [Google Scholar]
- 12.Post E, Forchhammer MC. 2008. Climate change reduces reproductive success of an arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B 363, 2369–2375. ( 10.1098/rstb.2007.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Høye TT, Post E, Meltofte H, Schmidt NM, Forchhammer MC. 2007. Rapid advancement of spring in the High Arctic. Curr. Biol. 17, R449–R451. ( 10.1016/j.cub.2007.04.047) [DOI] [PubMed] [Google Scholar]
- 14.Ernakovich JG, Hopping KA, Berdanier AB, Simpson RT, Kachergis EJ, Steltzer H, Wallenstein MD. 2014. Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob. Change Biol. 20, 3256–3269. ( 10.1111/gcb.12568) [DOI] [PubMed] [Google Scholar]
- 15.Cahoon SMP, Sullivan PF, Post E. 2016. Carbon and water relations of contrasting Arctic plants: implications for shrub expansion in West Greenland. Ecosphere 7, e01245 ( 10.1002/ecs2.1245) [DOI] [Google Scholar]
- 16.Sparks TH, Gorska-Zajaczkowska M, Wojtowicz W, Tryjanowski P. 2011. Phenological changes and reduced seasonal synchrony in western Poland. Int. J. Biometeorol. 55, 447–453. ( 10.1007/s00484-010-0355-8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Screen JA, Simmonds I. 2010. Increasing fall-winter energy loss from the Arctic Ocean and its role in Arctic temperature amplification. Geophys. Res. Lett. 37, 1–5. ( 10.1029/2010GL044136) [DOI] [Google Scholar]
- 18.Koenigk T, Caian M, Nikulin G, Schimanke S. 2016. Regional Arctic sea ice variations as predictor for winter climate conditions. Clim. Dyn. 46, 317–337. ( 10.1007/s00382-015-2586-1) [DOI] [Google Scholar]
- 19.Ovaskainen O, Skorokhodova S, Yakovleva M, Sukhov A, Kutenkov A, Kutenkova N, Shcherbakov A, Meyke E, Delgado MD. 2013. Community-level phenological response to climate change. Proc. Natl Acad. Sci. USA 110, 13 434–13 439. ( 10.1073/pnas.1305533110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reyes-Fox M, Steltzer H, Trlica MJ, McMaster GS, Andales AA, LeCain DR, Morgan JA. 2014. Elevated CO2 further lengthens growing season under warming conditions. Nature 510, 259–262. ( 10.1038/nature13207) [DOI] [PubMed] [Google Scholar]
- 21.Steltzer H, Post E. 2009. Seasons and life cycles. Science 324, 886–887. ( 10.1126/science.1171542) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available online at NSF's Arctic Data Center (https://arcticdata.io).