Abstract
In some eusocial insect societies, adaptation to the division of labour results in multimodal size variation among workers. It has been suggested that variation in size and growth among non-breeders in naked and Damaraland mole-rats may similarly reflect functional divergence associated with different cooperative tasks. However, it is unclear whether individual growth rates are multimodally distributed (as would be expected if variation in growth is associated with specialization for different tasks) or whether variation in growth is unimodally distributed, and is related to differences in the social and physical environment (as would be predicted if there are individual differences in growth but no discrete differences in developmental pathways). Here, we show that growth trajectories of non-breeding Damaraland mole-rats vary widely, and that their distribution is unimodal, contrary to the suggestion that variation in growth is the result of differentiation into discrete castes. Though there is no evidence of discrete variation in growth, social factors appear to exert important effects on growth rates and age-specific size, which are both reduced in large social groups.
Keywords: growth, division of labour, cooperative breeding, eusociality
1. Introduction
In most social vertebrates, the growth of individuals is affected by variation in the physical and social environment, and variation in age-specific size and growth is unimodally distributed across individuals [1,2]. Mole-rat societies have been suggested to resemble those of eusocial insects more than those of other vertebrates [3–5] and, like some eusocial insects, may show variation of growth and body mass that is associated with consistent differences in cooperative behaviour [3,6]. Recent studies have revealed the presence of unusually large variation in growth and age-specific size in both naked (Heterocephalus glaber) and Damaraland mole-rats (Fukomys damarensis) [7,8], but it is not yet known whether size is multimodally distributed, as would be expected if variation in growth represents functional specialization, or whether it is unimodally distributed, and reflects the effects of variation in physical and social environments on the growth of individuals.
Here, we describe the distribution in growth patterns in a population of 171 laboratory-born Damaraland mole-rats housed in groups in artificial tunnel systems. We first develop a Gompertz growth model from which we derive, for each individual, their predicted maximum body mass, the growth rate at the inflection point of their growth curve and a displacement factor. Subsequently, we use the function to estimate the age at which individuals reach 90% of their maximum body mass and the mass at the age of 1 year. If mole-rat growth resembles other cooperatively breeding vertebrates and less specialized social insects, we would expect the distributions of values extracted from the growth function to be unimodally distributed while if it was more similar to highly specialized eusocial insects, some parameters should show different modalities [9,10].
2. Material and methods
(a). Study animals and husbandry
The Damaraland mole-rat is a highly social rodent which occurs in groups containing a reproductive pair and a number of non-breeding animals of both sexes [11]. Recent evidence suggests that groups exhibit an age-based polyethism with faster growing individuals contributing more to cooperative tasks and that behavioural phenotypes are continuously distributed across non-breeding individuals [12,13].
The animals in this study were the offspring of wild caught Damaraland mole-rats, which were born and reared in captivity in a laboratory facility at the Kuruman River Reserve, South Africa. They remained in their natal group and carried a PIT-tag for identification. Depending on group size, total tunnel length of the polyvinyl chloride cages varied between 4 and 16 m. Twice daily groups were fed ad libitum with sweet potatoes and cucumbers as well as given clean sand (cf. [12])
(b). Data collection and analysis
Data were collected between October 2013 and July 2016. Individuals were of known age, being weighed weekly until the age of 90 days and fortnightly thereafter, using a Sartorius TE4100 electronic scale. We excluded individuals that died before the age of 1 year.
We fitted a Gompertz growth curve for each individual using the parametrization (as per ‘SSGompertz’ in the nlme package [14,15]):
| 2.1 |
where y(t) is the body mass at age t, a is the asymptotic body mass (maximum body mass), b is a displacement factor that controls the displacement along the x-axis (the inflection point of the growth curve occurs at t = −ln(b)/ln(c)) and c controls the relative maximum rate of growth (the maximum growth rate is –a × e−1 × ln(c), at the inflection point). From the fitted model, we also calculated the predicted body mass at 1 year of age and the latency to reach 90% of the maximum body mass. In total, our dataset included 14 211 weight records across 181 individuals. After excluding 10 individuals where secondary growth spurts produced estimates of growth parameter predictions outside the range known for this species, all models include 171 individuals from 87 litters born in 37 groups.
We tested for sex differences in growth by fitting linear mixed models (LMMs) with body mass at the age of 90, 180 and 365 days as response, sex as fixed effect, and litter and group identity as random effects. Subsequently, all analyses were conducted separately for each sex.
To evaluate whether the distribution of growth patterns among subordinate mole-rats represented unimodal distributions, or whether they were likely to result from a sample with bi- or multimodal distribution, we tested each of the five aforementioned growth-related values for multimodality using Hartigan's dip test implemented in the package ‘diptest’ [16]. To analyse how body mass at the age of 1 year, maximum body mass and latency to reach 90% of the maximum body mass are predicted by the social environment, we fitted each as a response in LMMs with Gaussian error structure and fitted mean group size, litter sex ratio, litter succession number (i.e. being first, second or third litter born to this females) and litter size (total number of individuals in this litter at birth) as covariates. Group and litter identity were set as random terms. We employed stepwise, backward model simplification until only significant terms remained in the final model. Terms dropped in the course of model selection are presented with the estimates, standard errors and the p-values with which they were last included in the model selection process. All analyses were conducted using R and the package lme4 [17].
3. Results
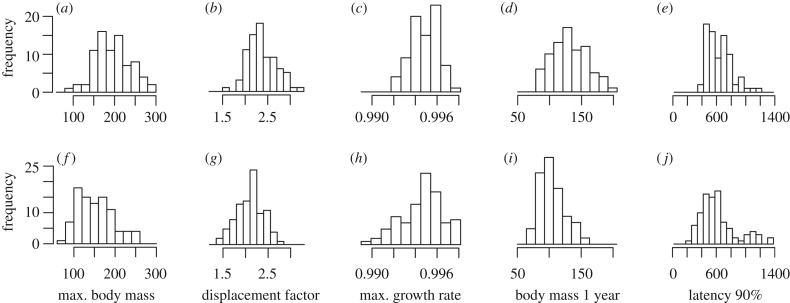
As in previous studies of Damaraland mole-rats, growth varied widely between individuals. Males were larger than females throughout ontogeny (LMM, mass at age 90, 180 and 365 days: estimate = 2.9, s.e. = 0.69, p < 0.001; estimate = 9.14, s.e. = 1.5, p < 0.001; estimate= 25.3, s.e. = 2.9, p < 0.001, respectively) and achieved higher predicted maximum body masses (LMM estimate = 46.9, s.e. = 5.9, p < 0.001; figure 1), but needed longer to reach maximum values (LMM, latency to reach 90% of maximum body mass, estimate = 0.1, s.e. = 0.04, p = 0.02). The distributions of maximum body mass, maximum growth rate and the displacement factor of the individual growth curves in the population were unimodally distributed in both sexes (figure 2; Hartigan's dip test, maximum body mass: females, D = 0.02, p = 0.99, males, D = 0.04, p = 0.71; displacement factor: females, D = 0.03, p = 0.95, males, D = 0.03, p = 0.99; maximum growth rate at inflection point: females, D = 0.03, p = 0.84, males, D = 0.04, p = 0.32). Neither the estimates of body mass at 1 year nor the estimates of latency to reach 90% of the maximum body mass appeared to originate from a bi- or multimodal distribution (figure 2; Hartigan's dip test, mass at the age of 1 year: females, D = 0.03, p = 0.76, males, D = 0.02, p = 0.99; latency to reach 90% of maximum body mass: females, D = 0.03, p = 0.85, males, D = 0.05, p = 0.2).
Figure 1.
Growth trajectories as projected by the Gompertz model for 171 subordinate mole-rats of our study population split into (a) females (N = 92) and (b) males (N = 79). (c) Illustrates the mean maximum body mass difference between males and females.
Figure 2.
Distribution of growth parameters in males (N = 79, panels (a–e)) and in females (N = 92, (f–j)), including data from 171 individuals.
Individuals in larger groups exhibited slower growth rates. In large groups, the body mass of males and females at the age of 1 year was lower than in small groups (table 1). Males reached lower predicted maximum body mass in large groups whereas predicted maximum body mass was independent of group size in females (table 1). Additionally, males needed more time to reach 90% of their maximum body mass when they were born into a late litter (produced by a female that raised many litters before), while this effect was absent in females (table 1).
Table 1.
Social factors explaining growth components in Damaraland mole-rats. Summarized are LMMs with Gaussian error structure including litter and group identity as random factors. Sample size is N = 92 for females and N = 79 for males. Terms in italics represent the minimal model.
| estimate | s.e. | p-values | |
|---|---|---|---|
| body mass at 1 year: females | |||
| intercept | 4.80 | 0.06 | |
| group size | −0.02 | 0.007 | 0.01 |
| litter size | −0.01 | 0.01 | 0.62 |
| litter succession | −0.02 | 0.02 | 0.25 |
| sex ratio (litter) | −0.11 | 0.07 | 0.12 |
| body mass at 1 year: males | |||
| intercept | 5.19 | 0.13 | |
| group size | −0.03 | 0.01 | <0.001 |
| litter size | −0.03 | 0.02 | 0.27 |
| litter succession | −0.03 | 0.02 | 0.19 |
| sex ratio (litter) | −0.03 | 0.09 | 0.77 |
| predicted maximum body mass: females | |||
| intercept | 144.36 | 9.99 | |
| group size | 0.32 | 1.74 | 0.87 |
| litter size | −0.16 | 4.36 | 0.98 |
| litter succession | 2.89 | 4.19 | 0.50 |
| sex ratio (litter) | −0.67 | 18.48 | 0.95 |
| predicted maximum body mass: males | |||
| intercept | 233.9 | 16.48 | |
| group size | −4.17 | 1.78 | 0.02 |
| litter size | −5.12 | 5.4 | 0.35 |
| litter succession | 0.27 | 4.14 | 0.94 |
| sex ratio (litter) | −11.55 | 16.03 | 0.45 |
| latency to maximum body mass: females | |||
| intercept | 6.13 | 0.14 | |
| group size | 0.02 | 0.01 | 0.11 |
| litter size | −0.01 | 0.04 | 0.80 |
| litter succession | 0.01 | 0.04 | 0.77 |
| sex ratio (litter) | 0.14 | 0.14 | 0.36 |
| latency to maximum body mass: males | |||
| intercept | 6.34 | 0.58 | |
| group size | −0.003 | 0.01 | 0.83 |
| litter size | 0.001 | 0.03 | 0.96 |
| litter succession | 0.5 | 0.02 | 0.04 |
| sex ratio (litter) | −0.08 | 0.09 | 0.39 |
4. Discussion
Although growth trajectories in Damaraland mole-rat populations vary widely among individuals, our results suggest that individual variation in growth is unimodally distributed and differences may be caused by growth reductions resulting from competition with other members of the group. Despite superficial similarities with eusocial insects, mole-rats do not appear to exhibit discrete growth trajectories that predispose them to their role later in life, or preclude the expression of particular life-history trajectories, as in some of the more specialized eusocial insects [9,10,18]. Variation in growth in Damaraland mole-rats appears to resemble that in other cooperatively breeding vertebrates and eusocial insect species where specialization of workers does not result in discrete body size polymorphism. In vertebrates, division of labour is rare and individual differences in behaviour often result from age-related variation rather than from specialization of individuals to fixed roles [12,19].
In line with previous research, Damaraland mole-rats in larger groups grew more slowly, and mothers that had previously raised many litters produced males that needed longer to reach maximum body mass [7,8]. We found no evidence that sex ratio at birth or litter size has long lasting effects on growth. This suggests that competition among subordinates in large groups may be the major social factor reducing growth, while direct resource availability (our study population receives ad libitum food) and interactions with the dominant breeders are unlikely to generate the observed growth patterns. This contrasts with the situation in some cooperative vertebrates where interactions with breeders or more dominant individuals inhibit growth [20] and group size positively influences growth rates [2].
Like the males of many other polygynous and polygynandrous mammals, male Damaraland mole-rats grew faster, achieved higher maximum body masses and needed more time to reach maximum body mass than females. Additionally, male maximum body mass was lower in large groups, which was not the case in females. Although those characteristics are common among mammals [1], they are unusual for cooperatively breeding species, such as mole-rats and meerkats, where intense competition among females leads to longer periods of growth and to secondary growth spurts in females [21–24]. This may suggest that patterns of intra-sexual competition in Damaraland mole-rats are more similar to those in conventional mammals where males are the more competitive (and larger) sex than to those in other cooperatively breeding species where females are the more competitive sex [21–23].
Acknowledgements
We thank Philippe Vullioud, Rute Mendonça, Adam Mitchell, Katy Goddard and all volunteers for help with the data collection, and Andrew Bateman, Arik Kershenbaum, Dominic Cram, Nichola Raihani and Philippe Vullioud for helpful comments and discussions. We thank the Kalahari Research Trust for access to the research facilities and Marta Manser for her contribution to maintaining the Kalahari Research Centre. We are grateful to the Northern Cape Department of Environment and Nature Conservation for permission to conduct research in the Northern Cape.
Ethics
The study has been approved by the ethics committee of the University of Pretoria (permits EC-089-12, EC-009-13 and SOP-004-13) and by Northern Cape Department of Environment and Nature Conservation.
Data accessibility
The data are available in Dryad Digital Repository [25].
Authors' contributions
M.Z. and T.C.-B. conceived the idea. All authors contributed to the organization of the study and/or the data collection. M.Z. and J.T. analysed the data. M.Z. drafted the manuscript and all authors contributed to the final version of the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This study was funded by a European Research Council grant to T.C.-B. (294494).
References
- 1.Clutton-Brock T. 2016. Mammal societies. New York, NY: John Wiley & Sons. [Google Scholar]
- 2.English S, Bateman AW, Mares R, Ozgul A, Clutton-Brock TH. 2014. Maternal, social and abiotic environmental effects on growth vary across life stages in a cooperative mammal. J. Anim. Ecol. 83, 332–342. ( 10.1111/1365-2656.12149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarvis JU. 1981. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science 212, 571–573. ( 10.1126/science.7209555) [DOI] [PubMed] [Google Scholar]
- 4.O'Riain M, Jarvis J, Alexander R, Buffenstein R, Peeters C. 2000. Morphological castes in a vertebrate. Proc. Natl Acad. Sci. USA 97, 13 194–13 917. ( 10.1073/pnas.97.24.13194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Riain MJ, Jarvis JUM, Faulkes CG. 1996. A dispersive morph in the naked mole-rat. Nature 380, 619–621. ( 10.1038/380619a0) [DOI] [PubMed] [Google Scholar]
- 6.Scantlebury M, Speakman J, Oosthuizen M, Roper T, Bennett N. 2006. Energetics reveals physiologically distinct castes in a eusocial mammal. Nature 440, 795–797. ( 10.1038/nature04803) [DOI] [PubMed] [Google Scholar]
- 7.Bennett N, Navarro R. 1997. Differential growth patterns between successive litters of the eusocial Damaraland mole-rat, Cryptomys damarensis, from Namibia. J. Zool. Lond. 241, 465–473. ( 10.1111/j.1469-7998.1997.tb04838.x) [DOI] [Google Scholar]
- 8.Young AJ, Jarvis JU, Barnaville J, Bennett NC. 2015. Workforce effects and the evolution of complex sociality in wild Damaraland mole rats. Am. Nat. 186, 302–311. ( 10.1086/682048) [DOI] [PubMed] [Google Scholar]
- 9.Gouws EJ, Gaston KJ, Chown SL. 2011. Intraspecific body size frequency distributions of insects. PLoS ONE 6, e16606 ( 10.1371/journal.pone.0016606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson EO. 1953. The origin and evolution of polymorphism in ants. Q. Rev. Biol. 28, 136–156. ( 10.1086/399512) [DOI] [PubMed] [Google Scholar]
- 11.Bennett NC, Jarvis JU. 1988. The social structure and reproductive biology of colonies of the mole-rat, Cryptomys damarensis (Rodentia, Bathyergidae). J. Mammal. 69, 293–302. ( 10.2307/1381379) [DOI] [Google Scholar]
- 12.Zöttl M, Vullioud P, Mendonça R, Ticó MT, Gaynor D, Mitchell A, Clutton-Brock T. 2016. Differences in cooperative behavior among Damaraland mole rats are consequences of an age-related polyethism. Proc. Natl Acad. Sci. USA 113, 10 382–10 387. ( 10.1073/pnas.1607885113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Šklíba J, Lövy M, Burda H, Šumbera R. In press. Variability of space-use patterns in a free living eusocial rodent, Ansell's mole-rat indicates age-based rather than caste polyethism. Sci. Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Development Team. 2012. nlme: linear and nonlinear mixed effects models. R package version 3.1-104 https://cran.r-project.org/web/packages/nlme/index.html (accessed 26 March 2016).
- 15.Begall S. 1996. The application of the Gompertz model to describe body growth. Growth Dev. Aging 61, 61–67. [PubMed] [Google Scholar]
- 16.Maechler M. 2015. Hartigan's dip test statistic for unimodality. See https://cran.r-project.org/web/packages/diptest/index.html: CRAN (accessed 28 March 2016).
- 17.Bates D, Maechler M, Bollinge B. 2011. lme4: linear mixed-effects models using S4 classes. R package v. 0.999375-42 ed https://cran.r-project.org/web/packages/lme4/index.html (accessed 26 March 2016).
- 18.Wheeler DE. 1991. The developmental basis of worker caste polymorphism in ants. Am. Nat. 138, 1218–1238. ( 10.1086/285279) [DOI] [Google Scholar]
- 19.Clutton-Brock TH, Russell AF, Sharpe LL. 2003. Meerkat helpers do not specialize in particular activities. Anim. Behav. 66, 531–540. ( 10.1006/anbe.2003.2209) [DOI] [Google Scholar]
- 20.Heg D, Bender N, Hamilton I. 2004. Strategic growth decisions in helper cichlids. Proc. R. Soc. Lond. B 271, S505–S508. ( 10.1098/rsbl.2004.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauber ME, Lacey EA. 2005. Bateman's principle in cooperatively breeding vertebrates: the effects of non-breeding alloparents on variability in female and male reproductive success. Integr. Comp. Biol. 45, 903–914. ( 10.1093/icb/45.5.903) [DOI] [PubMed] [Google Scholar]
- 22.Clutton-Brock TH, Hodge SJ, Spong G, Russell AF, Jordan NR, Bennett NC, Sharpe LL, Manser MB. 2006. Intrasexual competition and sexual selection in cooperative mammals. Nature 444, 1065–1068. ( 10.1038/nature05386) [DOI] [PubMed] [Google Scholar]
- 23.Young AJ, Bennett NC. 2013. Intra-sexual selection in cooperative mammals and birds: why are females not bigger and better armed? Phil. Trans. R. Soc. B 368, 20130075 ( 10.1098/rstb.2013.0075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huchard E, English S, Bell MB, Thavarajah N, Clutton-Brock T. 2016. Competitive growth in a cooperative mammal. Nature 533, 532–534. ( 10.1038/nature17986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zöttl M, Thorley J, Gaynor D, Bennett NC, Clutton-Brock T.2016. Data from: Variation in growth of Damaraland mole-rats is explained by competition rather than by functional specialisation for different tasks. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available in Dryad Digital Repository [25].